Abstract
Purpose
Discovery of agents that protect or mitigate normal tissue from radiation injury during radiotherapy, accidents, or terrorist attacks, is of importance. Specifically, bone marrow insufficiency, with possible infection due to immune suppression, can occur after total body irradiation (TBI) or regional irradiation and is a major component of the acute radiation syndrome. The purpose of this study was to identify novel radioprotectors and mitigators of the hematopoietic system.
Experimental Design
High throughput screening of small molecule libraries was performed using viability of a murine lymphocyte line as a read out with further validation in human lymphoblastoid cells. The selected compounds were then tested for their ability to counter TBI lethality in mice.
Results
All of two major classes of antibiotics, tetracyclines and fluoroquinolones, which share a common planar ring moiety, were radioprotective. Furthermore, tetracycline protected murine hematopoietic stem/progenitor cell populations from radiation damage and allowed 87.5% of mice to survive when given before and 35% when given 24 h after lethal TBI. Interestingly, tetracycline did not alter the radiosensitivity of Lewis lung cancer cells. Tetracycline and ciprofloxacine also protected human lymphoblastoid cells, reducing radiation-induced DNA double strand breaks by 33% and 21%, respectively. The effects of these agents on radiation lethality are not due to the classical mechanism of free radical scavenging but potentially through activation of the histone acetyl transferase Tip60 and altered chromatin structure.
Conclusions
Tetracyclines and fluoroquinolones can be robust radioprotectors and mitigators of the hematopoietic system with potential utility in anti-cancer radiotherapy and radiation emergencies.
Introduction
Total body irradiation (TBI) with 5–10 Gy doses results in an acute radiation syndrome (ARS) with possible lethality due primarily to hematopoietic failure and/or infection caused by immune impairment (1). Indeed, immunohematopoietic cells are very sensitive to radiation, dying mainly in interphase by apoptosis (2).
The peaceful and military use of atomic power after World War II spurred efforts to find agents for the prophylaxis, mitigation, or treatment of radiation injury; efforts that have been re-intensified recently by an increased threat of terrorist use of radiation sources. Numerous compounds have radioprotective effects (3, 4). Examples are tempol, antioxidant vitamins and melatonin, with the best studied being the thiol Amifostine (WR2721). Most are free radical scavengers that reduce initial radiation-induced DNA damage and work best if added just before or at the time of irradiation. Because of this, and their poor toxicity profile, amifostine and similar compounds are not practical countermeasures in a radiation incident (5). More recently, targeting superoxide dismutase (MnSOD) (3, 4) and activation of toll-like receptor 5 /NF-κB pathway by flagellin suggest that alternative approaches may be of value (6). Furthermore, certain cytokines such as G-CSF, SCF, and GM-CSF can accelerate recovery of the hematopoietic system after TBI (3, 4). However, the dearth of agents with robust, prolonged efficacy, broad specificity, and minimal toxicity that could protect a large population in the event of a radiological emergency, or that could increase the radiotherapeutic benefit of cancer treatment, warrants further searches.
We chose an unbiased high throughput screening (HTS) approach to identify modulators of radiation response, with the hypothesis that effective agents might form classes that share molecular signatures i.e. common chemical structures and biological pathways. Agents were given either before or after radiation to determine if they prevented or mitigated against radiation toxicity, respectively, or both. Radiation-induced apoptosis of a murine T lymphocyte cell line (Til1) was the primary screening endpoint and human lymphoblastoid cells (LCLs) were used for validation of compounds that could act across species barriers. Finally, agents were tested for their ability to protect mice and their immunohematopoietic system following TBI.
From screening of 3,600 bioactive compounds with known biological activity, all members of two classes of antibiotics, tetracyclines and fluoroquinolones, 18 in number, stood out as possessing radioprotective properties. In general, these compounds had low toxicity and representative compounds could improve progenitor cell and whole animal survival after lethal TBI. Some were effective in vivo even when given after TBI. We conclude that HTS, although unable to fully recapitulate many aspects of the complex in vivo ARS response, can be used to identify agents that modulate radiation responses.
Materials and Methods
Small molecule libraries
3,600 bioactive compounds from Prestwick (Prestwick, Washington DC), Biomol (Biomol International, Inc., Plymouth Meeting, PA) and Spectrum (MicroSource Discovery Systems, Gaylordsville, CT) libraries were tested at a 10 µM final concentration in 1% DMSO using an automated Biomek FX Workstation (Beckman Coulter, Inc., Fullerton, CA).
Cell lines and irradiation
A CD4+CD8+ murine T lymphocyte cell line [Til1, (7)] was cultured in DMEM with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin G, and 100 µg/ml streptomycin. Human lymphoblastoid cell lines (LCLs) derived from peripheral blood lymphocytes by transformation with Epstein Bar virus (8) were cultured as published (9). Cells were irradiated with a Mark I Cs137 irradiator at a dose rate of 5 Gy/min.
HTS of libraries
Ten thousand Til1 cells were dispensed into each well of 384-well plates using a Multidrop384 (Thermo Scientific, Waltham, MA). To identify radioprotectors, cells were pre-incubated with compounds for 3 h prior to irradiation (2 Gy). For mitigators, cells were irradiated 1 h prior to compound loading. Cell viability was determined at 24 h post-irradiation by luminescence-based measurement of ATP production (ATPlite reagent, Perkin-Elmer, Waltham, MA) with a SpectraMax M5 microplate reader (Molecular Devices, Sunnyvale, CA). Each compound was represented 4 times and the average was used for data processing. The Z’ factor (10) for the assay was > 0.5. To qualify for validation, the average values for the compound normalized to the vehicle controls had to be > 130 %.
Similarity and substructure analysis
Similarity and substructure searches were performed on the Collaborative Drug Discovery (CDD)™ platform. The entire library was ranked according to its structural similarity to a referenced hit based upon the Tanimoto coefficient, excluding coefficients < 0.7. Hits and non-hits within the library with similar structure were identified and a substructure analysis performed to determine minimal elements.
Secondary screening with annexin V/Propidium Iodide staining
Human LCLs were incubated for 2 h with the compounds at the final concentrations indicated, irradiated with 5 Gy, and 48 h later apoptosis assessed using annexin V/propidium iodide (BioVision, Inc., Mountain View, CA) followed by flow cytometry.
Animal survival assay
C3Hf/Kam mice were bred and maintained in a strict defined-flora, pathogen-free environment in the American Association of Laboratory Animal Care-accredited Animal Facilities of Department of Radiation Oncology, University of California (Los Angeles, CA). The UCLA Animal Care and Use Committee approved all experiments, which were performed in accordance with all local and national guidelines for the care and use of animals. Male mice, 10–15 weeks old, received 8 Gy TBI from a Gamma cell 40 irradiator (Cs137 source; Atomic Energy of Canada, Ltd.) at a dose rate of 67 cGy/ min. For radioprotection, drug or vehicle was injected i.p. 24 h and 1 h prior to TBI. For mitigation, they were given five times daily starting 24 h after TBI. Mice were monitored for 30 d using standard criteria for humane euthanasia as an endpoint.
Colony formation assay
Bone marrow cells (BMC) were harvested from femurs of C3Hf/Kam mice (n=4 per group) treated with vehicle, tetracycline, TBI with vehicle, or TBI with tetracycline. Water or tetracycline at 150 mg/kg was given i.p. 24 h and 1h prior to TBI and BMC collected 3 d later. Red blood cells (RBC) were lysed using ACK buffer (Lonza, Walkersville, MD), BMC were resuspended in IMDM containing Methocult M3234 (StemCell Technologies, Vancouver, Canada) and 10 ng/ml recombinant mGM-CSF (Invitrogen, Carlsbad, CA), and 2×104 BMC were plated into a 35 mm dish in triplicate. Colonies were counted after 9 d.
Clonogen survival assay
Exponentially growing murine Lewis lung cancer cells were pretreated with tetracycline at 5, 10, or 20 µM for 4 h, trypsinized, irradiated with 2, 4, 6 Gy, and plated in 100-mm dishes in triplicate. After 10 d, colonies were stained with crystal violet in 50% ethanol. Colonies consisting of >50 cells were counted to determine clonogen survival.
γ-H2AX immunofluorescence
Human LCLs were collected after 18 h incubation with the indicated compound and irradiated with 2 Gy. Fixing and staining procedures were performed as published (9).
Reactive oxygen species (ROS) measurement
Intracellular ROS were measured using 2’-7’-dichlorofluorescein diacetate (DCFH-DA, Invitrogen) (11, 12). TiL1l cells were incubated with compound for 2 h, 25 µM of DCFH-DA probe was added for 1 h, and irradiated with a high dose (10 Gy) to generate significant ROS. Fluorescence was measured by flow cytometery (13).
Tip60 HAT assay
Hela cells or LCLs were incubated with compounds for 4 h, extracts prepared and Tip60 immunoprecipitated (Upstate Biotechnology, Waltham, MA). Precipitates were incubated with biotinylated histone H4 peptide and AcetylCoA for 15 min (14), an aliquot was immobilized onto streptavidin plates and acetylation was detected by ELISA using acetyl-lysine specific antibody (Upstate Biotechnology).
Results
Primary screening and structure activity relationships (SAR)
Libraries of 3,600 bioactive compounds were screened at 10 µM for their ability to cytoprotect Til1 cells. Pilot experiments determined that the most suitable experimental design was adding compounds either 3 h before or 1 h after 2 Gy, which reduced viability of vehicle-treated cells to approximately 30% at 24 h. Compounds were considered as possible candidates if they increased this value by 130%. When added before irradiation, 22 hits were obtained (0.61%); after irradiation, 18 hits (0.5%). Hits were confirmed using the same screening assay over the dose range 195 nM to 100 µM. As a result, 10 (0.28%) and 7 (0.19%) compounds were chosen as the most reliable radioprotectors and mitigators, respectively (P < 0.05, Table 1, left column). The EC50 of these compounds generally ranged between 0.2 to 10 µM with a large therapeutic window of 1 to 2 logs.
Table 1.
EC50s for positive compounds
| primary screening | EC50 (µM) |
similarity analysis | EC50 (µM) |
|---|---|---|---|
| Prevention | Prevention | ||
| norfloxacin | 3.67 | rolitetracycline | 4.03 |
| 5’-AMP* | 6.05 | oxytetracycline | 2.49 |
| doxycycline | 0.5 | methacycline | 1.44 |
| tetracycline | 0.33 | gatifloxacin | 2.19 |
| chlorotetracycline | 1.15 | levofloxacin | 2.19 |
| minocycline | 0.24 | enoxacin | 13.87 |
| meclocycline | 0.55 | flumequine | 58.2 |
| ciprofloxacin | 1.09 | lomefloxacin | 27.77 |
| moxifloxacin | 0.8 | ofloxacin | 12.55 |
| CPA† | 2.67 | sarafloxacin | 0.7 |
| Mitigation | |||
| linoleic acid | 8.51 | ||
| scopolamine | 9.86 | ||
| rifabutin | 4.11 | ||
| vidarabine | 7.83 | ||
| acivicin | 1.13 | ||
| deoxyadenosine | 8.23 | ||
| tilorone | 2.49 |
adenosine 5’-monophosphate
cyclopiazonic acid
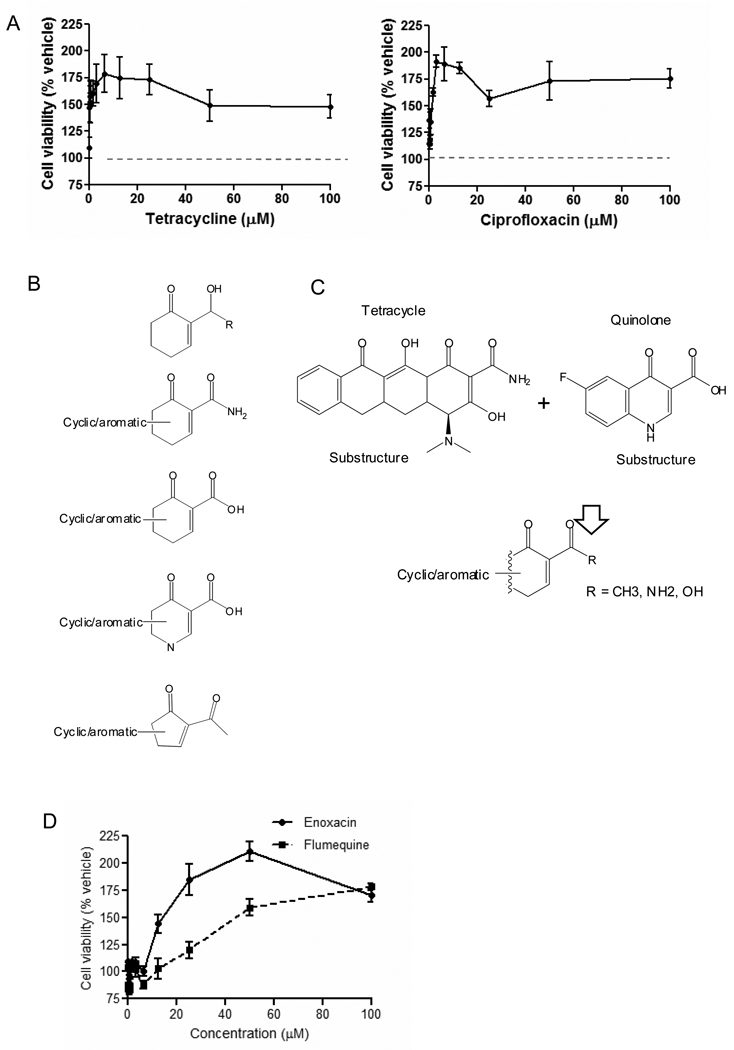
Of the 10 compounds that radioprotected Til1 cells, 8 were tetracycline derivatives or fluoroquinolones (representatives in Fig. 1A). No other class of antibiotics were radioprotective even though the Spectrum library alone contains more than 194 bactericidals out of 2,000 compounds and there were large representations of β-lactam based drugs such as penicillin G and ampicillin and macrolides such as erythromycin. To make sure that this was not simply a dosage effect, penicillin G, ampicillin, and erythromycin were additionally assayed at multiple doses up to 100 µM and were negative (P > 0.05, data not shown). Radioprotection by antibiotics seems therefore to be a sole property of tetracyclines and fluoroquinolones and is obviously separate and distinct from their bactericidal properties.
Figure 1.
Dose-response of representative radioprotectors and SAR/substructure analysis. (A) tetracycline and ciprofloxacin. (B) The first substructure was present only in compounds that were negative in the initial screen (11 compounds in total). All parent compounds containing this substructure possessed no adjacent cyclic/aromatic planar character and were all conjugated at the acyl functionality (R) to various other molecules. The second structure was present in all 12 tetracyclines. No compound within the libraries screened contains the third substructure. Eighteen compounds contained the fourth substructure, categorized as the fluoroquinolone class and one compound (cyclopiazonic acid) contained the fifth substructure. (C) The core substructures contained in all positive hits for tetracyclines and fluoroquinolones with a common structural theme shown. (D) Examples from similarity analysis (Table 1, right column). The percent cell viability normalized to vehicle control value is plotted. Note that the decrease in protective activity is presumably due to drug toxicity.
All positive hits within and across libraries were computationally compared using the Tanimoto rule of similarity to identify possible SAR and common substructures. The results are in Fig. 1B, with a general formula indicating a common cyclic/planar aromatic ring in Fig. 1C. Of interest is that reverse analysis of all active substructures within negative data yield the first substructure in Fig 1B, in which all of the compounds do not possess the adjacent ring character and all are conjugated at the acyl functionality. The only other group of positive compounds with structural similarity was three nucleotide derivatives, deoxyadenosine (mitigation), vidarabine (mitigation), and 5’-AMP (prevention), which has previously been reported to be radioprotective (15–16)(Table 1-left column).
“Similarity searches” were also used to identify false negative tetracyclines and fluoroquinolones in the libraries. Of the 10 tetracyclines, 5 had already been identified by the primary screening and of the 12 fluoroquinolones, 3 had been recognized as hits. To determine if the others had been miscategorized, for example because the dosage was suboptimal, or were true negatives, they were retested in the primary screen over a wider dose range. Only 10 of the 14 antibiotics were commercially available, but of these all 3 tetracyclines and all 7 fluoroquinolones retested as positive by the original criteria (cell viability > 130% and P < 0.05 compared to the vehicle control; Table 1, right column). Four of these had not been detected earlier because their EC50 was above the 10 µM test dose (representatives in Fig. 1D). In other words, all 18 members of these 2 classes of antibiotics that could be tested were radioprotective.
Since each compound was tested for both preventative and mitigating activity, we were able to determine if there was any correlation between these activities and if any were active when given both before and after irradiation. There was a weak correlation of 0.344 for Prestwick, 0.177 for Spectrum, 0.524 for Biomol-enzyme, and 0.272 for Biomol-lipid libraries (Fig. S1). The overall correlation was however statistically highly significant (P < 0.001) due to the large number of compounds. A few compounds fell outside the 95% confidence level ellipse in the upper quadrant indicating that they had both preventative and mitigating activity. These will be the subject of another publication.
Secondary screening using human LCLs
For further validation, the ability of 20 radioprotectors from the primary screens (Table 1) to radioprotect EBV-transformed wild type (W-T) LCLs was assessed using Annexin V and propidium iodide staining. Ataxia-telangiectasia (A-T) LCLs were included because even moderately effective radioprotectors could be beneficial for these patients given their hypersensitivity to radiation (17). Overall, 10 of the 20 compounds showed activity (Table 2), which was remarkable since the cells were from a different species, were EBV-transformed B cells as opposed to non-transformed T cells, and were tested in a different assay using a different radiation protocol. However, there was no consistent pattern of ATM dependence of the potential radioprotective effect. Four of the 20 significantly reduced radiation-induced apoptosis (P < 0.05) in both W-T and A-T; 3 only in W-T; and 3 only in A-T. The data therefore speak to the universality of the effects of these compounds, but further studies are needed to explore the role of ATM in this form of radioprotection.
Table 2.
Secondary screening by AnnexinV/PI staining % conferred reduction compared to vehicle controls is shown. Only the compounds with statistically significant reduction in apoptosis in W-T or A-T LCLs are shown (P < 0.05).
| Compound (µM) | W-T | A-T |
|---|---|---|
| tetracycline (20) | 19 | NS |
| norfloxacin (40) | 28 | 28 |
| levofloxacin (40) | 16 | 34 |
| doxycycline (10) | NS | 31 |
| chlorotetracycline (20) | NS | 48 |
| moxifloxacin (10) | NS | 50 |
| ciprofloxacin (5) | 21 | NS |
| flumequine (100) | 25 | 37 |
| enoxacin (100) | 18 | NS |
| 5’-AMP (20) | 34 | 13 |
NS=not significant
Tetracycline protects the immunohematopoietic system and allows mice to survive lethal TBI
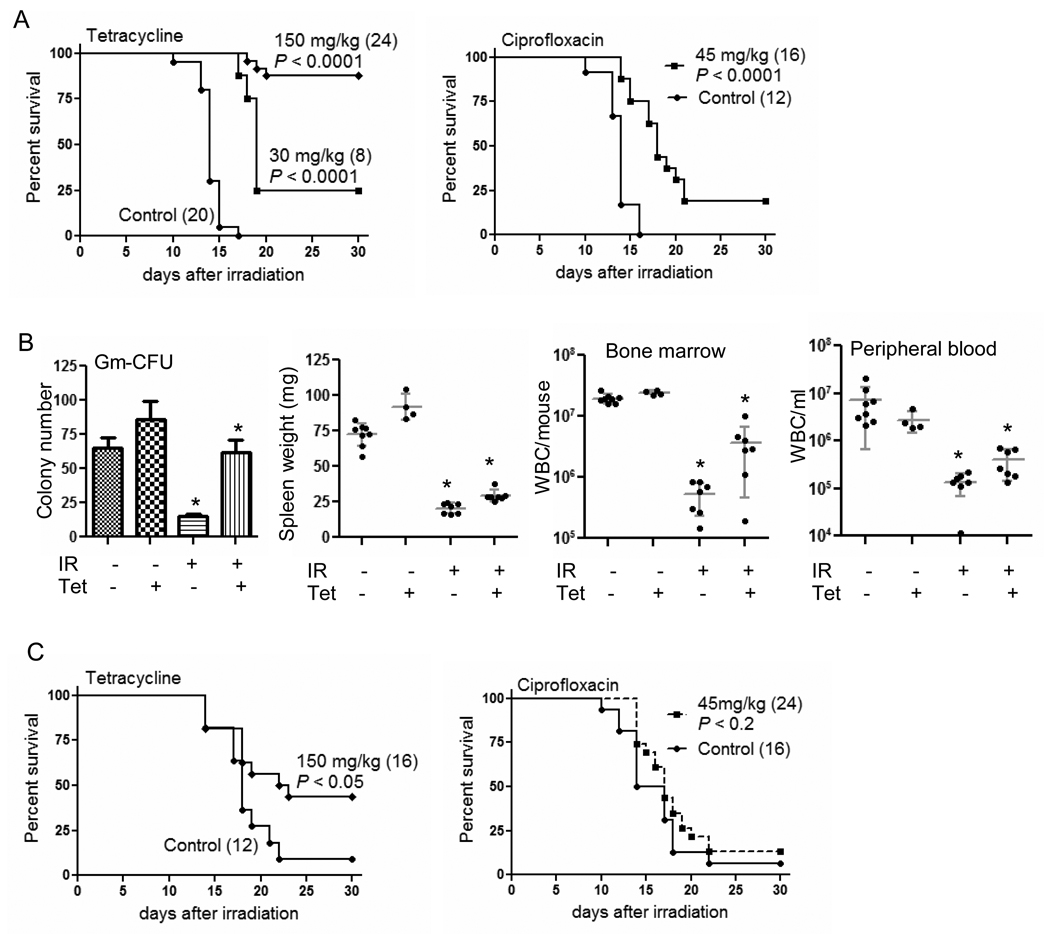
Tetracycline and ciprofloxacin were chosen as representatives of the two classes of antibiotics for in vivo studies, in part because they have been clinically most widely used and were active in both murine and human assays. When tetracycline (150 mg/kg) was given 24 h and 1 h before a lethal dose of 8 Gy TBI, 87.5% of mice survived while all vehicle-treated controls died (Fig. 2A, P < 0.0001). Tetracycline at 30 mg/kg and ciprofloxacin at 45 mg/kg showed some, but less, activity. The same tetracycline schedule that improved animal survival (150 mg/kg) caused an increase in hematopoietic stem/progenitor cells as shown by a granulocyte/macrophage colony formation assay, higher spleen weights, and higher white blood cell counts in bone marrow and peripheral blood after lethal TBI (Fig. 2B). Remarkably, when tetracycline was given as 5 daily injections (150 mg/kg) starting 24 h after 8 Gy TBI, survival was also significantly enhanced (Fig. 2C). Ciprofloxacin given at 45 mg/kg by the same schedule failed to mitigate radiation-induced lethality.
Figure 2.
Effect of tetracycline and ciprofloxacin in vivo against lethal TBI. (A) Two intraperitoneal injections of tetracycline or ciprofloxacin at 24 h and 1 h prior to 8 Gy TBI protected mice from lethality. This effect was most prominent with tetracycline at 150 mg/kg (87.5% survival). (B) The same schedule of tetracycline treatment at 150 mg/kg as Fig. 2A protects the immunohematopoietic system from lethal TBI. Spleen weights and white cell counts were performed 3 d later and bone marrow cells were pooled from 4 mice per treatment group for assessment of granulocyte-macrophage colony forming units (Gm-CFU). The data from two separate experiments were shown (combined n=7 except tetracycline-treated group: n=4). * indicates P < 0.05 (IR vs IR+tetracycline). (C) 5 daily injections of tetracycline starting 24 h after 8 Gy TBI improves animal survival (P < 0.05), while ciprofloxacin failed as a mitigator. Number of mice in each treatment group is in parenthesis.
Tetracycline does not interfere with radiation treatment of mouse tumor cells
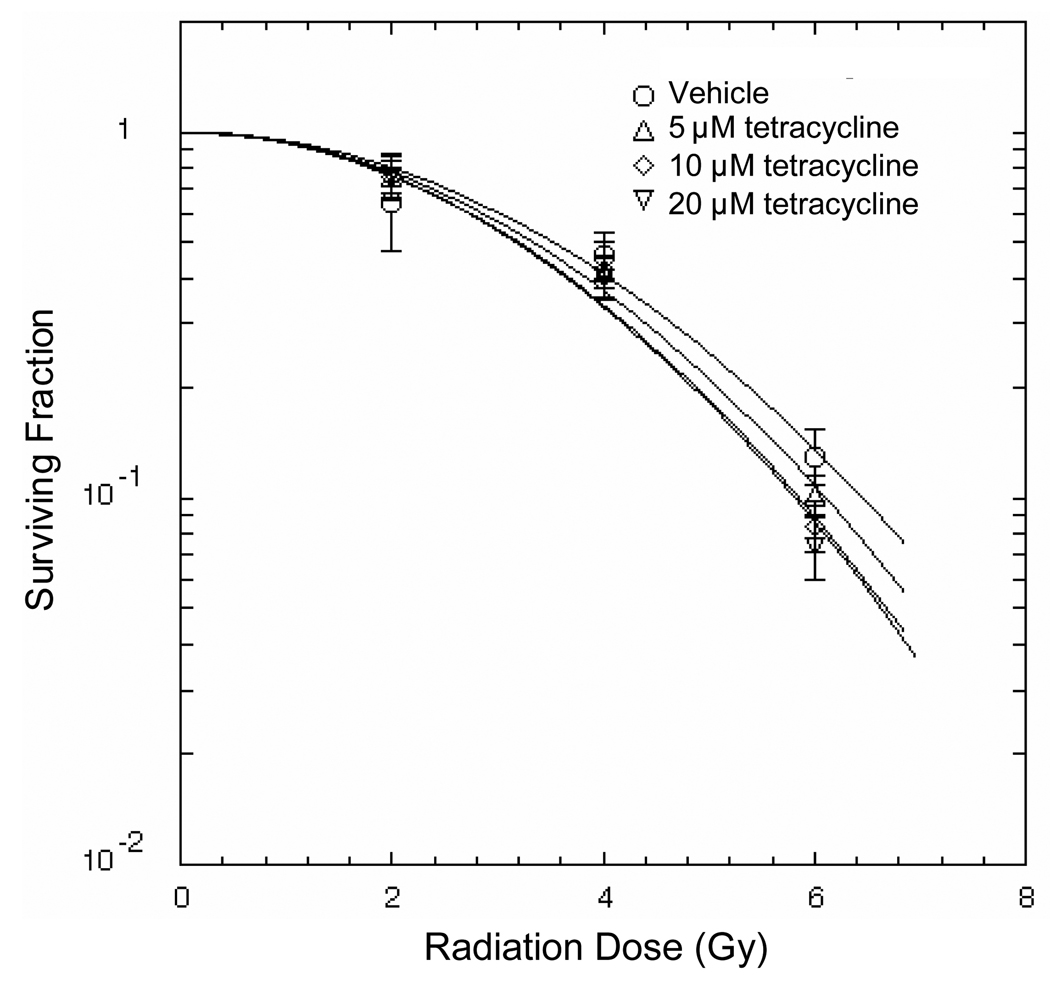
To determine if tetracycline would protect cancer as well as normal cells, it was added to murine Lewis lung cancer cells (LLC) at doses equal or higher than those that radioprotected Til1 and human LCLs and a clonogen survival assessed after various radiation doses (Fig. 3). Tetracycline did not protect LLC from radiation treatment, indicating that this drug may have potential in cancer radiotherapy.
Figure 3.
Effect of tetracycline on the radiosensitivity of Lewis lung cancer cell (LLC). Clonogenic assays with different doses of tetracycline were performed. The range of tetracycline doses used in this assay did not interfere with radiation treatment on LLC.
Tetracycline and ciprofloxacin protect LCLs from radiation-induced DNA double-strand breaks (DSBs)
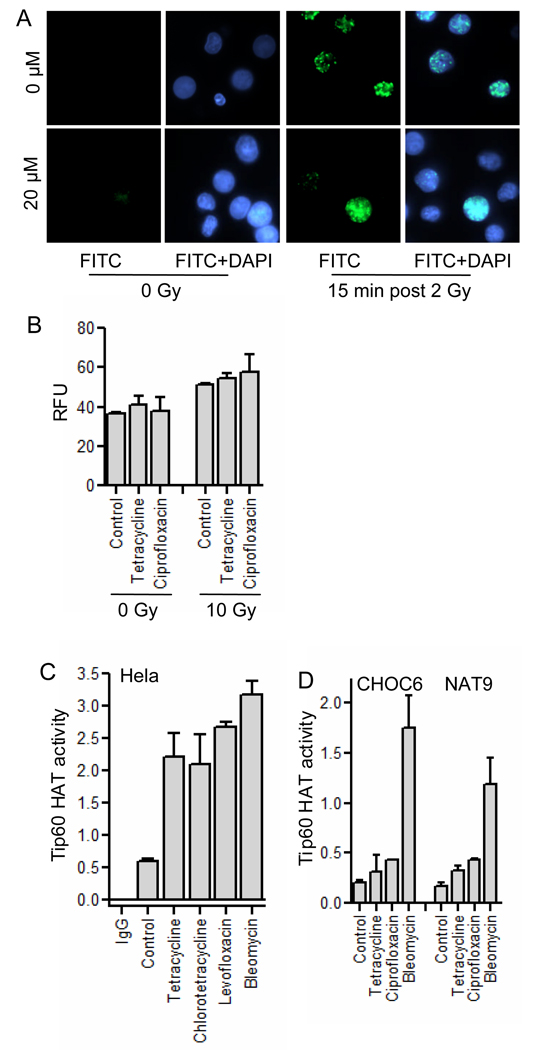
Having confirmed the radioprotective activity of tetracycline and ciprofloxacin both in vitro and in vivo, we explored their mechanism of radioprotection. First, the incidence of radiation-induced DNA DSBs in W-T LCLs after 2 Gy, as measured by phosphorylation of histone H2AX (γ-H2AX), was decreased by prior treatment with tetracycline (Fig. 4A and quantified in Table S1), ciprofloxacin, chlorotetracycline and moxifloxacin (Table S1), suggesting that initial DNA DSB formation or repair was affected. Although there are no apparent redox centers within these structures, the inability of these compounds to act as free radical scavengers was confirmed by measuring levels of reactive oxygen species (ROS) by flow cytometry in Til1 cells immediately after irradiation using DCFH (Fig. 4B).
Figure 4.
Protection against radiation-induced DNA DSBs. (A) The effect of tetracycline on radiation-induced DNA DSBs was assessed by γ-H2AX immunofluorescence foci formation at 15 min after irradiation at 2 Gy in human W-T LCLs. Tetracycline at 20 µM reduced radiation-induced foci by 33%. (B) Tetracycline (10 µM) or ciprofloxacin (5 µM) did not reduce radiation-induced ROS in Til1 cells. Tetracyclines and fluoroquinolone antibiotics stimulated Tip60 HAT activity in Hela cells to the level similar to the radiomimetic agent bleomycin (C) and in two human W-T LCLs (CHOC6 and NAT9) to a lesser degree (D). 5 µM bleomycin, 25 µM tetracycline, 10 µM ciprofloxacin, 10 µM levofloxacin, and 10 µM chlorotetracycline were utilized for the HAT assay.
The impact of tetracycline on the radiation-induced DNA damage downstream response was then assessed by Western blotting for phosphorylated forms of ATM, Chk2, DNA-PKcs, p53, and SMC1 proteins in the cell extracts of W-T and A-T LCLs following irradiation with 10 Gy (Fig. S2). Tetracycline treatment did not robustly alter the phosphorylation status of these molecules, however activation of a putative radiation target upstream of ATM protein, specifically Tip60 histone acetyltransferase (HAT), was affected. Tip60 HAT plays a central role in the DNA damage response being activated by ionizing radiation or bleomycin (14), the latter being used as the radiomimetic agent in our assay. All tested antibiotics upregulated Tip60 HAT activity strongly in Hela cells (4–5 fold of control) to a level similar to that of bleomycin (Fig. 4C), and tetracycline and ciprofloxacin did the same in human W-T LCLs, yet to a lesser degree (Fig. 4D, 2–3 fold of control).
Discussion
Tetracyclines and fluoroquinolones are broad-spectrum antibiotics that act against gram-positive and gram-negative bacteria. Non-antimicrobial activities of tetracyclines include inhibiting inflammation, angiogenesis, and apoptosis, as well as chelating divalent metal cations (18). Of possible relevance to this study, minocycline prevented neuronal cell apoptotic death in mice, reducing tissue injury and neurological deficits (19). Minocycline was similarly shown to delay mortality in a transgenic mouse model of Huntington disease (20) and to hinder progression of amyotrophic lateral sclerosis in mice (21). Fluoroquinolones inhibit DNA gyrase (prokaryotic topoisomerase II) and topoisomerase IV in bacteria through direct chromosome binding, but certain members of this family also display activity against eukaryotic topoisomerase II. They can therefore be toxic to proliferating cells and are being explored as anticancer drugs (22). They have been documented as having anti-inflammatory properties, decreasing the synthesis of pro-inflammatory cytokines like IL-1 and TNF (23, 24). Furthermore, fluoroquinolones including ciprofloxacin, sparfloxacin, and clinafloxacin have been reported to stimulate hemotopoiesis and slightly prolong the survival of sublethally irradiated mice (25), while aiding survival of TBI mice transplanted with bone marrow cells by decreasing the systemic spread of bacteria (26). It should be noted that the mice used in our study have a limited flora and lack culturable gram-negative organisms. Because of this, and the direct radioprotection afforded murine T lymphocytes and human B lymphocytes in vitro, we believe that the anti-microbial action of these compounds does not contribute to our in vivo findings.
The common planar ring structure that is shared by tetracyclines and quinolones and absent from other classes of antibiotics might explain their common radioprotective activity and provide a lead scaffold for compounds with improved efficacy. How this structure holds the core radioprotective activity however needs further investigation. Free radical scavenging, which is a common attribute of most radioprotectors (3), is clearly not involved. Both tetracycline and ciprofloxacin did reduce radiation-induced γH2AX foci formation although activation of downstream DNA damage signaling molecules such ATM, DNA-PKcs, Chk2, p53 and SMC1 did not seem to be robustly altered. We were able to show a radioprotective effect of some compounds in A-T LCLs, but not others and a clear picture did not emerge as to the ATM-dependency. This may be because DNA DSBs in A-T LCLs after irradiation are more extensive than in W-T LCLs (data not shown) and more difficult to repair. However, these compounds did activate the HAT Tip60 (Fig. 4C & D), indicating that they might directly influence chromatin structure and DNA damage responses.
Tip60 is a key component in the remodeling of chromatin structure during the repair of DNA DSBs (27–30). Since the extent of chromatin condensation influences radiosensitivity (31, 32), and genetic or chemical inactivation of Tip60 increases the sensitivity of cells to DNA damage (14, 33), this could be a mechanism of radioprotection. Tip60 HAT recruitment and histone acetylation surrounding DSBs have been found to be mediated by the HAT cofactor Traap (34, 35). Traap depletion impairs DNA damage-induced H4 acetylation and recruitment of RAD51 and BRCA1, leaving γ-H2AX accumulation and ATM-dependent DNA damage signaling intact. This could explain why, although ATM is one target of Tip60 (14), ATM did not appear to be consistently activated in our study. On the other hand, the homologous DNA repair machinery involving RAD51 and BRCA1 seems to require Tip60 dependent chromatin relaxation (34, 35) that the intercalating properties of tetracycline and ciprofloxacin might induce. A similar mechanism was proposed for the action of chloroquine, which can act as a radioprotector, but this drug activates ATM (36, 37).
Our finding that tetracycline promotes survival of mice even if given after TBI raises questions as to whether it ameliorates persisting radiation damage (38), activates signaling pathways leading to accelerated recovery of the immunohematopoietic system, or mitigates by other mechanisms. Minocycline has recently been shown to inhibit release of the non-histone DNA-binding high mobility group box-1 (HMGB1) protein in oxygen-glucose deprived PC12 cells and trigger p38mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinases (ERK1/2) pro-survival pathways (39), which leaves options open for further research.
Finally, it should be noted that antibiotics are already an important component of the treatment of radiation injuries (3, 40). Indeed, quinolone antibiotics and penicillin were attributed to reducing mortality in the Chernobyl nuclear accident (41). Our findings suggest that the choice of antibiotics in such emergencies, as well as in cancer patients receiving radiotherapy, could benefit from consideration of more than purely microbiological criteria since not all classes of antibiotics are active. Further, although tetracyline and ciprofloxacin have long been utilized in the clinic and there is no evidence of long-term deleterious effects, their ability to inhibit or enhance radiation carcinogenesis should be investigated, since modulation of radiation responses could have either outcome depending on the mechanistic pathway through which they work.
Translational Relevance
The sensitivity of the hematopoietic system to ionizing radiation results in adverse side effects following exposure and there is a lack of agents able to prevent or mitigate this damage. Our study using high throughput screening of small molecule bioactive compounds identified two major classes of antibiotics, specifically tetracyclines and fluoroquinolones, as being radioprotective of normal hematopoietic tissue both in vitro and in vivo. These drugs therefore have potential to improve the outcome of radiation exposure in a number of different scenarios and, as they do not seem to affect tumor responses, this may include cancer radiotherapy. Our findings suggest that the choice of antibiotics in settings of radiation exposure may benefit from consideration of more than purely anti-microbial criteria. In addition, the common structural moiety shown to be shared by both classes of antibiotics might serve as a lead scaffold for the discovery of better radioprotectors and mitigators.
Supplementary Material
Acknowledgements
This work was supported by UCLA’s Center for Biological Radioprotectors U19 AI067769/NIAID and Dana-Farber/Harvard Center for Medical Counter Measures against Radiation U19 AI067751.
References
- 1.Daly A, McAfee S, Dey B, et al. Nonmyeloablative bone marrow transplantation: Infectious complications in 65 recipients of HLA-identical and mismatched transplants. Biol Blood Marrow Transplant. 2003;9:373–382. doi: 10.1016/s1083-8791(03)00100-9. [DOI] [PubMed] [Google Scholar]
- 2.Nogami M, Huang JT, Nakamura LT, Makinodan T. T cells are the cellular target of the proliferation-augmenting effect of chronic low-dose ionizing radiation in mice. Radiation Res. 1994;139:47–52. [PubMed] [Google Scholar]
- 3.Murray D, McBride WH. Radioprotective Agents. In: Klayman DL, Copeland ES, editors. Kirk-Othmer Encyclopedia of Chemical Technology. 3rd. ed. ECT: John Wiley & Sons, Inc; pp. 801–832. [Google Scholar]
- 4.Greenberger JS. Radioprotection. In vivo. 2009;23:323–336. [PMC free article] [PubMed] [Google Scholar]
- 5.Seed TM. Radiation protectants: current status and future prospects. Health physics. 2005;89:531–545. doi: 10.1097/01.hp.0000175153.19745.25. [DOI] [PubMed] [Google Scholar]
- 6.Burdelya LG, Krivokrysenko VI, Tallant TC, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Droll A, Dougherty ST, Chiu RK, et al. Adhesive interactions between alternatively spliced CD44 isoforms. J Biol Chem. 1995;270:11567–11573. doi: 10.1074/jbc.270.19.11567. [DOI] [PubMed] [Google Scholar]
- 8.Gatti RA, Jonsson J. Detection of antibodies against virally-induced tumor-associated antigens by mixed hemadsorption. Int J cancer. 1974;13:795–807. doi: 10.1002/ijc.2910130608. [DOI] [PubMed] [Google Scholar]
- 9.Pollard JM, Reboucas JS, Durazo A, et al. Radioprotective effects of manganese-containing superoxide dismutase mimics on ataxia-telangiectasia cells. Free radical biology & medicine. 2009;47(3):250–260. doi: 10.1016/j.freeradbiomed.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 11.Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983;130:1910–1917. [PubMed] [Google Scholar]
- 12.Benov L, Sztejnberg L, Fridovich I. Critical evaluation of the use of hydroethidine as a measure of superoxide anion radical. Free Radical Biol Med. 1998;25:826–831. doi: 10.1016/s0891-5849(98)00163-4. [DOI] [PubMed] [Google Scholar]
- 13.Hafer K, Iwamoto KS, Schiestl RH. Refinement of the dichlorofluorescein assay for flow cytometric measurement of reactive oxygen species in irradiated and bystander cell populations. Radiation Res. 2008;169:460–468. doi: 10.1667/RR1212.1. [DOI] [PubMed] [Google Scholar]
- 14.Sun Y, Jiang X, Chen S, Fernandes N, Price BD. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc Natl Acad Sci USA. 2005;102:13182–13187. doi: 10.1073/pnas.0504211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofer M, Mazur L, Pospisil M, Weiterova L, Znojil V. Radioprotective action of extracellular adenosine on bone marrow cells in mice exposed to gamma rays as assayed by the micronucleus test. Radiation Res. 2000;154:217–221. doi: 10.1667/0033-7587(2000)154[0217:raoeao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Pospisil M, Hofer M, Netikova J, et al. Elevation of extracellular adenosine induces radioprotective effects in mice. Radiation Res. 1993;134:323–330. [PubMed] [Google Scholar]
- 17.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nature Rev. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 18.Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258–265. doi: 10.1016/j.jaad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez Mejia RO, Ona VO, Li M, Friedlander RM. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery. 2001;48:1393–1399. doi: 10.1097/00006123-200106000-00051. [DOI] [PubMed] [Google Scholar]
- 20.Chen M, Ona VO, Li M, et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nature Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- 21.Zhu S, Stavrovskaya IG, Drozda M, et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- 22.Sissi C, Palumbo M. The quinolone family: from antibacterial to anticancer agents. Current Med Chem. 2003;3:439–450. doi: 10.2174/1568011033482279. [DOI] [PubMed] [Google Scholar]
- 23.Dalhoff A, Shalit I. Immunomodulatory effects of quinolones. Lancet Inf Dis. 2003;3:359–371. doi: 10.1016/s1473-3099(03)00658-3. [DOI] [PubMed] [Google Scholar]
- 24.Dalhoff A. Immunomodulatory activities of fluoroquinolones. Infection. 2005;33 Suppl 2:55–70. doi: 10.1007/s15010-005-8209-8. [DOI] [PubMed] [Google Scholar]
- 25.Shalit I, Kletter Y, Weiss K, Gruss T, Fabian I. Enhanced hematopoiesis in sublethally irradiated mice treated with various quinolones. Eur J Haematol. 1997;58:92–98. doi: 10.1111/j.1600-0609.1997.tb00930.x. [DOI] [PubMed] [Google Scholar]
- 26.Kletter Y, Singer A, Nagler A, Slavin S, Fabian I. Ciprofloxacin enhances hematopoiesis and the peritoneal neutrophil function in lethally irradiated, bone marrow-transplanted mice. Exp Hematol. 1994;22:360–365. [PubMed] [Google Scholar]
- 27.Bird AW, Yu DY, Pray-Grant MG, et al. Acetylation of histone H4 by Esa1 is required for DNA double-strand break repair. Nature. 2002;419:411–415. doi: 10.1038/nature01035. [DOI] [PubMed] [Google Scholar]
- 28.Kusch T, Florens L, Macdonald WH, et al. Acetylation by Tip60 is required for selective histone variant exchange at DNA lesions. Science. 306:2084–2087. doi: 10.1126/science.1103455. [DOI] [PubMed] [Google Scholar]
- 29.Squatrito M, Gorrini C, Amati B. Tip60 in DNA damage response and growth control: many tricks in one HAT. Trends Cell Biol. 2006;16:433–442. doi: 10.1016/j.tcb.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Carrozza MJ, Utley RT, Workman JL, Cote J. The diverse functions of histone acetyltransferase complexes. Trends Genet. 2003;19:321–329. doi: 10.1016/S0168-9525(03)00115-X. [DOI] [PubMed] [Google Scholar]
- 31.Chapman JD, Stobbe CC, Gales T, et al. Condensed chromatin and cell inactivation by single-hit kinetics. Radiation Res. 1999;151:433–441. [PubMed] [Google Scholar]
- 32.Biade S, Stobbe CC, Boyd JT, Chapman JD. Chemical agents that promote chromatin compaction radiosensitize tumour cells. Int J Radiat Biol. 2001;77:1033–1042. doi: 10.1080/09553000110066068. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, Jiang X, Chen S, Price BD. Inhibition of histone acetyltransferase activity by anacardic acid sensitizes tumor cells to ionizing radiation. FEBS letters. 2006;580:4353–4356. doi: 10.1016/j.febslet.2006.06.092. [DOI] [PubMed] [Google Scholar]
- 34.Murr R, Loizou JI, Yang YG, et al. Histone acetylation by Trrap-Tip60 modulates loading of repair proteins and repair of DNA double-strand breaks. Nature Cell Biol. 2006;8:91–99. doi: 10.1038/ncb1343. [DOI] [PubMed] [Google Scholar]
- 35.Downey M, Durocher D. Chromatin and DNA repair: the benefits of relaxation. Nature Cell Biol. 2006;8:9–10. doi: 10.1038/ncb0106-9. [DOI] [PubMed] [Google Scholar]
- 36.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimmer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 37.Kastan MB. DNA damage responses: mechanisms and roles in human disease. Mol Cancer Res. 2008:517–524. doi: 10.1158/1541-7786.MCR-08-0020. [DOI] [PubMed] [Google Scholar]
- 38.Klokov D, MacPhail SM, Banath JP, Byrne JP, Olive PL. Phosphorylated histone H2AX in relation to cell survival in tumor cells and xenografts exposed to single and fractionated doses of X-rays. Radiother Oncol. 2006;80:223–229. doi: 10.1016/j.radonc.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 39.Kikuchi K, Kawahara KI, Biswas KK, et al. Minocycline attenuates both OGD-induced HMGB1 release and HMGB1-induced cell death in ischemic neuronal injury in PC12 cells. Biochem Biophys Research Commun. 2009;385:132–136. doi: 10.1016/j.bbrc.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 40.Brook I, Elliott TB. Quinolone therapy in the prevention of mortality after irradiation. Radiation Res. 1991;128:100–103. [PubMed] [Google Scholar]
- 41.Gale RP. Immediate medical consequences of nuclear accidents. Lessons from Chernobyl. Jama. 1987;258:625–628. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.