Abstract
Previous work has shown that a diet enriched with antioxidants and mitochondrial co-factors improves cognition in aged dogs, which was accompanied by a reduction oxidative damage in the brain. The objective of the present study was to assess the effects of supplementation with mitochondrial co-factors on cognition and plasma protein carbonyl levels in aged dogs. Specifically, we aimed to test whether the individual or combined action of lipoic acid (LA) and acetyl-L-carnitine (ALCAR) could account for the beneficial effects of the enriched diet that contained both plus antioxidants. Dogs were given LA or ALCAR, alone and then in combination and cognition was assessed using a spatial learning task and two discrimination and reversal paradigms. Dogs receiving the ALCAR supplement showed an increase in protein carbonyl levels that was associated with increased error scores on the spatial task, and which was reduced upon additional supplementation with LA. We did not observe significant positive effects on cognition. The present findings suggest that short-term supplementation with LA and ALCAR is insufficient to improve cognition in aged dogs, and that the beneficial effects of the full spectrum diet arose from either the cellular antioxidants alone or their interaction with LA and ALCAR.
Keywords: acetyl-L-carnitine, lipoic acid, oxidative damage, protein carbonyls, canine, spatial learning
Introduction
The accumulation of oxidative damage to lipids, proteins and nucleic acids in the brain contributes to neuronal dysfunction and the development of age-related neuropathology (Liu, 2008). The CNS is particularly vulnerable to the cumulative effects of oxidative damage because of its high rate of metabolism relative to other tissues (Ames et al., 1993). As is the case in other mammalian species, canines accumulate oxidative damage in their brains with age. Aged dogs show damage to protein carbonyl groups (Head et al., 2002; Opii et al., 2008; Skoumalova et al., 2003) and oxidative damage to lipids (Head et al., 2002; Papaioannou et al., 2001; Rofina et al., 2004; Rofina et al., 2006) and DNA/RNA (Rofina et al., 2006). Further, endogenous antioxidant enzyme activity, including glutamine synthetase and superoxide dismutase, is reduced in the brains of aged canines (Head et al., 2002; Kiatipattanasakul et al., 1997), and these changes are associated with age-related cognitive decline (Opii et al., 2008).
We have shown previously that treatment with a diet enriched in antioxidants (vitamins E and C and fruit and vegetable extracts) and mitochondrial co-factors (lipoic acid and acetyl-L-carnitine) induces rapid improvements in learning and slows cognitive decline in old dogs (Cotman et al., 2002; Milgram et al., 2002a; Milgram et al., 2004; Milgram et al., 2002b). Dogs fed this enriched diet over a two-year period had decreased markers of oxidative stress as well as increased expression of antioxidant and cell maintenance protein markers in the brain (Opii et al., 2008). Therefore, reducing excessive ROS and/or increasing levels of antioxidants may mitigate cognitive aging in the dog. In the present study, our aim was to determine whether the mitochondrial co-factors lipoic acid (LA) and/or acetyl-L-carnitine (ALCAR), without cellular antioxidants, would improve cognition in aged beagles. We predicted that supplementation with LA and ALCAR would improve cognition to a similar extent as the full spectrum diet used in the previous longitudinal study, which would indicate that LA and ALCAR are sufficient for improving brain function in aged beagles. No improvement in cognition would indicate that either the cellular antioxidants were the necessary components of the full spectrum diet, or that some synergistic effect of the mitochondrial co-factors with vitamins E and C and the fruit and vegetable extracts was acting to improve cognition. A negative effect of LA and/or ALCAR on cognition would suggest that either or both mitochondrial co-factors, when administered without cellular antioxidants, are detrimental to brain function in aged beagles.
Mitochondria are the major source of reactive oxygen species (ROS) generation, and mitochondrial dysfunction may play a significant role in aging and neurodegeneration (Ames et al., 1993). Accordingly, improving mitochondrial function is a focus of antioxidant strategies to combat brain aging. Mitochondrial cofactors, particularly alpha-lipoic acid (LA) and acetyl-L-carnitine (ALCAR), are effective in reducing age-related mitochondrial dysfunction and their combination may decrease oxidative damage to neurons and improve cognitive deficits (reviewed in Liu, 2008).
LA is a powerful antioxidant, acting as an inducer of phase 2 antioxidant enzymes and up-regulating endogenous cellular antioxidant defences (reviewed in Liu, 2008). In aged rats, LA administration restored long-term potentiation (LTP) (McGahon et al., 1999), and improved spatial memory in a water maze task, while decreasing oxidative damage in the CA4 region of the hippocampus (Liu et al., 2002a) and lipid peroxidation (Liu et al., 2002b). LA given to Tg2576 Alzheimer disease (AD) mice improved acquisition and memory performance on a water maze task and memory for contextual fear conditioning (Quinn et al., 2007), although a similar study reported no detectable improvements on a Y-maze task (Siedlak et al., 2009). In humans, randomized, placebo-controlled clinical studies are needed, however, a recent long-term, open-label study reported that LA supplementation in probable AD patients lead to stabilization of cognitive function over a 48-month follow-up period (Hager et al., 2007).
ALCAR, an acetyl derivative of L-carnitine, is required for the transport of long-chain fatty acids into the mitochondria for β-oxidation, ATP production, and removal of excess short- and medium-chained fatty acids (Rebouche, 1992), and thus helps to maintain efficient mitochondrial function. In aged rats, ALCAR administration improves memory on a water maze task (Liu et al., 2002a; Taglialatela et al., 1996), and partially restores ambulatory activity (Hagen et al., 1998a). In humans, a meta-analysis of 21 double blind prospective studies found improved function in patients with mild cognitive impairment and mild AD (Montgomery et al., 2003) with ALCAR supplementation. By contrast, a one-year clinical trial on the effects of supplementation with ALCAR (1g tid) in early onset AD patients failed to find significant effects on rate of cognitive decline, although scores on the Mini Mental State Examination (MMSE) were slightly higher in treated patients (Thal et al., 2000). These equivocal findings highlight how, even with placebo-controlled studies, there are often differences in dosage, duration or regularity of use, and form or source of supplement intake, making interpretation of the results difficult. Further, there is the additional challenge of determining baseline dietary intake of antioxidants in participants as well as their level of social engagement and physical activity, which may vary and affect the outcome (for example, Bennett et al., 2006; Colcombe et al., 2004).
In rodents, combined supplementation with LA and ALCAR appears to be more effective than using either alone. LA+ALCAR improves both acquisition and memory performance in a water maze task in aged rats, while each supplement given on its own improved only memory performance (Liu et al., 2002a). In this same study, the authors observed reduced oxidative damage to RNA in all regions of the hippocampus following supplementation with LA+ALCAR, whereas LA alone reduced oxo8G immunoreactivity only in the CA4 region and ALCAR alone did not have a significant impact on any region measured (Liu et al., 2002a). Consistent with these previous studies, recent reports show that LA+ALCAR increases the proliferation of intact neuronal mitochondria, decreases the number of damaged mitochondria (Aliev et al., 2009), reverses age-related decreases in complex I mitochondrial activity, reduces malondialdehyde and protein carbonyl levels, and increases reduced glutathione (GSH) and superoxide dismutase (SOD) activity in the brains of old rats (Long et al., 2009). To date, results from clinical trials examining the effectiveness of combined supplementation with LA and ALCAR in preventing cognitive decline with age or AD have not been published, although a few are currently in progress. Accordingly, well-controlled studies in higher animal models can help guide future human studies.
A recent pilot study conducted in aged canines suggests that supplementation with a combination of LA and ALCAR may be effective on some cognitive tests (Milgram et al., 2007). Thus, in the current study, dogs were given either LA or ALCAR alone, and then in combination to assess the effects of short-term supplementation with mitochondrial co-factors on cognitive performance in a larger sample of aged beagles with added cognitive outcome measures. Here we report that although supplementation produced significant changes in plasma protein carbonyl levels, an assay of oxidative damage, the intervention did not improve cognition as expected.
Materials and Methods
Subjects
A total of 30 beagles (21 females, 9 males; 7.8 to 11.2 years of age; 7.1 to 13.9kg) were provided with baseline cognitive testing. Beagles reach sexual maturity at approximately 35 to 41 weeks of age (James et al., 1979), their 10% survival rate is 16 years, and their median life span is 12 years (Albert et al., 1994). Comparative longevity research suggests that this age range roughly corresponds to 50 to 70 year-old humans (Patronek et al., 1997). We have previously shown that beagle dogs show significant age-related cognitive decline beginning as early as middle age (i.e. 6 years of age) on a spatial learning and memory task (Studzinski et al., 2006). Further, at 8 years of age, beagles show significant impairments compared to young dogs on acquisition of the landmark task used in this study (Christie et al., 2005). Thus, it was reasonable to expect that a significant number of dogs were experiencing age-related cognitive decline with sufficient severity to be able to detect any positive effects of supplementation.
Dogs were obtained from CanCog Technologies Inc. (Toronto, Canada), and had similar cognitive backgrounds, which included pre-training, as described previously (Milgram et al., 1994), and, in some cases, testing on additional tasks. Inclusion in the study required successful acquisition of the pre-training protocol and consistent responsiveness during baseline cognitive testing. Veterinary examinations were conducted on all dogs prior to the start of the study to assess their general health and to ensure visual, auditory and motor functioning were not compromised.
Dogs were housed indoors in groups of 2 or 4 and had free access to water. They were fed a standard adult maintenance diet once daily (Purina Pro Plan® Chicken and Rice) so as to maintain a consistent body weight and appropriate body condition, which was assessed every two weeks. Housing temperature was held at 21±6°C and relative humidity levels ranged between 15 and 75%.
Twenty-nine dogs completed the study; one male was removed from the study because of cervical disc disease.
Experimental design
Prior to allocation into experimental groups, all dogs were tested on a spatial memory task (delayed non-matching to position or DNMP, described below). Subsequently, dogs were divided equally into three experimental groups such that the groups were matched for memory performance and age. For all phases, dogs in group I served as placebo controls, and dogs in groups II and III received the mitochondrial co-factor supplements, as described below.
A summary of the experimental design is shown in Table 1. The single supplement phase began following the completion of baseline testing. In this phase, group II received 11mg/kg alpha-lipoic acid (LA) and group III received 27.5mg/kg acetyl-L-carnitine (ALCAR) for a total of 129 days, including a 14-day wash-in period in which no cognitive testing was conducted. These doses were chosen because they are similar, on a per kilogram body weight basis, to those previously used in human studies (Hager et al., 2007; Thal et al., 2000).
| # days | Task | Group I Age: 9.1 (8.2–10.3) |
Group II Age: 9.4 (8.0–11.2) |
Group III Age: 9.2 (7.8–11.1) |
|
|---|---|---|---|---|---|
| Baseline | 15 | DNMP | N=4M/6F placebo |
N=2M/8F placebo |
N=3M/7F placebo |
| 14 day Wash-in | |||||
| Single Supplement Phase | 129 | Landmark | N=4M/6F placebo |
N=2M/8F 11mg/kg LA |
N=3M/7F 27.5mg/kg ALCAR |
| 14 day Wash-in | |||||
| Combined Supplement Phase | 79 | Black/white Discrimination & Reversal | N=4M/6F placebo |
N=2M/8F 11mg/kg LA +27.5mg/kg ALCAR |
N=3M/7F 27.5mg/kg ALCAR +11mg/kg LA |
| 14 day Wash-out | |||||
| Wash-out Phase | 50 | Size Discrimination & Reversal | N=4M/6F placebo |
N=2M/8F placebo |
N=2M/7F placebo |
The combined supplement phase was conducted next. In this phase, group II continued to receive 11mg/kg LA and in addition was given 27.5mg/kg ALCAR while group III received 27.5mg/kg ALCAR plus 11mg/kg LA. Again, this phase began with a 14-day wash-in period and lasted for 79 days. This final phase was followed by a 14-day washout period, after which the study’s final cognitive testing was conducted.
Supplementation
For all phases, the ALCAR and LA supplements consisted of powders purchased from US Pharma Lab Inc. (North Brunswick, NJ). Daily oral doses were provided in capsules placed in small cubes of wet dog food. Each animal was dosed twice daily, once prior to cognitive testing at 8 a.m. and once after cognitive testing at 4:30 p.m., with each capsule containing half the daily dose. Group I received an equivalent amount of methylcellulose using the same procedures. Throughout the study, individuals conducting cognitive testing were blind to the treatment status of the animals.
Blood sampling and protein carbonyl assay
Blood samples were collected at four time-points throughout the study; (1) at baseline, (2) during the single supplement phase, (3) during the combined supplement phase, and (4) following washout. At each time-point, 20 ml of blood was collected in EDTA tubes and then centrifuged. Plasma from each subject was separated into aliquots of 0.5 ml, transferred to cryogenic vials and stored at −80 °C until the ELISA assay was conducted.
Protein carbonyl levels were assayed by a colorimetric ELISA method (Cell Biolabs Protein Carbonyl ELISA kit STA-310). Briefly, 10μg/ml plasma samples were loaded into the wells of an ELISA plate and incubated overnight on a protein binding plate at 4°C. After a series of washings, dinitrophenyl hydrazine (DNPH) working solution was added and the samples were incubated in the dark for 45 minutes at room temperature. Wells were then washed with 250μL of 1X PBS/Ethanol (1:1, v/v), blocking solution was added and 1 to 2 hour incubation at room temperature was carried out. After washing, samples were incubated with anti-dinitrophenyl (DNP) antibody for 1 hour followed by diluted horseradish peroxidase conjugated secondary antibody for another hour, both at room temperature. Warm substrate solution was then added. Following 20min incubation, stop solution was added to each well and absorbance was read immediately on a plate reader using 450nm as the primary wavelength and fully reduced bovine serum albumin (BSA) standard as the absorbance blank. The protein carbonyl content was then determined by comparing the dog samples with a standard curve prepared from oxidized and reduced BSA standards.
Cognitive testing
All cognitive testing was conducted in a canine adaptation of the Wisconsin general testing apparatus, as described previously (Milgram et al., 1994). Briefly, the apparatus consisted of a large holding area where the dog was housed during testing, which was separated from the experimenter by a wooden screen containing a one-way mirror and a hinged door at the bottom. A Plexiglas stimulus tray containing three food wells was pushed through the hinged door by the experimenter so that the dog could access the stimuli and food rewards by sticking its head through adjustable stainless steel gates at the front of the holding area. The tray was removed out of sight during delay and inter-trial intervals.
Food reward for correct responses during cognitive testing consisted of approximately 1g of wet dog food. To mask the presence of the food reward in the negative food wells, the undersides of all stimuli were baited with the same food such that, while able to smell it, the animals could not see or eat it. For all tasks, a partial correction procedure was used in which the dogs were permitted to correct their response after making an error once each session.
Delayed non-matching to position task
The DNMP task was used to assess baseline cognitive abilities and responsiveness prior to supplement administration. The task provides a measure of short-term spatial memory and has been described previously (Chan et al., 2002). Briefly, dogs received 18 trials daily with an inter-trial interval of 30 seconds. Each trial consisted of two phases. In the sample phase, a white block was presented over one of three food wells. The dog was required to displace the block and retrieve the food in the well below the block. The block was then removed from view and a delay was initiated. At the end of the delay, the choice phase began. In this phase, the dog was presented with two identical blocks; one over the initial well and a second over one of the two remaining wells. Dogs were required to displace the block in the novel spatial position to obtain the food reward. For the present study, DNMP testing was followed by a variable delay protocol in which delays of 5, 55, and 105 seconds occurred equally over the 18 daily test trials. Testing was conducted daily for 15 days.
The percentage of correct trials across delays was calculated and used to rank the dogs in ascending order. Dogs with the three lowest scores were randomly and evenly allocated to the three treatment groups. The animals with the next three lowest scores were then randomly allocated, and so forth, until all 30 dogs were assigned. Once all animals were assigned based on their overall performance, the group means were compared at each of the delay intervals to ensure that spatial memory performance was matched.
Landmark task
The landmark task is a spatial attention task that requires dogs to respond to one of two white discs based on its proximity to a vertical yellow block (the landmark). The landmark task assesses allocentric spatial learning and has been shown previously to be sensitive to both age and antioxidant diet intervention (Milgram et al., 2002a). In the present study, landmark testing was during the single supplement phase.
Two phases of the task were administered; land-0 and land-1. In land-0, the landmark was in direct contact with the white disc. Dogs had to learn that the white disc with the landmark was associated with food reward and the white disc without the landmark was associated with no food reward. This phase of the task assessed simple discrimination ability. For land-0, dogs were given 10 trials a day with an inter-trial interval of 30 seconds for a maximum of 25 test days or until a predetermined set of criteria for passing land-0 was reached. To pass, dogs were required to choose correctly on 9 out of 10 trials on one test day or 8 out of 10 trials on two consecutive test days, and then had to obtain a score of at least 70% correct (21/30) over the following three consecutive test days. Dogs that did not pass land-0 continued on to land-1 after they had completed the maximum 25 sessions of testing.
In land-1, the landmark was moved 1 cm medially and diagonally away from the white disc and the dogs had to learn to respond to the white disc that was closest to the landmark. This phase of the task assessed allocentric spatial learning ability. Again, 10 trials per day over 25 test days maximum were given to reach the same criteria as described above for land-0.
Black/white discrimination and reversal tasks
The black/white discrimination task evaluates an animal’s ability to learn to distinguish two objects on the basis of color, and the reversal task assesses the animal’s ability to change response strategies once the stimulus-reward association is reversed (Milgram et al., 2005). These tasks were used because we have previously found them to be age- and diet- sensitive (Milgram et al., 2005). The tasks were administered during the combined supplement phase of the present study.
Stimuli consisted of identically shaped black and white wooden blocks. Prior to discrimination testing, a preference test was administered to establish if a preference for either block existed. 10 trials were given and the number of choices for each stimulus was recorded. The preferred object served as the rewarded object for discrimination testing.
For both the discrimination and reversal tasks, animals were given 10 trials per day, with an inter-trial interval of 30 seconds. An error was counted when the dog displaced the non-rewarded stimulus, and a food reward was revealed when the dog displaced the rewarded stimulus. In order to pass the discrimination phase and continue to reversal testing, dogs had to meet a two-stage set of criteria. For the first stage, the animal had to average 80% correct over two test sessions, or at least 90% on a single session. For the second stage, the dog had to respond correctly on at least 70% of the trials over three successive sessions. Subjects had a maximum of 25 sessions to attain criterion on the discrimination phase.
Black/white reversal testing procedures were implemented the day after dogs reached criterion levels of responding on the discrimination problem. Reversal learning procedures were identical to the discrimination task, except that the reward contingencies of the positive and negative choice were reversed. Thus, if an animal was rewarded for approaching the white block during the discrimination testing, it was rewarded for approaching the black block during reversal testing.
Size discrimination and reversal tasks
The size discrimination task evaluates an animal’s ability to learn to distinguish two objects that differ only in height by selectively responding to either the smaller or larger of the two objects (Head et al., 1998). The size reversal task assesses the ability to switch from responding to one of the objects to the other, and provides a test of executive function (Tapp et al., 2003). This task was used because we have previously found it to be age (Tapp et al., 2003) and diet-sensitive (Milgram et al., 2004) and because performance varies as a function of beta amyloid protein accumulation in the brain (Head et al., 1998). The tasks were administered following the 14-day washout period.
Stimuli for the size discrimination and reversal tasks consisted of identically shaped red wooden blocks that differed only in height; the large block was twice the height of the small block. Prior to size discrimination testing, a preference test was administered to establish if a preference for either block existed. 10 trials were given and the number of choices for each stimulus was recorded. The preferred object served as the rewarded object for size discrimination testing.
For both the size discrimination and reversal tasks, animals were given 10 trials per day, with an inter-trial interval of 30 seconds. An error was counted when the dog displaced the non-rewarded stimulus, and a food reward was revealed when the dog displaced the rewarded stimulus. In order to pass the size discrimination task and continue to reversal testing, dogs had to meet the same two-stage set of criteria described above for the black/white discrimination and reversal tasks.
Size reversal testing procedures were identical to the size discrimination task, except that the reward contingencies of the positive and negative block were reversed.
Statistics
All statistical analyses were conducted using SPSS 15.0 for Windows. Repeated measures analysis of variance (ANOVA) was used to assess the effects of treatment on plasma protein carbonyls and cognition. Post hoc Tukey’s HSD tests were conducted to examine group differences when a significant overall effect was found.
For the DNMP task administered at baseline, the percentage of correct trials at each delay interval was used as the dependent variable. For all other cognitive tasks, the total number of errors made to reach the criteria described above was used as the main measure.
Results
Baseline DNMP performance and group assignment
The percentage of correct trials across delays on the DNMP task was calculated and used to assign the dogs to the three experimental groups, as described above. Table 1 lists the characteristics of the groups for each of the five phases of the study.
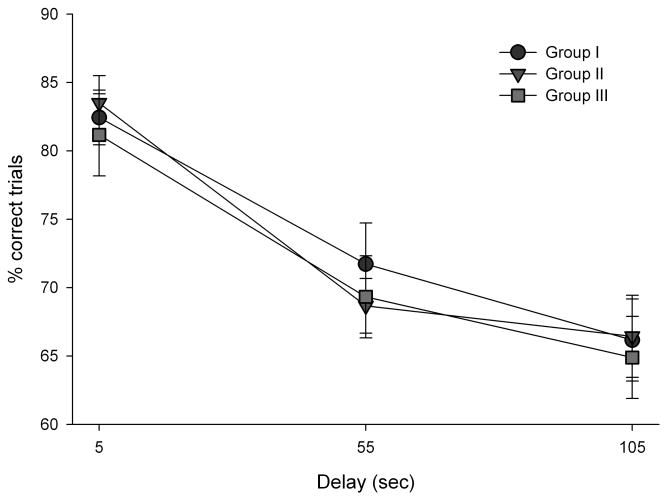
To further confirm that the groups were matched for cognitive performance prior to initiation of supplement treatment, a repeated measures ANOVA was conducted with delay as the within-subjects factor and group as the between-subjects factor. Overall, there was no significant effect of group (p = 0.89) or group X delay interaction (p = 0.69). There was a significant effect of delay, F(2, 54) = 103.02, p < 0.001; performance accuracy decreased as delay increased across all groups (Fig. 1).
Figure 1.
Delayed non-matching to position (DNMP) baseline performance. Prior to allocation into experimental groups, dogs were tested on the DNMP task, a test of spatial memory, and then divided equally into three experimental groups matched for performance on the task. There was a significant effect of delay, with accuracy decreasing as delay interval increased. Error bars show ± 1 S.E.M.
Treatment effects on plasma protein carbonyl levels
A repeated measures ANOVA with study phase as the within-subjects factor and group as the between-subjects factor was conducted to examine the effects of supplement treatment on protein carbonyl levels at the four time points at which plasma samples were collected. A significant interaction of group X study phase was found, F(6,81) = 2.784, p = 0.016.
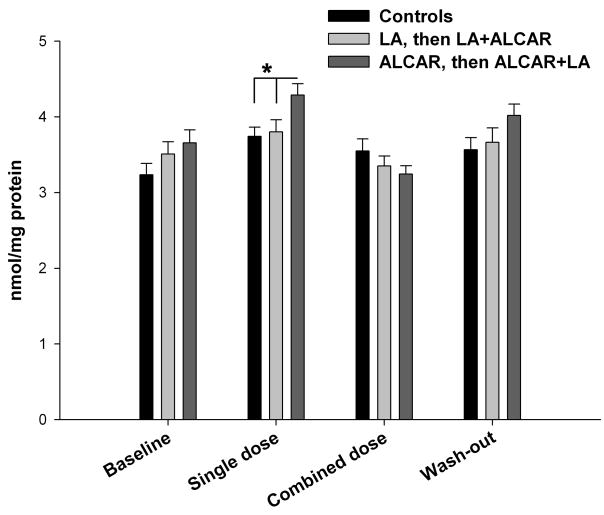
In order to deconstruct this effect, individual ANOVA tests were run to compare the three groups at each time point. An overall significant effect of group was found in the single supplement phase of the study F(2,27) = 4.874, p = 0.016. By contrast, at baseline and at the end of the combined supplement and washout phases, no significant group effect was found.
Post hoc Tukey HSD tests for the significant overall group effect during the single supplement phase showed that the ALCAR group differed significantly from both the control group (p = 0.022) and the LA group (p = 0.044). As shown in Figure 2, the ALCAR group had higher plasma protein carbonyl levels compared to both the control group and the LA group, suggesting that treatment with 27.5mg/kg ALCAR may have increased oxidative stress.
Figure 2.
Mean plasma protein carbonyl levels, as assessed by ELISA, in nmol/mg. Dogs receiving the 27.5mg/kg dose of ALCAR showed significantly increased protein oxidation levels compared to those receiving 11mg/kg LA and placebo control. This difference was no longer present when this group was given the combined ALCAR+LA supplement. Error bars show ± 1 S.E.M.
Cognitive results
Single supplement phase
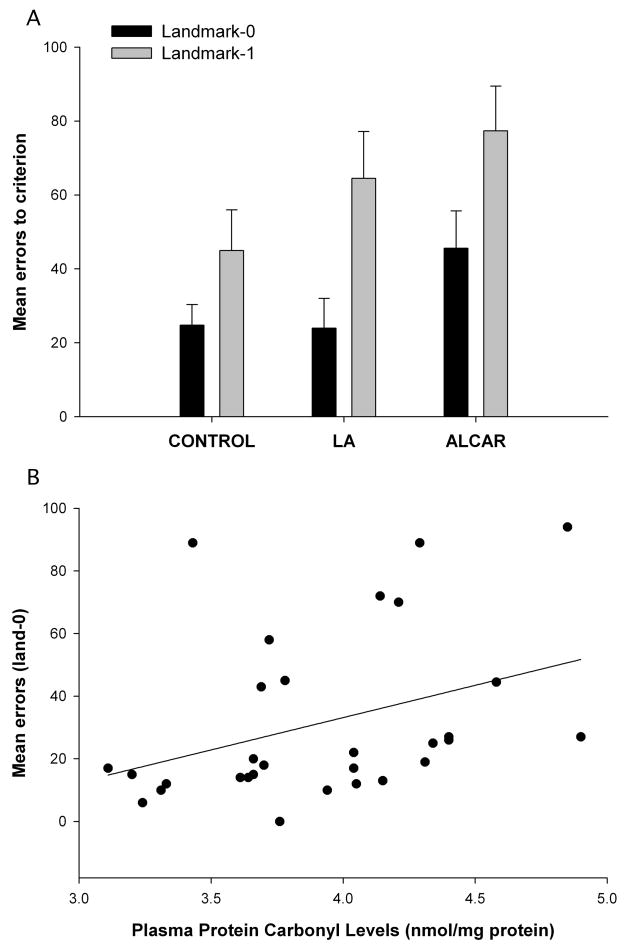
The landmark task was administered during the first treatment phase of the study when group II received 11mg/kg LA and group III received 27.5mg/kg ALCAR. A repeated measures ANOVA with landmark level (land-0 vs. land-1) as the within-subjects factor showed a significant effect of landmark level F(1, 27) = 16.8, p < 0.001; animals made more errors on land-1 than land-0. In addition, a trend for an overall group effect F(2, 27) = 2.94, p = 0.07 was observed for both landmark tests. A post hoc Tukey HSD test revealed that animals receiving ALCAR made slightly more errors to criterion compared to the control group, although this effect did not reach statistical significance (p = 0.06; Fig. 3A). Further, a significant positive correlation was found between error scores on land-0 and protein carbonyl levels in plasma collected just after the task was administered (Fig. 3B; r=0.37; p=0.05); dogs who made more errors during land-0 testing tended to have higher plasma protein carbonyl levels. No significant correlation was found between carbonyl levels and land-1 errors.
Figure 3.
A. Performance on the landmark task did not significantly differ between control animals, those receiving 27.5mg/kg ALCAR or 11mg/kg LA during the single supplement phase. Dogs receiving ALCAR tended to make more errors compared to control dogs on land-0 and land-1, but this effect did not reach statistical significance. There was a significant effect landmark level; all three groups made more errors on land-1 compared to land-0. B. A significant positive correlation between land-0 error scores and plasma protein carbonyl levels was found (r=0.37; p=0.05) during the single supplement phase. Error bars show ± 1 S.E.M.
Combined supplement phase
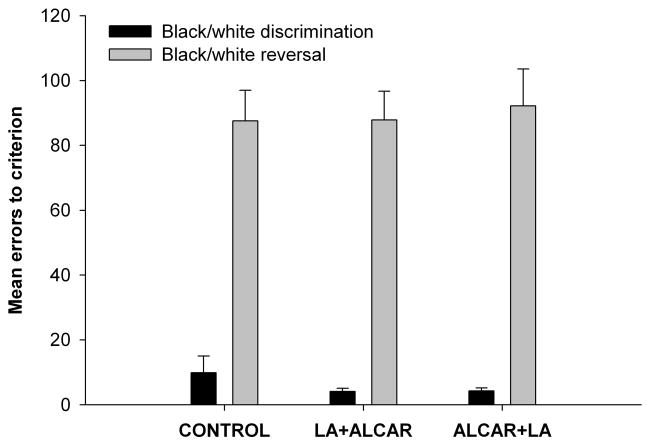
The black/white discrimination and reversal tasks were administered during the combined supplement phase, when group II had ALCAR added to their LA supplements and group III had LA added to their ALCAR supplements. A significant effect of task was found by repeated measures ANOVA F(1, 27) = 69.83, p < 0.001; animals in all three groups made more errors on the reversal task than the initial discrimination task. No significant group effect or group X task interaction effect was found (Fig. 4).
Figure 4.
Performance on black/white discrimination and reversal task did not differ between control animals, those receiving ALCAR+LA or LA+ALCAR during the combined supplement phase of the study. There was a significant effect task; all three groups made more errors in acquiring the reversal task compared to initial discrimination task. Error bars show ± 1 S.E.M.
Washout phase
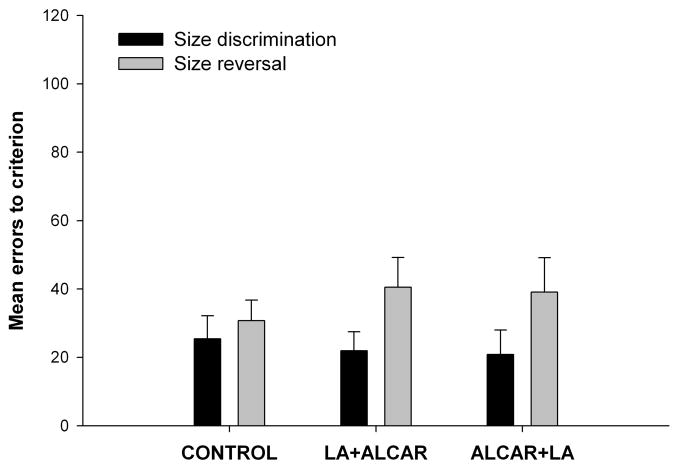
Repeated measures ANOVA on the size and size reversal task results from the washout phase of the study produced no significant group effect or group X task interaction effect. A significant effect of task was found F(1, 26) = 4.51, p = 0.04; animals in all three groups made more errors on the reversal task compared to the discrimination task (Fig. 5).
Figure 5.
Performance on size discrimination and reversal task did not differ between the three groups during the wash-out phase of the study. There was a significant effect task; all three groups made more errors in acquiring the reversal task compared to initial discrimination task. Error bars show ±1 S.E.M.
Discussion
The present study assessed the effects of short-term mitochondrial co-factor supplementation on protein carbonyl levels and cognition in aged canines. We found that short-term administration of ALCAR increases plasma protein oxidation in aged dogs compared to placebo-treated control animals, while LA alone did not alter carbonyl levels. We found no significant differences between treated and untreated animals on multiple tests of learning and memory, although allocentric spatial performance tended to be worse in dogs receiving ALCAR alone.
Dogs given 27.5 mg/kg ALCAR for one month showed increased plasma protein carbonyl levels compared to animals receiving placebo and LA. This is a paradoxical finding given that ALCAR was predicted to reduce ROS production by improving mitochondrial function based on previous findings in aged rodents (Hagen et al., 1998a; Liu et al., 2002a; Liu et al., 2002b). However, consistent with our present results, Hagen and colleagues (1998b) report an increase in rate of oxidant production and reduced cellular glutathione and ascorbate levels in cells isolated from ALCAR-supplemented old rats compared to untreated animals. In the same study, the authors report that ALCAR supplementation significantly reversed the age-associated decline of mitochondrial membrane potential and cardiolipin levels, which suggests that ALCAR may prevent age-related impairments in mitochondrial function but may also increase the amount of oxidants produced as by-products in mitochondrial electron transport (Hagen et al., 1998b). The tendency for high doses of ALCAR to increase oxidative stress in the brain is also reported in a dose-response study conducted in old rats (reviewed in Liu et al., 2002c). The authors report that 1.5% wt/vol ALCAR added to drinking water (≈1400mg/kg daily, assuming 1 ounce of water consumed per day by a 350g rat) increased brain levels of protein carbonyls and was not effective at reducing malondialdehyde levels (Liu et al., 2002c). This may partially explain why ALCAR-treated dogs had increased protein carbonyl levels in the present study, although our dose of 27.5mg/kg was significantly lower than the high doses used in rat studies.
By contrast, in aged rats, a 0.15% wt/vol dose (≈140mg/kg daily) effectively reduced brain levels of lipid, protein and nucleic acid oxidation in aged rats and improved ambulatory activity (Liu et al., 2004). Consistent with these findings, a recent study found decreases in protein oxidation in the hippocampus of rats given the equivalent of 150mg/kg of ALCAR daily, again via their drinking water (Poon et al., 2009), providing further evidence that supplementation with ALCAR decreases brain oxidation. Thus, in aged rats, high but not low doses of ALCAR appear to increase oxidation by increasing the amount of oxidant production per oxygen consumed (Hagen et al., 1998a; Liu et al., 2004). Currently, there are no formal ALCAR dose-response studies in canines, and so the doses that will increase or decrease oxidant production in the dog are unknown. The dose of ALCAR used in the present study was similar or higher, on a per kilogram body weight basis, to doses used in human studies (Hager et al., 2007; Thal et al., 2000). Further, the dose was five times higher than that used in our original longitudinal dietary intervention study in canines (Cotman et al., 2002; Milgram et al., 2002a; Milgram et al., 2004; Milgram et al., 2002b); we had hypothesized that ALCAR and LA were playing a major role in the beneficial effects of the diet on cognition and so we chose higher doses of both of these compounds to be given in capsules in the present study. Other than potential differences in dose-response across species, it is also difficult to compare the doses used in rats versus dogs because of varying routes of administration. Differences in absorption with delivery via water in rats versus twice daily capsules given to dogs in the present study may have significantly impacted the amount of ALCAR in both plasma and brain, although additional studies will be necessary to confirm this hypothesis.
Plasma carbonyl levels in ALCAR-treated animals decreased when LA was co-administered with ALCAR. Dogs given 27.5 mg/kg ALCAR plus 11 mg/kg LA did not have significantly different plasma carbonyl levels compared to the LA+ALCAR or control groups. This suggests that the increase in protein oxidation seen in ALCAR treated animals was attenuated by co-administration of LA. This finding is consistent with previous studies examining the effects of ALCAR and LA in combination (Aliev et al., 2009; Hagen et al., 2002; Liu et al., 2002a; Liu et al., 2002b; Long et al., 2009), and has been taken as justification for using ALCAR and LA together for the prevention of age-related cognitive decline.
Despite the effects observed on plasma protein carbonyl levels, we did not see any significant effects of supplementation on cognition in the present study. LA did not improve allocentric spatial memory performance, as assessed by our landmark task. Similarly, ALCAR supplementation did not improve landmark performance, and in fact, there was a trend for animals receiving ALCAR alone to make more errors compared to untreated controls. A significant correlation was found between land-0 errors and protein carbonyl levels during the single supplement phase; dogs that made more errors tended to have higher protein carbonyl levels, suggesting that error scores were reflective of increased oxidative stress. A recent study in dogs found positive effects of combined LA+ALCAR treatment on landmark performance using the same doses used in the present study (Milgram et al., 2007). In this study, beagles aged 7.6 to 8.8 years were given either LA+ALCAR or placebo and tested on the landmark task using similar procedures. After a short wash-in period, the LA+ALCAR group made significantly fewer errors in acquiring land-0 and land-1 compared to the control group (Milgram et al., 2007). Therefore, there is evidence from this study (Milgram et al., 2007) along with our previous longitudinal study in animals receiving a diet enriched with LA and ALCAR, vitamins E, C and fruit and vegetable pomace (Milgram et al., 2002a) that LA+ALCAR may be sufficient to improve landmark performance; the current study suggests that supplementation with LA and ALCAR separately cannot.
One important difference between the present study and the previous LA+ALCAR supplement study (Milgram et al., 2007) is that animals had varying levels of prior cognitive testing experience in the present study. Some of the animals had previous exposure to several cognitive tasks, including the landmark task, although none of the animals had been tested for a minimum of six months prior to the study start. However, we did not expect test sophistication to influence the results of the present study based on a past study in aged canines showing that prior cognitive testing experience did not influence the positive effects of an antioxidant-enriched diet on cognitive outcome measures (Ikeda-Douglas et al., 2004). Lack of test experience in dogs can actually mask age-related effects on cognitive performance (Milgram, 2003), which suggests that using cognitively experienced animals is more appropriate in a study evaluating the effects of a dietary intervention on age-related cognitive decline. Further, results from studies using animals with varied background experience and cognitive test history will be more translatable to human studies than those using naïve animals.
Our previous longitudinal study in dogs showed that a diet enriched with a broad spectrum of antioxidants along with LA and ALCAR induced rapid improvements in landmark performance and prevented age-related cognitive decline in old dogs (Milgram et al., 2002a). Therefore, it is unlikely that we would have found improvements on the landmark task had we administered the supplements for a longer period of time before initiating landmark testing. Supplementation with LA+ALCAR together did not significantly improve discrimination and reversal learning in the current study. Our previous longitudinal studies found positive effects of the enriched diet on discrimination and reversal learning only after an extended period of time; size discrimination and reversal learning was improved after one year (Milgram et al., 2004) and black/white discrimination and reversal learning was improved after two years (Milgram et al., 2005), which is consistent with a protective effect of the diet on the ability of animals to perform these tasks. The design of the current study only allowed us to assess acute effects of LA+ALCAR supplementation on discrimination and reversal learning, as the supplements were given for a two-week wash-in prior to discrimination testing and then throughout the 79-day testing protocol. Taken together, the results suggest that LA+ALCAR supplementation may improve discrimination and reversal learning only if administered for extended periods of time. However, the effect of long-term LA+ALCAR alone on discrimination and reversal learning remains to be determined since these compounds were effective in our previous longitudinal study in the context of dietary supplementation with additional cellular antioxidants.
Our results are consistent with studies reporting only minimal or no effects of LA (Siedlak et al., 2009) and ALCAR (Thal et al., 2000) supplementation on cognition in aged or AD subjects. However, they are conflicting with several other studies (Hager et al., 2007; Liu et al., 2002a; Quinn et al., 2007; Shenk et al., 2009; Stoll et al., 1993; Taglialatela et al., 1996) that do report positive effects. For example, Liu and colleagues (2002) report that aged rats given a combination of LA and ALCAR show improved spatial memory compared to controls, and that combining LA and ALCAR was more effective than administering either alone. One notable difference between the present study and this previous study was the delivery method of the supplements; we used capsules given twice daily, while rats received ALCAR in their water and LA in their chow (Liu et al., 2002a). The latter delivery methods may have resulted in more constant serum and thus brain concentrations of the supplements compared with our capsule delivery method. Indeed, it has been shown previously in dogs that maximum serum concentrations of LA vary as a function of delivery method (Gross and Zicker, 2008). Our previous studies on the effects of antioxidant- and mitochondrial co-factor-supplementation were conducted using an enriched diet, which may partially explain why we found robust cognitive effects in our prior studies (Cotman et al., 2002; Milgram et al., 2002a; Milgram et al., 2004; Milgram et al., 2002b) and not the present study. Time-course concentration of the supplements was not determined in the current or previous study, which precludes us from determining the link between blood levels and cognitive effects.
The present results suggest that dietary enrichment with a combination of antioxidants, LA and ALCAR will be more effective than supplementation with LA and ALCAR alone in improving cognitive function. Alternatively, the cellular antioxidants (vitamins E, C, fruit and vegetable extracts) given as part of the diet in our previous longitudinal study might be much more important for improving cognition and age-related pathology in dogs than LA and ALCAR. Additional studies comparing the effectiveness of the individual components of the diet are needed to directly test these hypotheses. Further, LA and ALCAR may have been less effective given in supplement form than when given as part of a formulated diet due to absorptive and/or pharmacokinetic differences; a hypothesis that also needs further evaluation.
Acknowledgments
Funding from the NIH/NIA AG12694 supported this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aliev G, Liu J, Shenk JC, Fischbach K, Pacheco GJ, Chen SG, Obrenovich ME, Ward WF, Richardson AG, Smith MA, Gasimov E, Perry G, Ames BN. 2009 [Google Scholar]
- Albert RE, Benjamin SA, Shukla R. Life span and cancer mortality in the beagle dog and humans. Mech Ageing Dev. 1994;74:149–59. doi: 10.1016/0047-6374(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–22. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuronal mitochondrial amelioration by feeding acetyl-L-carnitine and lipoic acid to aged rats. J Cell Mol Med. 13:320–33. doi: 10.1111/j.1582-4934.2008.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 2006;5:406–12. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- Chan AD, Nippak PM, Murphey H, Ikeda-Douglas CJ, Muggenburg B, Head E, Cotman CW, Milgram NW. Visuospatial impairments in aged canines (Canis familiaris): the role of cognitive-behavioral flexibility. Behav Neurosci. 2002;116:443–454. [PubMed] [Google Scholar]
- Christie LA, Studzinski CM, Araujo JA, Leung CS, Ikeda-Douglas CJ, Head E, Cotman CW, Milgram NW. A comparison of egocentric and allocentric age-dependent spatial learning in the beagle dog. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:361–369. doi: 10.1016/j.pnpbp.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–21. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Head E, Muggenburg BA, Zicker S, Milgram NW. Brain aging in the canine: a diet enriched in antioxidants reduces cognitive dysfunction. Neurobiol Aging. 2002;23:809–818. doi: 10.1016/s0197-4580(02)00073-8. [DOI] [PubMed] [Google Scholar]
- Gross KL, Zicker SC. Pharmacokinetics of alpha-lipoic acid in dogs. FASEB J. 2008;22:1117.5. [Google Scholar]
- Hagen TM, Ingersoll RT, Wehr CM, Lykkesfeldt J, Vinarsky V, Bartholomew JC, Song MH, Ames BN. Acetyl-L-carnitine fed to old rats partially restores mitochondrial function and ambulatory activity. Proc Natl Acad Sci U S A. 1998b;95:9562–6. doi: 10.1073/pnas.95.16.9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen TM, Liu J, Lykkesfeldt J, Wehr CM, Ingersoll RT, Vinarsky V, Bartholomew JC, Ames BN. Feeding acetyl-L-carnitine and lipoic acid to old rats significantly improves metabolic function while decreasing oxidative stress. Proc Natl Acad Sci U S A. 2002;99:1870–5. doi: 10.1073/pnas.261708898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen TM, Wehr CM, Ames BN. Mitochondrial decay in aging. Reversal through supplementation of acetyl-L-carnitine and N-tert-butyl-alpha-phenyl-nitrone. Ann N Y Acad Sci. 1998a;854:214–23. doi: 10.1111/j.1749-6632.1998.tb09904.x. [DOI] [PubMed] [Google Scholar]
- Hager K, Kenklies M, McAfoose J, Engel J, Munch G. Alpha-lipoic acid as a new treatment option for Alzheimer’s disease--a 48 months follow-up analysis. J Neural Transm Suppl. 2007:189–93. doi: 10.1007/978-3-211-73574-9_24. [DOI] [PubMed] [Google Scholar]
- Head E, Callahan H, Muggenburg BA, Cotman CW, Milgram NW. Visual-discrimination learning ability and beta-amyloid accumulation in the dog. Neurobiology of aging. 1998;19:415–425. doi: 10.1016/s0197-4580(98)00084-0. [DOI] [PubMed] [Google Scholar]
- Head E, Liu J, Hagen TM, Muggenburg BA, Milgram NW, Ames BN, Cotman CW. Oxidative damage increases with age in a canine model of human brain aging. J Neurochem. 2002;82:375–381. doi: 10.1046/j.1471-4159.2002.00969.x. [DOI] [PubMed] [Google Scholar]
- Ikeda-Douglas CJ, Zicker SC, Estrada J, Jewell DE, Milgram NW. Prior experience, antioxidants, and mitochondrial cofactors improve cognitive function in aged beagles. Vet Ther. 2004;5:5–16. [PubMed] [Google Scholar]
- James RW, Crook D, Heywood R. Canine pituitary-testicular function in relation to toxicity testing. Toxicology. 1979;13:237–47. [PubMed] [Google Scholar]
- Kiatipattanasakul W, Nakamura S, Kuroki K, Nakayama H, Doi K. Immunohistochemical detection of anti-oxidative stress enzymes in the dog brain. Neuropathology. 1997;17:307–312. [Google Scholar]
- Liu J. The effects and mechanisms of mitochondrial nutrient alpha-lipoic acid on improving age-associated mitochondrial and cognitive dysfunction: an overview. Neurochem Res. 2008;33:194–203. doi: 10.1007/s11064-007-9403-0. [DOI] [PubMed] [Google Scholar]
- Liu J, Atamna H, Kuratsune H, Ames BN. Delaying brain mitochondrial decay and aging with mitochondrial antioxidants and metabolites. Ann N Y Acad Sci. 2002c;959:133–66. doi: 10.1111/j.1749-6632.2002.tb02090.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, Cotman CW, Ames BN. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha -lipoic acid. Proc Natl Acad Sci U S A. 2002a;99:2356–61. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Head E, Kuratsune H, Cotman CW, Ames BN. Comparison of the effects of L-carnitine and acetyl-L-carnitine on carnitine levels, ambulatory activity, and oxidative stress biomarkers in the brain of old rats. Ann N Y Acad Sci. 2004;1033:117–31. doi: 10.1196/annals.1320.011. [DOI] [PubMed] [Google Scholar]
- Liu J, Killilea DW, Ames BN. Age-associated mitochondrial oxidative decay: improvement of carnitine acetyltransferase substrate-binding affinity and activity in brain by feeding old rats acetyl-L- carnitine and/or R-alpha -lipoic acid. Proc Natl Acad Sci U S A. 2002b;99:1876–81. doi: 10.1073/pnas.261709098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J, Gao F, Tong L, Cotman CW, Ames BN, Liu J. Mitochondrial decay in the brains of old rats: ameliorating effect of alpha-lipoic acid and acetyl-L-carnitine. Neurochem Res. 2009;34:755–63. doi: 10.1007/s11064-008-9850-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGahon BM, Martin DS, Horrobin DF, Lynch MA. Age-related changes in LTP and antioxidant defenses are reversed by an alpha-lipoic acid-enriched diet. Neurobiol Aging. 1999;20:655–64. doi: 10.1016/s0197-4580(99)00050-0. [DOI] [PubMed] [Google Scholar]
- Milgram NW. Cognitive experience and its effect on age-dependent cognitive decline in beagle dogs. Neurochem Res. 2003;28:1677–1682. doi: 10.1023/a:1026009005108. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Araujo JA, Hagen TM, Treadwell BV, Ames BN. Acetyl-L-carnitine and alpha-lipoic acid supplementation of aged beagle dogs improves learning in two landmark discrimination tests. FASEB J. 2007;21:3756–62. doi: 10.1096/fj.07-8531com. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Head E, Muggenburg B, Holowachuk D, Murphey H, Estrada J, Ikeda-Douglas CJ, Zicker SC, Cotman CW. Landmark discrimination learning in the dog: effects of age, an antioxidant fortified food, and cognitive strategy. Neurosci Biobehav Rev. 2002a;26:679–695. doi: 10.1016/s0149-7634(02)00039-8. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Head E, Weiner E, Thomas E. Cognitive functions and aging in the dog: acquisition of nonspatial visual tasks. Behav Neurosci. 1994;108:57–68. doi: 10.1037//0735-7044.108.1.57. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Head E, Zicker SC, Ikeda-Douglas C, Murphey H, Muggenberg BA, Siwak CT, Tapp PD, Lowry SR, Cotman CW. Long-term treatment with antioxidants and a program of behavioral enrichment reduces age-dependent impairment in discrimination and reversal learning in beagle dogs. Exp Gerontol. 2004;39:753–765. doi: 10.1016/j.exger.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Head E, Zicker SC, Ikeda-Douglas CJ, Murphey H, Muggenburg B, Siwak C, Tapp D, Cotman CW. Learning ability in aged beagle dogs is preserved by behavioral enrichment and dietary fortification: a two-year longitudinal study. Neurobiol Aging. 2005;26:77–90. doi: 10.1016/j.neurobiolaging.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Zicker SC, Head E, Muggenburg BA, Murphey H, Ikeda-Douglas CJ, Cotman CW. Dietary enrichment counteracts age-associated cognitive dysfunction in canines. Neurobiol Aging. 2002b;23:737–745. doi: 10.1016/s0197-4580(02)00020-9. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Thal LJ, Amrein R. Meta-analysis of double blind randomized controlled clinical trials of acetyl-L-carnitine versus placebo in the treatment of mild cognitive impairment and mild Alzheimer’s disease. Int Clin Psychopharmacol. 2003;18:61–71. doi: 10.1097/00004850-200303000-00001. [DOI] [PubMed] [Google Scholar]
- Opii WO, Joshi G, Head E, Milgram NW, Muggenburg BA, Klein JB, Pierce WM, Cotman CW, Butterfield DA. Proteomic identification of brain proteins in the canine model of human aging following a long-term treatment with antioxidants and a program of behavioral enrichment: relevance to Alzheimer’s disease. Neurobiol Aging. 2008;29:51–70. doi: 10.1016/j.neurobiolaging.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaioannou N, Tooten PC, van Ederen AM, Bohl JR, Rofina J, Tsangaris T, Gruys E. Immunohistochemical investigation of the brain of aged dogs. I. Detection of neurofibrillary tangles and of 4-hydroxynonenal protein, an oxidative damage product, in senile plaques. Amyloid. 2001;8:11–21. doi: 10.3109/13506120108993810. [DOI] [PubMed] [Google Scholar]
- Patronek GJ, Waters DJ, Glickman LT. Comparative longevity of pet dogs and humans: implications for gerontology research. J Gerontol A Biol Sci Med Sci. 1997;52:B171–8. doi: 10.1093/gerona/52a.3.b171. [DOI] [PubMed] [Google Scholar]
- Poon HF, Calabrese V, Calvani M, Butterfield DA. Proteomics analyses of specific protein oxidation and protein expression in aged rat brain and its modulation by L-acetylcarnitine: insights into the mechanisms of action of this proposed therapeutic agent for CNS disorders associated with oxidative stress. Antioxid Redox Signal. 2006;8:381–94. doi: 10.1089/ars.2006.8.381. [DOI] [PubMed] [Google Scholar]
- Quinn JF, Bussiere JR, Hammond RS, Montine TJ, Henson E, Jones RE, Stackman RW., Jr Chronic dietary alpha-lipoic acid reduces deficits in hippocampal memory of aged Tg2576 mice. Neurobiol Aging. 2007;28:213–25. doi: 10.1016/j.neurobiolaging.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Rebouche CJ. Carnitine function and requirements during the life cycle. FASEB J. 1992;6:3379–86. [PubMed] [Google Scholar]
- Rofina JE, Singh K, Skoumalova-Vesela A, van Ederen AM, van Asten AJ, Wilhelm J, Gruys E. Histochemical accumulation of oxidative damage products is associated with Alzheimer-like pathology in the canine. Amyloid. 2004;11:90–100. doi: 10.1080/13506120412331285779. [DOI] [PubMed] [Google Scholar]
- Rofina JE, van Ederen AM, Toussaint MJ, Secreve M, van der Spek A, van der Meer I, Van Eerdenburg FJ, Gruys E. Cognitive disturbances in old dogs suffering from the canine counterpart of Alzheimer’s disease. Brain Res. 2006;1069:216–26. doi: 10.1016/j.brainres.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Shenk JC, Liu J, Fischbach K, Xu K, Puchowicz M, Obrenovich ME, Gasimov E, Alvarez LM, Ames BN, Lamanna JC, Aliev G. The effect of acetyl-L-carnitine and R-alpha-lipoic acid treatment in ApoE4 mouse as a model of human Alzheimer’s disease. J Neurol Sci. 2009 doi: 10.1016/j.jns.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedlak SL, Casadesus G, Webber KM, Pappolla MA, Atwood CS, Smith MA, Perry G. Chronic antioxidant therapy reduces oxidative stress in a mouse model of Alzheimer’s disease. Free Radic Res. 2009;43:156–64. doi: 10.1080/10715760802644694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoumalova A, Rofina J, Schwippelova Z, Gruys E, Wilhelm J. The role of free radicals in canine counterpart of senile dementia of the Alzheimer type. Exp Gerontol. 2003;38:711–9. doi: 10.1016/s0531-5565(03)00071-8. [DOI] [PubMed] [Google Scholar]
- Stoll S, Hartmann H, Cohen SA, Muller WE. The potent free radical scavenger alpha-lipoic acid improves memory in aged mice: putative relationship to NMDA receptor deficits. Pharmacol Biochem Behav. 1993;46:799–805. doi: 10.1016/0091-3057(93)90204-7. [DOI] [PubMed] [Google Scholar]
- Studzinski CM, Christie LA, Araujo JA, Burnham WM, Head E, Cotman CW, Milgram NW. Visuospatial function in the beagle dog: an early marker of cognitive decline in a model of human aging and dementia. Neurobiol Learn Mem. 2006;86:197–204. doi: 10.1016/j.nlm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Taglialatela G, Caprioli A, Giuliani A, Ghirardi O. Spatial memory and NGF levels in aged rats: natural variability and effects of acetyl-L-carnitine treatment. Exp Gerontol. 1996;31:577–87. doi: 10.1016/0531-5565(96)00052-6. [DOI] [PubMed] [Google Scholar]
- Tapp PD, Siwak CT, Estrada J, Head E, Muggenburg BA, Cotman CW, Milgram NW. Size and reversal learning in the beagle dog as a measure of executive function and inhibitory control in aging. Learn Mem. 2003;10:64–73. doi: 10.1101/lm.54403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal LJ, Calvani M, Amato A, Carta A. A 1-year controlled trial of acetyl-l-carnitine in early-onset AD. Neurology. 2000;55:805–10. doi: 10.1212/wnl.55.6.805. [DOI] [PubMed] [Google Scholar]