Abstract
Light chain amyloidosis (AL) is associated with high mortality. The aim was to identify echocardiographic parameters that predict AL long-term mortality.
Methods/Results
42 biopsy-proven AL subjects (43% females; 61±12 years) had echocardiography and followed 29±16 (median 29.4) months. Standard echocardiographic and clinical parameters and heart failure (HF) class were tested using univariate/multivariable Cox proportional hazard regression analyses to identify markers of mortality. 23 subjects died with 1-year mortality of 44%. Univariate predictors of mortality were HF class (p<0.001), left ventricular systolic ejection time (ET, p=0.002), alkaline phosphatase (p<0.001), aspartate and alanine aminotransferase (p=0.003 each). On multivariable analysis, only HF Class (hazards ratio, 95% confidence interval, p-value: 4.86, 1.58-14.9, p=0.006), ET (10 ms increase, 0.87, 0.78-0.97, p=0.01) and alkaline phosphatase (10 U/L increase, 1.04, 1.01-1.06, p=0.01) were prognostic. ET≤240 ms had sensitivity/specificity of 61/90% in predicting 1-year mortality and 73/90% in predicting 1-year cardiac mortality.
Conclusions
AL amyloidosis was associated with high long-term mortality. Among echocardiographic and clinical parameters, only ET and alkaline phosphatase had incremental value to HF class in predicting mortality. This may be useful to identify high-risk patients.
Light chain or primary amyloidosis (AL) is a plasma cell dyscrasia with monoclonal production and extracellular deposition of immunoglobulin light chains in multiple organs 1-3. The accumulation of fibrillar amyloid deposits in the heart, kidneys, liver and nervous system leads to organ failure and death. It is associated with poor prognosis with median survival reduced to 4 months in patients with heart failure 4, 5. The early identification of high risk patients is important for risk stratification. Echocardiography is an important noninvasive tool to assess cardiac involvement with classic findings of ventricular thickening and diastolic dysfunction with often preserved left ventricular ejection fraction 6-8. However, early systolic dysfunction is increasingly recognized with more sensitive techniques such as strain imaging 9, 10 and regional systolic dyssynchrony or hypersynchrony has recently been reported in AL subjects 11, 12. Prior retrospective studies of AL patients demonstrated prognostic value for echocardiography-derived abnormal deceleration time, ratio of peak early mitral inflow to late mitral inflow velocity (E/A ratio) as well as the Tei index that incorporates ejection time and isovolumetric relaxation/contraction time 13, 14. Recently, with a median follow up of 13 months, Bellavia and colleagues reported that the most important echocardiographic predictor of mortality was reduced left ventricular systolic ejection time (ET) although the underlying mechanistic basis behind the increased risk remains unknown 11. We hypothesized that standard echocardiographic structural, systolic and diastolic measures can identify AL patients at risk of dying on long-term follow up. The aim of our study was to determine echocardiographic indices that have independent additive value to presenting heart failure class in predicting long-term mortality in AL subjects.
MATERIALS AND METHODS
Patient Population
Between May 2005 and June 2009, 42 consecutive subjects with biopsy-proven diagnosis of AL amyloidosis and elevated kappa or lambda immunoglobulin light chains on serum or urine seen at our institution were included. The study was approved by the local Institutional Review Board (IRB). Thirty seven subjects gave informed consent as part of a longitudinal study of AL amyloidosis. Five subjects suspected of AL amyloidosis undergoing diagnostic workup expired before recruitment to the study and waiver of consent authorization was obtained from the IRB for data collection. All five had biopsy evidence of amyloidosis and elevated light chains on serum or urine. They were included in the analysis to capture all consecutive patients with AL seen at our institution. The survival status of subjects was verified from hospital or outpatient clinic records as well as by Social Security Death Index 29±16 months (median 29.4 months) from the echo examination.
Echocardiography
Routine clinical echocardiography was performed using General Electric Vivid 7 (Waukesha WI) or Philips IE 33, 7500 and 5500 (Bothell WA) echo using adult cardiac probe. The diastolic anteroseptal and inferolateral thickness were measured from parasternal long axis view at the level of the tips of the mitral leaflets. Left ventricular mass was derived using the formula: 0.8[1.04{(It+LVID+At)3-LVID3}]+0.6 15 and indexed to body surface area. Left atrial volume index was calculated using area length method in the four and two chamber views 16. Ejection fraction was obtained using area length method from the 4 chamber view 16. ET was measured as the duration of flow using standard pulsed wave Doppler with sample volume in the left ventricular outflow tract just below the aortic valve leaflets 11. ET corrected to cycle length was obtained by dividing ET with cycle length. Preejection period (PEP) was measured as the time interval between R wave on ECG and beginning of left ventricular outflow tract flow. The ratio of PEP to ET was also obtained. Diastolic function was evaluated using pulsed Doppler of mitral inflow velocity measuring peak early mitral inflow velocity (E), peak late mitral inflow velocity during atrial contraction (A), E/A ratio and time from peak early mitral inflow velocity (E) to zero baseline (deceleration time, DT). Pulsed-wave tissue Doppler was performed at the lateral mitral annulus using a sample volume gate of 0.4 cm in the apical four chamber view to measure the early annulus velocity E’. The ratio of peak early mitral inflow velocity (E) and mitral annular velocity (E’), or E/E’ was calculated. Mitral annular peak systolic velocity (S’) was also obtained. The images were loaded into an Xcelera workstation (version 1.2 L4-1.2.4 140, Philips Medical, Bothell WA) for offline analyses. It is standard clinical practice in our laboratory to obtain at least 3 Doppler waveforms for patients in sinus rhythm and 5-10 in patients with atrial fibrillation. For the purpose of this study, Doppler measurements were performed in 1 representative beat (except in 4 patients with atrial fibrillation where 5 measurements were averaged). To determine consistency of Doppler measurements, 15 AL patients in sinus rhythm were randomly selected and reanalyzed. For left ventricular ejection time, the coefficients of variability were as follows: beat to beat per patient 0.3%, intraobserver 0.7% and interobserver 2.9%.
Clinical Parameters
Presenting New York Heart Association heart failure class (I-IV) was assessed at the time of echocardiography and adjudicated by a cardiologist based on presenting symptoms and signs according to established clinical standards 17. Data on age, gender, systolic blood pressure, heart rate, creatinine, alkaline phosphatase, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were obtained.
Data Analyses and Statistics
Data are expressed as mean ±standard deviation. Continuous variables were compared between groups using Student’s t-test (for normal distribution) or Mann-Whitney rank sum test (for non-normal distribution) using SigmaStat 3.5 software (Systat Software, Inc., Point Richmond CA). Categorical variables were compared using Fisher’s exact test. Significant p-value was set at 0.05. Stratified estimates of survival probabilities were computed using Kaplan-Meier estimators 18, with log-rank tests used to test for group differences in survival curves (MedCalc 9.6.4.0, Mariakerke, Belgium). Primary analysis involved all-cause mortality but cardiac mortality (heart failure, arrhythmia, heart block and myocardial infarction) was also used as another outcome. Cox stepwise multivariable analysis was performed and echocardiographic and clinical indices with univariate p<0.1 and heart failure class were included in the model using SAS 9.2 (The SAS Institute, Cary NC). Receiver operating characteristic curve analysis was performed to determine the sensitivity, specificity and likelihood ratio of ejection time in predicting 1-year mortality among patients who were censored or alive after 1-year follow up (MedCalc 9.6.4.0, Mariakerke, Belgium). Likelihood ratio analysis was used to determine the ejection time cutoff that would optimally provide the best combination of sensitivity and specificity. For observer variability, coefficient of variability was calculated as mean difference between readings divided by mean of all readings multiplied by 100%.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
There were 18 females and the mean age was 61±12 years old. Biopsy was positive for amyloid in the heart (N= 6), kidneys (N=20), bone marrow (N=12), abdominal fat pad (N=6), liver, hip bone and gastrointestinal tract (N= 2 each), tongue, clavicle and axillary mass (N=1 each). All subjects had abnormal elevation of lambda or kappa light chains in serum, urine or bone marrow. New York Heart Association functional classes were as follows: I: 18 (43%), II: 13 (31%), III: 3 (7%) and IV: 8 (19%). Thirty eight (90%) received chemotherapy and 13 (31%) received autologous stem cell transplantation. Two patients refused chemotherapy while two subjects were ineligible for chemotherapy due to advanced heart failure.
Echocardiographic and clinical parameters are shown in Table 1. Overall left ventricular systolic function (ejection fraction) was preserved. Similarly, mitral annular peak systolic velocity (S’) showed values closer to published values in normal subjects than subjects with heart failure from systolic dysfunction 19, 20. There was moderate thickening of the left ventricle and increased estimated left ventricular mass index. There was evidence of diastolic dysfunction in the cohort. There was increased left atrial volume index, reduced peak mitral annular early diastolic velocity (E’) and increased ratio of peak early diastolic mitral inflow to peak mitral annular early diastolic velocity (E/E’) suggesting elevated left atrial pressure. Overall the cohort had reduced renal function while mean alkaline phosphatase, aspartate and alanine aminotransferase were within normal limits.
Table 1.
Clinical and echocardiographic parameters by heart failure classification
| Variable | All | NYHA HF Class I (N=18) |
NYHA HF Class II-IV (N=24) |
p-value (I vs. II-IV) |
|---|---|---|---|---|
| Heart rate (beats per minute) | 81±20 | 80±24 | 81±16 | 0.9 |
| Systolic blood pressure (mm Hg) | 122±23 | 126±28 | 119±18 | 0.7 |
| Chemotherapy (N/%) | 38 (90) | 16 (89) | 22 (92) | 1 |
| Stem cell transplant (N/%) | 13 (31) | 10 (56) | 2 (8) | 0.001 |
| Left ventricular ejection fraction (%) | 57±13 | 59±12 | 56±13 | 0.4 |
| Mitral annular peak systolic velocity (cm/s) | 8.8±3.6 | 9.8±3.1 | 8.2±3.9 | 0.056 |
| Anteroseptal thickness (cm) | 1.6±0.4 | 1.5±0.4 | 1.6±0.3 | 0.1 |
| Inferolateral thickness (cm) | 1.5±0.3 | 1.3±0.3 | 1.6±0.4 | 0.047 |
| Left ventricular mass index (g/m2) | 119±50 | 107±56 | 127±44 | 0.049 |
| Left atrial volume index (mL/m2) | 34±11 | 30±10 | 37±10 | 0.03 |
| E/A ratio* | 1.3±0.6 | 1±0.4 | 1.6±0.7 | 0.02 |
| E’ (cm/s) | 7±3 | 8.6±3 | 5.8±3 | 0.006 |
| E/E’ | 18±15 | 15±14 | 20±15 | 0.05 |
| Deceleration time (ms) | 216±76 | 246±88 | 194±59 | 0.03 |
| Left ventricular ejection time (ms) | 266±49 | 282±42 | 254±51 | 0.07 |
| Ejection time/cycle length | 0.35±0.09 | 0.37±0.08 | 0.33±0.1 | 0.2 |
| Preejection period (ms) | 61±27 | 68±24 | 56±29 | 0.1 |
| Preejection period/ejection time | 0.24±0.12 | 0.22±0.1 | 0.23±0.1 | 0.6 |
| Creatinine (mg/dL) | 1.6±1.1 | 1.3±0.7 | 1.8±1.3 | 0.2 |
| Alkaline phosphatase (IU/L) | 133±134 | 83±25 | 171±168 | 0.1 |
| Aspartate aminotransferase (IU/L) | 41±67 | 27±15 | 51±88 | 0.4 |
| Alanine aminotransferase (IU/L) | 39±61 | 30±20 | 45±80 | 0.9 |
4 patients with atrial fibrillation did not have E/A data
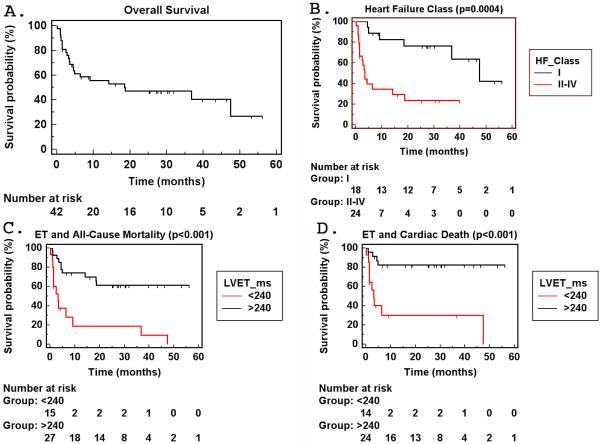
On follow-up, 23 subjects died with 1-year mortality rate of 44% (Table 2, Figure 1). The most frequent cause of death was heart failure or arrhythmias. Fifteen (71%) of the known causes of death involved a cardiac cause. Heart failure class was a significant predictor of mortality from univariate and multivariate analyses (Table 3, Figure 1).
Table 2.
Causes of mortality in AL subjects
| Subject | Cause | Interval from Echo (months) |
|---|---|---|
| 1 | Multiorgan failure | 0.9 |
| 2 | Renal failure | 1.0 |
| 3 | Sudden cardiac death | 2.8 |
| 4 | Pulmonary and heart failure | 18.7 |
| 5 | Multiorgan failure | 1.4 |
| 6 | Electromechanical dissociation, heart failure | 2.5 |
| 7 | Refractory multiple myeloma | 18.5 |
| 8 | Multiorgan failure | 47.7 |
| 9 | Unknown* | 4.5 |
| 10 | Heart failure | 6.4 |
| 11 | Sudden cardiac death | 1.1 |
| 12 | Pulseless electrical activity | 36.8 |
| 13 | Heart failure, ventricular tachycardia, pneumonia | 1.4 |
| 14 | Heart failure, ventricular tachycardia | 0.1 |
| 15 | Unknown* | 14.1 |
| 16 | Progressive multiple myeloma | 9 |
| 17 | Sepsis | 3.5 |
| 18 | Heart failure, ventricular tachycardia, sepsis | 3.2 |
| 19 | Ventricular tachycardia, heart block | 1.4 |
| 20 | Multiorgan failure, polymicrobial sepsis | 4.4 |
| 21 | Multiorgan failure | 3.4 |
| 22 | Multiorgan failure | 2.3 |
| 23 | Multiorgan failure | 0.9 |
death verified from Social Security Death Index
Figure 1.
Kaplan-Meier survival curves. A. Overall survival demonstrating 41% 1-year mortality. B. There is significant difference in survival in AL subjects presenting with NYHA Class I heart failure compared to those with Class II-IV. C-D. There is a significant increase in all-cause mortality as well as cardiac death in AL subjects with left ventricular ejection time ≤ 240 ms versus those with >240 ms.
Table 3.
Univariate and multivariable analysis of predictors of mortality
| Variable | χ2 | Hazard Ratio (95% C.I.) |
p-value |
|---|---|---|---|
| NYHA Heart Failure Class (1 df, linear) | 12.8 | 1.89 (1.33-2.68) | <0.001 |
| NYHA Heart Failure Class (1 df, binary II, III, IV vs. I)* | 10.2 | 5.31 (1.91-14.8) | 0.001 |
| Left ventricular ejection time | 9.2 | 0.985 (0.975-0.995) | 0.002 |
| Left ventricular ejection time (by 10 ms change)* | 9.2 | 0.857 (0.78-0.95) | 0.002 |
| Alkaline phosphatase | 16.5 | 1.006 (1.003-1.009) | <0.001 |
| Alkaline phosphatase (by 10 IU change)* | 16.5 | 1.059 (1.03-1.09) | <0.001 |
| Aspartate aminotransferase* | 8.96 | 1.008 (1.003-1.014) | 0.003 |
| Alanine aminotransferase* | 8.4 | 1.009 (1.003-1.015) | 0.004 |
| Left atrial volume index* | 3.4 | 1.04 (1.0-1.08) | 0.07 |
| Male gender* | 3.4 | 2.4 (0.94-6.2) | 0.07 |
| Systolic blood pressure* | 3.0 | 0.98 (0.96-1.0) | 0.08 |
| Deceleration time* | 2.7 | 0.995 (0.99-1.0) | 0.1 |
| Anteroseptal thickness | 2.2 | 2.2 (0.78-6.1) | 0.14 |
| E/E’ | 1.9 | 1.0 (0.99-1.04) | 0.17 |
| Inferolateral thickness | 1.8 | 2.2 (0.68-7.4) | 0.18 |
| E/A ratio | 1.8 | 1.61 (0.8-3.3) | 0.18 |
| Heart rate | 1.0 | 1.0 (0.99-1.03) | 0.3 |
| Lateral E’ | 0.89 | 0.94 (0.82-1.07) | 0.34 |
| Left ventricular mass index | 0.5 | 1 (0.996-1.0009) | 0.5 |
| Left ventricular ejection fraction | 0.4 | 0.99 (0.96-1.02) | 0.5 |
| Preejection period/ET | 0.2 | 0.5 (0.01-17.7) | 0.7 |
| Age | 0.1 | 1.0 (0.97-1.04) | 0.7 |
| Creatinine | 0.1 | 1.07 (0.7-1.6) | 0.7 |
| Mitral annular peak systolic velocity | 0.1 | 0.98 (0.87-1.1) | 0.7 |
| ET/cycle length | 0.01 | 0.8 (0.008-72) | 0.9 |
| Multivariable model | |||
| NYHA Heart Failure Class (binary, II,III,IV versus I) | 7.7 | 4.9 (1.58-14.9) | 0.006 |
| Left ventricular ejection time (by 10 ms change) | 6.5 | 0.87 (0.775-0.967) | 0.01 |
| Alkaline phosphatase (by 10 IU change) | 6.0 | 1.036 (1.007-1.065) | 0.01 |
variables included in multivariable model; NYHA-New York Heart Association; E’ mitral annular velocity; E/E’ ratio of mitral inflow to mitral annular velocity
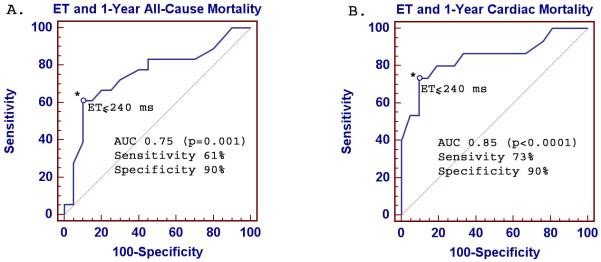
Liver function tests, heart failure class presentation and ET were significant univariate predictors of mortality (Table 3). Among echocardiographic indices, only ET was a univariate predictor of mortality. When comparing echocardiographic indices between AL patients who did not present with heart failure (NYHA Class I) versus those who did (NYHA Class II-IV), E’, deceleration time, E/A ratio, left atrial volume index, left ventricular mass index and inferolateral wall thickness were different between the two groups. ET, on the other hand, was not significantly different between the two groups with a trend towards lower ET in Class II-IV (Table 1). On multivariable analysis, heart failure class, ET and alkaline phosphatase were the only independent predictors of mortality in AL (Table 3). AL subjects with ET≤240 ms had significantly higher all-cause mortality as well as cardiac death than those with ET>240 ms (Figure 1). On receiver operating characteristic curve analysis, ET≤240 ms had 61% sensitivity and 90% specificity for predicting 1-year all-cause mortality in AL subjects (p<0.001, Figure 2). Using different cutoff values, the sensitivity and specificity are 67/80% (ET≤250 ms) and 72/70% (ET≤260 ms), respectively. For predicting 1-year cardiac death, ET≤240 ms had 73% sensitivity and 90% specificity (p<0.001, Figure 2).
Figure 2.
Receiver operating characteristic curve analysis of ejection time. The graph demonstrates good sensitivity and specificity in predicting 1-year all-cause mortality (A) and 1-year cardiac death (B) in AL subjects when using left ventricular ejection time.
Patients with ET≤240 ms had significantly higher ventricular thickness and left ventricular mass index compared to those with ET>240 ms (Table 4). Ejection fraction and diastolic function indices were similar between the two groups.
Table 4.
Clinical and echocardiographic characteristics by left ventricular ejection time
| Variable | ET ≤240 (N=15) | ET > 240 (N=27) | p-value |
|---|---|---|---|
| Age (years) | 58±12 | 62±12 | 0.3 |
| Gender (females, %) | 6 (40) | 12 (44) | 1.0 |
| Heart rate (beats per minute) | 89±20 | 76±18 | 0.03 |
| Systolic blood pressure (mm Hg) | 118±17 | 124±26 | 0.7 |
| NYHA Heart Failure Class (N/%) | 0.08 | ||
| I | 3 (20) | 15 (56) | |
| II | 5 (33) | 8 (30) | |
| III | 2 (13) | 1 (4) | |
| IV | 5 (33) | 3 (11) | |
| Chemotherapy (N/%) | 13 (87) | 25 (92) | 0.6 |
| Stem cell transplant (N/%) | 2 (13) | 10 (37) | 0.2 |
| Anteroseptal thickness (cm) | 1.8±0.3 | 1.5±0.4 | 0.02 |
| Inferolateral thickness (cm) | 1.6±0.2 | 1.4±0.4 | 0.01 |
| Left ventricular mass index (g/m2) | 136±43 | 109±52 | 0.02 |
| Left ventricular ejection fraction (%) | 55±16 | 58±11 | 0.5 |
| Left atrial volume index (mL/m2) | 38±11 | 32±10 | 0.09 |
| E’ (cm/s) | 6.2±3 | 7.4±3 | 0.3 |
| E/E’ | 19±10 | 18±17 | 0.3 |
| Deceleration time (ms) | 193±82 | 230±71 | 0.1 |
DISCUSSION
In this cohort of light chain amyloidosis subjects, presenting heart failure class was a strong and independent predictor of mortality. Among echocardiographic structural, systolic and diastolic functional indices, only left ventricular ejection time had additive independent prognostic value to heart failure class. Ejection time of ≤240 ms was associated with increased mortality and had good sensitivity and specificity for predicting 1-year all-cause mortality as well as 1-year cardiac death. Liver function assessed by alkaline phosphatase level was also an independent predictor of mortality in AL.
Our findings are consistent with prior studies that reported adverse consequences in the presence of heart failure, with median survival of less than 5 months 4, 21. In AL, extent of cardiac involvement is the most important determinant of clinical outcome 22. Cardiac involvement is thought to be present in ~50% of light chain amyloidosis cases 2, but recent studies using late gadolinium enhancement magnetic resonance imaging suggest cardiac involvement could be as high as 70% or more 23, 24. Echocardiography is an established noninvasive means to evaluate the presence and extent of cardiac involvement. On 2D echo, thickened myocardium with “granular sparkling” appearance and reduced left ventricular systolic function are seen in amyloid patients 8. Doppler echo demonstrate diastolic dysfunction with increased E/A ratio as a result of impairment in ventricular compliance leading to shorter deceleration time, reduced pulmonary vein peak systolic velocity and progressively more restrictive physiology 6, 7. Pulsed tissue Doppler also show impaired longitudinal myocardial velocity 9, 10. More recently, early systolic dysfunction has been reported in AL patients in the form of intraventricular regional dyssynchrony measured by strain 11 or 3-dimensional echocardiography 12.
The prognostic value of Doppler assessment of diastolic function has been described by Klein and colleagues when they reported increased cardiac mortality in AL patients with deceleration time less than 150 ms (mean duration of follow-up of 18 months) and found that reduced deceleration time together with increased E/A ratio were stronger prognosticators than ventricular thickness and fractional shortening using bivariate analyses 13. Their findings were consistent with studies showing the lack of prognostic value of ventricular thickness and ejection fraction 5. Mitral annular peak systolic velocity is a measure that parallels left ventricular ejection fraction; the values obtained in our cohort is closer to the values reported by previous investigators in normal subjects than to the values in subjects with systolic dysfunction and heart failure 19, 20, in parallel to the normal ejection fraction observed in the group. Our results from a longer duration of follow-up were similar to Klein and colleagues’ findings in terms of the importance of heart failure class in prognostication but also differ because deceleration time and E/A were not found to be independent predictors of mortality on multivariable analysis. This difference can perhaps be explained by the fact that abnormal deceleration time and E/A were associated with degree of heart failure (Table 1); when placed in a multivariable model, these diastolic indices fell out as having any additive prognostic value to presenting heart failure class. In contrast, ET did not differ significantly in patients without and with heart failure (Table 1) and multivariable analysis confirmed its independent and additive valu e to heart failure class. Among 18 patients who initially presented with no heart failure (Class I), 3/3 (100%) with ET≤240 ms and 3/15 (20%) with ET>240 ms died during follow up, suggesting the potential of ET for early identification of at-risk AL patients without overt clinical heart failure.
Recently, Bellavia and colleagues followed AL subjects (median follow up 13 months) and found that the only independent predictors of mortality were ET and level of brain natriuretic peptide with greater reduction in ET in more advanced cases 11. Our study differs from the study by Bellavia and colleagues in having a longer median time to follow-up (29.4 months); our study therefore complements the previous work in showing the robustness of ET as an independent predictor of AL mortality for both short term and long term follow-up. Our study, together with the study of Klein and colleagues 13 point to heart failure class as the strongest predictor of adverse outcome in this disease. This differs from the finding of Bellavia and colleagues showing heart failure class did not predict mortality. This difference may be related to two things: one, brain natriuretic peptide is potentially correlated to heart failure class and multivariable analysis may have resulted in this surrogate marker of heart failure to come out instead of heart failure class as an independent factor. Second, a greater proportion of AL patients in our cohort had advanced heart failure (NYHA Class II-IV, 57% versus 33% from previous study) and this may account for some of the study differences. Our results taken together with the previous study strengthen the robustness of ET as a predictor of mortality in both advanced and less advanced forms of AL. We similarly demonstrated that compared to conventional echo indices of cardiac amyloidosis (mass, thickness, ejection fraction, degree of diastolic function), ET is the most important predictor of adverse outcome. In the current study, ET ≤240 ms had good sensitivity (61%) and high specificity (90%) for predicting 1-year all-cause mortality, with even better prediction of 1-year cardiac death (73% sensitivity and 90% specificity). It is not surprising that ET has superior ability to predict 1-year cardiac death versus 1-year all cause mortality because it is a measure of cardiac function. AL is a multiorgan disease and as shown by our cohort, patients’ mode of exit is frequently cardiac plus other organ failure. From a clinical standpoint, it is useful that ET as a noninvasive measure can predict not only cardiac mortality but also all-cause mortality. Because a 1-point change in ET is a small change, it is expected that the change in hazard or risk is also small. However, when we consider a 10 ms change, likely a more clinically meaningful change, an increase in ET by this amount reduces risk of mortality by 13%. To put this into context of other cardiovascular diseases, a recent large hypertension study involving 180,000 patients showed that every 10 mm Hg increase in systolic blood pressure is associated with a 9% increased risk of death in older patients 25 and health professionals consider this degree of risk sufficient to warrant aggressive public health promotion program aimed at lowering blood pressure. In a disease such as AL that is associated with >40% 1-year mortality, a noninvasive test that can predict 13% reduction in risk is extremely relevant.
The utility of ET may also potentially be applicable to other forms of cardiac amyloidosis. In a retrospective study of 45 patients with cardiac amyloid (6 of whom had senile or familial amyloidosis), the Tei index which incorporated ET together with isovolumetric contraction and isovolumetric relaxation time was shown to be the only parameter aside from heart failure class that was independently predictive of survival 14.
The mechanism underlying the adverse prognostic consequence of reduced left ventricular ejection time is unknown and our prospective observational study is limited in providing mechanistic insight. Left ventricular ejection time is an old established measure of ventricular performance and is known to be sensitive to changes in inotropic state although it is also affected by preload and afterload 26-28. It has been used in the diagnosis and assessment of valvular disease, coronary artery disease, pericardial disease and hypertensive heart disease 27. The severity of ET abnormality paralleled the increase in functional class and reduction of resting cardiac index 29. Decreased ET was noted in subjects with hypovolemia 30. In cardiac amyloidosis, reduced ET was described using phonocardiogram and carotid pulse wave tracing 31 as well as Doppler echocardiography 14. The physiologic basis may be related to the fact that since systole is fixed in duration, myocardial dysfunction from amyloidosis can prolong preejection period leading to reduced ejection time. Indeed, prior studies on amyloid patients demonstrated both prolonged preejection period and decreased ejection time 14, 31. In addition, it has been postulated that the inability of the infiltrated myocardium to increase end-diastolic volumes shortens ejection time leading to reduced stroke volume and adverse clinical outcomes 14. A reduction in ET was recently found useful in identifying abnormal ventriculoarterial coupling in stable heart failure subjects 32. Reduced ET was also found in advanced AL subjects who demonstrate hypersynchronization of regional systolic function, in contrast to dyssynchrony in less advanced stages of the disease 11. These recent studies suggest that ET may be a sensitive marker of dysfunction in contractile synergy among ventricular segments and between atria and ventricles and that mechanical synchrony may play an importa nt role in the pathophysiology of AL amyloidosis.
It remains to be seen whether ET can be used to risk-stratify AL subjects in terms of aggressive treatment that currently includes chemotherapy, autologous stem cell transplantation or cardiac transplantation. Ejection time is currently not used to evaluate candidacy for stem cell transplantation (patients with advanced cardiac involvement are often ineligible for stem cell transplantation because of increased transplant related mortality 33-35). Therefore its role in the risk-adapted approach 33, 36 that is currently utilized by oncologists to determine eligibility for stem cell transplantation needs to be further studied. Similarly, ET may have potential usefulness in evaluating physiologic response to treatment on serial follow up.
Limitations
A major limitation of the study was the small sample size. Light chain amyloidosis, although increasingly recognized clinically, nevertheless remains a comparatively rare disease 2. The longer follow up period of the study and frequency of outcome mitigated the relatively small sample size. The sample size limited the ability to add more clinical variables to test in a Cox model because of resulting unstable parameter estimates. Furthermore the small sample size, the temporal delay between echocardiography and chemotherapy/stem cell transplantation and the risk-adapted approach that oncologists use to decline stem cell transplantation in patients with advanced cardiac involvement due to excessive risk 33, 36 precluded performing sophisticated and complicated multivariable modeling to assess the independent role of treatment options in patients’ survival. The results of this study therefore need to be validated further in a larger prospective study. We also lack data on myocardial strain that was shown to be abnormal in light chain amyloidosis 9, 10, 37 as well as right ventricular diastolic function, so these variables were not included in the multivariable analysis. However, a prior study revealed that myocardial strain was not an independent predictor of outcome in AL in a multivariate analysis that included ET; the latter, on the other hand, was found to be prognostic of outcome 11.
Summary and clinical implications
Light chain amyloidosis in this cohort was associated with high 1-year and long-term mortality. Left ventricular ejection time measured by pulsed Doppler echocardiography predicted long-term mortality in light chain amyloidosis independent of heart failure status. It was a sensitive and specific test in assessing 1-year all-cause mortality as well as cardiac death. Ejection time may be useful in risk-stratification of AL amyloid subjects by identifying vulnerable patients who may benefit from aggressive treatment or enhanced surveillance.
Acknowledgement
The study was supported by the American Heart Association Grant in Aid 0855683G, NIH R21HL092344-01A1, GCRC M01-RR00058 and Kirschtein National Research Service Award, Amyloidosis Research Foundation, American Cancer Society and Greater Milwaukee Foundation. The Biostatistics Consulting Service is supported by the Division of Biostatistics and the Clinical Translational Science Institute of Southeast Wisconsin. We acknowledge the assistance of Megan Bright, Vi Nguyen-Liu, Paulette Jacobs and Kwang Woo Ahn.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Comenzo RL, Vosburgh E, Simms RW, Bergethon P, Sarnacki D, Finn K, Dubrey S, Faller DV, Wright DG, Falk RH, Skinner M. Dose-intensive melphalan with blood stem cell support for the treatment of AL amyloidosis: one-year follow-up in five patients. Blood. 1996;88(7):2801–2806. [PubMed] [Google Scholar]
- 2.Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005;112(13):2047–2060. doi: 10.1161/CIRCULATIONAHA.104.489187. [DOI] [PubMed] [Google Scholar]
- 3.Sanchorawala V, Wright DG, Seldin DC, Dember LM, Finn K, Falk RH, Berk J, Quillen K, Skinner M. An overview of the use of high-dose melphalan with autologous stem cell transplantation for the treatment of AL amyloidosis. Bone Marrow Transplant. 2001;28(7):637–642. doi: 10.1038/sj.bmt.1703200. [DOI] [PubMed] [Google Scholar]
- 4.Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol. 1995;32(1):45–59. [PubMed] [Google Scholar]
- 5.Dispenzieri A, Gertz MA, Kyle RA, Lacy MQ, Burritt MF, Therneau TM, McConnell JP, Litzow MR, Gastineau DA, Tefferi A, Inwards DJ, Micallef IN, Ansell SM, Porrata LF, Elliott MA, Hogan WJ, Rajkumar SV, Fonseca R, Greipp PR, Witzig TE, Lust JA, Zeldenrust SR, Snow DS, Hayman SR, McGregor CG, Jaffe AS. Prognostication of survival using cardiac troponins and N-terminal pro-brain natriuretic peptide in patients with primary systemic amyloidosis undergoing peripheral blood stem cell transplantation. Blood. 2004;104(6):1881–1887. doi: 10.1182/blood-2004-01-0390. [DOI] [PubMed] [Google Scholar]
- 6.Klein AL, Hatle LK, Burstow DJ, Seward JB, Kyle RA, Bailey KR, Luscher TF, Gertz MA, Tajik AJ. Doppler characterization of left ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol. 1989;13(5):1017–1026. doi: 10.1016/0735-1097(89)90254-4. [DOI] [PubMed] [Google Scholar]
- 7.Klein AL, Hatle LK, Taliercio CP, Taylor CL, Kyle RA, Bailey KR, Seward JB, Tajik AJ. Serial Doppler echocardiographic follow-up of left ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol. 1990;16(5):1135–1141. doi: 10.1016/0735-1097(90)90545-z. [DOI] [PubMed] [Google Scholar]
- 8.Siqueira-Filho AG, Cunha CL, Tajik AJ, Seward JB, Schattenberg TT, Giuliani ER. M-mode and two-dimensional echocardiographic features in cardiac amyloidosis. Circulation. 1981;63(1):188–196. doi: 10.1161/01.cir.63.1.188. [DOI] [PubMed] [Google Scholar]
- 9.Koyama J, Davidoff R, Falk RH. Longitudinal myocardial velocity gradient derived from pulsed Doppler tissue imaging in AL amyloidosis: a sensitive indicator of systolic and diastolic dysfunction. J Am Soc Echocardiogr. 2004;17(1):36–44. doi: 10.1016/j.echo.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Koyama J, Ray-Sequin PA, Davidoff R, Falk RH. Usefulness of pulsed tissue Doppler imaging for evaluating systolic and diastolic left ventricular function in patients with AL (primary) amyloidosis. Am J Cardiol. 2002;89(9):1067–1071. doi: 10.1016/s0002-9149(02)02277-4. [DOI] [PubMed] [Google Scholar]
- 11.Bellavia D, Pellikka PA, Abraham TP, Al-Zahrani G, Dispenzieri A, Oh J, Espinosa RE, Scott CG, Miyazaki C, Miller FA., Jr. “Hypersynchronization” by Tissue Velocity Imaging in Patients with Cardiac Amyloidosis. Heart (British Cardiac Society) 2008;95(3):234–240. doi: 10.1136/hrt.2007.140343. [DOI] [PubMed] [Google Scholar]
- 12.Migrino RQ, Harmann L, Woods T, Bright M, Truran S, Hari P. Intraventricular dyssynchrony in light chain amyloidosis: a new mechanism of systolic dysfunction assessed by 3-dimensional echocardiography. Cardiovascular ultrasound. 2008;6:40. doi: 10.1186/1476-7120-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein AL, Hatle LK, Taliercio CP, Oh JK, Kyle RA, Gertz MA, Bailey KR, Seward JB, Tajik AJ. Prognostic significance of Doppler measures of diastolic function in cardiac amyloidosis. A Doppler echocardiography study. Circulation. 1991;83(3):808–816. doi: 10.1161/01.cir.83.3.808. [DOI] [PubMed] [Google Scholar]
- 14.Tei C, Dujardin KS, Hodge DO, Kyle RA, Tajik AJ, Seward JB. Doppler index combining systolic and diastolic myocardial performance: clinical value in cardiac amyloidosis. J Am Coll Cardiol. 1996;28(3):658–664. doi: 10.1016/0735-1097(96)00202-1. [DOI] [PubMed] [Google Scholar]
- 15.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57(6):450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 16.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 17.The Criteria Committee of the New York Heart Association . Nomenclature and criteria for diagnosis of diseases of the heart and blood vessels. Little Brown; Boston: 1964. [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 19.Alam M, Wardell J, Andersson E, Nordlander R, Samad B. Assessment of left ventricular function using mitral annular velocities in patients with congestive heart failure with or without the presence of significant mitral regurgitation. J Am Soc Echocardiogr. 2003;16(3):240–245. doi: 10.1067/mje.2003.52. [DOI] [PubMed] [Google Scholar]
- 20.Nikitin NP, Loh PH, Silva R, Ghosh J, Khaleva OY, Goode K, Rigby AS, Alamgir F, Clark AL, Cleland JG. Prognostic value of systolic mitral annular velocity measured with Doppler tissue imaging in patients with chronic heart failure caused by left ventricular systolic dysfunction. Heart (British Cardiac Society) 2006;92(6):775–779. doi: 10.1136/hrt.2005.067140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubrey SW, Cha K, Anderson J, Chamarthi B, Reisinger J, Skinner M, Falk RH. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. Qjm. 1998;91(2):141–157. doi: 10.1093/qjmed/91.2.141. [DOI] [PubMed] [Google Scholar]
- 22.Sirohi B, Powles R. Epidemiology and outcomes research for MGUS, myeloma and amyloidosis. Eur J Cancer. 2006;42(11):1671–1683. doi: 10.1016/j.ejca.2006.01.065. [DOI] [PubMed] [Google Scholar]
- 23.Maceira AM, Joshi J, Prasad SK, Moon JC, Perugini E, Harding I, Sheppard MN, Poole-Wilson PA, Hawkins PN, Pennell DJ. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111(2):186–193. doi: 10.1161/01.CIR.0000152819.97857.9D. [DOI] [PubMed] [Google Scholar]
- 24.Migrino RQ, Bright M, Pajewski N, Truran S, Gutterman DD, Hari P. American Heart Association (submitted) New Orleans LA: 2008. Late gadolinium enhancement on MRI is a prognostic marker of poor survival in light chain amyloidosis: results from long term follow up. [Google Scholar]
- 25.Murakami Y, Hozawa A, Okamura T, Ueshima H. Relation of blood pressure and all-cause mortality in 180,000 Japanese participants: pooled analysis of 13 cohort studies. Hypertension. 2008;51(6):1483–1491. doi: 10.1161/HYPERTENSIONAHA.107.102459. [DOI] [PubMed] [Google Scholar]
- 26.Chan GS, Middleton PM, Celler BG, Wang L, Lovell NH. Automatic detection of left ventricular ejection time from a finger photoplethysmographic pulse oximetry waveform: comparison with Doppler aortic measurement. Physiological measurement. 2007;28(4):439–452. doi: 10.1088/0967-3334/28/4/009. [DOI] [PubMed] [Google Scholar]
- 27.Lewis RP. Diagnostic value of systolic time intervals in man. Cardiovascular clinics. 1975;6(3):245–264. [PubMed] [Google Scholar]
- 28.Lewis RP, Rittogers SE, Froester WF, Boudoulas H. A critical review of the systolic time intervals. Circulation. 1977;56(2):146–158. doi: 10.1161/01.cir.56.2.146. [DOI] [PubMed] [Google Scholar]
- 29.Weissler AM, Harris WS, Schoenfeld CD. Bedside technics for the evaluation of ventricular function in man. Am J Cardiol. 1969;23(4):577–583. doi: 10.1016/0002-9149(69)90012-5. [DOI] [PubMed] [Google Scholar]
- 30.Geeraerts T, Albaladejo P, Declere AD, Duranteau J, Sales JP, Benhamou D. Decrease in left ventricular ejection time on digital arterial waveform during simulated hypovolemia in normal humans. The Journal of trauma. 2004;56(4):845–849. doi: 10.1097/01.ta.0000063406.34651.e5. [DOI] [PubMed] [Google Scholar]
- 31.Wizenberg TA, Muz J, Sohn YH, Samlowski W, Weissler AM. Value of positive myocardial technetium-99m-pyrophosphate scintigraphy in the noninvasive diagnosis of cardiac amyloidosis. Am Heart J. 1982;103(4 Pt 1):468–473. doi: 10.1016/0002-8703(82)90331-3. [DOI] [PubMed] [Google Scholar]
- 32.Cheng HM, Yu WC, Sung SH, Wang KL, Chuang SY, Chen CH. Usefulness of systolic time intervals in the identification of abnormal ventriculo-arterial coupling in stable heart failure patients. Eur J Heart Fail. 2008 doi: 10.1016/j.ejheart.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Gertz MA, Lacy MQ, Dispenzieri A, Hayman SR, Kumar S. Transplantation for amyloidosis. Current opinion in oncology. 2007;19(2):136–141. doi: 10.1097/CCO.0b013e32801494c6. [DOI] [PubMed] [Google Scholar]
- 34.Jantunen E, Itala M, Lehtinen T, Kuittinen O, Koivunen E, Leppa S, Juvonen E, Koistinen P, Wiklund T, Nousiainen T, Remes K, Volin L. Early treatment-related mortality in adult autologous stem cell transplant recipients: a nation-wide survey of 1482 transplanted patients. European journal of haematology. 2006;76(3):245–250. doi: 10.1111/j.1600-0609.2005.00605.x. [DOI] [PubMed] [Google Scholar]
- 35.Vesole DH, Perez WS, Akasheh M, Boudreau C, Reece DE, Bredeson CN. High-dose therapy and autologous hematopoietic stem cell transplantation for patients with primary systemic amyloidosis: a Center for International Blood and Marrow Transplant Research Study. Mayo Clinic proceedings. 2006;81(7):880–888. doi: 10.4065/81.7.880. [DOI] [PubMed] [Google Scholar]
- 36.Comenzo RL, Gertz MA. Autologous stem cell transplantation for primary systemic amyloidosis. Blood. 2002;99(12):4276–4282. doi: 10.1182/blood.v99.12.4276. [DOI] [PubMed] [Google Scholar]
- 37.Bellavia D, Abraham TP, Pellikka PA, Al-Zahrani GB, Dispenzieri A, Oh JK, Bailey KR, Wood CM, Novo S, Miyazaki C, Miller FA., Jr. Detection of left ventricular systolic dysfunction in cardiac amyloidosis with strain rate echocardiography. J Am Soc Echocardiogr. 2007;20(10):1194–1202. doi: 10.1016/j.echo.2007.02.025. [DOI] [PubMed] [Google Scholar]