Abstract
Atherothrombotic vascular diseases, such as myocardial infraction and stroke, are the leading causes of death in the industrialized world. The immediate cause of these diseases is acute occlusive thrombosis in medium-sized arteries feeding critical organs. Thrombosis is triggered by the rupture or erosion of a minority of atherosclerotic plaques that have advanced to a particular stage of “vulnerability.” Vulnerable plaques are characterized by certain key features, such as inflammation, thinning of a protective collagenous cap, and a lipid-rich necrotic core consisting of macrophage debris. A number of cellular events contribute to vulnerable plaque formation, including secretion of pro-inflammatory, procoagulant, and proteolytic molecules by macrophages as well as the death of macrophages, intimal smooth muscles cells, and possibly endothelial cells. The necrotic core in particular is a key factor in plaque vulnerability, because macrophage debris promotes inflammation, plaque instability, and thrombosis. Plaque necrosis arises from a combination of lesional macrophage apoptosis and defective clearance of these dead cells, a process called efferocytosis. This review focuses on how macrophage apoptosis, in the setting of defective efferocytosis, contributes to necrotic core formation and how a process known to be prominent in advanced lesions—activation of ER stress signal-transduction pathways—contributes to macrophage apoptosis in these plaques. Antioxid. Redox Signal. 11, 2333–2339.
The Life-and-Death Cycle of the Macrophage in Atherogenesis and the Consequences of Lesional Macrophage Apoptosis
Atherogenesis begins with the focal retention of plasma apolipoprotein B–containing lipoproteins by extracellular matrix molecules in focal areas of the subendothelial intima of medium-sized arteries (42). This event, which is essential for atherogenesis, triggers a maladaptive inflammatory response, which likely occurs after the retained lipoproteins have been modified by oxidation, aggregation, and other means. The inflammatory response is dominated by the influx of bone marrow–derived monocytes, which diapedese through the endothelium and take up residence in the intima (13, 24). Once there, the monocytes differentiate into macrophages, which then ingest the retained and modified lipoproteins.
In early- through mid-stage atherogenesis, the most prominent macrophage process is foam cell formation, which involves the ingestion and metabolism of lipoprotein-derived cholesterol. Specifically, the lipoprotein-cholesterol is trafficked from late endosomes to the endoplasmic reticulum (ER), where the enzyme acyl-CoA/cholesterol acyl transferase (ACAT) esterifies the cholesterol to cholesteryl fatty acyl esters (CEs) (3). The CEs coalesce into membrane-bound cytoplasmic lipid droplets, which give the cells a foamy appearance by microscopy.
The functional consequence of early lesional foam cell formation on atherogenesis has been debated (38), but genetic studies in mice in which monocytes are depleted or monocyte entry into lesions is blocked suggest that these macrophages contribute to the progression of atherosclerosis (37). The mechanism may involve secretion of proatherogenic molecules, such as inflammatory cytokines and proretentive extracellular matrix molecules, by macrophage foam cells (13, 24, 42). The overall concept that early- to mid-stage macrophages are proatherogenic has also been suggested by studies investigating the consequences of macrophage apoptosis in these lesions (40). Although macrophages are relatively long-lived cells, a finite incidence of macrophage apoptosis occurs throughout atherogenesis. In studies in which early lesional macrophage apoptosis was blocked by genetic engineering in mice, the lesions became more cellular, and atherogenesis was accelerated (23). The converse has been observed when early lesional macrophage apoptosis is enhanced (i.e., a decrease in lesion progression) (1). These data further support the notion that macrophages play a proatherogenic role and suggest that the turnover of macrophages by apoptosis limits lesion cellularity in early atherogenesis.
In advanced lesions, macrophage apoptosis increases, and the functional consequences appear to be markedly different from those in earlier lesions (40). In particular, increasing evidence suggests that advanced lesional macrophage apoptosis is associated with the development of a key feature of so-called “vulnerable plaques”: plaque necrosis (2). Vulnerable plaque is a term used to describe the minority of atherosclerotic lesions that progress to the point at which they can trigger acute, occlusive luminal thrombosis, which is the terminal event that precipitates acute coronary syndromes like unstable angina, acute myocardial infarction, sudden cardiac death, and stroke. These vulnerable plaques are characterized not by their larger size but rather by certain key characteristics, such as plaque necrosis, a heightened state of inflammation, and thinning of the “protective” collagenous scar, or “fibrous cap,” that forms in advanced atheromata (45). These properties render the plaques more prone to rupture or erosion, which then exposes the overlying luminal blood to procoagulant and prothrombotic molecules in the necrotic intima.
A number of cellular events contribute to vulnerable plaque formation, including secretion of pro-inflammatory, procoagulant, and proteolytic molecules by macrophages as well as the death of macrophages, intimal smooth muscles cells, and possibly endothelial cells. However, plaque necrosis per se is thought to be particularly important, because it is thought to promote plaque disruption and luminal thrombosis through activation of inflammatory signaling, proteolysis of fibrous cap collagen, and promotion of coagulation and thrombosis by cell-released proteins and lipids (20). In addition, the physical nature of the lipid-rich fibrous cap places mechanical stress on the fibrous cap that is thought to contribute to its disruption (16).
The Critical Function of Efferocytosis Throughout Atherogenesis
Why does macrophage apoptosis decrease lesion cellularity and progression in early lesions but promote plaque necrosis in more-advanced lesions? One likely explanation is that the normal physiologic process of phagocytic clearance (“efferocytosis”) of apoptotic cells is more efficient in earlier than in later lesions (32, 40). To understand this concept, it is important to define the term “necrosis.” At the cellular level, necrosis refers to a type of cell damage in which cellular membranes, including the plasma membrane, become leaky, and organelles swell, resulting in cell death and leakage of cellular contents into the extracellular environment (7). The released cellular debris is a potent inducer of inflammation in neighboring immune cells, such as macrophages and T cells (9).
Necrotic cell death is to be distinguished from apoptotic cell death, which is characterized by intact membranes and condensed organelles and which does not usually incite inflammation unless the cells are not rapidly cleared by phagocytes (see later) (7). At the tissue level, necrosis refers to areas that have accumulated necrotic cell-derived debris (e.g., “caseating necrosis” in tuberculosis lesions in the lung). For the purpose of this review on atherosclerosis, I shall refer to cell necrosis as “macrophage necrosis” and to tissue necrosis as “plaque necrosis.”
The most common cause of cell necrosis in vivo is so-called postapoptotic, or secondary, necrosis, which results from the lack of efficient phagocytic clearance (“efferocytosis”) of apoptotic cells (15). In normal physiology, during which apoptosis occurs at a very high rate with estimates as high as hundreds of billions of cells per day, apoptotic cells are rapidly cleared by both professional and “nonprofessional” phagocytes. Importantly, the clearance of apoptotic cells elicits an active antiinflammatory response in efferocytes (8). When efferocytosis is not efficient, as is thought to occur in disease states such as systemic lupus erythematosus and advanced atherosclerosis (see later), apoptotic cells become necrotic, as defined earlier, and an inflammatory response ensues (15).
The lack of plaque necrosis and inflammation associated with macrophage apoptosis in early atherosclerotic lesions implies efficient efferocytosis, although this has never formally been proven. In advanced lesions, the converse appears to be the case, and here supporting experimental evidence exists. In particular, Schrijvers and Martinet (33) meticulously scored advanced human lesions for free and phagocyte (macrophage)-associated apoptotic cells, which were identified by the terminal deoxynucleotidyl transferase biotin-dUTP nick-end labeling (TUNEL) stain. In comparison with human tonsils, an organ with efficient efferocytosis, advanced atheromata had a larger number of non–macrophage-associated TUNEL-positive cells. Furthermore, a number of molecular genetic causation studies in mice have been performed in which genes involved in efferocytosis, such as apolipoprotein E, transglutaminase 2, milk fat–binding globulin, and Mertk, have been targeted (41). In many of these models, disruption of efferocytosis is associated with plaque necrosis and, when examined, an increase in the number of non–macrophage-associated TUNEL-positive cells. Thus, it is likely that a defect in efferocytosis in advanced atheromata, the cause of which is not yet known, leads to postapoptotic macrophage necrosis and eventually plaque necrosis.
The Role of Endoplasmic Reticulum Stress in Advanced Lesional Macrophage Apoptosis
As summarized earlier, advanced lesional plaque necrosis likely involves a combination of macrophage apoptosis and defective efferocytosis. Thus, understanding the mechanisms of these two processes is a critical goal in atherosclerosis research. In this section, I discuss a key area related to mechanisms of advanced lesional macrophage apoptosis. For a discussion of possible mechanisms of defective efferocytosis, the reader is referred to previous reviews on this topic (32, 40).
All cells possess a highly conserved and integrated set of pathways called the unfolded protein response (UPR) or integrated stress response (ISR) that are designed to protect the endoplasmic reticulum (ER) from a variety of physiologic and pathophysiologic perturbations (18, 31). These perturbations include increased protein synthesis (“client load”); disturbances in ER calcium homeostasis; alterations in the physical property of the ER membrane bilayer (e.g., by certain types of lipids); disturbances in the redox environment of the ER; and misfolded proteins.
Three major branches of the UPR are known, each with specific functions related to correcting the aforementioned perturbations of the ER. Of great interest, evidence indicates that these branches also regulate the delicate balance between cell survival and apoptosis in cells (22, 50). From a teleologic viewpoint, one may imagine that evolution has designed the UPR to keep cells alive to enable the repair process to proceed but also to have a “fail-safe” mechanism to trigger cell death if the perturbation is irreparable. The branch of the UPR that has been associated most with apoptosis involves the UPR effector CHOP, or GADD153 (50). In particular, whereas short-term induction of CHOP is an important element of the protective UPR, prolonged expression of CHOP can lead to cell death. Many mechanisms of CHOP-induced apoptosis have been explored in cultured cells, including generation of reactive oxygen species and downregulation of Bcl-2 (39), but evidence for a dominant proapoptotic mechanism in vivo is lacking. UPR-induced apoptosis can also be coordinated with suppression of the other two branches of the UPR (the IRE1-XBP1 and ATF6 branches), which can, under certain circumstances, promote cell survival (22).
Austin and colleagues (29) raised the idea that the UPR may play a role in atherosclerosis by showing that homocysteine, a potentially proatherogenic molecule, causes ER stress–induced growth arrest in endothelial cells. Our group was interested in mechanisms of advanced lesional macrophage apoptosis. Based on the finding that macrophages in advanced atheromata accumulate large amounts of free cholesterol (FC) (17), we used a model in which apoptosis of cultured macrophages was induced by loading the cells with large amounts of lipoprotein-derived unesterified, or “free,” cholesterol (46–48). Under normal conditions, lipoprotein-derived FC is trafficked to the ER, where it is fatty acyl-esterified by the enzyme acyl-CoA:cholesterol acyl transferase (ACAT). FC accumulation in the macrophage model, therefore, requires inhibition or genetic targeting of ACAT (46). In vivo, a mouse model of advanced atherosclerosis in which macrophage ACAT was gene-targeted showed increased advanced lesional macrophage death (10), and several studies in which ACAT inhibitors were used in humans revealed worsening of atherosclerosis or heart disease in the drug-treated group (28, 43).
In searching for mechanisms of FC-induced apoptosis, we made the observations that FC loading led to UPR activation and CHOP induction in a manner that depended on cholesterol trafficking to the ER and that macrophages from Chop−/− mice showed ∼70% protection from FC-induced apoptosis (11). Moreover, strong correlations were found among FC accumulation in the ER membrane, increased-order parameter (“stiffening”) of the normally liquid-disordered (“fluid”) ER membrane, and inhibition of the ER calcium-pump sarcoplasmic/endoplasmic reticulum calcium-dependent ATPase (SERCA) (19). Thus, a plausible hypothesis was one in which FC loading, through biophysical changes in the ER membrane, led to depletion of ER calcium through inhibition of SERCA, subsequent dysfunction of calcium-dependent protein chaperones, and eventual activation of the UPR secondary to chaperone dysfunction.
In vivo data supporting a role for the UPR in advanced lesional macrophage apoptosis come from a number of studies. For example, aortic root lesions of Apoe−/− mice in which lipoprotein-cholesterol trafficking to the ER was compromised by a heterozygous mutation in the cholesterol-trafficking protein NPC1 showed a decrease in advanced lesional macrophage apoptosis and plaque necrosis (12). Austin and colleagues (49) found evidence of CHOP induction in the aortic root lesions of Apoe−/− mice, and we recently observed a decrease in advanced lesional macrophage apoptosis and plaque necrosis in Apoe−/− mice that are also deficient in CHOP (44a). Moreover, Myoishi et al. (27) found a striking correlation among ER stress markers, including CHOP; plaque vulnerability; and lesional apoptosis in samples of human coronary artery plaques. Together, these diverse pieces of experimental data support at least a partial role for UPR-induced apoptosis in the death of advanced lesional macrophages.
The Multihit Model of ER Stress-Induced Macrophage Apoptosis
We initially reasoned that FC-induced macrophage death was simply an example of UPR/CHOP-mediated apoptosis. Indeed, it is well known that if a very potent UPR inducer, such as high-dose tunicamycin (a protein glycosylation inhibitor) or thapsigargin (a SERCA inhibitor), is added to cultured cells, apoptosis will ensue. However, ER stress in vivo is probably more subtle and, by itself, insufficient to cause apoptosis. Rather, ER stress would reduce the threshold for one or more additional “hits” to trigger cell death. In that sense, we imagine that cells in vivo “sample” various subthreshold noxious stimuli in their environment and trigger the “suicide” response of apoptosis only when the combination of these stimuli signals irreparable damage.
Of interest in this regard, a number of compensatory cell-survival pathways, such as Akt, ERK, NF-κB, and p38α, are activated in the setting of ER stress, and some of the additional hits appear to function by suppressing these pathways (see later).
In the case of apoptosis in ER-stressed macrophages, this “multihit” concept was initially supported by our observation that apoptosis induced by lipoprotein-derived FC could be markedly suppressed by the absence or inhibition of two cell-surface pattern-recognition receptors (PRRs) that interact with atherogenic lipoproteins, the type A scavenger receptor (SRA) and Toll-like receptor 4 (TLR4) (6, 35). The inhibition of apoptosis in these experiments occurred despite unsuppressed UPR activation, indicating that the UPR alone was not sufficient for apoptosis. Moreover, apoptosis could be induced by combinations of a low-dose nonlipoprotein ER stressor, such as thapsigargin, plus an SRA/TLR4 ligand, such as fucoidan or acetyl-LDL, but not by either agent alone (35).
Many ER stressors and PRR ligands are known to exist in atheromata (34), and a number of these combinations can induce macrophage apoptosis—completely in the absence of FC loading (35). Thus, the multihit model broadens the applicability of ER stress-induced macrophage apoptosis beyond the FC model. Examples of lesional ER stressors include oxidant stress, peroxynitrite, insulin resistance, glucosamine, saturated fatty acids, hypoxia, homocysteine, oxidized phospholipids, oxysterols such as 7-ketocholesterol, and serum starvation (34). Examples of lesional PRR ligands that trigger macrophage apoptosis during ER stress include lesion-modified forms of LDL, advanced glycation end products (AGEs), β-amyloid, and oxidized phosphatidylcholine (34).
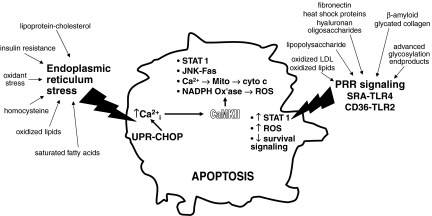
Beginning with the observation that combinations of subthreshold ER stress plus PRR engagement could trigger apoptosis, further experimental work revealed a complex web of signal-transduction pathways that lead to apoptosis (Fig. 1). In this model, a combination of activation of proapoptotic signaling and suppression of compensatory cell-survival pathways eventually leads to cell death.
FIG. 1.
The ER stress–PRR model of advanced lesional macrophage apoptosis. Macrophages in advanced atheromata are exposed to a number of factors that induce ER stress and also to multiple ligands for pattern-recognition receptors (PRRs). ER stress via CHOP induction and other mechanisms triggers ER calcium release. The resultant increase in cytosolic calcium, through activation of CaMKII, induces multiple proapoptotic pathways, including STAT1, JNK-Fas, calcium-induced mitochondrial depolarization, and NADPH oxidase–mediated ROS. When combined with combinatorial PRR signaling, such as SRA-TLR4 and CD36-TLR2, some of the proapoptotic pathways are amplified, and some compensatory cell-survival pathways are suppressed. The combination of all of these events tips the balance between subthreshold proapoptotic signaling and compensatory cell-survival signaling in favor of apoptosis. The key concept imbedded in this multihit model is that each of the proapoptotic hits are not, by themselves, sufficient to trigger apoptosis under the “subtle” conditions of the in vivo environment. Rather, the cells monitor the environment for a variety of noxious stimuli and do not trigger a suicide response unless the combination of these stimuli is deemed to indicate damage that is beyond repair. See text for details.
Mechanistic studies have revealed several key hubs in this web of pathways as well as a number of integrated amplification loops. Two of the key hubs are calcium/calmodulin-dependent protein kinase II (CaMKII) and NADPH oxidase–mediated reactive oxygen species (ROS) (21; J. Timmins, L. Ozcan, et al., unpublished data; G. Li, et al., unpublished data). Specifically, ER stress and CHOP, through various mechanisms, promote ER calcium release and activation of CaMKII, which in turn activates a number of proapoptotic pathways, including JNK-mediated Fas induction, mitochondrial membrane depolarization, STAT1 activation, and NADPH oxidase activation. NADPH oxidase-induced ROS generation, in turn, amplifies this pathway through effects on calcium release and also by suppressing compensatory Akt-mediated cell-survival signaling (G. Li, et al. and T. Seimon, et al., unpublished data). Engagement of PRRs is necessary for apoptosis because it further contributes to STAT1 activation and also silences a cell-survival pathway mediated by interferon-β (21, 35).
A remarkable feature of this network is that none of the proapoptotic steps by itself is alone sufficient for apoptosis, which reflects the subthreshold nature of the steps and, most important, the critical role of compensatory cell-survival signaling. This latter point was recently demonstrated in two in vitro projects, one linking ER stress and efferocytosis and the other linking ER stress/PRR-mediated apoptosis with insulin resistance in macrophages. In the first project, efferocytes that ingest FC-loaded apoptotic cells were found to be highly resistant to FC-induced death because NF-κB and Akt-mediated cell-survival pathways are activated in these cells (5). Conversely, macrophages with defective insulin signaling, a hallmark of type 2 diabetes, have suppressed NF-κB and Akt signaling. NF-κB suppression is mediated by increased nuclear localization of FoxO1, which induces that NF-κB inhibitor IκBɛ (36).
Different components of this integrated web have been tested in vivo. STAT1 deficiency in bone marrow–derived cells (macrophages in atheromata) in Western diet–fed Ldlr−/− mice suppresses advanced lesional macrophage apoptosis and plaque necrosis (21). Similar results have been obtained with CHOP deficiency (44a) and with deficiencies of two apoptosis-inducing PRRs, SRA and CD36 (25).
Two examples of how compensatory cell-survival signaling plays a role in vivo are Apoe−/− mice treated with pioglitazone, which suppresses the NF-κB cell-survival pathway in ER-stressed macrophages (44), and Apoe−/− mice with macrophage-deficient p38α MAPK, which activates an Akt-mediated cell-survival pathway in ER-stressed macrophages (35a). In both cases, the experimental mice showed increased advanced lesional macrophage apoptosis and plaque necrosis. Finally, in view of the increased sensitivity of insulin-resistant macrophages to ER stress–PRR-induced apoptosis (see earlier), Ldlr−/− mice reconstituted with macrophages lacking insulin receptors—a proof-of-concept model of macrophage insulin resistance—showed increased advanced lesional macrophage apoptosis and plaque necrosis (14). This finding may have relevance to the increasing epidemic of insulin resistance–associated atherothrombotic vascular disease, which is specifically associated with lesions having large necrotic cores (4).
Summary and Conclusions
Most individuals in industrialized societies have numerous atherosclerotic lesions in their coronary, carotid, and other susceptible peripheral-site arteries. Although only a small portion of these lesions will progress to plaques that have the potential to trigger acute occlusive luminal thrombosis, the toll of this progression on morbidity and mortality in our society is huge. Current cholesterol-lowering therapy has made a substantial impact on lessening plaque progression and clinical events, but atherothrombotic vascular disease still remains the leading cause of death in the industrialized world. The gains in cardiovascular disease prevention achieved by cholesterol lowering have been curtailed somewhat by the epidemic of obesity, insulin resistance, and type 2 diabetes, which are potent risk factors for atherothrombotic vascular disease and advanced plaque progression. In this context, complementary therapeutic approaches directed at the arterial wall in general, and plaque progression in particular, could have a tremendous effect on lessening disease burden, particularly given that type 2 diabetes will be an increasingly dominant force in promoting these processes for the foreseeable future.
However, to achieve this goal, two major advances are needed: readily available and clinically useful imaging techniques that focus on advanced plaque morphology and a fundamental knowledge of the pathophysiology of plaque progression. Plaque progression involves a number of complex processes, including the topics of this review—ER stress–induced macrophage apoptosis and defective efferocytosis—but also including other forms of macrophage death, death of intimal smooth muscle cells and possibly endothelium, and roles played by living macrophages in advanced plaques.
Within the focus of this review, the hope exists that knowledge of how the combination of ER stress–induced macrophage apoptosis and defective late lesional efferocytosis can some day lead to a novel therapeutic strategy. For example, in other settings of ER stress–induced pathology, so-called chemical chaperones have been investigated as therapeutic agents (30). Likewise, strategies to enhance efferocytosis have been explored in the setting of systemic lupus erythematosus, in which defective clearance of apoptotic neutrophils in joints is thought to contribute to the disease (26). Coordinated developments in the areas of advanced plaque imaging and the cellular and molecular biology of plaque progression offer the best hope of realizing these goals.
Abbreviations Used
- ACAT
acyl-CoA:cholesterol acyl transferase
- AGEs
advanced glycation end products
- CaMKII
calcium/calmodulin-activated protein kinase II
- CE
cholesteryl ester
- CHOP
CEBP-homologous protein
- ER
endoplasmic reticulum
- FC
free cholesterol
- LDL
low-density lipoprotein
- Ldlr
LDL receptor
- MAPK
mitogen-activated protein kinase
- NADPH
nicotinamide adenine dinucleotide phosphate
- PRR
pattern-recognition receptor
- ROS
reactive oxygen species
- SERCA
sarco/endoplasmic reticulum calcium ATPase
- SRA
type A scavenger receptor
- STAT
signal transducer and activator of transcription
- TLR
toll-like receptor
- TUNEL
terminal deoxynucleotidyl transferase biotin-dUTP nick-end labeling
- UPR
unfolded protein response
Acknowledgments
I acknowledge the outstanding experimental work, discussions, and insights of Drs. Bo Feng, Pin Mei Yao, Yankun Li, Tracie Seimon, Dongying Cui, Wahseng Lim, Edward Thorp, Jenelle Timmins, Gang Li, and Lale Ozcan. The research cited herein was supported by NIH grants HL54591 and HL75662 and U.S. Army Medical Research and Materiel Command (USAMRMC) grant W81XWH-06-1-0212.
References
- 1.Arai S. Shelton JM. Chen M. Bradley MN. Castrillo A. Bookout AL. Mak PA. Edwards PA. Mangelsdorf DJ. Tontonoz P. Miyazaki T. A role for the apoptosis inhibitory factor AIM/Spα/Api6 in atherosclerosis development. Cell Metab. 2005;1:201–213. doi: 10.1016/j.cmet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Ball RY. Stowers EC. Burton JH. Cary NR. Skepper JN. Mitchinson MJ. Evidence that the death of macrophage foam cells contributes to the lipid core of atheroma. Atherosclerosis. 1995;114:45–54. doi: 10.1016/0021-9150(94)05463-s. [DOI] [PubMed] [Google Scholar]
- 3.Brown MS. Ho YK. Goldstein JL. The cholesteryl ester cycle in macrophage foam cells: continual hydrolysis and re-esterification of cytoplasmic cholesteryl esters. J Biol Chem. 1980;255:9344–9352. [PubMed] [Google Scholar]
- 4.Burke AP. Kolodgie FD. Zieske A. Fowler DR. Weber DK. Varghese PJ. Farb A. Virmani R. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol. 2004;24:1266–1271. doi: 10.1161/01.ATV.0000131783.74034.97. [DOI] [PubMed] [Google Scholar]
- 5.Cui D. Thorp E. Li Y. Wang N. Yvan-Charvet L. Tall AR. Tabas I. Pivotal advance: macrophages become resistant to cholesterol-induced death after phagocytosis of apoptotic cells. J Leukoc Biol. 2007;82:1040–1050. doi: 10.1189/jlb.0307192. [DOI] [PubMed] [Google Scholar]
- 6.DeVries-Seimon T. Li Y. Yao PM. Stone E. Wang Y. Davis RJ. Flavell R. Tabas I. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edinger AL. Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Fadok VA. Bratton DL. Konowal A. Freed PW. Westcott JY. Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fadok VA. McDonald PP. Bratton DL. Henson PM. Regulation of macrophage cytokine production by phagocytosis of apoptotic and post-apoptotic cells. Biochem Soc Trans. 1998;26:653–656. doi: 10.1042/bst0260653. [DOI] [PubMed] [Google Scholar]
- 10.Fazio S. Major AS. Swift LL. Gleaves LA. Accad M. Linton MF. Farese RV., Jr Increased atherosclerosis in LDL receptor-null mice lacking ACAT1 in macrophages. J Clin Invest. 2001;107:163–171. doi: 10.1172/JCI10310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng B. Yao PM. Li Y. Devlin CM. Zhang D. Harding HP. Sweeney M. Rong JX. Kuriakose G. Fisher EA. Marks AR. Ron D. Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 12.Feng B. Zhang D. Kuriakose G. Devlin CM. Kockx M. Tabas I. Niemann-Pick C heterozygosity confers resistance to lesional necrosis and macrophage apoptosis in murine atherosclerosis. Proc Natl Acad Sci U S A. 2003;100:10423–10428. doi: 10.1073/pnas.1732494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glass CK. Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 14.Han S. Liang CP. DeVries-Seimon T. Ranalletta M. Welch CL. Collins-Fletcher K. Accili D. Tabas I. Tall AR. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 2006;3:257–266. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Henson PM. Bratton DL. Fadok VA. Apoptotic cell removal. Curr Biol. 2001;11:R795–R805. doi: 10.1016/s0960-9822(01)00474-2. [DOI] [PubMed] [Google Scholar]
- 16.Imoto K. Hiro T. Fujii T. Murashige A. Fukumoto Y. Hashimoto G. Okamura T. Yamada J. Mori K. Matsuzaki M. Longitudinal structural determinants of atherosclerotic plaque vulnerability: a computational analysis of stress distribution using vessel models and three-dimensional intravascular ultrasound imaging. J Am Coll Cardiol. 2005;46:1507–1515. doi: 10.1016/j.jacc.2005.06.069. [DOI] [PubMed] [Google Scholar]
- 17.Katz SS. Shipley GG. Small DM. Physical chemistry of the lipids of human atherosclerotic lesions: demonstration of a lesion intermediate between fatty streaks and advanced plaques. J Clin Invest. 1976;58:200–211. doi: 10.1172/JCI108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y. Ge M. Ciani L. Kuriakose G. Westover E. Dura M. Covey D. Freed JH. Maxfield FR. Lytton J. Tabas I. Enrichment of endoplasmic reticulum with cholesterol inhibits SERCA2b activity in parallel with increased order of membrane lipids: implications for depletion of ER calcium stores and apoptosis in cholesterol-loaded macrophages. J Biol Chem. 2004;279:37030–37039. doi: 10.1074/jbc.M405195200. [DOI] [PubMed] [Google Scholar]
- 20.Libby P. Geng YJ. Aikawa M. Schoenbeck U. Mach F. Clinton SK. Sukhova GK. Lee RT. Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol. 1996;7:330–335. doi: 10.1097/00041433-199610000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Lim WS. Timmins JM. Seimon TA. Sadler A. Kolodgie FD. Virmani R. Tabas I. STAT1 is critical for apoptosis in macrophages subjected to endoplasmic reticulum stress in vitro and in advanced atherosclerotic lesions in vivo. Circulation. 2008;117:940–951. doi: 10.1161/CIRCULATIONAHA.107.711275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin JH. Li H. Yasumura D. Cohen HR. Zhang C. Panning B. Shokat KM. Lavail MM. Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J. Thewke DP. Su YR. Linton MF. Fazio S. Sinensky MS. Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscler Thromb Vasc Biol. 2005;25:174–179. doi: 10.1161/01.ATV.0000148548.47755.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manning-Tobin JJ. Moore KJ. Seimon TA. Bell SA. Sharuk M. Varez-Leite JI. de Winther MP. Tabas I. Freeman MW. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler Thromb Vasc Biol. 2009;29:19–26. doi: 10.1161/ATVBAHA.108.176644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell S. Thomas G. Harvey K. Cottell D. Reville K. Berlasconi G. Petasis NA. Erwig L. Rees AJ. Savill J. Brady HR. Godson C. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J Am Soc Nephrol. 2002;13:2497–2507. doi: 10.1097/01.asn.0000032417.73640.72. [DOI] [PubMed] [Google Scholar]
- 27.Myoishi M. Hao H. Minamino T. Watanabe K. Nishihira K. Hatakeyama K. Asada Y. Okada K. Ishibashi-Ueda H. Gabbiani G. Bochaton-Piallat ML. Mochizuki N. Kitakaze M. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 28.Nissen SE. Tuzcu EM. Brewer HB. Sipahi I. Nicholls SJ. Ganz P. Schoenhagen P. Waters DD. Pepine CJ. Crowe TD. Davidson MH. Deanfield JE. Wisniewski LM. Hanyok JJ. Kassalow LM. Effect of ACAT inhibition on the progression of coronary atherosclerosis. N Engl J Med. 2006;354:1253–1263. doi: 10.1056/NEJMoa054699. [DOI] [PubMed] [Google Scholar]
- 29.Outinen PA. Sood SK. Liaw PC. Sarge KD. Maeda N. Hirsh J. Ribau J. Podor TJ. Weitz JI. Austin RC. Characterization of the stress-inducing effects of homocysteine. Biochem J. 1998;332:213–221. doi: 10.1042/bj3320213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozcan U. Yilmaz E. Ozcan L. Furuhashi M. Vaillancourt E. Smith RO. Gorgun CZ. Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ron D. Translational control in the endoplasmic reticulum stress response. J Clin Invest. 2002;110:1383–1388. doi: 10.1172/JCI16784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schrijvers DM. De Meyer GR. Herman AG. Martinet W. Phagocytosis in atherosclerosis: molecular mechanisms and implications for plaque progression and stability. Cardiovasc Res. 2007;73:470–480. doi: 10.1016/j.cardiores.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Schrijvers DM. De Meyer GR. Kockx MM. Herman AG. Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–1261. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 34.Seimon T. Tabas I. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J Lipid Res. doi: 10.1194/jlr.R800032-JLR200. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seimon TA. Obstfeld A. Moore KJ. Golenbock DT. Tabas I. Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. Proc Natl Acad Sci U S A. 2006;103:19794–19799. doi: 10.1073/pnas.0609671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35a.Seimon T. Wang Y. Kuriakose G. Han S. Senokuchi T. Tall A. Tabas I. Deficiency of p38α in macrophages promotes apoptosis and plaque necrosis in advanced murine atherosclerotic lesions. J Clin Invest. 2009;119:886–898. doi: 10.1172/JCI37262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Senokuchi T. Liang CP. Seimon TA. Han S. Matsumoto M. Banks AS. Paik JH. Depinho RA. Accili D. Tabas I. Tall AR. FoxOs promote apoptosis of insulin resistant macrophages during cholesterol-induced ER stress. Diabetes. doi: 10.2337/db08-0520. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith JD. Trogan E. Ginsberg M. Grigaux C. Tian J. Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci U S A. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinberg D. Lipoproteins and atherosclerosis: a look back and a look ahead. Arteriosclerosis. 1983;3:283–301. doi: 10.1161/01.atv.3.4.283. [DOI] [PubMed] [Google Scholar]
- 39.Szegezdi E. Logue SE. Gorman AM. Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 41.Tabas I. Mouse models of apoptosis and efferocytosis. Curr Drug Targets. 2008;8:1288–1296. doi: 10.2174/138945007783220623. [DOI] [PubMed] [Google Scholar]
- 42.Tabas I. Williams KJ. Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 43.Tardif JC. Gregoire J. L'Allier PL. Anderson TJ. Bertrand O. Reeves F. Title LM. Alfonso F. Schampaert E. Hassan A. McLain R. Pressler ML. Ibrahim R. Lesperance J. Blue J. Heinonen T. Rodes-Cabau J. Effects of the acyl coenzyme A: cholesterol acyltransferase inhibitor avasimibe on human atherosclerotic lesions. Circulation. 2004;110:3372–3377. doi: 10.1161/01.CIR.0000147777.12010.EF. [DOI] [PubMed] [Google Scholar]
- 44.Thorp E. Kuriakose G. Shah YM. Gonzalez FJ. Tabas I. Pioglitazone increases macrophage apoptosis and plaque necrosis in advanced atherosclerotic lesions of nondiabetic low-density lipoprotein receptor-null mice. Circulation. 2007;116:2182–2190. doi: 10.1161/CIRCULATIONAHA.107.698852. [DOI] [PubMed] [Google Scholar]
- 44a.Thorp E. Li G. Seimon TA. Kuriakose G. Ron D. Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab 2009. doi: 10.1016/j.cmet.2009.03.003. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Virmani R. Burke AP. Kolodgie FD. Farb A. Vulnerable plaque: the pathology of unstable coronary lesions. J Interv Cardiol. 2002;15:439–446. doi: 10.1111/j.1540-8183.2002.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 46.Warner GJ. Stoudt G. Bamberger M. Johnson WJ. Rothblat GH. Cell toxicity induced by inhibition of acyl coenzyme A: cholesterol acyltransferase and accumulation of unesterified cholesterol. J Biol Chem. 1995;270:5772–5778. doi: 10.1074/jbc.270.11.5772. [DOI] [PubMed] [Google Scholar]
- 47.Yao PM. Tabas I. Free cholesterol loading of macrophages induces apoptosis involving the fas pathway. J Biol Chem. 2000;275:23807–23813. doi: 10.1074/jbc.M002087200. [DOI] [PubMed] [Google Scholar]
- 48.Yao PM. Tabas I. Free cholesterol loading of macrophages is associated with widespread mitochondrial dysfunction and activation of the mitochondrial apoptosis pathway. J Biol Chem. 2001;276:42468–42476. doi: 10.1074/jbc.M101419200. [DOI] [PubMed] [Google Scholar]
- 49.Zhou J. Lhotak S. Hilditch BA. Austin RC. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2005;111:1814–1821. doi: 10.1161/01.CIR.0000160864.31351.C1. [DOI] [PubMed] [Google Scholar]
- 50.Zinszner H. Kuroda M. Wang X. Batchvarova N. Lightfoot RT. Remotti H. Stevens JL. Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]