Abstract
Exposures to predator odors are very effective methods to evoke a variety of stress responses in rodents. We have previously found that ferret odor exposure leads to changes in endocrine hormones (corticosterone and ACTH) and behavior. To distinguish the contributions of the main and accessory olfactory systems in these responses, studies were designed to interfere with these two systems either independently, or simultaneously. Male Sprague-Dawley rats were treated with 10% zinc sulfate (ZnSO4), which renders rodents anosmic (unable to smell) while leaving the accessory olfactory areas intact, or saline, in experiment 1. In experiment 2, the vomeronasal organs of rats were surgically removed (VNX) to block accessory olfactory processing, while leaving the main olfactory system intact. And in the 3rd experiment both the main and accessory olfactory areas were disrupted by combining the two procedures in the same rats. Neither ZnSO4 treatment or VNX alone reliably reduced the increased corticosterone response to ferret odor compared to strawberry odor, but in combination, they did. This suggests that processing through the main or the accessory olfactory system can elicit the endocrine stress response to ferret odor. VNX alone also did not affect the behavioral responses to the ferret. ZnSO4 treatment, alone and in combination with VNX, led to changes in behavior in response to both ferret and strawberry odor, making the behavioral results less clearly interpretable. Overall these studies suggest that both the main and accessory olfactory systems mediate the neuroendocrine response to predator odor.
Keywords: acute stress, corticosterone, olfaction, predator, vomeronasal organ, zinc sulfate
1. Introduction
The presentation of predators or cues associated with them elicits increases in the stress hormones, corticosterone and adrenocorticotropin (ACTH), defensive behavioral responses, and autonomic nervous system activation [8,9,11–14,16,25,26,32,36]. Predators and their cues offer a unique model to study stress and anxiety, and this class of stimuli have been suggested as important models to study the development of mood disorders. Predator stress may model aspects of PTSD including changes in hypothalamic-pituitary-adrenal axis function, widespread central nervous system effects, and long-term anxiety-like behavior [1–3]. There are several advantages for the use of predators and especially their associated cues for the study of stress and anxiety. Predator odor itself is not noxious and does not cause physical pain [7,10,32]. This is a significant advantage when studying the neural circuitry underlying stress/anxiety responses.
It has been suggested that predator odors are processed through a pheromonal, allomonal, or kairomonal pathway [6,13,31,39,48–50]; these odors activate the vomeronasal organ (VNO), which sends afferents to the accessory olfactory bulb (AOB) and ultimately project to the posteroventral, anterodorsal, and posterodorsal medial amygdala, among others, which are considered the ‘vomeronasal amygdala’ [18,19,23,33,37]. McGregor and colleagues (2004) found higher Fos protein immunoreactivity in the posterior AOB and posteroventral medial amygdala after cat odor compared to control odor exposure, but additionally reported higher Fos levels in the glomerular layer of the main olfactory bulb in the cat odor-exposed compared to control-odor exposed rats [31]. Likewise, higher c-fos mRNA expression was found in the posteroventral and posterodorsal medial amygdala after ferret odor exposure compared to a control odor exposure, but ferret odor exposed rats also had significantly higher levels of c-fos mRNA in several regions generally considered to be part of the main olfactory system, including the piriform cortex [25]. Traditionally, pheromones were thought to be processed exclusively by the accessory olfactory system, but recent studies have shown that the main olfactory system is also activated by known pheromonal odors [17,20,22,38,51], and the accessory olfactory system has been found to be reactive to non-pheromonal odors as well [41,44]. These recent findings lead to the conclusion that immediate-early gene expression alone may not provide the answer to the question of whether the main or accessory olfactory system independently or in combination mediate the effects of predator odors. The immunohistochemical and in situ hybridization techniques used only show that predator odors may be detected by these two olfactory systems. The techniques do not distinguish whether it is the accessory olfactory or main olfactory bulb activation that functionally induces the constellation of responses elicited by predator odors. A recent study in mice suggests that the dorsal (D1) domain of the main olfactory bulb mediates some of the effects of predator odors [20]; unfortunately, no functional data were presented with regard to putative activation, or lack thereof, of the accessory olfactory system with predator odor exposure in these specific olfactory knock-out mice.
The goal of the present study therefore, was to distinguish the contributions of the main and accessory olfactory systems in the defensive behavioral and neuroendocrine responses, as measured with plasma corticosterone levels, to ferret odor by blocking these two systems independently, or simultaneously. In our laboratory we have demonstrated that the fur/skin odor from a ferret is a highly potent stimulus that has long-lasting effects on behavior, HPA axis, and autonomic responses [9,25,26]. Zinc sulfate (ZnSO4), which renders rodents anosmic while leaving the accessory olfactory areas intact, was used to temporarily disrupt main olfactory processing. Accessory olfactory processing was disrupted by surgically removing the vomeronasal organ. Defensive behavioral responses to a ferret odor stimulus were examined in a defensive withdrawal paradigm and plasma corticosterone response was examined in a home cage exposure paradigm. Surprisingly, neither manipulation alone reliably reduced the neuroendocrine and behavioral responses, suggesting that both systems can independently contribute to predator odor-induced responses. Some of these data have been presented in abstract form [27,28].
2. Methods
2.1. Experiment 1
2.1.1. Subjects
Forty-eight male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 200 – 250 g at the time of arrival to the colony were used. They were group-housed (4 / cage) in a room kept on a controlled light-dark cycle (lights on 7:00 a.m. and off 7:00 p.m.) under constant humidity and temperature conditions. The rats were acclimated to the animal colony for a period of 7 days after arrival from the supplier, prior to any experimental manipulation. Rats were provided with food (rat chow) and water ad libitum. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Colorado and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.1.2. Zinc sulfate treatment
One day before anosmia and behavioral testing, the rats were treated with zinc sulfate (ZnSO4), to render them anosmic (n = 22), or saline (n = 18), as a control procedure. The rats were anesthetized with halothane and placed on their backs on an inclined surface with their heads facing downward. A curved 20-gauge syringe was put into the pharynx at the caudal end of the palate and slowly retracted rostrally to allow the tip to enter the nasal cavity via the posterior choanae. ZnSO4 (10% w/v in 0.9% saline; Ricca Chemical Co., Arlington, TX) was slowly perfused until a few drops were seen draining out of the external nares. The mouth of the rat was aspirated to remove saliva and excess solution during the procedure and during recovery from the anesthesia to prevent swallowing that could lead to sickness or death [5]. The sham-treated control rats were anesthetized and the nasal cavity perfused with saline instead of zinc sulfate. Post-treatment, all rats were individually housed in polycarbonate plastic cages (49.5 × 27.9 × 20.3 cm).
2.1.3. Anosmia testing
The next morning, to ensure that the rats were anosmic after ZnSO4 treatment, a cookie-finding task was administered. An Oreo cookie with a diameter of 4.5 cm (Nabisco), which the rats were previously familiarized with during acclimation to the animal colony, was placed in a polycarbonate plastic cage (49.5 × 27.9 × 20.3 cm) and woodchip bedding poured on top of the cookie and spread in the cage to a depth of 3 cm. A rat was placed in the middle of the cage and a stopwatch started. If the ZnSO4 treated rat did not actively find the cookie in 10 minutes, the rat was considered anosmic and included in the study. Previous studies have revealed that normal rats typically find the cookie within 2 minutes [24].
2.1.4. Odor Stimuli
Ferret odor was collected by placing a small hand towel in a cage with breeding adult ferrets for approximately 1 month (courtesy Mile High Ferret Club). The towel was cut into 5 × 5 cm squares and kept in an −80° C freezer until use. The towels were then thawed in a glass bell jar for 30 min before use. Strawberry odor was used as a novel control odor [25,26]. Towels were scented with strawberry odor by pipetting 100 μl of strawberry extract (McCormick & Co., Inc., Hunt Valley, MD) onto clean 5 × 5 cm towels.
2.1.5. Behavior Testing
The rats were previously habituated to the defensive withdrawal apparatus by placing each rat in the open field for 10 min on three consecutive days at approximately the same time each day. The apparatus consisted of a 58 × 58 × 39 cm Plexiglas open field chamber with a metal 29 × 20 × 14 cm chamber in one corner with an opening on the long side that is 9 cm wide and 8 cm tall. The floor and sides of the open field were black and white tape was used to delineate 16 equal sized squares on the floor, of which the smaller chamber occupies 2 full squares and half of two squares. The room that contains the apparatus was dimly lit by a 75-watt red light bulb and white noise (60 dB Sound Pressure Level) was provided by an AM7 Grass Medical Instruments audio monitor (Quincy, MA).
The rats were tested the evening after ZnSO4 or saline treatment after anosmia testing during the rats’ dark phase. A piece of towel (ferret or strawberry odor) was taped to the floor of the open field diagonally opposite the withdrawal chamber. The rat was placed in the open field chamber, across from the withdrawal chamber and behavior was videotaped (Sony VHS recorder) for 10 min by a Panasonic WV-BP130 video camera (Ontario, Canada) that was mounted directly above the apparatus (approximately 2.4 meters). An additional control group with naïve rats that had neither zinc sulfate nor saline treatment (n = 8) were placed in the defensive withdrawal paradigm without any towel stimulus to determine normal behavior without an odor influence.
Two researchers blind to the experimental conditions analyzed the videotaped behavior. Behaviors analyzed included: time spent in withdrawal chamber (seconds), time spent in the corner containing the towel (front paws in towel square), and number of rears (front paws leave floor). The scores of the two researchers were averaged. Scorers normally achieve a level of 95 – 99% agreement depending on the measure.
2.1.6. Home cage odor exposures
Immediately following behavior testing, the rats were placed back in their individual home cages, which were then placed in wood sound-attenuating chambers over night (kept on the same light cycle) to avoid manipulation and transport of the rats immediately prior to odor exposure. The next morning (rats’ light/inactive phase), 4 pieces of towel (ferret or strawberry odor) were carefully placed in each corner of the cages without disturbing the rats by hooking the towels to the wire cage lid with paper clips, so the towels hung inside the cage approximately 5 cm from the floor. Rats were exposed to the opposite odor in their home cage as they had been exposed to in the defensive withdrawal paradigm, so that each rat received each odor once. Immediately following the 30 min towel exposure, the rats were taken to an adjacent room, decapitated, and trunk blood was collected.
2.1.7. Corticosterone ELISA
Blood was collected into ice-chilled tubes containing EDTA (20 mg/ml). Blood samples were then centrifuged at 2000 rpm for 10 min, the plasma pipetted into 0.5 ml Eppendorf microcentrifuge tubes, and stored at −80° C until assayed. The corticosterone assay was performed according to the manufacturer’s instructions (kit #901-097 – AssayDesigns, Ann Arbor, MI). Levels were then quantified on a BioTek Elx808 microplate reader and calculated against a standard curve generated concurrently.
2.1.8. Data analysis
Corticosterone data was analyzed using multivariate analyses of variance (MANOVA; Pillai’s Trace) with treatment (ZnSO4 or saline) and odor (ferret or strawberry) as the between-subjects factors. Behavioral data were analyzed using multivariate analyses of variance (MANOVA; Pillai’s Trace) with treatment (ZnSO4, saline, or no treatment) and odor (ferret, strawberry, or no odor) as the between-subjects factors. Post-hoc comparisons were made using a Bonferroni test (p < 0.05).
2.2. Experiment 2
2.2.1. Subjects
Thirty-six male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 200 – 250 g at the time of arrival to the colony were used. Animals were housed as described in Experiment 1.
2.2.3. Vomeronasal organ removal surgery (VNX)
At least one week before testing, rats had their vomeronasal organs (VNO) surgically removed. The surgical procedure for the removal of the VNO was adapted from Wysocki and Wysocki (1995) [51]. The rats were anesthetized with an intramuscular injection of a solution of xylazine (60 mg/kg) and ketamine (21 mg/kg). The rats were then placed ventral side up, the lower jaw of the rat was retracted and an incision was made in the palate just caudal to the incisors to the second palatal ridge using a drill. When the VNO was visualized under a dissecting microscope (Leica MZ6) it was removed with micro-forceps. Throughout the surgery blood was gently aspirated. The cavity was filled with GelFoam (UpJohn, Kalamazoo, MI) to arrest bleeding and the soft palatal tissue sutured back. The sham-operated control rats were drilled and sutured but the VNO was not removed.
2.2.4. Behavior & home cage testing
One week following surgery, defensive withdrawal behavior and home cage towel exposure testing were conducted as described in Experiment 1. The one difference in the procedure was that blood was collected via tail nicks after the 30 min home cage towel exposure, instead of collecting trunk blood, to allow for later VNX verification. Data was collected and analyzed in the same way as described in Experiment 1 using multivariate analyses of variance (MANOVA; Pillai’s Trace) with treatment (VNX or Sham surgery) and odor (ferret or strawberry) as the between-subjects factors. Post-hoc comparisons were made using a Bonferroni test (p < 0.05). Data from the additional naïve/no surgery control rats (from experiment 1) that were tested in the defensive withdrawal paradigm without any odor towel stimulus were used for comparison purposes as an indicator of normal behavior without odor influences in the paradigm.
2.2.5. VNX histological verification
One week after the home cage towel exposure, the rats were given an additional 30 min ferret towel exposure. Sixty minutes after the end of the odor exposure, the rats were given an intraperitoneal overdose of sodium pentobarbital (1 mL). They were then perfused transcardially with ice-cold solutions of 100 ml of 0.9% saline containing heparin, and then by 500 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). In general, after removal of the brains from the cranial cavity, they were postfixed for 60 min in the same 4% paraformaldehyde solution at 4°C, and then placed in a 10% sucrose/0.1 M phosphate buffer/heparin solution at 4°C until the brains sank. The perfused brains were sectioned (30 μm) through the accessory olfactory areas for both soybean agglutinin – horseradish peroxidase (SBA-HRP) staining and Fos immunohistochemical (IHC) verification.
The SBA-HRP staining procedure used was adapted from Wysocki & Wysocki (1995) [52]. Briefly, the slides were rinsed in 0.1 M phosphate buffered saline (PBS), incubated in a 1% hydrogen peroxide solution for 40 min, and then 75 μl of 15 μg/mL SBA-HRP solution (Sigma #L2650) was pipetted over each slide and incubated at room temperature for 3 hours. The tissue was then rinsed again with PBS. Slides were then placed in chromagen 3,3′-diaminobenzidene (DAB) and hydrogen peroxide (Vector Laboratories, Burlingame, CA) solution for approximately 4 minutes and then rinsed with water. The slides were air-dried and then coverslipped with Permount (Fisher Scientific). A complete VNX leads to a loss of staining in the AOB. VNX and a random sample of sham-operated controls were processed together for comparison.
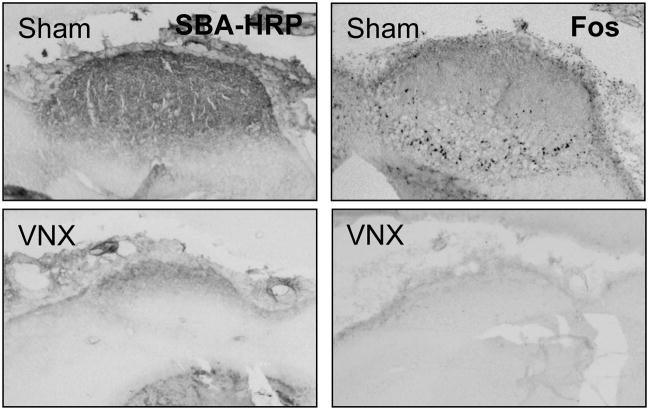
Fos immunohistochemistry was also employed as an additional verification method for the VNX surgery. All incubations were carried out with gentle agitation at room temperature. Sections were washed in 0.1 M phosphate buffered saline (PBS) between incubations in different solutions. Sections on the slides were first washed and then incubated in a 0.1 M PBS solution containing 0.3% hydrogen peroxide for 30 minutes. Slides were then blocked for 20 minutes each in avidin and biotin blocking solutions (blocking kit #SP-2001, Vector Laboratories, Burlingame, CA). After a 1-hr incubation in the immunohistochemical diluent (0.1 M PBS containing 0.25% carageenan lambda and 0.5% Triton X-100), slides were incubated in the immunohistochemical diluent containing a polyclonal rabbit antibody against Fos (SC-52; 1:8,000; Santa Cruz Biotechnology, Santa Cruz, CA) 40 – 50 hours at 4° C. Slides were then incubated in a solution containing the appropriate biotinylated secondary antibody (1:500 goat anti-rabbit; Vector Laboratories, Burlingame, CA) for 2 hours and then with the ABC complex (Vectastain Elite ABC peroxidase kit; Vector Laboratories, Burlingame, CA) for 2 hours. A peroxidase reaction was then performed using the chromagen 3,3′-diaminobenzidene (DAB) and hydrogen peroxide to form a brown precipitation product. Adjacent Fos labeled slides and SBA-HRP slides were examined together (see Figure 1 for example of complete VNX lesion). Additionally, Fos labeled cells were counted and calculated per mm2 using a Zeiss Z1 Axioimager microscope and AxioVision 4.7 software in both the main olfactory granular cell layer (immediately lateral to the internal plexiform layer of the olfactory bulb) and accessory olfactory bulb (small cell group ventral to the lateral orbital cortex) near 5.20 mm anterior from bregma (see Figure 2) according to Paxinos and Watson (1998) [35]. These regions correspond to areas previously reported to contain Fos-labeled cells in response to cat odors [31].
Figure 1.
Examples of SBA-HRP staining and Fos immunohistochemistry verification for complete VNX and sham surgery. Note the dark staining in sham-operated rats in the accessory olfactory bulb, which is nearly completely absent in the VNX rats.
Figure 2.
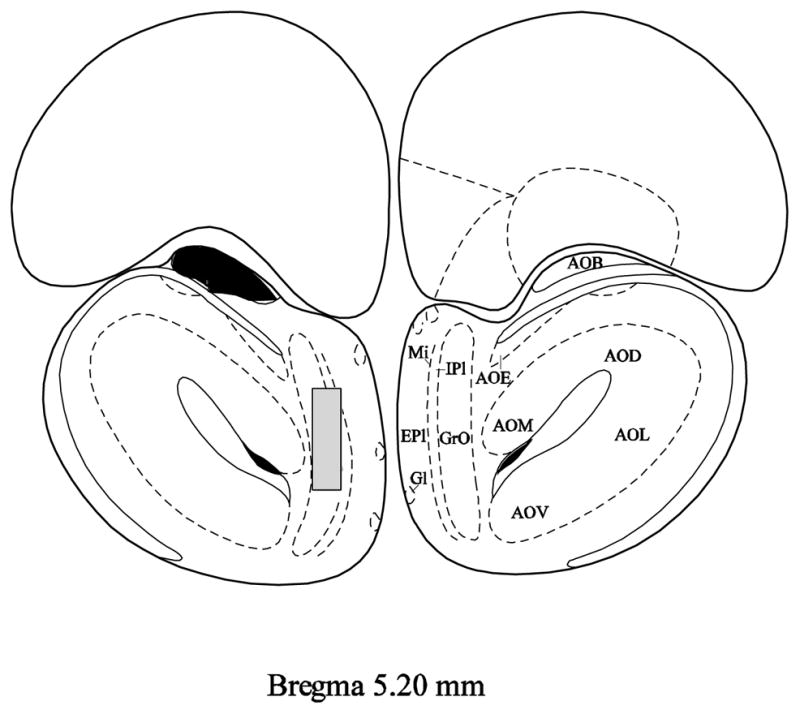
Depiction of areas of Fos quantification in the accessory olfactory bulb (AOB; dark filled area) and main olfactory bulb granular cell layer (GrO; gray filled rectangle) as shown in Figure 4 (bregma 5.20 mm) from Paxinos and Watson’s The Rat Brain In Stereotaxic Coordinates, 4th Edition. And whereas only the left side is depicted here with the right side displaying anatomical nomenclature, bilateral cell counts were performed. AOB = accessory olfactory bulb, AOD = dorsal anterior olfactory nucleus, AOE = external anterior olfactory nucleus, AOL = lateral anterior olfactory nucleus, AOM = medial anterior olfactory nucleus, AOV = ventral anterior olfactory nucleus, EPl = external plexiform layer of olfactory bulb, Gl = glomerular layer of olfactory bulb, GrO = granular cell layer of olfactory bulb, IPl = internal plexiform layer of olfactory bulb, Mi = mitral cell layer of olfactory bulb.
2.3. Experiment 3
Twenty-eight male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 200 – 250 g at the time of arrival to the colony were used. Experiment 3 was carried out exactly as described in Experiment 2 with one exception; all rats (VNX and sham-operated controls) were additionally treated with ZnSO4 before testing, as described in Experiment 1. Again the additional no surgery control group (from experiments 1 and 2) was included to visually compared normal defensive withdrawal behavior without odor influences to the experimental groups behavior.
3. Results
3.1. Experiment 1
To test for anosmia, a cookie-finding task was employed. Saline treated rats (n = 18) found the buried cookie in an average of 81.667 seconds (+/− 11.864 SEM) and all of these rats found the cookie in less than 3.5 minutes. ZnSO4 treated rats that found the cookie before the conclusion of the 10-minute (600 sec) test were excluded from the study.
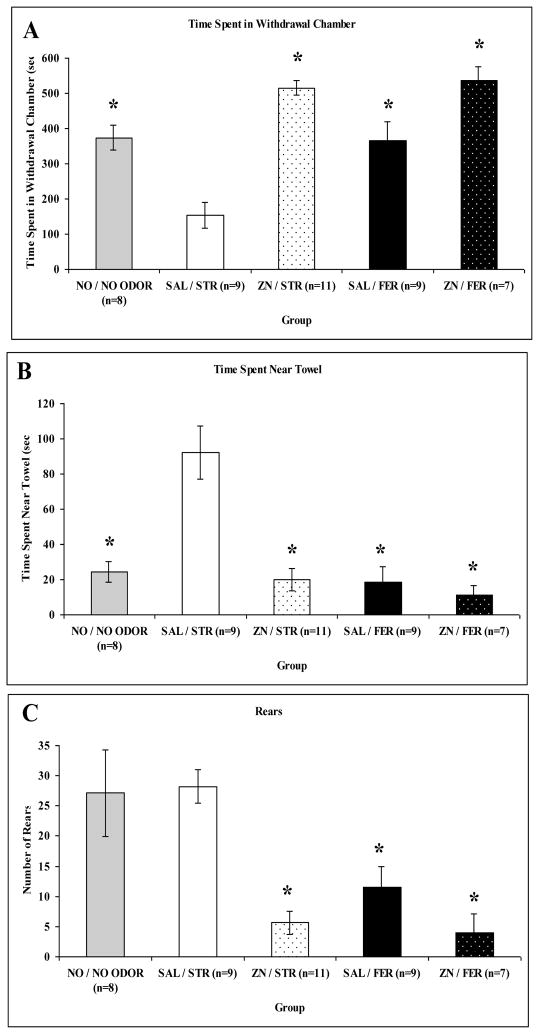
Defensive behavioral responses to ferret or strawberry odors were examined following ZnSO4 or saline treatments. These responses were also examined in naïve rats in the defensive withdrawal paradigm without any odor stimulus to determine normal responses. An overall MANOVA on the behaviors revealed significant main effects of odor: F(3,37) = 6.771, p = 0.001, treatment: F(3,37) = 19.165, p = 0.0001, and an interaction, F(3,37) = 4.160, p = 0.012. Individual ANOVAs uncovered an odor effect on time spent in chamber: F(1,39) = 10.048, p = 0.003, time spent near towel: F(1,39) = 19.209, p = 0.0001, and rears: F(1,39) = 5.601, p = 0.023. The same ANOVAs also uncovered treatment effects for time spent in chamber: F(1,39) = 51.348, p < 0.0001, time spent near towel: F(1,39) = 17.871, p = 0.0001, and rears: F(1,39) = 15.191, p = 0.0001. And, significant interaction effects were found for time spent in chamber: F(1,39) = 6.624, p < 0.014 and time spent near towel: F(1,39) = 11.814, p = 0.001. Post-hoc analyses revealed that saline treated rats exposed to strawberry odor spent less time in the withdrawal chamber than ZnSO4 treated, ferret odor, and no odor exposed rats (Bonferroni, p < 0.05), see Figure 3 panel A. Saline treated rats exposed to strawberry odor also spent significantly more time near the towel and reared more than the other groups (Bonferroni, p < 0.05), see Figure 3 panels B and C.
Figure 3.
Graphs showing mean (+/− SEM) behavior of rats treated with saline (SAL) or ZnSO4 (ZN) in the defensive withdrawal apparatus during exposure to ferret odor (FER) or strawberry odor (STR) for 10 min. A: Time spent in withdrawal hide box (in seconds). B: Time spent in area near towel stimulus (in seconds). C: Number of rears. Asterisks indicate a significant difference from SAL/STR group (p < 0.05).
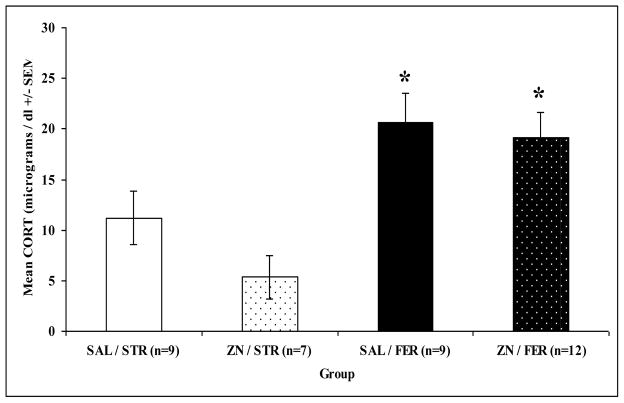
Plasma corticosterone responses to ferret or strawberry odors were examined following ZnSO4 or saline treatments. An overall univariate ANOVA on plasma corticosterone levels revealed significant main effects of odor: F(1,33) = 18.450, p = 0.0001 but no treatment effect: F(1,33) = 1.847, p = 0.183, or interaction, F(1,33) = 0.654, p = 0.424. Figure 4 shows that strawberry odor exposed rats had lower plasma corticosterone levels compared to ferret odor exposed rats, regardless of ZnSO4 or saline treatment (Bonferroni, p < 0.05).
Figure 4.
Mean plasma corticosterone (CORT; +/− SEM) for saline treated (SAL) or ZnSO4 treated (ZN) rats exposed to strawberry (STR) or ferret (FER) odor for 30 min in their home cages. Asterisks indicate a significant difference from ZN/STR group (p < 0.05).
3.2. Experiment 2
The complete removal of the vomeronasal organs were verified using SBA-HRP staining and additionally by examining the lack of ferret odor induced Fos protein expression in the AOB. Table 1 displays the means (+/− SEM) cell counts of VNX and sham rats from experiments 2 and 3. The brains of randomly chosen sham-operated controls were collected, therefore the n’s of the Fos counts do not correspond precisely with the behavioral and corticosterone results, but provide a close approximation to the Fos results for that group. VNX rats displayed significantly less ferret odor induced Fos expression in the AOB compared to the sham operated control rats: t(25) = 6.481, p < 0.00001. Sixteen out of 21 VNX rats were deemed complete VNX and used in the statistical analyses.
Table 1.
Mean Fos cell counts / mm2 (+/− SEM) in sham surgery (Sham) or vomeronasal organ removed (VNX) rats in the accessory olfactory bulb glomerular (AOB) and main olfactory bulb granular cell layers (MOB).
| AOB | MOB | |||
|---|---|---|---|---|
| SHAM | 399.789 (+/− 54.175) | n = 10 | 3445.988 (+/− 326.237) | n = 9 |
| VNX | 91.607 (+/− 18.371)* | n = 17 | 2689.815 (+/− 289.208) | n = 12 |
Significantly different from SHAM; t(25) = 6.481, p < 0.00001
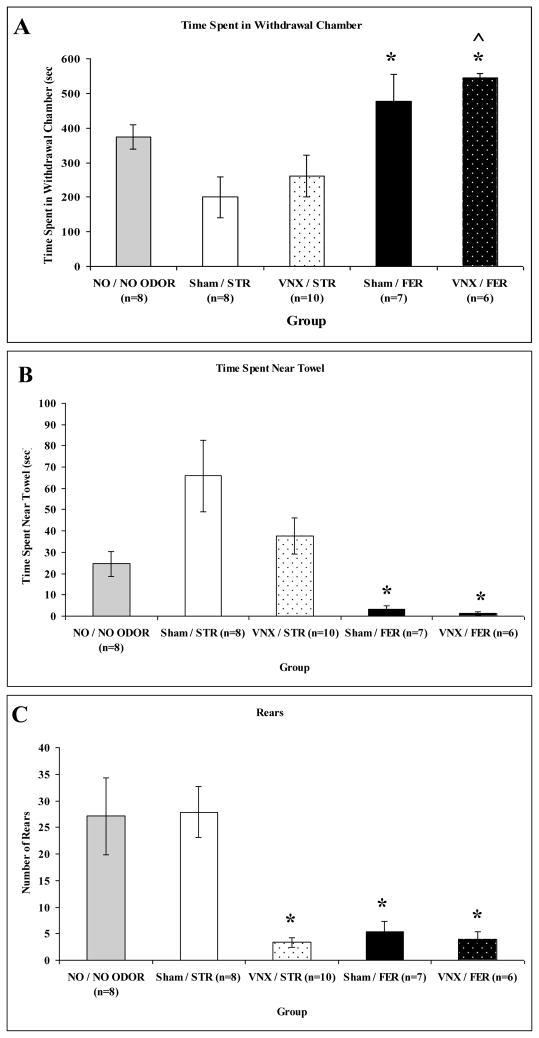
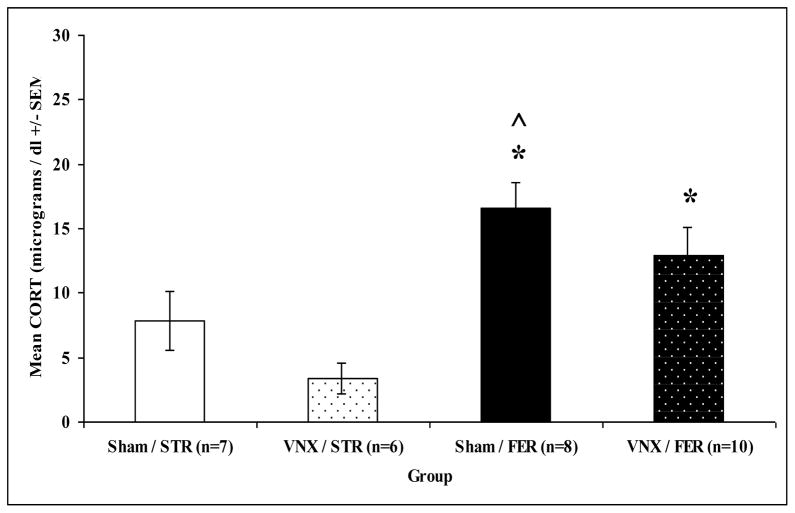
In Experiment 2, defensive behavioral and plasma corticosterone responses to ferret odor or strawberry odor were examined following vomeronasal organ removal or sham surgery to determine the effects of accessory olfactory disruption. An overall MANOVA revealed a significant main effect of odor (ferret or strawberry): F(5,23) = 11.051, p = 0.0001 but not treatment (VNX or sham surgery): F(5,23) = 1.847, p = 0.143 or interaction, F(5,23) = 1.372, p = 0.271. Additional significant ANOVAs on odor were found for time spent in chamber: F(1,27) = 20.461, p = 0.0001, time spent near towel: F(1,27) = 21.986, p = 0.0001, rears: F(1,27) = 21.476, p = 0.0001, and corticosterone response: F(1,27) = 18.750, p = 0.0001. Ferret odor exposed rats (both VNX and sham surgery) compared to strawberry odor exposed rats spent more time in the withdrawal chamber (Fig. 5A), spent less time with the towel (Fig. 5B), and reared less (Bonferroni, p’s < 0.05; Fig. 5C). Likewise, ferret odor exposed rats displayed higher plasma corticosterone responses than strawberry odor exposed rats, regardless of surgery (Bonferroni, p < 0.05), as shown in Figure 6.
Figure 5.
Graphs showing mean (+/− SEM) behavior of sham surgery (Sham) or vomeronasal organ removed (VNX) rats in the defensive withdrawal apparatus during exposure to ferret odor (FER) or strawberry odor (STR) for 10 min. A: Time spent in withdrawal hide box (in seconds). B: Time spent in area near towel stimulus (in seconds). C: Number of rears. Asterisks indicated a significant difference from Sham/STR group (p < 0.05). ^ indicate a significant difference from VNX/STR group (p < 0.05). Note that the NO / NO ODOR group has been reproduced from Figure 2 for comparison purposes.
Figure 6.
Mean plasma corticosterone (CORT; +/− SEM) for sham surgery (Sham) or vomeronasal organ removed (VNX) rats exposed to strawberry (STR) or ferret (FER) odor for 30 min in their home cages. Asterisks indicated a significant difference from VNX/STR group (p < 0.05). ^ indicate a significant difference from Sham/STR group (p < 0.05).
3.3. Experiment 3
Complete vomeronasal organ removal was verified using SBA-HRP staining and significant reduction of ferret odor induced Fos protein expression in the AOB was observed (means (+/− SEM) cell counts shown in Table 1). Nine out of 16 VNX were deemed complete VNX and used in the statistical analyses.
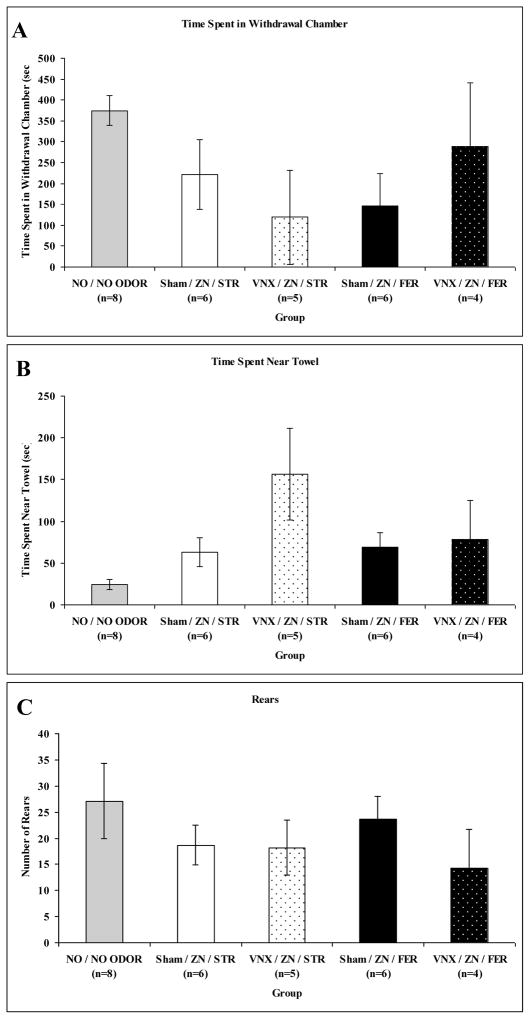
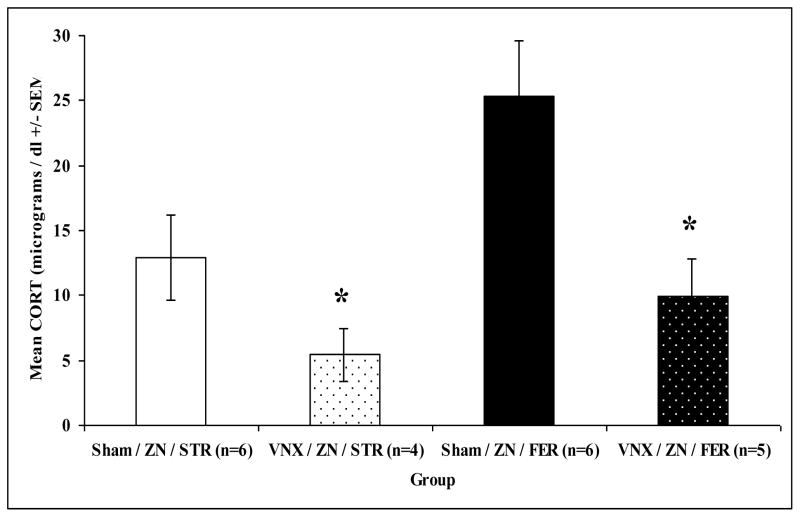
In this experiment, both accessory and main olfaction pathways were disrupted by combining the vomeronasal organ surgery and ZnSO4 treatment in some rats. In this experiment, an overall MANOVA on behaviors and plasma corticosterone levels revealed a significant main effect of treatment (Zn/VNX or Zn/sham surgery): F(13,5) = 9.286, p = 0.011 but not odor (ferret or strawberry): F(13,5) = 0.515, p = 0.845, or interaction effects, F(13,5) = 1.354, p = 0.392. Significant individual ANOVAs for odor and treatment were only found for the corticosterone response: F(1,17) = 10.437, p = 0.005 and F(1,17) = 5.703, p = 0.029, respectively (see Figure 7 for behavior). Combined VNX and ZnSO4 treatment significantly decreased the corticosterone response to ferret odor (Bonferroni, p = 0.033; Fig. 8).
Figure 7.
Graphs showing mean (+/− SEM) behavior of sham surgery (Sham) or vomeronasal organ removed (VNX) rats that were also treated with ZnSO4 (ZN) in the defensive withdrawal apparatus during exposure to ferret odor (FER) or strawberry odor (STR) for 10 min. A: Time spent in withdrawal hide box (in seconds). B: Time spent in area near towel stimulus (in seconds). C: Number of rears. Note that the NO / NO ODOR group has been reproduced from Figure 2 for comparison purposes.
Figure 8.
Mean plasma corticosterone (CORT; +/− SEM) for sham surgery (Sham) or vomeronasal organ removed (VNX) rats that were also treated with ZnSO4 (ZN) and exposed to strawberry (STR) or ferret (FER) odor for 30 min in their home cages. Asterisks indicate a significant difference from Sham/ZN/FER group (p < 0.05).
4. Discussion
These studies were designed to identify the olfactory system/s that is/are necessary for the detection of predator odors that leads to the ferret odor-evoked HPA axis and behavioral responses in rats. Experiment 1 utilized intranasal ZnSO4 treatment to render the rats anosmic. When used properly, ZnSO4 treatment leads to a temporary, about 3 to 5 days, but complete degeneration of the olfactory epithelium [4,5,30,43,47]. A cookie finding test was utilized before behavioral and endocrine response testing to ensure that the rats were anosmic. Ferret odor exposed saline treated rats compared to saline treated rats exposed to strawberry odor spent more time in the defensive withdrawal chamber (hide box) and less time with the towel stimulus, as expected. They also reared (front paws leaving floor) less, which may be interpreted as a reduction in exploration or locomotion. But ZnSO4 treated rats that were tested with a strawberry odor towel also spent significantly more time in the hide box and less time near the towel and rearing. Because normal rats placed in the defensive withdrawal paradigm without any odor stimulus (NO/NO ODOR group) also spend more time in the withdrawal chamber and less time in the area where an odor stimulus would have been placed, this leads us to believe that anosmia reduced interest in the environmental stimulus (strawberry odor towel) rather than enhanced basal defensive behaviors in the ZnSO4 treated rats. The home cage exposure paradigm is used to determine the neuroendocrine response to towels that cannot be avoided because of the limited space in the home cage. When exposed to strawberry odor towels for 30 min, saline and ZnSO4 treated rats exhibit relatively low levels of plasma corticosterone, as previously seen in normal rats [25,26]. Plasma corticosterone is routinely used as an indicator of hypothalamo-pituitary-adrenocortical (HPA) axis activation and therefore, an important component of the stress response [15,21,42]. Ferret odor compared to strawberry odor exposure, in both saline and ZnSO4 treated rats, led to significantly higher levels of plasma corticosterone. This suggests that main olfactory disruption does not block the ferret odor induced endocrine stress response.
Experiment 2 tested the effects of vomeronasal organ removal (VNX) on ferret odor induced stress responses. The vomeronasal sensory neurons, originating in the vomeronasal organ, project to the accessory olfactory bulb (AOB), the neurons of which then relay information to the limbic areas of the brain [23,33,37,40,46]. The traditional view that vomeronasal/accessory olfaction is solely involved with the detection of pheromones has been debated in recent years (see review of literature [38]). To test the role of this system on responses to predator odor, the vomeronasal organ was removed in rats. Rats that were exposed to ferret odor, both sham operated and VNX, spent significantly more time in the hide box and less time near the towel stimulus than strawberry odor exposed rats. Both sham operated and VNX rats that were exposed to ferret odor spent less time near the towel stimulus than naïve rats (NO/NO ODOR group) spent in the area where a towel stimulus would be placed, suggesting that the VNX and sham rats actively avoided the ferret towel. Likewise, VNX and sham operated rats exposed to ferret odor in their home cages also displayed high plasma corticosterone responses compared to strawberry odor exposed rats. These data suggest that disruption of the accessory olfactory system does not independently block the ferret odor induced behavioral and endocrine stress responses.
Because the previous studies suggested that neither main olfactory nor accessory olfactory disruption independently blocked the ferret odor induced stress responses, it was deemed necessary to show that the combined blockade of these systems would block the responses. Not surprisingly, blockade of both systems severely altered behavior. There were no differences between groups (sham or VNX surgery + ZnSO4 treatment) for either odor (strawberry or ferret) on any of the behavioral measures. Figure 7 shows that the behaviors examined in these animals were highly variable. Zinc sulfate treatment, depending on procedure and dose, has been shown to have effects on locomotor activity and rearing [29,43]. The combination of both VNX and ZnSO4 treatment has not been, to our knowledge, previously used. The combination of surgery (sham or VNX) then zinc sulfate treatment altered behavior more than zinc sulfate treatment alone. Zinc sulfate treated rats that were exposed to a strawberry towel (Exp. 1) spent more time in the withdrawal chamber, less time near the towel, and displayed fewer rears compared to the sham operated zinc sulfate treated rats exposed to a strawberry towel (Exp. 3). Likewise, the sham operated zinc sulfate treated rats that were exposed to ferret towels (Exp. 3) exhibited similar differences in behavior from the zinc sulfate treated rats exposed to ferret towels from Exp. 1. These procedures led to behavioral effects independent of the odor and different from the respective single procedures alone. The augmented corticosterone response to ferret odor compared to strawberry odor in the home cage exposure, however, was blocked by the combined disruption of both systems. This suggests at the very least that the neuroendocrine response to predator odor requires either both systems or each system can independently compensate for the loss of the other.
These data strongly support the idea that the roles of the main and accessory olfactory systems are much more complex than previously thought [17,20,22,38,41,45,51]. The two systems may jointly contribute to the effects, or perhaps these systems have overlapping roles. Clearly, this study reveals that both olfactory systems can mediate stress responses to predator odor.
Acknowledgments
We would like to thank Diego Restrepo of the University of Colorado in Denver for teaching us the vomeronasal organ removal (VNX) surgery and the Mile High Ferret Club for providing the ferret odor towels. These studies were supported by a grant from the National Institute of Mental Health, R01 MH065327 (SC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adamec R. Transmitter systems involved in neuroplasticity underlying increased anxiety and defense following traumatic stress. Neurosci Biobehav Rev. 1997;21:755–765. doi: 10.1016/s0149-7634(96)00055-3. [DOI] [PubMed] [Google Scholar]
- 2.Adamec R, Kent P, Anisman H, Shallow T, Merali Z. Neural plasticity, neuropeptides and anxiety in animals: implications for understanding and treating affective disorder following traumatic stress in humans. Neurosci Biobehav Rev. 1998;23:301–318. doi: 10.1016/s0149-7634(98)00032-3. [DOI] [PubMed] [Google Scholar]
- 3.Adamec R, Muir C, Grimes M, Pearcey K. Involvement of noradrenergic and corticoid receptors in the consolidation of the lasting anxiogenic effects of predator stress. Behav Brain Res. 2007;179:192–207. doi: 10.1016/j.bbr.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Alberts JR. Producing and interpreting experimental olfactory deficits. Physiol Behav. 1974;12:657–670. doi: 10.1016/0031-9384(74)90216-9. [DOI] [PubMed] [Google Scholar]
- 5.Alberts JR, Galef BG. Acute anosmia in the rat: a behavioral test of a peripherally-induced olfactory deficit. Physiol Behav. 1971;6:619–621. doi: 10.1016/0031-9384(71)90218-6. [DOI] [PubMed] [Google Scholar]
- 6.Blanchard DC, Blanchard RJ. Cocaine potentiates defensive behaviors related to fear and anxiety. Neurosci Biobehav Rev. 1999;23:981–991. doi: 10.1016/s0149-7634(99)00031-7. [DOI] [PubMed] [Google Scholar]
- 7.Blanchard DC, Li CI, Hubbard D, Markham CM, Yang M, Takahashi LK, Blanchard RJ. Dorsal premammillary nucleus differentially modulates defensive behaviors induced by different threat stimuli in rats. Neurosci Lett. 2003;345:145–148. doi: 10.1016/s0304-3940(03)00415-4. [DOI] [PubMed] [Google Scholar]
- 8.Blanchard RJ, Nikulina JN, Sakai RR, McKittrick C, McEwen B, Blanchard DC. Behavioral and endocrine change following chronic predatory stress. Physiol Behav. 1998;63:561–569. doi: 10.1016/s0031-9384(97)00508-8. [DOI] [PubMed] [Google Scholar]
- 9.Campeau S, Nyhuis TJ, Sasse SK, Day HE, Masini CV. Acute and chronic effects of ferret odor exposure in Sprague-Dawley rats. Neuroscience and Biobehav Rev. 2008;32:1277–1286. doi: 10.1016/j.neubiorev.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canteras NS, Goto M. Fos-like immunoreactivity in the periaqueductal gray of rats exposed to a natural predator. Neuroreport. 1999;10:413–418. doi: 10.1097/00001756-199902050-00037. [DOI] [PubMed] [Google Scholar]
- 11.Day HEW, Masini CV, Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5,-dihydro-2,4,5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res. 2004;1025:139–151. doi: 10.1016/j.brainres.2004.07.079. [DOI] [PubMed] [Google Scholar]
- 12.Dielenberg RA, Arnold JC, McGregor IS. Low-dose midazolam attenuates predatory odor avoidance in rats. Pharmacol Biochem Behav. 1999;62:197–201. doi: 10.1016/s0091-3057(98)00064-1. [DOI] [PubMed] [Google Scholar]
- 13.Dielenberg RA, Carrive P, McGregor IS. The cardiovascular and behavioral response to cat odor in rats: unconditioned and conditioned effects. Brain Res. 2001;897:228–237. doi: 10.1016/s0006-8993(01)02227-2. [DOI] [PubMed] [Google Scholar]
- 14.Dielenberg RA, Leman S, Carrive P. Effect of dorsal periaqueductal gray lesions on cardiovascular and behavioral responses to cat odor exposures in rats. Behav Brain Res. 2004;153:487–496. doi: 10.1016/j.bbr.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Endroczi E. Limbic system, pituitary-adrenal axis, and adaptive behavior. In: Selye H, editor. Selye’s Guide to Stress Research. Vol. 2. Scarborough, Ontario: Van Nostrand Reinhold Co. Inc.; 1983. pp. 249–270. [Google Scholar]
- 16.File SE, Zangrossi H, Sanders FL, Mabbutt PS. Dissociation between behavioral and corticosterone responses on repeated exposures to cat odor. Physiol Behav. 1993;54:1109–1111. doi: 10.1016/0031-9384(93)90333-b. [DOI] [PubMed] [Google Scholar]
- 17.Johnston RE. Pheromones, the vomeronasal system, and communication. Ann N Y Acad Sci. 1998;855:333–348. doi: 10.1111/j.1749-6632.1998.tb10592.x. [DOI] [PubMed] [Google Scholar]
- 18.Keverne EB. The vomeronasal organ. Science. 1999;286:716–720. doi: 10.1126/science.286.5440.716. [DOI] [PubMed] [Google Scholar]
- 19.Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. I. Efferents of the ‘vomeronasal amygdala’. J Comp Neurol. 1981;197:81–98. doi: 10.1002/cne.901970107. [DOI] [PubMed] [Google Scholar]
- 20.Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, Mori K, Sakano H. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–510. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- 21.Levine S. Influence of psychological variables on the activity of the hypothalamic-pituitary-adrenal axis. Eur J Pharmacol. 2000;405:149–160. doi: 10.1016/s0014-2999(00)00548-3. [DOI] [PubMed] [Google Scholar]
- 22.Lin W, Arellan J, Slotnick B, Restrepo D. Odors detected by mice deficient in cyclic nucleotide-gated channel subunit A2 stimulate the main olfactory system. J Neurosci. 2004;24:3703–3710. doi: 10.1523/JNEUROSCI.0188-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez-Marcos A, Halpern M. Differential projections from the anterior and posterior divisions of the accessory olfactory bulb to the medial amygdala in the opossum, Monodelphis domestica. Eur J Neurosci. 1999;11:3789–3799. doi: 10.1046/j.1460-9568.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- 24.Masini CV. Doctoral Dissertation. University of Georgia; Athens, GA: 2002. Depression, drug abuse, and dopamine: in vivo microdialysis of dopamine in ventral striatum and self-administration of d-amphetamine in the olfactory bulbectomized rat. [Google Scholar]
- 25.Masini CV, Sauer S, Campeau S. Ferret odor as a processive stress model in rats: neurochemical, behavioral, and endocrine evidence. Behav Neurosci. 2005;119:280–292. doi: 10.1037/0735-7044.119.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masini CV, Sauer S, White J, Day HEW, Campeau S. Non-associative defensive responses of rats to ferret odor. Physiol Behav. 2006;87:72–78. doi: 10.1016/j.physbeh.2005.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masini CV, Nyhuis TJ, Day HEW, Campeau S. Primary Olfactory Areas Mediating Ferret Odor-Induced Stress Responses in Rats. Abstract from poster presentation, 15th Annual International Symposium on Olfaction and Taste; San Francisco, CA. July 21 – 26, 2008. [Google Scholar]
- 28.Masini CV, Nyhuis TJ, Sasse SK, Day HEW, Campeau S. Accessory and main olfactory areas do not independently mediate predator odor-induced stress responses in rats. 38th Annual Meeting for Society for Neuroscience; Washington D.C.. November 15–19, 2007. [Google Scholar]
- 29.Mayer AD, Rosenblatt JS. Effects of intranasal zinc sulfate on open field and maternal behavior in female rats. Physiol Behav. 1977;18:101–109. doi: 10.1016/0031-9384(77)90100-7. [DOI] [PubMed] [Google Scholar]
- 30.McBride K, Slotnick B, Margolis FL. Does intranasal application of zinc sulfate produce anosmia in the mouse? An olfactometric and anatomical study. Chem Senses. 2003;28:659–670. doi: 10.1093/chemse/bjg053. [DOI] [PubMed] [Google Scholar]
- 31.McGregor IS, Hargreaves GA, Apfelbach R, Hunt GE. Neural correlates of cat odor-induced anxiety in rats: region-specific effects of the benzodiazepine midazolam. J Neurosci. 2004;24:4134–4144. doi: 10.1523/JNEUROSCI.0187-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGregor IS, Schrama L, Ambermoon P, Dielenberg RA. Not all ‘predator odours’ are equal: cat odour but not 2,4,5 trimethylthiazoline (TMT; fox odour) elicits specific defensive behaviours in rats. Behav Brain Res. 2002;129:1–16. doi: 10.1016/s0166-4328(01)00324-2. [DOI] [PubMed] [Google Scholar]
- 33.Mohedano-Moriano A, Pro-Sistiaga P, Ubeda-Banon I, Crespo C, Insausti R, Martinez-Marcos A. Segregated pathways to the vomeronasal amygdala: differential projections from anterior and posterior divisions of the accessory olfactory bulb. Eur J Neurosci. 2007;25:2065–2080. doi: 10.1111/j.1460-9568.2007.05472.x. [DOI] [PubMed] [Google Scholar]
- 34.Mayer AD, Rosenblatt JS. Peripheral olfactory deafferentiation of the primary olfactory system in rats using ZnSO4 nasal spray with special reference to maternal behavior. Physiol Behav. 1993;53:589–592. doi: 10.1016/0031-9384(93)90157-b. [DOI] [PubMed] [Google Scholar]
- 35.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego, CA: 2005. [Google Scholar]
- 36.Perrot-Sinal TS, Ossenkopp KP, Kavaliers M. Brief predator odour exposure activates the HPA axis independent of locomotor changes. Neuroreport. 1999;10:775–780. doi: 10.1097/00001756-199903170-00021. [DOI] [PubMed] [Google Scholar]
- 37.Pro-Sistiaga P, Mohedano-Moriano A, Ubeda-Banon I, Arroyo-Jimenez M, Marcos P, Artacho-Perula E, Crespo C, Insausti R, Martinez-Marcos A. Convergence of olfactory and vomeronasal projections in the rat basal telencephalon. J Comp Neurol. 2007;504:346–362. doi: 10.1002/cne.21455. [DOI] [PubMed] [Google Scholar]
- 38.Restrepo D, Arellano J, Oliva AM, Schaefer ML, Lin W. Emerging views on the distinct but related roles of the main and accessory olfactory systems in responsiveness to chemosensory signals in mice. Horm Behav. 2004;46:247–256. doi: 10.1016/j.yhbeh.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez I. Nosing into pheromone detectors. Nat Neurosci. 2003;6:438–440. doi: 10.1038/nn0503-438. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez I, Feinstein P, Mombaerts P. Variable patterns of axonal projections of sensory neurons in the mouse vomeronasal system. Cell. 1999;97:199–208. doi: 10.1016/s0092-8674(00)80730-8. [DOI] [PubMed] [Google Scholar]
- 41.Sam M, Vora S, Malnic B, Ma W, Novotny MV, Buck LB. Odorants may arouse instinctive behaviours. Neuropharmacology. 2001;412:142. doi: 10.1038/35084137. [DOI] [PubMed] [Google Scholar]
- 42.Selye H. The Stress of Life. New York: McGraw-Hill Book Company, Inc.; 1956. [Google Scholar]
- 43.Sieck MH, Baumbach HD. Differential effects of peripheral and central anosmia producing techniques on spontaneous behavioral patterns. Physiol Behav. 1974;16:407–425. doi: 10.1016/0031-9384(74)90096-1. [DOI] [PubMed] [Google Scholar]
- 44.Slotnick B, Glover P, Bodyak N. Does intranasal application of zinc sulfate produce anosmia in the rat? Behav Neurosci. 2000;114:814–829. [PubMed] [Google Scholar]
- 45.Trinh K, Storm DR. Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nat Neurosci. 2003;6:519–525. doi: 10.1038/nn1039. [DOI] [PubMed] [Google Scholar]
- 46.Ubeda-Banon I, Novejarque A, Mohedano-Moriano A, Pro-Sistiaga P, Insausti R, Martinez-Garcia F, Lanuza E, Martinez-Marcos A. Vomeronasal inputs to the rodent ventral striatum. Brain Res Bull. 2008;75:467–473. doi: 10.1016/j.brainresbull.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 47.Wang Z, Sindreu CB, Li V, Nudelman A, Chan G, Storm DR. Pheromone detection in male mice depends on signaling through type 3 adenylyl cyclase in the main olfactory epithelium. J Neurosci. 2006;26:7375–7379. doi: 10.1523/JNEUROSCI.1967-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watson RT, Mathis A, Thompson R. Influence of physical stress, distress cues, and predator kairomones on the foraging behavior of Ozarkzigzag salamanders, Plethodon angusticlavius. Behav Process. 1980;65:201–209. doi: 10.1016/j.beproc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Weldon PJ. In defense of “kairomone” as a class of chemical releasing stimuli. J Chem Ecol. 1980;6:719–725. [Google Scholar]
- 50.Wilson EO, Bossert WH. Chemical communication among animals. In: Houck LD, Drickamer LC, editors. Foundations of Animal Behavior. Classic Papers with Commentaries. Chicago: University of Chicago Press; 1963. pp. 602–645. 1996. [Google Scholar]
- 51.Xu F, Schaefer M, Kida I, Schafer J, Liu N, Rothman DL, Hyder F, Restrepo D, Shepherd GM. Simultaneous activation of mouse main and accessory olfactory bulbs by odors or pheromones. J Comp Neurol. 2005;489:491–500. doi: 10.1002/cne.20652. [DOI] [PubMed] [Google Scholar]
- 52.Wysocki CJ, Wysocki LM. Surgical removal of the mammalian vomeronasal organ and its verification. In: Spielman AI, Brand JG, editors. Experimental Cell Biology of Taste and Olfaction. Boca Raton: CRC Press, Inc.; 1995. pp. 49–57. [Google Scholar]