Abstract
Gamma-aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the brain, and the responsiveness of neurons to GABA can be modulated by sex steroids. To better understand how ovarian steroids influence GABAergic system in the primate brain, we evaluated the expression of genes encoding GABA receptor subunits, glutamic acid decarboxylase (GAD) and a GABA transporter in the brains of female rhesus macaques. Ovariectomized adults were subjected to a hormone replacement paradigm involving either 17β-estradiol (E), or E plus progesterone (E+P). Untreated animals served as controls. Using GeneChip® microarray analysis and real-time RT-PCR (qPCR), we examined gene expression differences within and between the amygdala (AMD), hippocampus (HPC) and arcuate nuclei of the medial basal hypothalamus (MBH). The results from PCR corresponded with results from representative GeneChip® probesets, and showed similar effects of sex steroids on GABA receptor subunit gene expression in the AMD and HPC, and a more pronounced expression than in the MBH. Exposure to E+P attenuated GAD1, GAD2 and SLC32A1 gene expression in the AMD and HPC, but not in the MBH. GABA receptor subunit gene expression was generally higher in the AMD and HPC than in the MBH, with the exception of receptor subunits ε and γ2. Taken together, the data demonstrate differential regulation of GABA receptor subunits and GABAergic system components in the MBH compared to the AMD and HPC of rhesus macaques. Elevated ε and reduced δ subunit expression in the MBH supports the hypothesis that the hypothalamic GABAergic system is resistant to the modulatory effects of sex steroids.
Keywords: gamma-aminobutyric acid, GABA receptor, Macaca mulatta, microarray
1. Introduction
Gamma-aminobutyric acid (GABA) is the most widespread inhibitory neurotransmitter in the vertebrate central nervous system (CNS) (Sivilotti and Nistri, 1991), where its release by both neurons (Zilberter et al., 1999) and glial cells (Angulo et al., 2008) affects various physiological functions. Changes in receptor subunit composition may alter properties and density of GABA receptor protein (Weiland and Orchinik, 1995) and may dramatically alter the effect the responsiveness of the cell to GABA (Steiger and Russek, 2004). Consequently, differential regulation of GABA receptor subtypes represents an important mechanism for maintaining or altering CNS excitation/inhibition (E/I) balance during homeostasis or in response to physiological challenge (Bethmann et al., 2008; Fritschy, 2008; Olsen and Sieghart, 2008). Fluctuations in the sex-steroid environment during reproductive cycles are highly associated with changes in GABA receptor subunit composition in the brain (Brussaard et al., 1997; Byrnes et al., 2007; Jorge et al., 2002; Lovick, 2006) and circulating steroids also affect expression of the rate limiting enzyme required for GABA synthesis, glutamic acid decarboxylase (GAD), (Herbison et al., 1992; McCarthy et al., 1995; Sagrillo and Selmanoff, 1997), as well as GABA transporters (Herbison et al., 1995).
The influence of ovarian steroids on GABA receptor subunit composition has been studied in detail in cellular models and in the rodent CNS, but not in primates. After ovulation, the corpus luteum (Stouffer, 2006) of rhesus macaque monkeys and humans, lasts much longer than in rodents and so continues to produce P for extended periods (Knobil, 1973; Weik et al., 1973), whereas in the rat and mouse, the ultra short-lived (Stouffer, 2006) corpus luteum is not well developed (Hilliard, 1973).
Progesterone can be metabolized in the brain to neuroactive metabolites which include 5α-dihydroprogesterone, 3α, 5α-tetrahydroprogesterone (allopregnanolone), and 3β, 5α-tetrahydroprogesterone (pregnanolone). These P derivatives are collectively referred to as THP (Compagnone and Mellon, 2000), and are potent positive modulators of GABAA receptors (Callachan et al., 1987; Gee et al., 1987). In addition to synthesis through modification of circulating steroids (Corpechot et al., 1983; Robel et al., 1987), these neuroactive steroids (neurosteroids) (Baulieu, 1998; Compagnone and Mellon, 2000) can also be synthesized de novo from cholesterol in rodent brains.
In humans, the links between P and neurosteroid levels may correlate anxiety and mood disorders with hormonal effects on the CNS during menstruation (Backstrom, 1976; Guille et al., 2008; N-Wihlback et al., 2006). The connections between hormonal fluctuations of reproductive cycles and neurosteroid modulation of GABAergic systems in primates are focal points in the study of human premenstrual syndrome (Rapkin et al., 1997).
The goal of the present study was to examine the effects of sex-steroids on GABAergic CNS physiology in a nonhuman primate, the rhesus macaque. Using Affymetrix® GeneChip® Rhesus Macaque Genome Arrays and quantitative real-time RT-PCR (qPCR), we examined how the expression of GABAergic genes in the amygdala (AMD), hippocampus (HPC) and arcuate nucleus of the medial basal hypothalamus (MBH) was affected by exposure to 17β-estradiol (E) or 17β-estradiol + progesterone (E+P); these hormone replacement paradigms mimic the follicular and luteal phases of the menstrual cycle, respectively.
2. Results
2.1 GeneChip® microarrays
A summary of the GeneChip® expression profiles for representative subunit probesets is provided in Table 1. Detailed information for all probesets annotated for GABA receptor subunits, GAD, and the GABA transporter SLC32A1 are provided in Table 2. Treatment-related expression differences within tissues were observed for genes encoding GABAA receptor subunits α1, α4, α5, β1, β3, γ2, and GABAB receptor subunits 1 and 2. Gene expression levels in the MBH were lower than in the AMD and HPC, with the exception of GABAA receptor subunit ε, where gene expression in the MBH was higher (Table 1). The most reliable probe sets for genes encoding GABAA receptor subunits α6, γ3, θ and π, as well as GABAC receptor subunits returned “absent” calls for multiple animals in multiple treatments, and were thus regarded as non-detectable using MAS 5.0 global scaling analysis. Expression of the gene encoding the vesicular GABA transporter SLC32A1 was comparable across all three brain regions in OVX animals but tended to be reduced by E and E+P in the AMD and HPC. In the AMD, vesicular GABA transporter expression was significantly reduced with E+P treatment (P<0.01). Similar results were observed with respect to expression of GAD1 (67kDa) and GAD2 (65 kDa) mRNA expression (Table 1).
Table 1.
Microarray analysis summary for expression of genes encoding GABAergic system components in the rhesus macaque.
| non subunit |
GABA Receptor | Brain Region | Treatment | Probeset ID | |||||
|---|---|---|---|---|---|---|---|---|---|
| Family | subunit | MBH | AMD | HPC | Ovx | E | E+P | ||
| A | α1 | ↑↑EP | ↓↓EP | ↓E, ↓↓EP | ↑↑H | --- | ↓A | MmuSTS.2385.1.S1_at | |
| α2 | --- | --- | --- | ↑H, ↑↑A | --- | --- | MmuSTS.4812.1.S1_at | ||
| α3 | --- | --- | --- | --- | --- | ↓H | MmuSTS.4431.1.S1_at | ||
| α4 | ↑↑EP | --- | ↓E, ↓EP | ↑H, ↑A | ↑H, ↑A | ↑H, ↑↑A | MmugDNA.20347.1.S1_at | ||
| α5 | --- | ↑↑EP | --- | ↑H, ↑↑A | ↑H, ↑A | ↑H | MmugDNA.3506.1.S1_at | ||
| α6 | ND | ND | ND | ND | ND | ND | MmugDNA.20349.1.S1_at | ||
| β1 | --- | ↑↑EP | --- | ↑↑A | ↑↑A | ↑↑A | MmugDNA.18163.1.S1_at | ||
| β2 | --- | --- | --- | ↑H, ↑↑A | --- | --- | MmuSTS.2387.1.S1_at | ||
| β3 | --- | ↓EP | --- | ↑↑A | ↑↑A | ↑↑H | MmugDNA.2520.1.S1_at | ||
| γ1 | --- | --- | --- | ↑A | --- | --- | MmugDNA.14456.1.S1_at | ||
| γ2 | --- | --- | ↓EP | ↑A | --- | ↓H, ↓A | MmuSTS.2388.1.S1_at | ||
| γ3 | ND | ND | ND | ND | ND | ND | MmugDNA.36967.1.S1_at | ||
| δ | --- | --- | --- | ↑↑H, ↑A | ↑H, ↑A | ↑↑A | MmugDNA.30046.1.S1_at | ||
| ε | --- | --- | --- | ↓↓H | ↓↓H, ↓A | ↓↓H, ↓A | MmuSTS.4814.1.S1_at | ||
| θ | ND | ND | ND | ND | ND | ND | MmuSTS.2391.1.S1_at | ||
| π | ND | ND | ND | ND | ND | ND | MmuSTS.4815.1.S1_at | ||
| B | 1 | ↑↑EP | --- | --- | ↑↑A | ↑↑A | ↑↑A | MmugDNA.18163.1.S1_at | |
| 2 | ↑EP | --- | --- | ↑↑H | ↑↑H, ↑A | ↑H, ↑↑A | MmugDNA.42052.1.S1_at | ||
| C | ρ1 | ND | ND | ND | ND | ND | ND | MmugDNA.40686.1.S1_at | |
| ρ2 | ND | ND | ND | ND | ND | ND | MmuSTS.4462.1.S1_at | ||
| GAD1 | --- | ↓EP | ↓EP | --- | --- | ↓↓H, ↓A | MmugDNA.40699.1.S1_at | ||
| GAD2 | --- | ↓↓EP | ↓EP | --- | --- | ↓↓H | MmugDNA.40703.1.S1_at | ||
| SLC32A1 | --- | ↓EP | ↓↓EP | --- | --- | ↓↓H | MmugDNA.30515.1.S1_at | ||
The table summarizes analyses of RMA-normalized gene expression using representative probesets on the GeneChip® Rhesus Macaque Genome Array. Arrows indicate Newman-Keuls post hoc test results from Kruskal-Wallis one-way analysis of variance are shown. Selected probesets were prioritized by A-grade annotation and STS designation. For brain region, HPC = hippocampus; AMD = amygdala; and MBH= arcuate nucleus of the medial basal hypothalamus. For treatment, OVX = ovariectomized; E= estrogen treatment; and E+P = estrogen + progesterone treatment. For brain regions, differences are shown with respect to OVX animals. For Treatment, differences are shown with respect to the MBH. ↑= upregulation; ↓= downregulation. Number of arrows indicates post-hoc test P values with respect to the OVX or MBH group. One arrow indicates P < 0.05. Two arrows indicate P < 0.01. Dash (---) indicates no expression differences in a given category. ND = negative detection call (Affymetrix GCOS analysis) in multiple animals in at least two treatment groups.
Table 2.
GABAergic system component sorted by probeset annotation.
| Target description common names | Gene symbol (search) |
Gene symbol (listed) |
Probe set I.D. | AG | MBH | # Positive AMD |
HPC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | mean | 1 | 2 | 3 | mean | 1 | 2 | 3 | mean | |||||
| GABA-A receptor α 1 | Gabra1 | none | MmuSTS.2385.1.S1_at | A | 4 | 4 | 4 | 791 | 4 | 4 | 4 | 1348 | 4 | 4 | 4 | 1695 |
| GABA-A receptor α 1 | Gabra1 | GABRA1 | MmugDNA.15252.1.S1_at | B | 4 | 4 | 4 | 700 | 4 | 4 | 4 | 1389 | 4 | 4 | 4 | 1845 |
| GABA-A receptor α 2 | Gabra2 | GABRA2 | MmugDNA.41722.1.S1_at | A | 1 | 1 | 2 | 29 | 2 | 2 | 1 | 45 | 4 | 1 | 4 | 40 |
| GABA-A receptor α 2 | Gabra2 | GABRA2 | MmuSTS.4812.1.S1_at | B | 4 | 4 | 4 | 784 | 4 | 4 | 4 | 1467 | 4 | 4 | 4 | 1238 |
| GABA-A receptor α 3 | Gabra3 | LOC711230 | MmuSTS.4431.1.S1_at | B | 4 | 4 | 4 | 440 | 4 | 4 | 4 | 374 | 4 | 4 | 4 | 242 |
| GABA-A receptor α 4 | Gabra4 | LOC704008 | MmugDNA.20347.1.S1_at | A | 4 | 4 | 4 | 282 | 4 | 4 | 4 | 1040 | 4 | 4 | 4 | 908 |
| GABA-A receptor α 4 | Gabra4 | none | MmugDNA.6656.1.S1_at | A | 3 | 2 | 2 | 36 | 4 | 3 | 3 | 97 | 4 | 3 | 4 | 68 |
| GABA-A receptor α 5 | Gabra5 | GABRA5 | MmuSTS.2386.1.S1_at | B | 4 | 4 | 4 | 448 | 4 | 4 | 4 | 1437 | 4 | 4 | 4 | 1780 |
| GABA-A receptor α 5 | Gabra5 | GABRA5 | MmugDNA.3506.1.S1_at | A | 3 | 2 | 3 | 75 | 4 | 4 | 4 | 306 | 4 | 4 | 4 | 353 |
| GABA-A receptor α 5 | Gabra5 | GABRA5 | MmugDNA.28557.1.S1_s_at | A | 4 | 2 | 4 | 78 | 4 | 2 | 2 | 217 | 4 | 4 | 4 | 230 |
| GABA-A receptor α 6 | Gabra6 | GABRA6 | MmugDNA.20349.1.S1_at | A | 0 | 0 | 1 | 25 | 1 | 1 | 0 | 35 | 0 | 0 | 0 | 23 |
| GABA-A receptor β 1 | Gabrb1 | GABRB1 | MmugDNA.24091.1.S1_at | A | 4 | 4 | 4 | 3453 | 4 | 4 | 4 | 3680 | 4 | 4 | 4 | 3495 |
| GABA-A receptor β 1 | Gabrb1 | none | MmugDNA.37267.1.S1_at | A | 1 | 0 | 0 | 16 | 0 | 0 | 0 | 19 | 0 | 0 | 0 | 24 |
| GABA-A receptor β 1 | Gabrb1 | GABRB1 | Mmu.10481.1.S1_at | B | 4 | 4 | 4 | 612 | 4 | 4 | 4 | 540 | 4 | 4 | 4 | 563 |
| GABA-A receptor β 2 | Gabrb2 | LOC696767 | MmuSTS.2387.1.S1_at | A | 1 | 1 | 1 | 67 | 4 | 1 | 0 | 99 | 4 | 1 | 0 | 78 |
| GABA-A receptor β 2 | Gabrb2 | none | MmugDNA.3290.1.S1_at | B | 0 | 0 | 0 | 33 | 2 | 1 | 0 | 60 | 2 | 1 | 0 | 67 |
| GABA-A receptor β 3 | Gabrb3 | GABRB3 | MmuSTS.4813.1.S1_at | A | 4 | 4 | 4 | 2337 | 4 | 4 | 4 | 3416 | 4 | 4 | 4 | 3586 |
| GABA-A receptor β 3 | Gabrb3 | GABRB3 | MmugDNA.2520.1.S1_at | A | 4 | 4 | 4 | 1052 | 4 | 4 | 4 | 1535 | 4 | 4 | 4 | 1293 |
| GABA-A receptor β 3 | Gabrb3 | GABRB3 | MmugDNA.2522.1.S1_at | A | 4 | 4 | 4 | 958 | 4 | 4 | 4 | 1896 | 4 | 4 | 4 | 1803 |
| GABA-A receptor β 3 | Gabrb3 | GABRB3 | MmugDNA.24108.1.S1_at | A | 3 | 4 | 4 | 218 | 4 | 4 | 4 | 366 | 4 | 4 | 4 | 353 |
| GABA-A receptor β 3 | Gabrb3 | none | MmugDNA.23997.1.S1_at | A | 1 | 0 | 1 | 16 | 0 | 1 | 0 | 27 | 0 | 0 | 1 | 24 |
| GABA-A receptor β 3 | Gabrb3 | GABRB3 | MmugDNA.33250.1.S1_at | B | 0 | 0 | 0 | 60 | 1 | 1 | 0 | 79 | 0 | 0 | 0 | 65 |
| GABA-A receptor γ 1 | Gabrg1 | none | MmugDNA.14456.1.S1_at | A | 4 | 4 | 4 | 247 | 4 | 4 | 4 | 253 | 4 | 4 | 4 | 181 |
| GABA-A receptor γ 2 | Gabrg2 | LOC697248 | MmuSTS.2388.1.S1_at | A | 4 | 4 | 4 | 1787 | 4 | 4 | 4 | 1850 | 4 | 4 | 4 | 1583 |
| GABA-A receptor γ 2 | Gabrg2 | LOC697248 | MmugDNA.20370.1.S1_at | A | 4 | 4 | 4 | 491 | 4 | 4 | 4 | 718 | 4 | 4 | 4 | 493 |
| GABA-A receptor γ 3 | Gabrg3 | LOC720390 | MmugDNA.36967.1.S1_at | A | 0 | 2 | 2 | 46 | 0 | 0 | 0 | 33 | 1 | 0 | 0 | 40 |
| GABA-A receptor δ | Gabrd | LOC710062 | MmugDNA.30046.1.S1_at | A | 0 | 1 | 0 | 34 | 4 | 4 | 4 | 390 | 4 | 4 | 4 | 313 |
| GABA-A receptor δ | Gabrd | LOC710062 | MmugDNA.20351.1.S1_at | A | 0 | 0 | 0 | 15 | 4 | 4 | 4 | 307 | 4 | 4 | 3 | 235 |
| GABA-A receptor ε | Gabre | GABRE | MmuSTS.4814.1.S1_at | A | 4 | 4 | 4 | 2000 | 4 | 4 | 4 | 253 | 4 | 4 | 4 | 194 |
| GABA-A receptor θ | Gabrq | LOC705252 | MmuSTS.2391.1.S1_at | A | 0 | 0 | 2 | 47 | 0 | 0 | 0 | 38 | 0 | 0 | 0 | 22 |
| GABA-A receptor π | Gabrp | GABRP | MmuSTS.4815.1.S1_at | A | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 9 |
| GABA-B receptor 1 | Gabbr1 | LOC708987 | MmugDNA.18163.1.S1_at | A | 4 | 4 | 4 | 1535 | 4 | 4 | 4 | 4081 | 4 | 4 | 4 | 3108 |
| GABA-B receptor 1 | Gabbr1 | none | MmugDNA.40991.1.S1_at | R | 4 | 4 | 4 | 89 | 3 | 4 | 4 | 112 | 4 | 4 | 4 | 108 |
| GABA-B receptor 2 | Gabbr2 | GABBR2 | MmugDNA.42052.1.S1_at | A | 4 | 4 | 4 | 1985 | 4 | 4 | 4 | 5440 | 4 | 4 | 4 | 5901.12 |
| GABA-B receptor 2 | Gabbr2 | GABBR2 | MmuSTS.2471.1.S1_at | A | 4 | 4 | 4 | 1220 | 4 | 4 | 4 | 3485 | 4 | 4 | 4 | 3634.03 |
| GABA-B receptor 2 | Gabbr2 | GABBR2 | MmugDNA.42052.1.S1_s_at | A | 4 | 4 | 4 | 896.3 | 4 | 4 | 4 | 2884 | 4 | 4 | 4 | 3011.51 |
| GABA-B receptor 2 | Gabbr2 | GABBR2 | MmugDNA.13130.1.S1_s_at | A | 4 | 3 | 3 | 191.1 | 4 | 4 | 4 | 925 | 4 | 4 | 4 | 962.675 |
| GABA-B receptor 2 | Gabbr2 | GABBR2 | MmugDNA.29020.1.S1_at | A | 4 | 3 | 3 | 106.8 | 4 | 4 | 4 | 475 | 4 | 4 | 4 | 518.683 |
| GABA-C receptor ρ 1 | Gabrr1 | GABRR1 | MmugDNA.40686.1.S1_at | A | 0 | 0 | 0 | 28 | 0 | 0 | 0 | 13 | 0 | 0 | 0 | 14 |
| GABA-C receptor ρ 2 | Gabrr2 | GABRR2 | MmuSTS.4462.1.S1_at | A | 0 | 0 | 0 | 34 | 0 | 0 | 0 | 46 | 0 | 1 | 0 | 32 |
| GABA(A) receptor-associated protein |
GABARAP | GABARAP | MmugDNA.2269.1.S1_at | A | 4 | 4 | 4 | 2851 | 4 | 4 | 4 | 3742 | 4 | 4 | 4 | 3060.24 |
| GABA receptor-associated protein- like 1 |
GABARAPL1 | LOC702869 | MmugDNA.9557.1.S1_at | B | 4 | 4 | 4 | 4370 | 4 | 4 | 4 | 3002 | 4 | 4 | 4 | 3952.11 |
| glycine cleavage system protein H | GABARAPL1 | LOC698520 | MmugDNA.9557.1.S1_s_at | A | 4 | 4 | 4 | 3252 | 4 | 4 | 4 | 4484 | 4 | 4 | 4 | 4023.68 |
| GABA receptor-associated protein- like 1 |
GABARAPL1 | LOC702869 | MmugDNA.14486.1.S1_at | B | 4 | 4 | 4 | 1692 | 4 | 4 | 4 | 2448 | 4 | 4 | 4 | 2131.4 |
| glycine cleavage system protein H | GABARAPL1 | LOC698520 | Mmu.3418.2.S1_at | A | 3 | 4 | 4 | 1525 | 4 | 4 | 4 | 1928 | 4 | 4 | 4 | 1695.14 |
| GABA receptor-associated protein like 2 |
GABARAPL2 | LOC714413 | MmugDNA.18777.1.S1_at | A | 4 | 4 | 4 | 7882 | 4 | 4 | 4 | 6647 | 4 | 4 | 4 | 6617.59 |
| GABA receptor-associated protein like 2 |
GABARAPL2 | LOC714413 | Mmu.11093.1.S1_at | A | 4 | 4 | 4 | 4050 | 4 | 4 | 4 | 4094 | 4 | 4 | 4 | 3503.16 |
| coiled-coil domain containing 23 | SLC32A1 | LOC698308 | MmugDNA.30515.1.S1_at | A | 4 | 4 | 4 | 650.8 | 4 | 4 | 4 | 512.4 | 4 | 4 | 4 | 321.483 |
| solute carrier family 6 | SLC6A11 | MmugDNA.19358.1.S1_at | A | 2 | 3 | 3 | 101.1 | 0 | 0 | 0 | 70.66 | 0 | 0 | 0 | 50.85 | |
| SLC6A11 | MmugDNA.3052.1.S1_at | A | 0 | 0 | 0 | 13.56 | 1 | 0 | 2 | 23.1 | 0 | 0 | 0 | 12.525 | ||
| SLC6A11 | MmugDNA.31612.1.S1_at | B | 0 | 0 | 0 | 22.92 | 0 | 0 | 0 | 13.29 | 0 | 0 | 1 | 14.2667 | ||
| SLC6A1 | MmuSTS.3877.1.S1_at | A | 4 | 4 | 4 | 2314 | 4 | 4 | 4 | 3351 | 4 | 4 | 4 | 3241.03 | ||
| multiple coiled-coil GABABR1-BP | JAKMIP1 | MmugDNA.30250.1.S1_at | B | 4 | 4 | 4 | 761.5 | 4 | 4 | 4 | 990 | 4 | 4 | 4 | 1061.1 | |
| glutamate decarboxylase 1 | GAD1 | GAD1 | MmugDNA.40699.1.S1_at | A | 4 | 4 | 4 | 3501 | 4 | 4 | 4 | 2869 | 4 | 4 | 4 | 2663.33 |
| glutamate decarboxylase 1 | GAD1 | GAD1 | MmuAffx.22.1.S1_at | B | 3 | 3 | 3 | 331.3 | 4 | 2 | 1 | 263 | 4 | 2 | 0 | 180.9 |
| glutamate decarboxylase 1 | GAD1 | GAD1 | MmugDNA.20165.1.S1_at | A | 3 | 4 | 4 | 235.1 | 4 | 3 | 2 | 245.4 | 4 | 3 | 3 | 165.375 |
| poly(A) binding protein | GAD2 | LOC721857 | MmugDNA.40703.1.S1_at | A | 4 | 4 | 4 | 2084 | 4 | 4 | 4 | 2225 | 4 | 4 | 4 | 1708.69 |
| Glutamate decarboxylase 2 | GAD2 | MmugDNA.34507.1.S1_at | A | 4 | 4 | 4 | 634.4 | 4 | 4 | 4 | 390 | 4 | 4 | 4 | 445.408 | |
| Glutamate decarboxylase 2 | GAD2 | GAD2 | MmugDNA.12677.1.S1_at | A | 2 | 1 | 2 | 119.3 | 3 | 2 | 2 | 115.9 | 4 | 2 | 1 | 117.4 |
| poly(A) binding protein | GAD2 | LOC721857 | MmugDNA.3230.1.S1_at | B | 2 | 4 | 3 | 95.1 | 4 | 1 | 3 | 73.57 | 4 | 2 | 2 | 80.825 |
| GAD2 | MmugDNA.33087.1.S1_at | C | 2 | 3 | 3 | 54.56 | 3 | 0 | 1 | 30.36 | 3 | 0 | 0 | 37.15 | ||
| poly(A) binding protein | GAD2 | LOC721857 | MmugDNA.6977.1.S1_at | B | 0 | 1 | 1 | 48.1 | 1 | 0 | 0 | 35.5 | 1 | 0 | 0 | 31 |
| GAD2 | MmugDNA.9669.1.S1_at | A | 0 | 0 | 0 | 8.817 | 0 | 0 | 0 | 12.37 | 0 | 0 | 0 | 14.1417 | ||
The table summarizes MAS 5.0 analysis of GeneChip® Rhesus Macaque Genome Arrays showing mean detection signals and number of animals with positive detection calls for genes encoding GABAergic system components. The search gene symbol is the text string used to retrieve annotation information in the Affymetrix NetAffx analysis center. The listed gene symbols are NetAffx annotations for specific probe sets retrieved under the gene symbol search. "AG" = Annotation grade (NetAffx); "# Positive" = number of animals showing positive detection for the given gene; "MBH" = arcuate nucleus of the medial basal hypothalamus; "HPC" = Hippocampus; "AMD" = Amygdala; "1" = OVX; "2" = E; “3" = E+P; “mean" = mean detection signal across the three treatment groups for the given tissue (MBH, HPC or AMD).
2.2 Real-time RT-PCR (qPCR)
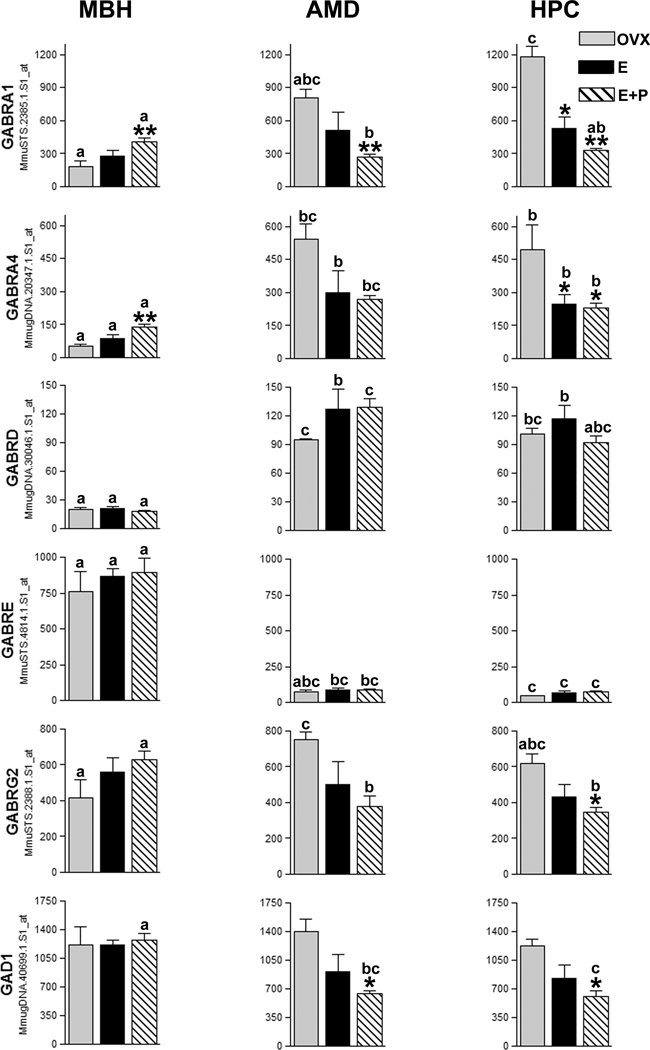
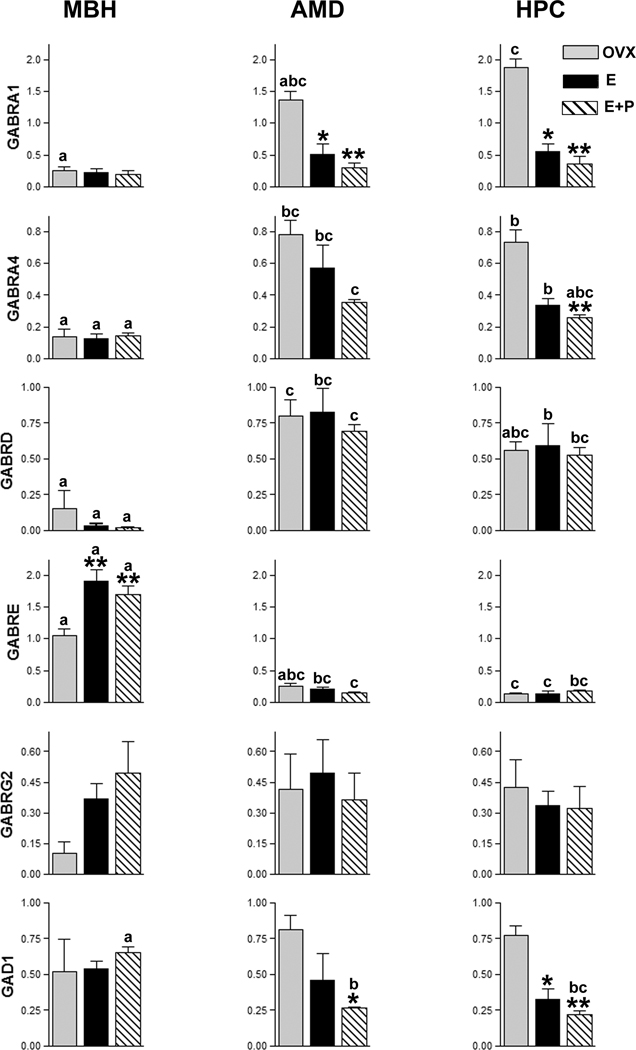
In general, gene expression patterns detected using the rhesus GeneChip® mirrored results from quantitative real-time RT-PCR (qPCR) analysis (Fig. 1). Effects of steroid hormone treatment on gene expression were observed in each brain region. In the MBH, E (P < 0.01) and E+P (P < 0.05)-treatment elevated GABRE expression above that of OVX controls. In the HPC, both E (P < 0.05) and E+P (P < 0.01) reduced the expression of GABRA1, and GAD1 compared to OVX controls. E+P-treatment also reduced GABRA4 expression (P < 0.01) in the HPC compared to OVX controls. In the AMD, E (P < 0.01) and E+P treatment reduced GABRA1 expression compared to OVX controls and E+P treatment reduced GAD1 expression (P < 0.05) relative to OVX controls (Fig. 2).
Figure 1. Relative expression of genes encoding GABA receptor subunits and GAD1 in the rhesus macaque.
Histograms show expression data from representative GeneChip® Rhesus Genome Array probesets corresponding to genes examined using qPCR. Probesets from sequence tagged sites (MmuSTS designation) were prioritized. Otherwise A-grade annotations were given priority. Where annotation grades were equal, probesets showing the highest expression were selected. Histograms show antilogarithms of RMA-normalized (log2) expression values, however, statistical analyses were conducted using log-transformed data.
Tissues: MBH = arcuate nucleus of the medial basal hypothalamus; AMD = amygdala; HPC = hippocampus.
Hormone treatments: OVX = Ovariectomized; E = Estrogen; E+P = Estrogen + Progesterone Asterisks denote statistical differences in expression for hormone effects within a brain region. Post-hoc test P values are listed with respect to the OVX group for that tissue only. * = P < 0.05; ** = P < 0.01.
Letters denote statistical differences in expression between brain regions under the same hormone treatment (compare bars with the same legend). Bars with the same legend sharing the same letter do not differ. “a vs. b” = P < 0.05; “a vs. c” = P < 0.01.
Figure 2. qPCR expression data for genes encoding GABA receptor subunit and GAD1 in the rhesus macaque.
Histograms show mean and SEM from qPCR quantity values normalized using arithmetic means of quantity values for expression of GAPDH, RPL13A and ALG9.
Brain regions: MBH = arcuate nucleus of the medial basal hypothalamus; AMD = amygdala; HPC = hippocampus.
Hormone treatments: OVX = Ovariectomized; E = Estrogen; E+P = Estrogen + Progesterone. Asterisks denote statistical differences in expression for hormone effects within a brain region. Post-hoc test P values are listed with respect to the OVX group for that tissue only. * = P < 0.05; ** = P < 0.01.
Letters denote statistical differences in expression between brain regions under the same hormone treatment (compare bars with the same legend). Bars with the same legend sharing the same letter do not differ. “a vs. b” = P < 0.05; “a vs. c” = P < 0.01.
Within treatments, gene expression levels in the AMD and HPC tended to be more similar to each other than to expression levels in the MBH. In the OVX animals, GABRA1, GABRA4 and GABRD gene expression in the AMD and HPC was generally higher than in the MBH. In E and E+P-treated animals, a similar regional expression pattern was observed for GABRA4 and GABRD. Regional expression trends for GABRE showed an inverse pattern compared to other subunit receptors examined, with higher expression in the MBH than in the AMD and HPC. E+P- treated animals also showed higher GAD1 gene expression in the MBH than in the AMD and HPC.
3. Discussion
In general, results from GeneChip® expression results were well corroborated by qPCR analysis, affirming the reliability of the expression data from selected probesets. However exceptions were noted for GABRG2, where AMD and HPC expression trends noted in the microarray analysis were not observed with qPCR. Similarly, E+P-treatment appeared to significantly change MBH expression levels for GABRA1 and GABRA4 in the microarray but not with qPCR analysis. Conversely for GABRE, MBH treatment effects detected with qPCR were not significantly different with microarray analysis.
In the current study, gene expression levels of most GABA receptor subunits tended to be lower in the MBH compared to the AMD and HPC. In a notable reversal of this trend, expression of the gene encoding the ε subunit was elevated in the MBH compared to expression in the AMD and HPC. Interestingly, elevated ε subunit expression in GABA receptors has been suggested as part of a mechanism by which the hypothalamus is buffered from neurosteroid fluctuations (Henderson, 2007). In the rat, hypothalamic neurons show high GABAA receptor ε subunit expression (Moragues et al., 2003; Sinkkonen et al., 2000; Whiting et al., 1997) and diminished responses to allopregnanolone compared to neurons with more typical ε subunit concentrations (Moragues et al., 2003; Sullivan and Moenter, 2003). Receptors incorporating the ε subunit are also insensitive to the GABA-modulatory actions of benzodiazepines and pregnane steroids (Belelli et al., 2002; Davies et al., 1997), but mechanistic data from several studies are difficult to reconcile (reviewed in Lambert et al., 2003) and the specific action of ε subunit inclusion may be highly dependent on receptor stoichiometry (Thompson et al., 2002)
In contrast to the ε subunit, δ subunit expression in the current study was lower in the MBH than in the AMD and HPC. Similarly, a low expression level of the δ subunit has also been observed in the rat hypothalamus (Fritschy et al., 1994; Pirker et al., 2000). Mice lacking the δ subunit show reduced steroid sensitivity (Mihalek et al., 1999; Shen et al., 2007; Spigelman et al., 2003), and presence of the δ subunit confers susceptibility to steroid modulation (Belelli et al., 2002; Bianchi and Macdonald, 2003; Brown et al., 2002) by increasing the maximum effect of steroids acting on receptors which express it (Wohlfarth et al., 2002).
Because the ε subunit reduces the GABAergic response to steroids whereas δ subunit expression enhances this GABAergic response, elevated ε and reduced δ subunit expression in the MBH may be an effective expression combination for dampening GABAergic responses to steroid fluctuations in the arcuate nucleus. These findings support the hypothesis that differential GABAA receptor subunit regulation is part of a mechanism that protects the hypothalamic GABAergic system from the modulatory influence of sex steroids (Henderson, 2007). The significance of this buffering may be related to GABAA receptor mediation of both stimulatory and inhibitory actions of GABA on hypothalamic release of gonadotropin-releasing hormone (GnRH) (Adler and Crowley, 1986; Akema et al., 1990; Jarry et al., 1991; Lamberts et al., 1983; Moguilevsky et al., 1991) which modulates steroid release from the gonads. With respect to GABA effects on GnRH release, GABA containing terminals synapse directly with GnRH neurons in the rat (Leranth et al., 1985), whereas synaptic contact between GABAergic neurons and GnRH neurons has not been detected in the rhesus macaque (Thind and Goldsmith, 1995). In the macaque, it is possible that other mechanisms may intercede between GABA and GnRH (Windsor-Engnell et al., 2007). Estrogen regulation of regional GABA concentrations has long been implicated in the feedback action of estrogen on gonadotropin release (Mansky et al., 1982). As opposed to direct modulation of GABAergic neurons by neurosteroids, estrogen likely modulates GABAergic inputs that affect the functionality of the neural networks regulating LH secretion (Herbison et al., 1991; Herbison and Dyer, 1991).
HPC δ receptor subunit expression is correlated with P levels during the mouse estrous cycle with enhanced expression during late diestrus (when circulating P is high), and reduced expression when P levels were low (Maguire et al., 2005). However, P-induced increases in hippocampal δ subunit expression were not observed in the current study.
Sex-steroid effects on α4 subunit expression were observed in the current study with tendencies (Fig 1 and Fig 2) in the AMD and HPC toward expression reductions with E and E+P treatment groups compared to OVX controls. Direct E (Zhou and Smith, 2007) and P (Pierson et al., 2005) administration independently increase α4 mRNA expression in NT2-N neuronal cells. Exposure of rats to P transiently increases α4 subunit expression after 48–72 hrs (Gulinello et al., 2001; Hsu et al., 2003) but this increase is followed by a return to control levels after 4–5 days (Gulinello et al., 2001). Note also that P or THP withdrawal increases α4 subunit expression in the rat HPC (Smith et al., 1998a) and AMD (Gulinello et al., 2003), highlighting the possibility that steroid removal through ovariectomy may elevate expression of some GABA receptor subunits, although short-term steroid exposure may also produce transient expression increases. Such studies also raise the question of whether subunit expression changes may be used to adapt to hormonal conditions over time. Examinations of temporal changes in primate subunit expression will require more enhanced time-course experiments than used in the current study.
The α1β2γ2 GABA receptor is estimated to represent the most widespread subunit combination in the mammalian brain (Fritschy and Mohler, 1995; McKernan and Whiting, 1996; Somogyi et al., 1996). In the current study, although probeset data showing hippocampal reductions in α1 subunit expression with E and E+P treatment were strong, β2 subunit expression in 64% of the study animals was not safely above global background levels (absent call) (Table 2). Reductions in γ2 subunit expression seen with E+P treatment in the in the HPC on the array analysis were not corroborated with qPCR. The γ2 subunit influences postsynaptic clustering of GABAA receptors (Essrich et al., 1998) through palmitoylation, and represents an additional mechanism for regulation of trafficking, cell-surface expression and postsynaptic clustering (Keller et al., 2004; Rathenberg et al., 2004).
The metabotropic GABAB receptors mediate slow synaptic inhibition and consist of heterodimers of B1 and B2 subunits (Bettler et al., 2004) which share structural homology with class-III G protein-coupled receptors (Binet et al., 2004; Kaupmann et al., 1997). The only known molecular diversity of the GABAB receptors arises from two GABAB1 isoforms (Kaupmann et al., 1998) whose few variants seem unable to explain the pharmacological heterogeneity of GABAB receptors observed in many functional studies (Huang, 2006; Raiteri, 2008). In the current study, E and E+P upregulation of the gene encoding the GABAB receptor subunit 1 in the AMD was not correlated to expression of the gene encoding subunit 2.
Ionotropic GABAC receptors are the least studied of the GABA receptor classes (Johnston et al., 2003), and expression of genes encoding GABAC receptor subunits were below the limits of microarray detection in the current study. The ρ subunits that make up the GABAC receptor have been identified in the retina (Enz et al., 1995), HPC (Enz et al., 1995; Rozzo et al., 2002) and pituitary (Boue-Grabot et al., 2000) of the rat.
Treatment with E downregulates GAD protein expression in rat hippocampal neurons (Murphy and Segal, 2000). Although this trend was noted for mRNA levels in the current study, significant reductions in GAD1 expression were only noted in the AMD with E+P treatment.
Steroidogenic regulation of GABAergic systems has been extensively studied in cell culture and rodent models. However, comparisons between the primate and rodent models may require caution. Even if conversion of circulating steroids to neurosteroids in brains of primates and rodents were equal (this question requires investigation), sheer differences in circulating physiological concentrations between the two orders likely place respective brains under different physiological pressures. Compared to rhesus macaques, rats have higher levels of plasma P, with baseline concentrations of around 12ng/ml and peak concentrations of 55ng/ml. Moreover rats have a short (24 hour) peak to trough concentration oscillation (Freeman, 2006). In addition, the dominant circulating progestin in the rat is 20α-hydroxyprogesterone (Eto et al., 1962; Hashimoto et al., 1968; Piacsek et al., 1971), as opposed to P. In contrast, the serum P levels of the rhesus macaque are an order of magnitude lower than in the rat, with baseline concentrations of less than 0.5ng/ml and peak concentrations on the order of 5ng/ml across a 12 day oscillation (Downs and Urbanski, 2006; Knobil, 1974; Macdonald and Greep, 1972). Humans show similar P profiles to rhesus monkeys but with higher mean P levels, and sustained peaks in the range of 8–20 ng/ml (Hale et al., 2007; Neill et al., 1967; Vande Wiele et al., 1970; Veldhuis et al., 1988). However, short-term samples (taken at 10–15 minute intervals) show mid-luteal human P peaks as high as the 30–60 ng/ml range (Filicori et al., 1984; Rossmanith et al., 1990).
GABAergic effects of circulating steroids are likely mediated by direct conversion to neurosteroids in neurons. Examples such as rat hippocampal cultures that show a 60 fold increase in THP after 24 hrs of P treatment (Murphy and Segal, 2000), support the hypothesis that circulating P modulates GABA receptor composition through neurosteroid action (Gulinello et al., 2001; Gulinello et al., 2003; Shen et al., 2005; Smith et al., 1998a; Smith et al., 1998b; Smith et al., 2007).
The THP P-metabolite, allopregnanolone, is the most abundant and efficacious endogenous positive allosteric modulator of the action of GABA at GABAA receptors (Pinna et al., 2008; Puia et al., 2003) and increases the efficacy of other positive allosteric modulators of GABA action (Belelli and Lambert, 2005; Pinna et al., 2000). Circulating levels of THP made by the corpus luteum parallel P levels throughout the menstrual cycle (Rapkin et al., 1997; Schmidt et al., 1994), and circulating steroids produced in the adrenals and ovaries are important modulators of GABAergic function (Paul and Purdy, 1992; Purdy et al., 1991; Reddy, 2003a; Reddy, 2003b). However, allopregnanolone is also produced in the brain from circulating progestins (Dong et al., 2001). In addition, production of allopregnanolone and other neurosteroids occurs in various brain regions of castrated adrenalectomized rats long after peripheral sources are removed (Cheney et al., 1995), and synthesis occurs in the brain through non-genomic mechanisms within seconds of behavioral stimulus (Frye et al., 2006).
In mice, THP concentrations in the brain are high enough to overwhelm potential peripheral contributions, and circadian fluctuations in brain concentrations overwhelm peripheral estrous cycle fluctuations (Corpechot et al., 1997). This suggests that the mouse may be more dependent on neurosteroid modulation of GABAergic inhibition than the primate (Smith et al., 2007). Assessing differences in the hormone physiology of primates and rodents may be made more complex because hormone bioavailability is modulated by steroid binding proteins which may be internalized into neuronal cells (Caldwell et al., 2006), and the interplay between hormone concentrations and steroid binding protein expression in the brain (Mopert et al., 2006; Sendemir et al., 2006) may differ between the two orders.
To a lesser extent, similar arguments of species-specific physiological considerations may be made regarding differences in estrogen physiology between primates and rodents. Rhesus macaque plasma E has baseline levels around 10–100 pg/ml with periovulatory concentrations exceeding 300 pg/ml (Downs and Urbanski, 2006; Freeman, 2006; Knobil, 1973; Weik et al., 1973). Rats show E baseline concentrations of 5–10 pg/ml with a proestrus peak just below 50 pg/ml (Freeman, 2006). Estradiol down regulates expression of brain-derived neurotrophic factor (BDNF) (Murphy et al., 1998b), which in turn down regulates GAD and GABA expression (Murphy et al., 1998a). This mechanism may explain some of estradiol’s rapid anti-GABAergic properties (Murphy and Segal, 2000) and GABAergic inhibition through GAD regulation (McCarthy et al., 1995; Rudick and Woolley, 2001; Weiland, 1992)
In women, the AMD and hypothalamus are among the brain regions where concentrations of P and its neurosteroid metabolites, show variation according to ovarian steroid production (Bixo et al., 1997). Also in humans, circulating levels of THP made by the corpus luteum parallel P levels throughout the menstrual cycle (Rapkin et al., 1997; Schmidt et al., 1994), making the human cycle associated with a much longer period of THP exposure compared to short THP peaks in the mouse (Mannan and O'Shaughnessy, 1988). Effects of physiological hormone concentrations on GABAergic activity have been studied in humans in conjunction with certain medical conditions. For example, the condition known as catamenial epilepsy is influenced by steroid modulation of GABAergic activity (Reddy, 2009). Seizure likelihood increases at mid-cycle and during the luteal phase (Backstrom, 1976; Herzog et al., 1997) and rodent models show increased seizure susceptibility or severity with P or THP withdrawal (Frye and Bayon, 1998; Reddy and Rogawski, 2001; Rogawski, 2003; Smith et al., 1998a). Challenges using benzodiazepines as GABAergic agonists, and measures of CNS activity such as saccadic eye velocity have been used to evaluate GABAergic effects resulting from human cyclic hormone fluctuation (McAuley and Friedman, 1999; Rukstalis and de Wit, 1999; Sundstrom et al., 1997; Sundstrom et al., 1998; Sundstrom and Backstrom, 1998). However, consensus regarding steroid-induced changes in GABA receptors has been complicated by difficulties in distinguishing effects due to changes in sensitivity to GABAergic drugs from effects due to changes in circulating hormone levels (reviewed in N-Wihlback et al., 2006). In addition, studies examining relationships between neuroactive steroid levels and premenstrual syndrome (PMS) or premenstrual dysphoric disorder (PMDD) have yielded discordant results (reviewed in Guille et al., 2008).
In light of physiological differences between primates and species typically used to study GABAergic system regulation, primate models may be most appropriate for elucidating effects of hormone cyclicity or hormone-replacement therapy on neurological changes in women. Findings in the rhesus macaque represented by data from the current study represent steps in establishing effects of physiological ovarian hormone concentrations on gene regulation of proteins modulating GABAergic function in brain areas influencing cognition and reproduction.
4. Experimental Procedure
4.1 Animals
Young (mean age = 9.67 years) adult rhesus macaques (Macaca mulatta) were obtained from the ONPRC Division of Animal Resources and were cared for in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals by the Division of Animal Resources at the Oregon National Primate Research Center (ONPRC). The animals were housed in a temperature-controlled environment and Primate Chow (Purina Mills Inc., St. Louis, MO) was made available to the animals twice daily, at 8:00 h and again at 15:00 h. This diet was supplemented with fresh fruit and vegetables, and the animals had continuous access to drinking water.
The animals were subjected to a hormone replacement paradigm designed to tightly mimic the sex-steroid changes that occur naturally across their menstrual cycle. All of the animals were bilaterally ovariectomized (OVX), and some of them then subjected to 17β-estradiol (E) replacement or E + progesterone (P) (E+P) replacement; the latter two paradigms (E and E+P) corresponded to the late follicular and mid luteal phases of the menstrual cycle respectively. On average (mean ± SEM) the animals were 9.4 ± 0.3 years old and weighed 5.5 ± 0.18 kg. The post-OVX interval was 6.7 ± 0.6 mo. Each of the three groups comprised four animals. Each animal received a SILASTIC implant (Dow Corning, Midland, MI), subcutaneously, in the periscapular area while under ketamine anesthesia (5 mg/kg, im; ketamine HCl, Parke Davis, Morris Plains, NJ). OVX control animals received an empty capsule. E- and E+P-treaded animals received SILASTIC capsule filled with crystalline 17β-estradiol (Steraloids, Wilton, NH). Fourteen days later, the E+P-treated animals received a SILASTIC capsule filled with crystalline P (Steraloids) (Kohama and Bethea, 1995). To verify the circulating hormone levels in these animals, serum samples were assayed for E and P. The OVX control group received no hormone replacement had undetectable serum E and P levels. The E group had serum concentrations of E = 117 ± 6.7 pg/ml, and the E+P group had serum E levels of 130 ± 9 pg/ml and P levels of 3.8 ± 0.4ng/ml. The animals were subjected to the E replacement paradigm for 4 weeks, with P treatment only in the final 2 weeks.
The animals were euthanized using sodium pentobarbital, and after a transcardial flush with 1 liter of 0.9% saline, their brains were immediately removed and cut into blocks. The brain blocks were preserved in RNAlater® (Ambion; Austin, TX) and areas of interest (MBH, HPC and AMD) were subsequently dissected from the surrounding tissue, resulting in a total of 36 tissue samples.
4.2 RNA extraction and cDNA preparation
AMD, HPC and MBH were homogenized with a PowerGen rotor-stator homogenizer (Fisher Scientific, Pittsburgh, PA) and total RNA was isolated using a Qiagen RNeasy Mini Kit, according to the manufacturer’s instructions (QIAGEN, Valencia, CA). Briefly, tissues were homogenized in RLT buffer with 1% β-mercaptoethanol. RNA was collected onto RNeasy mini columns and eluted in water. The concentration of RNA was measured by spectroscopy, with an expected A260/A280 ratio close to 2, denoting an acceptably pure nucleic acid sample. RNA integrity and quality was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara CA), and met Affymetrix requirements for hybridization to the Rhesus Genome GeneChip® microarray.
Using random hexamers, 500ng of RNA from each sample was converted to cDNA using manufacturer’s instructions for the SuperScript™ III First-Strand Synthesis System for RT-PCR (Invitrogen™ life technologies, Carlsbad CA www.Invitrogen.com).
4.3 Primers and probes
Rhesus macaque sequences for GAPDH and RPL13A were confirmed in-house using RT-PCR primers against source sequences to amplify rhesus macaque hippocampal cDNA pooled from several individuals. These PCR products were sequenced using an ABI 3730×l DNA sequencer (Applied Biosystems, Foster City, CA) according to manufacturer’s instructions and corresponded highly to the annotated source sequences. qPCR primers and probes for prospective housekeeping genes ALG9, GAPDH and RPL13A as well as GABA subunit receptors GABRA1, GABRA4, GABRG2, GABRD and GABRE and the enzyme GAD1 were designed using Primer Express 2.0 software (Applied Biosystems, Foster City, CA) from sequence sources listed described elsewhere (Noriega et al., submitted) To confirm that selected q PCR primers amplified the desired sequences, RT-PCR was conducted using Platinum® PCR SuperMix (Invitrogen™ life technologies, Carlsbad CA www.Invitrogen.com) and reaction products were analyzed on a 2% agarose gel.
4.4 qPCR
qPCR and data collection were conducted using the 7900HT Fast Real-Time PCR thermal cycler and sequence detection systems (Software version 2.2.1) from Applied Biosystems (Foster City, CA). No-template controls were included for each gene. Standard curves were constructed using cDNA pooled equally from all tissues and all animals. The curves were constructed from serial 5- fold serial dilutions and covered a cDNA dilution range of 0.2 to 0.0016 (larger fold dilutions produced unacceptably high cycle times for some genes in the study).
Reactions for qPCR were conducted in sealed 384-well optical plates in a total volume of 5 µl per well, using 1.0 µl of 1:5-diluted cDNA, 0.25 µM Taqman probe and 0.3µM each of forward and reverse primers. Reactions were conducted using thermal cycler conditions of: 2 min at 50°C, 10 min at 95°C and 50 cycles of 15s at 95°C (DNA melting) and 1 min at 60°C (primer annealing/ extension). Baseline and threshold levels for amplification plots were determined automatically using the ABI sequence detection systems software version 2.2.1. Validation of the reference genes GAPDH, RPL13A and ALG9 for normalization was conducted using geNorm, NormFinder and BestKeeper software as described elsewhere (Noriega et al., submitted). Normalization of linear quantity values from qPCR was performed using the arithmetic mean of linear quantity values for GAPDH, RPL13A and ALG9. i.e., we used a normalizer index comprised of multiple internal reference genes.
4.5 Gene microarrays
RNA quality was assessed using an Agilent 2100 Bioanalyzer. An assay performance assessment was conducted to confirm that the quality of the RNA used was acceptable and that genome hybridizations performed well. The performance assessment (conducted by AMC) was based on the GCOS analysis, and pair-wise scatter plots of Microarray Suite version 5.0 (MAS 5.0) absolute analyses. The RNA quality (35/36 samples) met the level recommended by Affymetrix for hybridization to the Rhesus Genome GeneChip® (micro)array, with the exception of an one sample (MBH from an OVX animal) that showed a left-ward shift in bioanalyzer profiles indicative of RNA degradation .
We examined gene expression in the MBH, AMD and HPC of rhesus macaques treated with a hormone replacement paradigm designed to test effects of E and E+P manipulation. In order to identify treatment-related changes in gene expression, we used the transcriptome (52,024 probe sets to monitor 47,000 transcripts) available on the GeneChip® Rhesus Macaque Genome Array which “leverages the homology of expressed sequences represented on the GeneChip® Human Genome U133 Plus 2.0 Array to annotate the Baylor School of Medicine’s rhesus macaque whole-genome shotgun assembly” (Affymetrix, 2005)
Microarray assays were performed in the Affymetrix Microarray Core (AMC) of the OHSU Gene Microarray Shared Resource. Samples were prepared using the AMC One-cycle cDNA IVT (in vitro transcription) amplification/labeling protocol (Standard Labeling). Each sample target was hybridized to a Rhesus Genome GeneChip® microarray and image processing and expression analysis were performed using Affymetrix GeneChip® Operating Software (GCOS) version 1.2.
Absolute analyses were run using global scaling to an average target intensity of 200. In this way, scaling allowed for direct comparison of the hybridization values from the two targets. Statistical algorithms from the Affymetrix Microarray Suite version 5.0 (MAS 5.0) were used to generate signal metrics. The parameters α1 and α2, which set the point at which the probe set is called present, marginally present, or undetectable, were set to 0.05 and 0.065 (Affymetrix defaults) respectively. These parameters were used to determine expression levels for each probe set based on the detection P-value of the probe set.
We used Affymetrix probe set ID numbers to search the GeneChip® microarray for oligonucleotide signal intensities associated with genes for GABA receptor subunits (Dingledine et al., 1999). Probe set ID assignments were referenced according to listings for the Rhesus GeneChip® microarray and searched using the Affymetrix online Netaffx™ Analysis Center software (query function).
4.6 Statistics
We used Kruskal-Wallis one-way analysis of variance on GeneSifter™ software (VizX Labs, Seattle WA, http://www.genesifter.net/) to identify GABA subunit receptors showing differences in expression. This software incorporated Robust Multichip Analysis (RMA) algorithms to normalize expression data across all chips. In order to directly compare normalized expression values in finer detail than allowable in GeneSifter™, we used Affymetrix® Expression Console™ software (Affymetrix Inc Santa Clara, CA) to independently perform RMA where normalized expression values for each sample could be used to examine treatment-related differences in finer detail than allowable in GeneSifter™.
Kruskal-Wallis one-way non-parametric analysis of variance was used to compare logarithmically scaled RMA expression results within each tissue group (MBH, HPC or AMD) across the three treatments (OVX, E and E+P). Similarly, the Kruskal-Wallis test was used to compare linearly scaled relative quantity data from qPCR. Treatment-related differences in mRNA expression were identified using Newman-Keuls post hoc tests. Tissue differences were assessed using one way parametric ANOVA (12 samples per group).
Acknowledgements
This work was supported by National Institutes of Health grants: AG026472, AG029612, HD029186 and RR000163. Gene microarray assays were performed in the Affymetrix Microarray Core of the Gene Microarray Shared Resource facility at OHSU.
Abbreviations
- ALG9
gene encoding asparagine-linked glycosylation 9 homolog
- AMC
Affymetrix Microarray Core
- AMD
amygdala
- E
17β-estradiol (treatment)
- E+P
17β-estradiol + progesterone (treatment)
- GABA
gamma-aminobutyric acid
- GABRA1
gene encoding GABAA receptor subunit alpha 1
- GABRA4
gene encoding GABAA receptor subunit alpha 4
- GABRD
gene encoding GABAA receptor subunit delta
- GABRE
gene encoding GABAA receptor subunit epsilon
- GABRG2
gene encoding GABAA receptor subunit gamma 2
- GAD1
gene encoding glutamic acid decarboxylase 1 (67 kDa)
- GAPDH
gene encoding glyceraldehyde 3-phosphate dehydrogenase
- GCOS
GeneChip® Operating Software
- HPC
hippocampus
- MBH
arcuate nucleus of the medial basal hypothalamus
- OHSU
Oregon Health & Science University
- ONPRC
Oregon National Primate Research Center
- OVX
ovariectomized
- qPCR
quantitative real-time RT-PCR
- RPL13A
gene encoding ribosomal protein L13a
- SLC32A1
gene encoding solute carrier family 32 (GABA vesicular transporter), member 1, also known as VGAT
- THP
neurosteroid derivatives of progesterone, 5α-dihydroprogesterone, namely, 3α, 5α-tetrahydroprogesterone (allopregnanolone), and 3β, 5α-tetrahydroprogesterone (pregnanolone)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature references
- Adler BA, Crowley WR. Evidence for gamma-aminobutyric acid modulation of ovarian hormonal effects on luteinizing hormone secretion and hypothalamic catecholamine activity in the female rat. Endocrinology. 1986;118:91–97. doi: 10.1210/endo-118-1-91. [DOI] [PubMed] [Google Scholar]
- Affymetrix. GeneChip® Rhesus Macaque Genome Array Data Sheet. 2005 [Google Scholar]
- Akema T, Chiba A, Kimura F. On the relationship between noradrenergic stimulatory and GABAergic inhibitory systems in the control of luteinizing hormone secretion in female rats. Neuroendocrinology. 1990;52:566–572. doi: 10.1159/000125645. [DOI] [PubMed] [Google Scholar]
- Angulo MC, Le Meur K, Kozlov AS, Charpak S, Audinat E. GABA, a forgotten gliotransmitter. Prog Neurobiol. 2008;86:297–303. doi: 10.1016/j.pneurobio.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Backstrom T. Epileptic seizures in women related to plasma estrogen and progesterone during the menstrual cycle. Acta Neurol Scand. 1976;54:321–347. doi: 10.1111/j.1600-0404.1976.tb04363.x. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23:963–987. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABA(A) receptors. Neuropharmacology. 2005;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Bethmann K, Fritschy JM, Brandt C, Loscher W. Antiepileptic drug resistant rats differ from drug responsive rats in GABA A receptor subunit expression in a model of temporal lobe epilepsy. Neurobiol Dis. 2008;31:169–187. doi: 10.1016/j.nbd.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABA(A) receptor channels from low- to high-efficacy gating patterns. J Neurosci. 2003;23:10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binet V, Brajon C, Le Corre L, Acher F, Pin JP, Prezeau L. The heptahelical domain of GABA(B2) is activated directly by CGP7930, a positive allosteric modulator of the GABA(B) receptor. J Biol Chem. 2004;279:29085–29091. doi: 10.1074/jbc.M400930200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixo M, Andersson A, Winblad B, Purdy RH, Backstrom T. Progesterone, 5alpha-pregnane-3,20-dione and 3alpha-hydroxy-5alpha-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Res. 1997;764:173–178. doi: 10.1016/s0006-8993(97)00455-1. [DOI] [PubMed] [Google Scholar]
- Boue-Grabot E, Taupignon A, Tramu G, Garret M. Molecular and electrophysiological evidence for a GABAc receptor in thyrotropin-secreting cells. Endocrinology. 2000;141:1627–1632. doi: 10.1210/endo.141.5.7476. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussaard AB, Kits KS, Baker RE, Willems WP, Leyting-Vermeulen JW, Voorn P, Smit AB, Bicknell RJ, Herbison AE. Plasticity in fast synaptic inhibition of adult oxytocin neurons caused by switch in GABA(A) receptor subunit expression. Neuron. 1997;19:1103–1114. doi: 10.1016/s0896-6273(00)80401-8. [DOI] [PubMed] [Google Scholar]
- Byrnes EM, Lee JO, Bridges RS. Alterations in GABA(A) receptor alpha2 subunit mRNA expression following reproductive experience in rats. Neuroendocrinology. 2007;85:148–156. doi: 10.1159/000102535. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Suleman F, Chou SH, Shapiro RA, Herbert Z, Jirikowski GF. Emerging roles of steroid-binding globulins. Horm Metab Res. 2006;38:206–218. doi: 10.1055/s-2006-925328. [DOI] [PubMed] [Google Scholar]
- Callachan H, Cottrell GA, Hather NY, Lambert JJ, Nooney JM, Peters JA. Modulation of the GABAA receptor by progesterone metabolites. Proc R Soc Lond B Biol Sci. 1987;231:359–369. doi: 10.1098/rspb.1987.0049. [DOI] [PubMed] [Google Scholar]
- Cheney DL, Uzunov D, Costa E, Guidotti A. Gas chromatographic-mass fragmentographic quantitation of 3 alpha-hydroxy-5 alpha-pregnan-20-one (allopregnanolone) and its precursors in blood and brain of adrenalectomized and castrated rats. J Neurosci. 1995;15:4641–4650. doi: 10.1523/JNEUROSCI.15-06-04641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Corpechot C, Synguelakis M, Talha S, Axelson M, Sjovall J, Vihko R, Baulieu EE, Robel P. Pregnenolone and its sulfate ester in the rat brain. Brain Res. 1983;270:119–125. doi: 10.1016/0006-8993(83)90797-7. [DOI] [PubMed] [Google Scholar]
- Corpechot C, Collins BE, Carey MP, Tsouros A, Robel P, Fry JP. Brain neurosteroids during the mouse oestrous cycle. Brain Res. 1997;766:276–280. doi: 10.1016/s0006-8993(97)00749-x. [DOI] [PubMed] [Google Scholar]
- Davies PA, Hanna MC, Hales TG, Kirkness EF. Insensitivity to anaesthetic agents conferred by a class of GABA(A) receptor subunit. Nature. 1997;385:820–823. doi: 10.1038/385820a0. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H, Watanabe H, Costa E, Guidotti A. Brain 5alpha-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci U S A. 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JL, Urbanski HF. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macaca mulatta) Biol Reprod. 2006;75:539–546. doi: 10.1095/biolreprod.106.051839. [DOI] [PubMed] [Google Scholar]
- Enz R, Brandstatter JH, Hartveit E, Wassle H, Bormann J. Expression of GABA receptor rho 1 and rho 2 subunits in the retina and brain of the rat. Eur J Neurosci. 1995;7:1495–1501. doi: 10.1111/j.1460-9568.1995.tb01144.x. [DOI] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Eto T, Masuda H, Suzuki Y, Hosi T. Progesterone and pregn-4-en-20 a-ol-3-one in rat ovarian venous blood at different stages in reproductive states in the reproductive cycle. Jpn j Anim Reprod. 1962;8:34–40. [Google Scholar]
- Filicori M, Butler JP, Crowley WF., Jr. Neuroendocrine regulation of the corpus luteum in the human. Evidence for pulsatile progesterone secretion. J Clin Invest. 1984;73:1638–1647. doi: 10.1172/JCI111370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman ME. Neuroendocrine control of the ovarian cycle of the rat. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. Vol. 2. St. Louis: Academic Press; 2006. pp. 2327–2388. [Google Scholar]
- Fritschy JM, Paysan J, Enna A, Mohler H. Switch in the expression of rat GABAA-receptor subtypes during postnatal development: an immunohistochemical study. J Neurosci. 1994;14:5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Fritschy JM. Epilepsy, E/I Balance and GABA(A) Receptor Plasticity. Front Mol Neurosci. 2008;1:5. doi: 10.3389/neuro.02.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Bayon LE. Seizure activity is increased in endocrine states characterized by decline in endogenous levels of the neurosteroid 3 alpha,5 alpha-THP. Neuroendocrinology. 1998;68:272–280. doi: 10.1159/000054375. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Petralia SM, Walf AA, Sumida K, Edinger KL. 3alpha-hydroxy-5alpha-pregnan-20-one in the midbrain ventral tegmental area mediates social, sexual, and affective behaviors. Neuroscience. 2006;138:1007–1014. doi: 10.1016/j.neuroscience.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee KW, Chang WC, Brinton RE, McEwen BS. GABA-dependent modulation of the Cl- ionophore by steroids in rat brain. Eur J Pharmacol. 1987;136:419–423. doi: 10.1016/0014-2999(87)90317-7. [DOI] [PubMed] [Google Scholar]
- Guille C, Spencer S, Cavus I, Epperson CN. The role of sex steroids in catamenial epilepsy and premenstrual dysphoric disorder: implications for diagnosis and treatment. Epilepsy Behav. 2008;13:12–24. doi: 10.1016/j.yebeh.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to a neuroactive steroid increases alpha4 GABA(A) receptor subunit levels in association with increased anxiety in the female rat. Brain Res. 2001;910:55–66. doi: 10.1016/s0006-8993(01)02565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Orman R, Smith SS. Sex differences in anxiety, sensorimotor gating and expression of the alpha4 subunit of the GABAA receptor in the amygdala after progesterone withdrawal. Eur J Neurosci. 2003;17:641–648. doi: 10.1046/j.1460-9568.2003.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale GE, Zhao X, Hughes CL, Burger HG, Robertson DM, Fraser IS. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the Staging of Reproductive Aging Workshop (STRAW) staging system. J Clin Endocrinol Metab. 2007;92:3060–3067. doi: 10.1210/jc.2007-0066. [DOI] [PubMed] [Google Scholar]
- Hashimoto I, Hendricks DM, Anderson LL, Melampy RR. Progesterone and pregn-4-en-20 a-ol-3-one in ovarian venous blood during various reproductive states in the rat. Endocrinology. 1968;82:333–341. doi: 10.1210/endo-82-2-333. [DOI] [PubMed] [Google Scholar]
- Henderson LP. Steroid modulation of GABAA receptor-mediated transmission in the hypothalamus: effects on reproductive function. Neuropharmacology. 2007;52:1439–1453. doi: 10.1016/j.neuropharm.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE, Chapman C, Dyer RG. Role of medial preoptic GABA neurones in regulating luteinising hormone secretion in the ovariectomised rat. Exp Brain Res. 1991;87:345–352. doi: 10.1007/BF00231851. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Dyer RG. Effect on luteinizing hormone secretion of GABA receptor modulation in the medial preoptic area at the time of proestrous luteinizing hormone surge. Neuroendocrinology. 1991;53:317–320. doi: 10.1159/000125735. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Augood SJ, McGowan EM. Expression of glutamic acid decarboxylase messenger RNA in rat medial preoptic area neurones during the oestrous cycle and after ovariectomy. Brain Res Mol Brain Res. 1992;14:310–316. doi: 10.1016/0169-328x(92)90098-v. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Augood SJ, Simonian SX, Chapman C. Regulation of GABA transporter activity and mRNA expression by estrogen in rat preoptic area. J Neurosci. 1995;15:8302–8309. doi: 10.1523/JNEUROSCI.15-12-08302.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia. 1997;38:1082–1088. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Hilliard J. Corpus luteum function in guinea pigs, hamsters, rats, mice and rabbits. Biol Reprod. 1973;8:203–221. [Google Scholar]
- Hsu FC, Waldeck R, Faber DS, Smith SS. Neurosteroid effects on GABAergic synaptic plasticity in hippocampus. J Neurophysiol. 2003;89:1929–1940. doi: 10.1152/jn.00780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ. GABAB receptor isoforms caught in action at the scene. Neuron. 2006;50:521–524. doi: 10.1016/j.neuron.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Jarry H, Leonhardt S, Wuttke W. Gamma-aminobutyric acid neurons in the preoptic/anterior hypothalamic area synchronize the phasic activity of the gonadotropin-releasing hormone pulse generator in ovariectomized rats. Neuroendocrinology. 1991;53:261–267. doi: 10.1159/000125727. [DOI] [PubMed] [Google Scholar]
- Johnston GA, Chebib M, Hanrahan JR, Mewett KN. GABA(C) receptors as drug targets. Curr Drug Targets CNS Neurol Disord. 2003;2:260–268. doi: 10.2174/1568007033482805. [DOI] [PubMed] [Google Scholar]
- Jorge JC, McIntyre KL, Henderson LP. The function and the expression of forebrain GABA(A) receptors change with hormonal state in the adult mouse. J Neurobiol. 2002;50:137–149. doi: 10.1002/neu.10021. [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Huggel K, Heid J, Flor PJ, Bischoff S, Mickel SJ, McMaster G, Angst C, Bittiger H, Froestl W, Bettler B. Expression cloning of GABA(B)-receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386:239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, Karschin A, Bettler B. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- Keller CA, Yuan X, Panzanelli P, Martin ML, Alldred M, Sassoe-Pognetto M, Luscher B. The gamma2 subunit of GABA(A) receptors is a substrate for palmitoylation by GODZ. J Neurosci. 2004;24:5881–5891. doi: 10.1523/JNEUROSCI.1037-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobil E. On the regulation of the primate corpus luteum. Biol Reprod. 1973;8:246–258. [Google Scholar]
- Knobil E. On the control of gonadotropin secretion in the rhesus monkey. Recent Prog Horm Res. 1974;30:1–46. doi: 10.1016/b978-0-12-571130-2.50005-5. [DOI] [PubMed] [Google Scholar]
- Kohama SG, Bethea CL. Steroid regulation of tyrosine hydroxylase messenger ribonucleic acid in dopaminergic subpopulations of monkey hypothalamus. Endocrinology. 1995;136:1790–1800. doi: 10.1210/endo.136.4.7895692. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABAA receptors. Prog Neurobiol. 2003;71:67–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Lamberts R, Vijayan E, Graf M, Mansky T, Wuttke W. Involvement of preoptic-anterior hypothalamic GABA neurons in the regulation of pituitary LH and prolactin release. Exp Brain Res. 1983;52:356–362. doi: 10.1007/BF00238029. [DOI] [PubMed] [Google Scholar]
- Leranth C, MacLusky NJ, Sakamoto H, Shanabrough M, Naftolin F. Glutamic acid decarboxylase-containing axons synapse on LHRH neurons in the rat medial preoptic area. Neuroendocrinology. 1985;40:536–539. doi: 10.1159/000124127. [DOI] [PubMed] [Google Scholar]
- Lovick TA. Plasticity of GABAA receptor subunit expression during the oestrous cycle of the rat: implications for premenstrual syndrome in women. Exp Physiol. 2006;91:655–660. doi: 10.1113/expphysiol.2005.032342. [DOI] [PubMed] [Google Scholar]
- Macdonald GJ, Greep RO. Ability of luteinizing hormone (LH) to acutely increase serum progesterone levels during the secretory phase of the rhesus menstrual cycle. Fertil Steril. 1972;23:466–470. doi: 10.1016/s0015-0282(16)39071-9. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Mannan MA, O'Shaughnessy PJ. Ovarian steroid metabolism during post-natal development in the normal mouse and in the adult hypogonadal (hpg) mouse. J Reprod Fertil. 1988;82:727–734. doi: 10.1530/jrf.0.0820727. [DOI] [PubMed] [Google Scholar]
- Mansky T, Mestres-Ventura P, Wuttke W. Involvement of GABA in the feedback action of estradiol on gonadotropin and prolactin release: hypothalamic GABA and catecholamine turnover rates. Brain Res. 1982;231:353–364. doi: 10.1016/0006-8993(82)90372-9. [DOI] [PubMed] [Google Scholar]
- McAuley JW, Friedman CI. Influence of endogenous progesterone on alprazolam pharmacodynamics. J Clin Psychopharmacol. 1999;19:233–239. doi: 10.1097/00004714-199906000-00006. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Kaufman LC, Brooks PJ, Pfaff DW, Schwartz-Giblin S. Estrogen modulation of mRNA levels for the two forms of glutamic acid decarboxylase (GAD) in female rat brain. J Comp Neurol. 1995;360:685–697. doi: 10.1002/cne.903600412. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci U S A. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moguilevsky JA, Carbone S, Szwarcfarb B, Rondina D. Sexual maturation modifies the GABAergic control of gonadotrophin secretion in female rats. Brain Res. 1991;563:12–16. doi: 10.1016/0006-8993(91)91508-x. [DOI] [PubMed] [Google Scholar]
- Mopert B, Herbert Z, Caldwell JD, Jirikowski GF. Expression of corticosterone-binding globulin in the rat hypothalamus. Horm Metab Res. 2006;38:246–252. doi: 10.1055/s-2006-925344. [DOI] [PubMed] [Google Scholar]
- Moragues N, Ciofi P, Lafon P, Tramu G, Garret M. GABAA receptor epsilon subunit expression in identified peptidergic neurons of the rat hypothalamus. Brain Res. 2003;967:285–289. doi: 10.1016/s0006-8993(02)04270-1. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998a;18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Segal M. Brain-derived neurotrophic factor mediates estradiol-induced dendritic spine formation in hippocampal neurons. Proc Natl Acad Sci U S A. 1998b;95:11412–11417. doi: 10.1073/pnas.95.19.11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Segal M. Progesterone prevents estradiol-induced dendritic spine formation in cultured hippocampal neurons. Neuroendocrinology. 2000;72:133–143. doi: 10.1159/000054580. [DOI] [PubMed] [Google Scholar]
- N-Wihlback AC, Sundstrom-Poromaa I, Backstrom T. Action by and sensitivity to neuroactive steroids in menstrual cycle related CNS disorders. Psychopharmacology (Berl) 2006;186:388–401. doi: 10.1007/s00213-005-0185-2. [DOI] [PubMed] [Google Scholar]
- Neill JD, Johansson ED, Datta JK, Knobil E. Relationship between the plasma levels of luteinizing hormone and progesterone during the normal menstrual cycle. J Clin Endocrinol Metab. 1967;27:1167–1173. doi: 10.1210/jcem-27-8-1167. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABA(A) receptors: Subtypes provide diversity of function and pharmacology. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Piacsek BE, Schneider TC, Gay VL. Sequential study of luteinizing hormone (LH) and "Progestin" secretion on the afternoon of proestrus in the rat. Endocrinology. 1971;89:39–45. doi: 10.1210/endo-89-1-39. [DOI] [PubMed] [Google Scholar]
- Pierson RC, Lyons AM, Greenfield LJ., Jr. Gonadal steroids regulate GABAA receptor subunit mRNA expression in NT2-N neurons. Brain Res Mol Brain Res. 2005;138:105–115. doi: 10.1016/j.molbrainres.2004.10.047. [DOI] [PubMed] [Google Scholar]
- Pinna G, Uzunova V, Matsumoto K, Puia G, Mienville JM, Costa E, Guidotti A. Brain allopregnanolone regulates the potency of the GABA(A) receptor agonist muscimol. Neuropharmacology. 2000;39:440–448. doi: 10.1016/s0028-3908(99)00149-5. [DOI] [PubMed] [Google Scholar]
- Pinna G, Agis-Balboa RC, Pibiri F, Nelson M, Guidotti A, Costa E. Neurosteroid biosynthesis regulates sexually dimorphic fear and aggressive behavior in mice. Neurochem Res. 2008;33:1990–2007. doi: 10.1007/s11064-008-9718-5. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Puia G, Mienville JM, Matsumoto K, Takahata H, Watanabe H, Costa E, Guidotti A. On the putative physiological role of allopregnanolone on GABA(A) receptor function. Neuropharmacology. 2003;44:49–55. doi: 10.1016/s0028-3908(02)00341-6. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr., Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci U S A. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiteri M. Presynaptic metabotropic glutamate and GABAB receptors. Handb Exp Pharmacol. 2008:373–407. doi: 10.1007/978-3-540-74805-2_12. [DOI] [PubMed] [Google Scholar]
- Rapkin AJ, Morgan M, Goldman L, Brann DW, Simone D, Mahesh VB. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90:709–714. doi: 10.1016/S0029-7844(97)00417-1. [DOI] [PubMed] [Google Scholar]
- Rathenberg J, Kittler JT, Moss SJ. Palmitoylation regulates the clustering and cell surface stability of GABAA receptors. Mol Cell Neurosci. 2004;26:251–257. doi: 10.1016/j.mcn.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia. 2001;42:337–344. doi: 10.1046/j.1528-1157.2001.10200.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Is there a physiological role for the neurosteroid THDOC in stress-sensitive conditions? Trends Pharmacol Sci. 2003a;24:103–106. doi: 10.1016/S0165-6147(03)00023-3. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Pharmacology of endogenous neuroactive steroids. Crit Rev Neurobiol. 2003b;15:197–234. doi: 10.1615/critrevneurobiol.v15.i34.20. [DOI] [PubMed] [Google Scholar]
- Reddy DS. The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy. Epilepsy Res. 2009 doi: 10.1016/j.eplepsyres.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robel P, Bourreau E, Corpechot C, Dang DC, Halberg F, Clarke C, Haug M, Schlegel ML, Synguelakis M, Vourch C, et al. Neuro-steroids: 3 beta-hydroxy-delta 5-derivatives in rat and monkey brain. J Steroid Biochem. 1987;27:649–655. doi: 10.1016/0022-4731(87)90133-6. [DOI] [PubMed] [Google Scholar]
- Rogawski MA. Progesterone, neurosteroids, and the hormonal basis of catamenial epilepsy. Ann Neurol. 2003;53:288–291. doi: 10.1002/ana.10534. [DOI] [PubMed] [Google Scholar]
- Rossmanith WG, Laughlin GA, Mortola JF, Johnson ML, Veldhuis JD, Yen SS. Pulsatile cosecretion of estradiol and progesterone by the midluteal phase corpus luteum: temporal link to luteinizing hormone pulses. J Clin Endocrinol Metab. 1990;70:990–995. doi: 10.1210/jcem-70-4-990. [DOI] [PubMed] [Google Scholar]
- Rozzo A, Armellin M, Franzot J, Chiaruttini C, Nistri A, Tongiorgi E. Expression and dendritic mRNA localization of GABAC receptor rho1 and rho2 subunits in developing rat brain and spinal cord. Eur J Neurosci. 2002;15:1747–1758. doi: 10.1046/j.1460-9568.2002.02013.x. [DOI] [PubMed] [Google Scholar]
- Rudick CN, Woolley CS. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J Neurosci. 2001;21:6532–6543. doi: 10.1523/JNEUROSCI.21-17-06532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukstalis M, de Wit H. Effects of triazolam at three phases of the menstrual cycle. J Clin Psychopharmacol. 1999;19:450–458. doi: 10.1097/00004714-199910000-00009. [DOI] [PubMed] [Google Scholar]
- Sagrillo CA, Selmanoff M. Castration decreases single cell levels of mRNA encoding glutamic acid decarboxylase in the diagonal band of broca and the sexually dimorphic nucleus of the preoptic area. J Neuroendocrinol. 1997;9:699–706. doi: 10.1046/j.1365-2826.1997.00630.x. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Purdy RH, Moore PH, Jr., Paul SM, Rubinow DR. Circulating levels of anxiolytic steroids in the luteal phase in women with premenstrual syndrome and in control subjects. J Clin Endocrinol Metab. 1994;79:1256–1260. doi: 10.1210/jcem.79.5.7962316. [DOI] [PubMed] [Google Scholar]
- Sendemir E, Herbert Z, Caldwell JD, Jirikowski GF. Changes of sex hormone-binding globulin/SHBG expression in the hypothalamo-hypophyseal system of rats during pregnancy, parturition and lactation. Horm Metab Res. 2006;38:219–224. doi: 10.1055/s-2006-925330. [DOI] [PubMed] [Google Scholar]
- Shen H, Gong QH, Yuan M, Smith SS. Short-term steroid treatment increases delta GABAA receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology. 2005;49:573–586. doi: 10.1016/j.neuropharm.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. Reversal of neurosteroid effects at alpha4beta2delta GABAA receptors triggers anxiety at puberty. Nat Neurosci. 2007;10:469–477. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkkonen ST, Hanna MC, Kirkness EF, Korpi ER. GABA(A) receptor epsilon and theta subunits display unusual structural variation between species and are enriched in the rat locus ceruleus. J Neurosci. 2000;20:3588–3595. doi: 10.1523/JNEUROSCI.20-10-03588.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivilotti L, Nistri A. GABA receptor mechanisms in the central nervous system. Prog Neurobiol. 1991;36:35–92. doi: 10.1016/0301-0082(91)90036-z. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JM, Li X. GABA(A) receptor alpha4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998a;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu FC. Withdrawal from 3alpha-OH-5alpha-pregnan-20-One using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor alpha4 subunit in association with increased anxiety. J Neurosci. 1998b;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABA(A) receptors: Focus on the alpha4 and delta subunits. Pharmacol Ther. 2007;116:58–76. doi: 10.1016/j.pharmthera.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Fritschy JM, Benke D, Roberts JD, Sieghart W. The gamma 2 subunit of the GABAA receptor is concentrated in synaptic junctions containing the alpha 1 and beta 2/3 subunits in hippocampus, cerebellum and globus pallidus. Neuropharmacology. 1996;35:1425–1444. doi: 10.1016/s0028-3908(96)00086-x. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Liang J, Cagetti E, Samzadeh S, Mihalek RM, Homanics GE, Olsen RW. Reduced inhibition and sensitivity to neurosteroids in hippocampus of mice lacking the GABA(A) receptor delta subunit. J Neurophysiol. 2003;90:903–910. doi: 10.1152/jn.01022.2002. [DOI] [PubMed] [Google Scholar]
- Steiger JL, Russek SJ. GABAA receptors: building the bridge between subunit mRNAs, their promoters, and cognate transcription factors. Pharmacol Ther. 2004;101:259–281. doi: 10.1016/j.pharmthera.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Stouffer RL. Structure, Function and Regulatin of the Corpus Luteum. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. Vol. 1. St. Louis: Academic Press; 2006. pp. 475–526. [Google Scholar]
- Sullivan SD, Moenter SM. Neurosteroids alter gamma-aminobutyric acid postsynaptic currents in gonadotropin-releasing hormone neurons: a possible mechanism for direct steroidal control. Endocrinology. 2003;144:4366–4375. doi: 10.1210/en.2003-0634. [DOI] [PubMed] [Google Scholar]
- Sundstrom I, Ashbrook D, Backstrom T. Reduced benzodiazepine sensitivity in patients with premenstrual syndrome: a pilot study. Psychoneuroendocrinology. 1997;22:25–38. doi: 10.1016/s0306-4530(96)00035-2. [DOI] [PubMed] [Google Scholar]
- Sundstrom I, Andersson A, Nyberg S, Ashbrook D, Purdy RH, Backstrom T. Patients with premenstrual syndrome have a different sensitivity to a neuroactive steroid during the menstrual cycle compared to control subjects. Neuroendocrinology. 1998;67:126–138. doi: 10.1159/000054307. [DOI] [PubMed] [Google Scholar]
- Sundstrom I, Backstrom T. Patients with premenstrual syndrome have decreased saccadic eye velocity compared to control subjects. Biol Psychiatry. 1998;44:755–764. doi: 10.1016/s0006-3223(98)00012-2. [DOI] [PubMed] [Google Scholar]
- Thind KK, Goldsmith PC. Glutamate and GABAergic neurointeractions in the monkey hypothalamus: a quantitative immunomorphological study. Neuroendocrinology. 1995;61:471–485. doi: 10.1159/000126870. [DOI] [PubMed] [Google Scholar]
- Thompson SA, Bonnert TP, Cagetti E, Whiting PJ, Wafford KA. Overexpression of the GABA(A) receptor epsilon subunit results in insensitivity to anaesthetics. Neuropharmacology. 2002;43:662–668. doi: 10.1016/s0028-3908(02)00162-4. [DOI] [PubMed] [Google Scholar]
- Vande Wiele RL, Bogumil J, Dyrenfurth I, Ferin M, Jewelewicz R, Warren M, Rizkallah T, Mikhail G. Mechanisms regulating the menstrual cycle in women. Recent Prog Horm Res. 1970;26:63–103. doi: 10.1016/b978-0-12-571126-5.50006-6. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Christiansen E, Evans WS, Kolp LA, Rogol AD, Johnson ML. Physiological profiles of episodic progesterone release during the midluteal phase of the human menstrual cycle: analysis of circadian and ultradian rhythms, discrete pulse properties, and correlations with simultaneous luteinizing hormone release. J Clin Endocrinol Metab. 1988;66:414–421. doi: 10.1210/jcem-66-2-414. [DOI] [PubMed] [Google Scholar]
- Weik RF, Dierschke DJ, Karsch FJ, Butler WR, Hotchkiss J, Knobil E. Periovulatory time courses of circulating gonadotropic and ovarian hormones in the rhesus monkey. Endocrinology. 1973;93:1140–1147. doi: 10.1210/endo-93-5-1140. [DOI] [PubMed] [Google Scholar]
- Weiland NG. Glutamic acid decarboxylase messenger ribonucleic acid is regulated by estradiol and progesterone in the hippocampus. Endocrinology. 1992;131:2697–2702. doi: 10.1210/endo.131.6.1446611. [DOI] [PubMed] [Google Scholar]
- Weiland NG, Orchinik M. Specific subunit mRNAs of the GABAA receptor are regulated by progesterone in subfields of the hippocampus. Brain Res Mol Brain Res. 1995;32:271–278. doi: 10.1016/0169-328x(95)00087-9. [DOI] [PubMed] [Google Scholar]
- Whiting PJ, McAllister G, Vassilatis D, Bonnert TP, Heavens RP, Smith DW, Hewson L, O'Donnell R, Rigby MR, Sirinathsinghji DJ, Marshall G, Thompson SA, Wafford KA, Vasilatis D. Neuronally restricted RNA splicing regulates the expression of a novel GABAA receptor subunit conferring atypical functional properties [corrected; erratum to be published] J Neurosci. 1997;17:5027–5037. doi: 10.1523/JNEUROSCI.17-13-05027.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor-Engnell BM, Kasuya E, Mizuno M, Keen KL, Terasawa E. An increase in in vivo release of LHRH and precocious puberty by posterior hypothalamic lesions in female rhesus monkeys (Macaca mulatta) Am J Physiol Endocrinol Metab. 2007;292:E1000–E1009. doi: 10.1152/ajpendo.00493.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Smith SS. Steroid requirements for regulation of the alpha4 subunit of the GABA(A) receptor in an in vitro model. Neurosci Lett. 2007;411:61–66. doi: 10.1016/j.neulet.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberter Y, Kaiser KM, Sakmann B. Dendritic GABA release depresses excitatory transmission between layer 2/3 pyramidal and bitufted neurons in rat neocortex. Neuron. 1999;24:979–988. doi: 10.1016/s0896-6273(00)81044-2. [DOI] [PubMed] [Google Scholar]