Abstract
Physiological hypoxia results in a host of responses which include increased ventilation, constriction of the pulmonary artery, and a cellular transcriptional program which promotes glycolysis, angiogenesis, and erythropoiesis. Mitochondria are the primary consumers of cellular oxygen and have thus been speculated for years to be the site of cellular oxygen sensing. Many of the cellular responses to hypoxia are now known to be mediated by the production of reactive oxygen species at mitochondrial complex III. While the mechanism by which cytosolic oxidant concentration is increased during hypoxia is unknown, the importance of the maintenance of cellular oxygen supply requires further investigation into the role of ROS as hypoxia signaling molecules. The following is a brief overview of the current understanding of the role of mitochondrial-produced ROS in cellular oxygen signaling.
Introduction
Most eukaryotic cells utilize oxidative phosphorylation for the maintenance of cellular ATP stores. The importance of oxygen as the terminal electron acceptor of the electron transport chain has led to the evolution of multiple mechanisms by which cells and organisms maintain an adequate supply of oxygen. Acute exposure of mammals to hypoxic environments results in the calcium-dependent constriction of pulmonary arteries, allowing for increased blood oxygen perfusion [1]. Prolonged hypoxia stimulates multiple cell types and induces the Hypoxia Inducible Factors (HIFs) which mediate transcription of a large number of hypoxia-sensitive genes, including the production of erythropoietin in the kidney [2]. Although the importance of the hypoxic response cannot be understated, the mechanism by which cells detect oxygen levels to initiate the hypoxic response remains a subject of debate. Mitochondria are the largest consumers of cellular O2 and as such are likely candidates for the location of the cellular oxygen sensor [3]. Significant evidence has accumulated supporting the role of mitochondria as putative oxygen sensors; however, as a mitochondrial signal could involve cellular energy state, cytosolic redox state, or mitochondrial production of reactive oxygen species (ROS), the nature of the hypoxic signal provided by mitochondria is a contentious point. This review focuses on the role of mitochondrial-generated ROS in the propagation of the hypoxic response through calcium-mediated pulmonary artery constriction and through HIF-mediated transcription.
The mitochondrial electron transport chain generates reactive oxygen species
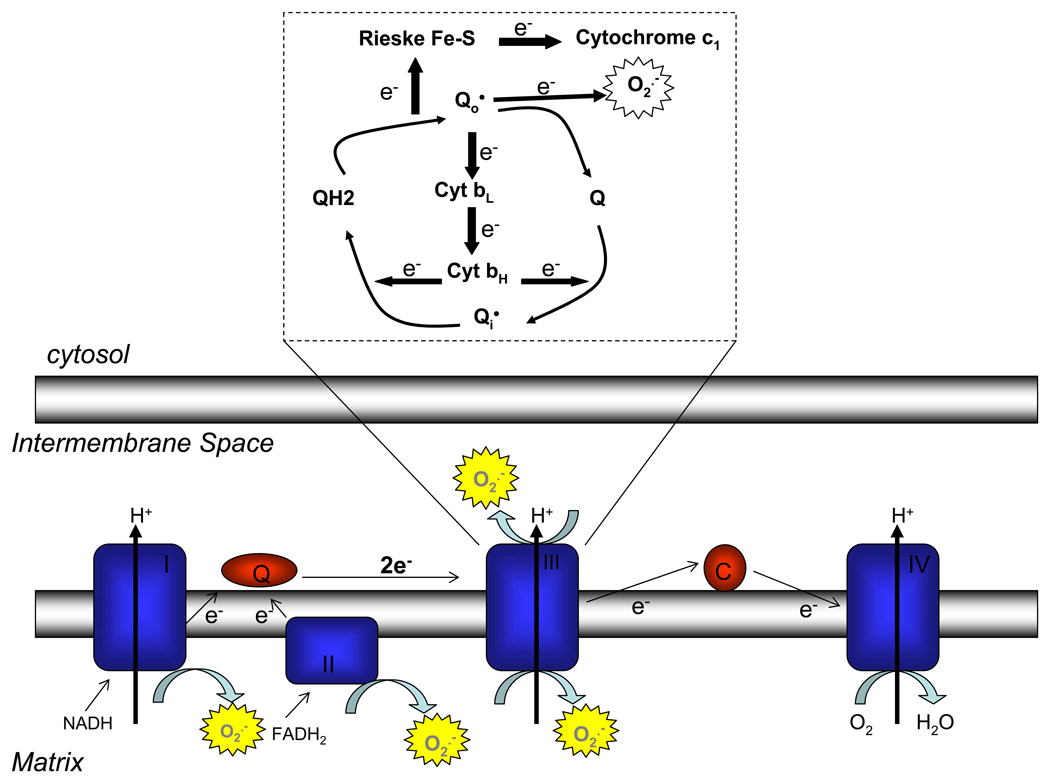
During mitochondrial respiration, electrons from NADH and FADH2 are transferred to mitochondrial complex I and complex II respectively. These electrons are then shuttled through mitochondrial complex III, and on to the final electron acceptor, oxygen, which is reduced to water at cytochrome oxidase (complex IV). The actions of mitochondrial complexes I, III, and IV result in a proton gradient, the free energy of which is used to drive ATP synthesis. It is estimated that 2–3% of O2 consumed by mitochondria is incompletely reduced, yielding reactive oxygen species (ROS) [4]. ROS are produced by the electron transport chain at complexes I, II, and III (Figure 1). While complexes I and II produce ROS only into the matrix, complex III is capable of producing ROS on both sides of the mitochondrial inner membrane [4,5]. ROS produced into the intermembrane space theoretically has an easier route to the cytosol to act as signaling molecules than do ROS produced into the matrix [6].
Figure 1. Mitochondrial generation of reactive oxygen species.
Mitochondrial complexes I, II, and III produce superoxide. While complexes I and II only produce superoxide into the mitochondrial matrix, complex III can produce superoxide on both sides of the mitochondrial inner membrane in a process termed the Q-cycle. Complexes I and II donate two electrons to coenzyme Q, forming ubiquinol. At complex III, the first of these electrons is transferred by the Rieske iron-sulfur protein (RISP) to cytochrome c1, leaving the radical ubisemiquinone. Subsequently, ubisemiquinone transfers the second electron to cytochrome b. Ubisemiquinone formed at the Qo site can donate its electron directly to oxygen, producing superoxide.
ROS are produced at complex III of the electron transport chain via formation of ubisemiquinone, a radical form of coenzyme Q. Mitochondrial complexes I and II donate two electrons to ubiquinone producing the reduced form, ubiquinol. Ubiquinol is oxidized at the Qo site of mitochondrial complex III in a two step process termed the Q-cycle (Figure 1). First, one electron from ubiquinol is transferred to the Rieske iron-sulfur protein (RISP). This electron is transferred sequentially from RISP to cytochrome c1, cytochrome c, and finally to complex IV. This one electron oxidation of ubiquinone results in the transient formation of ubisemiquinone. The free electron of ubisemiquinone is then transferred by RISP to cytochrome b, and is subsequently used to reduce another molecule of ubiquinone at the Qi site. Ubisemiquinone formed at the Qo site of complex III is capable of donating its free electron directly to oxygen, forming superoxide [7,8]. Superoxide dismutates either spontaneously, or through the action of superoxide dismutase (SOD), to form hydrogen peroxide [4].
While non-charged hydrogen peroxide can diffuse through the outer membrane to access the cytosol, superoxide reaches the cytosol through voltage-dependent anion channels (VDACs) [6]. In the cytosol, oxidation of cysteines remains the best-studied oxidative signaling modification [9]. Oxidation of the cysteine sulfhydryl group has been demonstrated to alter protein-protein interactions, the DNA binding activity of transcription factors, and the catalytic activity of enzymes, including phosphatases involved in signaling cascades [9,10]. Additionally, oxidation of two intra-, or intermolecular cysteines forms disulfide bridges allowing for conformational changes or oligomerization of proteins [11,12].
Hypoxia induces a cellular transcription program through induction of HIFs
One of the best-characterized physiological responses to sustained hypoxia is the induction of the glycoprotein hormone, erythropoietin (Epo), which binds to receptors on erythroid progenitor cells, enabling proliferation and differentiation into red blood cells [13]. Hypoxic induction of Epo was first demonstrated by Semenza and colleagues to be mediated in vitro by activation of the Hypoxia-Inducible transcription Factor HIF-1 [14,15]. HIF-1 is a hetero-dimeric transcription factor consisting of two basic helix-loop-helix/PAS proteins, HIF-1α and HIF-1β [16]. Epo induction in vivo has subsequently been demonstrated to be regulated by a second HIF-α subunit (HIF-2α), and to date, three HIF-α subunits have been identified (HIF-3α being the third). The single β-subunit, HIF-1β is constitutively present in the cell while HIF-α subunits are only present during hypoxia due to normoxic protein degradation through ubiquitin-mediated proteosomal degradation [17–19]. In addition to Epo, HIFs regulate the hypoxia-induced expression of glycolytic, angiogenic, cell cycle regulatory, and survival genes [20,21].
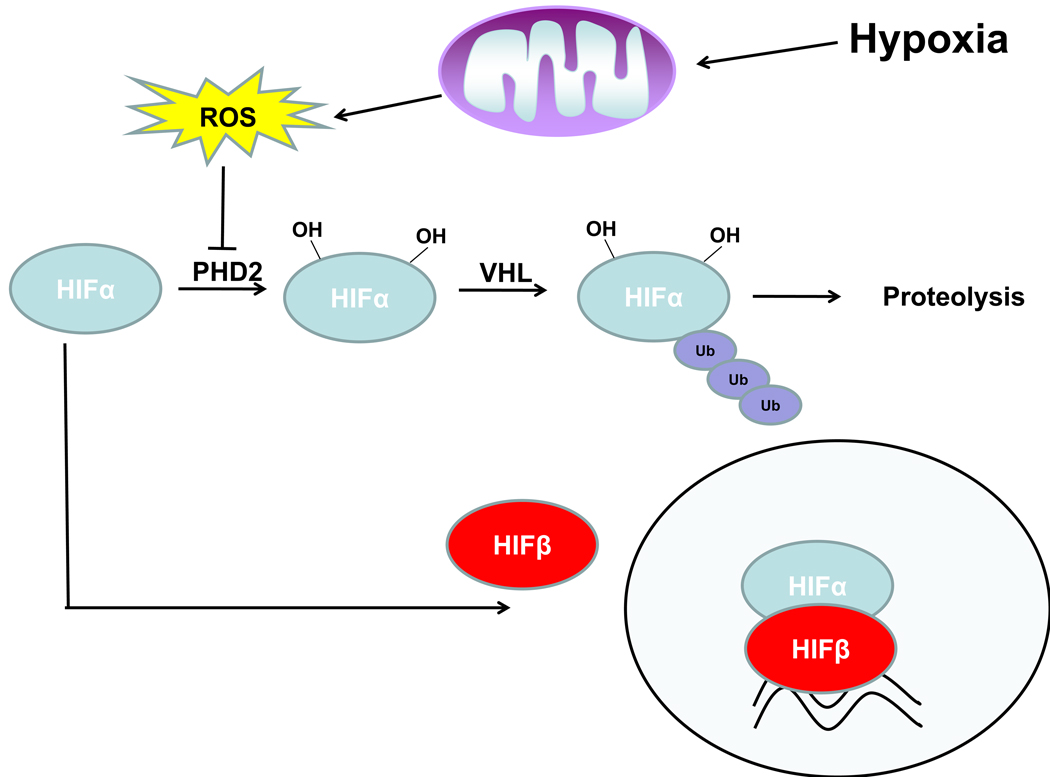
The mechanism of normoxic HIF-1 turnover was elucidated when it was demonstrated that hypoxia inhibits the interaction of HIF-1α with the von Hippel-Lindau (VHL) tumor suppressor protein and that VHL mediates the oxygen-dependent instability of HIF-1α (Figure 2) [22,23]. It was subsequently demonstrated that proline-directed hydroxylation of HIF-1α during normoxia targets HIF-1α for recognition by VHL and subsequent degradation [24,25]. This hydroxylation reaction is carried out by a family of 2-oxoglutarate-dependent dioxygenases termed prolyl hydroxylases 1, 2, and 3 (PHD1–3) [26,27]. In the hydroxylation reaction, PHDs catalyze the splitting of molecular oxygen, donating one atom to HIF prolines and the other to 2-oxoglutarate, forming succinate and CO2. Ferrous iron is used as a cofactor in the reaction and thus, the hydroxylation reaction is inhibited by the iron chelator desferrioxamine (DFO). As the hydroxylation reaction requires oxygen, it was widely speculated that PHDs act as the direct oxygen sensors of the HIF pathway [28].
Figure 2. Hypoxia-induced mitochondrial ROS inhibit HIFα subunit turnover.
Under normoxic conditions, HIFα subunits are hydroxylated on prolines by prolyl hydroxylases (PHDs). Hydroxylation tags HIFα for recognition by the von Hippel Lindau (VHL) tumor suppressor leading to ubiquitination and degradation of HIFα. During hypoxia, mitochondrial production of ROS inhibits the activity of PHDs allowing for stabilization of HIFα subunits and HIF-mediated transcription.
Mitochondrial ROS are required for hypoxic activation of HIFs
The first breakthrough suggesting a mitochondrial oxygen sensor came with the discovery that ρ0 Hep3B cells, which contain no mitochondrial DNA and thus no electron transport, are incapable of HIF-1 DNA binding activity and erythropoietin expression following hypoxia [29]. The observation that antioxidants abolish the hypoxic HIF response suggested that ROS generation at mitochondria is responsible for propagation of the hypoxic signal [29]. Correspondingly, treatment of cells with exogenous H2O2, treatment of cells with growth factors which induce H2O2 production, or cellular mutations which lead to H2O2 accumulation, are sufficient to stabilize HIF-1α during normoxia [30–32]. Further use of mitochondrial inhibitors suggested that ROS generation at mitochondrial complex III is critical for hypoxia signaling as complex I inhibitors (rotenone, MPTP) and Qo site inhibitors (myxothiazol, stigmatellin) abolish hypoxic HIF-1α induction, but the Qi site inhibitor antimycin A does not [29,30,33]. The use of pharmacological agents does not however prevent HIF stabilization in response to DFO, demonstrating that the HIF-1 pathway downstream of PHDs is intact. The results of these pharmalogical experiments were subsequently corroborated using shRNA or genetic targeting of mitochondrial proteins. Targeting of the Rieske iron-sulfur protein (RISP) using shRNAs prevents electron transfer from ubiquinol, inhibiting ubisemiquinone formation and hypoxic HIF-1 stabilization [34,35]. Similarly, embryonic stem cells lacking cytochrome c fail to stabilize HIF-1 during hypoxia as loss of cytochrome c leads to a complete reduction of cytochrome c1 and RISP, inhibiting transfer of electrons from ubiquinol at the Qo site [36].
As mitochondria are the primary cellular oxygen consumers, it was proposed that mitochondrial consumption of oxygen leaves the cytosol of a wild-type cell more hypoxic than that of a ρ0 cell for any given extracellular oxygen tension [37]. To further test the hypothesis that mitochondrial ROS production, but not oxygen consumption is required for hypoxic HIF-stabilization, genetic approaches were taken using cytoplasmic hybrid (cybrid) cell lines which consist of ρ0 cells reconstituted with either wild-type or mutant mitochondria. Cybrids harboring a deletion of the cytochrome b gene are respiratory deficient, yet are capable of generating ROS at the Qo site of complex III and stabilizing HIF-1α during hypoxia [38]. Targeting of RISP in these cytochrome b-deficient cybrids with shRNAs abolished ROS generation and hypoxic HIF induction. These experiments demonstrate that (1) it is not the ability of cells to consume oxygen or conduct oxidative phosphorylation that is important for the hypoxic signaling; (2) it is the ability of the cells to produce ROS at mitochondrial complex III which is crucial for hypoxic induction of HIF-α.
Mitochondrial ROS are required for hypoxic calcium signaling in pulmonary artery
While HIF-induced transcription occurs over a matter of hours, and erythrocytosis occurs within days of exposure to hypoxic environments, other physiological responses to hypoxia occur within seconds of exposure. In response to hypoxia, the arterial pressure of the pulmonary artery increases, allowing for improved gas exchange by diverting blood from poorly oxygenated regions of the lung. This response is termed hypoxic pulmonary vasoconstriction (HPV). Although the pulmonary endothelium can amplify this response, isolated pulmonary arterial smooth muscle cells (PASMCs) contract during hypoxia, indicating that the HPV response is initiated in PASMCs [39]. Hypoxia triggers contraction of PASMCs through an increase in cytosolic calcium from both intracellular and extracellular stores [40]. This calcium response is critical for cellular contraction due to hypoxia; however, the exact mechanism of this calcium elevation is unresolved [41].
Similar to HIF-1α expression in many tested cell lines, ρ0 PASMCs are deficient for contraction in response to hypoxia [42]. Perfusion of isolated lungs with mitochondrial inhibitors which act on or proximal to the Qo site of complex III inhibit the hypoxia-induced increase in arterial pressure while antimycin A and cyanide do not. Stimulation of cells with exogenous H2O2 was sufficient to induce PASMC contraction and an increase in arterial pressure of isolated lungs while hypoxia-induced vasoconstriction was inhibited by perfusion with antioxidants [42–44]. Furthermore, treatment of PASMCs with exogenous hydrogen peroxide is sufficient to induce calcium mobilization, while expression of catalase, which converts hydrogen peroxide to oxygen and water, inhibits hypoxia-induced cytosolic calcium accumulation and vasoconstriction [45–47]. Importantly, ρ0 PASMCs or isolated lungs treated with mitochondrial inhibitors were able to respond to the receptor-mediated vasoconstrictor U46619 [42].
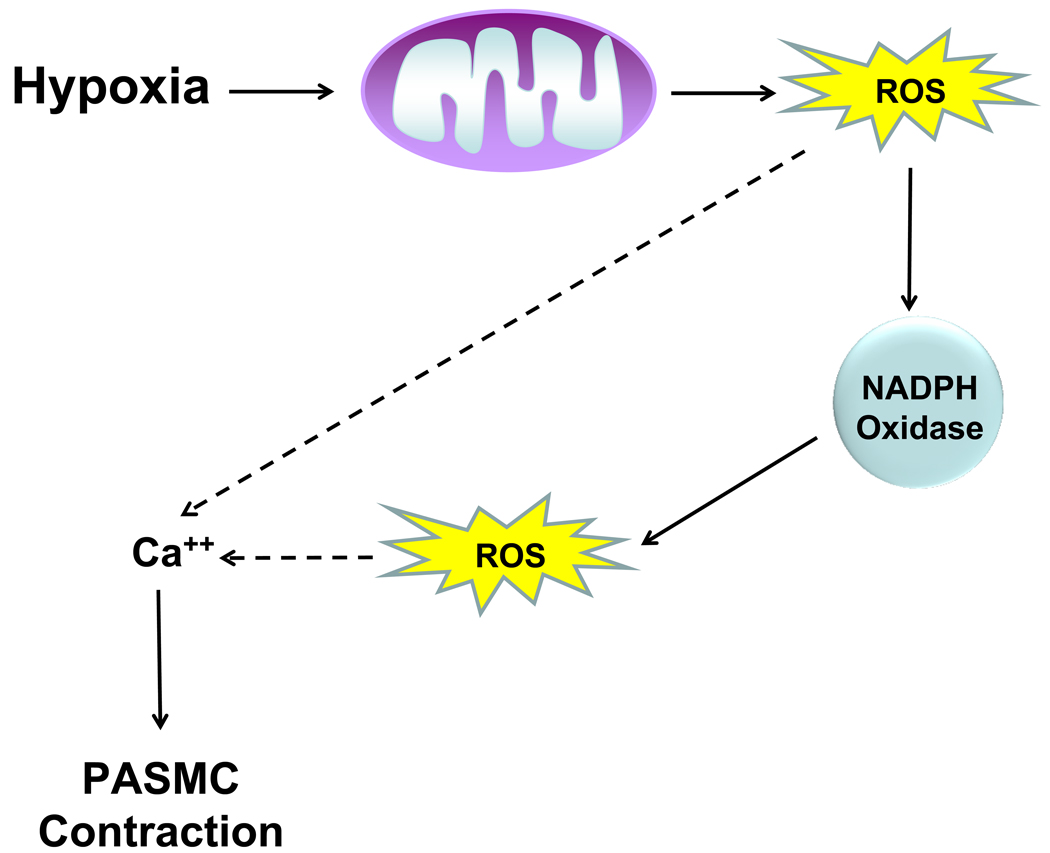
ROS required for HPV have also been suggested to result from the activation of NADPH oxidase in hypoxic PASMCs [48]. Recent studies have demonstrated that mitochondrial ROS generation is required for activation of NADPH oxidase in PASMCs during hypoxia [49]. While inhibition of NADPH oxidase activity attenuated the hypoxic increase in cytosolic calcium and contraction of PASMCs, antioxidants and mitochondrial inhibitors prevented hypoxic induction of NADPH oxidase activity. Thus, hypoxia is sensed in the mitochondria of PASMCs and ROS produced at complex III and NADPH oxidase act synergistically to elevate cytosolic calcium, inducing pulmonary vasoconstriction during hypoxia (Figure 3).
Figure 3. Hypoxia-induced mitochondrial ROS are required for elevation of cytosolic calcium and contraction of PASMCs.
During hypoxia, mitochondrial production of ROS leads to activation of NADPH oxidases, further amplifying the ROS signal. The combined production of ROS at mitochondria and NADPH oxidase allows for elevation of cytosolic calcium and contraction of PASMCs.
ROS and hypoxia signaling: Looking Forward
A major point of controversy regarding the role of ROS as hypoxic signaling molecules is that it is seemingly paradoxical that a decrease in a required substrate, O2, would result in an increase in ROS production. Although one might expect to observe a decrease in superoxide production as O2 concentrations drop, mitochondrial ROS production varies with both [O2] and [electron donors] and thus an increase in the reduction state of the electron transport chain could theoretically increase ROS production during hypoxia [50]. Ample evidence for both increased and decreased levels of ROS production during hypoxia exists; however, these studies rely on oxidation-sensitive dyes which can lack specificity, accumulate within organelles, exhibit autooxidation, and have limited intracellular access. Furthermore, none of these probes exhibits ratiometric fluorescence and as such are concentration dependent, adding further difficulty to accurate ROS measurements [51]. The use of new ratiometric fluorescent protein ROS probes offers better assessment of cellular redox status in real-time and have been used to demonstrate increased ROS production during hypoxia [45,52]. Furthermore, evidence of the accumulation of both DNA and lipid oxidation products during hypoxia suggest that cellular oxidant production increases during hypoxia [53,54].
Although the totality of the evidence suggests that increased production of mitochondrial ROS is required for both HIF-1-mediated transcription and for HPV, the direct targets of oxidation have yet to be determined. Some evidence suggests that oxidation of iron within the catalytic site of PHDs may play a role in ROS-mediated HIF-1 signaling; however, the role of post-translational modifications of PHDs has not been addressed [32]. For HPV, it has also been proposed that ROS act on ryanodine receptors, increasing their open probability, although the mechanism of this action is not determined [51]. In addition to the hypoxic responses presented above, evidence suggests that ROS may also mediate the hypoxic regulation of the Na/K ATPase and AMP-activated protein kinase, suggesting that mitochondrial ROS are a central mediator of hypoxic signaling [55,56]. The discovery of the mechanisms leading to hypoxic ROS production and the downstream effectors of ROS signaling is thus critical for understanding the cellular response to hypoxia.
Acknowledgements
This work was supported by a NIH Grant R01CA123067-03 and RO1GM60472-10 to N.S.C. R.B.H. was supported by a post-doctoral training grant T32CA070085-13.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weir EK, Lopez-Barneo J, Buckler KJ, Archer SL. Acute oxygen-sensing mechanisms. N Engl J Med. 2005;353:2042–2055. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 3. Ward JP. Oxygen sensors in context. Biochim Biophys Acta. 2008;1777:1–14. doi: 10.1016/j.bbabio.2007.10.010. An excellent review explaining oxygen sensors in multiple cell types
- 4. Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. An excellent overview of ROS production at various mitochondrial complexes
- 5.Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem. 2004;279:49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 6.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 7.Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 8.Sun J, Trumpower BL. Superoxide anion generation by the cytochrome bc1 complex. Arch Biochem Biophys. 2003;419:198–206. doi: 10.1016/j.abb.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 9. Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. An excellent comprehensive review of redox-based cellular signaling
- 10.Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 11.Delaunay A, Pflieger D, Barrault MB, Vinh J, Toledano MB. A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell. 2002;111:471–481. doi: 10.1016/s0092-8674(02)01048-6. [DOI] [PubMed] [Google Scholar]
- 12.Ahn SG, Thiele DJ. Redox regulation of mammalian heat shock factor 1 is essential for Hsp gene activation and protection from stress. Genes Dev. 2003;17:516–528. doi: 10.1101/gad.1044503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bunn HF, Gu J, Huang LE, Park JW, Zhu H. Erythropoietin: a model system for studying oxygen-dependent gene regulation. J Exp Biol. 1998;201:1197–1201. doi: 10.1242/jeb.201.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semenza GL, Nejfelt MK, Chi SM, Antonarakis SE. Hypoxia-inducible nuclear factors bind to an enhancer element located 3' to the human erythropoietin gene. Proc Natl Acad Sci U S A. 1991;88:5680–5684. doi: 10.1073/pnas.88.13.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 19.Gu YZ, Moran SM, Hogenesch JB, Wartman L, Bradfield CA. Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3alpha. Gene Expr. 1998;7:205–213. [PMC free article] [PubMed] [Google Scholar]
- 20.Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008;15:621–627. doi: 10.1038/cdd.2008.12. [DOI] [PubMed] [Google Scholar]
- 21.Patel SA, Simon MC. Biology of hypoxia-inducible factor-2alpha in development and disease. Cell Death Differ. 2008;15:628–634. doi: 10.1038/cdd.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 23.Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 24.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 25.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 26.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 27.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 28.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 29. Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. This is the first paper to demonstrate that HIF -mediated transcription depends on the mitochondrial production of ROS
- 30. Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. This is the first paper to demonstrate pharmacologically that ROS production at mitochondrial complex III is required for hypoxia-mediated transcription
- 31.Richard DE, Berra E, Pouyssegur J. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1alpha in vascular smooth muscle cells. J Biol Chem. 2000;275:26765–26771. doi: 10.1074/jbc.M003325200. [DOI] [PubMed] [Google Scholar]
- 32.Gerald D, Berra E, Frapart YM, Chan DA, Giaccia AJ, Mansuy D, Pouyssegur J, Yaniv M, Mechta-Grigoriou F. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell. 2004;118:781–794. doi: 10.1016/j.cell.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 33.Agani FH, Pichiule P, Chavez JC, LaManna JC. The role of mitochondria in the regulation of hypoxia-inducible factor 1 expression during hypoxia. J Biol Chem. 2000;275:35863–35867. doi: 10.1074/jbc.M005643200. [DOI] [PubMed] [Google Scholar]
- 34.Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 35. Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. Refs 34 and 35 use shRNA-mediated targeting of RISP to genetically demonstrate that mitochondrial ROS is required for HIF-mediated transcription
- 36. Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab. 2005;1:393–399. doi: 10.1016/j.cmet.2005.05.003. This paper uses genetic targeting of cytochrome c in ES cells to demonstrate that mitochondrial ROS is required for HIF-mediated transcription
- 37.Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1alpha. Science. 2003;302:1975–1978. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- 38. Bell EL, Klimova TA, Eisenbart J, Moraes CT, Murphy MP, Budinger GR, Chandel NS. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J Cell Biol. 2007;177:1029–1036. doi: 10.1083/jcb.200609074. This is the first paper to genetically demonstrate that production of mitochondrial ROS at complex III and not oxygen consumption is required for hypoxic signaling
- 39.Ward JP, Robertson TP. The role of the endothelium in hypoxic pulmonary vasoconstriction. Exp Physiol. 1995;80:793–801. doi: 10.1113/expphysiol.1995.sp003887. [DOI] [PubMed] [Google Scholar]
- 40.Ward JP, Snetkov VA, Aaronson PI. Calcium, mitochondria and oxygen sensing in the pulmonary circulation. Cell Calcium. 2004;36:209–220. doi: 10.1016/j.ceca.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 41.Aaronson PI, Robertson TP, Knock GA, Becker S, Lewis TH, Snetkov V, Ward JP. Hypoxic pulmonary vasoconstriction: mechanisms and controversies. J Physiol. 2006;570:53–58. doi: 10.1113/jphysiol.2005.098855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res. 2001;88:1259–1266. doi: 10.1161/hh1201.091960. This is the first paper to demonstrate a requirement of mitochondrial ROS for contraction of PASMCs and vasoconstriction of the pulmonary artery during hypoxia
- 43.Liu JQ, Sham JS, Shimoda LA, Kuppusamy P, Sylvester JT. Hypoxic constriction and reactive oxygen species in porcine distal pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2003;285:L322–L333. doi: 10.1152/ajplung.00337.2002. [DOI] [PubMed] [Google Scholar]
- 44.Weissmann N, Tadic A, Hanze J, Rose F, Winterhalder S, Nollen M, Schermuly RT, Ghofrani HA, Seeger W, Grimminger F. Hypoxic vasoconstriction in intact lungs: a role for NADPH oxidase-derived H(2)O(2)? Am J Physiol Lung Cell Mol Physiol. 2000;279:L683–L690. doi: 10.1152/ajplung.2000.279.4.L683. [DOI] [PubMed] [Google Scholar]
- 45.Waypa GB, Guzy R, Mungai PT, Mack MM, Marks JD, Roe MW, Schumacker PT. Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells. Circ Res. 2006;99:970–978. doi: 10.1161/01.RES.0000247068.75808.3f. [DOI] [PubMed] [Google Scholar]
- 46.Wang QS, Zheng YM, Dong L, Ho YS, Guo Z, Wang YX. Role of mitochondrial reactive oxygen species in hypoxia-dependent increase in intracellular calcium in pulmonary artery myocytes. Free Radic Biol Med. 2007;42:642–653. doi: 10.1016/j.freeradbiomed.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lin MJ, Yang XR, Cao YN, Sham JS. Hydrogen peroxide-induced Ca2+ mobilization in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1598–L1608. doi: 10.1152/ajplung.00323.2006. Refs 45–47 demonstrate that the role of mitochondria in PASMC contraction lies upstream of cytosolic calcium elevation, indicating that oxygen levels are sensed in the mitochondria which subsequently activates calcium signalling
- 48.Marshall C, Mamary AJ, Verhoeven AJ, Marshall BE. Pulmonary artery NADPH-oxidase is activated in hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 1996;15:633–644. doi: 10.1165/ajrcmb.15.5.8918370. [DOI] [PubMed] [Google Scholar]
- 49. Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, Wang YX. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med. 2008;45:1223–1231. doi: 10.1016/j.freeradbiomed.2008.06.012. This is the first paper to demonstrate that ROS produced at mitochondria induce NADPH oxidase to amplify the ROS signal resulting in an elevation of cytosolic calcium to induce contraction in pulmonary artery cells
- 50. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. An excellent, comprehensive review of how ROS are produced by the mitochondrial electron transport chain
- 51.Waypa GB, Schumacker PT. Oxygen sensing in hypoxic pulmonary vasoconstriction: using new tools to answer an age-old question. Exp Physiol. 2008;93:133–138. doi: 10.1113/expphysiol.2007.041236. [DOI] [PubMed] [Google Scholar]
- 52.Bell EL, Klimova TA, Eisenbart J, Schumacker PT, Chandel NS. Mitochondrial reactive oxygen species trigger hypoxia-inducible factor-dependent extension of the replicative life span during hypoxia. Mol Cell Biol. 2007;27:5737–5745. doi: 10.1128/MCB.02265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grishko V, Solomon M, Breit JF, Killilea DW, Ledoux SP, Wilson GL, Gillespie MN. Hypoxia promotes oxidative base modifications in the pulmonary artery endothelial cell VEGF gene. FASEB J. 2001;15:1267–1269. doi: 10.1096/fj.00-0755fje. [DOI] [PubMed] [Google Scholar]
- 54.Block ER, Patel JM, Edwards D. Mechanism of hypoxic injury to pulmonary artery endothelial cell plasma membranes. Am J Physiol. 1989;257:C223–C231. doi: 10.1152/ajpcell.1989.257.2.C223. [DOI] [PubMed] [Google Scholar]
- 55.Comellas AP, Dada LA, Lecuona E, Pesce LM, Chandel NS, Quesada N, Budinger GR, Strous GJ, Ciechanover A, Sznajder JI. Hypoxia-mediated degradation of Na,K-ATPase via mitochondrial reactive oxygen species and the ubiquitin-conjugating system. Circ Res. 2006;98:1314–1322. doi: 10.1161/01.RES.0000222418.99976.1d. [DOI] [PubMed] [Google Scholar]
- 56.Emerling BM, Weinberg F, Snyder C, Burgess Z, Mutlu GM, Viollet B, Budinger GR, Chandel NS. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic Biol Med. 2009;46:1386–1391. doi: 10.1016/j.freeradbiomed.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]