Abstract
Cobra venom factor (CVF) is a complement activating protein in cobra venom, which functionally resembles C3b, and has been used for decades for decomplementation of serum to investigate the role of complement in many model systems of disease. The use of CVF for clinical practice is considered impractical because of immunogenicity issues. Humanization of CVF was recently demonstrated to yield a potent CVF-like molecule. In the present study, we demonstrate that mice treated with recombinant humanized CVF (HC3-1496) are protected from myocardial ischemia-reperfusion (MI/R) injuries with resultant preservation of cardiac function. Also, C3 deposition in the myocardium following MI/R was not observed following treatment with HC3-1496. HC3-1496 led to complement activation and depletion of C3, but preserved C5 titers. These data suggest, unlike CVF, HC3-1496 does not form a C5 convertase in the mouse, similar to recent studies in human sera/plasma. These results suggest that humanized CVF (HC3-1496) protects the ischemic myocardium from reperfusion injuries induced by complement activation and represents a novel anti-complement therapy for potential clinical use.
Keywords: complement, inflammation, infarction
Introduction
Myocardial ischemia and reperfusion (MI/R) injury occurs after restoration of blood flow following ischemia in the heart (Walport, 2001b; Walport, 2001a). Complement was shown to be an important contributor to myocardial inflammation and tissue injury following MI/R for 30 years (Hill and Ward, 1971). Protease inhibitors, specific anti-complement biologics, and the use of genetically modified animals demonstrated that complement inhibition decreases inflammation and tissue injury following MI/R (Hill and Ward, 1971; Buerke et al., 1995; Walsh et al., 2005; Weisman et al., 1990; Pinckard et al., 1980). A recent clinical trial also suggests an important role of complement in humans (Armstrong et al., 2006; Granger et al., 2003). Thus, the development of biologics to inhibit complement activation following MI/R may represent a novel cardioprotective mechanism for the clinic.
Cobra venom factor (CVF) is a structural analog of the complement component C3 and is functionally similar to C3b. CVF is used to deplete complement to investigate the role of complement activation in many disease models (Vogel and Fritzinger, 2007; Vogel, 1991). Several studies demonstrated the therapeutic potential of CVF in the setting of MI/R (Hill and Ward, 1971; Pinckard et al., 1980; MacLean et al., 1978; Maroko et al., 1978), but the immunogenicity of this protein, as well as its ability to cleave C5, which generates the potent proinflammatory C5a anaphylatoxin, has not allowed its continuation into clinical development (Gowda et al., 1994; Gowda et al., 2001). Recently, we generated a human C3 derivative with CVF-like functions by replacing a short stretch of amino acids from the C-terminus of the C3 α-chain with the homologous sequences from CVF. The resulting hybrid proteins, termed “humanized CVF,” form a stable C3 convertase similar to CVF, which activates human complement component C3 but not C5 (Vogel and Fritzinger, 2007). Humanized CVF also exhibits partial resistance to the complement regulatory proteins factors H and I, and has been shown to deplete serum complement activity in vitro as well as in rodents and primates (Fritzinger et al., 2008a; Fritzinger et al., 2009; Fritzinger et al., 2008b). In the present study, we tested a humanized CVF protein (HC3-1496) for its ability to protect the mouse myocardium from complement activation and tissue injury following MI/R.
Material and Methods
All C57BL/6 mice used were 8–12 weeks old, weighed between 20–30g, and came from the Charles River Laboratories. The Institute’s Animal Care and Use Committee (IACUC) reviewed all procedures. We performed all experiments in accordance with the IACUC and the standards and principles set forth in the Guide for the Care and Use of Laboratory Animals (revised 1996).
Proteins for Decomplementation
CVF was prepared from lyophilized venom from N. kaouthia as previously described (Vogel and Muller-Eberhard, 1984). HC3-1496 is a human C3/CVF hybrid protein containing a 168 amino acid residue substitution of CVF sequence at the C-terminus of the α-chain of C3 (humanized CVF). The plasmid preparation, protein expression, and purification were performed essentially as previously described (Fritzinger et al., 2009; Vogel and Fritzinger, 2007).
Experimental myocardial ischemia-reperfusion (MI/R) model
C57BL/6 mice were treated with either 100µl of intra-peritoneal (IP) PBS, 250µg/kg of the recombinant humanized CVF (HC3-1496) or 250µg/kg purified CVF in 100µl of PBS two hours prior to induction of anesthesia. Experimental MI/R was performed as previously published (Walsh et al., 2005; Busche et al., 2008). Briefly: mice were intubated, ventilated, and anesthesia maintained with isoflurane. The chest was opened and a suture was placed around the left anterior descending coronary artery (LAD) and tightened. After 30 minutes of ischemia, the ligation was loosened and the myocardium reperfused for 4 hours. An electrocardiogram (modified lead III) was evaluated before, during, and after ischemia and was used to verify ischemia and reperfusion. Left ventricular discoloration/dyskinesis and its reversal were also visualized for additional documentation of ischemia and reperfusion, respectively.
Transthoracic Echocardiography (TTE)
Echocardiography (Philips Sonos 5500; Philips Medical Systems, Bothell, WA, USA) was performed (7–12 MHz animal transducer; Agilent Technologies, Santa Clara, CA, USA) following MI/R to assess cardiac function. We previously demonstrated myocardial injury via histological infarct analysis is directly correlated to loss of cardiac function as measured by echocardiography (Busche et al., 2008; Walsh et al., 2005). Ejection fraction (EF) was calculated via left ventricular M-mode measurements as well as by 2D imaging via long and short axis area measurements of the left ventricle (LV) (Sahn et al., 1978; Kenchaiah et al., 2004). Only M-mode data for EF are present, as both methods produced identical results, which we previously published (Busche et al., 2008).
Measurement of infarct size and area at risk (AAR)
Following reperfusion, a median sternotomy was performed and the LAD ligation suture re-tightened. A right oblique laparotomy was performed, the inferior vena cava isolated, 200µg of heparin administered and the vena cava transected for exsanguination. Then, vessels of the aortic arch were ligated, the descending aorta partially transected, polyethylene 10 tubing inserted, and 100–200µl of 1% Evans Blue injected for antegrade perfusion and negative staining of the AAR as we described (Busche et al., 2008; Walsh et al., 2005). Hearts were excised and cross-sectioned into 1-mm slices using a coronal acrylic matrix (Roboz). Sections were placed into 6-well plates (Costar) and incubated in 1% triphenyltetrazolium chloride (TTC) at 37°C for 15 minutes as we previously published (Walsh et al., 2005). Following TTC staining, sections were fixed in formalin, imaged, and analyzed using a Nikon SMZ800 stereoscopic zoom microscope and SPOT imaging software (Diagnostic Instruments). The myocardial infarct size was determined by calculating the total areas of the LV, as well as the nonischemic and ischemic area within the AAR. Infarct was expressed as a percentage of the total LV area or the AAR.
Immunochemistry
Following MI/R, some hearts were mounted in Tissue-Tek optimum cutting temperature (O.C.T.) compound, cut into 5µm sections and stained for C3 deposition. Primary and secondary antibodies consisted of goat anti-mouse C3 antibody (MP Biomedicals) and donkey anti-goat IRDye800 (Rockland), respectively. Sections were scanned and quantified by Odyssey (LiCor), as we published and validated previously (Busche et al., 2008; Walsh et al., 2005).
Functional mouse C5 analysis following CVF or HC3-1496 treatment
CVF is known to lead to the depletion of C3, as well as C5, resulting in production of C5a anaphylatoxin (Vogel, 1991). On the other hand, HC3-1496 does not form a C5 convertase in human sera (Vogel and Fritzinger, 2007). In order to establish whether C5 was present in mouse sera following PBS, CVF or HC3-146 treatment, sera were collected following the MI/R studies. Human serum (20%, 90 µl) depleted of C5 (Comptech, Tyler, TX) was supplemented with 10 µl of untreated mouse serum or serum from CVF, PBS or HC3-1496 treated mice following MI/R and immediately incubated with sensitized chicken RBCs (30 µl, Colorado Serum, Denver, CO) as described (Vakeva et al., 1998). Cells were separated from the sera after 30 min at 37°C and hemolytic activity was evaluated from serially diluted samples done in triplicate as described (Vakeva et al., 1998) .
Statistics
All statistical analysis was performed using Sigma Stat software version 3.0 (SPSS). All data were evaluated using one-way ANOVA and post hoc analysis using the Student-Newman-Keuls method. Values are expressed as the mean±SE.
Results
Analysis of cardiac function
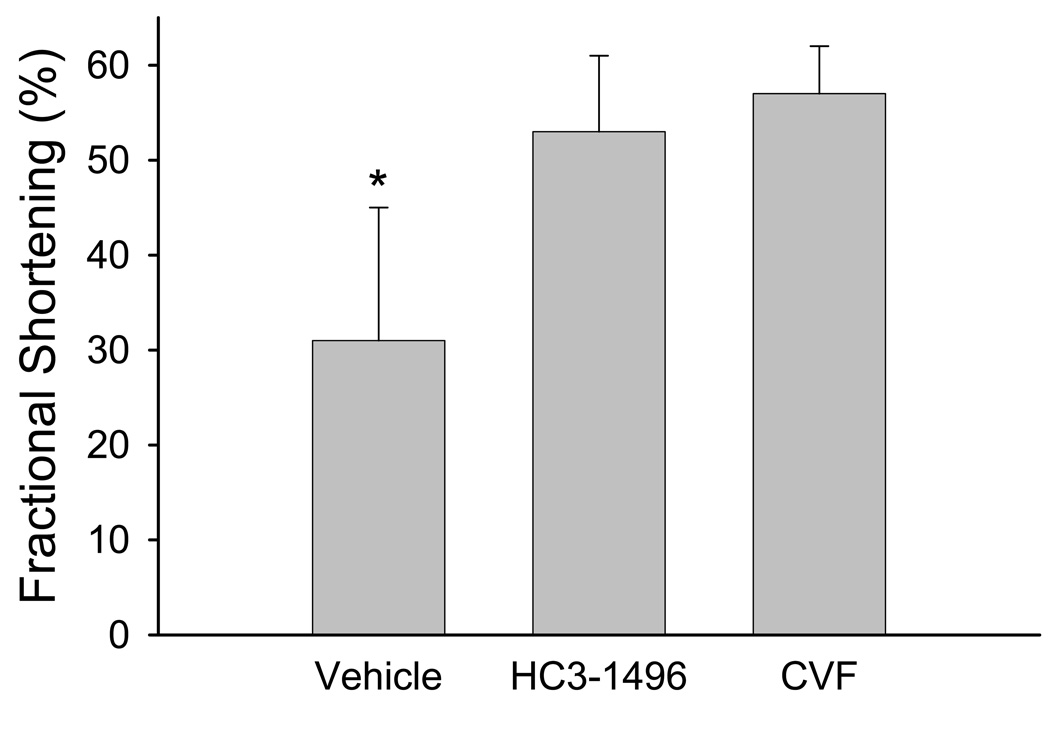
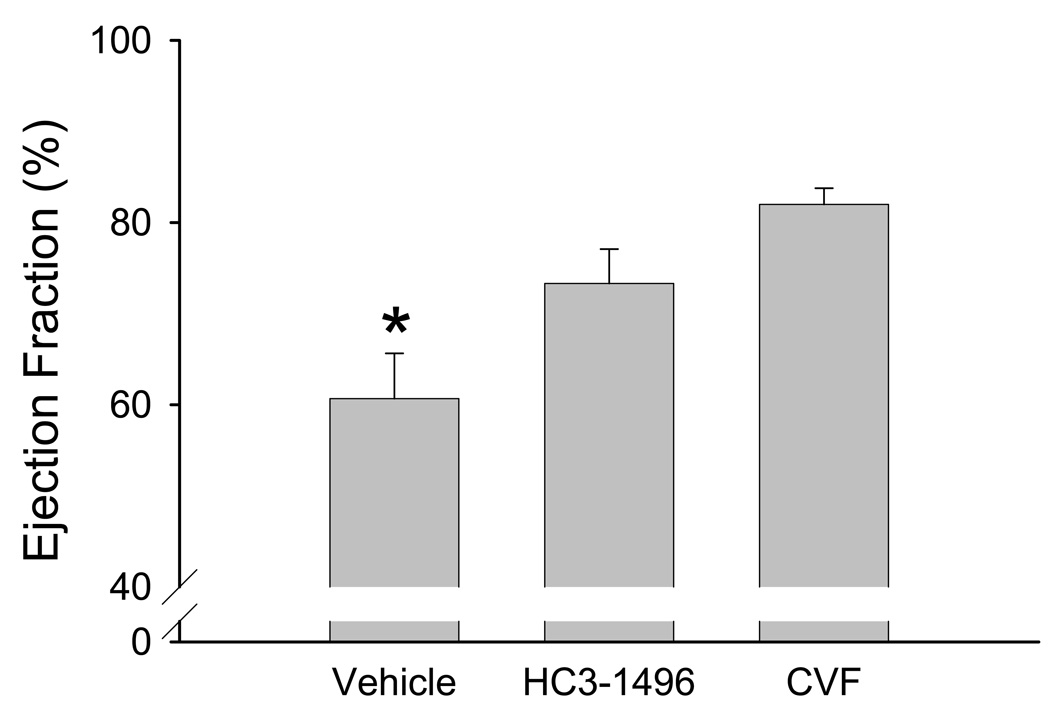
Following 30 minutes of ischemia and 4 hours of reperfusion, we observed a significant decrease in LV function in WT mice treated with PBS (i.e., vehicle) compared to HC3-1496 or CVF treatment (Figure 1). Utilizing M-mode echocardiography, fractional shortening in the long axis was significantly reduced following MI/R in the mice treated with PBS compared to CVF or HC3-1496 treatment. Similarly, when we evaluated LV ejection fraction by TTE, we obtained similar results (Figure 2). These data demonstrate that complement depletion with HC3-1496 or CVF significantly protected LV function following MI/R compared to PBS treatment.
Figure 1. Left ventricular function following MI/R.
Summary of fractional shortening measured by echocardiography for PBS (Vehicle; n=5), HC3-1496 (n=6), or CVF (n=5) treatment following MI/R. * p<0.05 compared to HC3-1496 or CVF. Bars and brackets represent mean±SE.
Figure 2. Left ventricular ejection fraction following MI/R.
Ejection fraction (EF) measured by TTE following MI/R in PBS (Vehicle; n=5), HC3-1496 (n=6), or CVF (n=5) treated mice. *p<0.05 compared to HC3-1496 or CVF. Bars and brackets represent mean±SE.
Infarct analysis
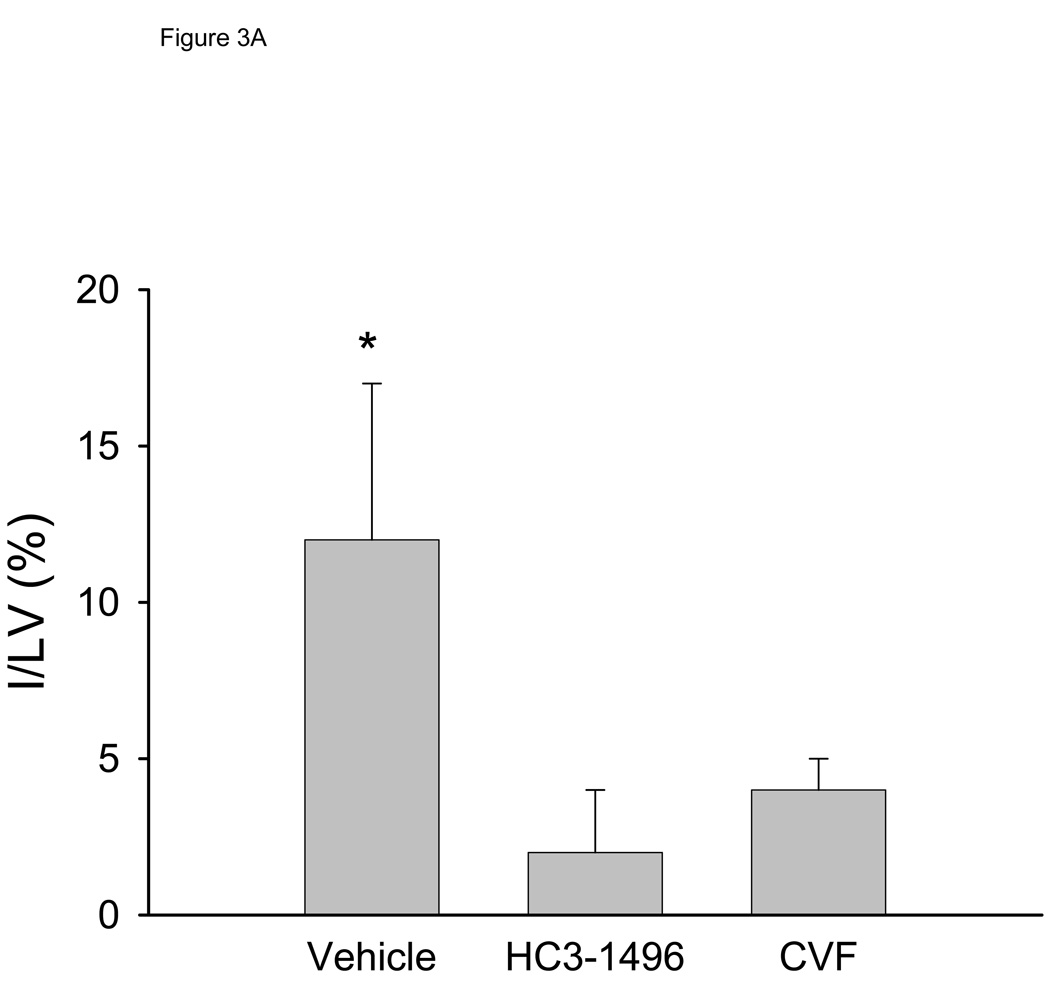
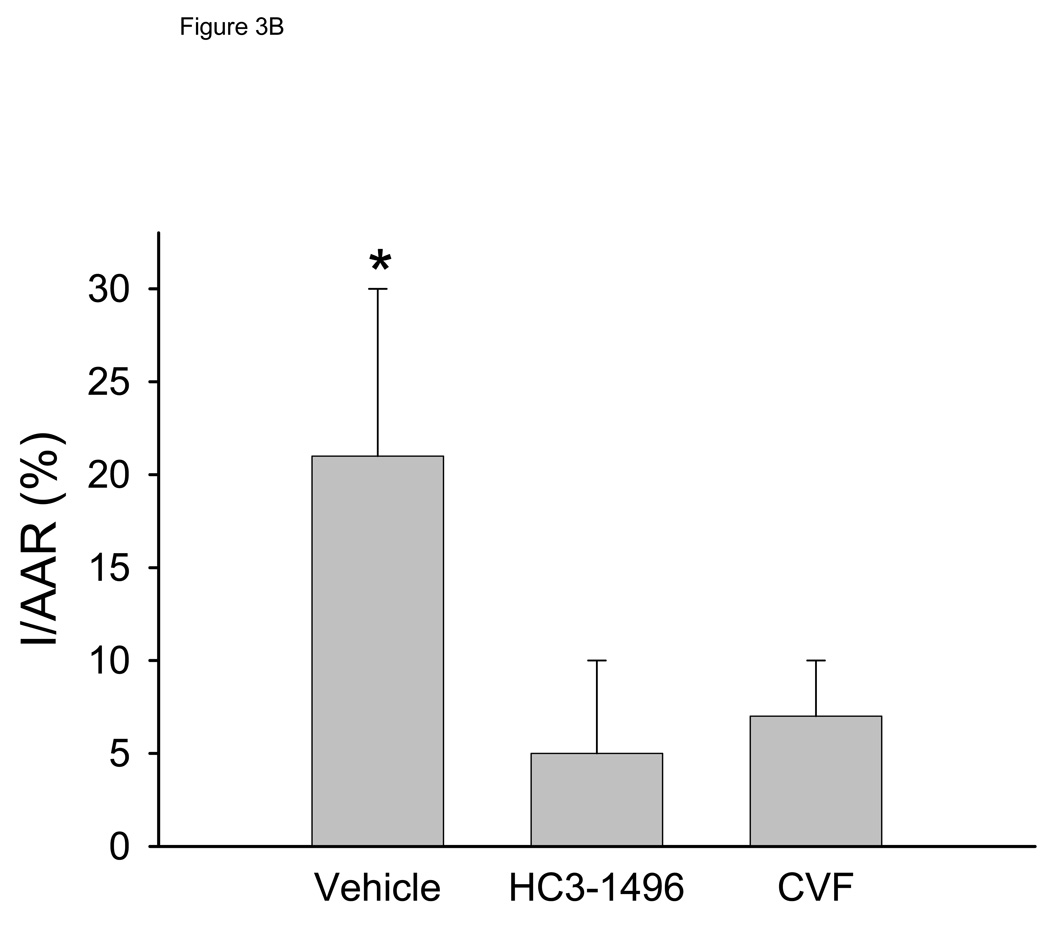
To investigate if the decrease in LV function was a result of tissue injury, we quantified infarct size. The area at risk (AAR) of ischemia compared to the entire LV was not statistically different between the groups (56±14%, 55±8%, and 48±14% for PBS, CVF, and HC3-1496, respectively). Infarct size, normalized to the LV (Figure 3A) or to the AAR (Figure 3B), was significantly larger in vehicle-treated mice compared to HC3-1496 or CVF treatment. Infarct size in HC3-1496 or CVF treated mice was not significantly different (Figure 3). Thus, infarct size was reduced by complement depletion with HC3-1496 to the same extent as CVF.
Figure 3. Myocardial infarct analysis.
Myocardial infarct assessment following MI/R in mice treated with PBS (Vehicle; n=5), CVF (n=5), and HC3-1496 (n=5).
Figure 3A. Infarct size to total left ventricle area. *p<0.001 compared to CVF or HC3-1496.
Figure 3B. Infarct size to area at risk. *p<0.002 compared to CVF or HC3-1496. Bars and brackets represent mean±SE.
Myocardial C3 deposition analysis
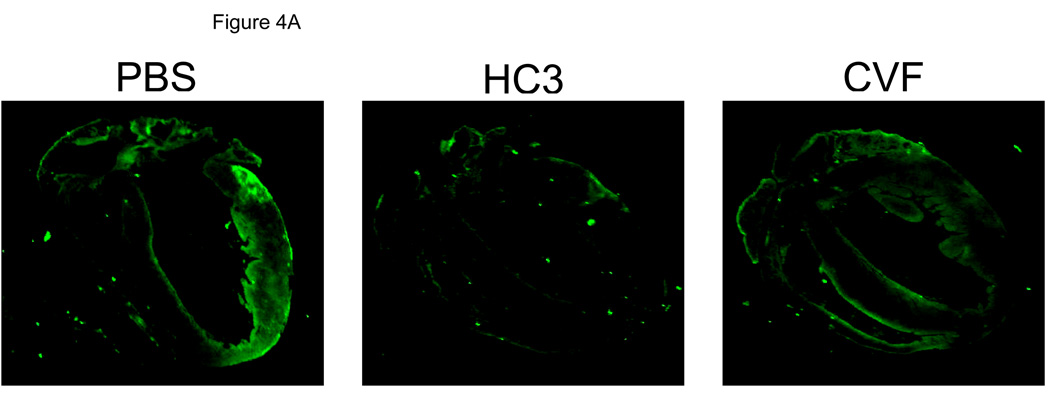
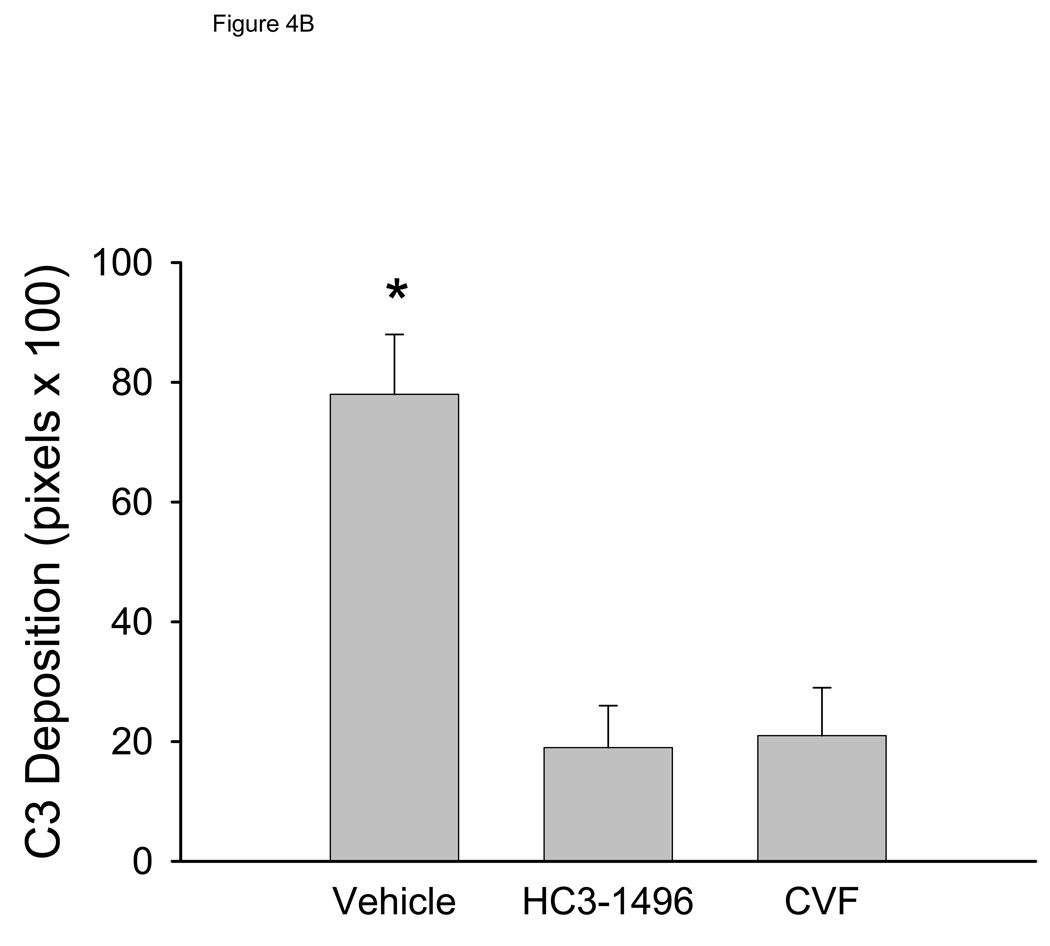
We evaluated complement C3 deposition in mice following MI/R. As shown in Figure 4A, control mice treated with PBS had more myocardial C3 deposition compared to HC3-1496 or CVF treated mice. Figure 4B summarizes quantitative evaluation of C3 deposition. Complement depletion with either HC3-1496 or CVF resulted in significantly less myocardial C3 deposition compared to PBS-treated mice. Thus, complement depletion with CVF or HC3-1496 results in decreased myocardial C3 deposition following MI/R.
Figure 4. Myocardial C3 deposition following MI/R.
Figure 4A. Representative myocardial C3 deposition following MI/R in PBS (Vehicle; n=3), HC3-1496 (n=3), or CVF (n=3) treated mice.
Figure 4B. Quantitative summary of myocardial C3 deposition. *p<0.001 compared to CVF or HC3-1496. Bars and brackets represent mean±SE.
HC3-1496 does not decrease mouse C5 levels
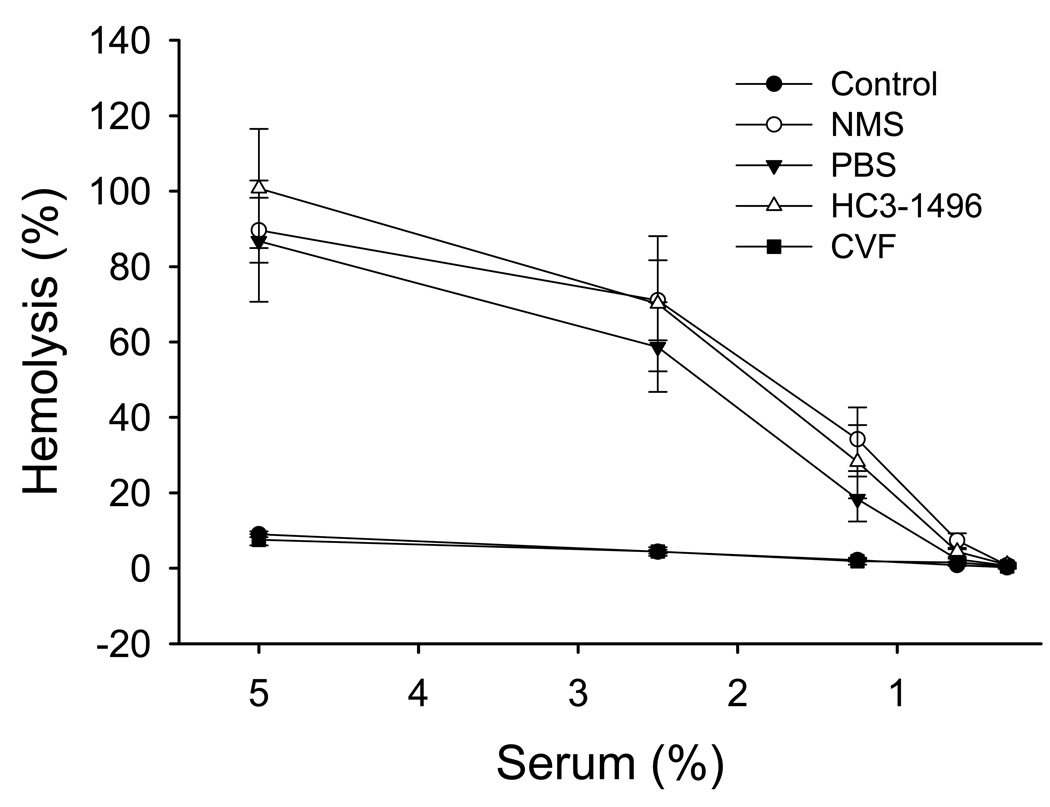
CVF is known to deplete complement through the formation of a stable C3/C5 convertase, which decreases C3 and C5 titers (Vogel, 1991). We recently demonstrated that the humanized CVF does not decrease C5 levels in human or primate sera (Vogel and Fritzinger, 2007; Fritzinger et al., 2008a; Fritzinger et al., 2009). To determine if HC3-1496 has similar properties in mouse serum, serum from PBS, HC3-1496 or CVF-treated mice were used as a source for C5 and added to C5-depleted human serum, which was then subjected to a CH50 assay. As shown in Figure 5, C5-depleted human serum does not result in significant hemolysis of sensitized chicken RBCs. Addition of 10 µl of normal mouse sera (NMS) or sera from mice treated with PBS or HC3-1496 results in significant hemolytic activity. In contrast, addition of 10 µl serum from CVF-treated mice had virtually no hemolytic activity. These data demonstrate that unlike mice treated with CVF, the amount of C5 in mice treated with HC3-1496 (250µg/kg) does not differ from normal mouse sera. Thus, HC3-1496 does not measurably activate C5 in mouse serum.
Figure 5. Preservation of murine C5 following HC3-1496 treatment in vivo.
Mouse sera were collected from mice treated with PBS, HC3-1496 (250µg/kg), or CVF (250µg/kg) following MI/R. Human serum depleted of C5 (Control) was used to lyse sensitized chicken RBCs following addition of 10µl of PBS (Control), normal mouse serum (NMS) or serum from mice treated with PBS, CVF or HC3-1496. Symbols and brackets represent mean±SE from 3 separate experiments per group.
Discussion
Complement depletion by CVF has long been the “gold standard” for the evaluation of complement playing a role in disease. Complement depletion with CVF allows the determination of the role of complement in disease models by generating a C3/C5 convertase that is both physiochemically stable and resistant to the complement inhibitors/proteases present within biological systems, leading to the depletion of these components and a temporary inactivation of the complement system. While CVF has been effective in demonstrating the role of complement in many settings, the immunogenicity of CVF has limited its ability to progress as a clinically viable biologic (Gowda et al., 1994).
CVF was shown to be effective in reducing the infarct size in a number of animal models of MI/R (Hill and Ward, 1971; Maroko et al., 1978; Crawford et al., 1988; Pinckard et al., 1980). In this study, we demonstrate that decomplementation with the humanized CVF protein, HC3-1496, is similarly effective in reducing infarct size in a mouse model of MI/R injury. Previous studies by our laboratory demonstrated that MBL initiates complement activation following I/R, which is then amplified by the alternative pathway leading to tissue inflammation and injury (Jordan et al., 2001; Stahl et al., 2003; Walsh et al., 2005; Hart et al., 2005). Interruption of the complement activation initiation by blocking MBL or inhibition of the amplification by the alternative pathway leads to similar protective actions in animal I/R models (Jordan et al., 2001; Stahl et al., 2003; Walsh et al., 2005; Hart et al., 2005). In the present study, we show that complement depletion via the formation of a stable C3 convertase containing the humanized CVF protein, HC3-1496, leads to similar cardioprotective action that we observed by inhibition of the alternative or MBL-dependent lectin complement pathways (Jordan et al., 2001; Stahl et al., 2003; Walsh et al., 2005; Hart et al., 2005).
While CVF is known to activate complement components C3 and C5, HC3-1496 does not activate human C5 in vitro (Vogel and Fritzinger, 2007; Fritzinger et al., 2008a; Fritzinger et al., 2009). In the present study, we developed an in vitro assay to test the hypothesis that HC3-1496, unlike CVF, does not activate murine C5. Using C5-depleted human serum, we used the C5 present in normal mouse serum to demonstrate that murine C5 can replace human C5 to lyse sensitized chicken RBCs. The hemolytic activity of C5-depleted human serum could be restored by normal mouse serum, or serum from mice that were treated with HC3-1496 or PBS. In contrast, serum from mice treated with CVF did not restore the hemolytic activity, indicating that little or no C5 was present. Thus, while both HC3-1496 and CVF can attenuate MI/R injury, HC3-1496 does this by only depleting C3 without formation of the potent anaphylatoxin, C5a.
In summary, we demonstrate that a humanized chimeric form of CVF provides similar cardioprotective actions in vivo following MI/R in mice. Unlike, CVF, HC3-1496 does not activate C5 and may represent a novel therapeutic biologic for the treatment of complement mediated diseases including myocardial infarction.
Acknowledgments
We gratefully acknowledge Margaret A. Morrissey for the preparation of the blinded cobra venom factor and HC3-1496 for the in vivo studies and for performing the CH50 assays. We would also like to acknowledge June Q. Lee for purifying HC3-1496 and CVF. We thank Heather Kearney for proofing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
GLS and CWV are members of the scientific advisory board for InCode Biopharmaceutics, Inc. (Thousand Oaks, CA). A portion of these studies was funded by a grant from InCode Biopharmaceutics, Inc.
Contributor Information
W. Brian Gorsuch, Center for Experimental Therapeutics and Reperfusion Injury, Brigham and Women’s Hospital, Harvard School of Medicine, 75 Francis Street, Boston, MA 02115.
Benjamin J. Guikema, Center for Experimental Therapeutics and Reperfusion Injury, Brigham and Women’s Hospital, Harvard School of Medicine, 75 Francis Street, Boston, MA 02115
David C. Fritzinger, Cancer Research Center of Hawaii, University of Hawaii at Manoa, 1236 Lauhala Street, Honolulu, HI 96813, USA.
Carl-Wilhelm Vogel, Cancer Research Center of Hawaii, University of Hawaii at Manoa, 1236 Lauhala Street, Honolulu, HI 96813, USA..
Gregory L. Stahl, Center for Experimental Therapeutics and Reperfusion Injury, Brigham and Women’s Hospital, Harvard School of Medicine, 75 Francis Street, Boston, MA 02115
References
- 1.Armstrong PW, Mahaffey KW, Chang WC, Weaver WD, Hochman JS, Theroux P, Rollins S, Todaro TG, Granger CB COMMA Investigators. Concerning the mechanism of pexelizumab's benefit in acute myocardial infarction. Am. Heart J. 2006;151:787–790. doi: 10.1016/j.ahj.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Buerke M, Murohara T, Lefer AM. Cardioprotective effects of a C1 esterase inhibitor in myocardial ischemia and reperfusion. Circulation. 1995;91:393–402. doi: 10.1161/01.cir.91.2.393. [DOI] [PubMed] [Google Scholar]
- 3.Busche MN, Walsh MC, McMullen ME, Guikema BJ, Stahl GL. Mannose-binding lectin plays a critical role in myocardial ischaemia and reperfusion injury in a mouse model of diabetes. Diabetologia. 2008;51:1544–1551. doi: 10.1007/s00125-008-1044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crawford MH, Grover FL, Kolb WP, McMahan CA, O'Rourke RA, McManus LM, Pinckard RN. Complement and neutrophil activation in the pathogenesis of ischemic myocardial injury. Circ. Res. 1988;78:1449–1458. doi: 10.1161/01.cir.78.6.1449. [DOI] [PubMed] [Google Scholar]
- 5.Fritzinger DC, Hew BE, Lee JQ, Newhouse J, Alam M, Ciallella JR, Bowers M, Gorsuch WB, Guikema BJ, Stahl GL, Vogel CW. Derivatives of human complement component C3 for therapeutic complement depletion: a novel class of therapeutic agents. Adv. Exp. Med. Biol. 2008a;632:293–307. [PubMed] [Google Scholar]
- 6.Fritzinger DC, Hew BE, Lee JQ, St.John W, Scaife M, Wilson S, Vogel C-W. Human C3/cobra venom factor hybrid proteins for therapeutic complement depletion: in vivo activity and lack of toxicity in primates. Mol. Immunol. 2008b;45:4112. [Google Scholar]
- 7.Fritzinger DC, Hew BE, Thorne M, Pangburn MK, Janssen BJ, Gros P, Vogel CW. Functional characterization of human C3/cobra venom factor hybrid proteins for therapeutic complement depletion. Dev. Comp. Immunol. 2009;33:105–116. doi: 10.1016/j.dci.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Gowda DC, Glushka J, Van Halbeek H, Thotakura RN, Bredehorst R, Vogel CW. N-linked oligosaccharides of cobra venom factor contain novel α(1–3)galactosylated Lex structures. Glycobiology. 2001;11:195–208. doi: 10.1093/glycob/11.3.195. [DOI] [PubMed] [Google Scholar]
- 9.Gowda DC, Petrella EC, Raj TT, Bredehorst R, Vogel C-W. Immunoreactivity and function of oligosaccharides in cobra venom factor. J. Immunol. 1994;152:2977–2986. [PubMed] [Google Scholar]
- 10.Granger CB, Mahaffey KW, Weaver WD, Theroux P, Hochman JS, Filloon TG, Rollins S, Todaro TG, Nicolau JC, Ruzyllo W, Armstrong PW. Pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to primary percutaneous coronary intervention in acute myocardial infarction: the COMplement inhibition in Myocardial infarction treated with Angioplasty (COMMA) trial. Circulation. 2003;108:1184–1190. doi: 10.1161/01.CIR.0000087447.12918.85. [DOI] [PubMed] [Google Scholar]
- 11.Hart ML, Ceonzo KA, Shaffer LA, Takahashi K, Rother RP, Reenstra WR, Buras JA, Stahl GL. Gastrointestinal ischemia-reperfusion injury is lectin complement pathway dependent without involving C1q. J. Immunol. 2005;174:6373–6380. doi: 10.4049/jimmunol.174.10.6373. [DOI] [PubMed] [Google Scholar]
- 12.Hill JH, Ward PA. The phlogistic role of C3 leukotactic fragments in myocardial infarcts in rats. J. Exp. Med. 1971;133:885–900. doi: 10.1084/jem.133.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordan JE, Montalto MC, Stahl GL. Inhibition of mannose-binding lectin reduces postischemic myocardial reperfusion injury. Circulation. 2001;104:1413–1418. doi: 10.1161/hc3601.095578. [DOI] [PubMed] [Google Scholar]
- 14.Kenchaiah S, Pfeffer MA, St.John Sutton M, Plappert T, Rouleau JL, Lamas GA, Sasson Z, Parker JO, Geltman EM, Solomon SD. Effect of antecedent systemic hypertension on subsequent left ventricular dilation after acute myocardial infarction (from the Survival and Ventricular Enlargement trial) Am. J. Cardiol. 2004;94:1–8. doi: 10.1016/j.amjcard.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 15.MacLean D, Fishbein MC, Braunwald E, Maroko PR. Long-term preservation of ischemic myocardium after experimental coronary artery occlusion. J. Clin. Invest. 1978;61:541–551. doi: 10.1172/JCI108965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maroko PR, Carpenter CB, Chiariello M, Fishbein MC, Radvany P, Knostman JD, Hale SL. Reduction by cobra venom factor of myocardial necrosis after coronary artery occlusion. J. Clin. Invest. 1978;61:661–670. doi: 10.1172/JCI108978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinckard RN, O'Rourke RA, Crawford MH, Grover FS, McManus LM, Ghidoni JJ, Storrs SB, Olson MS. Complement localization and mediation of ischemic injury in baboon myocardium. J. Clin. Invest. 1980;66:1050–1056. doi: 10.1172/JCI109933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardio-graphic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 19.Stahl GL, Xu Y, Hao L, Miller M, Buras JA, Fung M, Zhao H. Role for the alternative complement pathway in ischemia/reperfusion injury. Am. J. Pathol. 2003;162:455. doi: 10.1016/S0002-9440(10)63839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vakeva AP, Agah A, Rollins SA, Matis LA, Li L, Stahl GL. Myocardial infarction and apoptosis after myocardial ischemia and reperfusion. Role of the terminal complement components and inhibition by anti-C5 therapy. Circulation. 1998;97:2259–2267. doi: 10.1161/01.cir.97.22.2259. [DOI] [PubMed] [Google Scholar]
- 21.Vogel CW, Fritzinger DC. Humanized cobra venom factor: experimental therapeutics for targeted complement activation and complement depletion. Curr. Pharm. Des. 2007;13:2916–2926. doi: 10.2174/138161207782023748. [DOI] [PubMed] [Google Scholar]
- 22.Vogel CW, Muller-Eberhard HJ. Cobra venom factor: improved method for purification and biochemical characterization. J. Immunol. Meth. 1984;73:203–220. doi: 10.1016/0022-1759(84)90045-0. [DOI] [PubMed] [Google Scholar]
- 23.Vogel C-W. Cobra venom factor, the complement-activating protein of cobra venom. In: Tu AT, editor. Handbook of Natural Toxins: Reptile and Amphibian Venoms. New York: Marcel Dekker; 1991. pp. 147–188. [Google Scholar]
- 24.Walport MJ. Complement. First of two parts. N. Engl. J. Med. 2001a;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 25.Walport MJ. Complement. Second of two parts. N. Engl. J. Med. 2001b;344:1140–1144. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 26.Walsh MC, Bourcier T, Takahashi K, Shi L, Busche MN, Rother RP, Solomon SD, Ezekowitz RA, Stahl GL. Mannose-binding lectin is a regulator of inflammation that accompanies myocardial ischemia and reperfusion injury. J. Immunol. 2005;175:541–546. doi: 10.4049/jimmunol.175.1.541. [DOI] [PubMed] [Google Scholar]
- 27.Weisman HF, Bartow T, Leppo MK, Marsh HCJ, Jr, Carson GR, Concino MF, Boyle MP, Roux KH, Weisfeldt ML, Fearon DT. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990;249:146–151. doi: 10.1126/science.2371562. [DOI] [PubMed] [Google Scholar]