Abstract
Ultra high molecular weight polyethylene is widely used as a bearing surface in prosthetic arthroplasty. Over time the generation of implant-derived wear particles can initiate an inflammatory reaction characterized by periprosthetic inflammation and ultimately bone resorption at the prosthetic bone interface. Herein we present evidence that the different sized particles as well as the different length alkane polymers generated by implant wear leads to a two component inflammatory response. Polymeric alkane structures, with side chain oxidations, directly bind and activate the TLR-1/2 signaling pathway. Whereas micron and nanometer sized particulate debris are extensively phagocyted and induce enlargement, fusion and disruption of endosomal compartments. The resulting lysosomal damage and subsequent enzymatic leakage induces the NALP3 inflammasome activation as determined by cathepsins S and B cytosolic release, Caspase 1 activation and processing of pro-IL-1β, and pro-IL-18. These two processes synergistically results in the initiation of a strong inflammatory response with consequent cellular necrosis and extra-cellular matrix degradation.
Keywords: UHMWPE, Aseptic Osteolysis, Endosomal damage, Inflammation
INTRODUCTION
Total joint replacement has proven to be the most successful method of treating debilitating arthritis of many joints. One of the most commonly utilized bearing surface materials in joint replacements continues to be ultra high molecular weight polyethylene (UHMWPE). Polyethylene as it is utilized in joint replacements is essentially composed of long chains of hydrocarbon which are further cross-liked by irradiation for sterilization and/or increased wear resistance [1]). With time, motion across the surfaces of the implant generates particulate debris [2]. Estimates of the wear rates of UHMWPE of about 120 μm a year suggest that approximately 105 particles per step ranging in size from 0.2-200 microns are created [3]. The release of these particles into the surrounding tissues can lead to an inflammatory immune response within the joint. The progression of the inflammatory process to periprosthetic bone resorption known as osteolysis can result in substantial bone erosion, diminished implant stability which ultimately demand the revision of the prosthesis. Several studies suggest that the size of the particles generated at the articular interface is an important factor in determining the severity and character of the inflammatory response. In particular it appears that smaller size particles induce a stronger inflammatory response as compared to the larger ones [4, 6-14]. However, the molecular basis of this phenomenon is not currently understood.
We previously reported that the process of UHMWPE breakdown also generates short chain alkane polymers [5] in addition to the nanometers and micrometers size particles [4, 6-14]. Mass spectra analysis (acquired on a Varian Fourier transform Mass Spectrometer (FTMS) equipped with a MALDI source) of the polymers generated by UHMWPE breakdown indicates the existence of polymers consisting of a short alkane backbone without further modifications; however, the majority of the compounds identified contained side chain modification consisting of aldehyde, ketonic and hydroxyl groups [5]. It is likely, that under oxidative conditions in the presence of many enzymatic complexes, the free radicals formed by breakage of the C-C cross-linking react with oxygen giving rise to peroxide ROO• radicals. Subsequently the peroxide radicals are further converted to aldehyde, ketonic or hydroxyl groups as we previously determined by FTIR, and FTMS [5].
It is not well understood from an immunologic standpoint how UHMWPE debris (particles or polymers) initiates an inflammatory process and more importantly which molecular components are responsible for this inflammatory response. Herein we have determined that UHMWPE wear debris induces a two step inflammatory program. Alkane structure, polymeric in nature, bind and activate the TLR1/2 signaling pathway, whereas nanometer and micrometer size particles range are phagocyted by local myeloid lineage cells where they accumulate within the endosomal compartments and upon engorgement of the endosomal system they cause lysosomal disrupture and release of lysosomal enzymes.
MATERIALS AND METHODS
Imunohistochemistry
Periprosthetic soft tissue obtained at the time of hip revision surgery was fixed without decalcification. Histologic evaluation of formalin-fixed, paraffin embedded tissues was performed. Polarized light microscopy was utilized to identified birefringent polyethylene (UHMWPE) particles. B cell marker: CD20 monoclonal mouse anti-human (mAb) (clone L26; dilution 1:3000) (Dako Corp., Carpinteria, CA.), T cell marker: CD3 polyclonal rabbit anti-human (#A0452; dilution 1:200) (Dako Corp., Carpinteria, CA.), macrophage marker CD68 mAb (clone PGM-1; dilution 1:25) (Dako Corp., Carpinteria, CA.) were utilized for immunohistochemical studies (DakoCytomation Signet Acuity kit, Dako DAB +, and DakoCytomation Autostainer Universal Staining system). Formalin-fixed, paraffin embedded tissues were cut on a microtome at a thickness of 4um on Superfrost-plus microscope slides (Cardinal Health Care, Dublin, OH). Tissue slides were dried over night at room temperature, deparaffinize with xylene, cleared with graded ETOH (100% ×2, 95%, 70%), and rinsed with ddH2O. Antigen retrieval was performed by microwave (Biogenex E-Z Retriever microwave) HIER using Citrate buffer pH6.0 at 97° for 10 minutes. Slides were allowed to cool for 20 minutes, then rinsed in ddH2O and placed into a TBS buffer with Tween-20 (2.5 ml to 5 liters of TBS). Slides were incubated with the primary antibodies for 30 minutes, rinsed, and secondary antibodies with labeled polymer were applied for 25 minutes. Antigen-antibody reaction was visualized using diaminobenzidine chromogen (DAB) applied for 7 minutes. Sections were counterstained in Hematoxylin for 30 seconds, cleared in 2% Glacial acetic acid for 30 seconds, rinsed in hot water then in 0.2% Ammonia Water for 10 seconds, rinsed in water, then dehydrated in ETOH and in Xylene before manual cover slipping.
To determine surface expression of various cell surface markers, cells were washed with cold PBS, and labeled for 30 min on ice with saturating amounts of primary mAb in staining buffer (PBS/0.1 % BSA/ 0.01% NaN3). The following anti human mAbs were used; HLA-DR (clone TU36), CD 40 (clone 5C3), B7-1/CD80 (clone L307.4) , B7-2 /CD86 (clone 2231 ), CD51/61 (clone 23C3), CD14 (M5E2), CD11c (clone BLy6), CD16 (clone 3G8), CD19 (H1B19), CD83 (clone HB15e) and TCR (clone R73) from BD Biosciences, Pharmingen, San Diego, CA. Appropriate isotype controls were included in the experiment.
Fluorescence assisted cell sorting of the appropriately stained samples was performed at our in house facility using the FACSCalibur flow cytometer and cellquest software program (BD Biosciences Mountain View, CA, USA) was utilized for the acquisition of the histogram. Histogram analysis was performed by Flo Jo 7.2 and the mean fluorescence was calculated.
Cell culture
Peripheral blood was obtained from the New York Blood Bank. The monocyte population was separated using CD11C or CD14 conjugated MicroBeads (Miltenyi Biotec). Purified CD11C+ cells were cultured in GM-CSF/IL4 (30 ng/ml plus 10 ng/ml) (RD Systems) for dendritic cells, M-CSF (30 ng/ml) for macrophages or RANK-L (30 ng/ml) and M-CSF (30 ng/ml) for osteoclasts for 5 to 6 days in DMEM (GIBCO, Grand Island, NY, USA), supplemented with 50 U/ml penicillin, 50 g/ml streptomycin, 2 mM l-glutamine, 0.1 mM nonessential amino acids, 1 mM pyruvate, and 10 mM Hepes. CD14+ monocytes were used without further differentiation.
Microscopy
TEM
To the assess damage to endosomal compartments we utilized transmission electron microscopy. Patient tissue samples as well as cultured Monocytes, DC, MØ (treated or untreated with UHMWPE) were fixed with a mixture of 2% paraformaldehyde and 2.5 % glutaraldehyde in cacodylate buffer 0.1 M, pH 7.4 at 4°C Fixed cells were infiltrated in sequentially increasing concentrations of LX112-Araldite to 100%, embedded in BEEM capsules, and placed in a 60 °C oven for 4 days. Using a Dupont Sorvall Porter-Blum ultra microtome MT-1, semi-thin sections (0.5um) were stained with 1:1 mixture of 1.0% Methylene blue and 1.0% Azure B, observed with a light microscope, and subsequently selected regions were thin-sectioned and collected on 300 mesh copper grids. Tissue sections were stained with uranyl acetate followed by lead citrate, and viewed with a Jeol JEM-1200EX electron microscope at 80 kV.
SEM
Scanning electron microscopy was performed to view the morphology and particle size of patient sample purified particles. The samples were mounted on a stub but not sputter coated and viewed at 300X magnification.
Confocal
Primary dendritic cells were grown on culture slides with GMCSF for 6 days with and without UHMWPE. The cells were treated either with LysoTracker Green DND-26 (Molecular probes cat no L7526; excitation/emission 504/511 nm) at 75 nM concentration for 30 mins individually or in combination with cathepsin specific activity probe, GB123 (excitation/emission 646/666 nm) (15) washed, counterstained with DAPI (Molecular probes cat no D1306; excitation/emission 358/461 nm) and fixed in 4% paraformaldehyde.
The stained slides were finally mounted and examined under high-resolution laser scanning confocal microscope (Leica AOBS system). Images were captured at 450 nm excitation and emission at 510 nm at 63X under oil emersion objective. All images were collected under identical PMT detector settings. The images were exported into Adobe Photoshop for final processing.
β-hexosaminidase Assay
Analysis of ß-hexosaminidase release from human monocyte derived DC was determined by addition of 100ul of incubation buffer from UHMWPE treated or untreated cells to 100ul of reaction mixture (5ml 0.4M Sodium acetate pH 4.4, 5ml of 8mM 4-methylumbelliferyl-N-acetyl-B-D glucopyranoside in water, 0.250ml Triton X-100, 9.750 ml water) in a 96 well plate incubated at 370C for 30 minutes in an hybridization oven. The reaction will be stopped by addition of 75ul of 2M Na2CO3, and the fluorescence measured in a spectrofluorimeter (FluorStar Optima, BMG Labtechnologies Ltd, Durham, NC) at excitation 350nm/emission 450nm.
Western Blot Analysis of cathepsin S
Equal volumes (100 uL) from the supernatants of primary DCs untreated (control) or treated with different sized UHMWPE at 72 hours were separated on a 10% SDS-PAGE, blotted on nitrocellulose membrane (0.4 um), blocked for nonspecific binding with PBS/Tween 20 (5%) for one hour at room temperature, and further processed for incubation with anti-cathepsin S antibodies (Sc-6503 Santa Cruz). The incubation with anti-cathepsin S (goat polyclonal, 1/200 dilution from stock) in PBS/Tween 20 (0.5%) was performed over night at 4°C, further incubated with secondary antibody (anti-goat coupled with horseradish peroxidase (HRP)) for 1 hour at room temperature and developed with enhanced chemi-luminescent kit from Pierce.
Western Blot Analysis to determine inflammasome activation
Jaws cells untreated and treated 24 hours with UHMWPE in presence and absence of selective inhibitors mix of cathepsins, such as calpain inhibitor II (ALLM) and Pepstatin A were cultured. Western analysis of the total cell lysate was then probed with antibodies specific to Cathepsin B, Caspase 1 p10 fragment, IL-1β, II-18 proteins to evaluate the inflammasome activation. Cell lysates from human monocytes cultured with or without particles for 6 hours in the presence or absence of TLR stimulation were likewise immunoblotted to detect expression of Pro-IL1β.
Gene Chip Assay
Gene expression analysis was performed on control and 24 hour m-PE treated human DC. RNA was extracted using a Qiagen kit. Five micrograms of total RNA were hybridized on the Human Toll-like Receptor Oligo GEarray (SuperArray Biosciences Corporation). Data are reported as average hybridization numbers for each gene subtracted for background.
Luciferase assay
The HEK 293/ TLR 1/2 clones (Invivogen) were used to determine TLR1/2, activation by UHMWPE. The clone was transfected with the NF-Kβ cis- reporter enhancer (pNF- b-LUC, Stratagene) and an independent GFP containing plasmid using Fugene 6 transfection reagent (Roche). The expression of GFP was measured by FACS. Forty-eight hours post transfection the cells were treated with UHMWPE, as well as a positive control like PGN (peptidoglycan) and Zymosan for TLR 1/2 . The luciferase readout was measured at different time points using the standard Luciferase reporter assay kit (Promega).
In vitro processing of Collagen I by cathepsins
Collagen I processing was performed in vitro using human recombinant cathepsins (B, D, L and S) all purchased from Biomol. Briefly, native Collagen-I (Sigma) (5-10 ug/assay) was incubated with different units (0.2-0.5) of each enzyme in the buffer for each cathepsin assay: cathepsin B (120 mM sodium acetate, with 1mM DTT, pH 5.0); cathepsin D (100 mM sodium acetate, pH 3.5); cathepsin L (400 mM sodium acetate, with 8 mM DTT, pH 5.5); cathepsin S (50 mM sodium phosphate, 50 mM NaCl, 2 mM EDTA, pH 6.5). The reaction mixtures were incubated at 37°C and at 6 hours. 20 ul of the reaction mix were quenched with equal volume of SDS/beta-mercaptoethanol denaturing sample buffer for SDS polyacrylamide electrophoresis (PAGE). The products of the enzymatic digestion of collagen-I (different length fragments) were monitored using 7.5% SDS-PAGE. The gels were stained using standard procedures with colloidal blue or for higher sensitivity with silver stain. In order to identify fragments generated only from cathepsin processing of collagen-I, all enzymatic reactions were performed in parallel using selective inhibitors of cathepsins, such as calpain inhibitor II (ALLM) and Pepstatin A.
ELISA
The cytokine assay was performed with the control and PE treated (48hrs) dendritic cells using the Bio-rad human 17-plex panel (cat no#171-A11171) reagent kit. To the pre-wet wells the appropriate beads were added and washed adequately. Next the standards and the samples are added and incubated for 30 minutes. The wells are then washed three times and the appropriate detection antibody is added and incubation is continued for another 30 minutes. The wells were again washed and streptavidin-PE was added and after 10 mins washed again and re-suspended in 150 ul of PBS and the 96 well micro-plate is finally analyzed for fluorescence in a Luminex 200 system. IL1β production from human monocytes was determined using an OptEIA ELISA kit (BD Biosciences).
UHMWPE and cement particles
Ultra high molecular weight Polyethylene was purchased from Sigma (cat no #434272). The UHMWPE was surface modified with average size of 53-75 micron according to manufacturers datasheet. UHMWPE can only be dissolved at elevated temperatures in aromatic hydrocarbons, such as toluene or xylene. For our studies we resuspended 20 mg/ml of UHMWPE in PBS ( Phosphate buffered saline) to form a suspension solution. UHMWPE was always vortexed strongly just before addition and was added at a ratio of 20 ul of the vortexed suspension per ml of culture media. The particles of various sizes were obtained from Biomet Orthopedics Inc (Warsaw IN-46581) at a number of 1 × 105 particles in 1ml of distilled autoclaved water in flame sealed amber vials. Particles of Simplex Bone Cement (Howmedica Osteonics, Allendale, NJ) were prepared by filing using a Grobet Warding File 4 Cut: #4 Pattern:Swiss (MSC Industrial Supply Company). After filing, particles were soaked at 4°C in 70% EtOH overnight, then washed twice and stored in sterile PBS solution, pH 7.2. Endotoxin contamination of particles was excluded by limulus assay (E-Toxate; Sigma Chemical, St. Louis, Missouri).
RESULTS
Micrometer and nanometer size particles as well as short polymers are formed upon UHMWPE wear
Wear debries particles from periprosthetic tissue retreived at the time of revision surgery were purified as a first step in understanding the immunological response to alkane polymer UHMWPE [5]. A vast array of particles sizes were obtained from the surgically retrieved tissue. Particles ranged in size from several micrometer (Figure 1a) to sub-nanomenter size (Figure 1b). Furthermore various sized short alkane polymers were also retreived ranging from few carbon atoms to a hundred carbon atoms. Several of these polymers have been analyzed by FTMS [5] and structure predicted on the basis of the formula obtained. (Figure 1c). The majority of the retrieved polymers were carbonyl modified due to extensive oxidation of the alkane molecules as previously reported [5].
Figure 1. Different size particles and polymers are generated in vivo by the wear and tear of UHMWPE implant.
a and b) Transmission electron micrograph of micrometer (a) and nanometer (b) size UHMWPE wear debris recovered from the periprosthetic tissue.
c) Molecular formula representation of alkane polymer subunits recovered from the periprosthetic tissue.
Myelomonocytic infiltration and giant cell formation around micrometer size UHMWPE wear debris
Immunohistochemistry analysis was performed on periprosthetic tissue obtained at the time of implant failure revision surgery. Peri-articular tissue was stained with antibodies specific for different immune cell sub-populations. Infiltrate of predominantly macrophages (CD68) and dendritic cells (CD11c) were observed in all analyzed samples (Figure 2a). Only few T-cells (CD3) and B-cells (CD20) could be visualized in the inflamed tissue (Figure 2b). Most of the cellular infiltrates contained abundant multinuclear giant cells surrounding UHMWPWE particles (which appear birefringent under polarized light) (Figure 2c).
Figure 2. Macrophages and Dendritic cells are present in the peri-implant inflamed tissue as mononuclear or multinuclear giant cells.
a) CD11c and CD68 immunostaining (20X) demonstrates the presence of multinucleated giant cells forming around UHMWPE particles (identified by an *).
b) H&E staining demonstrating a multinucleated giant cell surrounding a clear UHMWPE particle. CD3 and CD20 immunostaining demonstrating few infiltrating T and B cells (20X).
c) Polarized light micrograph of intracellular birefringent polyethylene particles.
d) FACS quantification of cellular infiltrates present in the peri-osteolytic tissue.
To further confirm the nature of the infiltrates FACS analysis was performed on collagenase digested peri-prosthetic tissue. Analysis confirmed the previously documented immuno-histochemical findings that the majority of the infiltrates were myelomonocytic in nature with most of cells expressing DC markers (CD11c and CD83) or monocytes/macrophages markers (CD11c, CD14, and CD16) (Figure 2d).
Lysosomal damage and inflammasome activation by UHMWPE wear debris
Ultrastructural analysis of inflammatory infiltrates in the periprosthetic tissue indicated phagocytosis of nanometer-sized particles of UHMWPE (Figure 3a). Similarly, DC's cultured in vitro for 24 hours with different sized UHMWPE particles resulted in extensive phagocytosis of nanometer and sub-nanometer particles (Figure 3b). In every cell endosomal compartments were completely engorged with the alkane particles (Figure 3c). The failure of the endosomal enzymes to degrade UHMWPE resulted in a significant increase in the number of compartments, size of the compartments (Figure 3d, e and f) as well as increased fusion between compartments (Figure 3f and g). Finally, several compartments underlying the plasma membrane were also observed, some undergoing the process of exocytosis (Figure 3h).
Figure 3. UHMWPE wear debris are phagocyted by macrophages and dendritic cells.
a) Transmission electron micrograph of nanometer size UHMWPE particles phagocytosed by a lymph mononuclear present in the periprosthetic inflamed tissue.
b, c, d and e) Transmission electron micrograph of nanometer size UHMWPE particles phagocytosed by in vitro cultured dendritic cells. Individual cell endosomal compartments are completely engorged by the UHMWPE particles and are increased in number and size.
f and g) Transmission electron micrograph of endosomal compartment, engorged with nanometer size particles. Arrows indicate fusion between different compartments.
h) Transmission electron micrograph of endosomal compartment, engorged with nanometer size particles, underlying the plasma membrane. Arrows indicate a compartment undergoing the process of exocytosis.
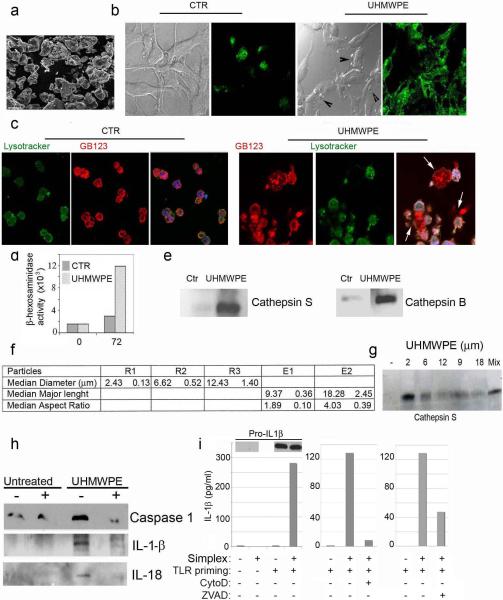
The next series of experiments was designed to determine whether the phagocytosis of particulate UHMWPE (Figure 4a) and subsequent endosomal engorgement would induce physical damage to the limiting membrane of the compartments with subsequent cytosolic release of endosomal enzymes. The integrity of endosomal compartments was assessed following endocytosis of the lysosensor DND 189, as well as a Cathepsin B,S,L specific activity probe [15]. Confocal analysis performed on control cells detected the presence of intact endosomal compartments (Figure 4b). On the other hand, DC cultured for 48 hours with UHMWPE demonstrated extensive damage of the endosomal compartments with release of the fluorescent tracer in the cytosol and the extracellular milieu (Figure 4c). To further confirm presence of endosomal enzymes extracellularly, the culture supernatant was collected and assayed for presence of lysosomal enzymes. A statistically significant increase of beta-hexosaminidase as well as cathepsin S and B was observed in the supernatant of DC cultured with UHMWPE (Figure 4d).
Figure 4. UHMWPE wear debris induce lysosomal damage and release of lysosomal enzymes.
a) Scanning electron micrograph of UHMWPE wear debris (300x) purified from the periprosthetic inflamed tissue. b) Confocal microscopic analysis of DC untreated and treated with UHMWPE for 48 hours after staining with lysosensor DND 189 to determine the distribution and integrity of endosomal compartments. c) Confocal micrograph of untreated and 48 hrs UHMWPE treated DC stained with DND189 (green) as well as cathepsin D ,L and S specific probe(red) to document the lysosomal destabilization. d) Quantification of β-hexosaminidase cathepsin S and cathepsin B released in the culture supernatant by primary human DC cultured with UHMWPE as compared with the untreated control. e) Tabular representation of the physical characteristics of size defined UHMWPE particles used in the study. f) Western blot analysis of Cathepsin S released in the culture supernatant by primary human DC cultured with UHMWPE as compared with the untreated control. g and h) Western blot analysis of g) Caspase1 p10 fragment, h) IL-1B and IL-18 in DC untreated and UHMWPE treated in presence (+) or absence (−) of cathepsin inhibitor. i) Western blot (cell lysates) and ELISA (conditioned media) analysis of monocytes with or without TLR stimulation and addition of Simplex bone cement (0.4mg/ml) in the presence or absence of cytochalasin D (0.2μM) or zVAD (20μM).
It has been previously reported that the size of the UHMWPE wear debris is important in determining the severity of the inflammatory reaction. To determine whether the size of the particles would play a role in the amount of lysosomal damage, DC were cultured for 72 hours with UHMWPE of known size and diameter (Figure 4e). Supernatant collected after 72 hours of culture indicated that smaller size particles induced more extensive endosomal damage as assessed by cathepsin S release into the supernatant Figure 4f).
Endosomal destabilization and release of cathepsin B has been reported to trigger activation of a protein complex designated as the NALP3 inflammasome, which consists of the proteins NALP3, ASC, and caspase 1. This leads to caspase 1 activation, which in turn catalyzes the conversion of IL-1β and II-18 from pro to active forms. Increased levels of Caspase 1 p10 Fragment (Figure 4g) and active IL-1β and II-18 (Figure 4h) were observed in UHMWPE treated DC. Caspase 1 cleavage and cytokine activation was down regulated in presence of cathepsin B specific inhibitor indicating that cytosolic release of cathepsin B following endosomal damage by UHMWPE particles plays a major role in the inflammasome activation (Figure 4g and h). Similar results were found with other disease-relevant myeloid cell types and particles. TLR stimulation of human monocytes (Figure 4i) and mouse bone-marrow derived macrophages (not shown) induced pro-IL1βexpression but not processing or release of mature IL1β. Further addition of particles of Simplex bone cement induced processing and release of this pro-IL1β via a cytochalasin D- and zvad-sensitive pathway, suggesting inflammasome activation by these particles.
Alkane polymers induce TLR1/2 activation
While inflammasome activation promotes IL1β processing and release, it does not support de novo generation of pro-IL1β. To effect IL1β production, parallel pathways of inflammasome activation and TLR stimulation are required. We previously identified that the process of UHMWPE breakdown generated short oxidized alkane polymers within a C12-C16 C length (besides the nanometermicrometer particles) that were able to bind and activate TLR1 and 2 [5].
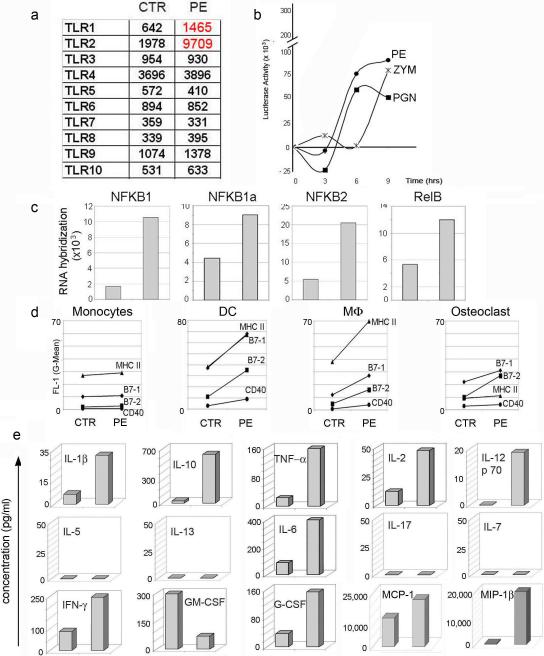
Significant increase in mRNA encoding for TLR1 and TLR2, as compared to other TLRs was observed in UHMWPE treated DC (Figure 5a). Similarly, RNA hybridization assay showed an up-regulation of the different NF-KB/ Rel subunits (Figure 5c) consistent with activation of a pro-inflammatory program induced by TLR1/2 engagement. TLR1/2 activation by UHMWPE polymers was further confirmed by a luciferase assay performed on stable HEK 3T3-TLR1/2 cell transfected with an NF-KB enhancer element (Figure 5b).
Figure 5. UHMWPE wear debris induces antigen presenting cell activation.
a) TLRs RNA hybridization assay of DC cells untreated or treated for 24 hrs with UHMWPE particles. b) Time course of luciferase activity expressed by HEK 3T-TLR1/2 cells treated with UHMWPE and positive controls PGN (Peptidoglycan) and Zymosan c) NFKB1a, NFKB2 and RelB RNA hybridization assay of DC untreated or UHMWPE treated d) Monocytes, DCs, Macrophages and Osteoclasts were cultured in presence or absence of UHMWPE particles for 48 hours. Cells were stained with antibodies specific for MHC II, B7-1, B7-2 and CD40 cell surface marker. Data are plotted as mean fluorescence index of untreated and UHMWPE treated cells. e) Elisa, for the reported cytokines, performed on the supernatant of UHMWPE treated DCs as compared to the untreated control. Cytokine concentration is reported in pg/ml
Alkane polymers induce myelomonocytic cell activation
To determine the extent of the pro-inflammatory program induced by UHMWPE wear debris we cultured different myeloid lineage cells, likely to play a role in the osteolytic process with a mixture of alkane particles and polymers. Macrophages and osteoclast, as the bone resident myeloid lineage cells and monocytes and DC, as APC's normally recruited to the site of inflammation (Figure 1c). After 48 hours of incubation cells were harvested and stained for surface activation markers. A significant up-regulation of surface MHC II, B7-1, B7-2 and CD40 was observed in dendritic cells and macrophages and to a lesser extent in monocytes and osteoclasts upon incubation with oxidized alkane but not in control treated cells (Figure 5d).
Supernatant from DC cell cultures was also harvested to measure cytokine production. Up-regulation of several pro-inflammatory cytokines including IL-1, IL-6, IL-10, IL-12, TNF-α, and IFN-γ was also observed in DC treated with UHMWPE wear debris as compared to the control treated cells. (Figure 5e).
Extracellular matrix degradation
Cytosolic and extracellular release of lysosomal enzymes is associated with cell necrosis. In the periprosthetic inflamed tissue several necrotic cells with pyknotic and fragmented nuclei could be observed (Figure 6a). Also, several myelin figures, characteristic of protein/lipid precipitation observed in necrotic tissue, were evident both intra and extracellularly (Figure 6b, c and d).
Figure 6. Tissue necrosis and collagen digestion are observed in periprosthetic tissue.

a) Transmission electron micrograph of necrotic cells with characteristic pyknotic and fragmented nuclei. b), c), d) Transmission electron micrograph representing several myelin figures typical of necrosis. e) Transmission electron micrograph of micrometer and nanometer size UHMWPE wear debris in the extra-cellular collagen matrix. f) Transmission electron micrograph of the collagen fibers next to a necrotic cell. g) Silver staining of a 12% SDS-PAGE. Collagen I undigested (first lane) or digested with different cathepsin enzymes (S,L, B and D) in presence (+) or absence (−) of cathepsins inhibitors.
Numerous micron and nanometer size particles could be detected in the extracellular collagen matrix (Figure 6e). Adjacent to necrotic cells extensive collagen damage could be visualized (Figure 6f). Collagen fibers appear disorganized, thinned and partially disbanded. These changes are consistent with an effect of the enzymes released by the necrotic cells on the extracellular matrix similar to the observed effects of endosomal cathepsins on matrix degradation observed in in vitro studies(Figure 6g).
DISCUSSION
Ultra high molecular weight polyethylene is considered to be a relatively biologically inert material [15]. Particles generated from UHMWPE wear range from the submicron to the multi-millimeter size and accumulate in the tissues surrounding the implant [16]. Histologically a classical foreign body reaction with multinucleated giant cell formation is generated around the micron size UHMWPE particles. Local and Infiltrating myeloid lineage cells attempt to eliminate the larger particles by fusing together into multinucleated giant cells and synergistically attempt to degrade and clear the wear debris, a phenomena often known as “frustrated phagocytosis”. The mechanisms controlling cellular fusion of the infiltrating mononuclear population are currently unknown. It has been suggested that a protein such as DC-STAMP, which is involved in formation of multinucleated giant osteoclasts [17], may also play a role in DC fusion in the periprosthetic tissue. In addition, other molecules such as IL-17A, which has recently being reported as one of the main cytokines involved in DC fusion during histiocytosis, may contribute to the formation of the polykaryons [18]. However, under our experimental conditions we did not detect IL-17 production by DC activated with alkane polymers.
A two step pro-inflammatory program, which relies on TLR1/2 and inflammasome activation, is initiated upon contact of UHMWPE wear debris with local antigen presenting cells.
As first step alkane polymers with side chain modifications, consisting of aldehyde, ketonic and hydroxyl groups directly interact and activate TLR1/2 surface receptor. Even though UHMWPE oxidation has been described by several groups [3,4,9,12] we originally reported that the oxidize alkane polymers, within 10 to 16 carbon atoms are by far more immunogenic in TLR1/2 stimulation that the non oxidize counterpart [5]. Herein we complement the previous data with further experiments indicating that TLR1/2 engagement induces a pro-inflammatory transcription program mediated by NFkB signaling pathways, inducing the expression of pro-IL-1β and pro-IL-18.
As second step UHMWPE particles, readily phagocyted by local cells, induce endosomal destabilization and inflammasome activation. It has been previously reported that lysosomal destabilization/damage and cathepsin release is perceived by the immune system as an endogenous danger signals inducing NALP3 inflammasome activation [6]. The NALP3 inflammasome is a multi-protein complex within inflammatory cells that regulates caspase-1 dependent processing and secretion of pro-inflammatory cytokines such as interleukin 1-beta (IL-1b) and interleukin 18 (IL-18). This pathway is involved in transduction of a remarkably wide range of danger signals, including ATP, toxins, gout crystals, calcium pyrophosphate dihydrate [19] and pathogens. Recently, it has emerged that another class of mediators, in the form of small phagocytosable silica particles also have the ability to activate the NALP3 inflammasome [20]. In this study we document that phagocytosis of UHMWPE wear debris or other orthopedic particles is also inducing inflammasome activation as determined by release of cathepsin B, as well as activation of caspase 1 and release of processed IL-1βand IL-18.
It was previously recognized that internalization of prosthetic wear debris may lead to cell necrosis that the size and properties of the particles was an important determinant of cell fate. Herein we determine the molecular basis for this phenomenon. We noted that smaller the particles size higher the amount of secreted cathepsin S. Released enzymes will be ultimately responsible for the extracellular matrix digestion, periprosthetic inflammation and contribute to the increased osteoclast-mediated bone resorption, which ultimately will generate aseptic osteolysis. We speculate that since the UHMWPE is biologically un-degradable the phagocytosed particles released by endosomal rupture and cell necrosis are re-engulfed by other cells to generate a sustained inflammatory condition resulting in perpetuation of the inflammatory process.
Our studies provide insights into the cellular events by which alkane polymers generated from UHMWPE work synergistically with phagocyted particles to activate TLR and inflammasome pathways leading to up-regulation of pro-inflammatory and osteoclastogenic cytokines involved in peri-implant granuloma formation and osteolysis. Furthermore, our results implicate cathepsin B and disruption of the integrity of the endosomal compartment in IL-1βand II-18 processing and release. We speculate that the development of UHMWPE implant prosthetic devices with less propensity to oxidation and debris formation will help reduce the incidence and morbidity associated with wear debris osteolysis.
Acknowledgements
We thankfully acknowledge Dr. G Blum for kindly providing us with the cathepsin based activity probe GB 123 for confocal analysis of endosomal damage by UHMWPE. The microscopic analysis both confocal and TEM were done at the AIF (Analytical Imaging Facility ) of AECOM. The mass spectroscopic data (FT/MS) were recorded at the LMAP (Laboratory for Macromolecular Analysis and Proteomics) AECOM. The FACS analysis were performed at the Flow Cytometry Core facility, Cancer Center, AECOM. We are grateful for all the help provided by the members of the aforementioned facilities. The antibodies against Caspase 1 p10 fragment and IL-18 were kindly donated by Dr. J. Brojatsch, of M & I, AECOM. We appreciate the critical comments and suggestions provided by Dr. S. Goldring of Hospital for special Surgery, New York.
Grant: This work was supported by NIH grant AI 48832; R.M. was supported by T32 NS 07098
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- 1.Geerdink CH, Grimm B, Vencken W, Heyligers IC, Tonino AJ. Cross-linked Compared with Historical Polyethylene in THA: An 8-year Clinical Study. Clin Orthop Relat Res. 2008 doi: 10.1007/s11999-008-0628-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baudriller H, Chabrand P, Moukoko D. Modeling UHMWPE wear debris generation. J Biomed Mater Res B Appl Biomater. 2007;80(2):479–85. doi: 10.1002/jbm.b.30620. [DOI] [PubMed] [Google Scholar]
- 3.Richards L, Brown C, Stone MH, Fisher J, Ingham E, Tipper JL. Identification of nanometre-sized ultra-high molecular weight polyethylene wear particles in samples retrieved in vivo. J Bone Joint Surg Br. 2008;90(8):1106–13. doi: 10.1302/0301-620X.90B8.20737. [DOI] [PubMed] [Google Scholar]
- 4.Kurtz SM, Peloza J, Siskey R, Villarraga ML. Analysis of a retrieved polyethylene total disc replacement component. Spine J. 2005;5(3):344–50. doi: 10.1016/j.spinee.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Maitra R, Clement CC, Crisi GM, Cobelli N, Santambrogio L. Immunogenecity of modified alkane polymers is mediated through TLR1/2 activation. PLoS ONE. 2008;3(6):e2438. doi: 10.1371/journal.pone.0002438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witkiewicz H, Vidovszky T, Turner RT, Rock MG, Morrey BF, Bolander ME. Fate of ultrahigh molecular weight polyethylene (UHMW-PE) wear debris in patients with hip implants. Tech Orthop. 1993;8(4):254–61. doi: 10.1097/00013611-199300840-00006. [DOI] [PubMed] [Google Scholar]
- 7.Williams S, Tipper JL, Ingham E, Stone MH, Fisher J. In vitro analysis of the wear, wear debris and biological activity of surface-engineered coatings for use in metal-on-metal total hip replacements. Proc Inst Mech Eng [H] 2003;217(3):155–63. doi: 10.1243/095441103765212659. [DOI] [PubMed] [Google Scholar]
- 8.Trindade MC, Affatato S, Fagnano C, Toni A. Proinflammatory mediator release in response to particle challenge: studies using the bone harvest chamber. J Biomed Mater Res. 1999;48(4):434–9. doi: 10.1002/(sici)1097-4636(1999)48:4<434::aid-jbm6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 9.Taddei P, Affatato S, Fagnano C, Toni A. Oxidation in ultrahigh molecular weight polyethylene and cross-linked polyethylene acetabular cups tested against roughened femoral heads in a hip joint simulator. Biomacromolecules. 2006;7(6):1912–20. doi: 10.1021/bm060007u. [DOI] [PubMed] [Google Scholar]
- 10.Ren WP, Markel DC, Zhang R, Peng X, Wu B, Monica H, Wooley PH. Association between UHMWPE particle-induced inflammatory osteoclastogenesis and expression of RANKL, VEGF, and Flt-1 in vivo. Biomaterials. 2006;27(30):5161–9. doi: 10.1016/j.biomaterials.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Lee AW, Santerre JP, Boynton E. Analysis of released products from oxidized ultra-high molecular weight polyethylene incubated with hydrogen peroxide and salt solutions. Biomaterials. 2000;21(8):851–61. doi: 10.1016/s0142-9612(99)00257-4. [DOI] [PubMed] [Google Scholar]
- 12.Kurth M, Eyerer P, Ascherl R, Dittel K, Holz U. An evaluation of retrieved UHMWPE hip joint cups. J Biomater Appl. 1988;3(1):33–51. doi: 10.1177/088532828800300102. [DOI] [PubMed] [Google Scholar]
- 13.Koseki H, Matsumoto T, Ito S, Doukawa H, Enomoto H, Shindo H. J Analysis of polyethylene particles isolated from periprosthetic tissue of loosened hip arthroplasty and comparison with radiographic appearance. Orthop Sci. 2005;10(3):284–90. doi: 10.1007/s00776-005-0896-6. [DOI] [PubMed] [Google Scholar]
- 14.Goodman S. Wear particulate and osteolysis. Orthop Clin North Am. 2005;36(1):41–8. vi. doi: 10.1016/j.ocl.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Blum G, von Degenfeld G, Merchant MJ, Blau HM, Bogyo M. Noninvasive optical imaging of cysteine protease activity using fluorescently quenched activity-based probes. Nat Chem Biol. 2007 Oct;3(10):668–77. doi: 10.1038/nchembio.2007.26. [DOI] [PubMed] [Google Scholar]
- 16.Oosterom R, Ahmed TJ, Poulis JA, Bersee HE. Adhesion performance of UHMWPE after different surface modification techniques. Med Eng Phys. 2006;28(4):323–30. doi: 10.1016/j.medengphy.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Brown TD, Stewart KJ, Nieman JC, Pedersen DR, Callaghan JJ. Local head roughening as a factor contributing to variability of total hip wear: a finite element analysis. J Biomech Eng. 2002;124(6):691–8. doi: 10.1115/1.1517275. [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto T. The dendritic cell-specific transmembrane protein DC STAMP is essential for osteoclast fusion and osteoclast bone-resorbing activity. Mod Rheumatol. 2006;16(6):341–2. doi: 10.1007/s10165-006-0524-0. [DOI] [PubMed] [Google Scholar]
- 19.Coury F, Annels N, Rivollier A, Olsson S, Santoro A, Speziani C, Azocar O, Flacher M, Djebali S, Tebib J, Brytting M, Egeler RM, Rabourdin-Combe C, Henter JI, Arico M, Delprat C. Langerhans cell histiocytosis reveals a new IL-17A-dependent pathway of dendritic cell fusion. Nat Med. 2008;14(1):81–7. doi: 10.1038/nm1694. [DOI] [PubMed] [Google Scholar]
- 20.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008 Aug;9(8):847–56. doi: 10.1038/ni.1631. Epub 2008 Jul 11. [DOI] [PMC free article] [PubMed] [Google Scholar]