Abstract
Fragments of hyaluronan released after injury bind and activate TLR4 in a complex with CD44. Here we investigated if the recognition of hyaluronan by CD44 and TLR4 alters lipopolysaccaride (LPS) responsiveness and thus could alter the septic response. In contrast to mice injected with LPS, mice exposed to hyaluronan prior to LPS had greatly decreased serum IL-6 and TNFα and were protected from symptoms of sepsis. The protective effect of HA was not seen in Cd44−/− mice. Consistent with our findings in vivo, addition of hyaluronan to macrophages before LPS exposure significantly decreased the release of IL-6 and TNFα and this effect was not seen in macrophages from Cd44−/− mice. Investigation of the mechanism responsible for inhibition of LPS activation showed hyaluronan treatment resulted in an increase in peritoneal macrophage A20 mRNA expression, and that this was significantly reduced in macrophages from Cd44−/− mice and Tlr4 −/− mice. Suppression of the A20 response with siRNA inhibited the ability of hyaluronan to protect against the cytokine response to LPS. Therefore, our results show that hyaluronan acts through TLR4, CD44 and A20 to stimulate a unique cellular response that can protect against the septic response to LPS.
Keywords: Hyaluronan, CD44, Toll-like receptor-4, endotoxic shock
1. Introduction
Septic shock is characterized by tissue and organ damage resulting from high production of cytokines and low molecular weight mediators in response to large amounts of Gram-negative bacteria or purified LPS (Cook, 1998). LPS is a major component of the Gram-negative bacteria cell wall and is a potent stimulus for recognition by the host innate immune system. LPS binds to and signals through a receptor complex, which consists of TLR4, CD14, and MD2 (Kawai et al., 1999; Poltorak et al., 1998). Activation of TLR4 promotes NF-κB-mediated production of proinflammatory cytokines in many cell types (Akira and Takeda, 2004). Prior exposure to LPS both in vitro and in vivo can lead to desensitization of immune cells to subsequent challenge with LPS, a phenomenon that has been referred to as ‘endotoxin tolerance.’
Progress has been made in understanding mechanisms involved in the negative regulation of TLR signaling (Liew et al., 2005), an essential event through which the host attenuates TLR signaling to avoid detrimental effects of an excess inflammatory response. Several intracellular proteins including TNFα-induced protein 3 (TNFAIP3/A20) (Boone et al., 2004; Lee et al., 2000) have been identified as negative regulators of TLR signaling. A20-deficient mice develop severe inflammation and cachexia, are hypersensitive to both LPS and TNF, and die due to the failure to regulate TNF-induced NF-κB activation (Boone et al., 2004; Lee et al., 2000). Furthermore, the ability of heterologous A20 expression to inhibit LPS-induced NF-κB activity suggests that endogenous A20 may be important for the termination of LPS signals (Cooper et al., 1996). However, the endogenous molecules responsible for A20 activation and subsequent suppression of TLR4 activation remain unknown.
Recent observations have shown that fragments of hyaluronan (HA) can serve as an endogenous trigger of TLR signaling and are able to activate innate immune defense, promoting the production of cytokines by a variety of cell types (Jiang et al., 2005; Termeer et al., 2002). Normally, HA is a high molecular mass (~107 Da) linear polysaccharide composed of repeating disaccharide units of β1–4 linked D-glucuronic acid (GlcA) and β1–3 linked N-acetyl-D-glucosamine (GlcNAc) residues (4GlcAβ1-3GlcNAc1β) (Kogan et al., 2007). HA is a major component of the extracellular matrix (Matsuno et al., 2008) and is thought to be involved in maintaining tissue hydration, the distribution and transport of plasma proteins, and in maintaining an intact matrix structure. Fragments of HA are generated during inflammation or injury through the activity of oxygen radicals or via enzymatic activity by hyaluronidase, β-glucuronidase, and hexosaminidase (Laurent et al., 1995). CD44, a receptor for HA, is involved in HA-induced cytokine release by forming a TLR4-CD44 complex (Taylor et al., 2007). In addition, there is an increasing body of evidence that CD44 is a negative regulator of TLR2- and TLR4-mediated inflammation (Kawana et al., 2008; Liang et al., 2007).
Some molecules that do not share structural homology with LPS can elicit a tolerant phenotype to LPS; a phenomenon that has been referred to as ‘cross-tolerance’ or ‘heterotolerance’. IL-1β, a mycoplasma lipopeptide (macrophage-activating lipopeptide-2; MALP-2) and lipoteichoic acid (LTA) have this ‘cross-tolerant’ capacity (Lehner et al., 2001; Medvedev et al., 2000; Sato et al., 2000). In this study, we show for the first time that HA and CD44 trigger alternate innate TLR4 signaling events to initiate a distinct response from LPS. These findings describe a previously unrecognized role for HA in protecting against unopposed LPS signaling.
2. Materials and methods
2.1. Reagents, cells, cell lines, mice
The mouse alveolar macrophage cell line MH-S was purchased from American Type Culture Collection (ATCC, catalog CRL-2019). Cells were maintained in RPMI1640 media supplemented with L-glutamine, 10% heat-inactivated fetal calf serum (FCS), penicillin/streptomycin (100 units/ml and 50 mg/ml, respectively) and 50 μM 2-Mercaptoethanol. HA was prepared by the method previously described (Taylor et al., 2004). Human umbilical cord HA was purchased from Sigma-Aldrich (St. Louis, MO) and contains at wide range of sizes up to 500 kDa based on HPLC analysis. HA preparations were free of DNA and protein contamination as preparations showed no absorbance at 260 nm and 280 nm. In addition, to ensure purity, 1 ml HA batches were boiled for one hour then run on two successive endotoxin-removal columns (Associates of Cape Cod Inc., East Falmouth, MA) to remove potential endotoxin contamination. LPS (from E. coli K12 D31m4) was obtained from LIST Biologics, Inc. (Campbell, CA). Tlr4−/− (C3H/HeJ), Tlr4+/+ controls, CD44-deficient mice (Cd44−/−), C57Bl/6J and Balb/c were all purchased from Jackson Laboratories. TRIF-deficient mice (Trif−/−) were originally generated by Yamamoto et al (Yamamoto et al., 2003). MyD88-deficient mice (Myd88−/−) were originally generated by Adachi et al.(Adachi et al., 1998) and were crossed >6 times onto a C57Bl/6 background. Cd44−/− mice lack all known CD44 isoforms and manifest no overt developmental phenotype (Schmits et al., 1997; Teder et al., 2002). Eight- to ten-wk-old/sex-matched animals were used for experiment.
2.2. Endotoxic shock and mouse behavioral studies
Eight to ten-wk-old male and female wild type and Cd44−/− mice were used. Mice were injected with HA (20 mg/kg) or PBS intraperitoneally (i.p.) as a pretreatment. One hour after pretreatment, we injected LPS (20 mg/kg) or PBS i.p. to induce the septic shock. Mice were then monitored over time for changes in behavior by blinded investigators and recorded by digital photography. At indicated time points, mice were transferred into a new cage environment with bedding on one end and food on the other end. Mice were observed for their behavior in the new environment, whether they explored their surroundings as a healthy mouse would or burrow under the bedding as they did when they were sick. Another indication of illness was ruffled and unkempt fur, squinty and sunken eyes, tremor, and diminished movement. Analysis of severity of mouse illness was based on criteria proposed by Nemzek et al (Nemzek et al., 2004). Blinded investigators evaluated mouse behaviors by 0–4 scales with 0 showing no change from normal behavior, 1 showing slightly spiky fur or squinty eyes but no apparent difference in activity, 2 showing decreased activity (25–50%) but mouse dose not burrow or hide, 3 with decreased activity (50–75%) but mouse dose not burrow or hide and 4 with complete loss of mobility. The increase of the numbers indicated the more severity of the septic shock symptoms.
2.3. Peritoneal macrophage isolation
Age- and sex- matched wild-type, Cd44−/−, Trif−/− and Myd88−/− mice were injected i.p. with 4% thioglycolate (Sigma-Aldrich, St. Louis, MO). After 72 h, peritoneal macrophages were isolated by peritoneal lavage and were plated onto 96-well tissue culture plates at 60,000 cells/well for 1h in RPMI1640. The adherent peritoneal macrophages were washed with PBS and cultured in 10% heat-inactivated FCS/RPMI1640 and rested for 24 h. After recovering, cells were stimulated and the supernatants and RNA were collected.
2.4. In vitro cell stimulation, tolerance induction and sample collection (macrophages)
Macrophages were grown to confluence in 96-well flat bottom plates (Corning Incorporated Life Sciences, Lowell, MA). For experiments, media was removed from cells and replaced with media containing either HA or LPS at the indicated concentration and the time point. All stimulations were done in low serum media (1% FCS). After stimulation, cells were allowed to incubate for indicated time, then media were collected and spun at 1,000g for 10 min at 4 °C to remove any debris. Cell cultured media were stored at −20°C until analysis. RNA was extracted from adherent cells after supernatant collection using TRIzol reagent (Invitrogen, Carlsbad, CA). RNA was stored at −80 °C. For tolerization, one set of macrophages was stimulated for 4 h with 25 μg/ml HA. After tolerization, the cells were washed with PBS to remove residual HA and were allowed to incubate for 16 hours in low serum media (1% FCS). Then, the supernatants of all wells were removed and replaced with media containing LPS 200 ng/ml, or medium alone as mock treatment. Following the second stimulation, supernatants and cells were harvested at the indicated time-points for readout at RNA and protein levels. The percent suppression of HA tolerance effect by A20 siRNA treatment (or control siRNA treatment) was calculated by dividing the total IL-6 decrease (difference between LPS alone treated cells and HA/LPS treated cells) of the A20 siRNA (control siRNA) treated cells by the total IL-6 decrease of the non-siRNA treated control cells, then multiplied by 100.
2.5. Quantitative RT-PCR
Real time PCR was used to determine the induction of A20 (Tnfaip3), Socs1, Tollip and Trim30 mRNA following HA stimulation. cDNA was synthesized from RNA by the iScript cDNA Synthesis Kit (BioRad) as described by the manufacture’s protocol. TaqMan™ Gene Expression Assays (Applied Biosystems) were used to analyze expression of A20, Socs1, Tollip and Trim30 as described by the manufacture instruction. Gapdh mRNA was used as an internal control to validate RNA for each sample. A20, Socs1, Tollip and Trim30 mRNA were calculated as relative expression to Gapdh mRNA, and all data are presented as normalized data against each control (mean of non-stimulated cells) (Yamasaki et al., 2009).
2.6. RNA interference
A20 specific siRNAs and mock siRNA (siGLO) were obtained from Dharmacon, Inc (Chicago, IL). Transfection of siRNAs were perfomed with siLentFect™ transfection reagent (BioRad). MH-S cells were cultured for 48 h, and replaced with 5% FCS containing media. siRNAs (final concentration at 10 nM) with transfection agent were added and incubated for 24 h. Then the media were replaced with low serum media (1% FCS) with HA (25 μg/ml) or PBS for 4 h. Sixteen hours later, MH-S cells were stimulated with LPS (200 ng/ml). Cultured media was collected for IL-6 ELISA at 24 h after LPS stimuli.
2.7. Analysis of supernatants and sera by ELISA
Supernatant or mouse serum cytokine concentrations were determined by species-specific IL-6 and TNFα ELISA (R&D Systems).
2.8. Statistical analysis
One-way or Two-way ANOVA was used to determine significance in the experiments, which was analyzed by GraphPad Prism 4 (GraphPad Software, Inc.).
3. Results
3.1. Hyaluronan (HA) and CD44 protects mice from LPS-induced shock
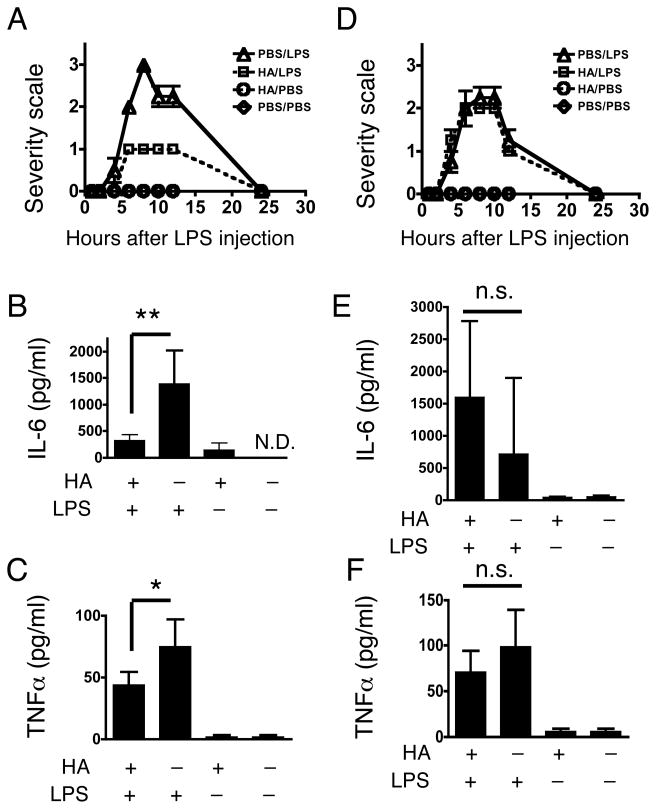
HA binds a complex of CD44 and TLR4 on the cell surface to initiate signaling (Taylor et al., 2004; Taylor et al., 2007; Yamasaki et al., 2009). We employed a mouse model of LPS-induced endotoxic shock to examine whether the unique receptor complex occupied by HA could affect the response to LPS. C57BL/6J mice were first injected i.p. with endotoxin-free HA (20 mg/kg) or PBS. One hour after this pretreatment, a following challenge of LPS (20 mg/kg) or PBS was i.p. injected into each group and then behavior and serum IL-6 and TNFα measured over time. An investigator blinded to the treatment conditions scored the septic behavioral response. Responses such as a lack of movement, ruffled fur and closed or squinting eyes were obvious between 5 and 12 h after LPS injection in all mice challenged by LPS and pretreated with PBS as a vehicle control (PBS/LPS). However, mice treated with HA prior to LPS were observed to demonstrate very little behavioral changes at 5–12 h, while mice treated the HA only (HA/LPS), or PBS only-treated mice (PBS/PBS), had no detectable behavioral changes. (Figure 1A). Consistent with observations of behavior, IL-6 and TNFα in serum at 10 h were significantly less in HA pretreated mice (HA/LPS) than PBS pretreated mice (PBS/LPS) (Figure 1B and C). Similar results were observed with Balb/c mice (data not shown). These findings suggested that HA pretreatment induces cross-tolerance, and subsequently protects mice from LPS-induced septic shock.
Fig. 1. HA protects mice from LPS-induced endotoxic shock and inhibits cytokine release in serum in a CD44 dependent manner.
(A) Mice were injected i.p. with HA (20 mg/kg) or PBS then injected with LPS (20 mg/kg) or PBS one hour later. Septic behavior severity responses were scored as described in Materials and Methods. PBS/LPS; PBS followed by LPS, HA/LPS; HA followed by LPS, HA/PBS; HA followed by PBS injection, PBS/PBS; PBS followed by PBS injection. Data are mean ± SEM of 4 mice per group of one experiment representative of three independent experiments. (B), (C) Serum IL-6 and TNFα from mice treated identically to those shown in Figure 1A measured by ELISA at 10 h after second injection. (D) Cd44−/− mice treated as in Figure 1A. Data are mean ± SEM of 4 mice per group of one experiment representative of three independent experiments. (E), (F) Serum IL-6 and TNFα from Cd44−/− mice treated identically to those shown in Figure 1A and 1D was measured by ELISA at 10 h after second injection. Data are presented as mean ± SEM. *; p < 0.05, **; p < 0.01, N.D., not detected, n.s., not significant.
Next, to determine the mechanism of protection, and test the hypothesis that association of HA with CD44 contributed to HA-LPS cross-tolerance, mice deficient in functional CD44 were treated similarly to those described in Figure 1A–C. In these mice, HA-pretreatment before LPS injection did not affect the septic behavior response compared to PBS/LPS, thus suggesting that the protective effects of HA against LPS was dependent on CD44 (Figure 1D). Serum from HA-pretreated Cd44−/− mice also showed elevations in serum IL-6 and TNFα after LPS injection similar to Cd44−/− and wild-type mice not treated with HA (PBS/LPS) (Figure 1E and F).
3.2. HA and CD44 modulates the response of macrophages to LPS
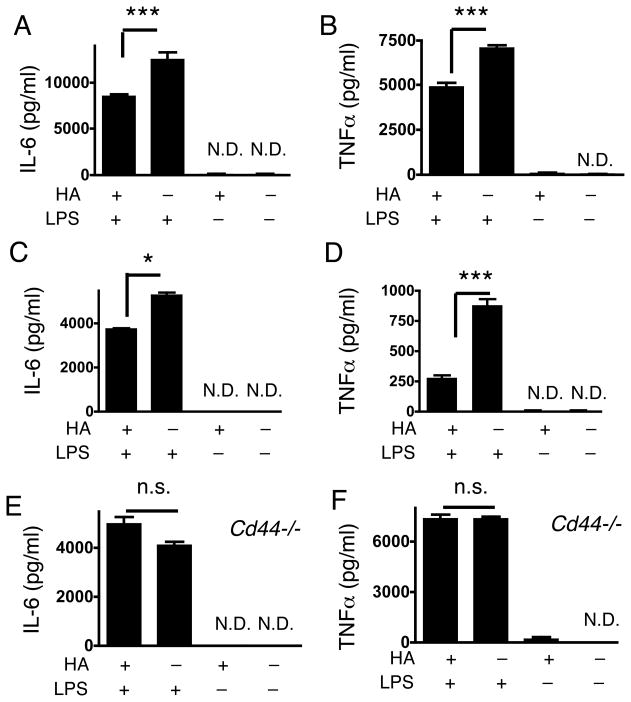
To further assess the mechanisms responsible for the effect of HA on LPS-induced septic responses, isolated mouse peritoneal macrophages or the MH-S macrophage cell line were cultured with combinations of LPS and/or HA, and IL-6 and TNFα expression were measured. Consistent with our findings in vivo, IL-6 and TNFα release from macrophages were significantly less after HA pretreatment compared to the cells treated with LPS without HA-pretreatment (Figure 2A–D). Furthermore, and also consistent with the findings in vivo, HA addition before LPS exposure did not suppress cytokine release stimulated by LPS treatment of peritoneal macrophages isolated from Cd44−/− mice (Figure 2E and F). These further support that HA-CD44 stimulation modulates cellular responses to LPS.
Fig. 2. HA inhibits cytokine release from macrophages in a CD44-dependent manner.
(A) IL-6 and (B) TNFα measured by ELISA in the cultured medium of mouse peritoneal macrophages. One set of macrophages was treated with 25 μg/ml HA. After 16 h, the macrophages were treated with 200 ng/ml LPS or medium alone for 24 h. (C) IL-6 and (D) TNFα, measured by ELISA in the cultured medium of MH-S cells treated as in A and B. (E) IL-6 and (F) TNFα, measured by ELISA in the cultured medium of peritoneal macrophages isolated from Cd44−/− mice and treated as in A and B. Data are mean ± SEM of 3 mice per group of one experiment representative of three independent experiments. *; p < 0.05, ***; p < 0.001, n.s.; not significant, N.D.; not detected.
3.3. HA-LPS heterotolerance acts through A20
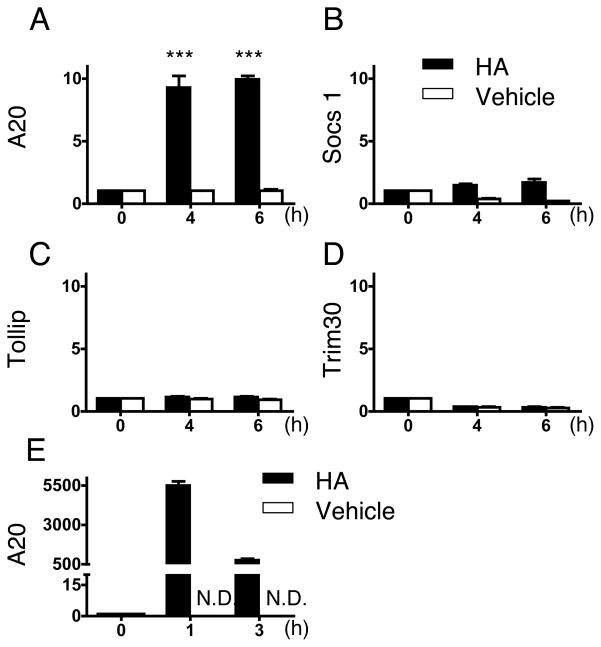
The finding of a HA-CD44 interaction as an inhibitor of LPS responsiveness led us to next examine the influence of HA on the expression of molecules known to act as negative regulators of TLR signaling. HA increased A20 (Tnfaip3) mRNA expression in MH-S cells (Figure 3A). The expression of other negative regulators of TLR signaling, Socs1, Tollip, and Trim30, were not dramatically changed by HA compared to the increase in A20 (Figure 3B, C, D). To determine if A20 was also induced in mice injected with HA, macrophages in the peritoneal cavity were collected 1 and 3 hours after HA injection (20 mg/kg), and these cells also showed a significant increase in A20 mRNA expression compared to control (Figure 3E).
Fig. 3. HA induces an increase in A20 expression.
(A) – (D) MH-S cells were treated with HA (25 μg/ml) (solid bars) or vehicle control (PBS) (open bars) for 4 h. (A), A20 (B), Socs1 (C), Tollip and (D), Trim30 mRNA expression was assessed by quantitative RT-PCR. Data are shown as relative expression compared to control (0 h) macrophages. ***; p < 0.001. (E) WT (C57Bl/6) mice were injected i.p. with HA (20 mg/kg) (solid bars) or PBS (open bars). After 1 and 3 h, the inflammatory cells in peritoneal cavity were isolated and A20 mRNA expression was assessed by quantitative RT-PCR. Data are shown as relative expression compared to control (0h; before injection). Data are presented as mean ± SEM of three mice per group of one experiment representative of three independent experiments.
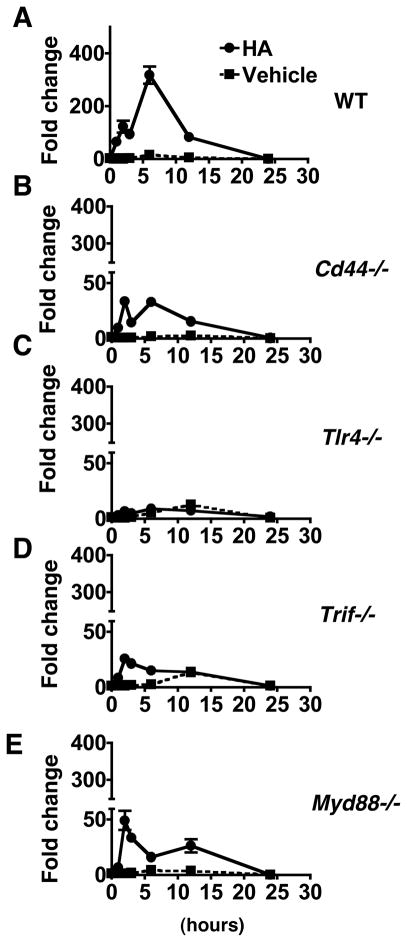
To determine if CD44 is involved in the increased expression of A20 induced by HA, peritoneal macrophages were isolated from Cd44−/− mice and treated with HA in culture. A20 was inducible by HA in macrophages from wild-type mice up to ca. 300-fold (Figure 4A). A20 mRNA expression was much less by HA in Cd44−/− mice in comparison to wild-type (Figure 4B). Since CD44 forms complex with TLR4 and TLR4 mediates recognition of HA (Taylor et al., 2007), we also tested the involvement of TLR4 in HA-dependent A20 expression. Macrophages from Tlr4−/− mice did not show a significant increase in A20 expression (Figure 4C). Similarly, peritoneal macrophages from mice lacking TRIF and MyD88, major adaptor molecules of TLR4, showed diminished responses to HA with increased A20 expression by up to 25.6-fold and 49.2-fold, respectively (Figure 4D and 4E). These data suggest that CD44, TLR4, and subsequent function of TRIF and MyD88 are required for optimal induction of A20 by HA
Fig. 4. HA induces A20 via CD44, TLR4, MyD88 and TRIF.
Peritoneal macrophages from wild type (A), Cd44−/− (B), Tlr4−/− (C), Trif−/− (D), Myd88−/− (E) were treated with HA. mRNA abundance for A20 was measured after HA exposure (closed circles) or vehicle control (closed squares). Quantitative RT-PCR data are shown as relative expression compared to control (0 h) macrophages. Data are presented as mean ± SEM of 3 mice per group of one experiment representative of three independent experiments.
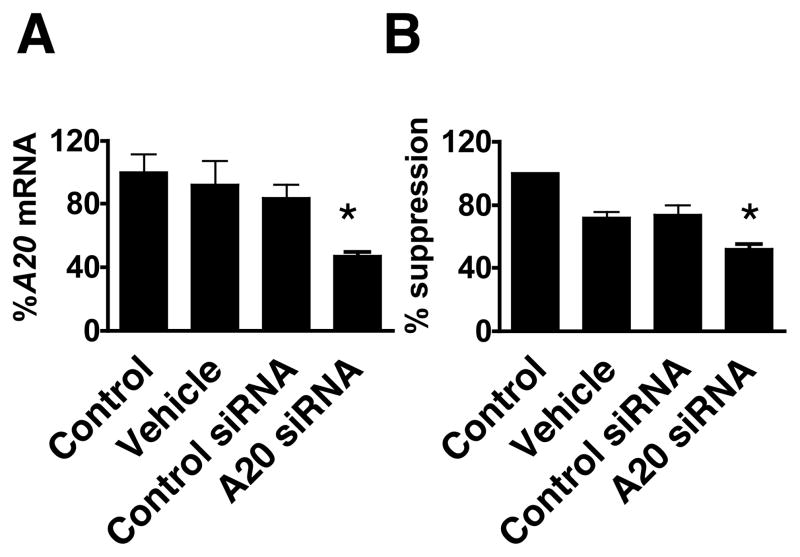
To determine the dependence on A20 for HA mediated suppression of LPS cytokine response, cells were treated with A20 siRNA. This procedure was successful in decreasing A20 gene expression to 47.0% of untreated cells (Figure 5A). A20 siRNA treatment induced a comparable and significant suppression of the ability of HA to inhibit IL-6 induction by LPS to 52.1% of siRNA untreated HA/LPS cells (Fig. 5B).
Fig. 5. Suppression of A20 inhibits HA-LPS heterotolerance.
(A) MH-S cells were treated with siRNAs for 24 h and A20 mRNA expression was examined by RT-PCR. (B) MH-S cells were treated with siRNAs for 24 h as in A, cells were then treated with 25 μg/ml of HA or PBS for 4 h followed by wash and incubation in fresh medium for 14 h. Cells were then stimulated with 200 ng/ml LPS for 24 hours and IL-6 in media measured by ELISA. %Suppression of HA effect was calculated as described in Materials and methods. Mean and SEM are shown. The agents used were; control: none, vehicle: transfection reagent alone, control siRNA: mock transfected with control siRNA, A20 siRNA: A20 siRNA-transfected. *; p < 0.05.
4. Discussion
Septic shock is one of the most challenging problems in critical care medicine, and accounts for significant morbidity and mortality in medical and surgical intensive care units (Parrillo, 2008). In this current study we demonstrate that HA pretreatment protects mice from the LPS-induced endotoxic shock phenomenon and suppresses cytokine elevations in serum. These observations shed new light on potential elements that regulate the septic shock response, and may have important therapeutic implications. Our data show that inhibition of the response to LPS by HA is mediated in part by induction of A20 through the unique CD44–TLR4–MyD88/TRIF pathway. The significance of this observation is that HA is an abundant glycosaminoglycan present in the extracellular matrix of many tissues and available for release in soluble form upon tissue injury (Taylor et al., 2007). HA is well known to bind to CD44 (Aruffo et al., 1990), and has been previously shown to engage a receptor complex of CD44 and TLR4 to act as an alternative to LPS in activation of TLR4 (Taylor et al., 2007). The current findings show for the first time that this CD44 and TLR4 complex induces A20, a known negative regulator of TLR4 signaling (Boone et al., 2004). Thus, the presence of free HA at sites of injury may modulate the host reaction to bacterial endotoxin and subsequently inhibit a septic response to incidental LPS exposure at the site of injury. This can be beneficial in situations where LPS exposure accompanies a bacterium innoculum that does not put the organism at risk of septicemia, but could be detrimental if the tolerance to LPS diminishes an inflammatory response that would otherwise have protected against development of bacteremia.
The phenomenon that prior exposure to LPS induces a transient state of cell refractoriness to subsequent LPS re-stimulation is known as endotoxin tolerance and has been well known for several years (Favorite and Morgan, 1942; Medvedev et al., 2002). In mammals, macrophages are major responders to LPS and they release numerous bioactive molecules such as inflammatory cytokines, H2O2 and NO (Hoffmann et al., 1999). Macrophages were identified as pivotal cellular participants in the acquisition of the tolerant phenotype by using LPS-resistant C3H/HeJ mice and LPS sensitive macrophages from C3H/HeN mice (Freudenberg and Galanos, 1988). In this study it was shown that if LPS sensitive macrophages were administered at the time of LPS pretreatment, LPS-resistant C3H/HeJ mice became resistant to a lethal challenge with LPS. Our findings are also consistent with a role for macrophages since these cells have high levels of CD44 expression (Camp et al., 1991), and can detect HA through CD44–TLR4 complex (Taylor et al., 2007). Although macrophages will increase release of some cytokines following HA stimulation alone (Taylor et al., 2007; Yamasaki et al., 2009), the current study shows that administration of HA alone has no significant effect on mouse behavior indicative of a septic response even at high concentrations of HA. Thus, the response to HA differs from the response to LPS despite sharing TLR4 as a recognition molecule. Furthermore, whole animals and cultured macrophages become less responsive to LPS challenge following HA pretreatment. Thus, here we describe for the first time that HA pretreatment induces cross-tolerance in macrophages to subsequent LPS stimulation. This observation suggests that HA could serve as an intrinsic modulator of the septic shock susceptibility, a finding that is also consistent with previous reports of HA exerting an anti-inflammatory effect on a variety of cell types (Bollyky et al., 2007; Jiang et al., 2005; Kogan et al., 2007).
The HA cross-tolerance effect is dependent on CD44, as seen in experiments showing the protective effect of HA was diminished in the absence of CD44. A recent publication also has suggested that CD44 provides a brake for innate immune inflammatory responses by promoting the expression and function of negative regulators in macrophages responding to pathogens (Liang et al., 2007). In the study the absence of CD44 leads to the suboptimal expression of A20, IRAK-M, and Tollip in response to LPS in vivo and macrophages in vitro. We showed that HA induces A20 significantly via CD44 and TLR4. Using siRNA technique knocking down A20, we were also able to show that A20 contributes to the ability of HA to protect against the cytokine induction by LPS. Taken together, these observations show that HA-CD44 interaction modulates a host response to septic shock by affecting negative regulators of innate immune receptors.
Two major pathways, MyD88-dependent and TRIF-dependent pathway are identified in TLR4 intracellular signaling. We found that the macrophages from both Trif−/− and Myd88−/− mice were impaired in the HA-induced A20 mRNA expression. Our results clearly demonstrated that the cooperation of MyD88-dependent and TRIF-dependent signaling pathways are required for the optimal induction of A20 by HA. Similarly, Yamamoto et al proposed that cooperation of MyD88-dependent and MyD88-independent (TRIF-dependent) signaling pathways are required for the TLR4-mediated inflammatory cytokine production such as IL-6 and TNFα (Yamamoto et al., 2003). A20 is required for the termination of TLR-induced activity of the transcription factor NF-κB and proinflammatory gene expression in macrophages, and this function protected mice from endotoxic shock. A20 accomplishes this biochemically by directly removing ubiquitin moieties from the signaling molecule TRAF6 (Boone et al., 2004). We used peritoneal macrophages from mice lacking TRIF and MyD88 to examine if HA-heterotolerance is diminished in Trif−/− or Myd88−/− macrophages. The secretion of IL-6 and TNFα from both Trif−/− and Myd88−/− macrophages were barely detectible after LPS treatment (data not show). Taken together, both TRIF and MyD88 pathways appear to be involved in the HA-heterotolerance to induce TNFAIP3/A20, and are negatively regulated by A20.
We have previously reported that HA increases cytokines CXCL2/MIP2 and IL-1β in the process of sterile injury (Taylor et al., 2007; Yamasaki et al., 2009). The initiation of a sterile intrinsic inflammatory event induces HA accumulation at the injured site. HA is recognized by a complex that involves TLR4, MD-2 and CD44. Taken together with other work associating HA and innate pattern recognition (del Fresno et al., 2005; Jiang et al., 2005; Taylor et al., 2004; Termeer et al., 2002), these observations have provided new insight into mechanisms responsible for sterile inflammation. HA has emerged as one of several danger signals that alert the host of tissue damage. Our results here further provide evidence that while HA provokes a signal of injury, it will modulate sequential challenges by endotoxins. Thus, the presence of HA suppresses the septic reaction by LPS. This is a logical process in the setting of skin injury, a relatively frequent event that will expose normally sterile tissue to external microbial challenge. In this process, abundant local innate immune defenses are triggered to suppress and eliminate microbial invasion (Nizet et al., 2001) and a systemic cytokine response would not be advantageous. Local HA release may therefore serve to focus the inflammatory response to the local environment while protecting the host from an unnecessary generalized septic response. Supporting this, we also observed that intradermal injection of HA decreased sequential cytokine induction by local injection of LPS (data not shown). Clinical observations also support this conclusion. Septic shock survivors have an increased incidence of bacterial infections and suppressed monocyte response to LPS (Ertel et al., 1995). Human macrophages derived from patients after hemorrhage, surgery, and trauma, (all events that can elevate the production of HA fragments), manifest suppressed responses to LPS ex vivo (Abraham and Freitas, 1989; Adib-Conquy et al., 2001; Moore et al., 1997). Thus, the status of intrinsic HA can serve as a sensor of tissue environmental changes and regulate the host inflammatory response.
HA size is critical for HA function (Stern et al., 2006). We used mixed HA obtained from human umbilical cords, which contain at wide range of HA sizes up to 500 kDa (Lago et al., 2005). HA fragments in the 200 – 500 kDa range induce inflammatory cytokines (Taylor et al., 2007; Yamasaki et al., 2009), and were seen in the current study to induce cross-tolerance against LPS dependent on CD44. However, smaller HA fragments less than 100 kDa have been reported to show different effects on host cells (Hodge-Dufour et al., 1997; Horton et al., 1998; Horton et al., 1999; McKee et al., 1997; McKee et al., 1996; Noble et al., 1993). Recently Kawana et al reported that the inhibitory effect of CD44 on TLR signaling is independent of HA (Kawana et al., 2008). They showed that LMW-HA (3 and 22 kDa) had inhibitory effects on TLR signaling, but these effects were independent of CD44 expression. Similarly, we have found that extracellular small HA oligosaccharides did not induce cytokines such as MIP2/Cxcl2 and IL-1β, but intracellular small HA oligosaccharides affect IL-1β release differently from extracellular small HA (Taylor et al., 2007; Yamasaki et al., 2009). These observations suggest that mechanisms of host responses to HA are dependent on HA size and localization. The molecular mechanisms of this size dependent HA action remains unknown.
In summary, the current findings support the conclusion that HA acts as a danger signal through recognition by CD44 and TLR4. Furthermore, it is now apparent that HA signaling can modify inflammatory reactions against sequential microbial challenge. This cross-tolerance appears to be mediated in part by the induction of A20. Therefore, HA has the unique capacity to modify host reactions to LPS and adjust the septic response. This event prevents excess inflammatory cytokine production that can harm the host. These observations further suggest that the regulation of HA catabolism may be a key variable to modify the host inflammatory response during injury and infection.
Acknowledgments
We thank Drs. Shizuo Akira and Allen F. Ryan for the Myd88−/− and Trif−/− mice. This work was supported by NIH grants P01HL057345, R01AR45676 and a VA Merit award R.L.G.
Abbreviations
- HA
hyaluronan
- LPS
Lipopolysaccharide
- IP
intraperitoneal or intraperitoneally
- HPLC
high performance liquid chromatography
- TLR
toll-like receptor
- MyD88
myeloid differentiation primary response gene 88
- TRIF
TIR domain-containing adaptor inducing IFN-β
- TRAF
TNF receptor associated factor
- SOCS
suppressor of cytokine signaling
- Tollip
Toll-interacting protein
- IFN
interferon
- IL-1β
interleukin 1β
- MIP-2
macrophage inflammatory protein-2
- IL-6
interleukin 6
- TNFα
tumor necrosis factor-α
- WT
wild type
- PBS
phosphate-buffered saline
- FCS
fetal calf serum
- ELISA
enzyme-linked immunosorbent assay
- RT-PCR
reverse transcription-PCR
- siRNA
small interfering RNA
Footnotes
Conflict of interest
The authors state no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham E, Freitas AA. Hemorrhage produces abnormalities in lymphocyte function and lymphokine generation. J Immunol. 1989;142:899–906. [PubMed] [Google Scholar]
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–50. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- Adib-Conquy M, Asehnoune K, Moine P, Cavaillon JM. Long-term-impaired expression of nuclear factor-kappa B and I kappa B alpha in peripheral blood mononuclear cells of trauma patients. J Leukoc Biol. 2001;70:30–8. [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–13. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Bollyky PL, Lord JD, Masewicz SA, Evanko SP, Buckner JH, Wight TN, Nepom GT. Cutting edge: high molecular weight hyaluronan promotes the suppressive effects of CD4+CD25+ regulatory T cells. J Immunol. 2007;179:744–7. doi: 10.4049/jimmunol.179.2.744. [DOI] [PubMed] [Google Scholar]
- Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, McNally E, Pickart C, Ma A. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5:1052–60. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- Camp RL, Kraus TA, Pure E. Variations in the cytoskeletal interaction and posttranslational modification of the CD44 homing receptor in macrophages. J Cell Biol. 1991;115:1283–92. doi: 10.1083/jcb.115.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JA. Molecular basis of endotoxin tolerance. Ann N Y Acad Sci. 1998;851:426–8. doi: 10.1111/j.1749-6632.1998.tb09020.x. [DOI] [PubMed] [Google Scholar]
- Cooper JT, Stroka DM, Brostjan C, Palmetshofer A, Bach FH, Ferran C. A20 blocks endothelial cell activation through a NF-kappaB-dependent mechanism. J Biol Chem. 1996;271:18068–73. doi: 10.1074/jbc.271.30.18068. [DOI] [PubMed] [Google Scholar]
- del Fresno C, Otero K, Gomez-Garcia L, Gonzalez-Leon MC, Soler-Ranger L, Fuentes-Prior P, Escoll P, Baos R, Caveda L, Garcia F, Arnalich F, Lopez-Collazo E. Tumor cells deactivate human monocytes by up-regulating IL-1 receptor associated kinase-M expression via CD44 and TLR4. J Immunol. 2005;174:3032–40. doi: 10.4049/jimmunol.174.5.3032. [DOI] [PubMed] [Google Scholar]
- Ertel W, Kremer JP, Kenney J, Steckholzer U, Jarrar D, Trentz O, Schildberg FW. Downregulation of proinflammatory cytokine release in whole blood from septic patients. Blood. 1995;85:1341–7. [PubMed] [Google Scholar]
- Favorite GO, Morgan HR. Effects Produced by the Intravenous Injection in Man of a Toxic Antigenic Material Derived from Eberthella Typhosa: Clinical, Hematological, Chemical and Serological Studies. J Clin Invest. 1942;21:589–99. doi: 10.1172/JCI101337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg MA, Galanos C. Induction of tolerance to lipopolysaccharide (LPS)-D-galactosamine lethality by pretreatment with LPS is mediated by macrophages. Infect Immun. 1988;56:1352–7. doi: 10.1128/iai.56.5.1352-1357.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge-Dufour J, Noble PW, Horton MR, Bao C, Wysoka M, Burdick MD, Strieter RM, Trinchieri G, Pure E. Induction of IL-12 and chemokines by hyaluronan requires adhesion-dependent priming of resident but not elicited macrophages. J Immunol. 1997;159:2492–500. [PubMed] [Google Scholar]
- Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–8. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- Horton MR, McKee CM, Bao C, Liao F, Farber JM, Hodge-DuFour J, Pure E, Oliver BL, Wright TM, Noble PW. Hyaluronan fragments synergize with interferon-gamma to induce the C-X-C chemokines mig and interferon-inducible protein-10 in mouse macrophages. J Biol Chem. 1998;273:35088–94. doi: 10.1074/jbc.273.52.35088. [DOI] [PubMed] [Google Scholar]
- Horton MR, Shapiro S, Bao C, Lowenstein CJ, Noble PW. Induction and Regulation of Macrophage Metalloelastase by Hyaluronan Fragments in Mouse Macrophages. J Immunol. 1999;162:4171–4176. [PubMed] [Google Scholar]
- Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–9. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–22. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- Kawana H, Karaki H, Higashi M, Miyazaki M, Hilberg F, Kitagawa M, Harigaya K. CD44 suppresses TLR-mediated inflammation. J Immunol. 2008;180:4235–45. doi: 10.4049/jimmunol.180.6.4235. [DOI] [PubMed] [Google Scholar]
- Kogan G, Soltes L, Stern R, Gemeiner P. Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol Lett. 2007;29:17–25. doi: 10.1007/s10529-006-9219-z. [DOI] [PubMed] [Google Scholar]
- Lago G, Oruna L, Cremata JA, Perez C, Coto G, Lauzan E, Kennedy JF. Isolation, purification and characterization of hyaluronan from human umbilical cord residues. Carbohydrate Polymers. 2005;62:321–326. [Google Scholar]
- Laurent TC, Fraser JR, Laurent UB, Engstrom-Laurent A. Hyaluronan in inflammatory joint disease. Acta Orthop Scand Suppl. 1995;266:116–20. [PubMed] [Google Scholar]
- Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289:2350–4. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner MD, Morath S, Michelsen KS, Schumann RR, Hartung T. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different Toll-like receptors independent of paracrine mediators. J Immunol. 2001;166:5161–7. doi: 10.4049/jimmunol.166.8.5161. [DOI] [PubMed] [Google Scholar]
- Liang J, Jiang D, Griffith J, Yu S, Fan J, Zhao X, Bucala R, Noble PW. CD44 is a negative regulator of acute pulmonary inflammation and lipopolysaccharide-TLR signaling in mouse macrophages. J Immunol. 2007;178:2469–75. doi: 10.4049/jimmunol.178.4.2469. [DOI] [PubMed] [Google Scholar]
- Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–58. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- Matsuno YK, Kakoi N, Kinoshita M, Matsuzaki Y, Kumada J, Kakehi K. Electrophoresis studies on the contaminating glycosaminoglycans in commercially available hyaluronic acid products. Electrophoresis. 2008;29:3628–35. doi: 10.1002/elps.200700941. [DOI] [PubMed] [Google Scholar]
- McKee CM, Lowenstein CJ, Horton MR, Wu J, Bao C, Chin BY, Choi AMK, Noble PW. Hyaluronan Fragments Induce Nitric-oxide Synthase in Murine Macrophages through a Nuclear Factor kappa B-dependent Mechanism. J Biol Chem. 1997;272:8013–8018. doi: 10.1074/jbc.272.12.8013. [DOI] [PubMed] [Google Scholar]
- McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, Noble PW. Hyaluronan (HA) Fragments Induce Chemokine Gene Expression in Alveolar Macrophages. The Role of HA Size and CD44. J Clin Invest. 1996;98:2403–2413. doi: 10.1172/JCI119054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev AE, Kopydlowski KM, Vogel SN. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and toll-like receptor 2 and 4 gene expression. J Immunol. 2000;164:5564–74. doi: 10.4049/jimmunol.164.11.5564. [DOI] [PubMed] [Google Scholar]
- Medvedev AE, Lentschat A, Wahl LM, Golenbock DT, Vogel SN. Dysregulation of LPS-induced Toll-like receptor 4-MyD88 complex formation and IL-1 receptor-associated kinase 1 activation in endotoxin-tolerant cells. J Immunol. 2002;169:5209–16. doi: 10.4049/jimmunol.169.9.5209. [DOI] [PubMed] [Google Scholar]
- Moore FA, Moore EE, Sauaia A. Blood transfusion. An independent risk factor for postinjury multiple organ failure. Arch Surg. 1997;132:620–4. discussion 624–5. [PubMed] [Google Scholar]
- Nemzek JA, Xiao HY, Minard AE, Bolgos GL, Remick DG. Humane endpoints in shock research. Shock. 2004;21:17–25. doi: 10.1097/01.shk.0000101667.49265.fd. [DOI] [PubMed] [Google Scholar]
- Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–7. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- Noble PW, Lake FR, Henson PM, Riches DW. Hyaluronate activation of CD44 induces insulin-like growth factor-1 expression by a tumor necrosis factor-alpha-dependent mechanism in murine macrophages. J Clin Invest. 1993;91:2368–77. doi: 10.1172/JCI116469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrillo JE. Septic shock--vasopressin, norepinephrine, and urgency. N Engl J Med. 2008;358:954–6. doi: 10.1056/NEJMe0800245. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Sato S, Nomura F, Kawai T, Takeuchi O, Muhlradt PF, Takeda K, Akira S. Synergy and cross-tolerance between toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J Immunol. 2000;165:7096–101. doi: 10.4049/jimmunol.165.12.7096. [DOI] [PubMed] [Google Scholar]
- Schmits R, Filmus J, Gerwin N, Senaldi G, Kiefer F, Kundig T, Wakeham A, Shahinian A, Catzavelos C, Rak J, Furlonger C, Zakarian A, Simard JJ, Ohashi PS, Paige CJ, Gutierrez-Ramos JC, Mak TW. CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood. 1997;90:2217–33. [PubMed] [Google Scholar]
- Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: An information-rich system. European Journal of Cell Biology. 2006;85:699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279:17079–84. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- Taylor KR, Yamasaki K, Radek KA, Di Nardo A, Goodarzi H, Golenbock D, Beutler B, Gallo RL. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem. 2007;282:18265–75. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science. 2002;296:155–8. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–3. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Muto J, Taylor KR, Cogen AL, Audish D, Bertin J, Grant EP, Coyle AJ, Misaghi A, Hoffman HM, Gallo RL. NLRP3/Cryopyrin Is Necessary for Interleukin-1{beta} (IL-1{beta}) Release in Response to Hyaluronan, an Endogenous Trigger of Inflammation in Response to Injury. J Biol Chem. 2009;284:12762–71. doi: 10.1074/jbc.M806084200. [DOI] [PMC free article] [PubMed] [Google Scholar]