Abstract
Much attention has been paid to the potential role of the immune system in the pathophysiology of major depression in humans. While activation of innate immune responses currently dominates the research landscape, early studies in depressed patients demonstrating impairment in acquired immune responses, in particular T cell responses, may warrant further consideration. Intriguing data suggest that activated T cells may play an important neuroprotective role in the context of both stress and inflammation. For example, generation of autoreactive T cells through immunization with central nervous system (CNS) specific antigens has been shown to reverse stress-induced decreases in hippocampal neurogenesis as well as depressive-like behavior in rodents. In addition, trafficking of T cells to the brain following stress, in part related to glucocorticoids, has been found to reduce stress-induced anxiety-like behavior. Data indicate that T regulatory cells may also play a role in depression through downregulation of chronic inflammatory responses. Based on the notion that T cells may subserve neuroprotective and anti-inflammatory functions during stress and inflammation, impaired T cell function may directly contribute to the development of depression. Indeed, increased sensitivity to apoptosis as well as reduced responsiveness to glucocorticoids, may not only decrease the availability of T cells in depressed patients, but also may reduce their capacity to traffic to the brain in response to relevant neuroendocrine or immune stimuli. Further elucidation of T cell pathology may lead to new insights into immune system contributions to depression. Moreover, enhancement of T cell function may represent an alternative strategy to treat depression.
Keywords: depression, T cell, cytokines, inflammation, stress, resilience, pathogenesis, immunization, autoimmunity
1. Introduction
Having spent much of my research career studying the impact of an activated immune system on depression and vice versa, it has been heartening for me to see the tremendous progress that has been made in terms of understanding not only how the immune system interacts with pathophysiologic pathways relevant to depression, but also how new therapies targeting the immune system might enrich the strategies which can be used to manage this devastating disease. It is strikingly clear that depression is one of the most disabling of chronic illnesses (Moussavi et al., 2007), leading to death by suicide in as many as 15% of patients (Sudak, 2005). To compound the problem, almost a third of depressed patients are unable to respond to or tolerate conventional antidepressant medications (Greden, 2001; Rush, 2007), and therefore the need for novel therapeutic approaches is especially timely. The urgency of non-response and evidence that treatment resistant depressed patients may be especially likely to exhibit immune system activation only heightens the interest in further developing approaches that involve the immune system to treat depression (Lanquillon et al., 2000; Miller et al., 2009).
Much of our recent focus has been on the role of the activated innate immune response and inflammation in neuronal integrity and neuropsychiatric disorders including depression (Dantzer et al., 2008; Miller et al., 2009). Indeed, each year, the number of presentations on these topics at the annual meeting of the Psychoneuroimmunology Research Society (PNIRS) has steadily increased. However, at the PNIRS meeting this year, we were reminded that acquired immune responses, in particular T cell responses, also contribute to stress- and depression-related vulnerabilities and resilience. Indeed, data was presented and discussed that T cells may play an important neuroprotective role in the context of stress and inflammation (Lewitus and Schwartz, 2009; Lewitus et al., 2009; Rook and Lowry, 2008). This more active role of T cells in nervous system function is in stark contrast to the theoretical notions of years past when T cells were viewed largely as innocent victims of the ravages of depression on the body, ultimately contributing to increased vulnerability in the context of a host of illnesses including infectious diseases and cancer. In my Presidential Address, I would like to take this opportunity to review where we have come in our understanding of the immune system and depression and revisit our early notions of the relevance of T cells in depression, especially in the light of new data on the impact of T cells on the brain and behavior. More specifically, I would like to elaborate the hypothesis that decreases in the number and function of relevant T cell subsets may directly to contribute to the development and maintenance of depression, and restoration and/or enhancement of relevant T cell functions may represent an interesting and novel approach to the treatment of this disorder.
2. Inflammation and Depression
There has been increasing interest in the role of an activated innate immune response and inflammation in the development of major depression (Dantzer et al., 2008; Miller et al., 2009). These findings are consistent with the notion that inflammation may serve as a common mechanism of disease for a number of disorders including cardiovascular disease, diabetes, and cancer (Aggarwal et al., 2006; Bisoendial et al., 2007; Bouzakri and Zierath, 2007). Patients with major depression have been shown to exhibit evidence of inflammation as manifested by increased inflammatory cytokines including tumor necrosis factor (TNF)-alpha, interleukin (IL)-1 and IL-6 in the peripheral blood and cerebrospinal fluid as well as increases in peripheral blood concentrations of acute phase proteins, chemokines and adhesion molecules (Miller et al., 2009). Peripheral blood elevations in the cytokine, IL-6, and the acute phase protein, c-reactive protein (CRP), appear to be some of the most reproducible findings in this regard (Howren et al., 2009; Mossner et al., 2007; Zorrilla et al., 2001). Interestingly, as noted above, depressed patients with treatment resistance may be most likely to exhibit increases in inflammatory markers (Benedetti et al., 2002; Lanquillon et al., 2000; Miller et al., 2009; Sluzewska et al., 1997). Administration of cytokines including interferon (IFN)-alpha and cytokine inducers such lipopolysaccharide (LPS) and typhoid vaccination have also been found to lead to a host of behavioral changes that overlap with those seen in depressed patients including depressed mood, anxiety, anorexia, fatigue, psychomotor retardation, impaired sleep and cognitive dysfunction (Brydon et al., 2008; Miller et al., 2009; Reichenberg et al., 2001; Wright et al., 2005). Indeed, in comparing cytokine-induced depressive syndromes with depression in otherwise medically healthy individuals, there is little differentiation in terms of specific symptom domains except for psychomotor retardation and anorexia, both of which are more frequent and/or severe in cytokine-treated individuals (Capuron et al., 2009). Finally, administration of drugs that inhibit the action of cytokines or their signaling pathways (e.g. etanercept and cyclooxygenase inhibitors) have been shown to improve mood in patients with inflammatory disorders, while enhancing responsiveness to antidepressants in patients with major depression (Mendlewicz et al., 2006; Muller et al., 2006; Tyring et al., 2006). Of note, treatment with conventional antidepressant medications has been shown to reduce inflammatory markers following successful therapy (Miller et al., 2009), and experiments have shown that antidepressants can inhibit the production of inflammatory cytokines in vitro (Kenis and Maes, 2002). Taken together, these findings have contributed to a major transformation in the understanding of the pathophysiology of major depression and have led to concerted efforts to identify inflammatory targets for the development of new depression therapies.
Regarding the mechanisms by which innate immune cytokines may impact behavior, studies have demonstrated that cytokines can influence the metabolism of the neurotransmitters, serotonin, norepinephrine and dopamine, and alter neuroendocrine function, leading to flattening of the cortisol curve and increased evening cortisol concentrations following chronic cytokine exposure (Anisman et al., 2008; Dantzer et al., 2008; Miller, 2008; Raison et al., 2009b; Rich et al., 2005). These cytokine-induced changes in neurotransmitter and neuroendocrine function have, in turn, been correlated with the development of both depression and fatigue (Dantzer et al., 2008; Felger et al., 2007; Miller et al., 2009; Raison et al., 2009a). Administration of cytokines and cytokine inducers in both laboratory animals and humans has also been shown to activate the enzyme, indoleamine 2,3 dioxygenase (IDO), leading to decreased peripheral blood concentrations of the serotonin precursor, tryptophan, while increasing concentrations of kynurenine, an IDO metabolite that can be catabolized into the neuroactive compounds, quinolinic acid and kynurenic acid (Bonaccorso et al., 2002; Capuron et al., 2003; Dantzer et al., 2008). IDO activation and kynurenine administration have been associated with depressive-like behavior in rodents (O'Connor et al., 2009; O'Connor et al., 2008). In terms of the brain circuits involved, administration of both IFN-alpha and typhoid vaccination to humans has been shown to alter mood relevant neurocircuits including the basal ganglia and the dorsal anterior cingulate cortex (dACC), brain regions that subserve behaviors related to motor activity and motivation (basal ganglia) as well as anxiety, arousal and alarm (dACC) (Brydon et al.,, 2008; Capuron et al., 2005; Capuron et al., 2007; Harrison et al., 2009b; Juengling et al., 2000). Recent data suggest that immune activation also activates more ventral aspects of the ACC including the subgenual region (Cg25) (Harrison et al., 2009a), which is implicated in emotion regulation and depression and is a primary target of antidepressant strategies using deep brain stimulation (Lozano et al., 2008).
There has been increasing interest in the role of growth factors, such as brain derived neurotrophic factor (BDNF) and neurogenesis in the development and treatment of depressive disorders (Duman and Monteggia, 2006), and a number of studies in rodents have demonstrated that stress-induced decreases in BDNF and neurogenesis (which are associated with depressive-like behavior) are related in part to the induction of innate immune cytokines, including IL-1 (Barrientos et al., 2004; Ben Menachem-Zidon et al., 2008; Goshen et al., 2008; Koo and Duman, 2008). Of note, stress-induced activation of microglia appear to play a role in this process, although a direct link between stress-induced microglial production of inflammatory cytokines and stress-induced decreases in BDNF and neurogenesis has yet to be established (Frank et al., 2007).
3. T cells and Depression
Given all the excitement regarding innate immunity, inflammation and depression, much less attention has been paid to the potential role of the adaptive immune response, especially T cells, in depressive disorders. Nevertheless, mounting data indicate that in addition to being ostensibly innocent victims of the pathophysiologic processes involved in depression, T cells, through their neuroprotective and anti-inflammatory effects, may play a pivotal role in both the development of depression as well as its treatment.
T cell Alterations in Patients with Major Depression
The first studies examining the impact of stress and depression on T cell responses in humans reported that in the context of bereavement or severe major depression (requiring hospitalization), proliferation of peripheral blood mononuclear cells in response to the T cell mitogens, phytohemagglutinin (PHA) and concanavalin A (Con A), was significantly reduced (Bartrop et al., 1977; Kronfol et al., 1983; Schleifer et al., 1983; Schleifer et al., 1984; Stein et al., 1991). A multitude of subsequent studies endeavored to repeat and expand these early findings on the inhibitory effects of stress and depression on T cell function, and although there were both successful and unsuccessful replication attempts, meta-analytic approaches to the literature in this area have reached the consensus that statistically reliable decreases in T cell responses exist in both stressed and depressed individuals (Irwin and Miller, 2007; Zorrilla et al., 2001). In addition, in vivo measures of cell-mediated immune function including skin responses to commonly encountered antigens, have suggested decreased T cell activity in depressed patients (Hickie et al., 1993; Sephton et al., 2009).
Complementing these functional assessments, meta-analytic analyses of the extant literature in this area have also revealed that depression and stress are associated with decreases in the percent of lymphocytes as well as the percent of T cells, respectively (Zorrilla et al., 2001). Of note, variability in the immunologic results regarding T cell number and function as they relate to depressed individuals is believed to be secondary to relevant demographic and clinical variables including age, sex, and the severity of depression (Irwin and Miller, 2007; Zorrilla et al., 2001).
The mechanisms of T cell alterations in stress and depression in humans have yet to be established, however, a number of possibilities have been identified. Interestingly, flow cytometric assessments have revealed that CD4+ T cells from depressed patients exhibit evidence of accelerated spontaneous apoptosis as well as increased expression of the receptor for Fas (CD95), which mediates apoptotic signaling by Fas-ligand (Eilat et al., 1999; Ivanova et al., 2007; Szuster-Ciesielska et al., 2008). Increased T cell apoptosis has also been observed as a function of chronic stress in both humans and laboratory animals (Sakami et al., 2002; Shi et al., 2003). One possibility that might explain increased T cell apoptosis in depression, especially in the context of increased immune activation, is tryptophan depletion. As noted above, a number of cytokines and cytokine signaling pathways have been shown to activate the enzyme, IDO, which breaks down tryptophan into kynurenine (Dantzer et al., 2008; Schwarcz and Pellicciari, 2002). Both activation of IDO and kynurenine have in turn been associated with the development of depression (Bonaccorso et al., 2002; Capuron et al., 2002; Dantzer et al., 2008). Relevant to T cell apoptosis, tryptophan is an essential proliferative stimulus for effector T cells, and in a tryptophan-deprived environment, T cells undergo apoptosis (Beissert et al., 2006; Mellor et al., 2003).
Another mechanism that has been considered regarding reduced T cell responses in major depression is inhibition of T cell function by glucocorticoids. Glucocorticoids have multiple effects on immune responses including inhibition of inflammation, mediation of cell trafficking and induction of apoptosis in multiple immune cell types including T cells, especially developing T cells in the thymus (McEwen et al., 1997). In addition, increased peripheral blood concentrations of the glucocorticoid, cortisol, is a hallmark of major depression (Pariante and Miller, 2001). Nevertheless, no relationship has been found between increased cortisol secretion and decreased in vitro proliferative responses to T cell mitogens in depressed patients (Kronfol et al., 1986). Moreover, several studies have demonstrated that peripheral blood lymphocytes from depressed patients, if anything, exhibit decreased responsiveness to the in vitro inhibitory effects of glucocorticoids on T cell proliferation (Bauer et al., 2003; Pariante and Miller, 2001; Raison and Miller, 2003). These findings are consistent with data showing that the synthetic glucocorticoid, dexamethasone, has a greater effect on T cell redistribution in controls than in patients with treatment resistant depression (Bauer et al., 2002). This failure of T cells to respond to neuroendocrine trafficking signals may have a major impact on the ability of T cells to mobilize to the brain and impart neuroprotective functions during stress (see below).
Decreased responsiveness of peripheral blood lymphocytes, including T cells, to glucocorticoids in depressed patients is believed to be related to a decreased expression of glucocorticoid receptors (GR) (Pariante and Miller, 2001; Raison and Miller, 2003). Several studies have shown that depressed patients exhibit reduced cytosolic GR binding in peripheral blood mononuclear cells (Pariante and Miller, 2001). Such changes have been shown to reverse after successful antidepressant treatment (Pariante and Miller, 2001). Changes in GR number and/or function in peripheral blood immune cells may also result from exposure to inflammatory cytokines (Pace et al., 2007). Inflammatory cytokines including IL-1 and IFN-alpha have been shown to reduce GR function through effects of downstream signaling molecules such as p38 mitogen activated protein kinase (MAPK) and signal transducer and activator of transcription (STAT)5 on GR translocation and GR DNA binding, respectively (Hu et al., 2009; Pace et al.,, 2007; Wang et al., 2004). Inflammatory cytokines have also been shown to increase the expression of the relatively inert beta isoform of the GR (Pace et al., 2007). Finally, depressed patients have been found to exhibit decreased expression of beta adrenergic receptors on peripheral blood mononuclear cells (likely involving T cells), which may further isolate immune cell subpopulations, including T cells, from the trafficking effects of neuroendocrine hormones such as catecholamines (Halper et al., 1988).
An additional potential mechanism whereby T cell function may be impaired in patients with depression is the disruption of T cell function by inflammatory cytokines, such as TNF-alpha, which, has been shown to be elevated in depressed patients (Miller et al., 2009). For example, both in vitro and in vivo studies have demonstrated that chronic exposure of T cells to TNF-alpha decreases T cell proliferation and cytokine production (Cope et al., 1997; Cope et al., 1994; Lee et al., 2008). The effects of TNF-alpha on T cell function can be reversed by injections of monoclonal antibodies to TNF-alpha in mouse models and in patients with rheumatoid arthritis (Bayry et al., 2007; Cope et al., 1994; Lee et al., 2008). The effects of TNF-alpha on T cell function are related in part to disruption of signaling through the T cell receptor (Cope et al., 1997; Cope et al., 1994). In addition, microarray analysis of the effects of chronic TNF-alpha administration on T cells indicates that genes regulating cell cycle, proliferation, ubiquitination, cytokine synthesis, calcium signaling, and apoptosis are also involved (Lee et al., 2008). Finally, chronic exposure to TNF-alpha impairs NF-κB and adaptor protein 1 transactivation, leading to T cell non-responsiveness (Lee et al., 2008).
It should also be noted that several genes that play a role in T cell function have been associated with major depression and the response to antidepressants (Wong et al., 2008). Single nucleotide polymorphisms (SNPs) in the genes, PSMB4 (proteasome beta4 subunit - which is important for antigen processing) and TBX21 (T bet - which is important in T cell differentiation), were shown to be associated in a dose dependent fashion with the likelihood of being diagnosed with depression (Wong et al., 2008). Indeed, subjects with three risk alleles were found to be almost 10 times more likely to carry a diagnosis of major depression (Wong et al., 2008). Regarding treatment response, SNPs in the genes that regulate T-cell development (CD3E, T-cell antigen receptor-e subunit of T3), antigen processing (PSMD9: proteasome 26S subunit, non-ATPase), and intracellular signaling (STAT3) were found to be significantly associated with the response to antidepressant medications (Wong et al., 2008). These genetic findings raise the intriguing possibility that T cell dysfunction in major depression may not only represent a consequence of the disease, but also may play an important role in its cause (see below).
Consequences of T cell Alterations in Depression
Given the data indicating T cell dysfunction in depression, there has been considerable interest in the possibility that depression may impart its negative effects on health outcome through its effects on T cells. A number of studies have demonstrated that depression is associated with a worse outcome in a number of diseases including both infectious diseases and cancer. For example, in patients with human immunodeficiency virus (HIV) infection, depression has been associated with an increased likelihood of progressing to Acquired Immune Deficiency Syndrome (AIDS) as well as an increased likelihood of AIDS-related death (Leserman, 2008). In patients with cancer, a history of depressive symptoms has been found to increase the likelihood of cancer-related death by 2.6 fold within the first 19 months following diagnosis (Stommel et al., 2002), and patients who became depressed following stem cell transplant were shown to have a three times greater risk of dying between 6 to 12 months following the procedure, even after adjusting for other prognostic factors (Loberiza et al., 2002).
Several investigators have directly addressed the impact of depression on T cells in the context of viral infection and cancer. Although results have been mixed, at least 3 large studies have demonstrated that depression is associated with a decreased CD4+ T cell count in patients with HIV (Leserman, 2008). For example, in a study of 765 HIV+ women spanning 7 years during the availability of highly activated antiretroviral therapies, women with chronic depressive symptoms exhibited a significantly greater decline in CD4+ T cell count over the course of the study and were approximately 2 times more likely to die from AIDS (Ickovics et al., 2001). T cell responses to varicella-zoster virus antigen have also been shown to be decreased in depressed patients (Irwin et al., 1998). In terms of patients with cancer, women with metastatic breast cancer who reported greater depressive symptoms exhibited suppressed T cell immunity as evidenced by a decreased response to a series of commonly encountered antigens administered intradermally (Sephton et al., 2009).
4. Autoreactive T cells and Neuroprotection
Although much of the focus on T cells in depression has been on the negative impact of depression on T cell function, emerging data suggests that T cells, especially T cells autoreactive to CNS antigens, may play an important role in neuroprotection and resilience against CNS pathology including neuropsychiatric disease (Lewitus and Schwartz, 2009).
Some of the earliest indication that autoreactive T cells may play a neuroprotective role in the CNS came from experiments demonstrating that the pathology of partial crush injury of the optic nerve in rats could be significantly attenuated by the administration of T cells activated by prior exposure to myelin basic protein (MBP)(Moalem et al., 1999). No protection was afforded by T cells immunized against non-self antigens or antigens not specific to the CNS. Labeling of these autoreactive CD4+ T cells demonstrated that they were trafficking to the site of CNS injury from the periphery following intraperitoneal injection. Although immunization against MBP has been shown to induce experimental autoimmune encephalomyelitis (EAE) in rodents, these data demonstrated that under certain circumstances, autoreactive T cells may be beneficial and limit CNS damage (Moalem et al., 1999). Subsequent studies have shown that the neuroprotective effects of CNS autoreactive T cells (i.e. “protective autoimmunity”) may generalize to a number of CNS pathologies including neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease (Avidan et al., 2004; Lewitus and Schwartz, 2009). CNS autoreactive T cells have also been shown to reverse the inhibitory effects of MK801 [a N-methyl-D-aspartate (NMDA) antagonist] on learning and memory, while additionally reversing the disruptive effects of MK801 and amphetamine on prepulse inhibition, a neurological phenomenon which is abnormal in patients with schizophrenia and other neuropsychiatric disorders (Kipnis et al., 2004b). Of note, these studies showed that upon encountering relevant CNS antigens, CNS autoreactive T cells are capable of producing neurotrophic factors such as BDNF (Kipnis et al., 2004b). As noted above, BDNF is believed to play an important role in depression through its effects on neural plasticity (Duman and Monteggia, 2006).
In terms of depressive-like behavior, immunization of rats with a modified MBP (which does not induce EAE, but induces weakly autoreactive T cells) was found to significantly reduce the development of anhedonia (reduced sucrose preference) and immobility in the forced swim test in response to 4 weeks of chronic mild stress (Lewitus et al., 2009). Immunization was also found to reverse stress-related decreases in hippocampal BDNF while increasing the generation of newly formed neurons as identified by BrdU and the early neuronal differentiation marker doublecortin, in the dentate gyrus of the hippocampus (Lewitus et al., 2009). Similar results were found in both Lewis and Sprague-Dawley rat strains (Lewitus et al., 2009).
To further explore the mechanisms and consequences of CNS T cell recruitment during stress, T cell trafficking to the brain was explored in mice exposed to predator odor (Cohen et al., 2006; Lewitus et al., 2008). Previous work has shown that behavioral adaptation following odor exposure (as measured by the acoustic startle response and the elevated plus maze) is significantly improved in the presence of mature effector T cells (Cohen et al., 2006). Interestingly, stressor (odor) exposure was associated with increased T cell infiltration to the choroid plexus, which was associated with a significant increase in the expression of the adhesion molecule, intercellular adhesion molecule (ICAM)-1, by choroid plexus cells (Lewitus et al., 2008). The role of glucocorticoids in this latter effect was established by demonstrating that systemic administration of corticosterone, the natural glucocorticoid of the mouse, also significantly increased ICAM-1 expression in the choroid plexus (Lewitus et al., 2008). These effects of glucocorticoids on adhesion molecule expression and T cell trafficking are consistent with a rich literature showing that stress-induced elevations in glucocorticoids play a key role in lymphocyte trafficking to sites throughout the body, where they are available to encounter relevant antigens and enhance immunologic responses to invading pathogens and cancer cells, while promoting wound healing (Dhabhar, 2009). Interestingly, compared to the Balb/c strain of mice, the C57BL/6J mouse strain, which has a reduced glucocorticoid response to stress, exhibited no stress-induced increases in ICAM-1 expression in the brain and minimal CNS T cell trafficking (Lewitus et al., 2008). C57BL/6J mice, which also have a greater behavioral sensitivity to stress, were shown to exhibit reduced stress-induced behavioral anxiety-like responses following immunization with a CNS-related antigen (Lewitus et al., 2008).
The role of T cells in the maintenance of normal cognitive function has also been examined (Brynskikh et al., 2008; Kipnis et al., 2004b; Wolf et al., 2009). For example, using the Morris Water Maze task, hippocampal-dependent visuo-spatial learning was measured in nude mice (which are devoid of T cells) with and without T cell replenishment (Kipnis et al., 2004b). Compared to nude mice without T cell replenishment, nude mice replenished with T cells exhibited significantly greater learning capacity in terms of the acquisition, extinction and reversal phases of the task (Kipnis et al., 2004b). Other memory tasks have been found to be in part dependent on intact T cell function including the Barnes Maze test and the Radial Arm Maze test (Brynskikh et al., 2008). T cells have also been shown to facilitate the expression of BDNF and hippocampal neurogenesis, including playing a role in the increased neurogenesis secondary to an enriched environment (Wolf et al., 2009; Ziv et al., 2006). As in the case of stressed animals, T cells are believed to mediate their effects in healthy animals through actions in the meningeal spaces (choroid plexus and subarachnoid spaces) (Kipnis et al., 2008). Of note, under non-pathological conditions, T cells do not penetrate the blood brain barrier and are rarely found in brain parenchyma (Kipnis et al., 2008). Taken together, these data indicate that in addition to playing a neuroprotective role during CNS pathology as well as in the context of behavioral alterations such as depression and anxiety following stress, T cells may subserve a more fundamental function in terms of the maintenance of neuronal integrity. Thus, inhibition of T cell function by stress and depression may have profound consequences on essential immunologic elements of healthy brain activity.
5. Regulatory T cells and Inflammation
Interesting conceptual notions regarding the potential role of T cells in psychiatric disease also have been elaborated from the “hygiene hypothesis” and the potential anti-inflammatory effects of regulatory T cells (Rook and Lowry, 2008). The “hygiene hypothesis” proposes that increased inflammation and inflammatory disorders in developed countries may in part be related to the lack of exposure to harmless organisms associated with soil, untreated water and “spoiled” vegetable matter in addition to a lack of infection with helminthes (Bach, 2002; Rook and Lowry, 2008). The result of deficiencies in environmental exposure to these “old friends” (prevalent in developing countries) is a defective development of counter-regulatory pathways to control inflammation, including the elaboration of regulatory T cells (Tregs). Tregs, also known as suppressor T cells (CD4+CD25+Foxp3+), function to inhibit inappropriate or excessive immune responses and mediate immune tolerance (Workman et al., 2009). Tregs have also been found to exhibit neuroprotective functions as well. For example, in a murine model of human immunodeficiency virus (HIV), adoptive transfer of CD3-activated Tregs attenuated astrogliosis and microglia inflammation with concomitant decreased proinflammatory cytokines and increased BDNF and glial cell-derived neurotrophic factor (Liu et al., 2009). Moreover, in a rat stroke model, Tregs were found to enhance survival of progenitor cells in the subventricular zone (Ishibashi et al., 2009). In addition to the regulation of antigen presenting cells (APC) (including activation of APC IDO)(Beissert et al., 2006), the expression and release of the inhibitory cytokines, IL-10 and transforming growth factor (TGF)-beta by Tregs is considered to be an important mechanism by which Tregs inhibit inflammatory responses (Bach, 2002; Workman et al., 2009). Given the potential role of inflammation in depression and the ability of Tregs to produce anti-inflammatory cytokines such as IL-10 (which has been shown to block the development of endotoxin-induced behavioral alterations in laboratory animals)(Dantzer et al., 2008), the possibility that decreased Treg activity may play a role in depression is an intriguing consideration. Nevertheless, there is limited data addressing Treg numbers or function in depression, although a recent study in patients with post traumatic stress disorder (PTSD) indicated a 48% reduction in Treg percentage in PTSD subjects (Sommershof et al., 2009). Consistent with the notion that Treg function may be decreased in depression is the finding that the IFN-gamma/TGF-beta ratio in depressed patients was higher than controls, and there was a significant negative correlation between plasma TGF-beta concentrations and depressive symptoms (Myint et al., 2005). In addition, low serum IL-10 concentrations have been found relative to IL-6 in patients with major depression (Dhabhar et al., 2009).
Similar to the strategies noted above in the elaboration of autoreactive T cells, studies have been conducted using exposure to probiotics (cultures of potentially beneficial bacteria of healthy gut microflora) and mycobacteria extracts to increase Treg activity and reduce inflammatory responses, while treating/preventing inflammatory disorders such as atopic dermatitis and psoriasis (Bach, 2002; Kalliomaki et al., 2001; Rook and Lowry, 2008). For example, in a randomized clinical trial, prenatal administration of Lactobacillus GG (a constituent of healthy gut microflora) to mothers with a first degree relative with an atopic disorder was found to substantially reduce the development of atopic disease in their infants during the first year of life (Kalliomaki et al., 2001). Regarding mycobacteria, administration of heat killed Mycobacterium vaccae (M. vaccae) to mice has been shown to increase allergen-specific Tregs, which confer protection against airway inflammation through the release of IL-10 and TGF-beta (Zuany-Amorim et al., 2002). Interestingly, administration of killed M. vaccae to patients with non-small cell lung cancer was associated with improved quality of life (primarily involving emotional health, bodily pain and cognitive function) during chemotherapy (O'Brien et al., 2004), consistent with the notion that induction of counter-regulatory T cell responses may mitigate against the behavioral consequences of medical treatments that induce inflammation (Rook and Lowry, 2008). These data support the notion that the “hygiene hypothesis” and Tregs may have relevance to behavioral disorders (Rook and Lowry, 2008).
It should be noted, however, that suppression of Treg activity by bacterial DNA has been shown to have a neuroprotective effect after optic nerve injury, an effect mediated by restoration of autoreactive T cell activity (Johnson et al., 2007). Moreover, Tregs have been found to inhibit the mitigating effects of autoreactive T cells on stress-induced anxiety-like behaviors (Cohen et al., 2006). These data suggest that Tregs may inhibit the ability of autoreactive T cells to mediate neuroprotection under certain circumstances. In attempts to disentangle these contradictory and oppositional effects of autoreactive T cells and Tregs, mice with severe combined immunodeficiency (SCID) were administered either Treg-free CD4+ (CD25−) cells or Treg alone by passive transfer and subjected to optic nerve injury secondary to glutamate injection (Kipnis et al., 2004a). Interestingly, both Treg-free CD4+ cells and Tregs alone exhibited a beneficial effect on neuronal cell loss, indicating that both cell types have the capacity to mediate neuroprotection in the absence of the other (Kipnis et al., 2004a). It should be noted however, that Tregs exhibit significant plasticity and can lose regulatory activity and express effector cell function under certain circumstances (Zhou et al., 2009). Nevertheless, it may very well be that the relative balance between autoreactive T cells and Tregs may play a critical role in the T cell dynamics that ultimately determine whether and under what conditions T cell responses are clinically beneficial or detrimental.
6. Translational Implications and Future Directions
Based on emerging data of the role of T cells in the maintenance of neuronal integrity and neuroprotection, coupled with an older literature indicating that T cell function is impaired by virtue of stress and depression, there is ample reason to believe that T cell pathology may not only contribute to the development and/or maintenance of depression but also may be a novel target for the elaboration of treatment and prevention strategies (Figure 1). Further studies are clearly warranted to more fully characterize the potential rich array of T cell functions that mediate the resilience against the behavioral consequences of stress, with special attention to the relative contribution of autoreactive T cells and Tregs, which may have different roles at different time points in the unfolding of the disease process. Indeed, it may be that the induction of autoreactive T cells will be most relevant as a protective or preventative strategy in the context of disease development, whereas Tregs may be more important after stress-induced activation of inflammatory responses has been established. Related to the potential role of activated innate immune responses in depression, it should also be noted that anti-inflammatory therapeutic strategies for depression which involve immunosuppressive agents may additionally influence T cell function with potentially complementary or opposing actions (Wekerle, 2008). For example, the immunosuppressive drug, rapamycin, has been found to enhance in vitro Treg expansion, whereas calcineurin inhibitors, such as cyclosporine, are associated with reduced frequency of Tregs (Wekerle, 2008). Moreover, anti-cytokine therapies, particularly those targeting TNF-alpha, may be especially relevant in reversing T cell dysfunction in depression. Indeed, anti-TNF-alpha therapy has been shown to increase the number and function of Tregs in patients with Chrohn’s disease and rheumatoid arthritis (Bayry et al., 2007; Ricciardelli et al., 2008). Thus, treatments targeting the innate immune response should consider the potential impact on relevant T cell functions.
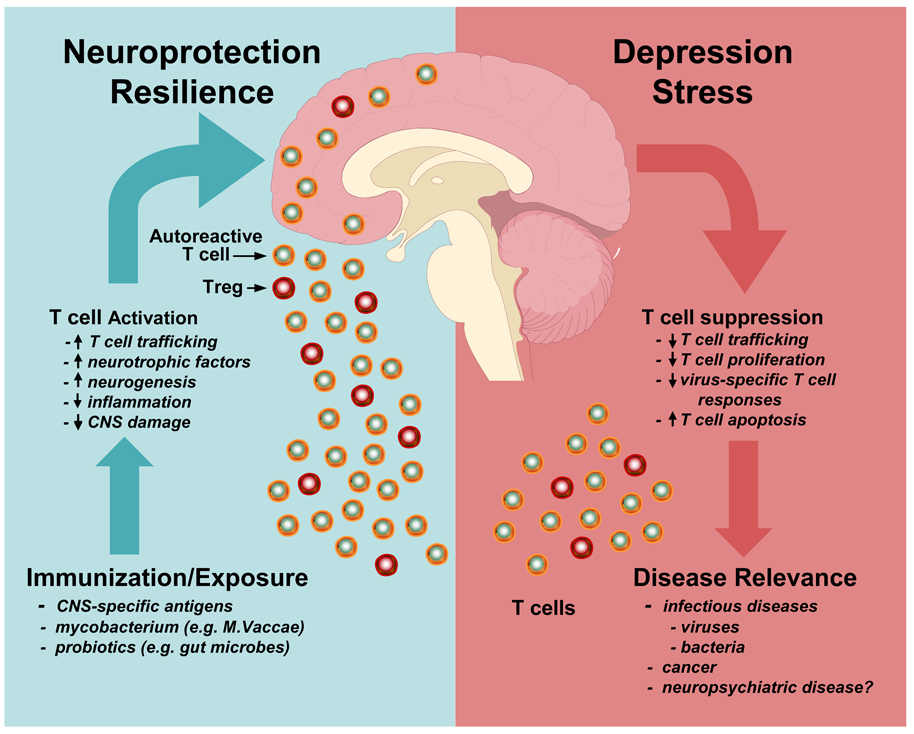
Figure 1. Brain-T cell Interactions in Health and Illness.
Depression and stress lead to activation of neuroendocrine and inflammatory pathways that can contribute to T cell dysfunction as manifested by reduced T cell trafficking in response to neuroendocrine hormones, decreased capacity to proliferate in response to non-specific and specific stimuli, and increased apoptosis. This T cell dysfunction may in turn contribute to disease development including infectious diseases and cancer as well as neuropsychiatric disorders. In contrast, immunization to central nervous system (CNS) specific antigens or exposure to ostensibly harmless or killed microorganisms such a mycobacterium vaccae (M. vaccae) has been found to activate T cell subpopulations including autoreactive T effector cells and regulatory T cells (Treg) which exhibit neuroprotective and anti-inflammatory characteristics, respectively. Once activated, these T cell subpopulations can traffic to the brain, produce local neurotrophic factors, increase neurogenesis and decrease inflammation, leading to reduced CNS pathology depending on the circumstances. Such T cell activities may also subserve resilience against stressors and maintain neuronal integrity during health and illness.
Aside from more fully elaborating the translational benefits that may be achieved by immunizing individuals with CNS-specific antigens or exposing them to probiotic bacteria or attenuated mycobacterium, more attention needs to be paid to the T cell changes that have already been described in patients with major depression. For example, the mechanisms by which T cells exhibit impaired mitogen-induced proliferation and increased spontaneous apoptosis have yet to be determined. Moreover, the relationship between increased inflammatory cytokines, such as TNF-alpha, and T cell function has not been established. Further work also is needed regarding the impact of inflammatory cytokines on GR function and the resulting impact of glucocorticoid resistance on the ability of glucocorticoids to mediate T cell trafficking to relevant bodily organs including the brain. Finally, given the data indicating that T cells are required for normal cognitive function as well as hippocampal neurogenesis in mice, studies are needed to examine the consequences of T cell dysfunction on specific cognitive behaviors in humans and laboratory animals, both in the context of depression and/or inflammatory disorders. Taken together, there appears to be great potential in further exploring the interaction of innate and acquired immune responses in major depression. Moreover, the data indicate that T cells may be a neglected player in the mix of immunologic processes that contribute to depression. As noted above, the initial studies on the immune system in depression focused on the impact of depression on T cell function. Now we have come full circle to return to where we began, appreciating the importance of T cells not only in the consequences of depression, but in its cause as well as its treatment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Anisman H, Merali Z, Hayley S. Neurotransmitter, peptide and cytokine processes in relation to depressive disorder: comorbidity between depression and neurodegenerative disorders. Prog Neurobiol. 2008;85:1–74. doi: 10.1016/j.pneurobio.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Avidan H, Kipnis J, Butovsky O, Caspi RR, Schwartz M. Vaccination with autoantigen protects against aggregated beta-amyloid and glutamate toxicity by controlling microglia: effect of CD4+CD25+ T cells. Eur J Immunol. 2004;34:3434–3445. doi: 10.1002/eji.200424883. [DOI] [PubMed] [Google Scholar]
- Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Sprunger DB, Campeau S, Watkins LR, Rudy JW, Maier SF. BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1beta administration. J Neuroimmunol. 2004;155:119–126. doi: 10.1016/j.jneuroim.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Bartrop RW, Luckhurst E, Lazarus L, Kiloh LG, Penny R. Depressed lymphocyte function after bereavement. Lancet. 1977;1:834–836. doi: 10.1016/s0140-6736(77)92780-5. [DOI] [PubMed] [Google Scholar]
- Bauer ME, Papadopoulos A, Poon L, Perks P, Lightman SL, Checkley S, Shanks N. Dexamethasone-induced effects on lymphocyte distribution and expression of adhesion molecules in treatment-resistant depression. Psychiatry Res. 2002;113:1–15. doi: 10.1016/s0165-1781(02)00243-3. [DOI] [PubMed] [Google Scholar]
- Bauer ME, Papadopoulos A, Poon L, Perks P, Lightman SL, Checkley S, Shanks N. Altered glucocorticoid immunoregulation in treatment resistant depression. Psychoneuroendocrinology. 2003;28:49–65. doi: 10.1016/s0306-4530(02)00009-4. [DOI] [PubMed] [Google Scholar]
- Bayry J, Siberil S, Triebel F, Tough DF, Kaveri SV. Rescuing CD4+CD25+ regulatory T-cell functions in rheumatoid arthritis by cytokine-targeted monoclonal antibody therapy. Drug Discov Today. 2007;12:548–552. doi: 10.1016/j.drudis.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Beissert S, Schwarz A, Schwarz T. Regulatory T cells. J Invest Dermatol. 2006;126:15–24. doi: 10.1038/sj.jid.5700004. [DOI] [PubMed] [Google Scholar]
- Ben Menachem-Zidon O, Goshen I, Kreisel T, Ben Menahem Y, Reinhartz E, Ben Hur T, Yirmiya R. Intrahippocampal transplantation of transgenic neural precursor cells overexpressing interleukin-1 receptor antagonist blocks chronic isolation-induced impairment in memory and neurogenesis. Neuropsychopharmacology. 2008;33:2251–2262. doi: 10.1038/sj.npp.1301606. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Lucca A, Brambilla F, Colombo C, Smeraldi E. Interleukine-6 serum levels correlate with response to antidepressant sleep deprivation and sleep phase advance. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1167–1170. doi: 10.1016/s0278-5846(02)00255-5. [DOI] [PubMed] [Google Scholar]
- Bisoendial RJ, Kastelein JJ, Stroes ES. C-reactive protein and atherogenesis: from fatty streak to clinical event. Atherosclerosis. 2007;195:e10–e18. doi: 10.1016/j.atherosclerosis.2007.04.053. [DOI] [PubMed] [Google Scholar]
- Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, Almerighi C, Verkerk R, Meltzer H, Maes M. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol. 2002;22:86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- Bouzakri K, Zierath JR. MAP4K4 gene silencing in human skeletal muscle prevents tumor necrosis factor-alpha-induced insulin resistance. J Biol Chem. 2007;282:7783–7789. doi: 10.1074/jbc.M608602200. [DOI] [PubMed] [Google Scholar]
- Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry. 2008;63:1022–1029. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brynskikh A, Warren T, Zhu J, Kipnis J. Adaptive immunity affects learning behavior in mice. Brain Behav Immun. 2008;22:861–869. doi: 10.1016/j.bbi.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Capuron L, Fornwalt FB, Knight BT, Harvey PD, Ninan PT, Miller AH. Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? Journal of Affective Disorders. 2009 doi: 10.1016/j.jad.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, Miller AH. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol Psychiatry. 2003;54:906–914. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili M, Woolwine BJ, Nemeroff CB, Berns GS, Miller AH. Anterior cingulate activation and error processing during interferon-alpha treatment. Biol Psychiatry. 2005;58:190–196. doi: 10.1016/j.biopsych.2005.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B, Berns GS, Nemeroff CB, Miller AH. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology. 2007;32:2384–2392. doi: 10.1038/sj.npp.1301362. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;7:468–473. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- Cohen H, Ziv Y, Cardon M, Kaplan Z, Matar MA, Gidron Y, Schwartz M, Kipnis J. Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4+CD25+ cells. J Neurobiol. 2006;66:552–563. doi: 10.1002/neu.20249. [DOI] [PubMed] [Google Scholar]
- Cope AP, Liblau RS, Yang XD, Congia M, Laudanna C, Schreiber RD, Probert L, Kollias G, McDevitt HO. Chronic tumor necrosis factor alters T cell responses by attenuating T cell receptor signaling. J Exp Med. 1997;185:1573–1584. doi: 10.1084/jem.185.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope AP, Londei M, Chu NR, Cohen SB, Elliott MJ, Brennan FM, Maini RN, Feldmann M. Chronic exposure to tumor necrosis factor (TNF) in vitro impairs the activation of T cells through the T cell receptor/CD3 complex; reversal in vivo by anti-TNF antibodies in patients with rheumatoid arthritis. J Clin Invest. 1994;94:749–760. doi: 10.1172/JCI117394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–317. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, Burke HM, Epel ES, Mellon SH, Rosser R, Reus VI, Wolkowitz OM. Low serum IL-10 concentrations and loss of regulatory association between IL-6 and IL-10 in adults with major depression. J Psychiatr Res. 2009;43:962–969. doi: 10.1016/j.jpsychires.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116, 1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Eilat E, Mendlovic S, Doron A, Zakuth V, Spirer Z. Increased apoptosis in patients with major depression: A preliminary study. J Immunol. 1999;163:533–534. [PubMed] [Google Scholar]
- Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, Kalin NH, Ratti E, Nemeroff CB, Miller AH. Effects of interferon-alpha on rhesus monkeys: a nonhuman primate model of cytokine-induced depression. Biol Psychiatry. 2007;62:1324–1333. doi: 10.1016/j.biopsych.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008;13:717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- Greden JF. The burden of disease for treatment-resistant depression. J Clin Psychiatry. 2001;62 Suppl 16:26–31. [PubMed] [Google Scholar]
- Halper JP, Brown RP, Sweeney JA, Kocsis JH, Peters A, Mann JJ. Blunted beta-adrenergic responsivity of peripheral blood mononuclear cells in endogenous depression. Isoproterenol dose-response studies. Arch Gen Psychiatry. 1988;45:241–244. doi: 10.1001/archpsyc.1988.01800270053006. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009a;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ, Critchley HD. Neural origins of human sickness in interoceptive responses to inflammation. Biol Psychiatry. 2009b;66:415–422. doi: 10.1016/j.biopsych.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickie I, Hickie C, Lloyd A, Silove D, Wakefield D. Impaired in vivo immune responses in patients with melancholia. Br J Psychiatry. 1993;162:651–657. doi: 10.1192/bjp.162.5.651. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Hu F, Pace TW, Miller AH. Interferon-alpha inhibits glucocorticoid receptor-mediated gene transcription via STAT5 activation in mouse HT22 cells. Brain Behav Immun. 2009;23:455–463. doi: 10.1016/j.bbi.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, Moore J. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285:1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- Irwin M, Costlow C, Williams H, Artin KH, Chan CY, Stinson DL, Levin MJ, Hayward AR, Oxman MN. Cellular immunity to varicella-zoster virus in patients with major depression. J Infect Dis. 1998;178 Suppl 1:S104–S108. doi: 10.1086/514272. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Maric D, Mou Y, Ohtani R, Ruetzler C, Hallenbeck JM. Mucosal tolerance to E-selectin promotes the survival of newly generated neuroblasts via regulatory T-cell induction after stroke in spontaneously hypertensive rats. J Cereb Blood Flow Metab. 2009;29:606–620. doi: 10.1038/jcbfm.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova SA, Semke VY, Vetlugina TP, Rakitina NM, Kudyakova TA, Simutkin GG. Signs of apoptosis of immunocompetent cells in patients with depression. Neurosci Behav Physiol. 2007;37:527–530. doi: 10.1007/s11055-007-0047-y. [DOI] [PubMed] [Google Scholar]
- Johnson TV, Camras CB, Kipnis J. Bacterial DNA confers neuroprotection after optic nerve injury by suppressing CD4+CD25+ regulatory T-cell activity. Invest Ophthalmol Vis Sci. 2007;48:3441–3449. doi: 10.1167/iovs.06-1351. [DOI] [PubMed] [Google Scholar]
- Juengling FD, Ebert D, Gut O, Engelbrecht MA, Rasenack J, Nitzsche EU, Bauer J, Lieb K. Prefrontal cortical hypometabolism during low-dose interferon alpha treatment. Psychopharmacology (Berl) 2000;152:383–389. doi: 10.1007/s002130000549. [DOI] [PubMed] [Google Scholar]
- Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076–1079. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- Kenis G, Maes M. Effects of antidepressants on the production of cytokines. Int J Neuropsychopharmacol. 2002;5:401–412. doi: 10.1017/S1461145702003164. [DOI] [PubMed] [Google Scholar]
- Kipnis J, Avidan H, Caspi RR, Schwartz M. Dual effect of CD4+CD25+ regulatory T cells in neurodegeneration: a dialogue with microglia. Proc Natl Acad Sci U S A. 2004a;101 Suppl 2:14663–14669. doi: 10.1073/pnas.0404842101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci U S A. 2004b;101:8180–8185. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Derecki NC, Yang C, Scrable H. Immunity and cognition: what do age-related dementia, HIV-dementia and 'chemo-brain' have in common? Trends Immunol. 2008;29:455–463. doi: 10.1016/j.it.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronfol Z, House JD, Silva J, Jr, Greden J, Carroll BJ. Depression, urinary free cortisol excretion and lymphocyte function. Br J Psychiatry. 1986;148:70–73. doi: 10.1192/bjp.148.1.70. [DOI] [PubMed] [Google Scholar]
- Kronfol Z, Silva J, Jr, Greden J, Dembinski S, Gardner R, Carroll B. Impaired lymphocyte function in depressive illness. Life Sci. 1983;33:241–247. doi: 10.1016/0024-3205(83)90382-x. [DOI] [PubMed] [Google Scholar]
- Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22:370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Lee LF, Lih CJ, Huang CJ, Cao T, Cohen SN, McDevitt HO. Genomic expression profiling of TNF-alpha-treated BDC2.5 diabetogenic CD4+ T cells. Proc Natl Acad Sci U S A. 2008;105:10107–10112. doi: 10.1073/pnas.0803336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosom Med. 2008;70:539–545. doi: 10.1097/PSY.0b013e3181777a5f. [DOI] [PubMed] [Google Scholar]
- Lewitus GM, Cohen H, Schwartz M. Reducing post-traumatic anxiety by immunization. Brain Behav Immun. 2008;22:1108–1114. doi: 10.1016/j.bbi.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Lewitus GM, Schwartz M. Behavioral immunization: immunity to self-antigens contributes to psychological stress resilience. Mol Psychiatry. 2009;14:532–536. doi: 10.1038/mp.2008.103. [DOI] [PubMed] [Google Scholar]
- Lewitus GM, Wilf-Yarkoni A, Ziv Y, Shabat-Simon M, Gersner R, Zangen A, Schwartz M. Vaccination as a novel approach for treating depressive behavior. Biol Psychiatry. 2009;65:283–288. doi: 10.1016/j.biopsych.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Liu J, Gong N, Huang X, Reynolds AD, Mosley RL, Gendelman HE. Neuromodulatory activities of CD4+CD25+ regulatory T cells in a murine model of HIV-1-associated neurodegeneration. J Immunol. 2009;182:3855–3865. doi: 10.4049/jimmunol.0803330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loberiza FR, Jr, Rizzo JD, Bredeson CN, Antin JH, Horowitz MM, Weeks JC, Lee SJ. Association of depressive syndrome and early deaths among patients after stem-cell transplantation for malignant diseases. J Clin Oncol. 2002;20:2118–2126. doi: 10.1200/JCO.2002.08.757. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Biron CA, Brunson KW, Bulloch K, Chambers WH, Dhabhar FS, Goldfarb RH, Kitson RP, Miller AH, Spencer RL, Weiss JM. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res Brain Res Rev. 1997;23:79–133. doi: 10.1016/s0165-0173(96)00012-4. [DOI] [PubMed] [Google Scholar]
- Mellor AL, Munn D, Chandler P, Keskin D, Johnson T, Marshall B, Jhaver K, Baban B. Tryptophan catabolism and T cell responses. Adv Exp Med Biol. 2003;527:27–35. doi: 10.1007/978-1-4615-0135-0_3. [DOI] [PubMed] [Google Scholar]
- Mendlewicz J, Kriwin P, Oswald P, Souery D, Alboni S, Brunello N. Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: a pilot open-label study. Int Clin Psychopharmacol. 2006;21:227–231. doi: 10.1097/00004850-200607000-00005. [DOI] [PubMed] [Google Scholar]
- Miller AH. Mechanisms of cytokine-induced behavioral changes: Psychoneuroimmunology at the translational interface. Brain Behav Immun. 2009;23:149–158. doi: 10.1016/j.bbi.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen IR, Schwartz M. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- Mossner R, Mikova O, Koutsilieri E, Saoud M, Ehlis AC, Muller N, Fallgatter AJ, Riederer P. Consensus paper of the WFSBP Task Force on Biological Markers: biological markers in depression. World J Biol Psychiatry. 2007;8:141–174. doi: 10.1080/15622970701263303. [DOI] [PubMed] [Google Scholar]
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370:851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B, Spellmann I, Hetzel G, Maino K, Kleindienst N, Moller HJ, Arolt V, Riedel M. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11:680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- Myint AM, Leonard BE, Steinbusch HW, Kim YK. Th1, Th2, and Th3 cytokine alterations in major depression. J Affect Disord. 2005;88:167–173. doi: 10.1016/j.jad.2005.07.008. [DOI] [PubMed] [Google Scholar]
- O'Brien ME, Anderson H, Kaukel E, O'Byrne K, Pawlicki M, Von Pawel J, Reck M. SRL172 (killed Mycobacterium vaccae) in addition to standard chemotherapy improves quality of life without affecting survival, in patients with advanced non-small-cell lung cancer: phase III results. Ann Oncol. 2004;15:906–914. doi: 10.1093/annonc/mdh220. [DOI] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, Andre C, Briley EM, Szegedi SS, Lestage J, Castanon N, Herkenham M, Dantzer R, Kelley KW. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J Immunol. 2009;182:3202–3212. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, Vogt GJ, Massung B, Miller AH. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 2009a;65:296–303. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Woolwine BJ, Massung B, Vogt G, Miller AH. Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior. Mol Psychiatry. 2009b doi: 10.1038/mp.2008.58. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Ricciardelli I, Lindley KJ, Londei M, Quaratino S. Anti tumour necrosis-alpha therapy increases the number of FOXP3 regulatory T cells in children affected by Crohn's disease. Immunology. 2008;125:178–183. doi: 10.1111/j.1365-2567.2008.02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich T, Innominato PF, Boerner J, Mormont MC, Iacobelli S, Baron B, Jasmin C, Levi F. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clin Cancer Res. 2005;11:1757–1764. doi: 10.1158/1078-0432.CCR-04-2000. [DOI] [PubMed] [Google Scholar]
- Rook GA, Lowry CA. The hygiene hypothesis and psychiatric disorders. Trends Immunol. 2008;29:150–158. doi: 10.1016/j.it.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Rush AJ. STAR*D: what have we learned? Am J Psychiatry. 2007;164:201–204. doi: 10.1176/ajp.2007.164.2.201. [DOI] [PubMed] [Google Scholar]
- Sakami S, Nakata A, Yamamura T, Kawamura N. Psychological stress increases human T cell apoptosis in vitro. Neuroimmunomodulation. 2002;10:224–231. doi: 10.1159/000068326. [DOI] [PubMed] [Google Scholar]
- Schleifer SJ, Keller SE, Camerino M, Thornton JC, Stein M. Suppression of lymphocyte stimulation following bereavement. JAMA. 1983;250:374–377. [PubMed] [Google Scholar]
- Schleifer SJ, Keller SE, Meyerson AT, Raskin MJ, Davis KL, Stein M. Lymphocyte function in major depressive disorder. Arch Gen Psychiatry. 1984;41:484–486. doi: 10.1001/archpsyc.1984.01790160070008. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- Sephton SE, Dhabhar FS, Keuroghlian AS, Giese-Davis J, McEwen BS, Ionan AC, Spiegel D. Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain Behav Immun. 2009 doi: 10.1016/j.bbi.2009.07.007. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Shi Y, Devadas S, Greeneltch KM, Yin D, Allan Mufson R, Zhou JN. Stressed to death: implication of lymphocyte apoptosis for psychoneuroimmunology. Brain Behav Immun. 2003;17 Suppl 1:S18–S26. doi: 10.1016/s0889-1591(02)00062-4. [DOI] [PubMed] [Google Scholar]
- Sluzewska A, Sobieska M, Rybakowski JK. Changes in acute-phase proteins during lithium potentiation of antidepressants in refractory depression. Neuropsychobiology. 1997;35:123–127. doi: 10.1159/000119332. [DOI] [PubMed] [Google Scholar]
- Sommershof A, Aichinger H, Engler H, Adenauer H, Catani C, Boneberg EM, Elbert T, Groettrup M, Kolassa IT. Substantial reduction of naive and regulatory T cells following traumatic stress. Brain Behav Immun. 2009 doi: 10.1016/j.bbi.2009.07.003. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Stein M, Miller AH, Trestman RL. Depression, the immune system, and health and illness. Findings in search of meaning. Arch Gen Psychiatry. 1991;48:171–177. doi: 10.1001/archpsyc.1991.01810260079012. [DOI] [PubMed] [Google Scholar]
- Stommel M, Given BA, Given CW. Depression and functional status as predictors of death among cancer patients. Cancer. 2002;94:2719–2727. doi: 10.1002/cncr.10533. [DOI] [PubMed] [Google Scholar]
- Sudak HS. Suicide. In: Sadock BJ, Sadock VA, editors. Kaplan & Sadock's Comprehensive Textbook of Psychiatry. Eighth Edition. New York: Lippincott, Williams & Wilkins; 2005. pp. 2442–2453. [Google Scholar]
- Szuster-Ciesielska A, Slotwinska M, Stachura A, Marmurowska-Michalowska H, Dubas-Slemp H, Bojarska-Junak A, Kandefer-Szerszen M. Accelerated apoptosis of blood leukocytes and oxidative stress in blood of patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:686–694. doi: 10.1016/j.pnpbp.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, Cella D, Krishnan R. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- Wang X, Wu H, Miller AH. Interleukin 1alpha (IL-1alpha) induced activation of p38 mitogen-activated protein kinase inhibits glucocorticoid receptor function. Mol Psychiatry. 2004;9:65–75. doi: 10.1038/sj.mp.4001339. [DOI] [PubMed] [Google Scholar]
- Wekerle T. T-regulatory cells-what relationship with immunosuppressive agents? Transplant Proc. 2008;40:S13–S16. doi: 10.1016/j.transproceed.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Steiner B, Akpinarli A, Kammertoens T, Nassenstein C, Braun A, Blankenstein T, Kempermann G. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. J Immunol. 2009;182:3979–3984. doi: 10.4049/jimmunol.0801218. [DOI] [PubMed] [Google Scholar]
- Wong ML, Dong C, Maestre-Mesa J, Licinio J. Polymorphisms in inflammation-related genes are associated with susceptibility to major depression and antidepressant response. Mol Psychiatry. 2008;13:800–812. doi: 10.1038/mp.2008.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman CJ, Szymczak-Workman AL, Collison LW, Pillai MR, Vignali DA. The development and function of regulatory T cells. Cell Mol Life Sci. 2009;66:2603–2622. doi: 10.1007/s00018-009-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CE, Strike PC, Brydon L, Steptoe A. Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav Immun. 2005;19:345–350. doi: 10.1016/j.bbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA. Plasticity of CD4(+) FoxP3(+) T cells. Curr Opin Immunol. 2009;21:281–285. doi: 10.1016/j.coi.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
- Zuany-Amorim C, Sawicka E, Manlius C, Le Moine A, Brunet LR, Kemeny DM, Bowen G, Rook G, Walker C. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat Med. 2002;8:625–629. doi: 10.1038/nm0602-625. [DOI] [PubMed] [Google Scholar]