Abstract
Hypopituitarism is defined as the deficiency of one or more of the hormones secreted by the pituitary gland. Several developmental factors necessary for pituitary embryogenesis and hormone secretion have been described, and mutations of these genes in humans provide a molecular understanding of hypopituitarism. Genetic studies of affected patients and their families provide insights into possible mechanisms of abnormal pituitary development, however, mutations are rare. This review characterizes several of these developmental proteins and their role in the pathogenesis of hypopituitarism. Continuing research is required to better understand the complexities and interplay between these pituitary factors and to make improvements in genetic diagnosis that may lead to early detection and provide a future cure.
Hypopituitarism
Pituitary hormone deficiency is the partial or complete loss of single or multiple pituitary hormones from the anterior (AP) and/or posterior pituitary (PP). The etiology, often multifactorial and secondary to neurological insult, includes head injury, neurosurgical sequelae, infiltrative disorders and cranial radiotherapy. A subset of patients may present either at birth with congenital hormone deficiency or develop deficiency with no previous neurological injury or pathology. These patients are diagnosed with idiopathic hypopituitarism and often have multiple or combine pituitary hormone deficiency (CPHD). The incidence and prevalence of hypopituitarism is still unclear. One report, which summarizes data from separate European populations, cites an incidence of 11.9 to 42.1 per million inhabitants per year, while the prevalence is estimated from 300 to 455 per million inhabitants [1].
Pituitary hormone deficiencies most commonly occur in the AP or adenohypophysis. The mature adenohypophysis contains five major pituitary cell types that produce six hormones; corticotrophs - adrenocorticotrophic hormone (ACTH), thyrotrophs– thyroid stimulating hormone (TSH), lactotrophs – prolactin (PRL), somatotrophs – growth hormone (GH), and gonadotrophs – lutenizing hormone (LH) and follicle stimulating hormone (FSH). A balanced and orchestrated sequence of events dependent on the temporal and spatial appearance developmental factors is necessary for normal pituitary development. Disruption of this cascade due to mutations in any of these gene products affects the ontogeny of one or several of the pituitary cell types and ultimately leads to hormone deficiency.
Experimental in vivo model systems have provided valuable insight into the importance of these factors and have defined signaling pathways in the developmental process. Gene mutations in these factors have been identified and linked to the development of hypopituitarism in animal models and humans. There is a wide spectrum of clinical presentations of hypopituitarism in humans; therefore, research continues to better understand the role of these gene products and their interplay during pituitary development. This review highlights the normal development of the pituitary, the transcription factors necessary for proper embryogenesis, and the major pituitary developmental factors studied in patients with hypopituitarism.
Clinical presentation of hypopituitarism
The clinical manifestations of hypopituitarism are often nonspecific and insidious. Regardless of whether the initial presentation and diagnosis include one or several hormone deficiencies, follow-up of these patients should be continuous, because of the risk for developing additional deficiencies [2, 3]. Table 1 lists some of the most common recognized findings in patients with hypopituitarism, according to age of presentation.
Table 1.
Clinical manifestations of hypopituitarism according to age
| Age | Features |
|---|---|
| Newborn/Infant | Hypoglycemia |
| Micropenis | |
| Conjugated hyperbilirrubinemia | |
| Adrenal crisis (Electrolyte abnormalities) | |
| Neurological abnormalities | |
| (Including Septo-optic dysplasia, Holoprosencephaly) | |
| Midline defects (cleft palate) | |
| Failure to thrive | |
| Child/Adolescent | Reduced growth velocity/ short stature |
| Pubertal delay/ No pubertal development | |
| Central obesity/ generalized weight gain | |
| Prominent forehead | |
| Delayed dentition | |
| Fatigue / Loss of appetite |
Among the AP hormones, deficiency of GH (GHD) is most commonly diagnosed and usually does not manifest until after two years of age with short stature or decreased growth velocity [4]. Newborns with hypopituitarism may present in the first days of life with hypoglycemia, electrolyte abnormalities, conjugated hyperbilirubinemia and hypothermia. Evaluation includes measuring serum GH, cortisol, ACTH, TSH and T4 levels. Both GHD and/or ACTH deficiency can cause significant morbidity and mortality on presentation. Boys born with gonadotropin deficiency or GHD may present with micropenis [4, 5]. The presentation of midline defects, including holoprosencephaly, cleft lip/palate or radiological evidence of absent corpus callosum or septum pellucidum should also prompt an evaluation of pituitary hormone secretion.
GHD in older children presents as either short stature or decreased growth velocity. Patients with severe GHD can develop a characteristic appearance with a prominent forehead, a depressed midface and delayed dentition [6]. GHD in older children and adults can lead to abnormalities of protein, fat and carbohydrate metabolism, which manifests with an increased propensity for central obesity, decreased lean mass, and low bone mineral density [5]. A diagnosis of GHD is dependent on the evaluation of growth charts, low IGF-1 and IGFBP3 levels, and abnormal GH stimulation [4].
As children enter adolescence, there is variable presentation of gonadotropin deficiency depending on gender and age. In girls, gonadotropin deficiency usually manifests as delayed breast development and primary amenorrhea, while boys will have prepubertal testicular size. Evaluation includes measuring testosterone (males), estrogen (females), LH, FSH and GnRH. The manifestations of TSH deficiency, central hypothyroidism, are similar to those of primary hypothyroidism, but usually less severe. These include poor growth, delayed bone age, constipation, dry skin, fatigue, cold intolerance, developmental delay and weight gain [2]. The diagnosis is based on low T4, inappropriate TSH levels and the TRH test, if available. ACTH deficiency in children and adolescents often presents with non-specific symptoms (lethargy, fatigue, weight loss, abdominal pain) unlike the neonatal presentation, which can present in crisis. Diagnosis can be confirmed by measuring serum cortisol after ACTH stimulation testing. Finally, children with a confirmed or suspected diagnosis of hypopituitarism should have a brain MRI performed as some radiological findings have been associated with the pituitary developmental genes (Table 2).
Table 2.
Developmental Factors Associated with CPHD
| Factor | Features/Function | Affected Cells | Clinical Features | Radiological Finding | Inheritance | Refs |
|---|---|---|---|---|---|---|
|
HESX1 (Rpx ) |
Paired-like class of homeobox genes Forebrain and pituitary development Repression leads to expression of PROP1 |
S, L, T, G, C | Wide spectrum of hormone deficiency (isolated GH deficiency to CPHD) Septo Optic Dysplasia (midline neural defects, optic nerve hypoplasia, hypopituitarism) |
Pituitary hypoplasia Ectopic posterior pituitary |
AD, AR | [20, 24, 25] |
| LHX3 | LIM-type homeodomain protein Important for pituitary and motor neuron development Regulates Rpx transcription Works in concert with Pit1 to activate TSH-β promoter Act synergistically with Pitx factors to activate αGSU expression Interaction with SOX2 (inner ear/pituitary development) |
S, L, T, G, C (Note: C cell lineage occurs, but propiomelanocortin cells fail to proliferate) |
Reported patients with CPHD Associated with rigid and short cervical spine and limited head rotation |
Anterior pitutary hypoplasia, (normal posterior pituitary and midline structures) Reports also of enlarged anterior pituitary & microadenoma |
AR | [29–33] |
|
LHX4 (Gsh4 ) |
LIM protein related to Lhx3 Expressed in developing hind brain, cortex, pituitary & spinal cord Required for proliferation/differentiation of pituitary cell types Overlapping function with Prop1 in early development |
S, T, C, G | Wide spectrum seen in anterior hormone deficiencies Reported family with intronic mutation → short stature, pituitary/hindbrain defects, abnormality of central skull base |
Small sella turcica Hypoplastic anterior pitutiary Ectopic posterior pituitary |
AD (also dominant negative or haplo- insufficiency) |
[37–39] |
| OTX2 | Bicoid-type homeodomain gene important in forebrain and ocular development Works in concert with Otx1, Emx2 and Pax6 (early in development binds Hesx1 → necessary for activation during pituitary development) |
S, T, C (G probable ) |
Malformations of eye: micropthalmia → anopthalmia CPHD reported without eye findings Associated with deletion in the 14q22–23 region (leading to ophthalmia and hypothalamic- pituitary anomalies) |
Defects of optic nerve, optic chiasm and brain Hypoplastic anterior pituitary Ectopic posterior pituitary |
DN, possible loss of function mutations |
[43–46] |
|
PITX2(RI EG 1, ARP1) |
Member of three-gene Paired-like homeodomain transcription factor (PITX) subfamily of bicoid-related proteins. Expressed early in anterior pituitary 3 major RNA forms derive (Pitx2 A, B & C) Majority of Pitx2-positive cells coexpress gonadotropins/ thyrotropins (role in maintenance of cells) Stimulates activation of LHβ and FSH β-subunit gene |
S, T, G | Associated with Axenfeld-Rieger syndrome Heterogeneous AD disorder with anomalies of anterior eye chamber, dental hypoplasia, craniofacial dysmorphism and protuberant umbilicus Hypopituitarism is also variable (GHD, hypothyroidism, absent/delayed puberty) |
AD | [49–52] | |
|
POU1F1 (Pit1) |
Member of the POU transcription factor family May have role in regulating apoptosis Contains two protein domains: POU-specific & POU-homeo necessary for DNA binding and gene activation Activates GH1, PRL and TSHβ genes Gene activation requires induction by retinoic acid May act in concert with Lhx4 to regulate GH expression |
S, L, T | Severe growth deficiency accompanied with hypothyroidism Initial presentation may be central hypothyroidism |
Hypoplastic anterior pituitary | AD, AR, Dominant Negative |
[55, 60, 61, 57] |
|
PROP1 (prophet of Pit 1) |
Expression restricted to anterior pituitary Paired-like homedomain transcription factor required for Pit 1 expression |
S, T, G, C | CPHD including GH, TSH, Prl and late onset ACTH Most common cause of genetic CPHD (50% reported cases) Patients with normal/delayed puberty |
Hypoplastic anterior pitutiary Partially empty sella Also described → pituitary enlargement then involution |
AR | [62–66] |
|
SIX6 (Optx2) |
Member of the SIX/sine oculis family of homeobox genes Expressed in developing retina, optic nerve, hypothalamic & pituitary regions |
S, G | Associated with reports of deletion in deletion of chromosome 14q22–23 (patients with bilateral anophthalmia and pituitary anomalies) Association with Brachiootorenal Syndrome and Oculoauriculovertebral Spectrum |
Patients with 14q22–23 deletion: absent optic chiasm, hypoplastic pituitary and cortical atrophy |
Haploinsufficiency | [67–69] |
| SOX2 | Member of the SOX (SRY-related high mobility group (HMG) box) family of transcription factors Expressesed early in development |
S, G (T shown in animal models) |
Hypogonadotropic hypogonadism Bilateral anophthalmia/microphthalmia Sensorineural defects Esophageal atresia and learning difficulty |
Anterior pituitary hypoplasia Mid-brain defects (corpus callosum and hippocampus) |
de novo | [71, 72] |
| SOX3 | Member of the SOX (SRY-related high mobility group (HMG) box) family of transcription factors Expressed in the developing infundibulum and hypothalamus |
S, T, G, C (T also reported as evolving) |
Spectrum of hormone deficiency: GH → panhypopituitarism X-linked hypopituitarism - expansion of a polyalanine tract on Xq27.1 Female carriers unaffected Dosage of SOX3 may be critical for pituitary development and phenotype |
MRI: anterior pituitary hypoplasia, ectopic posterior pituitary & absent infundibulum |
X-linked recessive | [73, 74] |
Legend S- somatotrophs, L - lactotrophs, T - thyrotrophs, G - gonadotrophs, C - corticotrophs CPHD - Combinded Pituitary Hormone Deficiency, AD - Autosomal Dominant, AR - Autosomal Recessive, DN - Dominant Negative
Pituitary Embryogenesis
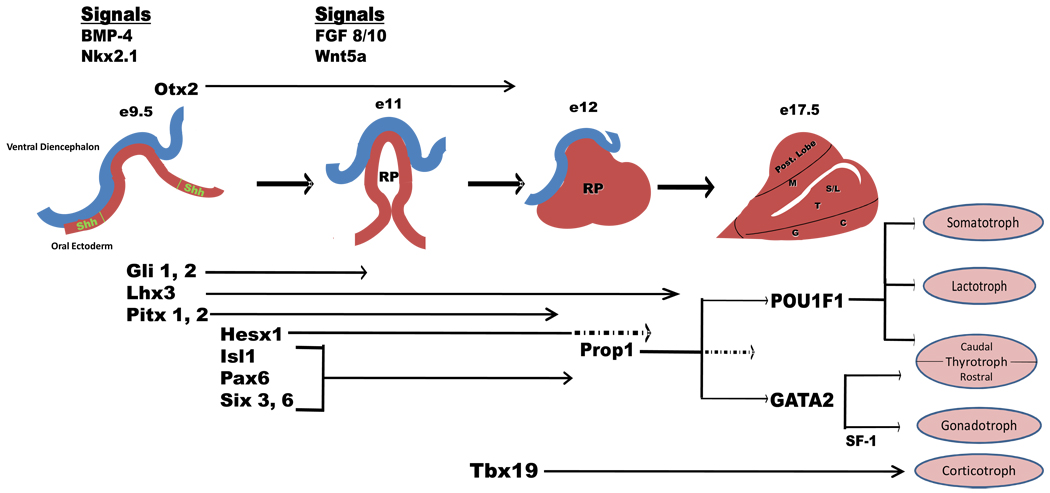
The development of the pituitary gland initiates with the juxtaposition of the oral ectoderm and the ventral diencephalon (neural ectoderm), which is guided by a series of inducing signals. This association results in a complex integrated sequence of events that will lead to the development of the different pituitary cell types and eventually a mature pituitary that is dynamic in adapting to physiological demands. Thus the identification of progenitor-like cells to better understand pituitary development and its plasticity has been a focus of many investigations [7, 8]. Figure 1 presents a modified overview of pituitary development and has been adapted from embryological studies performed and reported on murine species [9–12]. Hypothalamic factors have an important role in initiating the orchestration of these events and future pituitary regulation. This review focuses on important factors expressed in the pituitary during embryogenesis, especially on those implicated in human disease.
Figure 1.
A modified overview of pituitary development adapted from previous embryological studies performed in murine species. The development of the mature pituitary gland is dependent on the contact of the oral ectoderm with the ventral diencephalon (neural ectoderm) followed by a cascade of events consisting of both signaling molecules and transcription factors expressed in a specific temporal and spatial fashion. At approximately embryological day 9.5 (e9.5), BMP-4 and Nkx2.1 along with sonic hedgehog (Shh) participate with the initial evagination of ventral diencephalon and invagination of oral ectoderm to from the primordial Rathke’s pouch (RP). In addition, expression of Gli 1,2, Lhx3 and Pitx 1,2 plays an important role in the development of progenitor pituitary cell types. This is closely followed by the expression of Hesx1, Isl1, Pax6 and Six3, 6, which are also implicated in cellular development, proliferation and migration. Interactions between factors is illustrated by the attenuation of Hesx1 (hashed arrows at approximately e12.5) that is required for the expression of Prop1. By e12.5, RP has formed and by e17.5, differentiation of specific pituitary cell types has been completed. The expression of Pit1 is also marked with the attenuation of Prop1 expression (hashed arrows).The mature pituitary gland is marked by the differentiated cell types: somatotrophs (S), lactotrophs (L), thyrotrophs (T), gonadotrophs (G) and corticotrophs (C). Also shown are the posterior and intermediate lobe of the pituitary and the location of melanotropes (M) [6–9].
At approximately embryological day 9.5 (e9.5), the expression of bone morphogenetic protein 4 (BMP-4) and thyroid transcription factor (Ttf1; also called Nkx2.1) signaling in the ventral diencephalon along with Sonic Hedgehog (Shh) from the oral ectoderm initiates the formation of a primordial Rathke’s pouch (RP). Fibroblast growth factor signaling (Fgf 8 and Fgf10) and Wnt5a in combination with the early transcription factors Gli1 and -2, Lhx3 and Ptx1 and -2 further direct the progression of pituitary development beyond the invagination of RP and set the stage for pituitary progenitor cell proliferation [11]. The expression of Hesx1, Isl1, Pax6 and Six3 and 6 assist in the progression and proliferation of RP. Hesx1 expression continues exclusively in RP until its attenuation leads to the maximal expression Prop1 (prophet of Pit-1), which plays a role in both the spatial and terminal differentiation of specific pituitary cell types. As with Hesx1, Prop1 expression eventually becomes undetectable. Prop1 helps to induce expression of POU1F1 (Pit1) that leads to the terminal differentiation of somatotrophs (S), lactotrophs (L) and caudal thyrotrophs (T). Subsequently, ventral GATA binding protein 2 (GATA2) expression and steroidogenic factor 1 (SF-1) activation, play important roles in progenitor cell differentiation of both rostral thyrotrophs and gonadotrophs (G). Finally, the expression of Tbx19, expressed in POMC-producing cells, regulates the differentiation of corticotrophs (C).
As depicted in Figure 1, histological studies demonstrated that definitive AP cell types occupy specific positions once they emerge from proliferation zones. Although several of the major events required for proper development are represented in linear fashion, the precise orchestration of these events is much more interactive and complex. Epigenetic studies, for example, demonstrated that histone modification also plays an important role in controlling transcriptional programs during pituitary development [13]. Understanding the crosstalk between signals and the mechanisms for temporal regulation of transcriptional programs are future research challenges. In addition, more recent work demonstrated that cell positioning and cell-cell connection mechanisms in the pituitary exist to help determine pituitary hormone response and coordinate activities in physiological conditions [14, 15]. Therefore, understanding the integration of signaling molecules and activation of transcription factors will help provide further insight into the phenotypic variability seen in patients with hypopituitarism.
Mutations in Pituitary developmental factors resulting in CPHD
Several human gene mutations responsible for pituitary developmental defects in patients diagnosed with hormone deficiencies have been studied and associated with the pathogenesis of hypopituitarism. We describe the genes studied in patients with CPHD and review their role in the pathogenesis of hormone deficiency (see below and Table 2).
Hesx1
Hesx1, also referred to as Rpx (Rathke’s pouch homeobox), is a member of the paired-like class of homeobox genes and is essential for normal forebrain and pituitary formation [16]. It is one of the earliest known specific markers for the pituitary primordium and encodes for a developmental repressor that localizes to the Rathke’s pouch [17]. Hesx1 null mutant mice (Hesx −/−) demonstrate abnormalities in the forebrain, eyes and other anterior structures such as the pituitary [18]. These defects are similar to human phenotypes such as Septo-Optic Dysplasia (SOD) and CPHD. SOD is a heterogeneous condition that includes optic nerve hypoplasia, midline brain abnormalities and pituitary hormone deficiencies. Patients with SOD present with a wide spectrum of phenotypes associated with congenital hypopituitarism. Currently, 14 HESX1 mutations have been identified in patients with SOD and/or hypopituitarism; these mutations have both recessive and dominant inheritance [19–22]. Recently, two mouse models, each containing a reported human HESX1 mutation, have been developed and demonstrate pituitary abnormalities similar to those defects observed in Hesx−/− null mice [23]. This in vivo approach provides another tool to better understand mechanisms responsible for pituitary development and hypopituitarism. Despite the association of HESX1 with SOD, however, screening studies have attributed the incidence of coding region mutations to be less than 1% [24, 25]. Studies are continuing to further understand the importance of HESX1 in the complex and variable manifestations of pituitary hormone deficiency in humans.
Lhx3 (LIM-3, P-Lim)
Lhx3 is a member of the LIM-type homeodomain (HD) protein family of transcription factors that feature two LIM domains in their amino termini and a centrally located HD that interacts with specific DNA elements [26]. During development, Lhx3 expression, which persists in the adult pituitary, is expressed in the anterior and intermediate lobes of the pituitary, spinal cord and medulla [26]. Murine models with targeted disruption of Lhx3 have depleted thyrotrophs, gonadotrophs and somatotrophs, suggesting LHX3 is important for cell specification and proliferation [27]. Three LHX3 isoforms have been identified in humans, including hLHX3a, hLHX3b and hM2-LHX3 [28]. Of these, LHX3a displays the greatest ability to activate the promoters of pituitary genes. Patients with mutations in LHX3 have deficits of GH, PRL, TSH and gonadotropins, as well as abnormal pituitary morphology and a rigid cervical spine that limits head rotation [29]. LHX3 homozygous mutations are a rare cause of reported hypopituitarism, with an incidence in 2.2% in patients with CPHD [29]. Several other novel LHX3 mutations in patients with CPHD demonstrating autosomal recessive inheritance have been reported and characterized [30, 31].
Lhx4
Lhx4, another LIM homeodomain protein, is also expressed in the developing brain, including the cortex, pituitary and spinal cord [34]. Despite homology and similarities in protein structure to LHX3, LHX4’s role in development is distinct as demonstrated by single and combined gene deletion targeting in mice. Murine models with targeted deletion of Lhx4 (Lhx4−/−) form a definitive RP that arrests and results in a hypoplastic pituitary. Unlike Lhx3−/− , however, Lhx4−/− mice contain all five differentiated cell types [35, 36]. Furthermore, Lhx3 expression is impaired in Lhx4 mutants, suggesting that LHX4 is required for cell survival, expansion of the pouch and differentiation of pituitary-specific cell lineages [36]. Several reports described CPHD patients with hypoplastic pituitary harboring LHX4 mutations [37–39]. These heterozygous mutations result in proteins that are unable to bind DNA and activate pituitary gene expression [39]. Further studies demonstrate a functional relationship between Pit-1 and Lhx4’s role in regulating Pit-1 expression in specific pituitary cell types [37]. Also, several studies suggest that Lhx4 and Prop1 have overlapping functions in pituitary development [35]. Finally, in addition to pituitary hormone deficiencies, LHX4 mutations have also been implicated in structural abnormalities; patients with an LHX4 mutation had abnormal MRI findings including a hypoplastic AP, ectopic posterior lobe, poorly developed sella turcica and Chiari malformation [40].
Otx2
Otx2, a transcription factor belonging to the orthodenticle family, is a homeobox gene expressed earliest in the neuroectoderm cells of the forebrain and midbrain that also plays a role in ocular development [41]. There is evidence through mouse models to also suggest Otx2 may be an essential regulator of the identity and fate of neuronal progenitor domains in the ventral midbrain [42]. A heterozygous OTX2 mutation has been recently described in two unrelated patients with hypopituitarism [43]. Although initial studies demonstrate normal binding to HESX1 binding sites, the mutant OTX2 gene decreased activation of the HESX1 promoter suggesting a dominant negative effect leading to CPHD [43]. This inter-relationship between the genes OTX2 and HESX1 emphasizes the complexities of pituitary development and suggests multifactorial genetic etiologies.
Pitx2 (Ptx2, P-OTX2)
Pitx2 is a member of the bicoid-like homeobox transcription factor family closely related to the mammalian Otx genes. It is expressed in the rostral brain during development and required at multiple stages of pituitary development [47]. Ptx2 is expressed in thyrotrophs, gonadotrophs, somatotrophs and lactotrophs, but not in corticotrophs [48]. Mutations of PTX2, also known as RIEG in humans, have been described in patients diagnosed with Rieger Syndrome, an autosomal dominant condition with variable manifestations including anomalies of the anterior chamber of the eye, dental hypoplasia, a protuberant umbilicus, mental retardation, and pituitary abnormalities [49]. Six point mutations of Ptx2, located within the homeodomain responsible for DNA binding, have been described, and several of these mutations show loss of DNA binding capacity [49]. Furthermore, a heterozygous mutation that changes the lysine at position 50 to glutamic acid in the homeodomain has been described as imparting a dominant negative effect leading to an inability to not only bind DNA and transactivate the promoter in transfection assays, but also prevent synergism with Pit-1, which is important for somatotroph function [50].
POU1F1 (GHF-1, Pit-1)
POU1F1 is a member of the POU family of transcription factors that contains two protein domains, the POU-specific and POU-homeo. Both domains are necessary for DNA binding, activating GH and PRL genes and regulating PRL, TSH-β and Pit-1 genes [22]. Pit-1 regulates target genes by binding to response elements and recruiting coactivator proteins such as cAMP response element-binding protein (CREB) - binding protein (CBP) [53]. Its expression is restricted to the AP lobe and essential for the development of somatotrophs, lactotrophs and thyrotrophs [22]. Several patients diagnosed with CPHD with GH, PRL and TSH deficiencies have Pit-1 gene mutations [54]. The arginine to tryptophan mutation at codon 271 (R271W) located within the POU-homeodomain region is considered the most common mutation and has been described in several unrelated patients [54–56] This mutant Pit-1 gene is able to bind DNA; however, the mutant protein acts as a dominant inhibitor of target gene transcription [56]. Recent evidence confirmed the dominant negative effect of this mutation and also suggested a role for Pit-1 in cell survival [57]. A reported patient diagnosed with GH deficiency, along with dysregulation of PRL and TSH, was identified with a lysine to glutamic acid mutation at codon 216 (K216E) [58]. This mutant Pit-1 binds to DNA and does not inhibit basal activation of GH and PRL genes; however, the mutant is unable to support retinoic acid induction of Pit-1 gene expression [58]. A more recent report suggests that the cAMP response element-binding protein (CREB)-binding protein (CBP/p300) recruitment and Pit-1 dimerization are necessary for Pit-1 target gene activation; disruption of this process may account for the pathogenesis of CPHD [59].
Prop-1 (“Prophet of Pit-1”)
Prop1, a paired-like homeodomain transcription factor with expression restricted to the AP during development, has also been associated with CPHD [54]. Mutations of Prop-1 result in GH, PRL and TSH deficiencies, although failure in all cell lineages, including gonadotrophs and corticotrophs has been reported [62–64].The characterization of Prop1 mutations has been complex as the phenotypes are variable and dynamic since hormone deficiencies may develop over time even in patients with similar genetic backgrounds [62, 63]. A screen of 73 subjects (36 unrelated families) diagnosed with CPHD by Deladoey et al identified 35 patients with Prop-1 gene defects including three different missense mutations, two frameshift mutations and one splice site mutation. In 12 of the families, defects were located in the region nt 296–302, suggesting a possible hot spot for Prop-1 mutations in CPHD [65]. Although Prop-1 mutations appear to be rare in sporadic cases, its prevalence is 29.5% in familial cases of CHPD as reported by Turton et al [66].
Six6
Six6 is a member of the SIX/sine oculis family of homeobox genes that is expressed in the retina, optic nerve, hypothalamus and pituitary [67]. Murine expression studies of the TCF/LEF family of transcription factors during pituitary development demonstrate that Six6 plays a role in cell proliferation during early formation of RP [68]. Six6 maps to chromosome 14q22–23, and patients with deletions of this chromosomal region display bilateral anophthalmia and pituitary anomalies [69]. Patients with anophthalmia/microphthalmia have several frequent polymorphisms of Six6 and one potential causative missense mutation [67]. One case report implicates Six6 haploinsufficiency responsible for ocular and pituitary maldevelopment. Despite its importance in early development, further studies to determine the role of Six6 mutations in patients with pituitary hormone deficiency are necessary. Finally, another member of the SIX/sine oculis family, Six3, with embryological expression overlapping with Hesx1, plays an important role in pituitary morphogenesis [70].
Mutations in single cell-type genes resulting in pituitary hormone deficiency
In addition, to those genes that lead to CPHD, investigators also reported and characterized mutations in several genes responsible for the production of single hormones. Although a majority of these patients often present with a single pituitary hormone deficiency, several reports demonstrated the presence of either concomitant hormone deficiencies, anatomical defects or over time the development other hormone deficiencies. Table 3 and below summarizes several pituitary genes responsible for the development or function of a specific pituitary cell type. Mutations in these factors have been reported and the phenotypes characterized.
Table 3.
Developmental Factors Affecting Expression of Single Genes
| Factor | Features/Function | Affected Cells | Clinical Features | Radiological Finding | Inheritance | Refs |
|---|---|---|---|---|---|---|
| GH1 | Encodes GH peptide | S (C,T diagnosed over time in some patients) |
Isolated GH deficiency (sproadic or famililal) with short stature → wide variety of phenotypes Four types: IA: absence of GH and production human GH antibodies large deletion GH1 gene IB: homozygous splice site mutation in GH1 gene or GHRH1R gene II splice site mutation GH1 gene intron 3 III X-linked recessive, manifest agammaglobulinemia Bioinactive GH also described; elevated serum GH along with low basal serum IGF-1 |
MRI findings are variable: Normal pituitary Anterior pituitary hypoplasia (with or with ectopic posterior pituitary) |
AD, AR, X-linked | [5,75–78] |
| GHRHR | Ecodes G-protein-coupled receptor Contains seven transmembrane domains with a high binding affinity for GHRH Expression upregulated by Pit-1 Required for proliferation of somatotrophs |
S (G, delayed) | Severe postnatal growth failure Proportionate dwarfism Decreased cranial size (minimal or no facial hypoplasia) No history of hypoglycemia Delayed puberty Asymptomatic hypotension. Classified as IGHD Type IB |
Anterior pitutary hypoplasia | AR | [79–81] |
| GnRHR1 | Encodes the GnRH receptor on gonadotroph | G | Spectrum of phenotypes: Complete hypergonadotropic hypogonadism (including cryptorchidism) to mild pubertal delay. May account for large portion of familial and sporadic cases of hypogonadism No defects in olfaction |
AR (and sporadic) | [82] | |
|
TBX19 (TPIT) |
Member of T-box transcription factor family Expression restricted to pituitary POMC-expressing lineages: corticotrophs and melanotrophs Important for differentiation of POMC cells Documented mutations lead to loss-of-function |
C | Neonatal ACTH deficiency | Hypoplastic pitutary reported | AR | [83–86] |
| POMC | Encodes for pro-opiomelanocortin (POMC) | C (S, G, T diagnosed in some patients) |
Rare syndrome of isolated ACTH deficiency associated with red hair and severe early-onset obesity Recent report of early onset adrenal insufficiency associated with obesity, normal pigmentation and CPHD |
AR | [87] | |
| TSHβ | Encodes the β subunit of the thyrotropin molecule | T | Severe congenital hypothyroidism (non-goitrous cretinism) | Hyperplastic pituitary reported | AR | [88,89] |
| TRHr | Encodes the thyrotropin releasing hormone receptor on the thyrotroph |
T | Short stature and central hypothyroidism | AR | [90] | |
| LHβ/FSHβ | Encodes the β subunit of the LH or FSH molecule | G | Delayed or normal puberty, hypogonadism, infertility Most reported cases are familial |
AR | [91] |
Legend: S- somatotrophs, L - lactotrophs, T - thyrotrophs, G - gonadotrophs, C - corticotrophs CPHD - Combinded Pituitary Hormone Deficiency, ACTH - adrenocoroticotrophic hormone, AD - Autosomal Dominant, AR - Autosomal Recessive
GH1
The GH1 gene, part of the GH gene family located on the long arm of chromosome 17 (GH1, GH2, CSHP1, CSH CSH2), encodes for GH. One report estimates that 12.5% of patients diagnosed with isolated growth hormone deficiency (IGHD) have a mutation in GH1; however, ethnic and geographical differences and patient selection make the true incidence difficult to discern [75]. IGHD has been divided into three types based on clinical presentation and inheritance patterns. Type 1 IGHD, inherited with an autosomal recessive pattern, is further subdivided into two groups: Type 1A and Type 1B [5]. Patients with IGHD Type 1A, the most severe form of IGHD attributed to the deletion of the GH1 gene, present with no detectable serum GH. These patients may have decreased birth length, neonatal hypoglycemia and severe postnatal growth retardation [5]. The initial response to recombinant GH therapy is often robust; however, some patients may develop anti-GH neutralizing antibodies to the therapy [5]. IGHD Type IB is a milder form demonstrating low but detectable GH secretion after provocative stimulation and is often due to a homozygous splice site mutation in GH1. Mutations in the GH releasing hormone receptor (GHRHR) have also been categorized as type I IGHD. Type 2 IGHD, which is considered the common form, is an autosomal dominant disorder associated with GH1 gene mutations, which include splice site and missense mutations, Finally, Type 3 IGHD is an X-linked recessive disorder that has been associated with short stature and X-linked agammaglobulinemia.
Bioinactive GH has also been considered an etiology of short stature. These patients present with high serum GH levels and low serum IGF-1 concentrations. A recent report describes a mutation leading to the absence of a disulfide bridge in the GH1 gene resulting in decreased binding to its receptor and diminished activation of downstream signaling pathways [76]. Unlike the previously reported mutations that were all heterozygous, this mutation was found in the homozygous state in the patient studied.
The etiology of GH deficiency in the majority of patients with IGHD is often labeled as idiopathic and the phenotype of short stature may be quite variable. Some patients given the initial diagnosis of IGHD may develop corticotroph and thyrotroph deficiency later on in life. Finally, there is also a variable presentation of MRI findings that includes anterior pituitary hypoplasia. Anatomical defects, therefore, may account for GHD, although the etiology of abnormal pituitary development may be unknown.
Mutations in several other genes, including GHRHR, TBX19, POMC, TSH, LH and FSH have also been reported in patients presenting with single hormone deficiencies (Table 3). Finally, there are several genes expressed in the hypothalamus that are described with mutations leading to pituitary hormone deficiency; however, this review is targeted to simply focus on genes specific to the pituitary.
Conclusion
The sophisticated coordination of expression of a series of proteins occurring in a specific temporal and spatial pattern is required for both proper structural development and function of the anterior pituitary gland. This knowledge, combined with advances of genetic mapping in humans, has provided a genetic basis for hypopituitarism. Despite the improvements in hormone replacement that have reduced the morbidity associated with hormone deficiency, currently available treatments may be less than ideal for most patients. Furthermore, one of the challenges in understanding the genetics of hypopituitarism lies in the phenotypic and dynamic variability seen in patients. The attempt to target specific genes based on phenotype in an algorithmic approach at this time is unfortunately limited. In the future, however, improved diagnostic tools will be available to predict associated deficiencies as well as therapeutic choices to potentially reverse the associated hormone deficiencies. The use of viral vectors for gene transfer, for example, has already shown promise in treating murine tumor growth. Such technology may help repair gene abnormalities due to deficiencies associated with mutations in developmental genes in patients [92]. For this reason, progress in genetic screening in patients with hypopituitarism and their families will aid in the discovery of novel proteins and in the characterization of existing mutations in pituitary developmental factors, thus providing the necessary insight to improving the diagnosis and treatment of CPHD.
Glossary
- Agammaglobulinemia
an inherited disorder, primarily affecting males, in which the patient’s B lymphocytes fail to develop causing low levels of immunoglobulins.
- Amenorrhea
the absence of a menstrual period in women of reproductive age. This condition can be subdivided as primary amennorhea, the absence of menstruation in a female greater than 16 years of age, and secondary amenorrhea, the absence of menstruation in a female greater than six months from the last menstrual period.
- Anophthalmia
the absence of one or both eyes at birth.
- Chiari Malformation
a structural abnormality of the brain consisting of a downward displacement of the cerebellar tonsils and medulla through the foramen magnum that causes cerebrospinal fluid obstruction and leads to hydrocephalus.
- Corpus callosum
a structure in the brain consisting of white matter that connects the left and right hemispheres.
- Holoprosencephaly
a spectrum of cephalic disorders in which the forebrain fails to divide into two hemispheres. The phenotype may be mild including central brain defects to severe resulting in cyclopia.
- Lactotrophs
anterior pituitary cells that secrete prolactin. Suppression of prolactin in under regulation of dopamine secreted from the hypothalamus
- Rathke’s pouch
an embryological structure formed from the juxtaposition of the neural ectoderm and oral endoderm that will give rise to the anterior pituitary.
- Sella turcica
a “saddle-shaped” depression in the sphenoid bone of the skull. It is the site where the pituitary develops and located after development.
- Septum pellucidum
a thin membrane located centrally in the brain that separates the lateral ventricles in the brain.
- Somatotrophs
anterior pituitary cells that secrete growth hormone
- Thyrotrophs
anterior pituitary cells that secrete thyroid stimulating hormone.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ascoli P, Cavagnini F. Hypopituitarism. Pituitary. 2006;9:335–342. doi: 10.1007/s11102-006-0416-5. [DOI] [PubMed] [Google Scholar]
- 2.Toogood AA, Stewart PM. Hypopituitarism: clinical features, diagnosis, and management. Endocrinol Metab Clin North Am. 2008;37:235–261. doi: 10.1016/j.ecl.2007.10.004. x. [DOI] [PubMed] [Google Scholar]
- 3.Mehta A, et al. Congenital hypopituitarism: clinical, molecular and neuroradiological correlates. Clin Endocrinol (Oxf) 2009 doi: 10.1111/j.1365-2265.2009.03572.x. [DOI] [PubMed] [Google Scholar]
- 4.Ogilvy-Stuart AL. Growth hormone deficiency (GHD) from birth to 2 years of age: diagnostic specifics of GHD during the early phase of life. Horm Res. 2003;60:2–9. doi: 10.1159/000071219. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez LM, Lee PD, Camacho-Hubner C. Isolated growth hormone deficiency. Pituitary. 2007;10:351–357. doi: 10.1007/s11102-007-0073-3. [DOI] [PubMed] [Google Scholar]
- 6.Moseley CT, Orenstein MD, Phillips JA., 3rd GH Gene Deletions and IGHD type IA. Rev Endocr Metab Disord. 2002;3:339–346. doi: 10.1023/a:1020953608174. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, et al. Pituitary progenitor cells tracked down by side population dissection. Stem Cells. 2009;27:1182–1195. doi: 10.1002/stem.51. [DOI] [PubMed] [Google Scholar]
- 8.Vankelecom H. Non-hormonal cell types in the pituitary candidating for stem cell. Semin Cell Dev Biol. 2007;18:559–570. doi: 10.1016/j.semcdb.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Zhu X, et al. Signaling and epigenetic regulation of pituitary development. Curr Opin Cell Biol. 2007;19:605–611. doi: 10.1016/j.ceb.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelberman D, Dattani MT. The role of transcription factors implicated in anterior pituitary development in the aetiology of congenital hypopituitarism. Ann Med. 2006;38:560–577. doi: 10.1080/07853890600994963. [DOI] [PubMed] [Google Scholar]
- 11.Scully KM, Rosenfeld MG. Pituitary development: regulatory codes in mammalian organogenesis. Science. 2002;295:2231–2235. doi: 10.1126/science.1062736. [DOI] [PubMed] [Google Scholar]
- 12.Dasen JS, Rosenfeld MG. Signaling and transcriptional mechanisms in pituitary development. Annu Rev Neurosci. 2001;24:327–355. doi: 10.1146/annurev.neuro.24.1.327. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, et al. Opposing LSD1 complexes function in developmental gene activation and repression programmes. Nature. 2007;446:882–887. doi: 10.1038/nature05671. [DOI] [PubMed] [Google Scholar]
- 14.Fauquier T, et al. Hidden face of the anterior pituitary. Trends Endocrinol Metab. 2002;13:304–309. doi: 10.1016/s1043-2760(02)00616-1. [DOI] [PubMed] [Google Scholar]
- 15.Bonnefont X, et al. Revealing the large-scale network organization of growth hormone-secreting cells. Proc Natl Acad Sci U S A. 2005;102:16880–16885. doi: 10.1073/pnas.0508202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dattani MT, Robinson IC. HESX1 and Septo-Optic Dysplasia. Rev Endocr Metab Disord. 2002;3:289–300. doi: 10.1023/a:1020945406356. [DOI] [PubMed] [Google Scholar]
- 17.Carvalho LR, et al. A homozygous mutation in HESX1 is associated with evolving hypopituitarism due to impaired repressor-corepressor interaction. J Clin Invest. 2003;112:1192–1201. doi: 10.1172/JCI18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dattani MT, et al. Mutations in the homeobox gene HESX1/Hesx1 associated with septo-optic dysplasia in human and mouse. Nat Genet. 1998;19:125–133. doi: 10.1038/477. [DOI] [PubMed] [Google Scholar]
- 19.Coya R, et al. Panhypopituitarism: genetic versus acquired etiological factors. J Pediatr Endocrinol Metab. 2007;20:27–36. doi: 10.1515/jpem.2007.20.1.27. [DOI] [PubMed] [Google Scholar]
- 20.Sobrier ML, et al. Novel HESX1 mutations associated with a life-threatening neonatal phenotype, pituitary aplasia, but normally located posterior pituitary and no optic nerve abnormalities. J Clin Endocrinol Metab. 2006;91:4528–4536. doi: 10.1210/jc.2006-0426. [DOI] [PubMed] [Google Scholar]
- 21.Tajima T, et al. Sporadic heterozygous frameshift mutation of HESX1 causing pituitary and optic nerve hypoplasia and combined pituitary hormone deficiency in a Japanese patient. J Clin Endocrinol Metab. 2003;88:45–50. doi: 10.1210/jc.2002-020818. [DOI] [PubMed] [Google Scholar]
- 22.Cohen LE, Radovick S, Wondisford FE. Transcription Factors and Hypopituitarism. Trends Endocrinol Metab. 1999;10:326–332. doi: 10.1016/s1043-2760(99)00180-0. [DOI] [PubMed] [Google Scholar]
- 23.Sajedi E, et al. Analysis of mouse models carrying the I26T and R160C substitutions in the transcriptional repressor HESX1 as models for septo-optic dysplasia and hypopituitarism. Dis Model Mech. 2008;1:241–254. doi: 10.1242/dmm.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNay DE, et al. HESX1 mutations are an uncommon cause of septooptic dysplasia and hypopituitarism. J Clin Endocrinol Metab. 2007;92:691–697. doi: 10.1210/jc.2006-1609. [DOI] [PubMed] [Google Scholar]
- 25.Thomas PQ, et al. Heterozygous HESX1 mutations associated with isolated congenital pituitary hypoplasia and septo-optic dysplasia. Hum Mol Genet. 2001;10:39–45. doi: 10.1093/hmg/10.1.39. [DOI] [PubMed] [Google Scholar]
- 26.Hunter CS, Rhodes SJ. LIM-homeodomain genes in mammalian development and human disease. Mol Biol Rep. 2005;32:67–77. doi: 10.1007/s11033-004-7657-z. [DOI] [PubMed] [Google Scholar]
- 27.Sheng HZ, et al. Specification of pituitary cell lineages by the LIM homeobox gene Lhx3. Science. 1996;272:1004–1007. doi: 10.1126/science.272.5264.1004. [DOI] [PubMed] [Google Scholar]
- 28.Sloop KW, Dwyer CJ, Rhodes SJ. An isoform-specific inhibitory domain regulates the LHX3 LIM homeodomain factor holoprotein and the production of a functional alternate translation form. J Biol Chem. 2001;276:36311–36319. doi: 10.1074/jbc.M103888200. [DOI] [PubMed] [Google Scholar]
- 29.Pfaeffle RW, et al. Four novel mutations of the LHX3 gene cause combined pituitary hormone deficiencies with or without limited neck rotation. J Clin Endocrinol Metab. 2007;92:1909–1919. doi: 10.1210/jc.2006-2177. [DOI] [PubMed] [Google Scholar]
- 30.Rajab A, et al. Novel mutations in LHX3 are associated with hypopituitarism and sensorineural hearing loss. Hum Mol Genet. 2008;17:2150–2159. doi: 10.1093/hmg/ddn114. [DOI] [PubMed] [Google Scholar]
- 31.Bhangoo AP, et al. Clinical case seminar: a novel LHX3 mutation presenting as combined pituitary hormonal deficiency. J Clin Endocrinol Metab. 2006;91:747–753. doi: 10.1210/jc.2005-2360. [DOI] [PubMed] [Google Scholar]
- 32.Netchine I, et al. Mutations in LHX3 result in a new syndrome revealed by combined pituitary hormone deficiency. Nat Genet. 2000;25:182–186. doi: 10.1038/76041. [DOI] [PubMed] [Google Scholar]
- 33.Kristrom B, et al. A novel mutation in the LHX3 gene is responsible for combined pituitary hormone deficiency, hearing impairment, and vertebral malformations. J Clin Endocrinol Metab. 2009;94:1154–1161. doi: 10.1210/jc.2008-0325. [DOI] [PubMed] [Google Scholar]
- 34.Mullen RD, et al. Roles of the LHX3 and LHX4 LIM-homeodomain factors in pituitary development. Mol Cell Endocrinol. 2007;265–266:190–195. doi: 10.1016/j.mce.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raetzman LT, Ward R, Camper SA. Lhx4 and Prop1 are required for cell survival and expansion of the pituitary primordia. Development. 2002;129:4229–4239. doi: 10.1242/dev.129.18.4229. [DOI] [PubMed] [Google Scholar]
- 36.Sheng HZ, et al. Multistep control of pituitary organogenesis. Science. 1997;278:1809–1812. doi: 10.1126/science.278.5344.1809. [DOI] [PubMed] [Google Scholar]
- 37.Machinis K, Amselem S. Functional relationship between LHX4 and POU1F1 in light of the LHX4 mutation identified in patients with pituitary defects. J Clin Endocrinol Metab. 2005;90:5456–5462. doi: 10.1210/jc.2004-2332. [DOI] [PubMed] [Google Scholar]
- 38.Machinis K, et al. Syndromic short stature in patients with a germline mutation in the LIM homeobox LHX4. Am J Hum Genet. 2001;69:961–968. doi: 10.1086/323764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfaeffle RW, et al. Three novel missense mutations within the LHX4 gene are associated with variable pituitary hormone deficiencies. J Clin Endocrinol Metab. 2008;93:1062–1071. doi: 10.1210/jc.2007-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tajima T, et al. A novel missense mutation (P366T) of the LHX4 gene causes severe combined pituitary hormone deficiency with pituitary hypoplasia, ectopic posterior lobe and a poorly developed sella turcica. Endocr J. 2007;54:637–641. doi: 10.1507/endocrj.k06-200. [DOI] [PubMed] [Google Scholar]
- 41.Kurokawa D, et al. Regulation of Otx2 expression and its functions in mouse forebrain and midbrain. Development. 2004;131:3319–3331. doi: 10.1242/dev.01220. [DOI] [PubMed] [Google Scholar]
- 42.Puelles E, et al. Otx2 regulates the extent, identity and fate of neuronal progenitor domains in the ventral midbrain. Development. 2004;131:2037–2048. doi: 10.1242/dev.01107. [DOI] [PubMed] [Google Scholar]
- 43.Diaczok D, et al. A novel dominant negative mutation of OTX2 associated with combined pituitary hormone deficiency. J Clin Endocrinol Metab. 2008;93:4351–4359. doi: 10.1210/jc.2008-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chatelain G, et al. Molecular dissection reveals decreased activity and not dominant negative effect in human OTX2 mutants. J Mol Med. 2006;84:604–615. doi: 10.1007/s00109-006-0048-2. [DOI] [PubMed] [Google Scholar]
- 45.Ragge NK, et al. Heterozygous mutations of OTX2 cause severe ocular malformations. Am J Hum Genet. 2005;76:1008–1022. doi: 10.1086/430721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tajima T, et al. OTX2 loss of function mutation causes anophthalmia and combined pituitary hormone deficiency with a small anterior and ectopic posterior pituitary. J Clin Endocrinol Metab. 2009;94:314–319. doi: 10.1210/jc.2008-1219. [DOI] [PubMed] [Google Scholar]
- 47.Suh H, et al. Pitx2 is required at multiple stages of pituitary organogenesis: pituitary primordium formation and cell specification. Development. 2002;129:329–337. doi: 10.1242/dev.129.2.329. [DOI] [PubMed] [Google Scholar]
- 48.Drouin J, et al. The PTX family of homeodomain transcription factors during pituitary developments. Mol Cell Endocrinol. 1998;140:31–36. doi: 10.1016/s0303-7207(98)00026-4. [DOI] [PubMed] [Google Scholar]
- 49.Amendt BA, Semina EV, Alward WL. Rieger syndrome: a clinical, molecular, and biochemical analysis. Cell Mol Life Sci. 2000;57:1652–1666. doi: 10.1007/PL00000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saadi I, et al. Identification of a dominant negative homeodomain mutation in Rieger syndrome. J Biol Chem. 2001;276:23034–23041. doi: 10.1074/jbc.M008592200. [DOI] [PubMed] [Google Scholar]
- 51.Charles MA, et al. Pitx2 deletion in pituitary gonadotropes is compatible with gonadal development, puberty, and fertility. Genesis. 2008;46:507–514. doi: 10.1002/dvg.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cella W, et al. Structural assessment of PITX2, FOXC1, CYP1B1, and GJA1 genes in patients with Axenfeld-Rieger syndrome with developmental glaucoma. Invest Ophthalmol Vis Sci. 2006;47:1803–1809. doi: 10.1167/iovs.05-0979. [DOI] [PubMed] [Google Scholar]
- 53.Xu L, et al. Signal-specific co-activator domain requirements for Pit-1 activation. Nature. 1998;395:301–306. doi: 10.1038/26270. [DOI] [PubMed] [Google Scholar]
- 54.Kelberman D, Dattani MT. Hypopituitarism oddities: congenital causes. Horm Res. 2007;68 Suppl 5:138–144. doi: 10.1159/000110610. [DOI] [PubMed] [Google Scholar]
- 55.Turton JP, et al. Novel mutations within the POU1F1 gene associated with variable combined pituitary hormone deficiency. J Clin Endocrinol Metab. 2005;90:4762–4770. doi: 10.1210/jc.2005-0570. [DOI] [PubMed] [Google Scholar]
- 56.Cohen LE, et al. A "hot spot" in the Pit-1 gene responsible for combined pituitary hormone deficiency: clinical and molecular correlates. J Clin Endocrinol Metab. 1995;80:679–684. doi: 10.1210/jcem.80.2.7852536. [DOI] [PubMed] [Google Scholar]
- 57.Pellegrini I, et al. Involvement of the pituitary-specific transcription factor pit-1 in somatolactotrope cell growth and death: an approach using dominant-negative pit-1 mutants. Mol Endocrinol. 2006;20:3212–3227. doi: 10.1210/me.2006-0122. [DOI] [PubMed] [Google Scholar]
- 58.Cohen LE, et al. Defective retinoic acid regulation of the Pit-1 gene enhancer: a novel mechanism of combined pituitary hormone deficiency. Mol Endocrinol. 1999;13:476–484. doi: 10.1210/mend.13.3.0251. [DOI] [PubMed] [Google Scholar]
- 59.Cohen RN, et al. The role of CBP/p300 interactions and Pit-1 dimerization in the pathophysiological mechanism of combined pituitary hormone deficiency. J Clin Endocrinol Metab. 2006;91:239–247. doi: 10.1210/jc.2005-1211. [DOI] [PubMed] [Google Scholar]
- 60.Hashimoto Y, Cisternino M, Cohen LE. A novel nonsense mutation in the Pit-1 gene: evidence for a gene dosage effect. J Clin Endocrinol Metab. 2003;88:1241–1247. doi: 10.1210/jc.2002-021510. [DOI] [PubMed] [Google Scholar]
- 61.Arnhold IJ, et al. Clinical and molecular characterization of a Brazilian patient with Pit-1 deficiency. J Pediatr Endocrinol Metab. 1998;11:623–630. doi: 10.1515/jpem.1998.11.5.623. [DOI] [PubMed] [Google Scholar]
- 62.Lebl J, et al. Auxological and endocrine phenotype in a population-based cohort of patients with PROP1 gene defects. Eur J Endocrinol. 2005;153:389–396. doi: 10.1530/eje.1.01989. [DOI] [PubMed] [Google Scholar]
- 63.Vieira TC, da Silva MR, Abucham J. The natural history of the R120C PROP1 mutation reveals a wide phenotypic variability in two untreated adult brothers with combined pituitary hormone deficiency. Endocrine. 2006;30:365–369. doi: 10.1007/s12020-006-0015-2. [DOI] [PubMed] [Google Scholar]
- 64.Fluck C, et al. Phenotypic variability in familial combined pituitary hormone deficiency caused by a PROP1 gene mutation resulting in the substitution of Arg-->Cys at codon 120 (R120C) J Clin Endocrinol Metab. 1998;83:3727–3734. doi: 10.1210/jcem.83.10.5172. [DOI] [PubMed] [Google Scholar]
- 65.Deladoey J, et al. "Hot spot" in the PROP1 gene responsible for combined pituitary hormone deficiency. J Clin Endocrinol Metab. 1999;84:1645–1650. doi: 10.1210/jcem.84.5.5681. [DOI] [PubMed] [Google Scholar]
- 66.Turton JP, et al. Mutations within the transcription factor PROP1 are rare in a cohort of patients with sporadic combined pituitary hormone deficiency (CPHD) Clin Endocrinol (Oxf) 2005;63:10–18. doi: 10.1111/j.1365-2265.2005.02291.x. [DOI] [PubMed] [Google Scholar]
- 67.Gallardo ME, et al. Analysis of the developmental SIX6 homeobox gene in patients with anophthalmia/microphthalmia. Am J Med Genet A. 2004;129A:92–94. doi: 10.1002/ajmg.a.30126. [DOI] [PubMed] [Google Scholar]
- 68.Brinkmeier ML, et al. TCF4 deficiency expands ventral diencephalon signaling and increases induction of pituitary progenitors. Dev Biol. 2007;311:396–407. doi: 10.1016/j.ydbio.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nolen LD, et al. Deletion at 14q22-23 indicates a contiguous gene syndrome comprising anophthalmia, pituitary hypoplasia, and ear anomalies. Am J Med Genet A. 2006;140:1711–1718. doi: 10.1002/ajmg.a.31335. [DOI] [PubMed] [Google Scholar]
- 70.Gaston-Massuet C, et al. Genetic interaction between the homeobox transcription factors HESX1 and SIX3 is required for normal pituitary development. Dev Biol. 2008;324:322–333. doi: 10.1016/j.ydbio.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kelberman D, et al. SOX2 Plays a Critical Role in the Pituitary, Forebrain, and Eye during Human Embryonic Development. J Clin Endocrinol Metab. 2008;93:1865–1873. doi: 10.1210/jc.2007-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kelberman D, et al. Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. J Clin Invest. 2006;116:2442–2455. doi: 10.1172/JCI28658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Solomon NM, et al. Array comparative genomic hybridisation analysis of boys with X-linked hypopituitarism identifies a 3.9 Mb duplicated critical region at Xq27 containing SOX3. J Med Genet. 2007;44:e75. doi: 10.1136/jmg.2007.049049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woods KS, et al. Over- and underdosage of SOX3 is associated with infundibular hypoplasia and hypopituitarism. Am J Hum Genet. 2005;76:833–849. doi: 10.1086/430134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wagner JK, et al. Prevalence of human GH-1 gene alterations in patients with isolated growth hormone deficiency. Pediatr Res. 1998;43:105–110. doi: 10.1203/00006450-199801000-00016. [DOI] [PubMed] [Google Scholar]
- 76.Besson A, et al. Short stature caused by a biologically inactive mutant growth hormone (GH-C53S) J Clin Endocrinol Metab. 2005;90:2493–2499. doi: 10.1210/jc.2004-1838. [DOI] [PubMed] [Google Scholar]
- 77.Mullis PE, et al. Isolated autosomal dominant growth hormone deficiency: an evolving pituitary deficit? A multicenter follow-up study. J Clin Endocrinol Metab. 2005;90:2089–2096. doi: 10.1210/jc.2004-1280. [DOI] [PubMed] [Google Scholar]
- 78.De Bellis A, et al. Autoimmunity as a possible cause of growth hormone deficiency. J Endocrinol Invest. 2008;31:1132–1134. doi: 10.1007/BF03345664. [DOI] [PubMed] [Google Scholar]
- 79.Maheshwari HG, et al. Phenotype and genetic analysis of a syndrome caused by an inactivating mutation in the growth hormone-releasing hormone receptor: Dwarfism of Sindh. J Clin Endocrinol Metab. 1998;83:4065–4074. doi: 10.1210/jcem.83.11.5226. [DOI] [PubMed] [Google Scholar]
- 80.Murray RA, et al. Pituitary hypoplasia in patients with a mutation in the growth hormone-releasing hormone receptor gene. AJNR Am J Neuroradiol. 2000;21:685–689. [PMC free article] [PubMed] [Google Scholar]
- 81.Wajnrajch MP, et al. Haplotype analysis of the growth hormone releasing hormone receptor locus in three apparently unrelated kindreds from the indian subcontinent with the identical mutation in the GHRH receptor. Am J Med Genet A. 2003;120A:77–83. doi: 10.1002/ajmg.a.10209. [DOI] [PubMed] [Google Scholar]
- 82.Beranova M, et al. Prevalence, phenotypic spectrum, and modes of inheritance of gonadotropin-releasing hormone receptor mutations in idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2001;86:1580–1588. doi: 10.1210/jcem.86.4.7395. [DOI] [PubMed] [Google Scholar]
- 83.Pulichino AM, et al. Tpit mutations reveal a new model of pituitary differentiation and account for isolated ACTH deficiency. Med Sci (Paris) 2004;20:1009–1013. doi: 10.1051/medsci/200420111009. [DOI] [PubMed] [Google Scholar]
- 84.Vallette-Kasic S, et al. Congenital isolated adrenocorticotropin deficiency: an underestimated cause of neonatal death, explained by TPIT gene mutations. J Clin Endocrinol Metab. 2005;90:1323–1331. doi: 10.1210/jc.2004-1300. [DOI] [PubMed] [Google Scholar]
- 85.Vallette-Kasic S, et al. A neonatal form of isolated ACTH deficiency frequently associated with Tpit gene mutations. Endocr Res. 2004;30:943–944. doi: 10.1081/erc-200044166. [DOI] [PubMed] [Google Scholar]
- 86.Pulichino AM, et al. Human and mouse TPIT gene mutations cause early onset pituitary ACTH deficiency. Genes Dev. 2003;17:711–716. doi: 10.1101/gad.1065603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Clement K, et al. Unexpected endocrine features and normal pigmentation in a young adult patient carrying a novel homozygous mutation in the POMC gene. J Clin Endocrinol Metab. 2008;93:4955–4962. doi: 10.1210/jc.2008-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bonomi M, et al. Hyperplastic pituitary gland, high serum glycoprotein hormone alpha-subunit, and variable circulating thyrotropin (TSH) levels as hallmark of central hypothyroidism due to mutations of the TSH beta gene. J Clin Endocrinol Metab. 2001;86:1600–1604. doi: 10.1210/jcem.86.4.7411. [DOI] [PubMed] [Google Scholar]
- 89.Miyai K. Congenital thyrotropin deficiency--from discovery to molecular biology, postgenome and preventive medicine. Endocr J. 2007;54:191–203. doi: 10.1507/endocrj.kr-107. [DOI] [PubMed] [Google Scholar]
- 90.Collu R, et al. A novel mechanism for isolated central hypothyroidism: inactivating mutations in the thyrotropin-releasing hormone receptor gene. J Clin Endocrinol Metab. 1997;82:1561–1565. doi: 10.1210/jcem.82.5.3918. [DOI] [PubMed] [Google Scholar]
- 91.Trarbach EB, Silveira LG, Latronico AC. Genetic insights into human isolated gonadotropin deficiency. Pituitary. 2007;10:381–391. doi: 10.1007/s11102-007-0061-7. [DOI] [PubMed] [Google Scholar]
- 92.Quentien MH, et al. Pituitary transcription factors: from congenital deficiencies to gene therapy. J Neuroendocrinol. 2006;18:633–642. doi: 10.1111/j.1365-2826.2006.01461.x. [DOI] [PubMed] [Google Scholar]