Abstract
The presence of behavioral control over a stressor can blunt many of the effects of the stressor. We have recently reported that uncontrollable stress (inescapable electric tailshock shock, IS) reduces later social exploration of a juvenile whereas controllable stress (escapable shock, ES) does not. Activation of the ventral medial prefrontal cortex (vmPFC) is crucial to blunting the effects of IS on later escape behavior (“learned helplessness”). The goal of the current study was to test the role of the vmPFC in modulating the effects of stressor controllability on anxiety in the social exploration test. Thus, adult male rats were implanted with cannula guides for microninjection into the vmPFC. In experiment 1 temporary inactivation of the vmPFC with the GABAA agonist muscimol before exposure to ES prevented the protective effects of control, leading to reduced social exploration. In experiment 2 activation of the vmPFC prior to IS with the Cl(−) channel antagonist picrotoxin mimicked the stress resistance produced by control and prevented IS-induced reduction in social exploration. These results are consistent with prior work and identify the vmPFC as a critical component of the neural circuit mediating the effects of stressor control on later behaviors. The relationship between the vmPFC and the dorsal raphé nucleus, and other structures mediating stress-induced anxiety, are discussed.
Keywords: escapable stress, learned helplessness, social exploration, medial prefrontal cortex, anxiety, 5-HT
INTRODUCTION
The degree of behavioral control that an individual has over a stressor often determines the consequence of that stressor (Maier and Watkins 2005) and may play a critical role in the development of pathological behaviors after a traumatic event (Foa et al. 1992). Indeed, numerous behavioral outcomes have been reported to follow uncontrollable stress but not an equal exposure where the stressor is controllable (for reviews see (Maier and Watkins 2005; Shors 2004)). For example, exposure to a series of inescapable electrical tailshocks (IS) produces behavioral changes that include exaggerated fear conditioning, poor shuttle escape learning, and reduced social interaction, while exposure to tailshock that is escapable (ES) does not (Amat et al. 2005; Christianson et al. 2008; Short and Maier 1993).
Our laboratory has determined that one mechanism by which behavioral control operates is to prevent stress-induced activation and sensitization of the serotonergic dorsal raphé nucleus (DRN), the mechanism by which uncontrollable stress leads to the behavioral consequences cited above. Using in vivo microdialysis we have found that extracellular serotonin (5-HT) concentrations in the DRN nearly triple at the beginning of the shock session, whether the tailshocks are uncontrollable or controllable. However, when behavioral control is present 5-HT levels quickly return to baseline as the subject learns the escape response, even though tailshocks continue (Amat et al. 2005; Maswood et al. 1998). Extracellular 5-HT within the DRN reflects DRN 5-HT activation because activated DRN 5-HT neurons release 5-HT within the DRN from axon collaterals, as well as at axon terminals in projection regions (Matos et al. 1996). Extracellular DRN 5-HT may also reflect the activity of serotonergic inputs to the DRN, such as the median raphé, but we have demonstrated that this region is activated equally by ES and IS (Takase et al. 2004). Interestingly, both IS and ES activate brain regions that send excitatory input to the DRN (Amat et al. 2001) but as noted, only IS results in a sustained activation of 5-HT neurons. Thus, the differential net impact of IS and ES would seem to require ES-induced inhibitory inputs to the DRN.
In rat, the infralimbic/prelimbic region of ventromedial prefrontal cortex (vmPFC) sends glutamatergic efferents to the DRN (Gabbott et al. 2005) and this vmPFC region has been theorized to contribute to resilience after stress (Jordan et al. 1994). Efferent axons from the vmPFC to the DRN synapse preferentially on GABAergic interneurons that inhibit 5-HT cells (Jankowski and Sesack 2004). Thus, activation of descending vmPFC pyramidal neurons enhances inhibition within the DRN and decreases 5-HT neuronal activity (Celada et al. 2001). The foregoing suggested that perhaps the vmPFC detects behavioral control when it is present, leading to output to the DRN and inhibition of DRN 5-HT neurons. This hypothesis has been supported in a number of ways. First, inhibition of the vmPFC via microinjection of the GABAA agonist muscimol prior to stressor exposure had no effect on the DRN 5-HT neuronal response to IS, but led ES to produce DRN activation, later escape failure, and exaggerated post-shock freezing similar to IS, despite successful shock escape during the initial ES (Amat et al. 2005). Thus, preventing vmPFC activation during ES made the subject appear as if it had experienced IS. Conversely, pharmacological activation of the vmPFC by microinjection of the Cl(−) channel antagonist picrotoxin during IS reduced the stimulation of DRN 5-HT and prevented escape deficits after IS (Amat et al. 2008). That is, pharmacological activation of the vmPFC appeared to give rats the ‘illusion of control’. In sum, the evidence suggests that stress exposure per se activates the DRN, which may be a final common pathway whose projections lead to anxiety-like behaviors. However, when stress is controllable the vmPFC inhibits the DRN and prevents many of the sequelae of uncontrollable stress.
The data implicating the critical role of the vmPFC, however, all depend on post-shock freezing and shuttlebox shock escape failure as the behavioral endpoints. Thus, the generality of the vmPFC effects described above is unknown. We have recently reported that social exploration of a juvenile conspecific, a putative measure of anxiety (Christianson et al. 2008), is reduced by IS, but not ES. Furthermore, this effect of IS was dependent on activation of DRN 5-HT (Christianson et al. 2008). The goal of the present study was to determine whether the stress-protective effect of behavioral control on juvenile social exploration is the result of vmPFC activation. In Experiment 1, muscimol was microinjected into the vmPFC before ES or homecage control (HC) treatment to determine whether activation of this region by ES is necessary to the protective effects of behavioral control on stressor-induced reductions in social exploration. Inactivation of vmPFC by muscimol should release the DRN from inhibition by stressor control and generate a behavior profile similar to that produced by IS. In Experiment 2, picrotoxin was microinjected to the vmPFC before IS or HC treatment to determine whether activation of the region during stress is sufficient to prevent the usual reduction in social exploration produced by IS.
MATERIALS AND METHODS
Subjects
Adult (60–70 days old and weighing 275–350 gm at the time of testing) and juvenile (28–32 days old and weighing 90–100gm at the time of testing) male Sprague-Dawley (Harlan, Indianapolis, IN) rats were used. Rats were housed in plastic tub cages, 4 rats/cage with free access to food and water. The vivarium maintained a 12 h light/dark cycle with lights on at 7:00AM. All behavioral procedures were conducted in the first 5 h of the light cycle. The experimental protocols were reviewed and approved by the University of Colorado Institutional Animal Care and Use Committee and were in accordance with NIH guidelines.
Escapable and Inescapable Tailshock Procedures
Escapable tail shocks were administered in 14 × 11 ×17 cm acrylic wheel turn boxes enclosed in sound-attenuating chambers. Electric shock was delivered through copper electrodes augmented by electrode paste attached 2 and 4 cm from the base of the tail by a Precision Regulated Animal Shocker (Coulbourn Instruments, Allentown, PA). 100 tailshocks were presented on a variable interval-60 s schedule (VI-60). For ES subjects turning a wheel at the front of the chamber terminated each tailshock according to a protocol previously described (Amat et al. 2005). Shock intensity was 1.0mA for the first 33 trials, 1.3mA for the following 33 trials and 1.6mA for the remaining 34 trials. These parameters were used to maintain escape behavior in the ES subjects. For IS, 100, 5 s 1.6mA inescapable tailshocks were administered in clear plastic restraining tubes on a VI-60s schedule. This method has been reported previously, approximates the amount of shock received in yoked-IS, and elicits behavior that is indiscriminable from 100 trials of yoked-IS treatment on social exploration (Christianson et al. 2008). Naive, homecage control (HC) rats remained in their cages. A restraint stress-only control group was not included as this treatment was indescriminable from HC controls in a prior experiment (Christianson et al. 2008).
Juvenile Social Exploration Test
Each experimental adult rat was allocated a separate transparent plastic tubcage with shaved wood bedding and a wire lid located in a brightly lit testing room; food and water were not available in the testing cages. 24 h before stress exposure rats were removed from the vivarium and placed into the single cage. After 60 min a juvenile stimulus rat was added to the cage. Investigative behaviors, including sniffing, pinning and allogrooming, initiated by the adult rat were timed by an observer who was blind to group membership. After 3 min the juvenile was removed and the adult was returned to the homecage. Juvenile stimulus rats were used for multiple tests but were never exposed to the same adult more than once. This social exploration pretest was used to habituate the subjects to the procedure and to screen for rats with unusual baseline social exploration. Lipopolysaccharide, for example, reduces social exploration (Bluthe et al. 1992). In our experience, healty naïve rats typically explore for approximately 80 sec; thus, rats with pretrest social exploration times less than 50 sec were excluded as outliers. 24 hrs after stress, social exploration tests were conducted exactly as on the day before stress.
Cannula Placement and Microinjection Procedures
All surgeries were conducted under 2–3% isoflurane in oxygen inhalational anesthesia. Each rat was implanted with a single dual guide cannula (26g, 1 mm center-to-center distance; Plastics One, Roanoke, VA) so that bilateral injector tips would reach the border between infralimbic and prelimbic cortices (AP +2.9, LM +/− 0.5, DV −2.9). Coordinates were taken from bregma and dura according to Paxinos & Watson (Paxinos and Watson 1998). Cannulae were fixed to the skull with three screws and dental cement. A stylet was placed in the cannula extending 1 mm below the tip of the guide. After surgery each rat received prophylactic antibiotic, 0.25ml Twin-Pen (AgriLabs) per kg body weight. Microinjections were made by gently restraining the rat in a towel and replacing the stylet with a microinjector that extended 1mm beyond the cannula tip (33 g; Plastics One, Roanoke, VA) joined to a cannula connector. In Experiment 1, muscimol, 500ng/side, in 0.5µl 0.9% saline or saline alone was injected at a rate of 1µl/min through PE-50 tubing by a 25µl Hamilton syringe and a Kopf micromanipulator. Injectors remained in place for 2 min to permit diffusion. In Experiment 2, 0.5 µl of picrotoxin, 100ng/side, in 0.9% saline or saline alone was injected in the same way. Muscimol and picrotoxin doses were determined in pilot studies. At the end of each experiment rats were overdosed with sodium pentobarbital (60 mg/kg i.p.) and brains processed for cresyl violet verification of cannula placement using standard procedures.
Experimental Procedures
Experiment 1
After at least 7 days of acclimation to the vivarium, bilateral guide cannula were implanted. After 7–10 recovery days all rats were given a social exploration pretest and then randomly assigned to one of 4 groups in a (Stress: ES or HC) by (Drug: Muscimol or Saline) between-subjects factorial design (n = 10/group). IS rats were not included because the question posed was whether inactivation of the vmPFC during ES would now lead ES to reduce social exploration. IS subjects were not needed to answer this question, and in any event, intra-vmPFC muscimol has had no effect on IS subjects in prior studies (Amat et al. 2005; Amat et al. 2006; Baratta et al. 2007). On the following day, rats received microinjection of either muscimol or saline 60 min before ES. Rats in the HC group were returned to the vivarium after injection. 24 h after the onset of stress all rats were given the social exploration test.
Experiment 2
As in Experiment 1, rats were implanted with cannula, allowed 7–10 days to recover and given a social exploration pretest. Rats were randomly assigned to one of 4 groups in a (Stress: IS or HC) by (Drug: Picrotoxin or Saline) between-subjects factorial design (n = 10/group). On the following day, rats received microinjection of either picrotoxin or saline 60 min before IS. Rats in the HC group were returned to the vivarium after injection. 24 h after the onset of stress all rats were given the social exploration test. ES subjects were not included because the question posed was whether intra-vmPFC picrotoxin would block the reduction in social exploration produced by IS. Picrotoxin has had no effect in ES subjects in prior studies (Amat et al. 2008).
RESULTS
Experiment 1
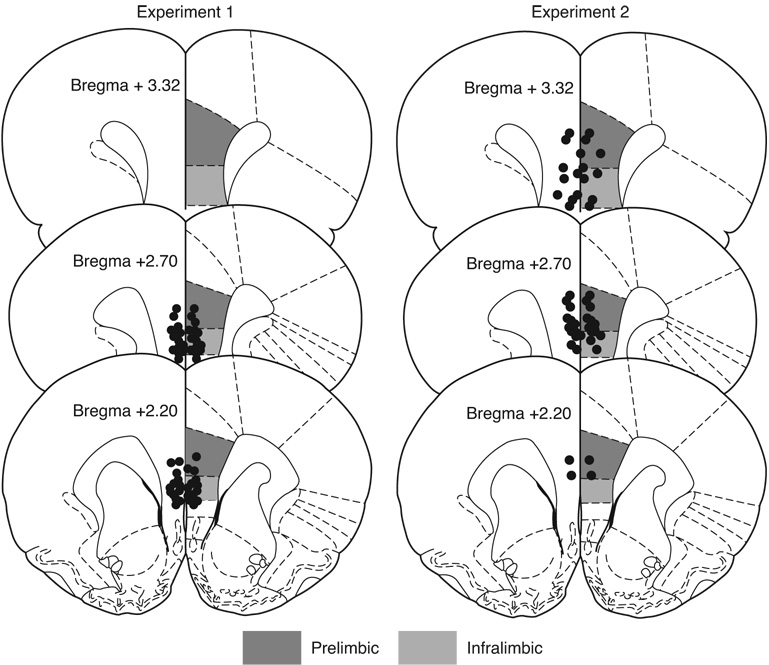
Exclusion of subjects with social exploration times less than 50 sec yielded the following group sizes: ES-Saline, n = 8, ES-Muscimol, n=10, HC-Saline, n = 8, HC-Muscimol, n =9. Pretreatment with muscimol did not interfere with acquisition of the wheel-turn escape response and all ES rats quickly reached the maximum escape requirement (4 full turns) with escape latencies under 5 s for the majority of trials and the data are comparable to those previously reported (Amat et al. 2005). Location of the cannula tips and mean social exploration time are shown in Figure 1 and Figure 2, respectively. In saline-treated rats, ES had no effect on social exploration relative to unstressed HC controls. However, inhibition of the vmPFC by muscimol led to a significant reduction in social exploration after ES but had no effect on the HC group. A two-way ANOVA identified a main effect of Stress, F (1, 30) = 5.754, p=0.023, and a significant Stress by Drug interaction, F(1, 30) = 14.65, p < 0.001. The main effect of Drug did not reach significance, p=0.14. Fisher’s protected least significant difference (PLSD) post-hoc comparisons indicated that mean social exploration in the ES-Muscimol group was significantly lower than ES-Saline and both HC groups, ps < 0.01. No other comparisons reached significance.
Figure 1.
Location of cannula injector tips in the ventromedial prefrontal cortex. Anatomical illustrations adapted from The Rat Brain in Stereotaxic Coordinates (Paxinos and Watson 1998).
Figure 2.
(a) Mean (±S.E.M.) time spent in social exploration of a juvenile during a 3 minute test. Escapable stress had no effect on social exploration (ES-Saline group) but inactivation of vmPFC during stress with muscimol interfered and significantly reduced social exploration in the ES-Muscimol group. *ES-Muscimol group significantly lower than all other groups, ps < 0.01.
Experiment 2
No rats had social exploration times less than 50 sec in the social exploration pretest. Location of cannula tips and mean social exploration time are shown in Figure 1 and Figure 2, respectively. IS reduced social exploration but activation of vmPFC before IS completely prevented this effect. A two-way ANOVA identified a marginally significant main-effect of Stress, F(1, 36)= 3.634, p = 0.065 and a significant Stress by Drug interaction, F(1, 36) = 6.16, p=0.018. The main effect of Drug did not reach significance, p=0.11. Fisher’s PLSD post-hoc comparisons revealed that mean social exploration time was significantly reduced in the IS-Saline group compared to HC-Saline and IS-Picrotoxin groups, ps < 0.01. No other comparisons reached significance.
DISCUSSION
The data from the present experiments further implicate the vmPFC in the neural circuit that mediates the resistance to the behavioral effects of a stressor that occurs when behavioral control over the stressor is present. Rats were exposed to escapable or inescapable tailshocks with concomitant pharmacological inactivation or activation, of the vmPFC during the stressor, respectively. Prior experiments have suggested that the presence of control activates vmPFC regions (Amat et al. 2005; Amat et al. 2006; Baratta et al. 2007). Thus, rats with active (ESSaline and IS-Picrotoxin) vmPFC during the stressor showed no effect of the stressor session in the social exploration test one day later. Based on previous observations in our lab, inactivation of the vmPFC in IS (IS-Muscimol) has no effect on IS subjects (Amat et al. 2005); likewise, activation of the mPFC (ES-picrotoxin) has no effect on ES subjects (Amat et al. 2008). Therefore, these experimental groups were unnecessary. These results complement prior work that utilized post-shock freezing and shuttle escape measures and illuminate the way by which control over stress relates to anxiety-like endpoints.
As noted, IS results in prolonged activation of the DRN, leading to downregulation of somatodendritic 5-HT1A autoreceptor mRNA, (Greenwood et al. 2003), resulting in sensitization of 5-HT neurons to subsequent aversive stimuli (Amat et al. 1998). Inhibition of DRN during IS prevents the subsequent reduction in social exploration (Christianson et al. 2008). Taken with the anatomical relationship between the vmPFC and the DRN described above, the present data further support the argument that control over stress regulates the activity of the DRN by a circuit involving the vmPFC.
The vmPFC cortex receives a convergence of thalamic and subcortical inputs and is considered an executive center suited to regulate a range of neural processes (Dalley et al. 2004). It is likely, therefore, that when the vmPFC receives sensory information that contains a contingent relationship between motor behaviors and shock termination (i.e., the escape response) that it is able to inhibit stress responsive nuclei such as the DRN. In contrast, when there is no apparent relationship between behavior and shock termination the vmPFC does not send inputs to stress-responsive regions, and so they remain unregulated. If this is the case, then the critical variable determining the outcomes of stressor controllability experiments is not whether the subject detects the non-contingency of behavior and shock as was originally proposed (Maier and Seligman 1976), but rather that behavioral control over stress activates vmPFC output.
These experiments implicate the vmPFC in the regulation of anxiety-like behavior. Social exploration is a widely used measure of anxiety-like behavior (File and Seth 2003) and DRN activity is correlated with reduced exploration (File et al. 1996; Higgins et al. 1992; Overstreet et al. 2006). The idea here is that activity within the DRN modulates anxiety behavior via projections to limbic regions such as the basolateral amygdala (Lowry et al. 2005). Therefore, social exploration may be reduced in IS-treated rats because the DRN is sensitized and is hyper-responsive in the mildly-anxiogenic context of a social encounter. Although the vmPFC might instead, or in addition, directly modulate amygdala activity (Quirk et al. 2003). The role of the DRN and vmPFC in anxiety are reinforced by two human studies. Lazenberger et al. reported a reduction in DRN 5-HT1A receptors in social anxiety disorder patients (Lanzenberger et al. 2007) and Monk et al. found an inverse relationship between prefrontal cortex and amygdala activity when patients with generalized anxiety were presented with anxiogenic stimuli (Monk et al. 2008). Because the present data do not directly address the relationship between the vmPFC and the amygdala this possibility remains open. The role of the vmPFC in anxiety-like behavior may be simply to prevent sensitization of the DRN.
Figure 3.
Mean (±S.E.M.) time spent in social exploration of a juvenile during a 3 minute test. Inescapable stress reduced social exploration compared to the homecage controls (IS-Saline group). However, activation of the vmPFC at the time of stress with picrotoxin completely prevented the effect of stress and social exploration time was equal to controls (IS-PIX group). *IS-Saline group significantly lower than all other groups, ps < 0.01.
ACKNOWLEDGEMENT
Funding for these experiments was provided by National Institutes of Health Grant MH050479 to S.F.M.
REFERENCES
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res. 1998;812:113–120. doi: 10.1016/s0006-8993(98)00960-3. [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Watkins LR, Maier SF. Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control. Neuroscience. 2008;154:1178–1186. doi: 10.1016/j.neuroscience.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosc. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Sparks PD, Matus-Amat P, Griggs J, Watkins LR, Maier SF. The role of the habenular complex in the elevation of dorsal raphe nucleus serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Res. 2001;917:118–126. doi: 10.1016/s0006-8993(01)02934-1. [DOI] [PubMed] [Google Scholar]
- Baratta MV, Christianson JP, Gomez DM, Zarza CM, Amat J, Masini CV, Watkins LR, Maier SF. Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neuroscience. 2007;146:1495–1503. doi: 10.1016/j.neuroscience.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthe RM, Dantzer R, Kelley KW. Effects of interleukin-1 receptor antagonist on the behavioral effects of lipopolysaccharide in rat. Brain Res. 1992;573:318–320. doi: 10.1016/0006-8993(92)90779-9. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: Involvement of serotonin-1A, GABA(A), and glutamate receptors. J Neurosci. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Paul ED, Irani M, Thompson BM, Kubala KH, Yirmiya R, Watkins LR, Maier SF. The role of prior stressor controllability and the dorsal raphe nucleus in sucrose preference and social exploration. Behav Brain Res. 2008;193:87–93. doi: 10.1016/j.bbr.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- File SE, Gonzalez LE, Andrews N. Comparative study of pre- and postsynaptic 5-HT1A receptor modulation of anxiety in two ethological animal tests. J Neurosci. 1996;16:4810–4815. doi: 10.1523/JNEUROSCI.16-15-04810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Foa EB, Zinbarg R, Rothbaum BO. Uncontrollability and unpredictability in post-traumatic stress disorder: an animal model. Psychol Bull. 1992;112:218–238. doi: 10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Jones BJ, Oakley NR. Effect of 5-HT1A receptor agonists in two models of anxiety after dorsal raphe injection. Psychopharmacology (Berl) 1992;106:261–267. doi: 10.1007/BF02801982. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, Sesack SR. Prefrontal cortical projections to the rat dorsal raphe nucleus: ultrastructural features and associations with serotonin and gamma-aminobutyric acid neurons. J Comp Neurol. 2004;468:518–529. doi: 10.1002/cne.10976. [DOI] [PubMed] [Google Scholar]
- Jordan S, Kramer GL, Zukas PK, Moeller M, Petty F. In vivo biogenic amine efflux in medial prefrontal cortex with imipramine, fluoxetine, and fluvoxamine. Synapse. 1994;18:294–297. doi: 10.1002/syn.890180404. [DOI] [PubMed] [Google Scholar]
- Lanzenberger RR, Mitterhauser M, Spindelegger C, Wadsak W, Klein N, Mien LK, Holik A, Attarbaschi T, Mossaheb N, Sacher J, Geiss-Granadia T, Kletter K, Kasper S, Tauscher J. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry. 2007;61:1081–1089. doi: 10.1016/j.biopsych.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- Maier SF, Seligman ME. Learned helplessness: theory and evidence. J Exp Psychol Gen. 1976;105:3–46. [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Maswood S, Barter JE, Watkins LR, Maier SF. Exposure to inescapable but not escapable shock increases extracellular levels of 5-HT in the dorsal raphe nucleus of the rat. Brain Res. 1998;783:115–120. doi: 10.1016/s0006-8993(97)01313-9. [DOI] [PubMed] [Google Scholar]
- Matos FF, Urban C, Yocca FD. Serotonin (5-HT) release in the dorsal raphe and ventral hippocampus: raphe control of somatodendritic and terminal 5-HT release. J Neural Transm. 1996;103:173–190. doi: 10.1007/BF01292626. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, Chen G, McClure-Tone EB, Ernst M, Pine DS. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Angel RA, Navarro M, Breese GR. Reduction in repeated ethanol-withdrawal-induced anxiety-like behavior by site-selective injections of 5-HT1A and 5-HT2C ligands. Psychopharmacology (Berl) 2006;187:1–12. doi: 10.1007/s00213-006-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th Ed. New York: Academic Press; 1998. [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ. Learning during stressful times. Learn Mem. 2004;11:137–144. doi: 10.1101/lm.66604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KR, Maier SF. Stressor controllability, social interaction, and benzodiazepine systems. Pharmacol Biochem Behav. 1993;45:827–835. doi: 10.1016/0091-3057(93)90128-g. [DOI] [PubMed] [Google Scholar]
- Takase LF, Nogueira MI, Baratta M, Bland ST, Watkins LR, Maier SF, Fornal CA, Jacobs BL. Inescapable shock activates serotonergic neurons in all raphe nuclei of rat. Behav Brain Res. 2004;153:233–239. doi: 10.1016/j.bbr.2003.12.020. [DOI] [PubMed] [Google Scholar]