Abstract
Previously we reported that plasma kallikrein and high molecular weight kininogen attach to the surface of dextran-coated superparamagnetic iron oxide nanoparticles (SPIONs) through the incompletely covered iron oxide core (Simberg et al., Biomaterials, 2009). Here we show that SPIONs also activate kallikrein-kinin system in vitro and in vivo. The serine protease activity of kallikrein was stably associated with SPIONs and could be detected on the nanoparticles even after extensive washing steps. The enzymatic activity was not detectable in kininogen-deficient and Factor XII-deficient plasma. The enzymatic activation could be blocked by precoating SPIONs with histidine-rich Domain 5 (D5) of kininogen. Importantly, the kallikrein activity was detectable in plasma of SPION-injected, but not of D5/SPION-injected mice. Tumor-targeted SPIONs when injected into kininogen-deficient and control mice, produced high levels of vascular clotting in tumors, suggesting that kallikrein activation is not responsible for the nanoparticle-induced thrombosis. These data could help in understanding the toxicity of nanomaterials and could be used in designing nanoparticles with controlled enzymatic activity.
Keywords: Kallikrein, iron oxide, nanoparticles, plasma, kininogen, tumor
1 Introduction
Basic understanding of interactions of nanomaterials with the body milieu is important for development of efficient drug delivery and imaging reagents. Upon injection into the blood, nanomaterials undergo extensive interactions with plasma proteins and cells [1] and become coated (opsonized) with various plasma proteins. The opsonization promotes nanoparticle clearance and toxicity. For example, absorption of complement factors makes the nanoparticles recognizable by macrophages through complement receptors, and in parallel induces toxicity by activation of downstream complement pathways [2].
Superparamagnetic iron oxide nanoparticles (SPIONs) are a widely used MR contrast imaging reagent [3]. SPIONs have been recently explored for targeted imaging of tumors. In these applications, SPIONS that were coated with targeting ligands achieved enhanced localization in the tumor tissue, greatly improving the imaging contrast by MRI [4] or by optical imaging [5]. Previously, we described the specific intravascular tumor clotting induced by SPIO coupled to homing peptide CREKA. This phenomenon is reminiscent of contact clotting activation of biomaterials [6], with the exception that thrombosis was restricted to tumors. We also recently reported [7] the specific assembly of histidine-rich plasma proteins, such as high molecular weight kininogen and histidine-rich glycoprotein, on the exposed iron oxide of dextran-coated SPIONs.
Here we show that following contact of SPIONs with mouse or human plasma the nanoparticles acquire kallikrein enzymatic activity through binding of kallikrein-kinin-system to the exposed iron oxide on the nanoparticle’s surface. Notably, this activity persists even after isolation of nanoparticles from plasma, and could be controlled through surface modification of nanoparticles. Although we demonstrate that the activation of kallikrein-kinin system does not play a role in the previously described prothrombogenic activity of SPIONs in tumor blood vessels [5], the controlled activation of kallikrein on the surface of SPIONs could be potentially used for design of smart proteolytic nanoparticles.
2 Materials and methods
2.1 Nanoparticles and plasma
Plain surface and aminated surface dextran-coated superparamagnetic iron oxide nanoparticles were synthesized in the laboratory of Dr. Sailor, UCSD [8]. Ferridex™ iron oxide nanoparticles were obtained from the UCSD Department of Radiology. The level of amination of SPIONs was varied by adding different concentrations of ammonia to epichlorohydrin-activated SPIONs [8]. The level of amination was determined as following. One hundred microliters of SPIONs solution in PBS (1 mg/ml) was mixed under vortexing with 10 μl of 0.1 mg/ml DMSO solution of FITC (Sigma). After 1h incubation, particles were precipitated once with 600 μl of 95% ethanol and spun at 14,000 rpm for 10 min. One hundred μl of the supernatant was mixed with 600 μl PBS and 10 μl ammonia, and the absorbance was measured at 492 nm. The amount of the reacted FITC was calculated by subtracting OD of the supernatant from OD of FITC solution (processed as above but with PBS added instead of the particles) and dividing by the molar extinction coefficient of FITC (78000). Pentapeptide CREKA labeled with fluorescein was conjugated using maleimide chemistry to the activated nanoparticles as described before [5]. Zeta potential was measured using Zetasizer instrument (Malvern). Fine glass microparticles were prepared by grinding pieces of a Pasteur pipette into powder with mortar and pestle.
Citrated human or mouse plasma were collected and stored at −80C. Factor XII deficient plasma was purchased from George King Bio-Medical. High molecular weight kininogen-deficient mouse plasma was from the laboratory of Dr. McCrae [9]. Glutathione-S-transferase (GST)-fusion histidine-rich domain 5 (D5) of mouse high molecular weight kininogen was from the same laboratory. For western blotting, the following antibodies were used: goat anti-mouse kallikrein antibody (R&D) and rabbit anti-mouse kininogen (provided by Dr. McCrae).
2.2 Nanoparticle interaction with plasma proteins
To determine whether nanoparticles attract specific clotting factors following incubation in citrated plasma, 0.3 ml of mouse plasma were incubated with 100 μg SPIONs, washed 4 times in PBS with recovery of SPIONs by ultracentrifugation at 100,000g for 10 min, and then eluted the bound proteins from SPIONs with 8M urea/1M NaCl. Eluted proteins were then separated by SDS-PAGE and identified by western blotting using specific antibodies.
2.3 Measurement of kallikrein activation in vitro
One hundred microliters of iron oxide nanoparticles (1 mg/ml) were incubated with 0.5 ml of a 1:10 diluted plasma in PBS for 5 min at room temperature. Kallikrein inhibitor aprotinin (Sigma) was used at a final concentration 0.5 mg/ml. For testing the inhibitory effect of kininogen D5 domain, 100 μg iron oxide were preincubated for 5 min with 20 μg of GST-D5 or GST, before adding plasma as above. To test the effect of washing on the enzymatic activity of the plasma protein-coated nanoparticles, the latter were washed 2 times with 1 ml PBS by ultracentrifugation. The supernatant was discarded, and the nanoparticle pellet or the same volume of control plasma (20 μl) was resuspended in 100 μl PBS. Following incubation with plasma, 100 μl of kallikrein chromogenic substrate (Chromogenix S-2302, DiaPharma) were added and the mixture was incubated on a water bath for 50 min at 37°C. SPIONs strongly absorb at 405 nm, which is also the absorbance wavelength of the kallikrein substrate. Therefore, for practical reasons, in the majority of the experiments the particles were pelleted by ultracentrifugation after the incubation, and the absorbance of the supernatant was measured using microplate reader. Whenever enzyme kinetics was measured, the cleavage of the substrate was monitored by reading the absorbance at 405 nm at 15 s intervals for 10 min using absorbance microplate reader (Molecular Devices, Sunnyvale, CA, U.S.A.). The initial absorbance of SPIONs was then subtracted.
2.4 Mouse experiments
All animal work was reviewed and approved by Burnham Institute’s Animal Research Committee. SPIONs were preincubated with kininogen D5, or with PBS. For intravenous injections, 8 week old BALB/c mice were anesthetized with intrapertioneal Avertin. Nanoparticles (1–4 mg Fe/kg body weight) were injected into the tail vein. Thirty minutes post-injection, mice were anesthetized and 200 μl of blood was collected with a syringe through the left ventricle, using 0.4% sodium citrate as an anticoagulant. Plasma was separated from cells by centrifugation at 5,000g for 10 min, diluted 10-fold in PBS and incubated with chromogenic substrate with or without aprotinin as described above. The kallikrein activity was measured as described for in vitro experiments.
For tumor homing experiments, we used mice with the disrupted Kng1 gene that lacked plasma kininogens. Eight-to-ten week-old kininogen-deficient and wild type female mice (C57BL/6 background) were injected with 1×106 B16/F1 melanoma cells s.c. After tumors had grown to 1cm diameter, mice were injected intravenously with CREKA-SPIONs as described [5]. Six hours later the mice we sacrificed and the tumors were processed for histological examination as described [5].
3 Results and Discussion
3.1 Kallikrein activation by SPIONs in vitro
We previously reported that SPIONs absorb significant amounts of kininogen and kallikrein [10] on their surface. To analyze the pattern of binding of these proteins to the nanoparticles, we performed immunoblotting with specific antibodies against kallikrein and kininogen, comparing the proteins attached to SPIONs with native plasma. Both kininogen and kallikrein were present on the particles but were cleaved into fragments, as indicated by their faster migration than that of the parent proteins, 120 kDa high molecular weight kininogen and 88 kDa kallikrein precursor (Fig. 1).
Figure 1. Binding of plasma proteins to iron oxide nanoparticles.
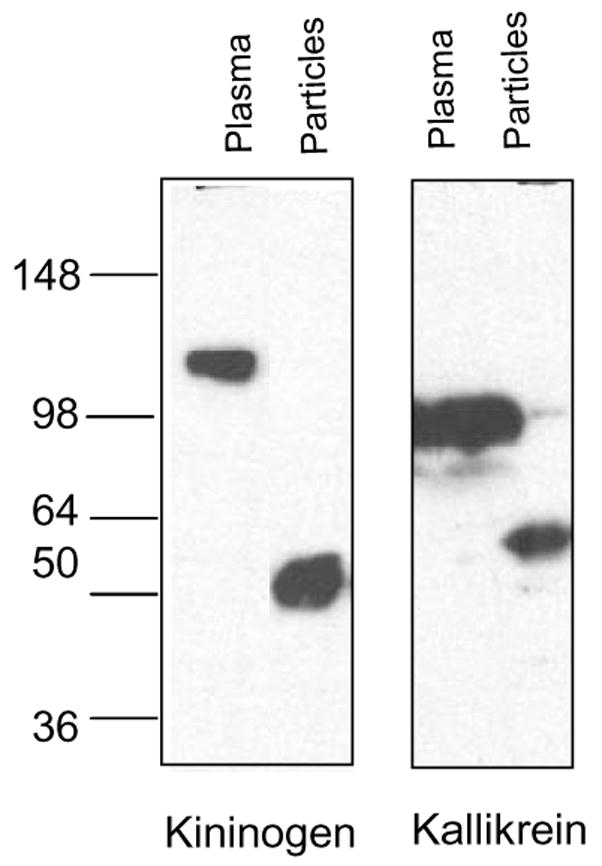
The nanoparticles were incubated with plasma or peptides as described in Methods. Particle-bound proteins were eluted and separated by SDS gel electrophoresis. The proteins were analyzed with specific antibodies against kininogen and kallikrein. Both antibodies were specific for the light chain of the proteins, detecting uncleaved 120 kDa kininogen and 88kDa kallikrein (arrows), or the cleaved light chain of both proteins (between 50 and 64 kDa size). One representative experiment out of 5 is shown.
These cleaved products were not detectable in normal plasma, only in plasma absorbed on SPIONs, suggesting that the cleavage took place as a result of binding to the nanoparticles.
The kallikrein-kinin system is known to assemble and become activated on foreign surfaces [11]. Kallikrein usually circulates in plasma in an equimolar complex with kininogen [11]. To measure the enzymatic activation of kallikrein, a serine protease, following the interaction between plasma and the nanoparticles, we used a specific chromogenic substrate (see Methods). The activation was tested in both mouse and human plasma. We used fine glass microparticles as a positive control for kallikrein activation. We observed that both glass particles and SPIONs nanoparticles were powerful activators of kallikrein in plasma (Fig. 2A). Next, we used aprotinin, which inhibits kallikrein, but not factor FXII, to exclude the contribution of FXII to the observed proteolytic activity. This activation was inhibited by approximately 90% by aprotinin, suggesting that kallikrein primarily contributed to the observed enzymatic activity (Fig. 2A). The same results were obtained when human plasma was used (not shown).
Figure 2. Kallikrein activation in human and mouse plasma.
Nanoparticles were incubated with diluted citrated plasma and with kallikrein chromogenic substrate as described in Methods. A, Effect of SPIONs on kallikrein activation in normal and kininogen-deficient mouse plasma; B, Kallikrein activity in human plasma and the role of Factor XII deficiency in the activation. The experiments were repeated 3 times.
In order to test whether kallikrein is activated by binding through high molecular weight kininogen to SPIONs, we used human plasma deficient for this protein [9]. The proteolytic activation of kallikrein by SPIONs was completely absent in plasma deficient for kininogen (Fig. 2A). Also, as shown in Figure 2B, kallikrein activation was absent in factor FXII deficient human plasma. Factor XII is known to bind to foreign surfaces where it is converted to factor XIIa, which is mainly responsible for cleavage and activation of kallikrein [11].
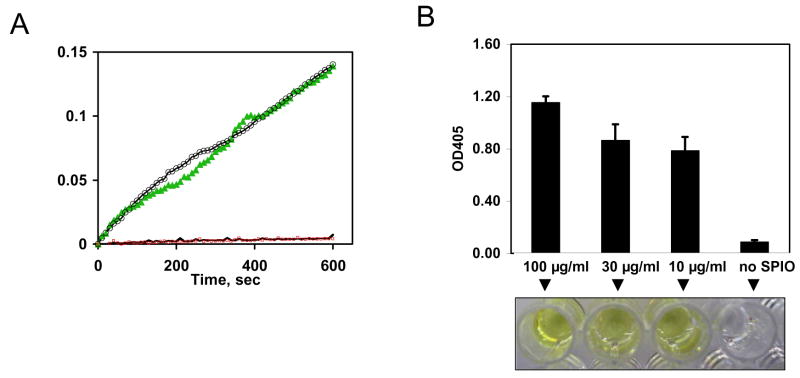
To demonstrate that the kallikrein activity results from particle-bound kallikrein, rather than from free plasma kallikrein, nanoparticles were incubated with plasma or PBS and washed as described in Methods. In parallel, plasma without SPIONs was washed in exactly the same way. The enzymatic conversion of the chromogenic substrate was measured for 10 min. According to the kinetic plot, high residual enzymatic activity was associated with the washed particles that were incubated in plasma, but not with the particles incubated in PBS (Fig. 3A).
Figure 3. Effect of SPION concentration and washing on the activation of kallikrein.
A, Effect of washing on kallikrein activity of SPIONs. Nanoparticles were incubated with plasma or PBS and washed as described in Methods section. In parallel, plasma without added nanoparticles was processed in exactly the same way. Ten microliters of SPIONs or control plasma were incubated with 50 μl of the chromogenic substrate. The substrate conversion was monitored by reading the absorbance in the kinetic mode over 10 min period. All the samples were run in duplicates. Plasma-coated SPIONs (green triangles and black circles show different replicates) showed very strong enzymatic activity, while control plasma replicate (blue trace) and control SPIONs (red trace) show no enzymatic activity (only one replicate of each sample is shown due to the overlapping curves); B, SPIONs nanoparticles were incubated with plasma at different concentrations, washed 3 times by ultracentrifugation, and the kallikrein activity was visualized by the intensity of yellow color of the chromogenic substrate as described in Methods.
Also, no residual enzymatic activity was found in the control plasma tube (without SPIONs) that was subjected to the same washings. To assess how much SPIONs is needed to produce measurable activity of kallikrein, three different concentrations of SPIONs were tested (Fig. 3B). Judging by the color intensity, the enzymatic cleavage of the chromogenic substrate after 50 min incubation was high for all the concentrations tested (Fig. 3B).
3.2 Effect of SPIONs surface properties on the activation of kallikrein in vitro
We next tested the role of the nanoparticle surface properties in kallikrein activation. SPIONs with different amination levels were prepared (zeta potential −4.9, +1.5, +4.8, respectively). The level of amination for each type of particle was also quantified as described in Methods (Fig. 4A).
Figure 4. Activation and cleavage of mouse plasma kallikrein by SPIONs nanoparticles with different amination level.
SPIONs with different amination levels (non-, low- or high- amine) or with conjugated peptide CREKA were incubated in plasma, and the kallikrein activity was measured as described in Methods. A, Measured levels of amination of SPIONs as described in Methods. B, Kallikrein activity in mouse plasma; C, nanoparticles were washed extensively and the bound proteins were analyzed on SDS-PAGE and visualized with anti-kininogen, left, and anti-kallikrein, right. Highly aminated surface prevents cleavage and activation of kallikrein. The experiments were repeated 2 times; D, Inhibition of activation of kallikrein by preincubation with Domain 5 of high molecular weight kininogen. The experiment was repeated three times.
In addition, SPIONs modified with CREKA, a potent tumor-homing peptide that recognizes clotted plasma [5], were prepared. Non-aminated SPIONs, low-aminated SPIONs and CREKA-SPIONs all produced high levels of kallikrein activation of mouse plasma kallikrein (Fig. 4B). Interestingly, Ferridex™ nanoparticles that are used clinically for liver MRI, also produced strong kallikrein activation (not shown). At the same time, highly aminated nanoparticles did not activate kallikrein. Western blotting analysis showed that kallikrein was bound to, but not cleaved on highly-aminated nanoparticles (Fig. 4C). This phenomenon could be attributed to the inactivation of kallikrein, FXII or both upon interaction with the highly basic surface of nanoparticles. Kininogen also remained uncleaved on the surface of highly aminated SPIONs (Fig. 4C).
Since kallikrein binds to the iron oxide through the histidine-rich domain 5 of high molecular weight kininogen [7], we tested whether precoating nanoparticles with recombinant D5 (described in Methods) could inhibit or decrease the activation of kallikrein. We previously found that D5 can inhibit binding of both kininogen and histidine-rich glycoprotein to the SPIONs iron oxide surface [7]. As demonstrated in Fig. 4D, kininogen D5 domain completely inhibited the observed enzymatic activity of kallikrein. Similar inhibition by D5 was observed in human plasma (not shown).
3.3 Kallikrein activation in vivo
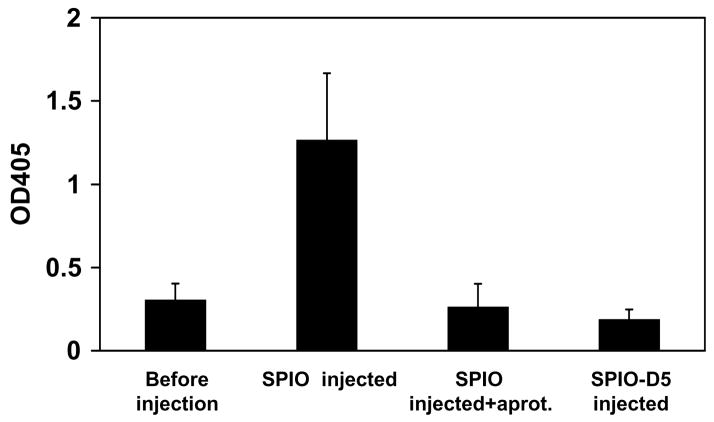
The artificial surface of nanoparticles provides a scaffold for binding and activation of an enzymatic machinery. In view of stable and efficient activation of kallikrein on the surface of SPIONs, it would be interesting to know whether intravenously injected SPIONs are also enzymatically active. To assess that hypothesis, we collected citrated plasma from mice that were injected with plain SPIONs or D5-coated SPIONs, and from non-injected control mice (Fig. 5). Plasma isolated from SPIONs-injected mice showed strong kallikrein activity that was almost completely inhibitable by aprotinin. Remarkably, plasma collected from mice injected with D5-coated nanoparticles was proteolytically inactive, suggesting that, similarly to the in vitro results, D5 was able to inhibit the kallikrein activation by nanoparticles in vivo.
Figure 5. Proteolytic activation of nanoparticles after injection in vivo.
SPIONs were injected intravenously into mice, the plasma sample was collected at 30 min and analyzed for kallikrein activation as described in Methods. Note that preincubation of SPIONs with D5 before injection prevented the activation of kallikrein. Addition of aprotinin to plasma collected from SPIO-injected mice also blocked the proteolytic activity. The average of two experiments is shown.
3. 4 Role of kallikrein-kinin system in the in vivo prothrombogenic properties of SPIONs
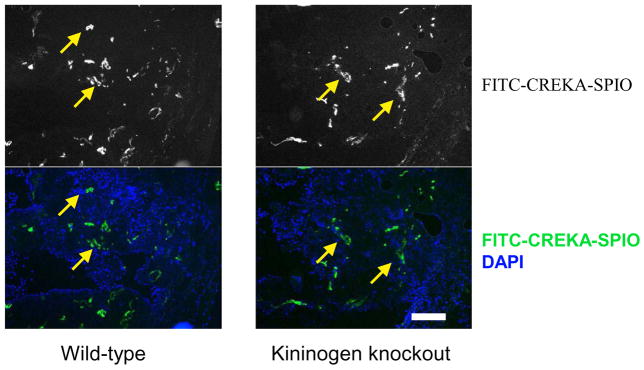
The role of the contact clotting pathway in the thrombogenic activity of nanomaterials is not clear [6]. SPIONs coupled to tumor-homing peptide CREKA promote thrombosis in tumor-specific thrombosis in blood vessels [5]. The thrombosis is initiated by local high concentration of SPIONs in the lumen and leads to the entrapment of additional nanoparticles in the growing intravascular thrombi, appearing as bright fluorescent aggregates inside tumor vasculature. To investigate the role of kallikrein-kinin system in the tumor clotting by CREKA-SPIO, we grew subcutaneous B16/F1 melanoma tumors in high molecular weight kininogen-deficient [9] and wild type mice. The experiments in kininogen-deficient mice did not show any significant decrease in the intravascular clotting (Fig. 6), with both kininogen-deficient and wild-type mice showing massive accumulation of the nanoparticles in the vascular clots. Although contact system is important for clotting activation in vitro [11], it is unlikely that activation of kallikrein on the surface of SPIONs plays a major role in the observed intravascular clotting.
Figure 6. Effect of kininogen deficiency on the thrombogenic activity of SPIONs in tumors.
CREKA-SPIONs nanoparticles were injected into tumor-bearing mice as described in Methods, and the tumor distribution of CREKA-SPIONs was examined 6 h post-injection. Upper images: accumulation of FITC-labeled nanoparticles in the tumors. Lower images: merge of DAPI (nuclei, blue) and fluorescein (nanoparticles, green) fluorescence. In both kininogen-deficient and control mice the entrapment of SPIONs in thrombi in blood vessels (yellow arrows) was observed. Size bar, 200 μm. One representative experiment out of 4 is shown.
There is a significant interest in delivering active enzymes to tumors for therapy and prodrug activation [12–14]. The biomimetic conversion of nanoparticles into enzymatically-active entities in vivo and its regulation by surface modifications is a novel addition to the known functions of nanoparticles, and to our best knowledge, has not been thoroughly investigated before. Tumor-targeted CREKA-SPIONs nanoparticles localize in the vascular luminal space in tumors (Fig. 6). Our next steps will be focused to determine if CREKA-SPIONs still maintain their kallikrein activity after homing in the tumor blood vessels. If positive, then the use of this phenomenon could be a prodrug therapy where nanoparticles lodged in tumor vasculature activate a subsequently injected prodrug. The ability of histidine-rich peptides to block the proteolytic activation of nanoparticles creates a possibility of engineering nanoparticles with controlled enzymatic activity.
Acknowledgments
This work was supported by National Cancer Institute Grant CA119335 and, in part, by CA124427.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moghimi SM, Hunter AC, Murray JC. Pharmacol Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 2.Moghimi SM, Patel HM. Biochim Biophys Acta. 1989;984:384–7. doi: 10.1016/0005-2736(89)90307-6. [DOI] [PubMed] [Google Scholar]
- 3.Bulte JW, Kraitchman DL. NMR Biomed. 2004;17:484–99. doi: 10.1002/nbm.924. [DOI] [PubMed] [Google Scholar]
- 4.Schellenberger EA, Bogdanov A, Jr, Hogemann D, Tait J, Weissleder R, Josephson L. Mol Imaging. 2002;1:102–7. doi: 10.1162/15353500200202103. [DOI] [PubMed] [Google Scholar]
- 5.Simberg D, Duza T, Park JH, Essler M, Pilch J, Zhang L, Derfus AM, Yang M, Hoffman RM, Bhatia S, Sailor MJ, Ruoslahti E. Proc Natl Acad Sci U S A. 2007;104:932–6. doi: 10.1073/pnas.0610298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorbet MB, Sefton MV. Biomaterials. 2004;25:5681–703. doi: 10.1016/j.biomaterials.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 7.Simberg D, Park JH, Karmali PP, Zhang WM, Merkulov S, McCrae K, Bhatia SN, Sailor M, Ruoslahti E. Biomaterials. 2009 doi: 10.1016/j.biomaterials.2009.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JH, von Maltzahn G, Zhang L, Schwartz MP, Ruoslahti E, Bhatia S, Sailor MJ. Adv Mater. 2008;20:1630–1635. doi: 10.1002/adma.200800004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merkulov S, Zhang WM, Komar AA, Schmaier AH, Barnes E, Zhou Y, Lu X, Iwaki T, Castellino FJ, Luo G, McCrae KR. Blood. 2008;111:1274–81. doi: 10.1182/blood-2007-06-092338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simberg D, Park JH, Karmali PP, Merkulov S, Zhang WM, McCrae KR, Sailor MJ, Bhatia S, Ruoslahti E. Biomaterials accepted. 2009 doi: 10.1016/j.biomaterials.2009.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colman RW, Schmaier AH. Blood. 1997;90:3819–43. [PubMed] [Google Scholar]
- 12.Malik DK, Baboota S, Ahuja A, Hasan S, Ali J. Curr Drug Deliv. 2007;4:141–51. doi: 10.2174/156720107780362339. [DOI] [PubMed] [Google Scholar]
- 13.Dass CR, Friedhuber AM, Khachigian LM, Dunstan DE, Choong PF. J Microencapsul. 2008;25:421–5. doi: 10.1080/02652040802033673. [DOI] [PubMed] [Google Scholar]
- 14.Fang J, Deng D, Nakamura H, Akuta T, Qin H, Iyer AK, Greish K, Maeda H. Int J Cancer. 2008;122:1135–44. doi: 10.1002/ijc.22982. [DOI] [PubMed] [Google Scholar]