Abstract
Most of our knowledge about chronic musculoskeletal pain is based on cutaneous pain models. To test the hypothesis that animals develop chronic muscular hyperalgesia following intramuscular acidic saline injections, primary hyperalgesia within the gastrocnemius muscle was analyzed and compared to secondary cutaneous hyperalgesia in the hind paw that develops following intramuscular acid saline injection. Two acidic saline (pH 4.0) injections were administrated into the gastrocnemius of female CF-1 mice. The results indicate that mice developed a robust hypersensitivity bilaterally in primary (gastrocnemius muscle) and secondary (cutaneous hind paw) sites that lasted up to 2 weeks. In addition, primary hyperalgesia correlated well with levels of Fos expression. Fos expression patterns in the spinal cord were different for primary and secondary site stimulation. Hind paw palpation stimulated ipsilateral Fos expression in the superficial spinal laminae at L4/L5 levels, and bilaterally in deep laminae at L2-L5 spinal levels. In contrast, gastrocnemius compression stimulated widespread Fos expression in all regions of the ipsilateral dorsal horn within L2-L6 spinal segments. These findings indicate that acidic saline injection induces primary hyperalgesia in muscle and that the patterns of Fos expression in response to primary versus secondary stimulation are strikingly different.
Keywords: Fos, spinal cord, muscle pain, acidic saline, mice, cutaneous pain
PERSPECTIVE
This study assesses primary site muscular pain, which is the main complaint of people with musculoskeletal conditions, and identifies spinal patterns activated by noxious mechanical stimuli to the gastrocnemius. This study demonstrates approaches to test nociception arising from muscle and aids to our understanding of spinal processing of primary and secondary site hyperalgesia.
Introduction
Pain associated with chronic musculoskeletal disorders poses a major clinical problem, affecting nearly one-third of the world's population and costing approximately $100 billion each year.4,13 Clinically, people report pain arising from joint, muscle, fascia or visceral tissues.18 However, most of our knowledge concerning the mechanism and measurement of pain is inferred from studies assessing cutaneous tissue. Little is known about muscle pain, in part because of the complexity of muscle nociception18 and the lack of reliable laboratory measures. Clinically, muscle pain is measured by the presence of edema and bruising, loss of strength, and pain with palpation. In animals, muscle strength tests have been used for testing muscle pain,19,26 but these techniques are more feasible to assessing forelimb muscles. An effective way to test hind limb deep tissue pain may be direct muscle compression,26,29,42 which parallels clinical assessment of muscle palpation. Yu et al.42 first described a study in which a compression was applied to the deep hind limb tissues using an instrumented forceps to control the compressive force applied. This device has become a widely accepted and beneficial tool for measuring deep tissue pain in various pain models.15,26,29,42
Animal models of muscle pain are induced by ischemia;8,9 various irritant chemicals;12,14,19,25 muscle contraction via either electrical stimulation34,39 or exercise;17 and acidic normal saline.30 The model of acidic saline hyperalgesia is unique in that it causes no muscle damage and the widespread hypersensitivity has been proposed to mimic fibromyalgia.30 Primary muscle hyperalgesia induced by pH 4.0 saline injection is dependent on the presence of acid sensing ion channel-3 (ASIC3)31 and mediated by central sensitization of spinal11,22,27,28,29,30,31,32 and supraspinal mechanisms.36 Following 2 acidic saline injections, animals develop widespread secondary hyperalgesia that spreads to bilateral cutaneous,7,11,27,28,30,32 visceral tissues22 and primary muscle hyperalgesia.36,41 The development of chronic cutaneous hyperalgesia has been reported in many studies, but development of chronic primary muscle hyperalgesia following intramuscular acid saline remains to be tested.
Fos has been extensively used to examine neuronal activation following cutaneous application of noxious chemical,5,14,16,21,33 thermal,40 and electrical stimuli.20,34 The activation and expression of Fos in the dorsal horn in response to gastrocnemius muscle stimulation is limited to two studies utilizing chemical or electrical stimuli.14,39 Here, we used immunohistochemistry to map the distribution Fos in the dorsal horn in response to mechanical stimulation of the gastrocnemius muscle and hind paw in the acid-induced non-inflammatory chronic pain model. The purpose of the present study was to assess primary gastrocnemius hyperalgesia using an instrumented forceps device and Fos activation in the acidic saline pain model and compare patterns of Fos expression in response to either hind paw palpation and gastrocnemius muscle compression. Investigating features of primary muscle hyperalgesia and its spinal activation may provide a broader understanding of nociception and add to our understanding of clinical testing of muscle palpation.
Materials and Methods
Animals
All experiments were approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center and adhered to the University's animal care guidelines. Twenty female CF-1 mice purchased from Charles River (Wilmington, MA) were randomly assigned to either acidic saline (experiment; pH 4.0) or neutral saline (control; pH 7.4) groups. Two 20 μl injections of either pH 4.0 or pH 7.4 normal saline were administered 2 days apart into the right gastrocnemius muscle to induce wide spread hypersensitivity. Injections were made with a 1 ml latex-free insulin syringe with 10 μl increments (Becton Dickinson, Franklin Lakes, NJ). The examiner was blinded to group assignment for all experiments.
Behavioral Assessments
Secondary Cutaneous Sensitivity
Cutaneous sensation of both hind paws was measured using 1.0 g von Frey monofilaments according to a previous study.7 For all animals, the monofilament was applied 5 times to each hind paw with 15-30 seconds between each application for a total of 3 trials. Three days prior to the initial testing, animals were acclimated under small plastic chambers on wire mash table for 30 minutes each day until the day of testing. The numbers of positive responses were recorded, and the percent withdrawal response for each paw was calculated. A positive response was defined as retraction of the paw. Animals were tested pre-injection and 1, 10 and 15 days following the 2nd acid injection to examine the chronic stage of cutaneous hyperalgesia.
Primary Muscle Sensitivity
Deep tissue hyperalgesia following acidic saline injections was assessed by a forceps compression device similar to the one described by Yu et. al.42 Our device is a modified version, built internally in our Neuromuscular Research laboratory. The device consists of a forceps, a pressure sensor, a signal amplifier, and a laptop computer. Yu et. al.42 used a strain gage sensor affixed in the middle portion of a forceps' arm. The deformation of the forceps arm was measured during the experiment and later converted to the compressive force applied at the tip of the forceps. Here, we used a commercial pressure sensor, (LCKD subminiature compression load cells, Omega Engineering, Inc. Stanford, CT, USA), which was mounted on the inner tip flat surface of the forceps. This modification makes the device more reliable and allows a direct measure of the compressive force applied to the tissue through the tip of the forceps. The signal from the load cells was amplified through a DMD-465 Bridgesensor AC Powered Signal Conditioner (Omega Engineering, Inc. Stanford, CT, USA), digitized using an A/D board, and stored in a laptop computer. The examiner applied manual force from a marked area using the forceps (Fig. 1). The contact area of the pressure sensor measured the direct compressive force applied to the tissue. The recorded force signal was processed using a custom-written Mat-Lab computer program (Matlab 6.5, The MathWorks Inc., Boston, MA, USA) to identify the force peaks and the respective loading time. The peak force was defined as the amount of force when the animal either withdrew its hind limb or vocalized.
Figure 1.
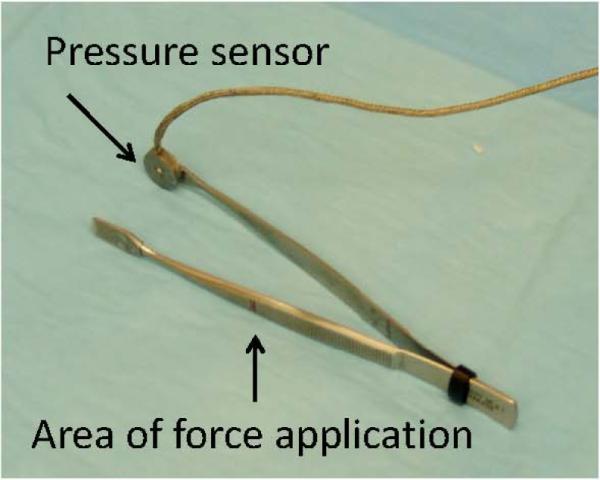
Photograph of the forceps device used to measure muscle compression threshold. Arrows indicate the pressure sensor (which is also the contact area with the tissue) and the site where force was applied by the examiner.
Three days prior to each testing session, mice were acclimated in a 50 ml restraining tube for 10 minutes 3 times a day. Each gastrocnemius muscle was compressed 3 times in 1 trial for a total of 3 trials. Only peak forces with a loading time (defined as the beginning of loading to the point of peak) of less than 1.0 second were used for the final calculations.Occasionally, a gastrocnemius muscle was compressed up to 5 times when the examiner sensed a longer loading period. Later these trials were included in analysis if they meet the criteria of 1.0 second loading time. Thus a mean of 9-11 peak forces over 3 trials was calculated for each hind limb. Testing was conducted prior to acidic saline injection and 3 and 16 days post-second acidic saline injection.
Hind Paw Palpation and Gastrocnemius Compression to Induce Fos Expression
Mice were further tested for differences in spinal cord neuronal activation in response to paw and gastrocnemius compression. In order to produce a strong enough mechanical stimulus to induce Fos expression in the spinal cord, a manual compression was given to either right paw (n=10; 5 with pH 7.4; control group and 5 with pH 4.0; experiment group) or the right gastrocnemius muscle (n=10; 5 with pH 7.4; control group and 5 with pH 4.0; experiment group) following 18 days post second acidic saline injection. For secondary hyperalgesia, mice were restrained in 50 ml conical tubes with their hind paws exposed, and the right hind paw was manually compressed with a circular motion for 2 minutes as described in a previous study.7 For primary hyperalgesia, mice were similarly restrained and the right gastrocnemius was squeezed with examiner's thumb pad and manually compressed with a circular motion for two minutes. The manual compression to hind paw and gastrocnemius muscle was applied by the same individual who was blinded to the group assignments. The amount of pressure for paw and gastrocnemius was attempted to be the same and determined by animal vocalization.
Immunohistochemistry
Two hours following gastrocnemius or hind paw compression,10,14,39 mice were deeply anesthetized with 500 μl 1.25 % Avertin (20 ul / g; 100% Avertin in 10 g 2,2,2-tribromoethyl alcohol and 10 ml tert-amyl alcohol) and perfused intracardically with 50 ml 1x PBS followed by 500 ml 4% paraformaldehyde (pH 7.4). The lumbar spinal cord was removed and the ventral surface of the spinal cord was nicked on one side to identify the right and left sides. The cord was postfixed overnight then cryoprotected overnight with 30% sucrose. Serial 20 μm sections of frozen lumbar cord were cut and mounted on microscope slides. Approximately every 3rd slide was chosen for Fos immunocytochemistry. The sides were incubated in 0.5% Triton X-100 for 20 minutes, followed by 2 washes and 10% normal horse serum for 10 minutes at room temperature. Sections were incubated overnight with a rabbit polyclonal Fos primary antibody (1:3000, Santa Cruz Biotechnology, Santa Cruz, Ca) at 4°C in a humidified tray. The sections were washed 3 times and incubated with the secondary antibody (donkey anti-rabbit CY3; 1:200 in 0.1M PBS; Jackson ImmunoResearch) for one hour at 4°C. Sections were washed in PBS and coverslipped before viewing.
To assess the rostrocaudal extent of spinal Fos activation in response to the primary site gastrocnemius or the secondary site paw stimulation, the lumbar spinal cord was subdivided into 3 segments: L2/3 (rostral), L4/5 (central) and L6 (caudal). Furthermore, each dorsal horn was divided into 3 regions on each side: lamina I and II (superficial region); lamina III and IV (intermediate region); lamina V and VI (deep region). Every 3rd section on each slide was used to count Fos-positive cells. The dorsal horns were photographed using a Nikon E800 microscope attached to a Magnafire digital camera (Optronics, Goleta, CA). An investigator blinded to the animals' grouping manually counted positive cells in each area of the ipsilateral and contralateral dorsal horns.
Statistical Analysis
For all measures, ipsilateral and contralateral sides were analyzed separately. A repeated measures analysis of variance (RM ANOVA) was used to analyze von Frey withdrawal responses and muscle compression withdrawal peak forces before and after acidic saline injections. In addition, a t-test was conducted to examine the group differences related to secondary (cutaneous) and primary (muscle) hyperalgesia at different time points when group differences or interactions (time*acid) were significant using RM ANOVA. Group differences in the number of Fos-positive cells in L4/L5 segment were also analyzed between and the two saline groups (pH 4.0; experiment and 7.4; control) using RM ANOVA to account for possible laminar interactions. Further analysis was conducted using t-tests to examine differences in specific laminae when either group or interactions were significant with RM ANOVA. Additionally, correlations were performed between behavioral measures and the number of Fos-positive cells in the ipsilateral superficial, intermediate and deep dorsal horns using Pearson's Correlation Coefficients. Values were considered significant at α level P < 0.05.
Results
Behavioral Assessments
Secondary Cutaneous Sensitivity
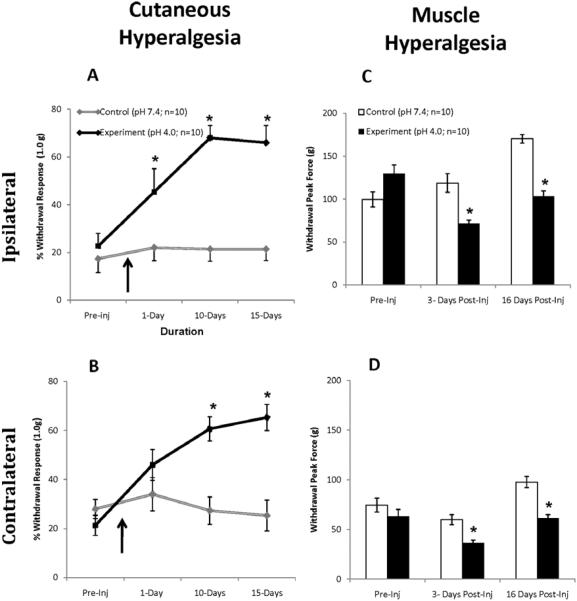
Saline injections of pH 4.0 (acidic saline) into the right gastrocnemius muscle produced secondary cutaneous hyperalgesia in both hind paws as assessed by paw withdraw in response to von Frey monofilaments. In pH 7.4 saline-injected mice (control group), withdrawal responses remained relatively constant on the ipsilateral and contralateral sides (Fig. 2A, B). Whereas the % withdrawal response in the ipsilateral paw of pH 4.0 saline-injected mice (experiment group) increased from 22.67% ± 5.28 SEM to 66.00% ± 7.20 SEM (P < 0.05) 15 days following acidic saline injection (Fig. 2A). The % withdrawal response in the contralateral paw of acidic saline-injected mice also increased from 21.33% ± 4.07 SEM to 65.33% ± 5.33 SEM (P < 0.05) 15 days following pH 4.0 saline injection (Fig. 2B). These results indicate changes in the withdrawal threshold level of ipsilateral and contralateral sides in animals following acidic saline injections and confirm the development of cutaneous hyperalgesia in the acidic saline-induced pain model.
Figure 2. Quantification of Hind Paw Withdrawal to Von Frey Stimulation of the Paw and Compression of the Gastrocnemius Muscle.
Mice displayed significant increase in cutaneous withdrawal responses from pre-injection to post-injection on the ipsilateral (A) and on the contralateral (B) sides following pH 4.0 saline injection. Likewise, mice displayed significant reductions in muscle withdrawal thresholds on both ipsilateral (C) and contralateral (D) sides following pH 4.0 saline injection. Arrows illustrate the time point at which pH 4.0 saline was injected into the right gastrocnemius. Data represented as mean ± S.E.M. Asterisks denote significant differences between neutral saline-and acidic saline-injected mice (P <0.05). Neutral saline-injection (pH 7.4) represents control group and acidic saline-injection (pH 4.0) represents experiment group.
Primary Muscle Sensitivity
The assessment of primary hyperalgesia was tested with a forceps compression device as shown inFig 1. In the ipsilateral limb of ph 7.4 saline-injected mice (neutral saline; control group), the compression withdrawal threshold increased from 99.58g ± 8.74 SEM pre-injection to 170.27g ± 5.04 SEM (P < 0.05Fig. 2C) 16 days following the 2nd injection. Likewise, the compression withdrawal thresholds in the contralateral limb of neutral saline-injected mice also increased (74.63g ± 7.12 SEM pre-injection to 97.71g ± 5.59 SEM post 16 days 2nd injection; P < 0.05;Fig. 2D). This suggests that the neutral saline-injected mice became acclimated to the test and were able to endure greater compression at later tests.
In contrast to neutral saline-injected mice, acidic saline-injected mice developed robust muscle hypersensitivity in response to pH 4.0 saline injection (Figs 2C, D). In the ipsilateral limb of acidic saline-injected mice, compression withdrawal thresholds significantly decreased from 129.39g ± 10.59 SEM pre-injection to 103.47g ± 6.13 SEM (P < 0.01) 16 days following pH 4.0 saline injections. In the contralateral limb of acidic saline-injected mice, the compression withdrawal thresholds also significantly decreased after pH 4.0 saline injection (63.43g ± 6.79 SEM to 61.09g ± 3.82 SEM 16 days post-acidic saline injections; P < 0.05). These decreases in compression withdrawal thresholds were also evident 3 days post acidic pH saline injection in both limbs and were significantly different from neutral saline-injected mice (P < 0.05,Fig. 2C, D). These results indicate that mice developed bilateral muscle hyperalgesia that lasted up to 16 days.
Acidic Saline-Induced Spinal Fos Expression From Hind Paw and Gastrocnemius
The early immediate gene c-Fos is upregulated in spinal cells following the administration of peripheral noxious stimuli. Here, the number of Fos-positive neurons in L4/5 segment was quantified in neutral saline- and acidic saline-injected animals in response to either rigorous hind paw palpation or compression of the gastrocnemius. Both stimuli were performed in the leg ipsilateral to pH 7.4 or 4.0 saline injection. Hind paw or gastrocnemius compression was performed 18 days following the second injection. As shown in Table 1 and Fig. 3, paw palpation induced the expression of a total of 39.89 ± 2.84 SEM Fos-positive cells in neutral saline-injected mice and 46.49 ± 2.88 SEM Fos-positive cells in acidic saline-injected mice. However, this increase was not statistically significant (P > 0.05).
Table 1.
| Superficial | Intermediate | Deep | Total | ||
|---|---|---|---|---|---|
| Paw Palpation | pH 7.4 | 12.46 ± 3.0 | 8.32 ± 1.88 | 19.11 ± 3.65 | 39.89 ± 2.84 |
| pH 4.0 | 16.19 ± 3.57 | 9.40 ± 1.88 | 20.90 ± 3.19 | 46.49 ± 2.88 | |
| Muscle Compression | pH 7.4 | 8.75 ± 2.47 | 5.31 ± 1.60 | 11.75 ± 1.21 | 25.80 ± 1.35 |
| pH 4.0 | *15.85 ± 2.10 | 7.12 ± 1.55 | 13.27 ± 1.58 | 36.24 ± 1.50 |
The table illustrates the mean ± S.E.M number of Fos-positive cells in each L4/L5 spinal laminae ipsilateral to the acidic saline-injected limb in response to either hind paw palpation (secondary hyperalgesia) or gastrocnemius compression (primary hyperalgesia).
denotes significant difference between neutral saline and acidic saline-injected mice.
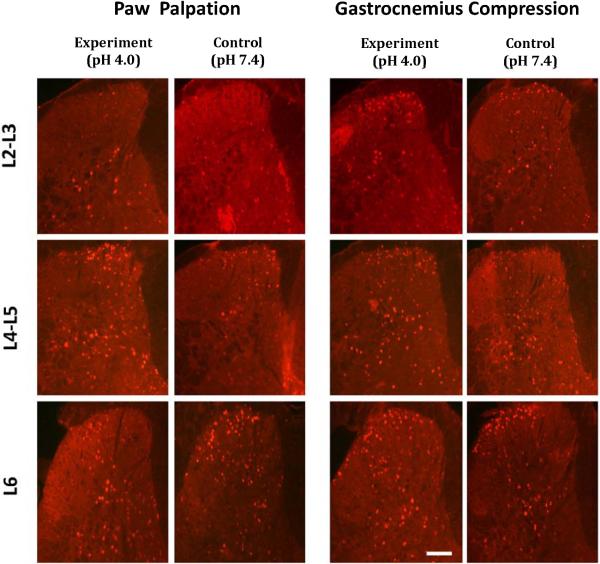
Figure 3. Acidic Saline-Induced Fos Expression in Ipsilateral Lumbar Dorsal Horn Following Paw Palpation or Gastrocnemius Compression.
Expression of Fos positive cells in ipsilateral L2/3, L4/5 and L6 dorsal horn of neutral and acidic saline-injected animals following paw palpation (left side) and gastrocnemius compression (right side). Images are displayed with scale bar equals 100 μms.
In contrast to paw palpation, gastrocnemius compression resulted in 25.80 ± 1.35 SEM Fos-positive cells in neutral saline-injected mice (Table 1, Fig. 3). However, Fos expression in acidic saline-injected mice was higher than neutral saline-injected mice (36.24 ± 1.50 SEM Fospositive cells), nearly reaching significant level (P = 0.054). Further analysis revealed that the effects of pH 4.0 saline on increased Fos expression were mainly restricted to the ipsilateral superficial laminae of L4/5 spinal level (P < 0.05). Thus fig 3 shows only ipsilateral dorsal horn of lumbar segments. This increased expression of Fos is consistent with the robust mechanical hypersensitivity and suggests that chronic primary hyperalgesia can be induced with acidic saline in mice. Moreover, even though no significant differences were observed following paw palpation, a similar trend was observed in that acidic saline increased Fos expression in the superficial laminae only, suggesting laminar-specific changes in response to acidic salineinduced hyperalgesia.
Fos Expression Patterns in Response to Mechanical Compression of Hind Paw or Gastrocnemius Muscle
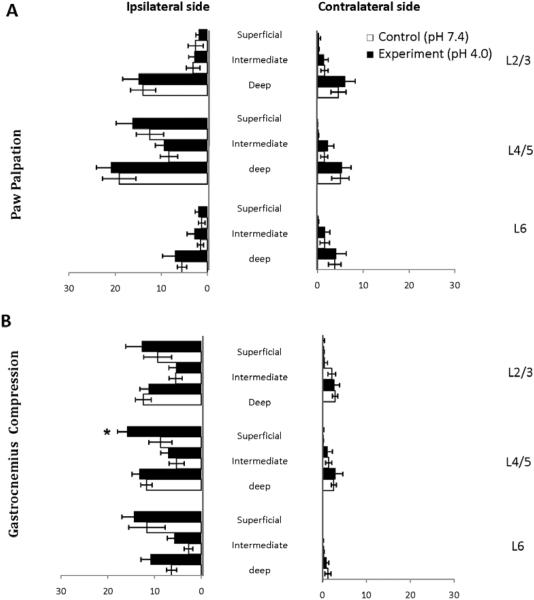
As part of the analysis of Fos expression, we mapped the rostrocaudal and ipsilateralcontralateral expression of Fos-positive cells in response to either secondary site paw palpation or primary site gastrocnemius compression. The number of Fos-positive cells from 3 different lumbar segments within each lamina from both ipsilateral and contralateral sides were quantified and represented schematically in Fig. 4. First, paw palpation induced Fos expression predominantly in deeper laminae in lumbar segments L2-L5; the greatest number of Fospositive cells were located in laminae V/VI. Second, paw palpation induced a noticeable bilateral expression of Fos. Finally, paw palpation induced Fos expression in superficial laminae that was restricted to lumbar segments L4/L5. In comparison to paw palpation, gastrocnemius compression led to a very different pattern of Fos expression (Fig. 4). First, gastrocnemius compression induced a relatively uniform pattern of Fos expression with superficial to deep laminae. This is in contrast to paw palpation in which the predominant pattern was in deep layers of the dorsal horn. Second, the Fos positive cells were restricted to the ipsilateral dorsal horn. Finally, gastrocnemius compression led to a much broader rostrocaudal pattern of expression that stretched from L2-L6. These results reveal that there are differences in the patterns of Fos expression with the dorsal horn (superficial to deep) in response to distal paw or proximal gastrocnemius compression sites. In addition, there are important differences in the rostrocaudal limits to Fos expression induced by paw or gastrocnemius compression.
Figure 4. Schematic Representation of Fos Expression in the Lumbar Spinal Cord.
A representation of the segmental distribution of the entire lumbar spinal cord following mechanical stimulation to hind paw (A) and gastrocnemius (B) anatomical sites. Following paw palpation, the largest number of Fos-positive cells was concentrated in superficial dorsal horn of segment L4/5 on ipsilateral side and deeper layers from L2-L5. Following gastrocnemius compression, widespread Fos activity was observed throughout the rostrocaudal extent of the lumbar spinal cord, L2-L6.
Correlations Between Behavioral Measures and Fos-Positive Cells
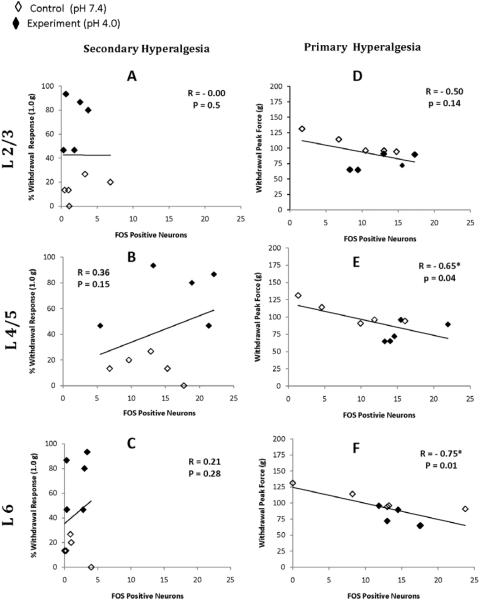
In addition, we performed an analysis of the correlation between behavioral responses and Fos expression of the superficial, intermediate and deep laminae at different spinal levels (Table 2, Fig. 5). The correlations between the superficial laminae and behavioral responses are also depicted in Fig. 5 as the superficial lamina displayed the most Fos activity from the primary site stimulation and significant changes in response to acidic saline injections. The analysis suggests that Fos expression in the dorsal horn correlates strongly with measures of primary site gastrocnemius behavioral sensitivity (Table 2, Right Panel; Fig. 5D, E, F). The correlations were significant at L4/5 superficial (p = 0.04) and L6 superficial (p = 0.01) and deep laminae (0.01; Table 2). Related to secondary site paw palpation, correlations were non-significant (Table 2, Left Panel; Fig. 5A, B, C), suggesting both measures of mechanical stimulus are distinct.
Table 2.
| Secondary hyperalgesia | Primary hyperalgesia | ||||||
|---|---|---|---|---|---|---|---|
| Superficial | Intermediate | Deep | Superficial | Intermediate | Deep | ||
| L2/3 | R | 0.00 | -0.05 | 0.07 | -0.05 | -0.34 | 0.11 |
| P | 0.99 | 0.87 | 0.83 | 0.14 | 0.34 | 0.77 | |
| L4/5 | R | 0.36 | 0.11 | 0.15 | -0.65* | -0.28 | 0.06 |
| P | 0.31 | .77 | 0.68 | .04 | 0.44 | 0.84 | |
| L6 | R | 0.21 | 0.18 | -0.03 | -0.75* | -0.75* | -0.27 |
| P | 0.57 | .61 | 0.92 | 0.01 | 0.01 | 0.27 | |
The table illustrates the correlations between von Frey-induced withdrawal responses and paw palpation-induced Fos positive cells (secondary hyperalgesia; Left panel) and between gastrocnemius compression peak forces and gastrocnemius compression-induced Fos positive cells (primary hyperalgesia; Right panel) in the ipsilateral superficial, intermediate and deep dorsal horn.
denotes significant correlations.
Figure 5. Correlation of Behavioral Responses and Fos Expression.
Correlations between von Frey-induced withdrawal responses and paw palpation-induced Fos expression (secondary hyperalgesia; A, B, C) and between gastrocnemius compression peak forces and gastrocnemius compression-induced Fos expression (primary hyperalgesia; D, E, F) in the ipsilateral superficial dorsal horn. Correlations were significant only with primary hyperalgesia at L4/5 and L6 segments.
Discussion
Muscular pain is relatively understudied and approaches to quantify muscle pain are limited. Here, we compared primary and secondary hyperalgesia that develops after acidic saline injection into the gastrocnemius muscle and analyzed the patterns of Fos expression following primary site gastrocnemius and secondary site paw stimulation. Our results suggest that acidic saline injection induces long-lasting primary muscle hyperalgesia and the patterns of Fos expression vary in response to gastrocnemius or paw stimulation. These results provide further evidence about our understanding of the acid-induced model of pain and give new insight about the patterns of spinal activation that arise from mechanical stimulation of different anatomical sites.
Acidic Saline-Induced Primary and Secondary Hyperalgesia
The intramuscular acidic saline model is proposed to mimic aspects of musculoskeletal pain associated with secondary hyperalgesia that spreads to the paws and viscera.22,30 This model does not rely on peripheral inflammation to elicit changes, and central mechanisms likely contribute to the maintenance of the hyperalgesia.11,27,30,32 Convergent input in the spinal cord from muscle/paw fibers and receptive field plasticity likely underlie the acidic saline-induced secondary hyperalgesia. For example, after acidic saline injection, wide dynamic range (WDR) neurons that respond to multiple peripheral stimuli and have broad receptive fields. After acidic saline injection, WDR neurons increase their responsiveness to mechanical stimuli and expand their receptive fields. These changes are thought to be critical for the appearance of the secondary hyperalgesia.31,38 As the use of intramuscular acidic saline injections increases, reports are emerging that pH 4.0 saline injection does not always generate demonstrable hypersensitivity. For example, a recent study by Ambalavanar et al.3 reported that pH 4.0 saline injection into the masseter muscle failed to induce nociceptive behavioral responses. This has led some to question the reliability of acidic saline to induce hyperalgesia, but this result could also be explained simply by the inherent differences in various muscles to acidic saline injection (masseter versus gastrocnemius). Our assessment of cutaneous secondary hyperalgesia is consistent with previous findings using this model, both from our laboratory and others.7,30
Our assessment of acidic saline-induced primary hyperalgesia adds to previous findings36,41 and suggests that pH 4.0 saline also induces muscle hyperalgesia that persists for at least 2 weeks. The response thresholds to gastrocnemius muscle compression significantly decreased bilaterally and remained decreased up to 2 weeks. Additional analysis of primary site hyperalgesia with Fos expression revealed significant differences in deep tissue sensitivity between neutral saline- and acidic saline-injected mice. These differences in Fos expression mirrored the behavioral assessments and suggest that pH 4.0 injection does lead to a primary hyperalgesia in mice and is consistent with previous reports in rats that propose acidic saline induces primary hyperalgesia.36,41 Our results extend their findings as the primary site hyperalgesia lasted for over 2 weeks, suggesting that this hyperalgesia is not transient.
The use of quantitative approaches to measure deep tissue pain thresholds is relatively new. Using similar measures in rats, investigators have reported average threshold around 15,000 - 23,000 mN29,42 under inflammatory conditions and 1500 mN following acidic saline-induced hyperalgesia.29 All of these studies used a flat contact surface with a relatively large area of pressure applied to the animals' muscle belly. In comparison, Schafers et. al.26 reported an average threshold of 1000 mN when pressure was applied to the muscle belly using a small contact area.
Our study is the first to report changes in muscle tissue pain thresholds using this approach in mice. Here, we used a small contact area to measure direct compressive force applied parallel to the muscle belly. We found the muscle compression threshold values for mice were approximately 75 to 100 g (1000 mN), indicating that mice may have similar threshold levels as rats when using the same size contact area. It is important to note that the mechanical threshold levels were greater for ipsilateral compared to the contralateral sides. This difference may be due to the testing paradigm, as the ipsilateral side was always tested before the contralateral side. It is possible that animals anticipated testing of the contralateral side and responded to a smaller amount of compression force. Moreover, mice were able to tolerate greater force over time, and these threshold changes could be attributed to acclimation to repeated testing and handling. Both of these effects may need to be accounted for in future studies.
Patterns of Fos Expression In Response to Hind Paw or Gastrocnemius Stimulation
Besides acidic saline-induced Fos analysis, we also assessed spinal cord activation pattern from two anatomical sites, hind paw and gastrocnemius nociception. These anatomical sites may have different central projections to the spinal cord. Previous studies have reported that afferent fibers from rat hind paw project predominantly to lamina I and II8 at L4/5 spinal segments1,35 with additional projections to adjacent segments (L3-L6).20 Accordingly, we observed the highest Fos expression in superficial laminae within spinal L4/L5 segments. In addition, we observed substantial Fos expression in deeper layers (laminae IV/V) following paw palpation, suggesting that mechanical palpation activates Fos expression significantly in deeper layers of the dorsal horn.
In comparison, patterns of Fos expression following gastrocneimus stimulation are not well understood. Wang and colleagues39 examined Fos expression following electrical stimulation to the gastrocnemius muscle and reported Fos expression predominantly in laminae I, II and V. Similarly, Hunt et. al.14 reported Fos expression in laminae I/II following the introduction of chemical irritants into the gastrocnemius. Our findings support the idea that gastrocnemius nociception activates Fos in laminae I, II and V, but also suggest that mechanical compression induces Fos in intermediate laminae as well. However, it is plausible that cutaneous afferents were also activated overlying the gastrocnemius, as our muscle compression included the overlying skin. Thus, the pattern of Fos reflects the anatomical site, the type of tissue stimulated, and the pain state of that tissue for acidic saline-induced animals.
One clear finding from this study is that compared to distal paw stimulation, proximal gastrocnemius stimulation induced Fos expression in a much broader pattern within the rostrocaudal axis. We observed relatively equal Fos expression in L2 and extending back to L6, suggesting that gastrocnemius compression leads to a broad activation of spinal neurons. This rostrocaudal expansion of Fos intensity into all dorsal horn layers within lumbar segments L2-L6 is in agreement with previous studies,6,34 supporting the idea that deep tissues project to multiple spinal segments compared to cutaneous projections.
Fos as a Correlate of Mechanical Sensitivity
Fos has been extensively used as a marker of activation of spinal cells in response to inflammatory1,2,5,12,14,16,21 and neuropathic pain.23,24 One important aspect of the current study is that we compared the von Frey monofilament-induced responses of mice with the expression of Fos following paw palpation. Interestingly, no correlation between these 2 measures of pain sensitivity was observed at any segmental level. In contrast, correlations between gastrocnemius muscle compression with forceps device and Fos expression induced by manual compression were strong and significant. This is expected as muscle compression is a noxious stimulation compared to von Frey monofilament stimulation, which is considered non-noxious. Our finding of acidic saline-induced primary hyperalgesia via behavioral measures and Fos activity indicates that the compression device used in the present study is a reliable tool to test deep tissue hyperalgesia in mice.
One important limitation to our study is that compressive force was manually delivered to induce Fos expression. A quantifiable method such as the forceps compression device used for behavioral testing in the present study should be utilized in future studies. Secondly, future studies should also consider anesthetizing skin overlying the gastrocnemius to minimize activation of cutaneous afferents during mechanical perturbation.
Finally, this study assessed whether acidic saline injection alters the patterns of Fos expression following compression of the gastrocnemius or paw. Surprisingly, acidic saline did not significantly change Fos expression of spinal cord in response to secondary hyperalgesia (paw palpation). However, select differences within the superficial dorsal horn were noted in association with the primary hyperalgesia (gastrocnemius compression). The inability of acidic saline to substantially increase overall spinal Fos patterns may support the view that spinal Fos expression is a better reflection of primary nociceptor drive from the periphery and may not indicate the pain status nearly as well in settings of central sensitization.
Conclusion
The present study assessed acidic saline-induced deep tissue hyperalgesia and spinal Fos expression following two anatomical sites, paw and gastrocnemius in mice. Our results confirms previous studies in rats that acidic saline does induce primary muscle hyperalgesia36,41, in addition to the cutaneous and visceral pain that has been reported.7,22,30 This study adds to the growing knowledge of muscle thresholds and primary hyperalgesia in this model of widespread pain and provides new information about Fos-related patterns of spinal activation in response to mechanically stimulated gastrocnemius afferent fibers.
Acknowledgements
Support contributed by NIH R01NS43314 (DEW) and the Foundation for Physical Therapy Research (POD II scholarship) (NKS). The authors would like to thank Megan Johnson and Karra Muller for helpful comments on the manuscript and Melissa Sindt for helping with behavioral testing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbadie C, Besson JM. c-fos expression in rat lumbar spinal cord during the development of adjuvant-induced arthritis. Neuroscience. 1992;48:985–993. doi: 10.1016/0306-4522(92)90287-c. [DOI] [PubMed] [Google Scholar]
- 2.Abbadie C, Honore P, Fournie-Zaluski MC, Roques BP, Besson JM. Effects of opioids and non-opioids on c-Fos-like immunoreactivity induced in rat lumbar spinal cord neurons by noxious heat stimulation. Eur J Pharmacol. 1994;258:215–227. doi: 10.1016/0014-2999(94)90483-9. [DOI] [PubMed] [Google Scholar]
- 3.Ambalavanar R, Yallampalli C, Yallampalli U, Dessem D. Injection of adjuvant but not acidic saline into craniofacial muscle evokes nociceptive behavious and neuropeptide expression. Neuroscience. 2007;149:650–659. doi: 10.1016/j.neuroscience.2007.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergman S. Management of musculoskeletal pain. Best Pract Res Clin Rheumatol. 2007;21(1):153–66. doi: 10.1016/j.berh.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Bon K, Wilson SG, Mogil JS, Roberts WJ. Genetic evidence for the correlation of deep dorsal horn Fos protein immunoreactivity with tonic formalin pain behavior. J Pain. 2002;3:181–189. doi: 10.1054/jpai.2002.123710. [DOI] [PubMed] [Google Scholar]
- 6.Clement CI, Keay KA, Podzebenko K, Gordon BD, Bandler R. Spinal sources of noxious visceral and noxious deep somatic afferent drive onto the ventrolateral periaqueductal gray of the rat. J Comp Neurol. 2000;425:323–344. doi: 10.1002/1096-9861(20000925)425:3<323::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi R, Ryals JM, Wright DE. Neurotrophin-3 reverses chronic mechanical hyperalgesia induced by intramuscular acid injection. J Neurosci. 2004;24:9405–9413. doi: 10.1523/JNEUROSCI.0899-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graven-Nielsen T, Arendt-Nielsen L, Mense S. Thermosensitivity of muscle: high-intensity thermal stimulation of muscle tissue induces muscle pain in humans. J Physiol. 2002;540:647–656. doi: 10.1113/jphysiol.2001.013336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graven-Nielsen T, Mense S. The peripheral apparatus of muscle pain: evidence from animal and human studies. Clin J Pain. 2001;17:2–10. doi: 10.1097/00002508-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Harris JA. Using c-fos as a neural marker of pain. Brain Res Bull. 1998;45:1–8. doi: 10.1016/s0361-9230(97)00277-3. [DOI] [PubMed] [Google Scholar]
- 11.Hoeger-Bement MK, Sluka KA. Phosphorylation of CREB and mechanical hyperalgesia is reversed by blockade of the cAMP pathway in a time-dependent manner after repeated intramuscular acid injections. J Neurosci. 2003;23:5437–5445. doi: 10.1523/JNEUROSCI.23-13-05437.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoheisel U, Mense S, Simons DG, Yu XM. Appearance of new receptive fields in rat dorsal horn neurons following noxious stimulation of skeletal muscle: a model for referral ofT muscle pain? Neurosci Lett. 1993;153:9–12. doi: 10.1016/0304-3940(93)90064-r. [DOI] [PubMed] [Google Scholar]
- 13.Holden JE, Pizzi JA. The challenge of chronic pain. Adv Drug Deliv Rev. 2003;55:935–948. doi: 10.1016/s0169-409x(03)00097-8. [DOI] [PubMed] [Google Scholar]
- 14.Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- 15.Ikeuchi M, Kolker SJ, Burnes LA, Walder RY, Sluka KA. Role of ASIC3 in the primary and secondary hyperalgesia produced by joint inflammation in mice. Pain. 2008;137:662–669. doi: 10.1016/j.pain.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jinks SL, Simons CT, Dessirier JM, Carstens MI, Antognini JF, Carstens E. C-fos induction in rat superficial dorsal horn following cutaneous application of noxious chemical or mechanical stimuli. Exp Brain Res. 2002;145:261–269. doi: 10.1007/s00221-002-1128-3. [DOI] [PubMed] [Google Scholar]
- 17.Kehl LJ. Eccentric exercise induces muscle hyperalgesia in rats. Am Pain Soc. 1996;641:A-51. Abstracts. [Google Scholar]
- 18.Kehl LJ, Fairbanks CA. Experimental animal models of muscle pain and analgesia. Exerc Sport Sci Rev. 2003;31:188–194. doi: 10.1097/00003677-200310000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Kehl LJ, Trempe TM, Hargreaves KM. A new animal model for assessing mechanisms and management of muscle hyperalgesia. Pain. 2000;85:333–343. doi: 10.1016/S0304-3959(99)00282-1. [DOI] [PubMed] [Google Scholar]
- 20.Lawrence J, Stroman PW, Bascaramurty S, Jordan LM, Malisza KL. Correlation of functional activation in the rat spinal cord with neuronal activation detected by immunohistochemistry. Neuroimage. 2004;22:1802–1807. doi: 10.1016/j.neuroimage.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Lighthall G, Liang DY, Clark JD. Alterations in spinal cord gene expression after hindpaw formalin injection. J Neurosci Res. 2004;78:533–541. doi: 10.1002/jnr.20274. [DOI] [PubMed] [Google Scholar]
- 22.Miranda A, Peles S, Rudolph C, Shaker R, Sengupta JN. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126:1082–1089. doi: 10.1053/j.gastro.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Molander C, Hongpaisan J, Grant G. Changing pattern of c-FOS expression in spinal cord neurons after electrical stimulation of the chronically injured sciatic nerve in the rat. Neuroscience. 1992;50:223–236. doi: 10.1016/0306-4522(92)90394-h. [DOI] [PubMed] [Google Scholar]
- 24.Munglani R, Hunt SP. Molecular biology of pain. Br J Anaesth. 1995;75:186–192. doi: 10.1093/bja/75.2.186. [DOI] [PubMed] [Google Scholar]
- 25.Radhakrishnan R, Moore SA, Sluka KA. Unilateral carrageenan injection into muscle or joint induces chronic bilateral hyperalgesia in rats. Pain. 2003;104:567–577. doi: 10.1016/s0304-3959(03)00114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schafers M, Sorkin LS, Sommer C. Intramuscular injection of tumor necrosis factor-alpha induces muscle hyperalgesia in rats. Pain. 2003;104:579–588. doi: 10.1016/S0304-3959(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 27.Skyba DA, King EW, Sluka KA. Effects of NMDA and non-NMDA ionotropic glutamate receptor antagonists on the development and maintenance of hyperalgesia induced by repeated intramuscular injection of acidic saline. Pain. 2002;98:69–78. doi: 10.1016/s0304-3959(01)00471-7. [DOI] [PubMed] [Google Scholar]
- 28.Skyba DA, Lisi TL, Sluka KA. Excitatory amino acid concentrations increase in the spinal cord dorsal horn after repeated intramuscular injection of acidic saline. Pain. 2005;119:142–149. doi: 10.1016/j.pain.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 29.Skyba DA, Radhakrishnan R, Sluka KA. Characterization of a method for measuring primary hyperalgesia of deep somatic tissue. J Pain. 2005;6:41–47. doi: 10.1016/j.jpain.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 31.Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–239. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- 32.Sluka KA, Rohlwing JJ, Bussey RA, Eikenberry SA, Wilken JM. Chronic muscle pain induced by repeated acid Injection is reversed by spinally administered mu- and delta-, but not kappa-, opioid receptor agonists. J Pharmacol Exp Ther. 2002;302:1146–1150. doi: 10.1124/jpet.102.033167. [DOI] [PubMed] [Google Scholar]
- 33.Svendsen O, Edwards CN, Lauritzen B, Rasmussen AD. Intramuscular injection of hypertonic saline: in vitro and in vivo muscle tissue toxicity and spinal neurone c-fos expression. Basic Clin Pharmacol Toxicol. 2005;97:52–57. doi: 10.1111/j.1742-7843.2005.pto_97108.x. [DOI] [PubMed] [Google Scholar]
- 34.Taguchi T, Matsuda T, Tamura R, Sato J, Mizumura K. Muscular mechanical hyperalgesia revealed by behavioural pain test and c-Fos expression in the spinal dorsal horn after eccentric contraction in rats. J Physiol. 2005;564:259–268. doi: 10.1113/jphysiol.2004.079483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi Y, Chiba T, Kurokawa M, Aoki Y. Dermatomes and the central organization of dermatomes and body surface regions in the spinal cord dorsal horn in rats. J Comp Neurol. 2003;462:29–41. doi: 10.1002/cne.10669. [DOI] [PubMed] [Google Scholar]
- 36.Tillu DV, Gebhart GF, Sluka KA. Descending facilitatory pathways from the RVM initiate and maintain bilateral hyperalgesia after muscle insult. Pain. 2007;136(3):331–9. doi: 10.1016/j.pain.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Todd AJ, Spike RC, Young S, Puskar Z. Fos induction in lamina I projection neurons in response to noxious thermal stimuli. Neuroscience. 2005;131:209–217. doi: 10.1016/j.neuroscience.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- 39.Wang SF, Chen CC, Liao WS, Shyu BC. Different types of variant muscle nociception after intermittent and continuous neuromuscular stimulation in rats. J Biomed Sci. 2005;12:467–479. doi: 10.1007/s11373-005-6595-7. [DOI] [PubMed] [Google Scholar]
- 40.Williams S, Evan GI, Hunt SP. Changing patterns of c-fos induction in spinal neurons following thermal cutaneous stimulation in the rat. Neuroscience. 1990;36:73–81. doi: 10.1016/0306-4522(90)90352-5. [DOI] [PubMed] [Google Scholar]
- 41.Yokoyama T, Maeda Y, Audette KM, Sluka KA. Pregabalin reduces muscle and cutaneous hyperalgesia in two models of chronic muscle pain in rats. J Pain. 2007;8:422–429. doi: 10.1016/j.jpain.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Yu YC, Koo ST, Kim CH, Lyu Y, Grady JJ, Chung JM. Two variables that can be used as pain indices in experimental animal models of arthritis. J Neurosci Methods. 2002;115:107–113. doi: 10.1016/s0165-0270(02)00011-0. [DOI] [PubMed] [Google Scholar]