Abstract
A critical period in respiratory network development occurs in the rat around postnatal days (P)12–13, when abrupt neurochemical, metabolic, and physiological changes were evident. As serotonin (5-HT) and its receptors are involved in respiratory modulation, and serotonergic abnormality is implicated in Sudden Infant Death Syndrome, we hypothesized that 5-HT receptors are significantly down-regulated during the critical period. This was documented recently for 5-HT2AR in several respiratory nuclei. The present study represents a comprehensive analysis of postnatal development of 5-HT1AR and 5-HT1BR in ten brain stem nuclei and 5-HT2AR in six nuclei not previously examined. Optical densitometric analysis of immunohistochemically-reacted neurons from P2 to P21 indicated four developmental patterns of expression: 1) Pattern I: a high level of expression at P2–P11, an abrupt and significant reduction at P12, followed by a plateau until P21 (5-HT1AR and 5-HT1BR in raphé magnus [RM], raphé obscurus [ROb], raphé pallidus [RP], pre-Bötzinger complex [PBC], nucleus ambiguus [Amb], and hypoglossal nucleus [XII; 5-HT1AR only]). 2) Pattern II: a high level at P2–P9, a gradual decline from P9 to P12, followed by a plateau until P21 (5-HT1AR and 5-HT1BR in the retrotrapezoid nucleus [RTN]/parafacial respiratory group [pFRG]). 3) Pattern III: a high level at P2–P11, followed by a gradual decline until P21 (5-HT1AR in the ventrolateral subnucleus of solitary tract nucleus [NTSVL] and the non-respiratory cuneate nucleus [CN]). 4) Pattern IV: a relatively constant level maintained from P2 to P21 (5-HT1AR in the commissural subnucleus of solitary tract nucleus [NTSCOM]; 5-HT1BR in XII, NTSVL, NTSCOM, and CN; and 5-HT2AR in RM, ROb, RP, RTN/pFRG, NTSVL, and NTSCOM). Thus, a significant reduction in the expression of 5-HT1AR, 5-HT1BR, and 5-HT2AR in multiple respiratory-related nuclei at P12 is consistent with reduced serotonergic transmission during the critical period, thereby rendering the animals less able to respond adequately to ventilatory distress.
Keywords: critical period, development, hypoglossal nucleus, nucleus ambiguus, Pre-Bötzinger complex, respiratory nuclei
It is well established that 5-hydroxytryptamine (5-HT, serotonin), an indoleaminergic neurotransmitter, is involved in respiratory modulation (Halliday et al, 1995; Hilaire and Duron, 1999; Hodges and Richerson, 2008). Brain stem serotonergic neurons are distributed mainly in medullary raphé nuclei and the ventrolateral medulla (parapyramidal area) (Holtman et al., 1984; Connelly et al., 1989; Smith et al., 1989; Thor and Helke, 1989; Manaker and Tischler, 1993). These serotonergic neurons innervate all of the brain stem nuclei that are involved in respiratory control as well as phrenic motor nuclei in the spinal cord (Steinbusch, 1981; Holtman et al., 1984; Connelly et al., 1989; Voss et al., 1990; Hodges and Richerson, 2008). Serotonin’s neuromodulatory effects on respiration are mediated by various receptor subtypes, such as 5-HT1AR, 5-HT1BR, and 5-HT2AR (Zifa, and Fillion, 1992; Bonham, 1995; Hilaire and Duron, 1999; Hodges and Richerson, 2008). These effects vary according to neuronal populations, pre-or post-synaptic sites, developmental stages, and environmental conditions (Zifa and Fillion, 1992; Hodges and Richerson, 2008). A developmental abnormality in the medullary 5-HT system has been implicated in certain pathological events occurring postnatally, such as in Sudden Infant Death Syndrome (SIDS) and obstructive sleep apnea (Hilaire et al., 1993; Panigrahy et al., 2000; Narita et al., 2001; Kinney, 2005).
Previously, we reported that a sudden drop in neuronal metabolic activity concomitant with reduced expression of excitatory neurochemicals (glutamate and NMDA receptors) and heightened expression of inhibitory neurochemicals (GABA, GABAB receptors, and glycine receptors) occurred at postnatal day (P) 12 in multiple brain stem respiratory nuclei of the rat (Liu and Wong-Riley, 2002; 2005; Wong-Riley and Liu, 2005). Both ventilatory and metabolic responses to hypoxia were also weakest at this time (P13; Liu et al, 2006; 2009). Thus, the end of the second postnatal week is a critical period of postnatal respiratory development in the rat.
Since 5-HT1AR, 5-HT1BR, and 5-HT2AR all play an important role in respiratory modulation, we hypothesized that their expressions also undergo distinct changes around the critical period. Our recent investigation indicated that 5-HT2AR expression was significantly reduced at P12 in the pre-Bötzinger complex (PBC), nucleus ambiguus (Amb), and hypoglossal nucleus (XII) (Liu and Wong-Riley, 2008, Wong-Riley and Liu, 2008). The present study represents a comprehensive, in-depth analysis of postnatal development of 5-HT1AR and 5-HT1BR in ten brain stem nuclei and 5-HT2AR in six nuclei not previously examined. These nuclei included the respiratory-related ones (the PBC, Amb, XII, the ventrolateral subnucleus of the solitary tract nucleus [NTSVL], the commissural subnucleus of the solitary tract nucleus [NTSCOM], and the retrotrapezoid nucleus [RTN]/parafacial respiratory group [pFRG]), serotonergic neuronal groups (nucleus raphé magnus [RM], nucleus raphé obscurus [ROb], and nucleus raphé pallidus [RP]), and a non-respiratory nucleus (the cuneate nucleus or CN) as a reference. CN is known for its relay function in somatosensory transduction but not with any respiratory function.
EXPERIMENTAL PROCEDURES
Tissue preparation
A total of 130 Sprague-Dawley rats from 16 litters were used. All experiments and animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publications No. 80-23, revised 1996), and all protocols were approved by the Medical College of Wisconsin Animal Care and Use Committee (approval can be provided upon request). All efforts were made to minimize the number of animals used and their suffering. Rat pups were sacrificed at each of postnatal days (P) 2–5, 7–17, and 21 (i.e., 16 time points), with ten rats from ten different litters for each day from P10 to P14, eight rats from eight different litters each day for P2–5, P7, P17, and P21, and six rats from six different litters each day for P8, P9, P15, and P16. Rats were deeply anesthetized with 4% chloral hydrate (1 ml/100 g IP; Fisher Scientific, Fair Lawn, NJ) and perfused through the aorta with 4% paraformaldehyde in 0.1 M sodium phosphate buffered saline (PBS), pH 7.4, with 4% sucrose. After perfusion, brain stems were removed and immersion fixed in the same fixative for 3 h at 4°C. They were then cryoprotected in increasing concentrations of sucrose (10, 20, and 30%) in 0.1 M PBS at 4°C, frozen on dry ice, and stored at −80°C until use.
Characterization of antibodies
Table 1 includes a brief summary of the antibodies used in the present study. All three antibodies (anti-5-HT1AR, anti-5-HT1BR, and anti-5-HT2AR) have been well characterized and their specificities established by the manufacturers and previous investigators. The amino acid sequence of each of the synthetic peptides bore no sequence homology with any other peptides, and there was no cross-reactivity with any other proteins. Moreover, the specificity of AB5406 antibodies against 5-HT1AR has been verified by preadsorption with the immunogen peptide, which effectively abolished the specific immunolabeling (Zhang et al., 2004). Huo et al. (2009) localized the same antibodies at the electron microscopic level to dendrites and cell bodies of neurons in the ventrolateral orbital cortex, where 5-HT1AR-agonists and antagonists elicited specific behavioral response. The AB5410 antibodies against 5-HT1BR was effectively used by Clark et al. (2002) to colocalize 5-HT1BR immunoreactivity with GFP-labeled plasmid expressing 5-HT1BR in the same dorsal raphé neurons. The same antibodies were documented in western blots by Carruthers et al. (2001) and the labeling was abolished by preadsorption with the immunogen peptide. The sc-15074 affinity purified IgG against 5-HT2AR was documented by Siddiqui et al. (2006) by western blots to a single band of 52 kDa in HT1376 cells as well as in the rat brain. Li et al. (2006) also localized a single band for sc-15074 on immunoblot in rat limbs.
Table 1.
Primary antibodies used.
| Antigen | Immunogen | Manufacturer, species, type, catalog number |
Dilution used |
|---|---|---|---|
| Serotonin 1A receptor | A synthetic peptide corresponding to a region located in the large third intracellular loop of the rat and mouse serotonin 1A receptor protein, with sequence specific to 5-HT1AR. | Chemicon (Temecula), guinea pigs polyclonal, #AB5406, | 1:100 |
| Serotonin 1B receptor | A synthetic peptide corresponding to a region located in the large third intracellular loop of the rat and mouse serotonin 1B receptor protein, with sequence specific to 5-HT1BR. | Chemicon (Temecula), guinea pigs polyclonal, #AB5410, | 1:100 |
| Serotonin 2A receptor | A synthetic peptide mapping within an internal region of human serotonin 2A receptor protein (accession number P28223), with sequence specific to 5-HT2AR. | Santa Cruz Biotech (Santa Cruz), goat polyclonal, affinity purified IgG, #sc-15074 | 1:100 |
Immunohistochemistry
Coronal sections of frozen brain stems were cut at 12-µm thickness with a cryostat. Six sets of serial sections were mounted on gelatin-coated slides. Sections from 3–4 rats at different ages were mounted on the same slides so that they might be processed together. Ages were typically grouped as follows: P2–10–21, P3–4–5–17, P7–8–9, P11–12–13, and P14–15–16. All sections from all animals were processed under identical conditions (i.e., time, temperature, and concentration of reagents). They were blocked overnight at 4°C with 5% nonfat dry milk-5% normal goat (or rabbit for 5-HT2AR) serum-1% Triton X-100 in 0.1 M PBS (pH 7.4). Sections were then incubated at 4°C for 36 h in the primary antibodies diluted at the proper concentration in the same solution as used for blocking: 1:200 anti-5-HT1AR polyclonal antibodies (pAb); 1:200 anti-5-HT1BR pAb; and 1:100 anti-5-HT2AR pAb. Sections were rinsed 3 times, 5 min each, in PBS, then incubated in the secondary antibodies: 1:100 goat anti-guinea pig IgG-HRP (Chemicon, Temecula, CA) for 5-HT1AR and 5-HT1BR, 1:100 rabbit anti-goat IgG-HRP (Chemicon) for 5-HT2AR, diluted in the modified blocking solution (without Triton X-100) for 4 h at room temperature. After rinsing twice with PBS and once with 0.1 M ammonium phosphate buffer (APB), pH 7.0, immunoreactivity was detected with 0.05% DAB-0.004% H2O2 in APB for 5 min, and the reaction was stopped with APB for 5 min and then rinsed in PBS three times, dehydrated, and coverslipped. Control sections were processed either without the primary antibodies or with a non immune serum in place of the primary antibodies.
For estimates of the percentage of immunoreactive neurons in a specific nucleus, alternate sections were processed with Nissl, which stained all neuronal cell bodies. Another set of alternate sections were reacted for neurokinin receptor subunit 1 with protocols described previously (Liu and Wong-Riley, 2002).
Semi-quantitative optical densitometry
The expression of different markers in the cytoplasm of neurons in various nuclei studied was semi-quantitatively analyzed by optical densitometric measurements of reaction product of immunohistochemistry, performed with a Zeiss Zonax MPM 03 photometer, a ×25 objective, and a 2-µm-diameter measuring spot. White (tungsten) light was used for illumination, and all lighting conditions were held constant for all of the measurements. Since light intensity can directly affect optical densitometric values, a stepped density filter (Edmund Industrial Optics, Barrington, NJ), with 10-step increments of 0.1 from 0.1 to 1, was used to precisely adjust the intensity of the light source to a standard value identical for all samples.
The boundary of each brain stem nucleus studied was determined with the aid of the Paxinos and Watson’s “The Rat Brain Atlas” (Academic Press, New York, 1986), and some of them, including the PBC that was identifiable with the neurokinin-1 receptor labeling, were described in our previous papers (Liu and Wong-Riley, 2002; 2003; 2005). The RTN was originally described by Smith et al. (1989). The pFRG was identified in the neonate with electrophysiological approach, and is located at the rostral ventrolateral medulla, ventrolateral to the facial nucleus and close to the ventral surface (Onimaru and Homma, 2003). It apparently overlaps with the RTN (Feldman and Del Negro, 2006). Since it is not known if the RTN and pFRG are anatomically and functionally distinct (Feldman and Del Negro, 2006), we opted to refer to this region as ‘RTN/pFRG’. The part of the nucleus ambiguus chosen for the present study (and our previous studies) was the semicompact formation and the rostral loose formation innervating upper airway muscles and representing pharyngolaryngomotor functions (Bieger and Hopkins, 1987). For the remaining nuclei, measurements were taken from the central main portion of each nucleus.
The optical densitometric value of each labeled neuron in the various brain stem nuclei studied was an average reading of two to four spots in the cytoplasm of each cell body. Only those neurons whose nuclei are clearly visible (i.e., sectioned through the middle of the cell body) were measured. To avoid measuring the same neuron more than once, values were taken from cells in sections at least 70 µm apart, as the largest neurons had a maximal diameter of 25–30 µm, with a maximal nuclear diameter of only 10 µm. The relative thinness and crisscrossing of the processes in the neuropil rendered them difficult to be measured with a 2-µm-diameter measuring spot. Thus, the processes and neuropil were described only qualitatively with respect to the intensities of their labeling. About 100 neurons in each brain stem nucleus were measured for each marker in each rat, and a total of about 1000 (for P10–14), 600 (P8, P9, P15, and P16), or 800 neurons (for the remaining ages examined) for each marker in each nucleus at each age were measured. For statistical analyses, each sample’s optical density value for each nucleus of each rat was the average of about 100 neurons. Thus, the sample number of each time point in each nucleus is ten, eight, or six in Figure 4, Figure 7, and Figure 9.
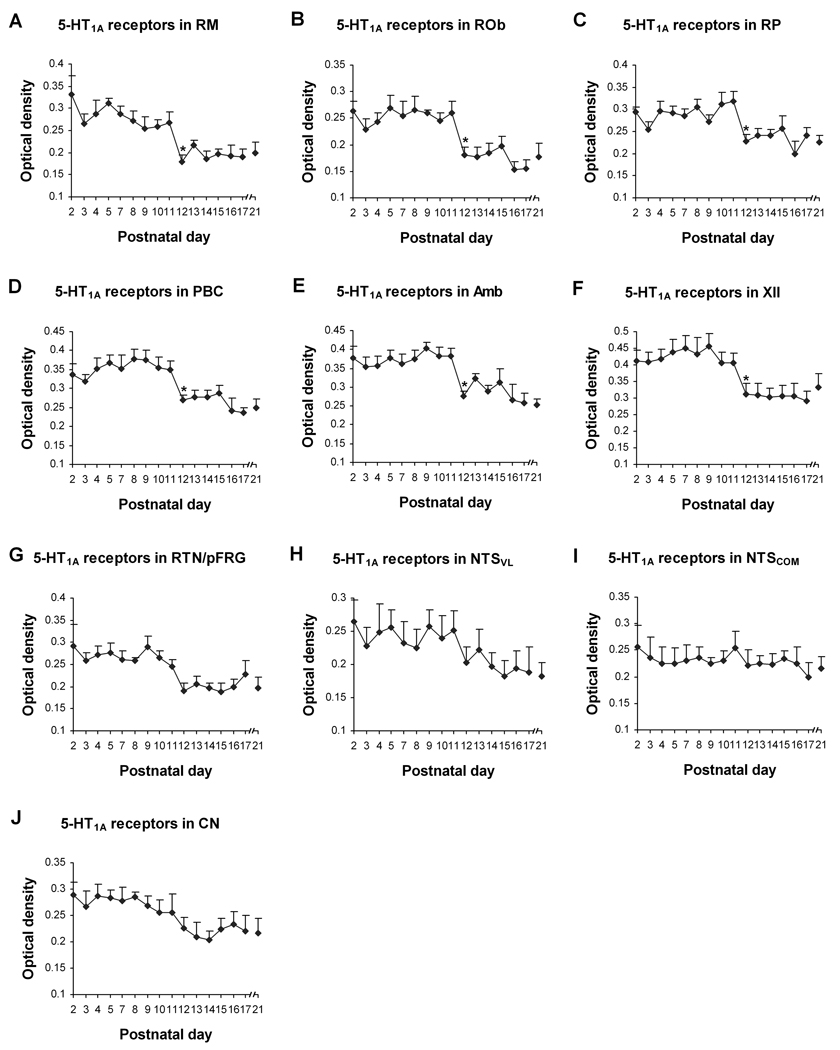
Fig. 4.
Optical densitometric measurements of immunoreactive product for 5-HT1AR in the cytoplasm of individual neurons in the RM (A), ROb (B), RP (C), PBC (D), Amb (E), XII (F), RTN/pFRG (G), NTSVL (H) , NTSCOM (I), and CN (J) from P2 to P21. Data points were presented as mean ± SEM. The first six nuclear groups (A–F) showed pattern I trend of development, with significant reduction of labeling at P12 (P < 0.05). The trend for RTN/pFRG (G) was that of pattern II, whereas those for the NTSVL (H) and CN (J) were of pattern III. In contrast, NTSCOM (I) assumed a pattern IV trend of development (see text for details). ANOVA yielded significant differences in the 5-HT1AR expression among ages in the RM, ROb, RP, PBC, Amb, XII, and RTN/pFRG (P < 0.01). Tukey’s Studentized test revealed a significant reduction in the 5-HT1AR expression in the RM, ROb, RP, PBC, Amb, and XII at P12, compared with their adjacent younger age groups (P11). *, P < 0.05 (Tukey’s Studentized test).
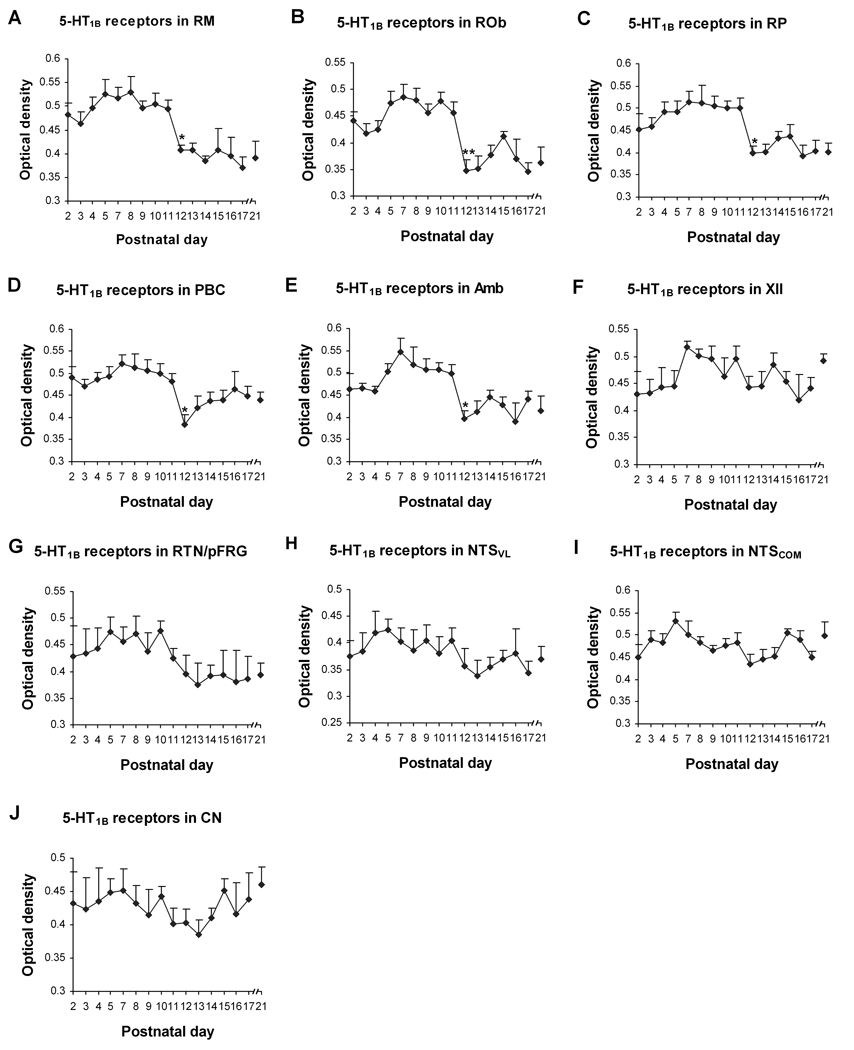
Fig. 7.
Optical densitometric measurements of immunoreactive product for 5-HT1BR in the cytoplasm of individual neurons in the RM (A), ROb (B), RP (C), PBC (D), Amb (E), XII (F), RTN/pFRG (G), NTSVL (H) , NTSCOM (I), and CN (J) from P2 to P21. Data points were presented as mean ± SEM. The labeling in the first five nuclear groups (A–E) followed pattern I trend of development, with a significant decrease at P12 (P < 0.05). The trend for the RTN/pFRG (G) was that of pattern II, whereas those for XII (F), NTSVL (H), NTSCOM (I), and CN (J) were of pattern IV (see text for details). ANOVA revealed significant differences in 5-HT1BR expression among ages in the RM, ROb, RP, PBC, and Amb (P < 0.01). Tukey’s Studentized test showed a significant reduction in the RM, ROb, RP, PBC, and Amb at P12, compared with their adjacent younger age groups (P11). *, P < 0.05 (Tukey’s Studentized test).
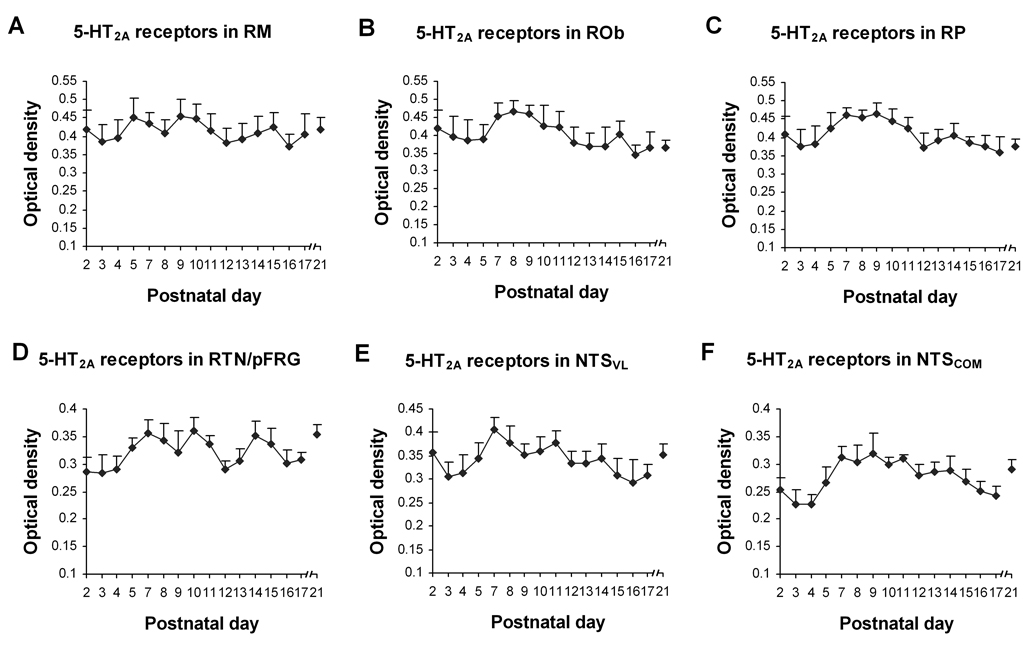
Fig. 9.
Optical densitometric measurements of immunoreactive product for 5-HT2AR in the cytoplasm of individual neurons in the RM (A), ROb (B), RP (C), RTN/pFRG (D), NTSVL (E), and NTSCOM (F) from P2 to P21. Data points represented the mean ± SEM. Labeling in all of the nuclear groups followed a pattern IV trend of development during the first 3 postnatal weeks. ANOVA did not reveal any significant differences in 5-HT2AR expression among ages in any of these nuclear groups.
Mean optical density values, standard deviations, and standard errors of the mean in each nucleus at each age were then obtained. Statistical comparisons were made among the age groups by using one-way ANOVA (to control for the type I comparisonwise error rate) and, when significant differences were found, comparisons were made between successive age groups (e.g., P2 vs. P3, P3 vs. P4, and P5 vs. P7) by using Tukey's Studentized range test (a post hoc multiple comparisons, to control for the type I experimentwise error rate). Significance was set at P < 0.01 for one-way ANOVA and P < 0.05 for Tukey's test.
RESULTS
I. 5-HT1AR-immunoreactive(-ir) neurons in the brain stem nuclei
In general, 5-HT1AR-ir products were clearly visible in subpopulations of neurons in each of the brain stem nuclei examined (Fig. 1A1–3). The dorsal raphé nucleus, known to express a significant amount of 5HT1AR {Zifa and Fillion, 1992), was also densely immunoreactive (see Supplementary Fig. 1). The size of 5-HT1AR-ir neurons increased with age and reached a relatively stable level after P10–P11 (Fig. 2). The expression of 5-HT1AR-ir product in the neuropil of various brain stem nuclei studied showed a relatively high level before P12 and a relatively low level at P12 and after. Control sections demonstrated no specific immunoreactive product above background (Fig. 1A4). The developmental trends of 5-HT1AR-ir expression in neurons of various brain stem nuclei can be divided into four patterns: 1) Pattern I: a relatively high level until P11, then a significant decrease at P12 (P < 0.05), followed by a plateau at a relatively low level, as exhibited in the RM, ROb, RP, PBC, Amb, and XII (Fig. 4A–F); 2) pattern II: a relatively high level until P9, then a gradual decline from P9 to P12, followed by a plateau at a relatively low level, as shown in RTN/pFRG (Fig. 4G); 3) pattern III: a gradual reduction from P2 to P21, as demonstrated in NTSVL and CN (Fig. 4H, J); and 4) pattern IV: a relatively stable level from P2 to P21 with minor fluctuations, as present in NTSCOM (Fig. 4I). ANOVA showed significant differences (P < 0.01) in the 5-HT1AR-ir expression among the ages in the RM, ROb, RP, PBC, Amb, XII, and RTN/pFRG, and Tukey’s Studentized range test that compared one age group with its adjacent younger age group indicated significant differences (reductions) at P12 for the first six nuclei (P < 0.05, as compared to the values at P11). P12 was the only time point that yielded statistical significance during the first three postnatal weeks (Fig. 4).
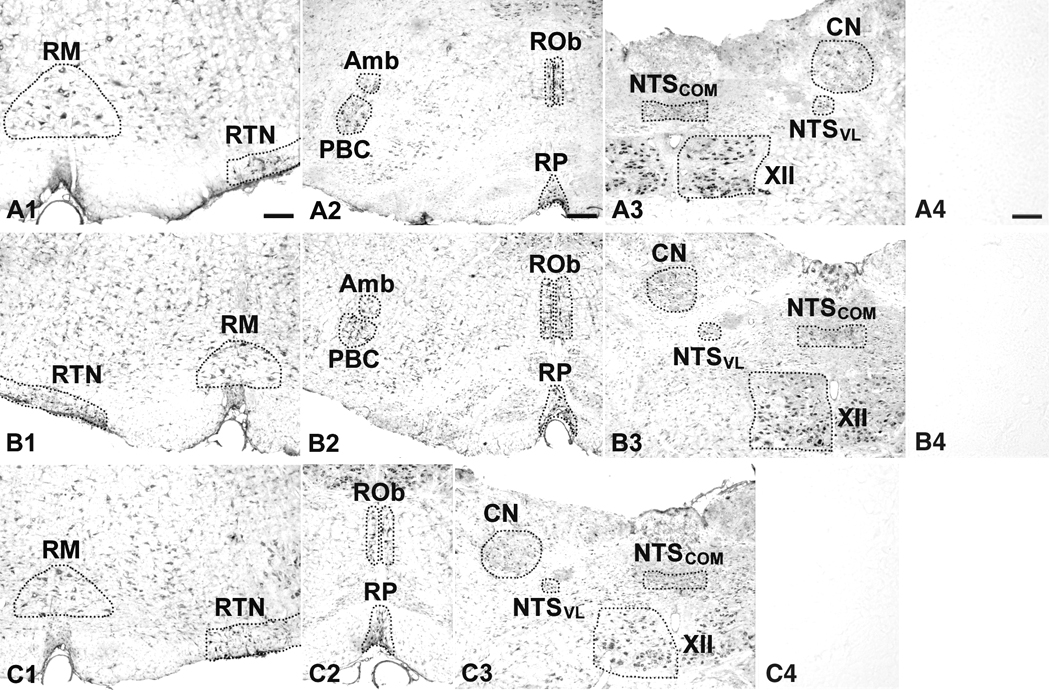
Fig. 1.
Low magnification photomicrographs of rat brain stem sections at postnatal day (P) 21 reacted for serotonin (5-HT) 1A receptors (5-HT1AR) (A1–3), 5-HT1BR (B1–3), and 5-HT2AR (C1–3). A4, B4, and C4 are control sections processed with non-immune serum instead of the respective primary antibody. Amb, nucleus ambiguus; CN, cuneate nucleus; NTSCOM, commissural subnucleus of the solitary tract nucleus; NTSVL, ventrolateral subnucleus of the solitary tract nucleus; PBC, pre-Bötzinger complex; RM, nucleus raphé magnus; ROb, nucleus raphé obscurus; RP, nucleus raphé pallidus; RTN, retrotrapezoid nucleus/parafacial respiratory group; XII, hypoglossal nucleus. Scale bar in A1: 200 µm for A1, A3, B1, B3, and C1–3. Scale bar in A2: 250 µm for A2 and B2. Scale bar in A4: 20 µm for A4, B4, and C4.
Fig. 2.
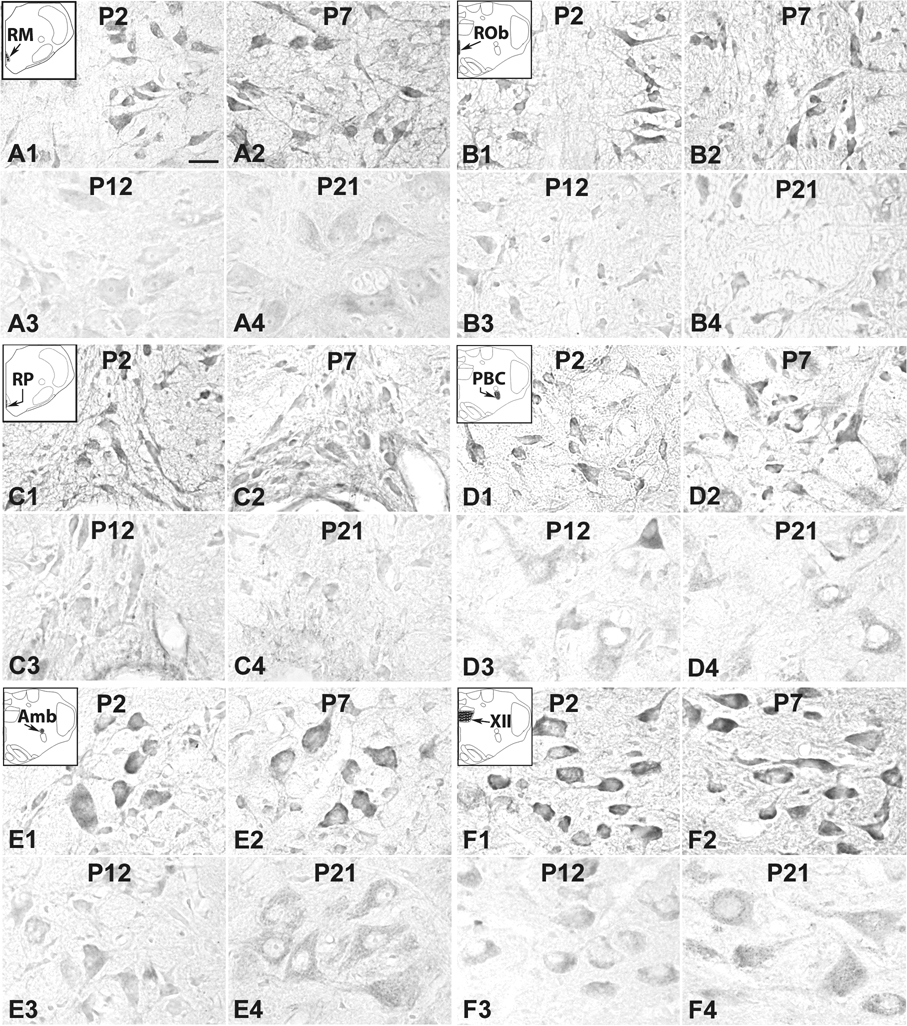
5-HT1AR-ir neurons in the RM (A), ROb (B), RP (C), PBC (D), Amb) (E, and XII (F) at representative postnatal P2 (A1–F1), P7 (A2–F2), P12 (A3–F3), and P21 (A4–F4). The insets in A1–F1 indicate the locations of each nucleus in a diagrammatic cross section of the medulla or pons. Labeling in neurons of all six nuclear groups followed the type I pattern of development, with relatively high levels from P2 (A1–F1) through P7 (A2–F2) to P11 (not shown), but significantly declined at P12 (A3–F3) and remained low thereafter until P21 (A4–F4). Scale bar: 20 µm for all.
A. Pattern I of developmental trend in the expression of 5-HT1AR in brain stem nuclei
a. 5-HT1AR-ir neurons in the RM
5-HT1AR-ir was present in ~ 70% – 80% of the RM neurons that were mainly medium or large in size, and multipolar or pyramid-like in shape. The size of medium neurons ranged from 10.5 to 14 µm in diameter at P2–P9 to 16–18 µm at P11–P21, whereas large neurons ranged from 16 to 20 µm at P2–P9 to 23–27 µm at P11–P21. Immunoreactivity was observed in the cell bodies and proximal processes, but at a much lower level in the rest of the neuropil (Fig. 2A1–4). Optical densitometric measurements of all the age groups examined indicated a developmental trend described here as pattern I, i.e., a plateau at a relatively high level from P2 to P11, a significant reduction at P12 (P < 0.05), followed by a plateau at a relatively low level from P13 to P21 (Fig. 4A).
b. 5-HT1AR-ir neurons in the ROb
About 70% – 90% of neurons in the ROb showed 5-HT1AR immunoreactivity. They were multipolar, granular, or fusiform in shape and mainly small in size. They ranged from 6.5 to 10 µm in diameter at P2–P9 to 7.5 – 12.5 µm at P11–P21. The distribution of immunoreaction product in neurons and neuropil was similar to those in the RM (Fig. 2B1–4), and the developmental trend followed that of pattern I (Fig. 4B). The fall at P12 was statistically significant (P <0.05), but not the dip at P16.
c. 5-HT1AR-ir neurons in the RP
Approximately 65% – 85% of neurons in the RP exhibited 5-HT1AR-ir, and the distribution was similar to those of RM and RP (Fig. 2C1–4). Immunoreactive neurons were mainly small in size, and multipolar, oval, or fusiform in shape. Sizes ranged from 5.5 to 9 µm in diameter at P2–P9 to 6.5 – 12.5 µm at P11–P21. Both the pattern of distribution (Fig. 2C1–4) and the developmental trend (Fig. 4C) were similar to those in RM and ROb.
d. 5-HT1AR-ir neurons in the PBC
5-HT1AR immunoreactivity was observed in ~ 50% – 65% of neurons in the PBC. They were small or medium in size, and multipolar, granular, or fusiform in shape. The size of small neurons ranged from 6.5 – 8.5 m in diameter at P2 to 8 – 10.5 m at P21, whereas medium-sized neurons ranged from 10 – 11.5 m at P2 to 11 – 16 m at P21. Immunoreactivity was observed in the cell bodies and proximal processes of neurons, but was at a low level in the rest of the neuropil (Fig. 2D1–4). Optical densitometric analysis showed a pattern I type of developmental trend, with a distinct reduction at P12 (P < 0.05) (Fig. 4D).
e. 5-HT1AR-ir neurons in the upper airway representation of the Amb
About 80% –100% of Amb neurons were 5-HT1AR-ir. Immunoreactivity was present in the cell bodies and proximal processes of neurons, but was low in the rest of the neuropil (Fig. 2E1–4). These neurons were multipolar or oval in shape, and small to mainly medium in size. The size of small neurons ranged from 7.5 – 9.5 µm in diameter at P2 to 9 – 12.5 µm at P21, whereas medium-sized neurons ranged from 10 – 12.5 µm at P2 to 13.5 – 16 µm at P21. The developmental trend of 5-HT1AR-ir in neurons followed pattern I, and the reduction at P12 was significant (P < 0.05) (Fig. 4E).
f. 5-HT1AR-ir neurons in the XII
About 75% – 90% of neurons in the XII were 5-HT1AR-ir, and they were mainly medium or large in size and multipolar, pyramid-like, or granular in shape The size of medium neurons ranged from 10.5 – 14 µm in diameter at P2 to 13 – 16 µm at P21, whereas large neurons ranged from 16.5 – 18 µm at P2 to 16.5 – 22 µm at P21. Immunoreactivity was present not only in the cell bodies and proximal processes of neurons, but was relatively high in the rest of the neuropil as well before P12 (Fig. 2F1–4), unlike the case of the other brain stem nuclei described above. The developmental trend of 5-HT1AR expression in neurons was a typical pattern I, with a significant reduction at P12 (P < 0.05) (Fig. 4F).
B. Pattern II of developmental trend in the expression of 5-HT1AR in brain stem nuclei
a. 5-HT1AR-ir neurons in the RTN/pFRG
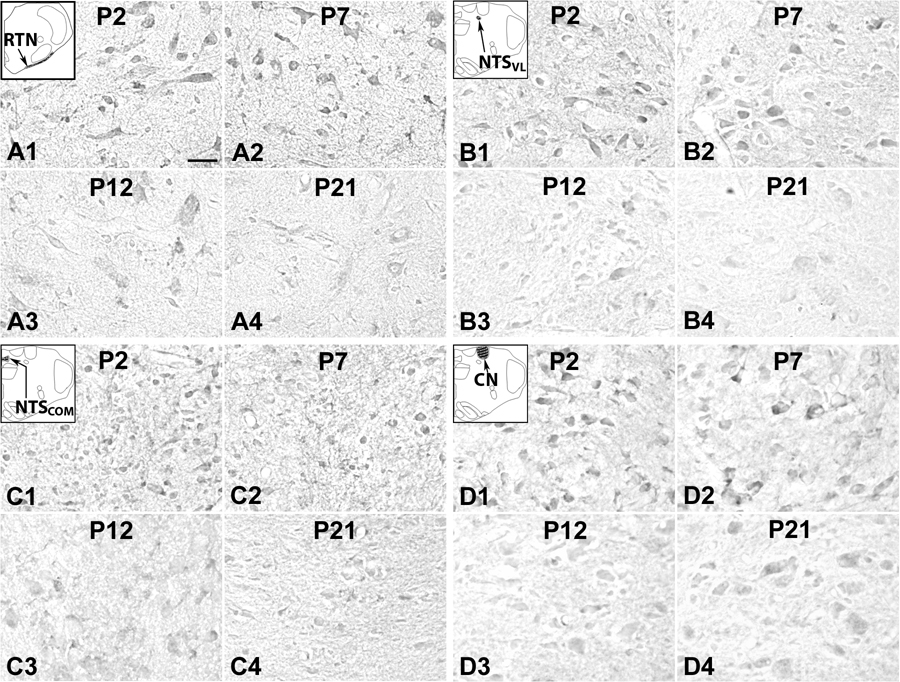
5-HT1AR immunoreactivity was present in ~ 40% – 55% of RTN/pFRG neurons, where both the cell bodies and some proximal processes were labeled, but the rest of the neuropil had relatively low level of labeling (Fig. 3A1–4). Immuno-positive neurons were mainly multipolar, granular or fusiform in shape and small and medium in size. The size of small neurons ranged from 6.5 to 10 µm in diameter at P2–P9 to 9 – 12.5 µm at P11–P21, whereas medium-sized neurons ranged from 11 to 14 µm at P2–P9 to 13–16 µm at P11–P21. The developmental trend of 5-HT1AR expression in neurons was a typical pattern II, with relatively high levels between P2 and P9, then a gradual decrease from P9 to P12, followed by a relative plateau of low expression (Fig. 4G).
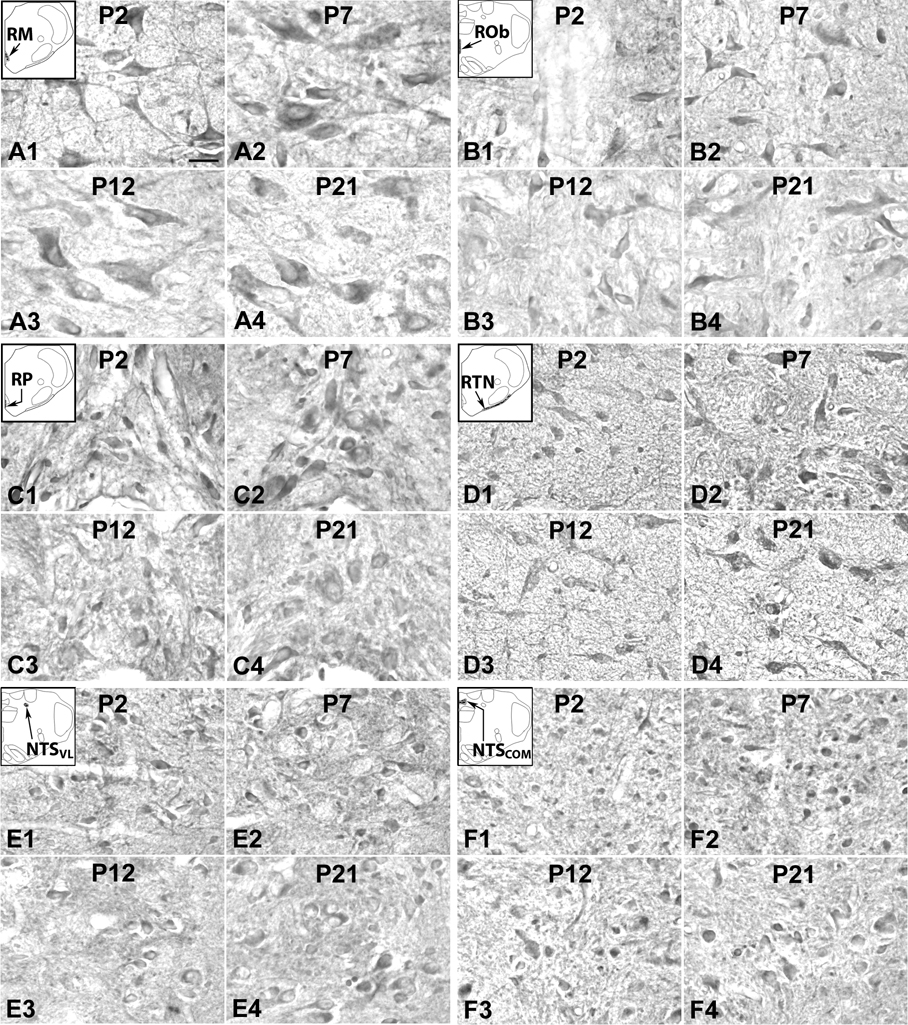
Fig. 3.
5-HT1AR-ir neurons in the RTN/pFRG (labeled as RTN for this region in the inset) (A), (NTSVL (B), NTSCOM (C), and CN (D) at P2 (A1–D1), P7 (A2–D2), P12 (A3–D3), and P21 (A4–D4). The insets in A1-D1 indicate diagrammatically the locations of these four nuclear groups. Labeling of neurons in the RTN/pFRG was relatively high from P2 (A1) through P7 (A2) to P9, then gradually decreased at P10 through P12 (A3), followed by a plateau at relatively low level until P21 (A4). Labeling of neurons in the NTSVL and CN exhibited a progressive reduction from P2 to P21 (B1–B4 and D1–D4, respectively). On the other hand, immunoreactivity remained quite constant through the first 3 postnatal weeks in the NTSCOM (C1–C4). Scale bar: 20 µm for all.
C. Pattern III of developmental trend in the expression of 5-HT1AR in brain stem nuclei
a. 5-HT1AR-ir neurons in the NTSVL
5-HT1AR-ir was observed in about 50% – 80% of neurons in the NTSVL. They were small in size and mainly multipolar, oval, or granular in shape. The size ranged from 5.5 to 8.5 µm in diameter at P2–P9 to 7.5 – 12.5 µm at P11–P21. Immunoreactivity was present in the cell bodies and some proximal processes of neurons (Fig. 3B1–4), and it followed a pattern III developmental trend, with a gradual decrease in expression with age from P2 to P21, albeit some fluctuations were observed (Fig. 4H).
b. 5-HT1AR-ir neurons in the CN
About 35% – 50% of the CN neurons exhibited 5-HT1AR-ir in the cell bodies, but less so in the processes or the rest of the neuropil (Fig. 3D1–4). Labeled neurons were mainly small in size and multipolar, granular, or oval in shape. The size of small neurons ranged from 5 – 8.5 µm in diameter at P2 to 7 – 10.5 µm at P21, whereas medium-sized neurons ranged from 10 – 12 µm at P2 to 11 – 14 µm at P21. The developmental trend of 5-HT1AR expression in neurons was that of pattern III, with gradual decline with age (Fig. 4J).
C. Pattern IV of developmental trend in the expression of 5-HT1AR in brain stem nuclei
a. 5-HT1AR-ir neurons in the NTSCOM
5-HT1AR-ir was observed in about 50% – 80% of NTSCOM neurons that were mainly multipolar, granular, or oval in shape and small in size (Fig. 3C1–4). The size ranged from 5 to 8 µm in diameter at P2–P9 to 8 – 11.5 µm at P11–P21. The developmental trend of 5-HT1AR expression in neurons was that of pattern IV, which remained relatively stable from P2 to P21, with only minor fluctuations throughout (Fig. 4I).
II. 5-HT1BR-ir neurons in the brain stem nuclei
In general, 5-HT1BR immunoreactivity was clearly visible in subpopulations of neurons in each of the brain stem nuclei examined (Fig. 1B1–3). The substantia nigra, known to express a significant amount of 5HT1BR {Zifa and Fillion, 1992), was also densely immunoreactive (see Supplementary Fig. 1). The size of labeled neurons increased with age and reached a relatively stable level after P10–P11 (Fig. 5 and 6). The size, shapes, and prevalence of labeled neurons were comparable to those of 5-HT1AR-ir neurons, and will not be described in detail below. The high percentage of neurons with similar sizes and shapes labeled for both 5-HT1AR and 5-HT1BR in some nuclei makes it very likely that many, if not most, of them expressed both receptors. The labeling in the neuropil was relatively high in the neonate but became lower during the third postnatal week. Control sections did not have any labeling above background (Fig. 1B4). The developmental trends of 5-HT1BR-ir in various brain stem nuclei can be divided into comparable patterns as for 5-HT1AR (except that pattern III was not observed): 1) Variations of pattern I was found in RM, ROb, RP, PBC, and Amb (Fig. 5A–E); 2) pattern II was present in RTN/pFRG (Fig. 6A); and 3) pattern IV was followed by the XII (Fig. 5F), NTSVL, NTSCOM, and CN (Fig. 6B–D). ANOVA indicated significant differences (P < 0.01) in 5-HT1BR-ir expression among ages in the RM, ROb, RP, PBC, and Amb, and Tukey’s Studentized range test showed significant reductions at P12 for these nuclei (P < 0.05, as compared to the values at P11). This was the only time point that yielded statistical significance when two adjacent age groups were compared during the first three postnatal weeks (Fig. 7).
Fig. 5.
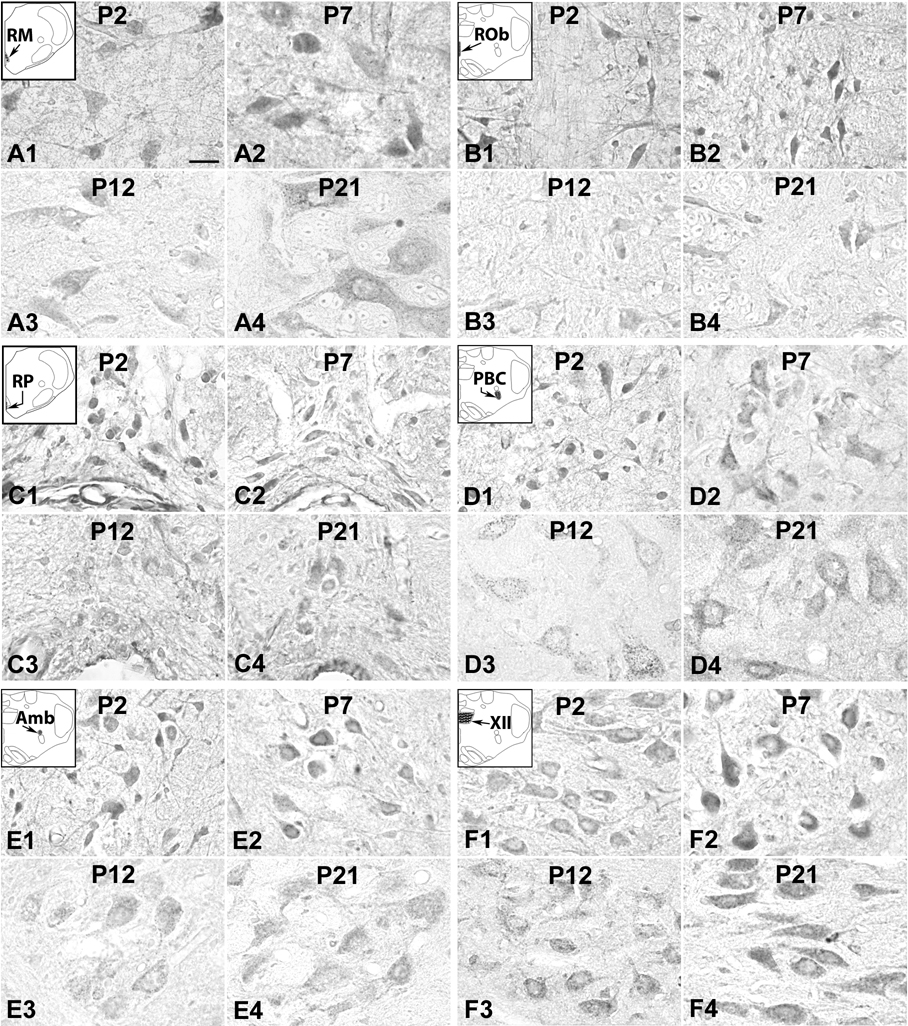
5-HT1BR-ir neurons in the RM (A), ROb (B), RP (C), PBC (D), Amb (E), and XII (F) at P2 (A1–F1), P7 (A2–F2), P12 (A3–F3), and P21 (A4–F4). The expression of 5-HT1BR in the first five nuclear groups (A–E) was relatively high from P2 (A1–E1) through P7 (A2–E2) to P11, then precipitously dropped at P12 (A3–E3), followed by some fluctuations until P21 (A4–E4). The labeling in the XII was relatively stable from P2 to P21 (F1–F4), with statistically insignificant fluctuations. Scale bar: 20 µm for all.
Fig. 6.
5-HT1BR-ir neurons in the RTN/pFRG (A; labeled as RTN in the inset), NTSVL (B), NTSCOM (C), and CN (D) at P2 (A1–D1), P7 (A2–D2), P12 (A3–D3), and P21 (A4–D4). The labeling in the RTN/pFRG exhibited a relatively high level from P2 (A1) through P7 (A2) to P10, then gradually fell from P10 through P12 (A3) to P13, followed by a plateau at a relatively low level until P21 (A4). The labeling in the NTSVL, NTSCOM, and CN was relatively constant from P2 to P21 (B1–B4, C1–C4, and D1–D4). Scale bar: 20 µm for all.
A. Pattern I of developmental trend in the expression of 5-HT1BR in brain stem nuclei
a. 5-HT1BR-ir neurons in the RM
About 60% – 80% of RM neurons had 5-HT1BR-ir in the cell bodies and some of the proximal processes, but labeling was much less in the rest of the neuropil (Fig. 5A1–4). 5-HT1BR expression in neurons followed a variation of pattern I during postnatal development, in that the level at P2–P3 was not as high as those from P4 to P11, then it took a drastic fall at P12 (P < 0.05), followed by a relatively low plateau until P21 (Fig. 7A).
b. 5-HT1BR-ir neurons in the ROb
5-HT1BR immunoreactivity was observed in the cell bodies and some proximal processes of 60% – 85% of ROb neurons, but it was much less in the rest of the neuropil (Fig. 5B1–4). The developmental trend of labeling in neurons was again a variation of pattern I, in that the level was lower between P2 to P4 than those between P5 and P11. It then plunged significantly at P12 (P < 0.01), and the level remained low until P21, with a small but statistically insignificant peak at P15 (Fig. 7B).
c. 5-HT1BR-ir neurons in the RP
5-HT1BR-ir was observed in the cell bodies and some proximal processes of about 50% – 80% of RP neurons (Fig. 5C1–4). The developmental trend was comparable to those of RM and ROb neurons, with moderate levels at P2 and P3, relatively high levels between P4 and P11, a striking reduction at P12 (P < 0.05), followed by relatively low levels until P21 (Fig. 7C).
d. 5-HT1BR-ir neurons in the PBC
About 40% – 60% of PBC neurons exhibited 5-HT1BR immunoreactivity distributed mainly in the cell bodies and some proximal processes (Fig. 5D1–4). The developmental expression was a variation of pattern I, with a slight dip at P2–P4, a relatively high level from P5 to P11, a sudden fall at P12 (P < 0.05), then a rise to a moderate level from P13 to P16, and plateaued thereafter until P21 (Fig. 7D).
e. 5-HT1BR-ir neurons in the upper airway representation of the Amb
5-HT1BR immunoreactivity was observed in the cell bodies and some proximal processes of ~ 70% – 90% of Amb neurons (Fig. 5E1–4). The developmental trend was a variation of type I that was very similar to that in ROb , i.e., a moderate level at P2–P4, a relatively high level between P5 and P11, then a precipitous fall at P12 (P < 0.05), followed by a plateau with some fluctuations until P21 (Fig. 7E).
B. Pattern II of developmental trend in the expression of 5-HT1BR in brain stem nuclei
a. 5-HT1BR-ir neurons in the RTN/pFRG
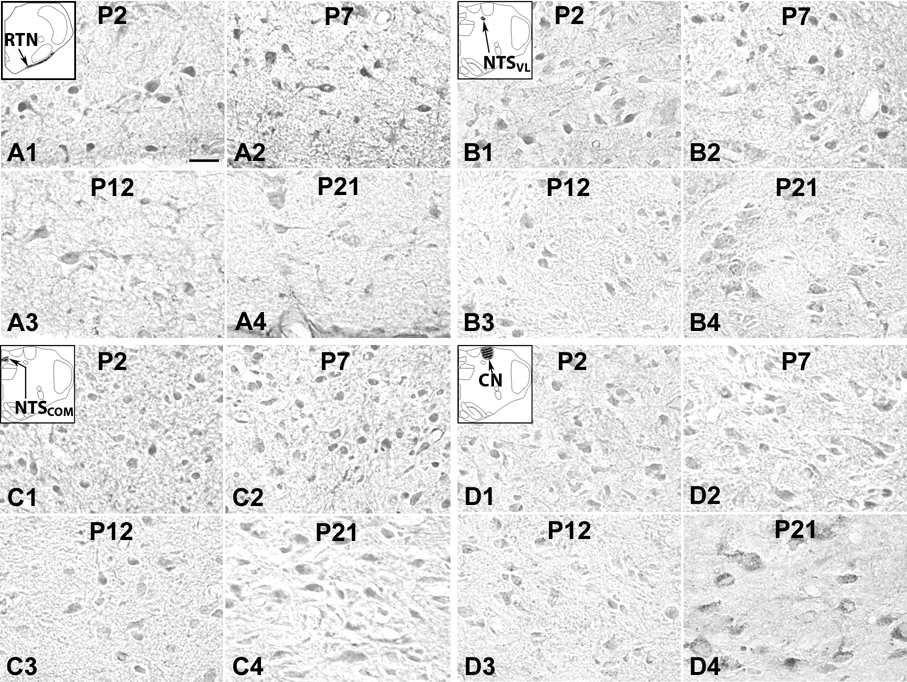
5-HT1BR immunoreactivity was observed in ~ 50% –60% of RTN/pFRG neurons (Fig. 6A1–4). The developmental trend was that of pattern II, comparable to that of 5-HT1AR-ir in the same region. Thus, the level was relatively high between P2 and P10, then it fell gradually from P10 to P13, and plateaued thereafter until P21 (Fig. 7G).
C. Pattern IV of developmental trend in the expression of 5-HT1BR in brain stem nuclei
a. 5-HT1BR-ir neurons in the XII
About 70% – 90% of XII neurons exhibited 5-HT1BR immunoreactivity in their cell bodies and some proximal processes, with relatively high levels in the rest of the neuropil (Fig. 5F1–4). Unlike the pattern I trend of development for 5-HT1AR in this nucleus, that of 5-HT1BR was a series of fluctuations from P2 to P21, more akin to that of pattern IV, although the levels were the lowest at P2–P5, P12–13, and P15–P17 (Fig. 7F).
b. 5-HT1BR-ir neurons in the NTSVL
About 50% – 60% of NTSVL neurons exhibited 5-HT1BR immunoreactivity in their cell bodies and some proximal processes, and labeling was also present in the rest of the neuropil (Fig. 6B1–4). The developmental trend was that of pattern IV, although the levels were the lowest at P12–P14 and P17 (Fig. 7H).
c. 5-HT1BR-ir neurons in the NTSCOM
5-HT1BR immunoreactivity was present in about 70% – 90% of NTSCOM neurons (Fig. 6C1–4). The progression of expression through the first 3 postnatal weeks was that of pattern IV, with some fluctuations among the ages and lowest at P12 (Fig. 7I).
d. 5-HT1BR-ir neurons in the CN
About 40% – 65% of CN neurons expressed 5-HT1BR immunoreactivity in their cell bodies and some proximal processes (Fig. 6D1–4). The developmental trend was reminiscent of type IV, with some fluctuations from P2 to P21 (Fig. 7J).
III. 5-HT2AR-ir neurons in the brain stem nuclei
Immunoreactivity for 5-HT2AR was clearly observed in subpopulations of neurons in each of the brain stem nuclei studied (Fig. 1C1–3). The size of labeled neurons increased with age and reached a relatively stable level after P10–P11. Labeling was present in the cell bodies and proximal processes of neurons and in the rest of the neuropil (Fig. 8). The shapes and sizes of immunoreactive neurons were similar to those with 5-HT1AR-ir and 5-HT1BR, and will not be described in detail below. These common features and the high percentage of neurons labeled for each of the markers suggest that many of the same neurons may express all three 5-HT receptors. Control sections had no labeling above background (Fig. 1C4). The developmental trends of 5-HT2AR expression in various brain stem nuclei examined in the present study followed variations of pattern IV, i.e., relatively stable levels from P2 to P21, but with fluctuations throughout (Fig. 9). This pattern was distinctly different from those of the PBC, Amb, and XII reported previously (Liu and Wong-Riley, 2008), in which a significant reduction in 5-HT2AR expression was seen at P12, despite a relatively stable level during the rest of the first three postnatal weeks, a pattern that can be referred to as pattern V. The non-respiratory CN had a pattern IV trend for 5-HT2AR (Liu and Wong-Riley, 2008) and will not be repeated here. ANOVA of the current study did not yield any significant differences among the ages in each of the nuclei examined.
Fig. 8.
5-HT2AR-ir neurons in the RM (A), ROb (B), RP (C), RTN/pFRG (D), NTSVL (E), and NTSCOM (F) at P2 (A1–F1), P7 (A2–F2), P12 (A3–F3), and P21 (A4–F4). The labeling was relatively constant with statistically insignificant fluctuations from P2 to P21. Scale bar: 20 µm for all.
A. 5-HT2AR-ir neurons in the RM
5-HT2AR immunoreactivity was present in the cell bodies and proximal processes of about 60% – 80% of RM neurons, but was lower in the rest of the neuropil (Fig. 8A1–4). Labeling followed a pattern IV trend from P2 to P21, with very minor fluctuations, being the lowest at P3, P12, and P16 (Fig. 9A).
B. 5-HT2AR-ir neurons in the ROb
About 80% – 90% of ROb neurons exhibited 5-HT2AR immunoreactivity in their cell bodies and proximal processes, but labeling was lower in the rest of the neuropil (Fig. 8B1–4). The trend of labeling in neurons was relatively stable during the first three postnatal weeks with some fluctuations, being lower at P3–5, P12–P13, and P16 (Fig. 9B).
C. 5-HT2AR-ir neurons in the RP
5-HT2AR immunoreactivity was found in the cell bodies and some proximal processes of ~ 65% – 80% of RP neurons (Fig. 8C1–4). The expression was relatively stable from P2 to P21, with the lowest points at P3–P4, P12, and P17 (Fig. 9C).
D. 5-HT2AR-ir neurons in the RTN/pFRG
About 60% – 85% of RTN/pFRG neurons had 5-HT2AR-ir in their cell bodies (Fig. 8D1–4). The developmental trend was periodic fluctuations, with the lowest expressions at P2–P4 and P12, none of which, however, reached statistical significance (Fig. 9D).
E. 5-HT2AR-ir neurons in the NTSVL
5-HT2AR immunoreactivity was evident in cell bodies and some proximal processes of ~ 50% – 80% of NTSVL neurons as well as in the rest of the neuropil (Fig. 8E1–4). Labeling was the lowest at P3–P4 and P16 against a relatively stable trend from P2 to P21, but there were no statistically significant day-to-day changes (Fig. 9E).
F. 5-HT2AR-ir neurons in the NTSCOM
About 55% – 70% of NTSCOM neurons exhibited 5-HT2AR immunoreactivity (Fig. 8F1–4). The labeling started relatively low at P2–P4, but increased to a steady level until P21. No statistical significance was found among the age groups examined (Fig. 9F).
DISCUSSION
The present in-depth study revealed four patterns of developmental trend for 5-HT receptor expressions in ten brain stem nuclei through the first three postnatal weeks in rats. Patterns I, II, and III all showed stronger labeling in the earlier stages of postnatal development (first week to P11) than later stages (from P12 to P21). The distinctive feature of pattern I is that the precipitous drop in 5-HT receptor immunoreactivity from P11 to P12 is the only time point during the entire first three postnatal weeks that a day-to-day change shows statistical significance. Pattern II is similar but not identical to that of I, but because the decrease toward P12 is more gradual (over 2–3 days rather than 1 day), the reduction at P12 is no longer statistically significant. Pattern III is also a gradual decline, but the trend starts during the first postnatal week and continues to P21. Pattern IV represents a relatively constant level from P2 to P21, with some fluctuations throughout. Pattern V is similar to pattern I, and is retroactively described for 5-HT2AR immunoreactivity reported previously for a few brain stem nuclei (Liu and Wong-Riley, 2008); in this case, the labeling returned to relatively high levels after the statistically significant drop at P12.
The present study focused on cell body labeling of 5-HT1A, 5-HT1B, and 5-HT2A receptors. The general patterns of distribution in the brain stem are comparable to those reported by others. For example, most brain stem motoneurons express 5-HT1AR and 5-HT2AR (Wright et al., 1995; Azmitia et al., 1996), and all three receptors were observed in the raphé nuclei (Voigt et al., 1991; Azmitia et al., 1996, Xie et al., 2002) and NTS (Manaker and Verderame, 1990; Raul, 2003). For 5-HT1BR, our findings of labeling in somas and dendrites are in agreement with those of others (Smith et al., 1998; Makarenko et al., 2002; 2005; Jacobs et al., 2007; Peddie et al., 2008), but we did not focus on its distribution in axons and terminals as reported previously (Sari et al., 1997; 1999; Riad et al., 2000; Sari, 2004). Labeling in cell bodies would be consistent with a postsynaptic distribution of receptors and autoreceptors in somas and dendrites (Sotelo et al., 1990), but it is also consistent with the existence of presynaptic autoreceptors in axons and terminals, as all receptors are synthesized in cell bodies.
Serotonin plays an important role in the regulation of cardiorespiratory, thermal, neuroendocrine, motor, and sensory functions, and is involved in arousal, cognitive, feeding, sexual, affective, and aggressive behaviors (Zifa and Fillion, 1991; Jacobs and Fornal, 1995; Leysen, 2004). The primary effect of serotonin on respiration is excitatory, for it exerts a tonic drive to maintain respiratory output during wakefulness (Richerson, 2004; Hodges and Richerson, 2008). 5-HT is also involved in respiratory rhythmogenesis, respiratory motoneuron excitability, and reportedly central chemosensitivity (Richerson, 2004; Richerson et al., 2005; Hodges and Richerson, 2008), upper airway reflexes (Haxhiu et al., 1998), and responses to changes in oxygen and carbon dioxide levels (Taylor et al., 2005; Penatti et al., 2006).
Among serotonergic receptors, 5-HT1AR is reportedly involved in the modulation of mood, emotions, and sexual behavior, as well as the regulation of temperature, feeding, respiration, and other motor activities (Zifa and Fillion, 1992; Richter et al., 2003). The 5-HT1A autoreceptors in dorsal raphé nuclei and other brain regions are involved in regulating the firing of serotonergic neurons (Zifa and Fillion, 1992). Activation of 5-HT1AR increases phrenic nerve activity (Wilken et al., 1997; Teng et al., 2003; Wang et al. 2007; Nucci et al., 2008; Valic et al., 2008) and induces an increase in phasic and tonic genioglossus muscle activity important for tongue protrusion and the prevention of airway obstruction (Besnard et al., 2007). 5-HT1AR also exerts excitatory modulation on respiratory rhythm generation (Hilaire et al., 1997), and, when co-activated with 5-HT2AR, it is reportedly involved in the depolarization of PBC neurons (Schwarzacher et al., 2002). In neonatal rats (P8 or younger), 5-HT1AR substantially increases minimal repetitive firing frequency in hypoglossal motoneurons, but the effect was gone at P20 and later (Talley et al., 1997). The expression of 5-HT1AR mRNA in XII peaks at P7 but is strikingly reduced at P14 and thereafter (Talley et al., 1997). The time points between P7 and P14 were not examined. Our findings of a relatively high level of 5-HT1AR expression in XII before P12 and much lower levels after P12 are consistent with their results, but the precipitous fall at P12 was missed in their study. The relatively strong expression of 5-HT1AR before P12 and much weaker expression after P12 in most of the brain stem respiratory nuclei examined in the present study indicate that 5-HT1AR plays a much more important role in modulating respiratory nuclear functions during the first 1 ½ postnatal weeks than after. Significantly, the expression of 5-HT1AR in the cerebellum is relatively high at P2–P8, but becomes markedly lower at P13 and in the adult, a pattern opposite to those in the cerebral cortex and hippocampus (Miquel et al., 1994).
5-HT1BR is the rodent homologue of 5-HT1Dβ receptors in human and other species (Hoyer et al., 1994). It shares some of the same functions as those for 5-HT1AR, such as thermoregulation, feeding, sexual, and emotional behaviors (Zifa and Fillion, 1992). Both 5-HT1A and 5-HT1B receptors are monomers with seven transmembrane domains and are negatively coupled to adenylyl cyclase via G proteins (Hoyer et al., 1994). Presynaptic 5-HT1B autoreceptors in dorsal raphé nuclei (and likely in medullary raphé in the present study) are involved in regulating the firing of serotonergic neurons and inhibit the release of 5-HT from serotonergic terminals (Zifa and Fillion, 1992; Hoyer et al., 1994). Postsynaptic receptors, on the other hand, can have various functions depending on the brain region. Activation of 5-HT1BR inhibits phrenic motoneurons (Di Pasquale et al., 1997) and XII motoneurons (Singer et al., 1996). This effect is opposite that of 5-HT1AR (see above). Thus, the 1A and 1B receptors may act in concert to balance each other’s actions. Indeed, the expression of 5-HT1BR is also relatively high in the first postnatal week (except for P2–P4) until P11, and falls significantly at P12 and thereafter in the PBC, Amb, RM, ROb, RP, and RTN/pFRG, just as in the case of 5-HT1AR. The expression of 5-HT1BR in XII is maintained at a relatively constant level throughout the first 3 postnatal weeks, indicating the importance of preserving its modulatory role even when 5-HT1AR expression is reduced. These results are consistent with those of Kubin and Volgin (2008), whereby the level of 5-HT1BR proteins in XII is quite constant from neonate to P60. The transient decrease in 5-HT1BR mRNA at P12–14 (Volgin et al., 2003) is matched in our study by a slight and statistically insignificant decrease in 5-HT1BR-ir at P12–P13. Overall, the influence of 5-HT1BR predominates over that of 5-HT1AR from P12 onward in XII. A constant level of 5-HT1BR expression is also observed in NTSVL, NTSCOM, and CN, strongly suggesting that this receptor plays an important role in the normal functioning of these nuclei through the first three weeks of postnatal development.
5-HT2AR is also involved in cardiorespiratory, motor, viscerosensory, sexual, and arousal functions (Zifa and Fillion, 1992). Activation of this receptor induces depolarization in the PBC neurons in neonatal rats (Schwarzacher et al., 2002). As in the case of 5-HT1AR, 5-HT2AR increases phasic and tonic genioglossus muscle activity (Besnard et al., 2007) and modulates respiratory rhythm generation (Peña and Ramirez, 2002). In addition, 5-HT2AR plays an important role in respiratory long-term facilitation (Baker-Herman and Mitchell, 2002) and gasping (Tryba et al, 2006). These critical functions may explain the maintained high levels of 5-HT2AR expression during the first three postnatal weeks in almost all of the brain stem nuclei that we have examined. A notable finding, however, is the precipitous and significant fall in 5-HT2AR immunoreactivity at P12 in the PBC, Amb, and XII (Liu and Wong-Riley, 2008), indicating that in these vital respiratory nuclei, serotonergic transmission via 5-HT2AR is down-regulated within a very narrow window (see Discussion below). This fall at P12 was not reported for 5-HT2AR mRNA (Volgin et al., 2003), but a dip in message was seen in their study at P14. Notable also is the prominent though statistically non-significant dip at P12 in RM, RP, and RTN/pFRG, as well as the lower expression at P2–P4 in all of the brain stem nuclei examined in the present study and in our recent report (Liu and Wong-Riley, 2008)
The presence of 5-HT1AR, 5-HT1BR, and 5-HT2AR immunoreactivity in a large number of neurons in each brain stem nucleus examined indicates that a sizeable population of neurons express two, if not all three, of the 5-HT receptors. Such colocalization of receptors is likely to ensure a proper balance between the excitatory and inhibitory effects of serotonin. For example, genioglossus motoneurons are excited by 5-HT1AR and/or 5-HT2AR, but they are inhibited by 5-HT1BR activation (Besnard et al., 2007). Likewise, phrenic motoneurons are activated by 5-HT1AR but inhibited by 5-HT1BR (Di Pasquale et al., 1997; Valic et al., 2008). The combined effects on individual brain stem neurons await future investigation. It is known, for example, that both 1A and 2A receptors are involved in depolarizing PBC neurons (Schwarzacher et al., 2002), and so they will have a synergistic effect. As a “level-setting” system (Holstege, 2009), 5-HT no doubt exerts a modulating influence on many functions, including cardiovascular and respiratory control.
The major finding of the present study is a consistent reduction in the expression of 5-HT1AR and 5-HT1BR at P12 that is statistically significant for RM, ROb, RP, PBC, Amb, and, in the case of XII, for 5-HT1AR. The expression of 5-HT2AR is also significantly reduced at P12 in PBC, Amb, and XII (Liu and Wong-Riley, 2008). P12 is the only time point in the entire first 3 postnatal weeks that a day-to-day change in receptor immunoreactivity reaches statistical significance. Even for those nuclei that did not show significance, the level of labeling is notably lower at P12 or P12–P13 (such as 5-HT1AR in RTN/pFRG; 5-HT1BR in XII, RTN/pFRG, NTSVL, and NTSCOM; and 5-HT2AR in RP and RTN/pFRG). This widespread reduction in 5-HT receptor expression would attenuate the ‘net stimulatory effect’ of 5-HT on respiratory output reported by a number of investigators (Lindsay and Feldman, 1993; Richerson, 2004; Hodegs and Richerson, 2008) and disrupt the normal interaction of serotonergic system with other neurotransmitter and neuromodulator systems, with potential adverse effect on respiratory control. Multiple nuclei in the respiratory network are affected. The PBC is one of the major centers of respiratory rhythmogenesis (Smith et al., 1991; Rekling and Feldman, 1998; Smith et al., 2000). The Amb and XII controls upper airway muscles and the tongue, respectively, that are associated with breathing and airway patency (Jordan, 2001; Sawczuk and Mosier, 2001). The NTSCOM and NTSVL receive peripheral chemosensitive afferents (Finley and Katz, 1992) and are involved in respiratory modulation (Paton et al., 1991; Bonham, 1995). The RTN/pFRG are engaged in central chemosensitivity (Guyenet et al., 2005; Richerson et al., 2005), peripheral chemosensitivity (Takakura et al., 2006), as well as a reported site for respiratory rhythmogenesis (Onimaru and Homma, 2003; Feldman and Del Negro, 2006; Janczewski and Feldman, 2006). The RM, ROb, and RP serotonergic neurons contribute to respiratory regulation (Richerson et al., 2005) and reportedly central chemosensitivity (Richerson, 2004; Richerson et al., 2005). The present study documents for the first time that all of these vital respiratory nuclei have reduced 5-HT receptor expression during the critical period, though to different extent in different nuclei, under normal conditions. This is significant in view of reports that the expression and/or binding of 5-HT receptors (including 5-HT1AR and 5-HT2AR) were notably reduced in several brain stem nuclei (such as the arcuate nucleus (or RTN) , ROb, and NTS) in SIDS (Panigrahy et al., 2000; Ozawa and Okado, 2002; Paterson et al., 2006). We propose that during the critical period (toward the end of the second postnatal week in rats), reduced 5-HT receptor expression results in compromised respiratory regulation in brain stem respiratory network. Together with the other neurochemical, metabolic, and physiological changes that we have observed in normal animals (reviewed in Wong-Riley and Liu, 2005, 2008), decreased 5-HT transmission renders the animals less capable of responding adequately to exogenous respiratory insults, such as hypoxia or hypercapnia. The insult would be more severe during sleep, when respiratory activities are normally depressed (Olson and Simon, 1996) and firing of serotonergic neurons is reduced (Veasey et al., 1996), thereby threatening airway patency (Horner, 2007)). Of relevance is the apparent correspondence in brain delopmental time scale between rodent postnatal days P11–14 (encompassing our critical period) and human postnatal 2 to 4 months (the peak incidence of SIDS) (Ballanyi, 2004). Whether the “abnormal” expression of the serotonergic system in SIDS infants actually reflects a normal phenomenon during postnatal development or whether it represents additional serotonergic defects in these victims awaits future investigations.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by NIH grant HD048954 and a grant from the Children's Hospital and Health System Foundation, Milwaukee, Wisconsin. It’s a pleasure to thank Dr. S. Shar for her helpful discussions.
Abbreviations
- 5-HT
serotonin
- XII
hypoglossal nucleus
- Amb
nucleus ambiguus
- ANOVA
analysis of variance
- APB
ammonium phosphate buffer
- CN
cuneate nucleus
- GABA
gamma-aminobutyric acid
- -ir
immunoreactive
- NMDA
N-methyl-D-aspartate
- NTS
solitary tract nucleus
- NTSCOM
commissural subnucleus of solitary tract nucleus
- NTSVL
ventrolateral subnucleus of solitary tract nucleus
- P
postnatal day
- PBC
pre-Bötzinger complex
- PBS
sodium phosphate buffered saline
- pFRG
parafacial respiratory group
- R
receptor
- RM
nucleus raphé magnus
- ROb
nucleus raphé obscurus
- RP
nucleus raphé pallidus
- RTN
retrotrapezoid nucleus
- SIDS
Sudden Infant Death Syndrome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Azmitia EC, Gannon PJ, Kheck NM, Whitaker-Azmitia PM. Cellular localization of the 5-HT1A receptor in primate brain neurons and glial cells. Neuropsychopharmacology. 1996;14:35–46. doi: 10.1016/S0893-133X(96)80057-1. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanyi K. Neuromodulation of the perinatal respiratory network. Curr Neuropharm. 2004;2:221–243. doi: 10.2174/1570159043476828. [DOI] [PubMed] [Google Scholar]
- Besnard S, Massé F, Verdaguer M, Cappelin B, Meurice JC, Gestreau C. Time-and dose-related effects of three 5-HT receptor ligands on the genioglossus activity in anesthetized and conscious rats. Sleep Breath. 2007;11:275–284. doi: 10.1007/s11325-007-0107-0. [DOI] [PubMed] [Google Scholar]
- Bieger D, Hopkins DA. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J Comp Neurol. 1987;262:546–562. doi: 10.1002/cne.902620408. [DOI] [PubMed] [Google Scholar]
- Bonham AC. Neurotransmitters in the CNS control of breathing. Respir Physiol. 1995;101:219–230. doi: 10.1016/0034-5687(95)00045-f. [DOI] [PubMed] [Google Scholar]
- Carruthers AM, Sellers LA, Jenkins DW, Jarvie EM, Feniuk W, Humphrey PPA. Adenosine A1 receptor-mediated inhibition of protein kinase A-induced calcitonin gene-related peptide release from rat trigeminal neurons. Mol Pharmacol. 2001;59:1533–1541. doi: 10.1124/mol.59.6.1533. [DOI] [PubMed] [Google Scholar]
- Clark MS, Sexton TJ, McClain M, Root D, Kohen R, Neumaier JF. Overexpression of 5-HT1B receptor in dorsal raphé nucleus using Herpes Simplex virus gene transfer increases anxiety behavior after inescapable stress. J Neurosci. 2002;22:4550–4562. doi: 10.1523/JNEUROSCI.22-11-04550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin M, Bäckberg M, Önnestam K, Meister B. 5-HT1A receptor immunoreactivity in hypothalamic neurons involved in body weight control. NeuroReport. 2002;13:945–951. doi: 10.1097/00001756-200205240-00009. [DOI] [PubMed] [Google Scholar]
- Connelly CA, Ellenberger HH, Feldman JL. Are there serotonergic projections from raphé and retrotrapezoid nuclei to the ventral respiratory group in the rat? Neurosci Lett. 1989;105:34–40. doi: 10.1016/0304-3940(89)90007-4. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Lindsay A, Feldman J, Monteau R, Hilaire G. Serotonergic inhibition of phrenic motoneuron activity: an in vitro study in neonatal rat. Neurosci Lett. 1997;230:29–32. doi: 10.1016/s0304-3940(97)00469-2. [DOI] [PubMed] [Google Scholar]
- Siddiqui EJ, Shabbir MA, Mikhailidis DP, Mumtaz FH, Thompson CS. The effect of serotonin and serotonin antagonists on bladder cancer cell proliferation. BJU. Int. 2006;97:634–639. doi: 10.1111/j.1464-410X.2006.06056.x. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley JCW, Katz DM. The central organization of carotid body afferent projection to the brain stem of the rat. Brain Res. 1992;572:108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA, Mulkey DK. Retrotrapezoid nucleus: a litmus test for the identification of central chemoreceptors. Exp Physiol. 2005;90:247–257. doi: 10.1113/expphysiol.2004.029637. [DOI] [PubMed] [Google Scholar]
- Halliday G, Harding A, Paxinos G. Serotonin and tachykinin systems. In: Paxinos G, editor. The Rat Nervous System. Sidney: Academic Press; 1995. pp. 929–974. [Google Scholar]
- Haxhiu MA, Erokwu B, Bhardwaj V, Dreshaj IA. The role of the medullary raphé nuclei in regulation of cholinergic outflow to the airways. J Auton Nerv Syst. 1998;69:64–71. doi: 10.1016/s0165-1838(98)00009-5. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Duron B. Maturation of the mammalian respiratory system. Physiol Rev. 1999;79:325–360. doi: 10.1152/physrev.1999.79.2.325. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Morin D, Lajard AM, Monteau R. Changes in serotonin metabolism may elicit obstructive apnoea in the newborn rat. J Physiol. 1993;466:367–381. [PMC free article] [PubMed] [Google Scholar]
- Hilaire G, Bou C, Monteau R. Serotonergic modulation of central respiratory activity in the neonatal mouse: an in vitro study. Eur J Pharmacol. 1997;329:115–120. [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Contributions of 5-HT neurons to respiratory control: Neuromodulatory and trophic effects. Respir Physiol Neurobiol. 2008;164:222–232. doi: 10.1016/j.resp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege G. The mesopontine rostromedial tegmental nucleus and the emotional motor system: role in basic survival behavior. J Comp Neurol. 2009;513:559–565. doi: 10.1002/cne.21990. [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr, Norman WP, Skirboll L, Dretchen KL, Cuello C, Visser TJ, Hökfelt T, Gillis RA. Evidence for 5-hydroxytryptamine, substance P, and thyrotropin-releasing hormone in neurons innervating the phrenic motor nucleus. J Neurosci. 1984;4:1064–1071. doi: 10.1523/JNEUROSCI.04-04-01064.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner RL. Respiratory motor activity: influence of neuromodulators and implications for sleep disordered breathing. Can J Physiol Pharmacol. 2007;85:155–165. doi: 10.1139/y06-089. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Huo FQ, Chen T, Lv BC, Wang J, Zhang T, Qu CL, Li YQ, Tang JS. Synaptic connections between GABAergic elements and serotonergic terminals or projecting neurons in the ventrolateral orbital cortex. Cerebral Cortex. 2009;19:1263–1272. doi: 10.1093/cercor/bhn169. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Serotonin and behavior. A general hypothesis. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the Fourth Generation of Progress. New York: Raven Press; 1995. pp. 461–469. [Google Scholar]
- Jacobs C, Van Den Broeck W, Simoens P. Neurons expressing serotonin-1B receptor in the basolateral nuclear group of the amygdala in normally behaving and aggressive dogs. Brain Res. 2007;1136:102–109. doi: 10.1016/j.brainres.2006.11.096. [DOI] [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol. 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan D. Central nervous pathways and control of the airways. Respir Physiol. 2001;125:67–81. doi: 10.1016/s0034-5687(00)00205-x. [DOI] [PubMed] [Google Scholar]
- Kinney HC. Abnormalities of the brainstem serotonergic system in the sudden infant death syndrome: a review. Pediatr Dev Pathol. 2005;8:507–524. doi: 10.1007/s10024-005-0067-y. [DOI] [PubMed] [Google Scholar]
- Kubin L, Volgin DV. Developmental profiles of neurotransmitter receptors in respiratory motor nuclei. Respir Physiol Neurobiol. 2008;164:64–71. doi: 10.1016/j.resp.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leysen JE. 5-HT2 receptors. Curr Drug Targets. 2004;3:11–26. doi: 10.2174/1568007043482598. [DOI] [PubMed] [Google Scholar]
- Li TS, Furutani A, Takahashi M, Ohshima M, Qin SL, Kobayashi T, Ito H, Hamano K. Imparied potency of bone marrow mononuclear cells for inducing therapeutic angiogenesis in obese diabetic rats. Am J Physiol Heart Circ Physiol. 2006;290:H1362–H1369. doi: 10.1152/ajpheart.00766.2005. [DOI] [PubMed] [Google Scholar]
- Lindsay AD, Feldman JL. Modulation of respiratory activity of neonatal rat phrenic motoneurones by serotonin. J Physiol. 1993;461:213–233. doi: 10.1113/jphysiol.1993.sp019510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT. Postnatal expression of neurotransmitters, receptors, and cytochrome oxidase in the rat pre-Bötzinger complex. J Appl Physiol. 2002;92:923–934. doi: 10.1152/japplphysiol.00977.2001. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT. Postnatal changes in cytochrome oxidase expressions in brain stem nuclei of rats: implications for sensitive periods. J Appl Physiol. 2003;95:2285–2291. doi: 10.1152/japplphysiol.00638.2003. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT. Postnatal developmental expressions of neurotransmitters and receptors in various brain stem nuclei of rats. J Appl Physiol. 2005;98:1442–1457. doi: 10.1152/japplphysiol.01301.2004. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Postnatal changes in the expression of serotonin 2A receptors in various brain stem nuclei of the rat. J Appl Physio. 2008;104:1801–1808. doi: 10.1152/japplphysiol.00057.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Lowry TF, Wong-Riley MT. Postnatal changes in ventilation during normoxia and acute hypoxia in the rat: implication for a sensitive period. J Physiol. 2006;577:957–970. doi: 10.1113/jphysiol.2006.121970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Fehring C, Lowry TF, Wong-Riley MT. Postnatal development of metabolic rate during normoxia and acute hypoxia in rats: implication for a sensitive period. J Appl Physiol. 2009;106:1212–1222. doi: 10.1152/japplphysiol.90949.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarenko IG, Meguid MM, Ugrumov MV. Distribution of serotonin 5-hydroxytriptamine 1B (5-HT(1B)) receptors in the normal rat hypothalamus. Neurosci Lett. 2002;328:155–159. doi: 10.1016/s0304-3940(02)00345-2. [DOI] [PubMed] [Google Scholar]
- Makarenko IG, Meguid MM, Gatto L, Chen C, Ramos EJ, Goncalves CG, Ugrumov MV. Normalization of hypothalamic serotonin (5-HT 1B) receptor and NPY in cancer anorexia after tumor resection: an immunocytochemical study. Neurosci Lett. 2005;383:322–327. doi: 10.1016/j.neulet.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Manaker S, Verderame HM. Organization of serotonin 1A and 1B receptors in the nucleus of the solitary tract. J Comp Neurol. 1990;301:535–553. doi: 10.1002/cne.903010405. [DOI] [PubMed] [Google Scholar]
- Manaker S, Tischler LJ. Origin of serotoninergic afferents to the hypoglossal nucleus in the rat. J Comp Neurol. 1993;334:466–476. doi: 10.1002/cne.903340310. [DOI] [PubMed] [Google Scholar]
- Miquel MC, Kia HK, Boni C, Doucet E, Daval G, Matthiessen L, Hamon M, Vergé D. Postnatal development and localization of 5-HT1A receptor mRNA in rat forebrain and cerebellum. Brain Res Dev Brain Res. 1994;80:149–157. doi: 10.1016/0165-3806(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Narita N, Narita M, Takashima S, Nakayama M, Nagai T, Okado N. Serotonin transporter gene variation is a risk factor for sudden infant death syndrome in the Japanese population. Pediatrics. 2001;107:690–692. doi: 10.1542/peds.107.4.690. [DOI] [PubMed] [Google Scholar]
- Nucci TB, Branco LG, Gargaglioni LH. 5-HT1A, but not 5-HT2 and 5-HT7, receptors in the nucleus raphé magnus modulate hypoxia-induced hyperpnoea. Acta Physiol (Oxf) 2008;193:403–414. doi: 10.1111/j.1748-1716.2008.01853.x. [DOI] [PubMed] [Google Scholar]
- Olson EJ, Simon PM. Sleep-wake cycles and the management of respiratory failure. Curr Opin Pulm Med. 1996;2:500–506. [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa Y, Okado N. Alteration of serotonergic receptors in the brain stems of human patients with respiratory disorders. Neuropediatrics. 2002;33:142–149. doi: 10.1055/s-2002-33678. [DOI] [PubMed] [Google Scholar]
- Panigrahy A, Filiano J, Sleeper LA, Mandell F, Valdes-Dapena M, Krous HF, Rava LA, Foley E, White WF, Kinney HC. Decreased serotonergic receptor binding in rhombic lip-derived regions of the medulla oblongata in the sudden infant death syndrome. J Neuropathol Exp Neurol. 2000;59:377–384. doi: 10.1093/jnen/59.5.377. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA. 2006;296:2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- Paton JF, Rogers WT, Schwaber JS. Tonically rhythmic neurons within a cardiorespiratory region of the nucleus tractus solitarii of the rat. J Neurophysiol. 1991;66:824–838. doi: 10.1152/jn.1991.66.3.824. [DOI] [PubMed] [Google Scholar]
- Peddie CJ, Davies HA, Colyer FM, Stewart MG, Rodríguez JJ. Dendritic colocalisation of serotonin1B receptors and the glutamate NMDA receptor subunit NR1 within the hippocampal dentate gyrus: an ultrastructural study. J Chem Neuroanat. 2008;36:17–26. doi: 10.1016/j.jchemneu.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Peña F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penatti EM, Berniker AV, Kereshi B, Cafaro C, Kelly ML, Niblock MM, Gao HG, Kinney HC, Li A, Nattie EE. Ventilatory response to hypercapnia and hypoxia after extensive lesion of medullary serotonergic neurons in newborn conscious piglets. J Appl Physiol. 2006;101:1177–1188. doi: 10.1152/japplphysiol.00376.2006. [DOI] [PubMed] [Google Scholar]
- Raul L. Serotonin2 receptors in the nucleus tractus solitarius: characterization and role in the baroreceptor reflex arc. Cell Mol Neurobiol. 2003;23:709–726. doi: 10.1023/A:1025096718559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Feldman JL. Pre-Bötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417:181–194. [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Hodges MR, Dohle CI, Diez-Sampedro A. Homing in on the specific phenotype(s) of central respiratory chemoreceptors. Exp Physiol. 2005;90:259–269. doi: 10.1113/expphysiol.2005.029843. [DOI] [PubMed] [Google Scholar]
- Richter DW, Manzke T, Wilken B, Ponimaskin E. Serotonin receptors: guardians of stable breathing. Trends Mol Med. 2003;9:542–548. doi: 10.1016/j.molmed.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev. 2004;28:565–582. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Sari Y, Lefèvre K, Bancila M, Quignon M, Miquel MC, Langlois X, Hamon M, Vergé D. Light and electron microscopic immunocytochemical visualization of 5-HT1B receptors in the rat brain. Brain Res. 1997;760:281–286. doi: 10.1016/s0006-8993(97)00400-9. [DOI] [PubMed] [Google Scholar]
- Sari Y, Miquel MC, Brisorgueil MJ, Ruiz G, Doucet E, Hamon M, Vergé D. Cellular and subcellular localization of 5-hydroxytryptamine1B receptors in the rat central nervous system: immunocytochemical, autoradiographic and lesion studies. Neuroscience. 1999;88:899–915. doi: 10.1016/s0306-4522(98)00256-5. [DOI] [PubMed] [Google Scholar]
- Sawczuk A, Mosier KM. Neural control of tongue movement with respect to respiration and swallowing. Crit Rev Oral Biol Med. 2001;12:18–37. doi: 10.1177/10454411010120010101. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Pestean A, Günther S, Ballanyi K. Serotonergic modulation of respiratory motoneurons and interneurons in brainstem slices of perinatal rats. Neurosci. 2002;115:1247–1259. doi: 10.1016/s0306-4522(02)00540-7. [DOI] [PubMed] [Google Scholar]
- Singer JH, Bellingham MC, Berger AJ. Presynaptic inhibition of glutamatergic synaptic transmission to rat motoneurons by serotonin. J Neurophysiol. 1996;76:799–807. doi: 10.1152/jn.1996.76.2.799. [DOI] [PubMed] [Google Scholar]
- Smith D, Shaw D, Hopkins R, McAllister G, Hill R, Sirinathsinghji D, Longmore J. Development and characterisation of human 5-HT1B-or 5-HT1D-receptor specific antibodies as unique research tools. J Neurosci Methods. 1998;80:155–161. doi: 10.1016/s0165-0270(97)00209-4. [DOI] [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brain stem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Butera RJ, Koshiya N, Negro CD, Wilson CG, Johnson SM. Respiratory rhythm generation in neonatal and adult mammals: the hybrid pacemaker-network model. Respir Physiol. 2000;122:131–147. doi: 10.1016/s0034-5687(00)00155-9. [DOI] [PubMed] [Google Scholar]
- Sotelo C, Cholley B, El Mestikawy S, Gozlan H, Hamon M. Direct immunohistochemical evidence of the existence of 5-HT1A autoreceptors on serotoninergic neurons in the midbrain raphe nuclei. Eur J Neurosci. 1990;2:1144–1154. doi: 10.1111/j.1460-9568.1990.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neurosci. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Sadr NN, Bayliss DA. Postnatal development of serotonergic innervation, 5-HT1A receptor expression, and 5-HT responses in rat motoneurons. J Neurosci. 1997;17:4473–4485. doi: 10.1523/JNEUROSCI.17-11-04473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Medullary serotonergic neurons modulate the ventilatory response to hypercapnia but not hypoxia in conscious rats. J Physiol. 2005;566:543–557. doi: 10.1113/jphysiol.2005.083873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YD, Bingaman M, Taveira-DaSilva AM, Pace PP, Gillis RA, Wrathall JR. Serotonin 1A receptor agonists reverse respiratory abnormalities in spinal cord-injured rats. J Neurosci. 2003;23:4182–4189. doi: 10.1523/JNEUROSCI.23-10-04182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor KB, Helke CJ. Serotonin and substance P colocalization in medullary projections to the nucleus tractus solitarius: dual-colour immunohistochemistry combined with retrograde tracing. J Chem Neuroanat. 1989;2:139–148. [PubMed] [Google Scholar]
- Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci. 2006;26:2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valic M, Pecotic R, Dogas Z. Phrenic nerve activity is enhanced by 5-HT1A receptor agonist 8-OH-DPAT in spontaneously breathing anesthetized rats. J Physiol Pharmacol. 2008;59:17–25. [PubMed] [Google Scholar]
- Veasey SC, Panckeri KA, Hoffman EA, Pack AI, Hendricks JC. The effects of serotonin antagonists in an animal model of sleep-disordered breathing. Am J Respir Crit Care Med. 1996;153:776–786. doi: 10.1164/ajrccm.153.2.8564132. [DOI] [PubMed] [Google Scholar]
- Voigt MM, Laurie DJ, Seeburg PH, Bach A. Molecular cloning and characterization of a rat brain cDNA encoding a 5-hydroxytryptamine1B receptor. EMBO J. 1991;10:4017–4023. doi: 10.1002/j.1460-2075.1991.tb04977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgin DV, Fay R, Kubin L. Postnatal development of serotonin 1B, 2 A and 2C receptors in brainstem motoneurons. Eur J Neurosci. 2003;17:1179–1188. doi: 10.1046/j.1460-9568.2003.02545.x. [DOI] [PubMed] [Google Scholar]
- Voss MD, De Castro D, Lipski J, Pilowsky PM, Jiang C. Serotonin immunoreactive boutons form close appositions with respiratory neurons of the dorsal respiratory group in the cat. J Comp Neurol. 1990;295:208–218. doi: 10.1002/cne.902950205. [DOI] [PubMed] [Google Scholar]
- Wang X, Dergacheva O, Kamendi H, Gorini C, Mendelowitz D. 5-Hydroxytryptamine 1A/7 and 4alpha receptors differentially prevent opioid-induced inhibition of brain stem cardiorespiratory function. Hypertension. 2007;50:368–376. doi: 10.1161/HYPERTENSIONAHA.107.091033. [DOI] [PubMed] [Google Scholar]
- Wilken B, Lalley P, Bischoff AM, Christen HJ, Behnke J, Hanefeld F, Richter DW. Treatment of apneustic respiratory disturbance with a serotonin-receptor agonist. J Pediatr. 1997;130:89–94. doi: 10.1016/s0022-3476(97)70315-9. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT, Liu Q. Neurochemical development of brain stem nuclei involved in the control of respiration. Respir Physiol Neurobiol. 2005;149:83–98. doi: 10.1016/j.resp.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT, Liu Q. Neurochemical and physiological correlates of a critical period of respiratory development in the rat. Respir Physiol Neurobiol. 2008;164:28–37. doi: 10.1016/j.resp.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- Xie H, Ma F, Zhang YQ, Gao X, Wu GC. Expression of 5-HT(2A) receptor mRNA in some nuclei of brain stem enhanced in monoarthritic rats. Brain Res. 2002;954:94–99. doi: 10.1016/s0006-8993(02)03347-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gray TS, D’Souza DN, Carrasco GA, Damjanoska KJ, Dudas B, Garcia F, Zainelli GM, Hanley NR, Battaglia G, Muma NA, Van de Kar LD. Desensitization of 5-HT1A receptors by 5-HT2A receptors in neuroendocrine neurons in vivo. J Pharmacol Exp Ther. 2004;310:59–66. doi: 10.1124/jpet.103.062224. [DOI] [PubMed] [Google Scholar]
- Zifa E, Fillion G. 5-Hydroxytryptamine receptors. Pharmacol Rev. 1992;44:401–458. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.