Abstract
Background
Despite numerous studies demonstrating efficacy of cellular adoptive transfer for therapeutic myocardial regeneration, problems remain for donated cells with regard to survival, persistence, engraftment, and long-term benefits. This study redresses these concerns by enhancing the regenerative potential of adoptively transferred cardiac progenitor cells (CPCs) via genetic engineering to overexpress Pim-1, a cardioprotective kinase that enhances cell survival and proliferation.
Methods and Results
Intramyocardial injections of CPCs overexpressing Pim-1 were given to infarcted female mice. Animals were monitored over 4, 12, and 32-weeks to assess cardiac function and engraftment of Pim-1 CPCs using echocardiography, in vivo hemodynamics, and confocal imagery. CPCs overexpressing Pim-1 show increased proliferation and expression of markers consistent with cardiogenic lineage commitment following dexamethasone exposure in vitro. Animals that received CPCs overexpressing Pim-1 also produce greater levels of cellular engraftment, persistence, and functional improvement relative to control CPCs up to 32-weeks post-delivery. Salutary effects include reduction of infarct size, greater number of c-kit+ cells, and increased vasculature in the damaged region.
Conclusions
Myocardial repair is significantly enhanced by genetic engineering of CPCs using Pim-1 kinase. Ex vivo gene delivery to enhance cellular survival, proliferation, and regeneration may overcome current limitations of stem cell-based therapeutic approaches.
Keywords: Gene therapy, infarction, echocardiography, hemodynamics
Introduction
Stem cell-based interventional approaches for myocardial regeneration have recently generated substantial enthusiasm as a novel treatment for heart failure. Despite initial optimism, application of regenerative medicine in the myocardium has been stymied by the marginal regenerative potential of adoptively transferred stem cell populations. Experimental studies routinely find the vast majority of adoptively transferred stem cells die or vanish shortly after delivery. Thus, benefits observed are likely mediated by a small population of surviving cells, leading to the postulate: improving cardiac progenitor cell (CPC) survival and proliferation will have dramatic consequences for enhancing myogenesis and empower therapeutically relevant implementation of myocardial regeneration.
CPCs expressing the stem cell marker c-kit+ reside within the heart1, 2, mediating maintenance and repair of damaged cardiac tissue in response to myocardial injury1, 3-6. CPCs have been isolated and used with modest success for treatment and regeneration of damaged heart tissue after infarction4, 7-9. Ideally, to enhance the regenerative process, adoptively transferred CPCs would benefit from modification to promote cellular survival and proliferation without inhibiting lineage commitment and differentiation provided by appropriate environmental cues.
Previous studies by our group have identified a kinase responsible for cardioprotection downstream of Akt signaling named Pim-110, a conserved serine/threonine protein kinase originally described as the proviral intergration site of the Moloney murine leukemia virus11. Primary downstream targets of Pim-1 include molecules responsible for regulation of cellular survival and mitotic activity11-15. The role of Pim-1 in promoting proliferation and transition through cell cycle has been extensively documented15-19. Unlike native Akt regulated by phosphorylation, Pim-1 is constitutively activated and is produced in response to stress or pathologic injury in the myocardium. Pim-1 is also expressed in activated stem cells20 as well as in endothelial21 and vascular smooth muscle cells14. While cardioprotective effects of Pim-1 are clear from our work10, 22, the impact of Pim-1 as a mediator of myocardial regeneration remains to be explored. Results presented herein demonstrate the beneficial capacity of Pim-1 to enhance cardiac regeneration, advancing the concept of ex vivo gene therapy with cultured CPCs to enhance cardiogenesis when reintroduced into infarcted myocardium.
Methods
Generation of Lentiviral Vectors
CPCs were genetically modified using a bicistronic lentiviral vector to deliver human Pim-1 gene under control of a myeloproliferative sarcoma virus LTR-negative control region deleted (MND) promoter and eGFP driven off a vIRES. Lentivirus was made as previously described23.
Cardiac Progenitor Cell Isolation, Culture, and Transductions
CPCs were isolated from syngenic male FVB mice as previously described5 and cultured for 3-weeks in cardiac stem cell media. CPCs were plated (.2×106 cells/well) in 48-well plates, transduced with lentivirus (MOI=10) with 4μg/ml polybrene, expanded, and analyzed by flow cytometry to determine percentage eGFP+ cells. CPCs were differentiated as previously described5 using 10-8M dexamethasone.
SuperArrays
mRNA and cDNA from CPCe and CPCeP was obtained using Triazol (Invitrogen) and harvested per manufacture's protocol. Apoptosis (PAMM-012) and cell proliferation (APMM-012) arrays were obtained from SuperArray and ran per manufacturer's protocol.
Trypan Blue Exclusion and MTT assay
Uninfected, CPCe, and CPCeP, plated in quadruplicate (10,000 cells/well) in 24 well plates. Viable cells determined by trypan blue exclusion. For MTT assay 5000 cells/well were plated in 96-well plates, incubated 4-5 hours with 50μg of MTT reagent (Fluka), treated with 100μl stopping reagent (.01N HCl + 10% SDS) overnight at 37°C, and analyzed on a spectrophotometer at 570nm. Quercetagetin (Calbiochem, #551590) was incubated with cells at 10μM where indicated.
Myocardial Infarction, Injections, Echocardiography, and Hemodynamics
Infarctions, echocardiography, and hemodynamics were performed as previously described10 with additional details in supplement.
Confocal Microscopy
Heart sections were deparaffinized, antigen retrieved in 1mM Citrate (pH 6.0), followed by 1 hour block in TNB. Primary antibodies were incubated overnight at 4°C at appropriate dilutions (see Supplemental Methods). Slides washed in 1X TN followed by secondary antibody incubation, 2 hours at room temperature. Subsequent tyramide amplification was performed as necessary.
Telomere Length
Paraffin sections were prepared for in-situ hybridization and stained for telomere length as per manufacturers protocol (Dako).
Statistics
Statistics were calculated using SPSS software. One way ANOVA and two way repeated measures ANOVA for echocardiography with Tukey's post-hoc test were calculated. Values with p<.05 were considered statistically significant. Generalized estimating equations with empirical (robust) covariance estimation were used to confirm two way ANOVA results.
Animal Studies
All animal studies were approved by IACUC.
Results
Pim-1 lentiviral vector expression in cardiac progenitor cells (CPCs)
Bicistronic lentiviral vectors expressing enhanced green fluorescent protein alone (Lv-egfp) or in combination with Pim-1 kinase (Lv-egfp+Pim1) (Figure S1A) were used to introduce cDNA constructs into c-kit+ CPCs isolated from male nontransgenic (NTG) FVB mice. CPC populations modified with Lv-egfp (CPCe) or with Lv-egfp+Pim1 (CPCeP) were subjected to immunoblot analyses to confirm protein expression of genomically integrated gene products. eGFP protein expression was present in CPCe and CPCeP and increased protein levels for Pim-1 in CPCeP samples (Figure S1B). CPC and CPCe showed low level endogenous Pim-1 protein expression.
Pim-1 increases CPC proliferation
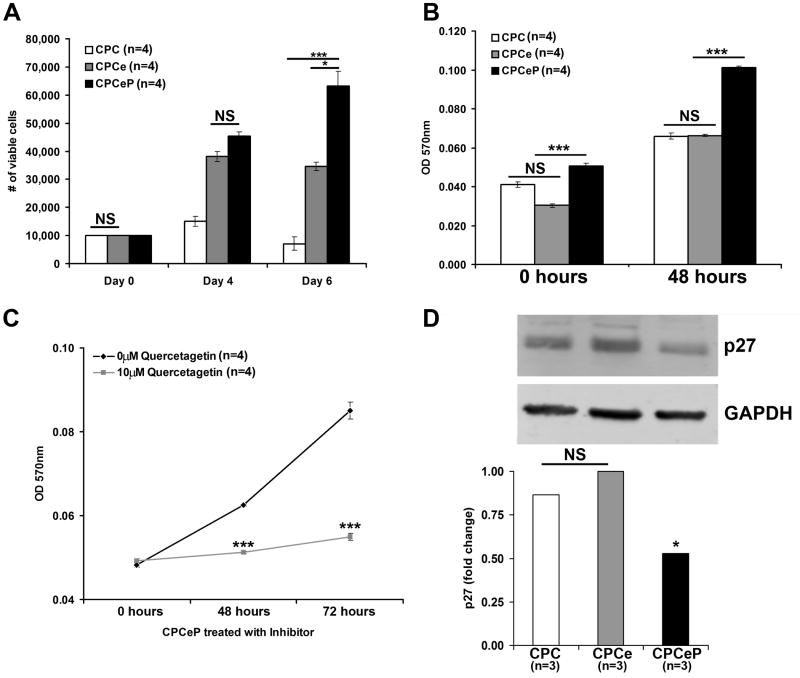
CPCeP exhibit increased proliferation compared to CPCe (p<.05) and CPC (p<.001) as assessed by Trypan Blue exclusion (Figure 1A). In comparison, increased CPCe cell numbers observed at day 4 were not maintained at day 6. CPCeP also possess increased metabolic rates relative to CPCe at time zero (p<.001) or 48 hours (p<.001) in vitro as determined by MTT assay (Figure 1B). The increase in CPCeP growth rate was abrogated by addition of Quercetagetin (a specific Pim-1 activity inhibitor) after forty-eight and seventy-two hours (p<.001) relative to vehicle treated CPCeP (Figure 1C). Several mRNAs encoded by genes responsible for cell cycle arrest are downregulated in CPCeP by array analysis (Figure S2). Immunoblot analyses of CPCeP also show decreased expression of p27, a cell cycle dependent kinase inhibitor (Figure 1D). Thus, enhanced proliferation of CPCeP is likely due to altered regulation of cell cycle inhibitors.
Figure 1. Pim-1 increases the proliferation rate of CPCs in vitro.
(A) The number of viable cells as determined by trypan blue exclusion was determined for CPCeP, CPCe, and CPCs over a six day time course (mean ± SEM, n=4/group). (B) Metabolic rate of CPCeP, CPCe, and CPCs was measured by MTT assay over a 48 hour time course (mean ± SEM, n=4/group). (C) Metabolic rate evaluated by MTT assay in CPCeP treated with and without 10μM Quercetagetin over a 72 hour time course (mean ± SEM, n=4/group). (D) Immunoblot analysis of p27 protein in CPCeP, CPCe, and CPCs (n=3/group). *p<.05, **p<.01, ***p<.001.
CPCeP differentiate into cardiac, endothelial, and smooth muscle lineages
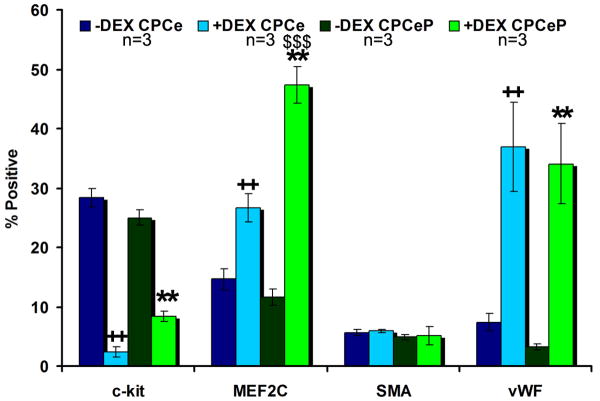
Flow cytometric analyses (Figure 2, Figure S3) confirmed CPCe and CPCeP had significant (p<.01) increases in MEF2C and vWF after treatment with dexamethasone (Dex)2. CPCeP also had a significant increase (p<.001) in percent of cells staining positive for MEF2C compared to CPCe. These results indicate genetically modified CPCs are amenable to lineage commitment. Additional phenotypic characterizations for cell markers consistent with cardiovascular lineages by immunolabeling and uptake of acetylated-low density lipoprotein (Ac-LDL) by cultured CPCe and CPCeP confirmed these findings (Supplemental Results, Figure S4).
Figure 2. Flow cytometric analysis of CPCe and CPCeP.
Analysis of cardiogenic lineages of CPCe and CPCeP before (dark blue and dark green) and after (light blue and light green) dexamethasone treatment (mean ± SEM, n=3/group). ++p<.01 compared to CPCe –Dex. **p<.01 compared to CPCeP -DEX, $$$p<.001 compared to CPCe +Dex.
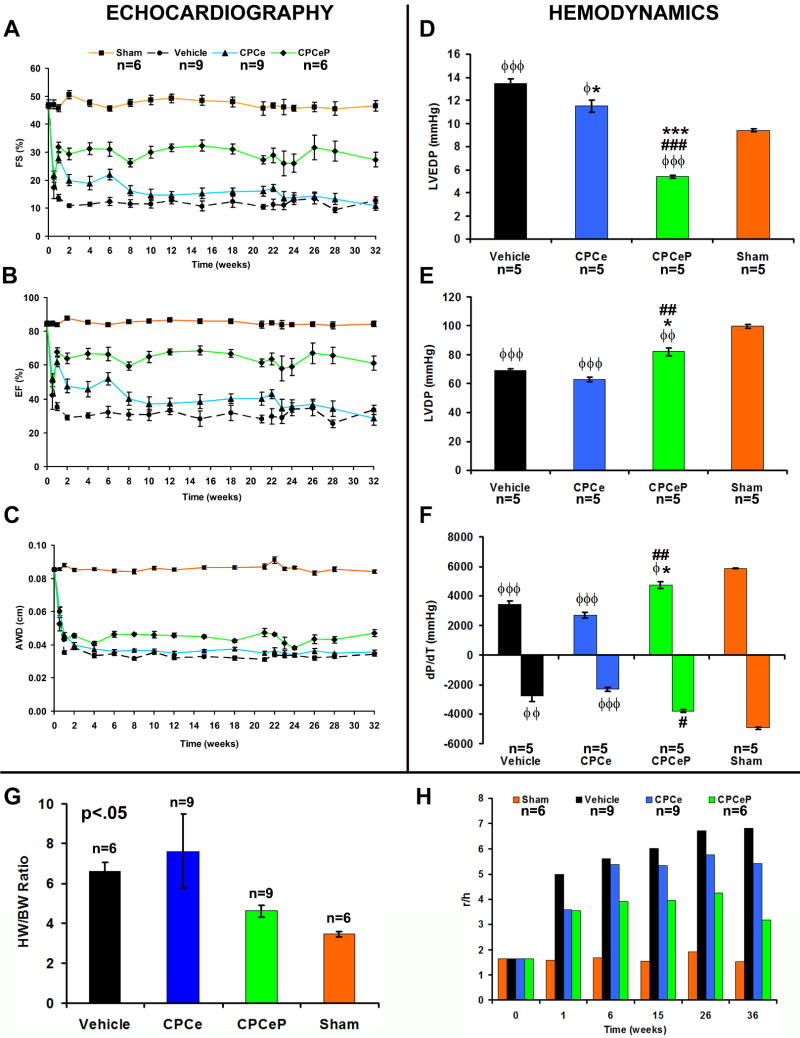
CPCeP improve cardiac function 12-weeks post myocardial infarction
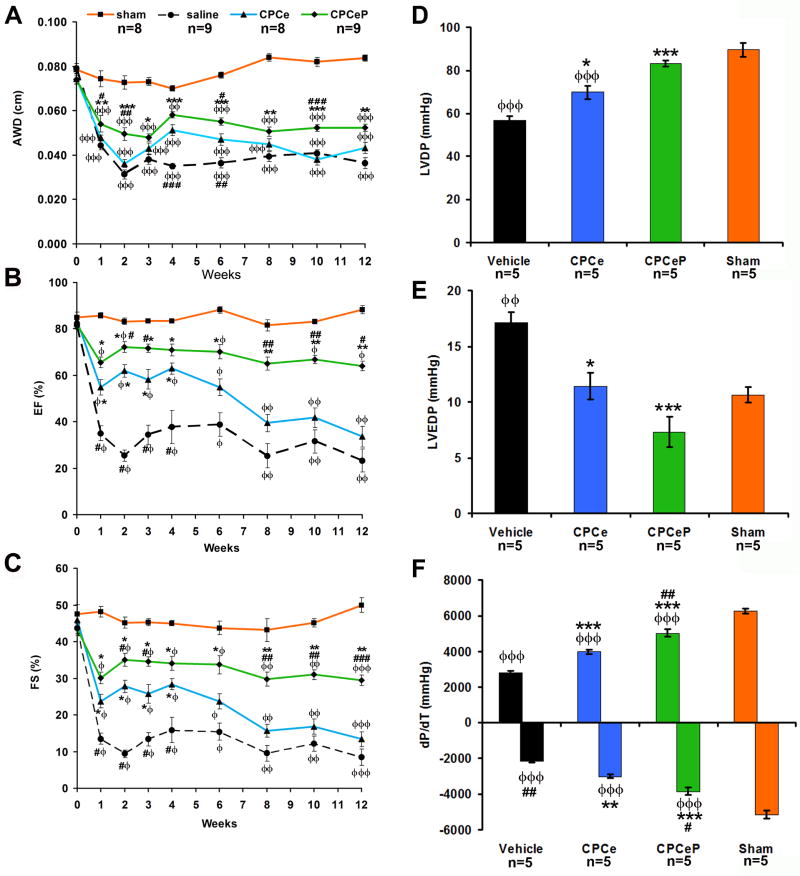
Transgenic mice with cardiac-specific Pim-1 expression are resistant to infarction challenge10, 22, therefore CPCeP should ameliorate pathological damage following adoptive transfer into infarcted myocardium. Twelve week old female mice, subjected to myocardial infarction, were treated with CPCe or CPCeP injected directly into the peri-ischemic border zone. Hearts of mice receiving CPCeP possessed thicker anterior wall dimension (AWD) compared to vehicle (p<.001) or CPCe (p<.01) injected mice (Figure 3A) by echocardiography at 2-weeks. Both groups receiving CPCs showed significant improvements in ejection fraction (EF) and fractional shortening (FS) at 4-weeks post-delivery (p<0.05) relative to vehicle injected mice (p<.05) (Figure 3B-C). However, EF, FS (Figure 3B-C), left ventricular developed pressure (LVDP), left ventricular end diastolic pressure (LVEDP), and maximum dp/dt (Figure S5A-C) were not significantly different (p>0.05) between CPCe and CPCeP groups.
Figure 3. Intramyocardial injection of CPCeP improves cardiac function 12-weeks post infarction.
(A-C) Electrocardiographic assessment of AWD (A), EF (B), and FS (C), in sham (■, orange, n=8), vehicle (●, black, n=9), CPCe (▲, blue, n=8), and CPCeP (◆, green, n=9), 12-weeks post-infarction (mean ± SEM). (D-F) Cardiac function of sham (orange, n=5), vehicle (black, n=5), CPCe (blue, n=5), and CPCeP (green, n=5) were evaluated using in vivo hemodynamic measurements of LVDP (D), LVEDP (E), and dP/dT (F) 12-weeks post-intramyocardial injection (mean ± SEM). φp<.05, φφp<.01, φφφp<.001 compared to sham; #p<.05, ##p<.01, ###p<.001 compared to vehicle, * p<.05, **p<.01, ***p<.001 compared to CPCe. Echocardiography significant (p<.05) by two-way repeated measures ANOVA.
CPCeP injections were compared to mice receiving CPCe injections over an extended 12 week time course following delivery into infarcted hearts to assess long lasting beneficial effects. Injection of CPCe conferred improved function at one week after delivery, however transitioned into decompensation at 6-weeks, becoming indistinguishable from vehicle controls by 8-weeks (Figure 3B-C). In comparison, CPCeP injected mice maintained EF and FS and had significantly improved function relative to CPCe injected mice at 6-weeks following delivery. At 12-weeks, CPCeP treatment preserved EF and FS, whereas CPCe treated hearts suffer from a 2-fold and 1.6-fold decrease in function, respectively (Figure 3B-C). Significantly enhanced cardiac function in mice receiving CPCeP was confirmed by measurement of LVDP, LVEDP, and changes in ±dp/dt (Figure 3D-F) relative to mice receiving CPCe.
Injection of CPCeP leads to infarct reduction, de novo myocyte formation, and neovascularization
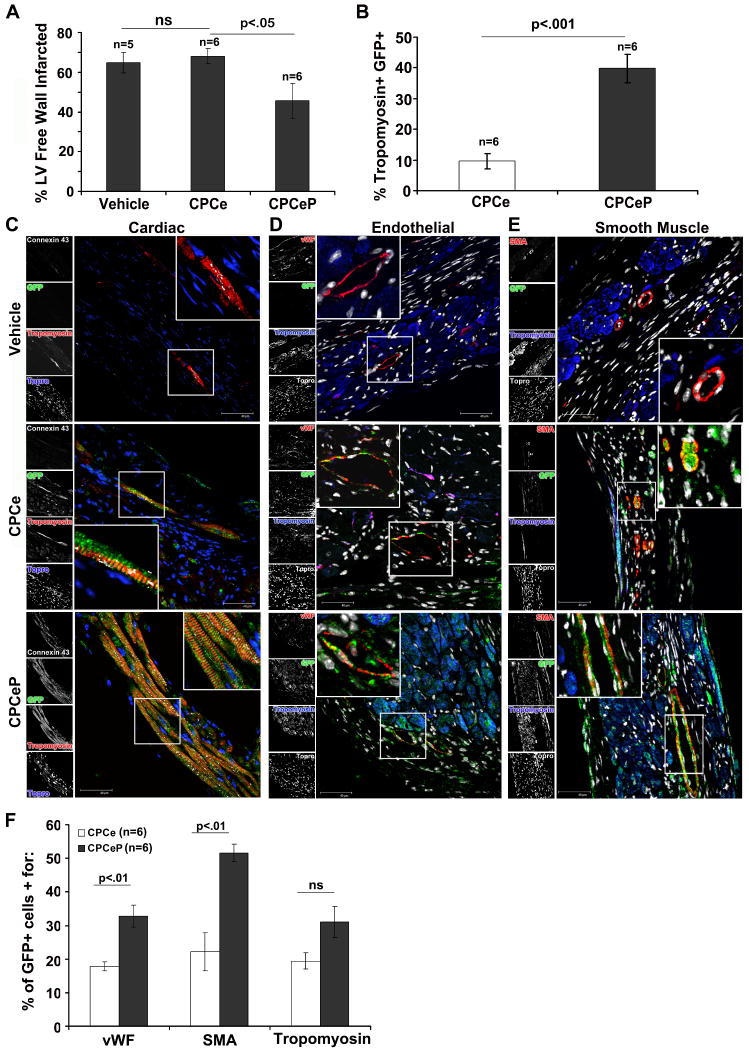
Hearts from CPCe and CPCeP injected animals were immunostained with sarcomeric tropomyosin to detect surviving myocardium at 12-weeks post-infarction. CPCeP injected hearts showed significant reductions in infarct size (1.5-fold; p<0.05; Figure 4A) and fibrosis (Figure S6) relative to CPCe treated. In CPCeP injected hearts 39.8% of the surviving myocardium within the infarct was eGFP+ (Figure 4B). This corresponded to a significant (p<.001) 4-fold increase compared to CPCe injected hearts.
Figure 4. De novo myocyte formation and neovascularization results in reduction of infarct size in CPCeP treated animals.
(A) Quantitation of infarction area in vehicle (n=5), CPCe (n=6), and CPCeP (n=6) treated hearts 12-weeks post injection (mean ± SEM). (B) Quantitation of Tropomyosin+ eGFP+ cells in CPCe (n=6) and CPCeP (n=6) animals (mean ± SEM). (C-E) Representative immunostaining for colocalization of eGFP (green) and cardiac myocytes (tropomyosin, red) and connexin-43 (white) (C), endothelial cells (vWF, red) (D), and smooth muscle cells (SMA, red) (E) in vehicle, CPCe, and CPCeP treated hearts 12-weeks post-intramyocardial injection. Enlarged areas are represented with indicated boxes. Scale bars represent 40μm. (F) Quantitation of eGFP+ population in CPCe (n=6) and CPCeP (n=6) for cardiac lineage markers.
Persistence and cellular phenotype of donated CPCs was examined at 12-weeks post-delivery by immunostaining myocardial sections with antibodies to eGFP co-localized with sarcomeric tropomyosin to detect myocytes as well as with connexin-43 suggesting formation of functional gap junctions with neighboring cells (Figure 4C). Coincident localization of eGFP and striated tropomyosin labeling is indicative of myocytes presumptively derived from the donated cell population in hearts receiving CPCeP. Interestingly, while de novo myocardium is formed in the central area of the infarcted region, the majority of regeneration from transplanted cells is at or adjacent to the border zone where cells are initially injected. A donor–derived origin for these eGFP+ myocytes is supported by confirmation of smaller cell size as well as longer telomere length; both consistent with the phenotype of a younger cell (Supplemental Results, Figure S7A). Additionally, immunostaining for Pim-1 was increased in CPCeP injected hearts (Supplemental Results, Figure S7B). Furthermore, presence of Y-chromosome, indicative of the donated cell population, was detected in samples from hearts receiving CPC treatment (Supplemental Results, Figure S7C-D).
Together with myocyte labeling, vessels coincident with eGFP were observed by colocalization with antibodies to VonWillebrands Factor (vWF) (Figure 4D) or smooth muscle actin (Figure 4E) from vehicle treated, CPCe, and CPCeP injected hearts 12-weeks post-infarction. Quantitative assessment of lineage markers demonstrated that in CPCeP injected hearts 32.8% and 51.6% of the eGFP+ population stained positive for vWF and SMA (Figure 4F), respectively. This correlated to a significant 1.8- and 2.3-fold increase compared to CPCe, further implicating the CPCeP population in formation of new vasculature.
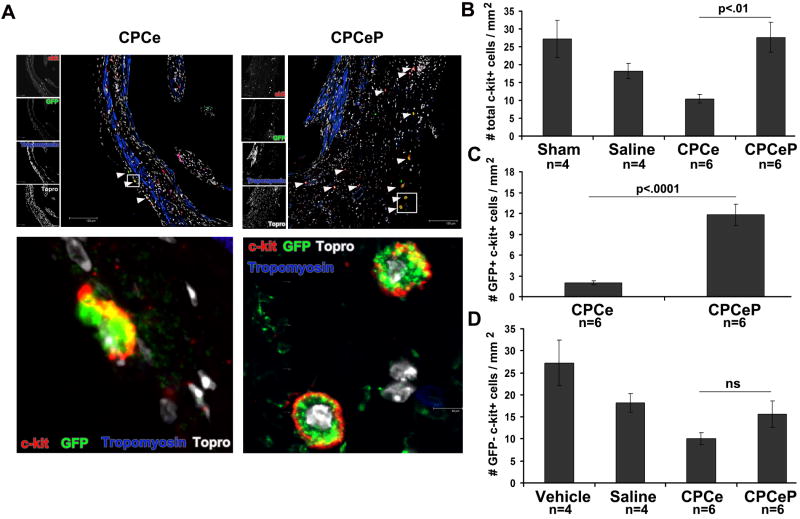
Increased number of c-kit+ cells in hearts receiving CPCeP show enhanced proliferation
Presence of c-kit+ cells within the infarct area was quantitated to assess enhanced recruitment of endogenous stem cells by CPCeP 6, 9, 24. CPCeP treated hearts had a 2.6-fold increase in total c-kit cells relative to CPCe at 12-weeks (Figure 5A and B). Quantitation of the number of eGFP+ c-kit+ cells in CPCeP injected hearts demonstrated CPCeP had a 6-fold increase compared to CPCe (Figure 5C). The number of eGFP- c-kit+ cells was not statistically different between CPCeP and CPCe (Figure 5D). Furthermore, CPCeP have increased proliferation in vivo as determined by quantitation of BrdU incorporation at 5 weeks and by PCNA immunolabeling at 12 weeks post-delivery (Supplemental Results, Figure S8A-B). Hearts receiving CPCeP also show decreased apoptotic cell death in resident myocardium (Supplemental Results, Figure S8C). Collectively, these results indicate CPCeP have an increased ability to survive and proliferate in the damaged myocardium compared to CPCe.
Figure 5. CPCeP treated hearts have increased numbers of c-kit+ cells 12-weeks post intramyocardial injection.
(A) Immunostaining for c-kit 7 and eGFP (green) in heart sections from mice treated with CPCe (n=6) or CPCeP (n=6) 12-weeks post-intramyocardial injection. Enlarged areas are represented with indicated boxes. (B) Quantitation of the number of total, (C) eGFP+, and (D) eGFP- c-kit+ cells in hearts of mice injected with CPCe (n=6) or CPCeP (n=6) (mean ± SEM). Scale bars represent 120μm in top panels.
Persistent improvement in myocardial performance is afforded by CPCeP
Beneficial effects of adoptively transferred cell populations are often measured within days or a few weeks after delivery6, 9, 25, 26, but genetic modification of CPC with Pim-1 may allow for long-term functional improvements not observed with normal cells. A 32-week longitudinal study was performed to determine myocardial function in infarcted mice receiving vehicle, CPCe, or CPCeP. Validity of infarction was verified at three days by statistically equivalent decreases in EF, FS, and AWD (Figure 6A-C) of all three infarcted experimental groups. CPCeP injected mice showed improved FS and EF by one week following delivery sustained throughout the course of the study (Figure 6A-B). CPCeP injected hearts also had significantly increased AWD relative to CPCe injected hearts at 32-weeks (Figure 6C). In vivo hemodynamic analysis confirmed improved myocardial performance following CPCeP treatment, evidenced by decreased LVEDP, increased DP, and ±dp/dt (Figure 6D-F) relative to mice receiving CPCe. Heart weight to body weight ratios in CPCeP treated mice were comparable to sham operated controls and significantly smaller relative to CPCe and vehicle-injected controls (Figure 6G; p<.05). Cardiac remodeling as determined by Law of Laplace revealed the ratio of left ventricular diameter (r) to wall thickness (h) was significantly decreased in mice receiving CPCeP at 36-weeks post-delivery (Figure 6H). Results from the 32-week time course validate results obtained in the 12-week study (Figure 3) and demonstrate persistent and significant improvement in myocardial performance and remodeling afforded only by CPCeP.
Figure 6. Long-term persistent cardiac functional recovery in animals treated with CPCeP.
(A-C) Electrocardiographic assessment of FS (A), EF (B), and AWD (C), in sham (■, orange, n=6), vehicle (●, black, n=9), CPCe (▲, blue, n=9), and CPCeP (◆, green, n=6), 32-weeks post-infarction (mean ± SEM). Statistics for each time point are provided in Table S1. (D-F) Cardiac function of sham (n=5), vehicle (n=5), CPCe (n=5), and CPCeP (n=5) were evaluated using in vivo hemodynamic measurements of LVEDP (D), LVDP (E), and dP/dT (F), 32-weeks post-intramyocardial injection (mean ± SEM). (G) Heart weight: body weight ratios. Statistically significant p<.05 (ANOVA). (H) Wall stress assessment comparing ratio of left ventricular diameter (r) to wall thickness (h) from sham (n=6), vehicle (n=9), CPCe (n=9), and CPCeP (n=6) treated animals (mean ± SEM). φp<.05, φφp<.01, φφφp<.001 compared to sham; #p<.05, ##p<.01, ###p<.001 compared to vehicle, * p<.05, **p<.01, ***p<.001 compared to CPCe. Echocardiography significant (p<.05) by two-way repeated measures ANOVA.
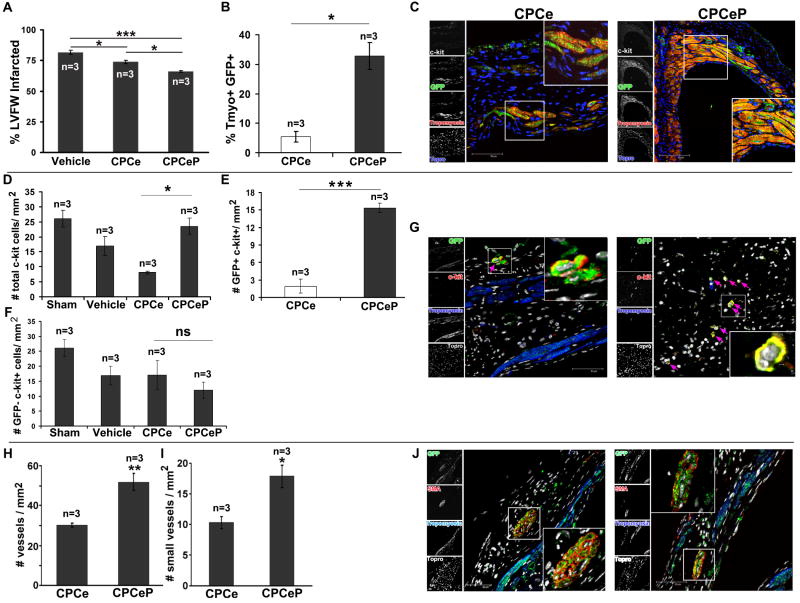
CPCeP engraftment and commitment persists up to 32-weeks post intramyocardial injection
Myocardial samples from mice receiving CPCe and CPCeP were examined for presence of adoptively transferred cells as evidenced by presence of immunoreactivity for eGFP coincident with markers of cardiogenic lineages at 32-weeks post-delivery. Myocytes expressing eGFP were detected in hearts receiving either CPCe or CPCeP. Similar to observations at 12-weeks (Figure 4A), hearts receiving CPCeP showed a significant 1.3-fold reduction in infarct size (Figure 7A). In CPCeP injected hearts 32.8% of the surviving myocardium was eGFP+ (Figure 7B,C). This corresponded to a significant (p<.001) 6-fold increase compared to CPCe injected hearts. CPCeP injected animals also had significant increases in total c-kit+ cells (2.9-fold) at 32-weeks relative to CPCe injected hearts. CPCeP injected hearts had a significant 7.9-fold increase in the number of eGFP+ c-kit+ cells compared to CPCe, while number of eGFP- c-kit+ cells was not statistically different between the two groups (Figure 7D-G). Hearts of mice receiving CPCeP showed a 71% increase in vascular density in the infarct region visualized by immunostaining for smooth muscle actin, with 74% more vessels characterized by small diameter of 1-2 cells evident in the circumference (Figure 7H-J).
Figure 7. Persistent engraftment and differentiation of Pim-1 expressing CPCs 32-weeks post intramyocardial injection.
(A) Infarct size measurement, (B) quantitation of Tropomyosin+ eGFP+ cells, (C) and confocal micrographs of hearts injected with CPCe (n=3) or CPCeP (n=3) 32-weeks post-infarction. (mean ± SEM). Sections were stained for eGFP (green), Tropomyosin 7, c-kit (white), and nuclear stain Topro-3-iodide (blue). Quantitation of total (D), eGFP+ (E), and eGFP- (F) c-kit+ cells in heart sections from mice treated with CPCe (n=3) or CPCeP (n=3) 32-weeks post-infarction. (G) Immunolabeling with eGFP (green), c-kit 7, tropomyosin (blue), and nuclear stain Topro-3-iodide (white). (H) Quantitation of total number of vessels, (I) small vessels and (J) immunolabeling for vasculature in hearts from mice treated with CPCe (n=3) or CPCeP (n=3) 32-weeks post-infarction. Sections were stained with eGFP (green), smooth muscle actin 7, tropomyosin (blue), and nuclear stain Topro-3-iodide (white). Mean ± SEM, n=3, * p<.05, **p<.01, ***p<.001. Scale bars represent 50μm.
Discussion
A revolution in the treatment of heart disease has begun, initiated by discovery of stem cell-mediated myocardial regeneration2, 27, 28. As the field matures key observations and recurrent themes continually emerge from experimental and clinical studies: 1) myocardial structure and function are consistently and reproducibly improved although degree of benefit is modest, 2) the vast majority of adoptively transferred stem cells are lost due to death or poor retention after delivery, 3) long-term efficacy of the regenerative process mediated by adoptively transferred cells remains debatable, due in part to their short-lived nature, 4) therapeutically relevant implementation of stem cell-based myocardial repair ultimately depends upon enhancing the modest regenerative effects currently observed, and 5) ex vivo modification or treatment of adoptively transferred stem cells significantly enhances reparative and regenerative processes. The first four observations point out present day stem cell-based myogenesis and regeneration approaches fall short of the efficacy desired for reconstitution of myocardial tissue following cardiomyopathic injury. However, the last point offers hopes in the form of engineering stem cells to enhance their regenerative capacity.
Several laboratories have demonstrated impressive gains in blunting infarction damage through use of stem cells modified by genetic engineering29-32 or exposure to environmental, chemical, and biological treatments prior to delivery29, 33-35. A consistent theme from these studies is that the ex vivo manipulation of the stem cell population has salutary effects for the adoptively transferred cells but also for the host myocardium upon reintroduction. The mechanistic basis of these treatments has been linked to enhanced cellular survival, secretion of paracrine factors, activation of endogenous repair processes, and contribution of the donated cells to formation of de novo myocardium. Among studies of ex vivo stem cell modification, activation of survival kinases such as Akt, is an effective strategy for potentiating stem cell regeneration, but severely limited or complete lack of direct participation in de novo tissue formation and marginal long-term engraftment of engineered cells remains a serious limitation31, 34, 36-39. Downstream of nuclear Akt signaling we identified Pim-110 kinase that stabilizes pro-proliferative proteins and influences their subcellular localization through phosphorylation and/or direct association. Examples include nuclear export and subsequent degradation of cyclin dependent kinase inhibitors p27 and p2118, 19, stabilization of c-myc15, and mitotic complex assembly with NuMA16. Additionally CPCs from Pim-1 transgenic mice show elevated levels of nucleostemin, a protein whose expression is associated with maintenance of proliferation in stem cells40. Consistent with Pim-1's role in increased proliferation, our studies demonstrate injection of CPCeP promotes increased density of eGFP+ c-kit+ cells in the region of infarction (Figure 5B, 7B). As well, anti-apoptotic41, 42 and pro-proliferative11 actions of Pim-1 were observed with CPCs in vitro (Figure 1 and S2) and in vivo (Figure S8). CPCeP promote salutary effects relative to CPCe for up to 32-weeks (Figure 3,6), presumably by conferring upon the limited number (100,000) of donated cells increased ability to survive and proliferate (Figure S8). CPCeP may also provide enhanced survival and increased cycling of the resident (GFP-) myocardium (Figure S8) through both paracrine effects and formation of de novo vasculature.
Stem cells derived from the heart, capable of generating myocytes and vasculature2, 7, 14, may be “primed” for differentiation into cardiac lineages as evidenced by expression of c-kit in conjunction with MEF2C and NKX2.5 and increased expression of vWF (Figure 2, 4, S3A,C, and S4). Consistent with the role of Pim-1 in endothelial cell differentiation21, CPCeP express vWF prior to differentiation (Figure S4A) and incorporate Ac-LDL (Figure S4B). CPCeP hearts also had significant increases in eGFP+ cells staining positive for vasculature markers vWF and SMA (Figure 4F). Pim-1 is also necessary for vascular smooth muscle cell (VSMC) proliferation, while knockdown inhibited capillary formation and reduced VSMC proliferation14. These results infer that CPCeP may be predisposed toward vasculature lineages and suggests CPCeP-mediated angiogenesis may enhance new myocyte formation as well as retention of existing myocytes. Intramyocardial injection of CPCs has been shown to enhance cardiac function 4-weeks after injection3, 6, 9, 26, but functionally relevant myocardial regeneration requires long-term repopulation of all three cardiac lineages: myocytes, endothelial, and VSMC. Collectively, our results indicate CPCeP possess the inherent ability to provide such persistent multifaceted cellular repair.
Evidence of engraftment and differentiation of adoptively transferred stem cells remains a major focus of contention, but persistent presence of the donated CPCeP population is supported by immunostaining for Pim-1 (Figure S7B) as well as presence of SRY and eGFP DNA by PCR in cells and infarcted hearts (Figure S7C-D). Increased telomere length in small eGFP+ myocytes relative to small eGFP- myocytes in hearts receiving CPCeP (Figure S7A) are consistent with de novo myocyte formation from the CPCeP population.
Autologous stem cell therapy suffers from critical limitations in cellular survival and persistence that may be amenable to ex vivo genetic modification for regenerative therapy. However, lentiviral modification allowing chromosomal integration and long-term persistence also poses regulatory and safety concerns. Although oncogenic transformation has not been observed with CPCeP, safe and efficacious viral modification of CPCs may require inducible Pim-1 expression. Taken together our data suggest Pim-1 mediated CPC reprogramming provides profound improvements in cardiac structure and function that may prove to be superior to current treatment modalities. The long-term goal is to advance these findings toward translational therapeutic implementation of ex vivo gene therapy to enhance stem cell-based cardiac myogenesis.
Supplementary Material
Acknowledgments
The authors thank Dr. Bruce Torbett at the Scripps Research Institute for use of the lentiviral vector, all members of the Sussman laboratory, and Alexandra Dial for their helpful discussions and technical support, and Dr. Lisa Kath for her help with statistical analysis. K.M.F. thanks the ARCS foundation for their support.
Funding Sources: K.M.F. and N.A.G. are supported by the Rees-Stealy Foundation. N.A.G. is supported by the American Heart Association Predoctoral Training Grant. M.A.S. is supported by NIH grants 5R01HL067245, 1R01HL091102, 1P01HL085577, and 1P01AG023071 (to P.A.).
Footnotes
Clinical Perspective: A revolution in the treatment of heart disease has begun, initiated by stem cell-mediated myocardial regeneration. Despite initial enthusiasm, multiple experimental and clinical studies indicate present day stem cell-based myogenesis and regeneration approaches fall short of the efficacy desired for reconstitution of myocardium following cardiomyopathic injury. Three significant problems to surmount are 1) poor initial survival of the donated cell population, 2) the number of cells required to mediate reparative processes, and 3) limited engraftment and persistence over time. Therefore, engineering stem cells to enhance their regenerative capacity by increasing their survival, proliferation, and persistence could provide for drastic improvements in both cardiac structure and function. Toward that goal, we compared cardiac progenitor cells engineered to overexpress Pim-1, a pro-survival and pro-proliferation kinase, relative to unmodified cardiac progenitor cells in order to assess potential long term benefits in an experimental infarction model. Results show long term structural and functional benefits are only gained through the use of cardiac progenitor cells expressing Pim-1. Increased de novo formation of both myocardium and vascular structures are observed using the Pim-1 engineered cells relative to control cells. Furthermore, the Pim-1 engineered cells persist for more than eight months in the recipient heart. Thus, our data demonstrate Pim-1 mediated cellular reprogramming demonstrably enhances myocardial repair and regeneration while using fewer cells than typically required for such interventions, offering a novel strategy for cellular cardiomyoplasty that is superior to current treatment modalities.
Disclosures: None
References
- 1.Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Bohm M, Quaini F, Urbanek K, Leri A, Hintze TH, Kajstura J, Anversa P. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 3.Leri A, Kajstura J, Anversa P, Frishman WH. Myocardial regeneration and stem cell repair. Curr Probl Cardiol. 2008;33:91–153. doi: 10.1016/j.cpcardiol.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez A, Rota M, Nurzynska D, Misao Y, Tillmanns J, Ojaimi C, Padin-Iruegas ME, Muller P, Esposito G, Bearzi C, Vitale S, Dawn B, Sanganalmath SK, Baker M, Hintze TH, Bolli R, Urbanek K, Hosoda T, Anversa P, Kajstura J, Leri A. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res. 2008;102:597–606. doi: 10.1161/CIRCRESAHA.107.165464. [DOI] [PubMed] [Google Scholar]
- 5.Fransioli J, Bailey B, Gude NA, Cottage CT, Muraski JA, Emmanuel G, Wu W, Alvarez R, Rubio M, Ottolenghi S, Schaefer E, Sussman MA. Evolution of the c-kit-positive cell response to pathological challenge in the myocardium. Stem Cells. 2008;26:1315–1324. doi: 10.1634/stemcells.2007-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, Silvestri F, Beltrami CA, Bussani R, Beltrami AP, Quaini F, Bolli R, Leri A, Kajstura J, Anversa P. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci U S A. 2005;102:8692–8697. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tillmanns J, Rota M, Hosoda T, Misao Y, Esposito G, Gonzalez A, Vitale S, Parolin C, Yasuzawa-Amano S, Muraski J, De Angelis A, Lecapitaine N, Siggins RW, Loredo M, Bearzi C, Bolli R, Urbanek K, Leri A, Kajstura J, Anversa P. Formation of large coronary arteries by cardiac progenitor cells. Proc Natl Acad Sci U S A. 2008;105:1668–1673. doi: 10.1073/pnas.0706315105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 9.Rota M, Padin-Iruegas ME, Misao Y, De Angelis A, Maestroni S, Ferreira-Martins J, Fiumana E, Rastaldo R, Arcarese ML, Mitchell TS, Boni A, Bolli R, Urbanek K, Hosoda T, Anversa P, Leri A, Kajstura J. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008;103:107–116. doi: 10.1161/CIRCRESAHA.108.178525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muraski JA, Rota M, Misao Y, Fransioli J, Cottage C, Gude N, Esposito G, Delucchi F, Arcarese M, Alvarez R, Siddiqi S, Emmanuel GN, Wu W, Fischer K, Martindale JJ, Glembotski CC, Leri A, Kajstura J, Magnuson N, Berns A, Beretta RM, Houser SR, Schaefer EM, Anversa P, Sussman MA. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med. 2007;13:1467–1475. doi: 10.1038/nm1671. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, Bhattacharya N, Weaver M, Petersen K, Meyer M, Gapter L, Magnuson NS. Pim-1: a serine/threonine kinase with a role in cell survival, proliferation, differentiation and tumorigenesis. J Vet Sci. 2001;2:167–179. [PubMed] [Google Scholar]
- 12.Aksoy I, Sakabedoyan C, Bourillot PY, Malashicheva AB, Mancip J, Knoblauch K, Afanassieff M, Savatier P. Self-renewal of murine embryonic stem cells is supported by the serine/threonine kinases Pim-1 and Pim-3. Stem Cells. 2007;25:2996–3004. doi: 10.1634/stemcells.2007-0066. [DOI] [PubMed] [Google Scholar]
- 13.Bachmann M, Kosan C, Xing PX, Montenarh M, Hoffmann I, Moroy T. The oncogenic serine/threonine kinase Pim-1 directly phosphorylates and activates the G2/M specific phosphatase Cdc25C. Int J Biochem Cell Biol. 2006;38:430–443. doi: 10.1016/j.biocel.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Katakami N, Kaneto H, Hao H, Umayahara Y, Fujitani Y, Sakamoto K, Gorogawa S, Yasuda T, Kawamori D, Kajimoto Y, Matsuhisa M, Yutani C, Hori M, Yamasaki Y. Role of pim-1 in smooth muscle cell proliferation. J Biol Chem. 2004;279:54742–54749. doi: 10.1074/jbc.M409140200. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Wang Z, Li X, Magnuson NS. Pim kinase-dependent inhibition of c-Myc degradation. Oncogene. 2008;27:4809–4819. doi: 10.1038/onc.2008.123. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharya N, Wang Z, Davitt C, McKenzie IF, Xing PX, Magnuson NS. Pim-1 associates with protein complexes necessary for mitosis. Chromosoma. 2002;111:80–95. doi: 10.1007/s00412-002-0192-6. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Bhattacharya N, Mixter PF, Wei W, Sedivy J, Magnuson NS. Phosphorylation of the cell cycle inhibitor p21Cip1/WAF1 by Pim-1 kinase. Biochim Biophys Acta. 2002;1593:45–55. doi: 10.1016/s0167-4889(02)00347-6. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Wang Z, Magnuson NS. Pim-1 kinase-dependent phosphorylation of p21Cip1/WAF1 regulates its stability and cellular localization in H1299 cells. Mol Cancer Res. 2007;5:909–922. doi: 10.1158/1541-7786.MCR-06-0388. [DOI] [PubMed] [Google Scholar]
- 19.Morishita D, Katayama R, Sekimizu K, Tsuruo T, Fujita N. Pim kinases promote cell cycle progression by phosphorylating and down-regulating p27Kip1 at the transcriptional and posttranscriptional levels. Cancer Res. 2008;68:5076–5085. doi: 10.1158/0008-5472.CAN-08-0634. [DOI] [PubMed] [Google Scholar]
- 20.Hammerman PS, Fox CJ, Birnbaum MJ, Thompson CB. Pim and Akt oncogenes are independent regulators of hematopoietic cell growth and survival. Blood. 2005;105:4477–4483. doi: 10.1182/blood-2004-09-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zippo A, De Robertis A, Bardelli M, Galvagni F, Oliviero S. Identification of Flk-1 target genes in vasculogenesis: Pim-1 is required for endothelial and mural cell differentiation in vitro. Blood. 2004;103:4536–4544. doi: 10.1182/blood-2003-11-3827. [DOI] [PubMed] [Google Scholar]
- 22.Muraski JA, Fischer KM, Wu W, Cottage CT, Quijada P, Mason M, Din S, Gude N, Alvarez R, Jr, Rota M, Kajstura J, Wang Z, Schaefer E, Chen X, Macdonnel S, Magnuson N, Houser SR, Anversa P, Sussman MA. Pim-1 kinase antagonizes aspects of myocardial hypertrophy and compensation to pathological pressure overload. Proc Natl Acad Sci U S A. 2008;105:13889–13894. doi: 10.1073/pnas.0709135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swan CH, Buhler B, Steinberger P, Tschan MP, Barbas CF, 3rd, Torbett BE. T-cell protection and enrichment through lentiviral CCR5 intrabody gene delivery. Gene Ther. 2006;13:1480–1492. doi: 10.1038/sj.gt.3302801. [DOI] [PubMed] [Google Scholar]
- 24.Kubo H, Jaleel N, Kumarapeli A, Berretta RM, Bratinov G, Shan X, Wang H, Houser SR, Margulies KB. Increased cardiac myocyte progenitors in failing human hearts. Circulation. 2008;118:649–657. doi: 10.1161/CIRCULATIONAHA.107.761031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyngbaek S, Schneider M, Hansen JL, Sheikh SP. Cardiac regeneration by resident stem and progenitor cells in the adult heart. Basic Res Cardiol. 2007;102:101–114. doi: 10.1007/s00395-007-0638-3. [DOI] [PubMed] [Google Scholar]
- 26.Urbich C, Rossig L, Dimmeler S. Restoration of cardiac function with progenitor cells. Novartis Found Symp. 2006;274:214–223. discussion 223-217, 272-216. [PubMed] [Google Scholar]
- 27.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 28.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai Y, Xu M, Wang Y, Pasha Z, Li T, Ashraf M. HIF-1alpha induced-VEGF overexpression in bone marrow stem cells protects cardiomyocytes against ischemia. J Mol Cell Cardiol. 2007;42:1036–1044. doi: 10.1016/j.yjmcc.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haider H, Sim EK, Lei Y, Ashraf M. Cell-based ex vivo delivery of angiogenic growth factors for cardiac repair. Arterioscler Thromb Vasc Biol. 2005;25:e144. doi: 10.1161/01.ATV.0000190665.72652.d7. [DOI] [PubMed] [Google Scholar]
- 31.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 32.Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, Dzau VJ, Pratt RE. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14:840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 33.Christoforou N, Gearhart JD. Stem cells and their potential in cell-based cardiac therapies. Prog Cardiovasc Dis. 2007;49:396–413. doi: 10.1016/j.pcad.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. Faseb J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 35.Haider HK, Ashraf M. Strategies to promote donor cell survival: Combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol. 2008 doi: 10.1016/j.yjmcc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deb KD, Sarda K. Human embryonic stem cells: preclinical perspectives. J Transl Med. 2008;6:7. doi: 10.1186/1479-5876-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang J, Slivova V, Harvey K, Valachovicova T, Sliva D. Ganoderma lucidum suppresses growth of breast cancer cells through the inhibition of Akt/NF-kappaB signaling. Nutr Cancer. 2004;49:209–216. doi: 10.1207/s15327914nc4902_13. [DOI] [PubMed] [Google Scholar]
- 38.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shujia J, Haider HK, Idris NM, Lu G, Ashraf M. Stable therapeutic effects of mesenchymal stem cell-based multiple gene delivery for cardiac repair. Cardiovasc Res. 2008;77:525–533. doi: 10.1093/cvr/cvm077. [DOI] [PubMed] [Google Scholar]
- 40.Siddiqi S, Gude N, Hosoda T, Muraski J, Rubio M, Emmanuel G, Fransioli J, Vitale S, Parolin C, D'Amario D, Schaefer E, Kajstura J, Leri A, Anversa P, Sussman MA. Myocardial induction of nucleostemin in response to postnatal growth and pathological challenge. Circ Res. 2008;103:89–97. doi: 10.1161/CIRCRESAHA.107.169334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aho TL, Sandholm J, Peltola KJ, Mankonen HP, Lilly M, Koskinen PJ. Pim-1 kinase promotes inactivation of the pro-apoptotic Bad protein by phosphorylating it on the Ser112 gatekeeper site. FEBS Lett. 2004;571:43–49. doi: 10.1016/j.febslet.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 42.Macdonald A, Campbell DG, Toth R, McLauchlan H, Hastie CJ, Arthur JS. Pim kinases phosphorylate multiple sites on Bad and promote 14-3-3 binding and dissociation from Bcl-XL. BMC Cell Biol. 2006;7:1. doi: 10.1186/1471-2121-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.