Abstract
A recent advance in the treatment and understanding of autoimmune disease has been the efficacy of B cell targeted therapy. Such therapies are effective for several such diseases, with systemic autoimmunity being a prototypical example. The mechanism of action is not fully defined, but blocking B cell Ag presentation to T cells is likely to be important. T-B interactions probably engender a positive feedback loop that amplifies and sustains autoimmunity. But how is self-tolerance first broken to initiate this loop? I propose, based on recent data, a model in which autoreactive B cells are activated first, independent of T cells, but dependent upon BCR and TLR signals. These activated B cells then break T celltolerance, initiating full-blown autoimmunity.
Introduction
For many years it was assumed that autoantibodies, particularly immune complexes of them, are the main pathogenic agents in a subclass of systemic autoimmune diseases. T cells in these diseases were eventually recognized to play an important role, but only as helpers of the B cell response [1]. In both systemic autoimmune diseases, as well as organ-specific diseases such as MS and T1D [2,3], it is becoming increasingly clear that B cells play additional roles aside from autoantibody secretion. Conversely, it seems that T cells in systemic autoimmunity do more than just promote the activation of Ab-secreting B cells [4,5].
This review will focus on B cells in systemic autoimmunity, emphasizing recent basic and clinical results that provide insight into how autoreactive B cells are activated and then promote autoimmunity. It will first cover evidence that B cells have Ab-independent functions in autoimmunity. These include the ability of B cells to present Ags to T cells, which suggests the existence of a positive feedback loop between activated autoreactive B and T cells. The potential initiating events for such a positive feedback cycle will be considered next. New data suggesting that B cells may initiate autoimmunity, being the first cell type to break tolerance, will be reviewed and discussed. Emphasis will be on how and where this initial B cell activation occurs, focusing in particular on the roles of TLRs, acting independently of T cells. Together, these emerging studies suggest a new view of how systemic autoimmunity is initiated and propagated, integrating information on the effects of B cell depletion, the roles of TLRs and model systems for study of the activation of autoreactive B cells.
Evidence for Ab independent roles for B cells
Early clues that B cells indeed do more than secrete autoantibodies came from the phenotype of B cell-deficient autoimmune-prone mice [6–8]. These mice had no T cell interstitial infiltrates in their kidneys, and had a ten-fold reduction in memory phenotype CD4 and CD8 T cells. The effects of B cells were independent of autoantibody secretion, as T cell infiltration and activation was restored by B cells that could present Ag to T cells, but could not secrete Ab [9]. These Ab-independent effects on T cells are presumably due to presentation of autoAg to T cells; another potential mechanism for Ab-independent B cell influence on T cells is secretion of cytokines, including TNF-α, IL-6, IL-2 and IFN-γ [10]. Such cytokines can influence T cells and might also have direct pathogenic effects.
Regardless of whether by antigen presentation, cytokine secretion, or both, effects of autoreactive B cells on T cells have also been seen in B cell depletion settings in both humans and mice. Even in diseases that are thought to have an Ab-mediated component of pathogenesis, clinical responses are often faster than drops in Ab levels. This seems to be true in at least some RA and SLE patients [11,12]. In mice, chronic high dose administration of anti-hCD20 in hCD20 Tg lupus-prone MRL/lpr mice eventually led to B cell depletion. In these mice, the frequencies of activated and memory CD4 T cells were reduced compared to controls, but the effects were modest [13]. The limited effect might have been due to the slow pace of B cell depletion or it may indicate that once autoreactive T cells are established, they are not so dependent on B cells for either their maintenance or further expansion. Such effects of B cell depletion have also been observed in reports of rituximab treatment of lupus patients [14], in which B depletion led to a reduction of circulating T cells with an activated phentoype. Activation of transferred glutamic acid decarboxylase-specific TCR Tg T cells (BDC2.5 clonotype) was also reduced in the pancreatic LN of diabetes-prone NOD mice subsequent to B cell depletion [15]. Nonetheless, in these cases a direct linkage between effects on T cells and impact on disease was not established. Thus, more work is required on the APC and other functions of B cells in promoting the initial activation and the continuous reactivation of autoreactive T cells.
Positive feedback model and the question of initiation
We previously proposed a model of positive feedback between T and B cells to explain these observation [4,5]. In this model, T cells help autoreactive B cells and promote their clonal expansion, somatic hypermutation, isotype switch, affinity maturation and differentiation into antibody-secreting cells. Cycles of such mutual T-B interactions would lead to abundant numbers of autoreactive lymphocytes that could mediate disease via a number of mechanisms, ranging from autoantibody deposition to T cell mediated cytotoxicity.
This model has provided a useful framework for predicting and then later explaining why B cell depletion is effective in modulating various types of autoimmunity, including T cell-mediated autoimmune diseases. Indeed, this model has been supported by a substantial body of murine studies and human clinical results, as discussed above. However, one key issue that remains unclear is what starts the positive feedback cycle. This is a critical question, both for our mechanistic understanding as well as for formulating diagnosis and treatment strategies. It has been presumed, due to the mutual nature of the interaction, that B and T cell tolerance would need to be lost at the same time and in the same place at initiation of the autoimmunity cycle. Alternatively, or in addition, it has been assumed that T cells would initially be activated by dendritic cells [16], not B cells, as naïve T cells are thought to be first activated in this fashion in normal circumstances [17]. In fact, the relative role of B cells and DCs in the initial activation of autoreactive T cells in autoimmunity is unknown. Further, it is not clear whether tolerance in the T or B cell compartment could be breached first, leading thereafter to breaking tolerance in the other compartment.
To help clarify this issue, BCR Tg mice with specificity for relevant autoAgs have been used to determine the requirements for initial activation and differentiation, as well as the ability of the B cells to present autoAg and secrete cytokines [18–20]. We have been studying the AM14 rheumatoid factor (RF) Tg mouse, which is specific for IgG2a of the “a” allotype (IgG2aa) [21]. This Tg mouse and specificity is unique in allowing genetic control of an authentic autoAg, as well as the measurement and exogenous administration of this Ag. In the past few years we and others have made observations that suggest that initial B cell activation may procede via TLR, and not T cell-mediated signals. These in turn lead to a proposal for how B cell activation precipitates the autoimmunity positive feedback cycle, as will be discussed below.
TLRs promote the in vitro and in vivo activation of B cells with classic lupus-associated specificities
The restriction in systemic autoimmunity of domiant autoantibody specificities to nuclear Ags, along with self-IgG, suggested that there were specific receptors for components of these self-Ags that contributed to B cell activation. However, until recently the identity of such receptors remained a mystery. A critical insight into this problem was the discovery by Rifkin et al and Leadbetter et al. that AM14 RF Tg B cells were robustly stimulated to proliferate in vitro by immune complexes (ICs) comprised of IgG anti-chromatin and chromatin shed from dead cells [22,23]. Other types of immune complexes lacked this property. Hence, it was reasoned that an essential component for activation must be a receptor in B cells specific for chromatin. Leadbetter et al went on to show that this receptor signaled via MyD88, and, via a series of inhibitors, further implicated TLR9—a DNA-specific TLR. These data suggested a scheme in which BCR ligation with these ICs provided both a strong BCR signaling event as well as a means to internalize the ICs and deliver the chromatin to an intracellular compartment where TLR9 could recognize the DNA component [24]. Subsequent work extended this concept to TLR9 and anti-DNA B cells, which can recognize chromatin directly. In addition, RNA containing immune complexes were shown to stimulate RF B cells in a TLR7-dependent fashion, thus further generalizing the concept [25,26].
To investigate whether nucleic acid-specific TLRs controlled activation of anti-nuclear and RF B cells in vivo, our lab produced F2 crosses between B6 TLR9-deficient and lupus prone MRL.Faslpr mice [27]. Most strikingly, mice lacking TLR9 also lacked serum Ab that causes the homogenous ANA pattern, an indicator of anti-DNA Ab. The absence of anti-chromatin Abs in TLR9-deficient mice was even more evident in the uniform absence of the mitotic chromatin staining pattern. Elements of these findings were confirmed subsequently by us in MRL.Faslpr mice with a fully backcrossed homozygous TLR9-deficiency [28], and in a variety of other models of autoimmunity by other labs [29–31].
TLR7 was also investigated in several models. We produced MRL.Faslpr mice homozygous for a Tlr7-null allele [28]. These mice lacked detectable Abs to RNA-containing Ags, including Sm, and RNP. Further insight was gained from mice with higher than normal TLR7 expression. It was recently shown that the Yaa allele, a Y-chromosome locus known to confer autoimmunity on a variety of genetic backgrounds, represented a duplication of a region of the X-chromosome that contained TLR7 [32,33]. Hence, mice carrying Yaa express an extra copy of TLR7; overexpression of TLR7 in Yaa mice led to increased anti-RNA Abs and systemic disease. This was reinforced by BAC Tg mice that overexpressed to varying degrees just the TLR7 locus and which had autoimmune disease characterized by anti-RNA type Abs [34].
While the effects of these TLRs is likely due at least in part to expression in B cells, their precise role in B cells and other cell-types still remains to be fully defined. Berland and colleagues studied the effects of TLR7 directly on B cells using a BCR knockin mouse with B cells specific for RNA [35]. The activation of these B cells, most of which had an anergic phenotype, was dependent on TLR7, confirming that RNA-specific B cells are regulated by TLR7. Though implicating B cells, this experiment does not formally establish a B cell-intrinsic role for TLR7. Such a role for TLR signaling in B cells was indicated in experiments with AM14 RF B cells that lacked MyD88; when transferred into wild type recipients, these cells not stimulated at all by chromatin-containing ICs in vivo [36].
It is possible that myeloid cells that express nucleic acid TLRs can contribute to B cell activation [37]. DCs provide important support for plasmablasts in vivo [38,39], though this has been called into question at least for certain T-independent responses [40]. TLR7 and TLR9 on conventional DCs (cDCs) and plasmacytoid DCs (pDCs) can elicit factors such as IFN-I and BAFF [41,42] that positively influence B cell responses [43–45] [46].
Further, signals from these TLRs can activate DCs, which may capture autoantigens by a variety of mechanisms including lectin receptors such as Mincle and DNGR-1 [47,48] or as part of ICs via activating FcRs [49,50]. These Ags can then be presented as peptides to T cells, which could provide cognate help to autoreactive B cells; in addition, DCs can present intact Ags to B cells [51,52] and may even be able to capture and present intact autoantigens, though there is no direct evidence of this to date.
TLR-dependent activation of B cells with lupus-associated specificities does not require T cells
We set out to assess the requirements for T cells in the activation of AM14 RF B cells in vivo. To test this idea directly, we used a system in which we provide IgG2a anti-chromatin Abs directly in vivo [20,36]. This is essentially an in vivo translation of the in vitro system originally described by Rothstein and colleagues [22,23]. This treatment results in a prompt and robust extrafollicular plasmablast response by the RF B cells, without a detectable GC response, thus mimicking the situation during spontaneous age-dependent activation in MRL/lpr mice. This in vivo activation pathway is TLR-dependent, enabled by both TLR7 and TLR9, and is absent when MyD88 is not expressed in responding B cells [36]. Most important for the current discussion, when IgG2a anti-chromatin Ab was administered to αβ T cell-deficient AM14 Tg MRL/lpr mice, the response was indistinguishable from that seen in T cell sufficient controls; nor did γδ T cell depletion in these animals have any impact. Hence T cells are not absolutely required for the extrafollicular AM14 RF response elicited by anti-chromatin Abs. Of interest in this regard, we found that B cell isotype switching and somatic hypermutation, while enhanced by T cells, nevertheless did not require them to achieve substantial levels ([36] and unpublished data). In a GC response, these two DNA modifications are thought to be entirely T cell-dependent. In recent years, though, it has become clear that a number of factors can promote T-independent isotype switch, at the least, including: IFN-I, TLR signals, and BAFF/APRIL signals [45,53–55]. Since these factors that promote class switch must elicit expression activation induced cytidine deaminase [56], they could also enable somatic hypermutation, which also relies on this enzyme. Presumably some of these factors are at play, substituting for T cells during the AM14 B cell response to chromatin ICs.
T-independent systemic autoimmunity
It is reasonable to question whether the lack of requirement for T cells in initial autoreactive B cells is particular to the AM14 system. One can speculate that other B cells that can gather TLR signals—most notably anti-DNA/chromatin B cells—should behave similarly, which seems to be the case at least in vitro [25,26] and least to some extent in vivo [29]. Two other groups have recently reported T cell independent activation of autoreactive B cells and even disease [57,58]. Mackay and colleagues have produced a model of autoimmunity based on Tg-mediated overexpression of BAFF [59]. These mice display an SLE-like phenotype, including autoantibody production that does not require T cells but does require expression of TLRs in B cells [58]. Tsao et al. [57] studied B6 mice carrying the 56R H chain site directed Tg, which confers high affinity for dsDNA to most B cells [60,61], and which also has high level spontaneous anti-DNA [57]. They found that such mice lacking either CD4 or all T cells still produced these autoantibodies, and that they were also isotype switched. These three recent studies point to T-independent pathways of autoreactive B cell activation in diverse systems.
B cells can break tolerance first
From these data, we propose that, for B cells that recognize self-sturctures that contain endogenous ligands for TLRs, B cell tolerance (or ignorance) can be broken without the help of T cells. This scheme is outlined in Fig. 1. We postulate that, for such autoantigens, T cell tolerance and B cell tolerance need not be broken at the same place and time in order for autoreactive B cell clonal expansion and differentiation to ensue. Rather, in our model, the self-reactive B cell specific for autoantigens that are endogenous TLR ligands are an Achilles’ heel of the immune system. Though the evolutionary advantage of TLR7-9 expression in B cells is as yet unclear, it could help to promote a rapid and robust extrafollicular plasmablast response to bacterial infection [62] that could occur prior to the steps needed for T cell priming and expansion. It was recently shown that TLR9 and BCR signals combined on a particulate Ag direct such responses in vivo [63]. The trade-off for the ability to respond without T cell help in such situations could be the possibility of autoimmunity. Indeed, this particular susceptibility to T-independent activation of DNA and RNA-specific B cells by virtue of expression of TLR9 and TLR7 could explain why systemic autoimmunity invariably targets DNA and RNA-related autoantigens to the exclusion of a vast array of other self-epitopes [64].
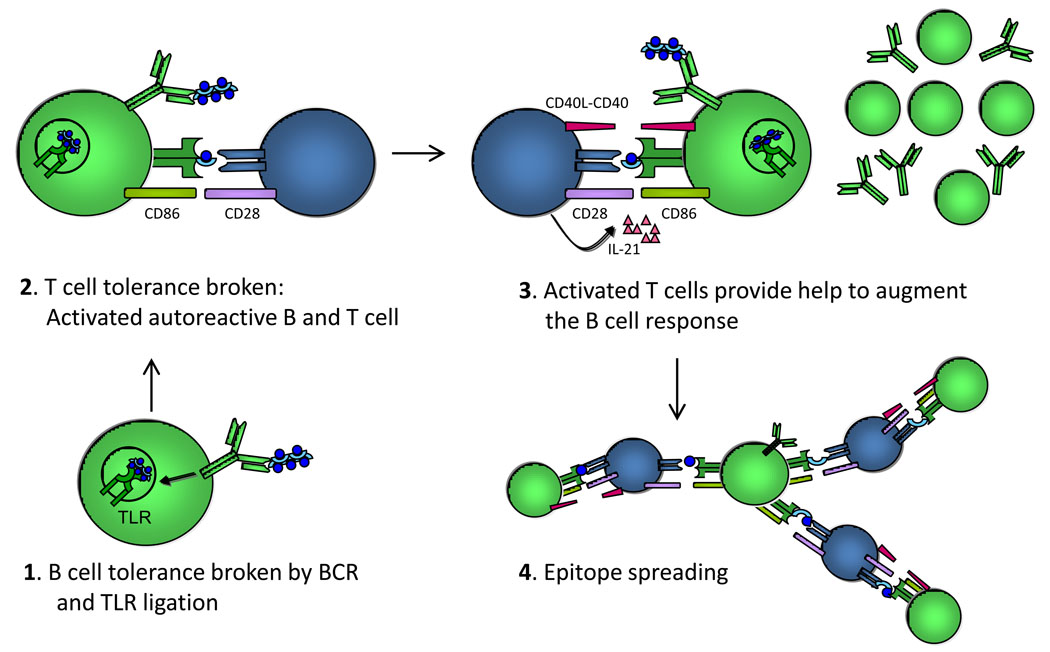
Figure 1. Proposed model for B cell-mediated, TLR-dependent initiation of the autoimmunity feedback cycle.
In step 1, B cell tolerance (or ignorance) is broken in autoAg-specific B cells that recognize self-Ags that carry an endogenous TLR ligand, such as DNA. In step 2, these B cells present autoAg to any self-reactive T cell that can recognize an epitope that is contained on the self-Ag recognized by the B cell. In this case, it is the “blue circle”. The T cell becomes activated, expressing CD40L and IL-21 (shown in step 3) along with other costimulatory molecules and cytokines (not shown). In step 3, these activated T cells provide help to the cognate B cells, leading to enhanced Ab production, isotype switching, clonal expansion and somatic hypermutation. In step 4, the activated B cells can present various different epitopes to T cells with different specificity allowing these in turn to promote the activation of additional autoreactive B cells. Some of these may not need a TLR ligand for activation as they have help now from previously activated T cells. Examples of this include cells specific for the “semi-circular light blue disk” on the right of the panel.
What about T cell contributions to autoreactive B cell activation and autoimmunity?
The notion that autoreactive B cells break tolerance in a T-independent fashion, and that this is an early step in the genesis of autoimmune disease, seems at odds with evidence demonstrating the importance of T cells in systemic autoimmunity [65–69]. (It should, however, be noted that lupus-prone mice lacking important T cell function, including ab T cells and/or CD40L, still demonstrated substantial, albeit reduced disease, perhaps consistent with a facultative role for T cells [66,68,69]). The key to resolving this paradox comes from recognizing the difference between the initial events that breach tolerance and those that are required subsequently to generate a full-blown autoimmune disease. While initiation of autoreactive B cell responses may be T-independent, for chronic disease to occur, it seems clear that T cell tolerance must also be broken. There are two non-exclusive mechanisms by which this can happen. Autoreactive T cells could be activated by myeloid APCs, such as DCs and macrophages, in a more classically accepted pathway for the activation of naïve T cells in normal immune responses [16]. Alternatively, naïve autoreactive T cells could be activated directly by cognate B cells that were previously activated in a T-independent, BCR and TLR-dependent fashion. Regardless of which mechanism causes T cell activation, after such T cells are activated, they may then modify or expand pre-existing autoreactive B cell reactions that were first incited without the help of T cells. Once this point is reached, a positive feedback cycle, as we have previously discussed [4,5], is achieved.
Aside from data showing that T-deficient or T-depleted lupus prone mice have reduced disease and autoantibody production, several studies suggest that activated T cells can promote further B cell activation in the second part of the feedback loop. When T cell help is provided to anergic anti-DNA B cells, they undergo brisk activation, with extrafollicular proliferation and AFC formation [18]. Similarly, anti-Sm T cells that had broken tolerance in an autoimmune prone background, upon transfer into normal mice that harbored anergic anti-Sm B cells, were able to promote their differentiation into AFCs [19]. In our work, we did find a partial role for T cells as well in both induced and spontaneous RF reactions, indicating that while T cells are not required for activation, when present they do modify the response. Recent work from Odegard et al. has identified a specialized T cell subset in MRL/lpr mice, with some resemblance to follicular helper T cells, but which reside at the extrafollicular site, which may be implicated this process naturally [70]. Interestingly, these T cells express high levels of ICOS and IL-21, and these two molecules may play key roles in promoting T-B interactions and the B cell response.
Can B cells activate naïve or anergic T cells?
Our model raises an important and controversial question: Can B cells present autoantigens to naïve T cells and activate these T cells from a tolerant or ignorant state? Classical studies reached the conclusion that naïve B cells cannot activate naïve T cells and may even turn them off [71,72]. However, the situations contrived in these experiments are probably not physiologically relevant to autoimmunity in vivo, as in these papers B cells were caused to present Ag without cross-linking their BCR. In autoimmunity with respect to Ag-specific B cells, it is axiomatic that B cells presenting to T cells will, at the least, have had their BCRs ligated by the relevant Ag.
In fact, a number of experiments have indicated that B cells can promote activation T cells in vivo when the B cells have received a BCR signal. CD4 T cell responses to protein Ags are impaired when B cells are eliminated, either via genetic mutation or induced depletion [15,73,74]. However, these studies have generally been interpreted to mean that B cells amplify responses initiated by other APCs. It is more controversial whether B cells can primarily activate naïve T cells and even self-reactive T cells, which presumably could be anergic. Mamula, et al. demonstrated that priming B cells with human cytochrome c in vivo, then immunizing with mouse cytochrome c, which can be recognized by the human cytochrome-specific B cells, will allow T cell tolerance to mouse cytochrome c to be broken [75]. Similar data were obtained with snRNP-specific responses, in both normal and BCR Tg mice [19,76]. In most of these experiments, a primary role for DCs or other APCs—perhaps enhanced by Ab that directed Ag to the FcR-bearing APCs—could not be excluded. Yan et al. however showed in mice that lacked secreted Ab that SmD protein targeted to hapten-specific B cells was effective in breaking T cell tolerance whereas the same Ag that was not targeted had no effect [77]. In elegant transfer studies, Rodriguez-Pinto and Moreno isolated APC capacity to B cells alone and showed that they could prime naïve CD4 T cells [78]. Hence, there is ample evidence that Ag-specific B cells can prime naïve T cells in vivo.
Conclusions
The cellular interactions that initiate and promote autoimmunity are still poorly understood, though both animal models and new clinical results demonstrate a key role for B cells. We have proposed that B cells could be activated before autoreactive T cells, by virtue of combined BCR and TLR signals delivered by selected autoAgs. B cells could then become a vector for activating autoreactive B cells, spreading, propagating and stabilizing autoimmunity. This second step may separate innocuous, transient autoimmunity from bona fide autoimmune disease. To what extent this scenario applies to various patients, animal models, autoimmune diseases, and autoantigens is unclear. There may be multiple routes to the same final positive feedback situation that defines autoimmunity. In addition to investigation of further models and more mechanistic patient data, it will be useful to determine directly the roles of other APCs, chiefly DCs, in systemic autoimmunity. Ultimately, it will be necessary to devise systems to track and visualize the activation of autoreactive T cells in systemic autoimmunity in order to understand linked T and B cell activation. The goal is a more comprehensive model that will not only be able to explain disease and responses to emerging cell and molecule-specific therapies, but also to predict new such therapies.
Acknowledgements
I thank the many members of my lab who have contributed data, ideas and discussions that are the basis of this review. I thank my longstanding collaborator Ann Marshak-Rothstein for the same. I thank Josephine Giles for preparing the figure. Supported by NIH grants R01-AI073722, R01-AR044077, and P01-AR050256.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kotzin B. Systemic lupus erythematosus. Cell. 1996;85:303–306. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 2.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383.Hauser: This report of a double blind clinical study of rituximab treatment for relapsing-remitting MS patients showed remarkable efficacy, implicating B cells in a human autoimmune disease previously thought to be T cell-mediated.
- 3.Hu CY, Rodriguez-Pinto D, Du W, Ahuja A, Henegariu O, Wong FS, Shlomchik MJ, Wen L. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117:3857–3867. doi: 10.1172/JCI32405.Hu: B cell depletion in NOD mice was shown to prevent diabetes and strikingly, to induce remission in newly diabetic animals, again showing the role of B cells in ongoing disease thought to be T cell-mediated
- 4.Chan OTM, Madaio MP, Shlomchik MJ. The central and multiple roles of B cells in lupus pathogenesis. Immunol. Rev. 1999;169:107–121. doi: 10.1111/j.1600-065x.1999.tb01310.x. [DOI] [PubMed] [Google Scholar]
- 5.Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: positive feedback in systemic autoimmune disease. Nat Rev Immunol. 2001;1:147–153. doi: 10.1038/35100573. [DOI] [PubMed] [Google Scholar]
- 6.Chan OT, Madaio MP, Shlomchik MJ. B cells are required for lupus nephritis in the polygenic, Fas-intact MRL model of systemic autoimmunity. J Immunol. 1999;163:3592–3596. [PubMed] [Google Scholar]
- 7.Chan O, Shlomchik MJ. A new role for B cells in systemic autoimmunity: B cells promote spontaneous T cell activation in MRL-lpr/lpr mice. J. Immunol. 1998;160:51–59. [PubMed] [Google Scholar]
- 8.Shlomchik MJ, Madaio MP, Ni D, Trounstein M, Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. J Exp Med. 1994;180:1295–1306. doi: 10.1084/jem.180.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008;20:332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Looney RJ. B cells as a therapeutic target in autoimmune diseases other than rheumatoid arthritis. Rheumatology (Oxford) 2005;44(Suppl 2):ii13–ii17. doi: 10.1093/rheumatology/keh618. [DOI] [PubMed] [Google Scholar]
- 12.Cambridge G, Leandro MJ, Edwards JC, Ehrenstein MR, Salden M, Bodman-Smith M, Webster AD. Serologic changes following B lymphocyte depletion therapy for rheumatoid arthritis. Arthritis Rheum. 2003;48:2146–2154. doi: 10.1002/art.11181. [DOI] [PubMed] [Google Scholar]
- 13.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B cells in murine lupus: efficacy and resistance. J Immunol. 2007;179:3351–3361. doi: 10.4049/jimmunol.179.5.3351.Ahuja: B cell depletion in MRL/lpr mice was severely impaired, implying a barrier to depletion associated with lupus. However, long-term treatment did deplete B cells, with resultant clinical improvement and reduction in T cell activation.
- 14.Sfikakis PP, Boletis JN, Lionaki S, Vigklis V, Fragiadaki KG, Iniotaki A, Moutsopoulos HM. Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand: an open-label trial. Arthritis Rheum. 2005;52:501–513. doi: 10.1002/art.20858. [DOI] [PubMed] [Google Scholar]
- 15.Bouaziz J-D, Yanaba K, Venturi GM, Wang Y, Tisch RM, Poe JC, Tedder TF. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proceedings of the National Academy of Sciences. 2007;104:20878–20883. doi: 10.1073/pnas.0709205105.Bouziz: With newly-developed anti-mCD20 mAbs, the authors depleted B cells and directly showed effects on induced and spontaneous T cell activation in autoimmune settings, such as diabetes
- 16.Banchereau J, Pascual V, Palucka AK. Autoimmunity through cytokine-induced dendritic cell activation. Immunity. 2004;20:539–550. doi: 10.1016/s1074-7613(04)00108-6. [DOI] [PubMed] [Google Scholar]
- 17.Inaba K, Metlay JP, Crowley MT, Witmer-Pack M, Steinman RM. Dendritic cells as antigen presenting cells in vivo. Int Rev Immunol. 1990;6:197–206. doi: 10.3109/08830189009056630. [DOI] [PubMed] [Google Scholar]
- 18.Seo SJ, Fields ML, Buckler JL, Reed AJ, Mandik-Nayak L, Nish SA, Noelle RJ, Turka LA, Finkelman FD, Caton AJ, et al. The impact of T helper and T regulatory cells on the regulation of anti-double-stranded DNA B cells. Immunity. 2002;16:535–546. doi: 10.1016/s1074-7613(02)00298-4. [DOI] [PubMed] [Google Scholar]
- 19.Yan J, Mamula MJ. Autoreactive T cells revealed in the normal repertoire: escape from negative selection and peripheral tolerance. J Immunol. 2002;168:3188–3194. doi: 10.4049/jimmunol.168.7.3188. [DOI] [PubMed] [Google Scholar]
- 20.Herlands RA, William J, Hershberg U, Shlomchik MJ. Anti-chromatin antibodies drive in vivo antigen-specific activation and somatic hypermutation of rheumatoid factor B cells at extrafollicular sites. European Journal of Immunology. 2007;37:3339–3351. doi: 10.1002/eji.200737752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shlomchik MJ, Zharhary D, Saunders T, Camper S, Weigert M. A Rheumatoid factor transgenic mouse model of autoantibody regulation. Int. Immunol. 1993;5:1329–1341. doi: 10.1093/intimm/5.10.1329. [DOI] [PubMed] [Google Scholar]
- 22.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 23.Rifkin IR, Leadbetter EA, Beaudette BC, Kiani C, Monestier M, Shlomchik MJ, Marshak-Rothstein A. Immune complexes present in the sera of autoimmune mice activate rheumatoid factor B cells [In Process Citation] J Immunol. 2000;165:1626–1633. doi: 10.4049/jimmunol.165.3.1626. [DOI] [PubMed] [Google Scholar]
- 24.Rifkin IR, Leadbetter EA, Busconi L, Viglianti G, Marshak-Rothstein A. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol Rev. 2005;204:27–42. doi: 10.1111/j.0105-2896.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 25.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, Christensen SR, Shlomchik MJ, Viglianti GA, Rifkin IR, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viglianti GA, Lau CM, Hanley TM, Miko BA, Shlomchik MJ, Marshak-Rothstein A. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–847. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 27.Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202:321–331. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Ehlers M, Fukuyama H, McGaha TL, Aderem A, Ravetch JV. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J Exp Med. 2006;203:553–561. doi: 10.1084/jem.20052438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lartigue A, Courville P, Auquit I, Francois A, Arnoult C, Tron F, Gilbert D, Musette P. Role of TLR9 in anti-nucleosome and anti-DNA antibody production in lpr mutation-induced murine lupus. J Immunol. 2006;177:1349–1354. doi: 10.4049/jimmunol.177.2.1349. [DOI] [PubMed] [Google Scholar]
- 31.Yu P, Wellmann U, Kunder S, Quintanilla-Martinez L, Jennen L, Dear N, Amann K, Bauer S, Winkler TH, Wagner H. Toll-like receptor 9-independent aggravation of glomerulonephritis in a novel model of SLE. Int Immunol. 2006;18:1211–1219. doi: 10.1093/intimm/dxl067. [DOI] [PubMed] [Google Scholar]
- 32.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 33.Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, Liang C, Bartov G, McDaniel LD, Zhou XJ, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deane JA, Pisitkun P, Barrett RS, Feigenbaum L, Town T, Ward JM, Flavell RA, Bolland S. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009.Deane: Overexpression of TLR7 in BAC Tg mice resulted in anti-RNA autoantibodies and lupus-like disease, as well as a myeloproliferative syndrome, in B6 mice. This directly implicated TLR7 as sufficient to cause autoimmunity, if overexpressed.
- 35.Berland R, Fernandez L, Kari E, Han JH, Lomakin I, Akira S, Wortis HH, Kearney JF, Ucci AA, Imanishi-Kari T. Toll-like receptor 7-dependent loss of B cell tolerance in pathogenic autoantibody knockin mice. Immunity. 2006;25:429–440. doi: 10.1016/j.immuni.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 36.Herlands RA, Christensen SR, Sweet RA, Hershberg U, Shlomchik MJ. T Cell-Independent and Toll-like Receptor-Dependent Antigen-Driven Activation of Autoreactive B Cells. Immunity. 2008;29:249. doi: 10.1016/j.immuni.2008.06.009.Herlands: This paper demonstrated a B cell-intrinsic requirement for TLR signals to activate the RF extrafollicular response to anti-chromatin ICs. Importantly, T cells were not required for this, but modified both the induced and spontaneous RF response. These results suggested the paradigm of TLR-dependent B cell initiation of autoimmunity.
- 37.Christensen SR, Shlomchik MJ. Regulation of lupus-related autoantibody production and clinical disease by Toll-like receptors. Seminars Immunol. 2007;19:11–23. doi: 10.1016/j.smim.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacLennan ICM, Vinuesa CG. Dendritic Cells, BAFF, and APRIL: Innate Players in Adaptive Antibody Responses. Immunity. 2002;17:235–238. doi: 10.1016/s1074-7613(02)00398-9. [DOI] [PubMed] [Google Scholar]
- 39.Sze DM, Toellner KM, de Vinuesa CG, Taylor DR, MacLennan IC. Intrinsic Constraint on Plasmablast Growth and Extrinsic Limits of Plasma Cell Survival. J Exp Med. 2000;192:813–822. doi: 10.1084/jem.192.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hebel K, Griewank K, Inamine A, Chang HD, Muller-Hilke B, Fillatreau S, Manz RA, Radbruch A, Jung S. Plasma cell differentiation in T-independent type 2 immune responses is independent of CD11c(high) dendritic cells. Eur J Immunol. 2006;36:2912–2919. doi: 10.1002/eji.200636356. [DOI] [PubMed] [Google Scholar]
- 41.Yasuda K, Richez C, Maciaszek JW, Agrawal N, Akira S, Marshak-Rothstein A, Rifkin IR. Murine dendritic cell type I IFN production induced by human IgG-RNA immune complexes is IFN regulatory factor (IRF)5 and IRF7 dependent and is required for IL-6 production. J Immunol. 2007;178:6876–6885. doi: 10.4049/jimmunol.178.11.6876. [DOI] [PubMed] [Google Scholar]
- 42.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatinimmunoglobulin G complexes. J Exp Med. 2004;199:1631–1640. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thibault DL, Chu AD, Graham KL, Balboni I, Lee LY, Kohlmoos C, Landrigan A, Higgins JP, Tibshirani R, Utz PJ. IRF9 and STAT1 are required for IgG autoantibody production and B cell expression of TLR7 in mice. J Clin Invest. 2008;118:1417–1426. doi: 10.1172/JCI30065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poeck H, Wagner M, Battiany J, Rothenfusser S, Wellisch D, Hornung V, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood. 2004;103:3058–3064. doi: 10.1182/blood-2003-08-2972. [DOI] [PubMed] [Google Scholar]
- 45.Coro ES, Chang WLW, Baumgarth N. Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. Journal of Immunology. 2006;176:4343–4351. doi: 10.4049/jimmunol.176.7.4343. [DOI] [PubMed] [Google Scholar]
- 46.Green NM, Laws A, Kiefer K, Busconi L, Kim YM, Brinkmann MM, Trail EH, Yasuda K, Christensen SR, Shlomchik MJ, et al. Murine B cell response to TLR7 ligands depends on an IFN-beta feedback loop. J Immunol. 2009;183:1569–1576. doi: 10.4049/jimmunol.0803899.Green: IFN-I signaling is shown to enhance autoreactive B cell responses by two distinct mechanisms, sensitization of BCR signaling and TLR7 upregulation, with immediate relevance to which Ags activate the B cells. This provides an important link between myeloid/innate activation and autoreactive B cell stimulation.
- 47.Sancho D, Joffre OP, Keller AM, Rogers NC, Martinez D, Hernanz-Falcon P, Rosewell I, Reis e Sousa C. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9:1179–1188. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]
- 49.Bave U, Magnusson M, Eloranta ML, Perers A, Alm GV, Ronnblom L. Fc gamma RIIa is expressed on natural IFN-alpha-producing cells (plasmacytoid dendritic cells) and is required for the IFN-alpha production induced by apoptotic cells combined with lupus IgG. J Immunol. 2003;171:3296–3302. doi: 10.4049/jimmunol.171.6.3296. [DOI] [PubMed] [Google Scholar]
- 50.Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest. 2005;115:407–417. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 52.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell Surface Recycling of Internalized Antigen Permits Dendritic Cell Priming of B Cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 53.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heer AK, Shamshiev A, Donda A, Uematsu S, Akira S, Kopf M, Marsland BJ. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J Immunol. 2007;178:2182–2191. doi: 10.4049/jimmunol.178.4.2182. [DOI] [PubMed] [Google Scholar]
- 55.He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, Shan M, Chadburn A, Villanacci V, Plebani A, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 56.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 57.Tsao PY, Jiao J, Ji MQ, Cohen PL, Eisenberg RA. T cell-independent spontaneous loss of tolerance by anti-double-stranded DNA B cells in C57BL/6 mice. J Immunol. 2008;181:7770–7777. doi: 10.4049/jimmunol.181.11.7770.Tsao: Activation of high affinity anti-DNA B cells on the permissive B6 background was surprisingly found not to require T cells for most of the effects, supporting the notion that in some settings breach of B cell tolerance can be a primary step.
- 58.Groom JR, Fletcher CA, Walters SN, Grey ST, Watt SV, Sweet MJ, Smyth MJ, Mackay CR, Mackay F. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J Exp Med. 2007;204:1959–1971. doi: 10.1084/jem.20062567.Groom: Mice overexpressing BAFF get lupus-like autoimmunity. In this work, Groom et al. show that the syndrome does not require T cells, but does require MyD88, again implicating this pathway in a different autoimmune setting.
- 59.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y, Louzoun Y, Weigert M. Editing Anti-DNA B Cells by V{lambda}x. J Exp Med. 2004;199:337–346. doi: 10.1084/jem.20031712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sekiguchi DR, Yunk L, Gary D, Charan D, Srivastava B, Allman D, Weigert MG, Prak ET. Development and selection of edited B cells in B6.56R mice. J Immunol. 2006;176:6879–6887. doi: 10.4049/jimmunol.176.11.6879. [DOI] [PubMed] [Google Scholar]
- 62.Cunningham AF, Gaspal F, Serre K, Mohr E, Henderson IR, Scott-Tucker A, Kenny SM, Khan M, Toellner KM, Lane PJ, et al. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J Immunol. 2007;178:6200–6207. doi: 10.4049/jimmunol.178.10.6200. [DOI] [PubMed] [Google Scholar]
- 63.Eckl-Dorna J, Batista FD. BCR-mediated uptake of antigen linked to TLR9 ligand stimulates B-cell proliferation and antigen-specific plasma cell formation. Blood. 2009;113:3969–3977. doi: 10.1182/blood-2008-10-185421.Eckl-Dorna: Using a defined system of antigen on particulate beads, with or without TL9 ligand CpG DNA, the authors demonstrated formally that BCR plus TLR9 ligation in vivo exclusively directs a robust extrafollicular plasmablast response, much as seen in the AM14 RF response.
- 64.von Muhlen CA, Tan EM. Autoantibodies in the diagnosis of systemic rheumatic diseases. Seminars in Arthritis and Rheumatism. 1995;24:323–358. doi: 10.1016/s0049-0172(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 65.Wofsy D, Seaman WE. Reversal of advanced murine lupus in NZB/NZW F1 mice by treatment with monoclonal antibody to L3T4. J Immunol. 1987;138:3247–3253. [PubMed] [Google Scholar]
- 66.Ma J, Xu J, Madaio MP, Peng Q, Zhang J, Grewal IS, Flavell RA, Craft J. Autoimmune lpr/lpr mice deficient in CD40 ligand: spontaneous Ig class switching with dichotomy of autoantibody responses. J Immunol. 1996;157:417–426. [PubMed] [Google Scholar]
- 67.Peng SL, Madaio MP, Hayday AC, Craft J. Propagation and regulation of systemic autoimmunity by gammadelta T cells. J Immunol. 1996;157:5689–5698. [PubMed] [Google Scholar]
- 68.Peng SL, Madaio MP, Hughes DP, Crispe IN, Owen MJ, Wen L, Hayday AC, Craft J. Murine lupus in the absence of alpha beta T cells. J Immunol. 1996;156:4041–4049. [PubMed] [Google Scholar]
- 69.Peng SL, McNiff JM, Madaio MP, Ma J, Owen MJ, Flavell RA, Hayday AC, Craft J. alpha beta T cell regulation and CD40 ligand dependence in murine systemic autoimmunity. J Immunol. 1997;158:2464–2470. [PubMed] [Google Scholar]
- 70.Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, Flavell RA, Craft J. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205:2873–2886. doi: 10.1084/jem.20080840.Odegard: This paper reports a detailed characterization of extrafollicular T cells and further implicates them in the direct bidirectional interaction with B cells at these sites, mediated by ICOS-ICOSL and IL-21.
- 71.Eynon EE, Parker DC. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J. Exp. Med. 1992;175:131. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fuchs EJ, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 73.Liu Y, Wu Y, Ramarathinam L, Guo Y, Huszar D, Trounstine M, Zhao M. Gene-targeted B-deficient mice reveal a critical role for B cells in the CD4 T cell response. International Immunology. 1995;7:1353–1362. doi: 10.1093/intimm/7.8.1353. [DOI] [PubMed] [Google Scholar]
- 74.Constant S, Schweitzer N, West J, Ranney P, Bottomly K. B lymphocytes can be competent antigen-presenting cells for priming CD4+ T cells to protein antigens in vivo. J. Immunol. 1995;155:3734–3741. [PubMed] [Google Scholar]
- 75.Mamula MJ, Lin R-H, Janeway CA, Jr, Hardin JA. Breaking T cell tolerance with foreign and self co-immunogens: a study of autoimmune B and T cell epitopes of cytochrome c. J. Immunol. 1992;149:789–795. [PubMed] [Google Scholar]
- 76.Mamula MJ, Fatenejad S, Craft J. B cells process and present lupus autoantigens that initiate autoimmune T cell responses. J. Immunol. 1994;152:1453–1461. [PubMed] [Google Scholar]
- 77.Yan J, Harvey BP, Gee RJ, Shlomchik MJ, Mamula MJ. B cells drive early T cell autoimmunity in vivo prior to dendritic cell-mediated autoantigen presentation. J Immunol. 2006;177:4481–4487. doi: 10.4049/jimmunol.177.7.4481. [DOI] [PubMed] [Google Scholar]
- 78.Rodriguez-Pinto D, Moreno J. B cells can prime naive CD4+ T cells in vivo in the absence of other professional antigen-presenting cells in a CD154-CD40-dependent manner. Eur J Immunol. 2005;35:1097–1105. doi: 10.1002/eji.200425732. [DOI] [PubMed] [Google Scholar]