Abstract
The outcome of liver injury is dictated by the effectiveness of repair. Successful repair (i.e., regeneration) results in replacement of dead epithelial cells with healthy epithelial cells, and reconstructs normal hepatic structure and function. Liver regeneration is known to involve replication of surviving mature hepatocytes and bile duct cells. This review discusses recent evidence for other mechanisms that might also replace dead hepatic epithelial cells and repair liver damage, particularly during chronic injury. According to this theory, certain epithelial cells in developing livers and/or injured adult livers undergo epithelial-to-mesenchymal transition (EMT) and move into the hepatic mesenchyme where they exhibit fibroblastic features. Some of these epithelia-derived mesenchymal cells, however, may be capable of undergoing subsequent mesenchymal-to-epithelial transition (MET), reverting to epithelial cells that ultimately become hepatocytes or cholangiocytes. Although these concepts remain to be proven, the theory predicts that the balance between EMT and MET modulates the outcome of chronic liver injury. When EMT activity outstrips MET, repair is mainly fibrogenic, causing liver fibrosis. Conversely, predominance of MET favors more normal liver regeneration. In this review, we summarize evidence that certain resident liver cells are capable of epithelial-mesenchymal transitions in vitro and during chronic liver injury.
Keywords: Cholangiocyte, Fibrosis, Hepatic stellate cell, myofibroblast, Regeneration
Similar to the skin, intestine, lung, and glandular tissues like the pancreas, the adult liver is comprised largely of epithelial cells and mesenchymal cells. In all of these organs, the ultimate outcome of epithelial injury is dictated by repair. Successful liver repair results in replacement of dead or damaged hepatic epithelial cells with healthy new epithelial cells, i.e., liver regeneration. Regenerative responses differ depending on the severity and chronicity of liver injury. For example, residual mature hepatocytes and cholangiocytes proliferate to restore liver mass after acute partial hepatectomy(1), while liver progenitors are involved in the repair of chronically injured livers(2). Repair of chronic liver injury also variably involves changes in mesenchymal cells. Presumably, alterations in hepatic “stromal” cells in some way contribute to epithelial repair. However, they may also lead to hepatic inflammation, vascular remodeling, and fibrosis, and result in hepatic architectural distortion and liver dysfunction, eventually culminating in cirrhosis(3). Therefore, efforts have focused on understanding the mechanisms that control potentially “fibrogenic” repair. The purpose of this review is to summarize evidence for and against the possibility that fibrogenic repair involves epithelial-to-mesenchymal, and mesenchymal-to-epithelial, transitions (EMT/MET) of resident liver cells.
Definitions
Epithelial cells are adherent cells that closely attach to each other, forming coherent layers in which cells exhibit apico-basal polarity. Mesenchymal cells, in contrast, are non-polarized cells, capable of moving as individual cells because they lack intercellular connections. EMT describes the process by which cells gradually lose typical epithelial characteristics and acquire mesenchymal traits. MET refers to the reverse process. It is important to emphasize that EMT/MET refer to changes in cell shape and adhesive properties. Cell fate (lineage) is specified by other mechanisms. Hence, EMT/MET are merely manifestations of the inherent plasticity of cells(4–6).
Key epithelial features that are eventually lost during EMT include typical epithelial expression and distribution of proteins that mediate cell-cell and cell-matrix contacts, as well as the cytoskeletal organization that is responsible for normal epithelial polarity. Key mesenchymal characteristics that are ultimately gained during EMT include the ability to migrate and invade the surrounding matrix. This migratory/invasive phenotype requires induction of mesenchymal filaments, cytoskeletal rearrangements, and increased production of factors that degrade extracellular matrix, as well as new matrix molecules themselves(5). Such global alterations in cellular phenotype do not occur simultaneously. Rather completion of EMT (or its reversal) requires a carefully-orchestrated series of events that eventually lead to wide-spread changes in gene expression. This is regulated both at the level of gene transcription and via various post-transcriptional mechanisms(7).
Situations associated with Epithelial-Mesenchymal Transitions (EMTs)
EMT/MET occur when tissues are being built or remodeled. Hence, EMT/MET are involved in 1) embryogenesis/development, 2) wound healing/tissue regeneration/organ fibrosis, and 3) neoplasia. Because the context and consequences of EMT/MET differ in these three settings, consensus is emerging that epithelial-mesenchymal transitions (EMTs) are best classified into 3 different biological subtypes based on the biological context in which they occur. A detailed description of similarities and differences in the 3 subtypes of EMTs is provided in recent review articles(4–7). Briefly, type 1 EMTs occur during implantation, embryogenesis and organ development. Among other outcomes, type 1 EMT generates mesodermal and endodermal mesenchyme that then undergoes MET to generate secondary epithelia that, in turn, undergo further rounds of EMT/MET to form various organs. Type 1 EMT does not cause fibrosis. In contrast, fibrosis is a potential outcome of type 2 EMT. The latter generally begins as a repair-associated event in adult tissues. Type 2 EMT is associated with inflammation and generally abates when inflammation subsides, presumably because superfluous fibroblastic cells that emerged during the process undergo apoptosis. However, when injury and inflammation persist, type 2 EMT generates fibroblastic cells that accumulate and cause progressive fibrosis, eventuating in organ destruction. Type 3 EMTs occur as a result of genetic and epigenetic changes in cancer cells and promote invasion and spread of tumor cells, as well as subsequent emmergence of metastastic tumor foci at sites distant from the primary tumor. Type 3 EMTs resemble type 1 EMTs in that generation of epithelia, rather than fibrosis, is typically the ultimate outcome. While these three classes of EMTs represent distinct biological processes and therefore, have various unique features, it has been proposed that “a common set of genetic and biochemical elements appears to underlie, and thus enable, these outwardly diverse phenotypic programs”(5, 7). Further research is needed to evaluate this concept, particularly in various adult tissues where it remains debated if (and to what extent) EMT occurs during chronic degenerative/fibrosing disorders(4).
Regulation of EMT
Most of the existing fundamental knowledge about EMT regulation has been generated by studying cultures of malignant and nonmalignant cells because EMT is relatively easy to induce in cultured epithelial cells(7). Additional knowledge has been gained by tracking and manipulating organogenesis during fetal development(6). A detailed discussion of this research is beyond the scope of this review. It is important to emphasize, however, that results in cell culture systems may not perfectly recapitulate the cues that control EMTs in intact tissues. In addition, unique modulatory mechanisms are likely to influence EMT depending on the biological context (i.e., development versus neoplasia versus adult repair) and specific cell type (e.g., normal progenitor versus malignantly transformed cell versus mature epithelial cells) in which EMT occurs. Existing evidence also already suggests that types 1 and 3 EMT might be more similar to each other than to type 2 EMT, which occurs mainly in injured/inflamed adult tissues. Suffice it to say, however, the work in cultured cells and developing embryos has led to the identification of common matrix molecules and a repertoire of soluble factors that are capable of triggering EMT in certain epithelial cell types(5). Activation of specific receptors by transforming growth factor-beta (TGF-β1) has been shown to provoke EMT in many types of epithelial cells in culture(8, 9). Hence, TGF-β1 is generally considered to be one of the master positive regulators of EMT. Conversely, another TGF-β family member, Bone morphogenetic protein (BMP)-7, is the prototypical negative regulator of EMT because it generally(8–11), although not always(12), opposes the EMT promoting actions of TGF-β receptor activation. Experimental manipulation of signaling events down-stream from TGF-β1 and BMP-7 receptors has, therefore, been used to tease out mechanisms that mediate distinct processes that occur during EMT, such as disruption of cell-cell adherence, loss of apico-basal polarity, matrix remodeling, and migration. As a result, it has become evident that although overlapping mechanisms mediate many of the different events that transpire as cells lose epithelial characteristics and gain mesenchymal features, distinct signals also regulate each of the different processes(13). Consequently, different aspects of EMT generally occur sequentially, with inhibition of cell-cell contact occurring before cytoskeletal rearrangement and acquisition of a motile/invasive phenotype. Thus, at any given moment, individual cells in a culture or tissue may be at different stages of EMT, depending upon which signals have already been launched, and which signals have not yet been initiated(8). This heterogeneity complicates efforts to identify cells that are undergoing EMT, particularly in intact tissues(4, 7). Efforts to “simplify” analysis by focusing attention on matrix-producing cells introduce bias, however, because many types of fibroblastic cells are capable of generating matrix molecules, and matrix production is not be an obligate feature of EMT(5, 6). Moreover, it remains to be determined to what (if any) extent EMT contributes to adult organ fibrosis, although type 2 EMT is defined by its association with this process.
Challenges to identifying cells that are undergoing EMT
Cells that are in the midst of EMT are sometimes referred to as “transitioning cells” or cells that have undergone “partial EMT”(8). Concomitant expression of epithelial and mesenchymal markers is often used to identify cells that are undergoing EMT(7, 8). However, it is important to recognize that EMT may be underway in epithelial cells that have not yet fully activated expression of mesenchymal genes. The phenotype of mesenchymal cells is also dynamic(14). Hence, not every mesenchymal marker is expressed concurrently, and this complicates efforts to identify a cell as being (or not being) mesenchymal. The issue is further confounded by evidence that EMT may be reversible by a process termed mesenchymal-to-epithelial transition (MET). MET presumably involves “reverse” sequential silencing of the mechanisms that led to EMT, thereby permitting a mesenchymal-type cell that was derived from an epithelial cell to gradually reacquire its epithelial phenotype(4, 6). The possibility that many cells are capable of undergoing both EMT and MET provides further evidence for the inherent plasticity of cells(13), but complicates efforts to prove either the origin or fate of individual fibroblastic cells, particularly in intact tissues. A consensus panel of EMT experts recently developed an array of parameters that are useful for “diagnosing” EMT in situations where cellular lineage-tracing (see below) is not possible, including analysis of human tissue samples. The likelihood of EMT increases with the number of individual criteria that are satisfied(7).
Evidence that certain adult liver cell types are capable of EMTs
Three types of adult liver cells, hepatocytes, cholangiocytes, and hepatic stellate cells (HSC), have been shown to undergo epithelial-mesenchymal transitions (i.e., EMT or MET) in culture. Several groups have demonstrated that treating primary rat hepatocytes or hepatocyte cell lines with sub-lethal doses of TGF-β causes them to down-regulate expression of epithelial genes, such as albumin, up-regulate expression of mesenchymal genes, including α-smooth muscle actin (α-sma), collagen, and fibroblast specific protein (FSP)-1, and/or to acquire a migratory phenotype(10, 15–18). Primary hepatocytes from rats with carbon tetrachloride (CCl4)-induced cirrhosis exhibit characteristics of mesenchymal cells(17). Hence, there is solid experimental evidence that hepatocytes can be induced to undergo EMT in culture, and some data that a similar process may occur during conditions that promote liver fibrosis in vivo.
Omenetti et al. reported that treating an immature cholangiocyte line with conditioned medium from myofibroblastic HSC (MF-HSC) caused the cholangiocytes to undergo complete EMT (i.e., to repress expression of epithelial genes, induce expression of mesenchymal genes, and acquire a migratory phenotype)(19). In addition, they demonstrated that primary cholangiocytes from rats with biliary fibrosis co-expressed epithelial and mesenchymal markers(19, 20), a characteristic of cells that are undergoing EMT. Rygiel et al. also documented co-expression of epithelial and mesenchymal markers in intrahepatic biliary epithelial cells in human tissues, and reported that cultured primary human cholangiocytes induced mesenchymal markers and became highly motile when treated with TGF-β(21). Finally, co-localization of CK19 (a marker of bile ductular cells) and various mesenchymal proteins was demonstrated by Diaz et al. in their studies of biliary atresia and several other liver diseases that are associated with bile ductular proliferation(22). Hence, there is strong evidence that, like hepatocytes, bile ductular cells are capable of EMT in vitro, and perhaps, in vivo.
The concept that cells that are involved in ductular reactions during chronic liver injury are capable of EMT is intriguing because subpopulations of these cells are thought to comprise liver epithelial progenitors(2, 23). Yovchev et al. recently reported co-expression of epithelial and mesenchymal markers in liver epithelial progenitors (oval cells) that they purified from rats that had been treated to increase liver progenitors(24). The investigators went on to prove that these transitional cells were true hepatic progenitors by transplanting them into rats with injured livers and demonstrating hepatic repopulation. That liver progenitors are capable of undergoing epithelial-mesenchymal transitions is further supported by evidence that progenitor cells from fetal livers undergo EMT/MET(25, 26).
Hepatic stellate cells might also be capable of mesenchymal-epithelial transitions. Recent publications from two independent groups suggest that HSC are derived from sub-mesothelial cells during liver development(27, 28). Submesothelial cells are thought to arise from EMT of adjacent primitive coelomic epithelium that invaginated to generate the epicardium and serosal linings (mesothelia) of the primitive lung, gut and liver(29, 30). Therefore, quiescent HSC might be transitional cells derived from epithelial cells that have undergone partial EMT. This concept is supported by studies of HSC from adult livers. Kordes et al. showed that a sub-population of adult, primary rat HSC that expressed the progenitor cell marker, CD133, could be induced to become either myofibroblastic, or hepatocytic when cultured under different conditions(31). These observations suggest that some adult HSC (which have a mesenchymal phenotype in situ) retain the capacity to undergo MET and revert back to an epithelial phenotype. During this MET process, HSC-derived hepatocytic cells were demonstrated to express alpha-fetoprotein, a marker of immature hepatocytes, and eventually albumin, a mature hepatocyte marker, raising the intriguing possibility that HSC and mature liver epithelial cells are derived from a common progenitor. A recent fate-mapping study in adult transgenic mice supports this concept (see below), as do other published data in cultured HSC. Sicklick et al., for example, reported co-expression of mesenchymal genes and oval cell markers (i.e., alpha-fetoprotein and CK19) in two distinct clones of MF-HSC that had been generated from primary HSC that were isolated from a rat with CCl4-induced cirrhosis(32). In their studies, cells that were derived from a clone with strong basal expression of multiple myofibroblastic markers could be induced to acquire epithelial gene expression.
Thus, there seems to be fairly good evidence that some seemingly-mature hepatocytes and cholangiocytes, as well as certain bipotent hepatic epithelial progenitors, can transiently acquire markers of myofibroblastic cells, and that some adult HSC can be induced to undergo at least partial MET under certain circumstances. However, given the dynamic nature of EMT, it has been challenging to prove (or disprove) that any of these cell types actually undergoes EMT (or MET) during chronic liver injury. Consequently, it remains unclear if (and how) epithelial-mesenchymal transitions of resident liver cells might be involved in liver repair.
Evidence for EMT during liver injury
Determining whether or not EMT occurs in situ, and how significant this process might be to outcomes of liver injury (e.g., regeneration or fibrosis), is inherently difficult(8). Unlike development or carcinogenesis, during which large populations of cells typically undergo relatively synchronous EMT(6), chronic epithelial degeneration is thought to provoke patchy epithelial-mesenchymal transitions that involve relatively small numbers of cells(8). Thus, assessment of EMT in adult liver repair has generally been addressed by immunohistochemistry. However, technical considerations limit the numbers of cellular proteins that can be demonstrated in any given cell at any point in time. Therefore, it is simply not feasible to obtain a “snap-shot” that captures global changes in the phenotype of individual cells using this approach. Moreover, even when staining suggests co-expression of individual epithelial and mesenchymal markers, it is often difficult to resolve whether or not the seemingly co-localized markers are actually expressed by one cell, as opposed to the possibility that one of the markers is being expressed by another adjacent/adherent cell. The latter is virtually impossible to exclude when serial sections are stained individually to generate photomicrographs that are then “overlaid” upon each other to estimate marker co-expression. Finally, without time-lapse photography, even superb immunohistochemistry is incapable of capturing cell movement, and the latter is generally considered to be a critical proof-of-concept that a cell has undergone complete EMT(8, 9).
Fate-mapping (also termed lineage-tracing) is another approach that has been used to track transitions in cell phenotype, including EMT/MET. This strategy uses cell-type-specific activation of gene regulatory elements to generate permanently expressed markers (e.g., LacZ) that specifically identify all of the different progeny of that cell type(33). For example, transgenic mice have been generated in which cis-acting elements that control albumin gene expression were used to drive expression of Cre-recombinase. In such mice, only cells that activated albumin gene transcription produced Cre-recombinase(34). Cre-recombinase cleaves loxP sites, removing “floxed” segments of DNA that are flanked by engineered loxP sites. Breeding such Albumin-cre mice with floxStopRepressorfloxLacZ transgenic mice resulted in double transgenic mice in which selective removal of the repressor element occurred only in cells that also expressed Cre-recombinase. Therefore, only progeny of cells that had activated albumin gene transcription at some point during their development became permanently “marked” by LacZ expression. LacZ-expressing cells exhibit beta-galactosidase (β-gal) activity and, thus, are demonstrated by histochemical techniques.
Zeisberg et al. took advantage of this model to assess the role of hepatocyte EMT in CCl4-induced hepatic fibrosis(10). A detailed description of their work is justified because it is the first of only three studies that have attempted to use fate-mapping to monitor cellular transitions during adult liver injury/repair. The authors tracked the accumulation of fibroblastic cells that expressed fibroblast specific protein (FSP)-1, a marker of fibroblastoid cells that are generated by EMT during renal models of fibrosis(14), and determined what proportion of these EMT-derived fibroblastic cells were LacZ-expressing (i.e., β-galactosidase positive). In healthy control livers, only few FSP(+)-fibroblastic cells were noted, predominately around terminal hepatic venules and within portal tracts. However, during CCl4 treatment, FSP(+)-fibroblastic cells accumulated in areas of collagen deposition. Cells that expressed α-sma also accumulated during liver fibrosis. The latter are generally considered myofibroblasts that are generated mostly from Q-HSC(3). Interestingly, populations of α-sma-expressing cells and FSP(+)-cells were largely discrete, with each cell population increasing as fibrosis progressed, such that each cell type comprised ~10–15% of the liver 6 weeks post-CCl4 treatment. Only about 10% of the fibroblastic cells co-expressed both markers at that time point. In contrast, almost half of the FSP-expressing fibroblastic cells co-stained with β-gal, leading to the conclusion that most of the EMT-derived fibroblastic cells were derived from hepatocytes. These findings have been widely cited and are generally considered to provide the most definitive proof of the concept that hepatocyte EMT contributes to liver fibrosis.
However, like all good research, this study generates as many questions as it resolves. First, it is notable that over half of the FSP(+)-fibroblastic cells in fibrotic livers did not exhibit β-gal activity. Therefore, these cells were not derived from mature hepatocytes that had activated albumin transcription. Indeed, only about 70% of the hepatocytes in the healthy Alb-Cre-LacZ mice exhibited β-gal activity pre-treatment, suggesting that progeny of almost one-third of the hepatocytic cells would not be identified by β-gal staining. While it is conceivable that technical artifacts account for the apparent lack of β-gal expression in sizeable subpopulations of hepatocytic and fibroblastic cells in this study, other explanations also merit consideration, particularly given strong in vitro evidence that other types of liver cells are capable of EMT/MET. The latter suggests that cholangiocytes and/or HSC might have undergone epithelial-mesenchymal transitions (EMT and/or MET) to generate β-gal-negative fibroblastic cells and/or hepatocytic cells in Alb-cre/LacZ transgenic mice. Unfortunately, information to resolve this issue is lacking. It was not specified if ductular-appearing cells expressed FSP-1 in CCl4-treated Alb-Cre/LacZ mice, although cholangiocyte expression of FSP-1 has been demonstrated in mice and humans with biliary-type fibrosis(19, 22). Also unknown is whether β-gal activity was demonstrated in any of the α-sma-expressing cells. Others have demonstrated that hepatocytes can express α-sma when they are induced to undergo EMT in culture(15), and shown that α-sma(+) MF-HSC can be induced to express markers of immature and mature hepatocytes(31, 32). Finally, because staining was not done to prove that β-gal(+) fibroblastic cells were actually producing matrix molecules, it remains unclear whether these EMT-derived cells directly contributed matrix deposition during liver fibrosis. Thus, even this sophisticated fate-mapping approach has limitations that preclude definitive assignment of the origin (or fate) of various fibroblastic cells during liver fibrogenesis, or their precise roles in liver repair.
The second attempt to use fate-mapping to track transitioning cells during adult liver injury also relied on cell-specific deletion of the floxedStopRepressor cassette. In this case, floxStopRepressorfloxgreen fluorescent protein (GFP) transgenic mice were bred with glial fibrillary acidic protein (GFAP)-Cre mice. The resultant double transgenic, GFAP-Cre/GFP mice expressed Cre-recombinase exclusively in cells that activated transcription of GFAP. GFAP is a marker of HSC in adult liver(3). Thus, these mice were designed to track the progeny of HSC in order to determine if HSC undergo MET to generate mature liver epithelial cells after diet-induced liver injury(35). In the livers of healthy adult GFAP-Cre/GFP mice, stellate-appearing sinusoidal cells expressed GFAP, Cre-recombinase, and GFP; each of these genes was also expressed in freshly isolated Q-HSC; when Q-HSC were cultured, the myofibroblastic progeny remained GFP(+) despite having down-regulated expression of GFAP and Cre-recombinase. Surprisingly, however, many bile ductular cells also expressed GFAP, Cre-recombinase and GFP in the healthy adult mice. The latter confounded efforts to interpret the dramatic findings that were noted in these mice during and after liver injury. Namely, roughly one third of the mature-appearing albumin(+) hepatocytes, and virtually all of the ductular cells in the regenerating livers of these mice expressed the fate-mapping marker, but it was impossible to determine if such cells were derived from HSC, ductular cells, or some other GFAP-expressing progenitor cell. Nevertheless, these data raise the intriguing possibility that hepatocytes, cholangiocytes and HSC are derived from a common progenitor that is capable of EMT/MET during certain types of liver injury.
The third fate-mapping study that investigated cell transitions during liver injury was published by Sackett et al.(36). FloxStopRepressorfloxLacZ transgenic mice were bred with transgenic mice that expressed Cre-recombinase under the control of regulatory elements for Foxl1, a gene that is expressed in mesenchymal progenitor cells in the intestine(37). Thus, all Foxl1-expressing cells and their progeny were expected to exhibit β-gal activity in Foxl1-cre/LacZ mice. These mice were then subjected to bile duct ligation (BDL) or treatment with a hepatotoxin that promotes oval cell accumulation. In healthy mice, β-gal-expressing cells were localized in portal tract ductular structures, and some β-gal(+)-ductular cells co-expressed CK19, a marker of mature cholangiocytes. After both types of liver injury, Foxl1-expressing cells generated progeny that differentiated mainly along the biliary lineage, although small numbers of β-gal(+) hepatocytes were also noted. β-gal did not co-localize with markers of portal fibroblasts (elastin), Q-HSC (desmin) or myofibroblasts (α-sma), suggesting that neither Foxl1-expressing progenitors nor their progeny acquired a mesenchymal phenotype at the time points that were examined. However, given the dynamic nature of EMT/MET, it is impossible to know if such processes might have occurred in Foxl1-expressing cells or their progeny at other times. Also, these findings do not exclude EMT/MET in non-Foxl1-derived cells.
Summary
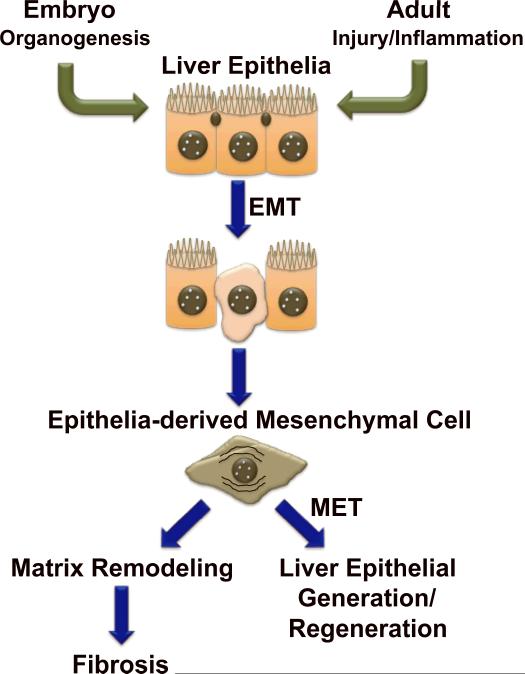
Epithelial-mesenchymal transitions (EMT/MET) are known to occur when tissues are constructed during embryogenesis/development. They are also thought to occur during adult tissue remodeling responses, including carcinogenesis and fibrosis. During culture, several resident adult liver cells appear capable of undergoing EMT and/or MET, raising the possibility that EMT/MET might be involved in liver regeneration. However, despite considerable effort to deploy state-of-the-art technology to determine if (and how) such phenotypic transitions influence the outcomes of liver injury, the issue remains quite confusing. The existing fate-mapping data that might prove to be helpful in resolving the role of epithelial-mesenchymal transitions in adult liver repair was derived from 3 different transgenic mice, each of which likely marked distinct types of liver cells and their resultant progeny. Data interpretation is further confounded by that fact that the published studies used different models of injury and examined outcomes at different time points. Nevertheless, a few take home messages have emerged. Two of the three studies (work in Alb-Cre/LacZ mice and GFAP-Cre/GFP mice) provide compelling in vivo evidence that EMT/MET does occur in certain types of adult liver injury, although the exact cell types that are capable of this response remain unclear. Also, when it occurs, EMT does appear to correlate with changes in hepatic matrix production/accumulation, although it has not yet been proved (or disproved) that the EMT-derived fibroblastic cells actually generate matrix. One of the three studies (work in GFAP-Cre/GFP mice) suggests that MET may have a significant role in hepatocyte regeneration. Despite the acknowledged technical limitations, there is also growing immunohistochemical evidence for EMT/MET in various human liver diseases, including primary biliary cirrhosis, biliary atresia, alcoholic and nonalcoholic fatty liver disease(19, 21, 22). In addition, therapeutic manipulation of known EMT regulators has generally been demonstrated to influence liver regeneration and fibrosis in rodents. For example, supplementing BMP-7 inhibited liver fibrosis in CCl4-treated mice(10, 38), and improved liver regeneration after partial hepatectomy(39), while inhibiting BMP-7 activity delayed normal, post-partial hepatectomy regeneration in mice(39). Likewise, pharmacological inhibition of cholangiocyte αvβ6 integrin, a receptor that is selectively induced in epithelial cells that are undergoing EMT, blocked biliary fibrosis in rodents(40). Coupled with strong in vitro data demonstrating that several types of cells in healthy adult liver are capable of undergoing EMT/MET(10, 15–19, 31, 32) all of this information suggests a novel model for repair of chronically injured livers, in which the balance between EMT and MET dictate whether or not repair is fibrogenic (Figure 1). Further research is needed to evaluate this theory. The resultant knowledge may be important in designing novel diagnostic and therapeutic strategies to prevent and treat liver damage.
Figure 1. Epithelial-mesenchymal transitions (EMT) are known to occur when tissues are constructed during embryogenesis/development and during adult tissue remodeling responses.
During adult liver injury/inflammation, EMT is one mechanism that promotes liver repair. EMT facilitates transient acquisition of a mesenchymal phenotype by certain types of liver epithelial cells. Some of these epithelia-derived mesenchymal cells may contirubte to liver fibrosis, but some are capable of undergoing mesenchyal-to-epithelial transition (MET), reverting to epithelial cells that ultimately become hepatoytes or cholangiocytes. Recent fate-mapping studies in transgenic mice suggest that MET may have a role in hepatocyte regeneration.
Acknowledgments
[Support] This work was supported by National Institute of Health Grants 1R01DK077794-01A2 (AMD) and the Barton F. Haynes Award from the Department of Medicine at Duke University Medical Center (SSC).
Abbreviations
- α-SMA
α-smooth muscle actin
- BDL
bile duct ligation
- BMP-7
bone morphogenetic protein 7
- EMT
epithelial-to-mesenchymal transition
- GFAP
glial fibrillary acidic protein
- Hh
hedgehog
- HSC
hepatic stellate cell
- MET
mesenchymal-to-epithelial transition
- MF
myofibroblast
- TGF-β
transforming growth factor-beta
References
- 1.Grisham JW. A morphologic study of deoxyribonucleic acid synthesis and cell proliferation in regenerating rat liver: Autoradiographic studies. Cancer Res. 1962;22:842–849. [PubMed] [Google Scholar]
- 2.Falkowski O, An HJ, Ianus IA, Chiriboga L, Yee H, West AB, Thiese ND. Regeneration of hepatocyte “buds” in cirrhosis from intrabiliary stem cells. J Hepatol. 2003;39:357–364. doi: 10.1016/s0168-8278(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 3.Wynn TA. Cellular and molelcular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalluri R. EMT: When epithelial cells decide to become mesenchymal-like cells. J Clin Invest. 2009;119:1417–1419. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–1448. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zavadil J, Bottinger EP. TGF-β and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 9.Lamouille S, Derynck R. Cell size and invasion in TGF-β-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol. 2007;138:437–451. doi: 10.1083/jcb.200611146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeisberg M, Yang C, Martino M, Duncan MB, Fieder F, Tanjore H, Kalluri R. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282:23337–23347. doi: 10.1074/jbc.M700194200. [DOI] [PubMed] [Google Scholar]
- 11.Valcourt U, Kowanetz M, Niimi H, Heldin C-H, Moustakas A. TGF-β and the smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal transition. Mol Biol Cell. 2005;16:1987–2002. doi: 10.1091/mbc.E04-08-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray LA, Hackett TL, Warner SM, Shaheen R, Argentieri RL, Dudas P, Farrell FX, et al. BMP-7 does not protect against bleomycin-induced lung or skin fibrosis. PLoS ONE. 2008;3:e4039. doi: 10.1371/journal.pone.0004039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Teng Y, Zeisberg M, Kalluri R. Transcriptional regulation of epithelial-mesenchymal transition. J Clin Invest. 2007;117:304–306. doi: 10.1172/JCI31200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaimori A, Potter J, Kaimori JY, Wang C, Mezey E, Koteish A. Transforming growth factor-beta1 induces an epithelial-to-mesenchymal transition state in mouse hepatocytes in vitro. J Biol Chem. 2007;282:22089–22101. doi: 10.1074/jbc.M700998200. [DOI] [PubMed] [Google Scholar]
- 16.Meindl-Beinker NM, Dooley S. Transforming growth factor-beta and hepatocyte transdifferentiation in liver fibrogenesis. J Gastroenterol Hepatol. 2008;23(Suppl1):S122–S127. doi: 10.1111/j.1440-1746.2007.05297.x. [DOI] [PubMed] [Google Scholar]
- 17.Nittta T, Kim JS, Mohuczy D, Behrns KE. Muring cirrhosis induces hepatocyte epithelial mesenchymal transition and laterations in survival signaling pathways. Hepatology. 2008;48:909–919. doi: 10.1002/hep.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dooley S, Hamzavi J, Ciucian L, Godoy P, Ilkavets I, Ehnert S, Ueberham E, et al. Hepatocyte-specific Smad7 expression attenuates TGF-beta-mediated fibrogenesis and protects against liver damage. Gastroenterology. 2008;135:642–659. doi: 10.1053/j.gastro.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 19.Omenetti A, Porrello A, Jung Y, Yang L, Popov Y, Choi SS, Witek RP, et al. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest. 2008;118:3331–3342. doi: 10.1172/JCI35875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Omenetti A, Yang L, Li YX, McCall SJ, Jung Y, Sicklick JK, Huang J, et al. Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation. Lab Invest. 2007;87:499–514. doi: 10.1038/labinvest.3700537. [DOI] [PubMed] [Google Scholar]
- 21.Rygiel KA, Robertson H, Marshall HL, Pekalski M, Zhao L, Booth TA, Jones DE, et al. Epithelial-mesenchymal transition contributes to portal tract fibrogenesis during human chronic liver disease. Lab Invest. 2008;88:112–123. doi: 10.1038/labinvest.3700704. [DOI] [PubMed] [Google Scholar]
- 22.Diaz R, Kim JW, Hui JJ, Li Z, Swain GP, Fong KS, Csiszar K, et al. Evidence for the epithelial to mesenchymal transition in biliary atresia fibrosis. Hum Pathol. 2008;39:102–115. doi: 10.1016/j.humpath.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 23.Thiese ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, Kumar A, et al. The canals of Hering and hepatic stem cells in humans. Hepatology. 1999;30:1425–1433. doi: 10.1002/hep.510300614. [DOI] [PubMed] [Google Scholar]
- 24.Yovchev MI, Grozdanov PN, Zhou H, Racheria H, Guha C, Daveva MD. Identification of adult hepatic progenitor cells capable of repopulating injured rat liver. Hepatology. 2008;47:636–647. doi: 10.1002/hep.22047. [DOI] [PubMed] [Google Scholar]
- 25.Pagan R, Martin I, Liobera M, Vilaro S. Epithelial-mesenchymal transition of cultured rat neonatal hepatocytes is differentially regulated in response to epidermal growth factor and dimethyl sulfoxide. Hepatology. 1997;25:598–606. doi: 10.1002/hep.510250318. [DOI] [PubMed] [Google Scholar]
- 26.Valdes F, Alvarez AM, Locascio A, Vega S, Herrera B, Fernandez M, Benito M, et al. The epithelial mesenchymal transtion confers resistance to the apoptotic effects of transforming growth factor Beta in fetal rat hepatocytes. Mol Cancer Res. 2002;1:68–78. [PubMed] [Google Scholar]
- 27.Loo CK, Wu XJ. Origin of stellate cells from submesothelial cells in a developing human liver. Liver Int. 2008;28:1437–1445. doi: 10.1111/j.1478-3231.2008.01788.x. [DOI] [PubMed] [Google Scholar]
- 28.Asahina K, Tsai SY, Li P, Ishii M, Maxson RE, Sucov HM, Tsukamoto H. Mesenchymal origin of hepatic stellate cells, submseothelial cells, and perivascular mesenchymal cells during mouse liver development. Hepatology. 2009;49:998–1011. doi: 10.1002/hep.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carmona R, Bonzalez-Iriarte M, Perez-Pomares JM, Munoz-Chapuli R. Localization of the Wilm's tumour protein WT1 in avian embryos. Cell Tissue Re. 2001;303:173–186. doi: 10.1007/s004410000307. [DOI] [PubMed] [Google Scholar]
- 30.Ijpenberg A, Perez-Pomares JM, Guadix JA, Carmona R, Portillo-Sanchez V, Macias D, Hohenstein P, et al. Wt1 and retinoic acid signaling are essential for stellate cell development and liver morphogenesis. Dev Biol. 2007;312:157–170. doi: 10.1016/j.ydbio.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Kordes C, Sawitza I, Muller-Marbach A, Ale-Agha N, Keitel V, Klonowski-Stumpe H, Haussinger D. CD133+ hepatic stellate cells are progenitor cells. Biochem Biophys Res Commun. 2007;352:410–417. doi: 10.1016/j.bbrc.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 32.Sicklick J, Choi SS, Bustamante M, McCall SJ, Perez EH, Huang J, Li YX, et al. Evidence for epithelial-mesenchymal transitions in adult liver cells. Am J Physiol Gastrointest Liver Physiol. 2006;291:G575–G583. doi: 10.1152/ajpgi.00102.2006. [DOI] [PubMed] [Google Scholar]
- 33.Mao X, Fujiwara Y, Orkin SH. Improved reporter strain for monitoring Cre recombinase- mediated DNA excisions in mice. Proc Natl Acad Sci USA. 1999;96:5037–5042. doi: 10.1073/pnas.96.9.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274:305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- 35.Yang L, Jung Y, Omenetti A, Witek RP, Choi SS, Vandongen HM, Alpini GD, et al. Fate mapping evidence that hepatic stellate cells are epithelial progenitors in adult mouse livers. Stem Cell. 2008;26:2104–2113. doi: 10.1634/stemcells.2008-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sackett SD, Li Z, Hurtt R, Gao Y, Wells RG, Brondell K, Kaestner KH, et al. Foxl1 is a markder of bipotential hepatic progenitor cells in mice. Hepatology. 2009;49:920–929. doi: 10.1002/hep.22705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sackett SD, Fulmer JT, Friedman JR, Kaestner KH. Foxl1-Cre BAC transgenic mice: a new tool for gene ablation in the gastrointestinal mesenchyme. Genesis. 2007;45:518–522. doi: 10.1002/dvg.20315. [DOI] [PubMed] [Google Scholar]
- 38.Kinoshita K, Iimuro Y, Otogawa K, Saika S, Inagaki Y, Nakajima Y, Kawada N, et al. Adenovirus-mediated expression of BMP-7 suppresses the development of liver fibrosis in rats. Gut. 2007;56:706–714. doi: 10.1136/gut.2006.092460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugimoto H, Yang C, LeBleu VS, Soubasakos MA, Giraldo M, Zeisberg M, Kalluri R. BMP-7 functions as a novel hormone to facilitate liver regeneration. FASEB J. 2007;21:256–264. doi: 10.1096/fj.06-6837com. [DOI] [PubMed] [Google Scholar]
- 40.Patsenker E, Popov Y, Stickel F, Jonczyk A, Goodman SL, Schuppan D. Inhibition of integrin alphavbeta6 on cholangiocytes blocks transforming growth factor-beta activation and retards biliary fibrosis progression. Gastroenterology. 2008;135:660–670. doi: 10.1053/j.gastro.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]