Abstract
Sleep disturbance is associated with inflammation and related disorders including cardiovascular disease, arthritis, and diabetes mellitus. Given sex differences in the prevalence of inflammatory disorders with stronger associations in females, this study was undertaken to test the effects of sleep loss on cellular mechanisms that contribute to proinflammatory cytokine activity. In 26 healthy adults (11 females; 15 males), monocyte intracellular proinflammatory cytokine production was repeatedly assessed at 08:00, 12:00, 16:00, 20:00, and 23:00 h during a baseline period and after partial sleep deprivation (awake from 11 PM to 3 AM). In the morning after a night of sleep loss, monocyte production of interleukin 6 and tumor necrosis factor- α differentially changed between the two sexes. Whereas both females and males showed a marked increase in the lipopolysaccharide (LPS) - stimulated production of IL-6 and TNF-α in the morning immediately after PSD, production of these cytokines during the early- and late evening was increased in the females as compared to decreases in the males. Sleep loss induces a functional alteration of monocyte proinflammatory cytokine responses with females showing greater cellular immune activation as compared to changes in males. These results have implications for understanding the role of sleep disturbance in the differential risk profile for inflammatory disorders between the sexes.
Introduction
Epidemiologic data indicate that sleep disturbance and short sleep duration adversely impact human physical health (Gangwisch et al., 2007) and mortality risk (Dew et al., 2003; Gangwisch et al., 2008; Shankar et al., 2008). Although the biological basis for these links between sleep and health are not known, risk of a wide spectrum of medical conditions including cardiovascular disease, arthritis, diabetes, certain cancers, obesity, and functional decline is associated with activation of cellular signals that initiate expression of inflammatory cytokines (Karin, 2005; Volpato et al., 2001).
Sleep loss has been found to have consequences on inflammatory mechanisms. Experimental sleep deprivation induces increases in circulating levels of inflammatory markers such as interleukin-6 (IL-6), tumor necrosis factor-α(TNF-α), and C-reactive protein (CRP) (Irwin et al., 2004; Meier-Ewert et al., 2004; Shearer et al., 2001). In addition, early night partial sleep deprivation (PSD) activates cellular expression of IL-6 and TNF-α, with effects on up-stream sources of cellular inflammatory cytokine expression (Irwin et al., 2006). Moreover, such up-regulation of inflammatory gene expression following such sleep loss (Irwin et al., 2006) is due to activation of nuclear factor (NF)-κB transcription control (Irwin et al., 2008) that plays a key role in controlling cellular expression of pro-inflammatory genes (Pascual and Glass, 2006). Activation of NF-κB following sleep loss, however, occurs primarily in females, but not in males (Irwin et al., 2008).
There are well-known sex differences in the prevalence of inflammatory disorders, with females being between 2-9 times as likely as males to develop autoimmune disorders including arthritis and diabetes (Whitacre, 2001). In addition, whereas cardiovascular disease is thought to have an inflammatory basis and is more prevalent in males than females, recent epidemiologic data indicate that subjective symptoms of disturbed sleep and short sleep duration were associated with a greater risk of cardiovascular disease in females than in males, even after control for relevant confounders such as body mass index and physical activity (Cappuccio et al., 2007; Newman et al., 2000). Moreover, Suarez (Suarez, 2008) recently found that poor sleep quality and self-reported prolonged sleep latency were associated with increases of CRP and IL-6 in females but not males. Hence, in this study we sought to determine sex differences in the effects of experimental sleep loss on the inflammatory response by measuring the production of proinflammatory cytokines by monocytes following ligation of the Toll-like receptor 4 (TLR4) with lipopolyssacharide (LPS). TLRs mediate innate immune responses to common pathogens (Cook et al., 2004), and aberrant increases of TLR activity has been linked to inflammatory diseases such as rheumatoid arthritis (Andreakos et al., 2004), Crohn’s disease (Andreakos et al., 2004), and heart failure (Satoh et al., 2005).
Methods
Twenty six subjects (eleven women and fifteen men) who were medically healthy, as determined by medical history, physical examination, and laboratory testing, were recruited between October 2006 and June 2008. All subjects fulfilled criteria for Never Mentally Ill as determined by Structured Clinical Interview for Diagnostic and Statistical Manual - IV (SCID). Females and males did not differ in mean ± SD age (37.2 ± 9.6 vs. 36.3 ± 10.4 years; t = 0.2, P = 0.82), education level (15.9 ± 1.8 vs. 15.3 ± 1.8; t = 0.9, P = 0.37) mean body mass index [BMI] (23.8 ± 3.8 vs. 24.9 ± 3.7; t = 0.7, P = 0.47), or ethnicity (nonwhite: 36.4% vs. 20.0%; χ2 = 0.86, P = 0.35) 8=26.9 [SD=3.6]; 35.7% were non-white). Without restricting assessments to a single point in the menstrual cycle, females and males did not differ in mean levels of progesterone (1.2 ± 0.6 ng/ml vs. 1.0 ± 0.6; t = 1.1, P = 0.30) or estradiol (67.1 ± 62.3 ng/ml vs. 37.5 ± 9.7 ng/ml; t = 1.9, P = 0.08). Past or current Axis I DSM-IV psychiatric disorder, use of psychotropic medication, regular use of nonsteroidal anti-inflammatory medications, and tobacco smoking were exclusionary. Subjects regularly slept between 22:30 and 7:30 h as confirmed by 2-week sleep diaries. The study was approved by the UCLA Institutional Review Board.
Subjects participated in a PSD protocol that was conducted on the UCLA General Clinical Research Center (GCRC) as previously described (Irwin et al., 2006). The PSD night immediately followed the baseline night and all subjects remained on the GCRC during the day with behavioral monitoring to prevent any napping behavior. Acquisition of blood samples occurred via an indwelling venous forearm catheter at 08:00, 12:00, 16:00, 20:00, and 23:00 h during baseline and after PSD. Hence, a total of 5 measures were obtained prior to sleep deprivation (i.e., baseline) with 5 additional measures obtained after PSD. Blood samples were held at room temperature between collection and assay, as we have found up to a 10 h- interval does not impact assay results; baseline and PSD samples were treated similarly in terms of hold times.
Monocyte intracellular production of IL-6 and TNF-α was assessed by flow cytometry using peridinin chlorophyll protein—labeled CD14 monoclonal antibody, allophycocyanin-labeled anti- TNF-a monoclonal antibody, and phycoerythrinlabeled IL-6 antibody. (Collado-Hidalgo et al., 2006). Heparin-treated blood (1 mL) was mixed with 100 pg/mL LPS (Sigma, St. Louis, MO) and 10 Ag/mL brefeldin A (Sigma) and incubated for 4 hours at 37°C in a platform mixer followed by an overnight incubation at 4°C. RBCs were lysed in fluorescence-activated cell sorting lysing solution (BD Biosciences), remaining cells were permeabilized in fluorescence-activated cell sorting permeabilizing buffer (BD Biosciences), and fluorescence conjugatedantibodies were added for 30 minutes at room temperature in the dark. Cells were then washed and resuspended in 1% paraformaldehyde for assay on a Coulter Elite flow cytometer using the Coulter Elite software. Forward scatter and side scatter were used to gate on monocytes and granulocytes. About 12,000 CD14+ events were counted to determine the percentage of cytokine-secreting monocytes, with quadrant coordinates set based on unstimulated monocytes cells. To determine percentage of stimulated cells expressing TNF-α, IL-6, or co-expressing TNF-α and IL-6, unstimulated cytokine-positive event percentages were subtracted from stimulated percentages to obtain net stimulated cytokine positive event percentages. We have previously reported that PSD does not alter absolute numbers of monocytes (Irwin et al. 1996).
Statistical Analyses
Data were analyzed using SAS version 9.13 for Windows. To determine the effects of PSD on monocyte intracellular proinflammatory cytokine expression in females and males, repeated measures mixed model ANOVA was performed using a 2 (sex: female, male) × 2 (condition: baseline, PSD) × 5 (time: 08:00, 12:00, 16:00, 20:00, 23:00 h) design, covarying for age, education, ethnicity, BMI, progesterone and estradiol. Analyses were conducted for monocytes expressing TNF-α, IL-6, or co-expressing TNF-α and IL-6, as well as the aggregate.
Results
Partial night sleep deprivation induced differential changes in the capacity of monocytes to express TNF-α and IL-6 as compared to the baseline condition, with a condition × time interactions for the cells expressing TNF-α, IL-6, co-expressing TNF-α and IL-6, and the aggregate measure (Figure 1) (F (4,183)= 3.9, P < 0.01; F (4,183) = 25.8, P < 0.001; F (4,183) = 8.2, P < 0.001; F (4,183) = 5.8, P < 0.001). Notably, both females and males showed a marked increase in the stimulated production of IL-6 and TNF-α in the morning immediately after PSD, consistent with our prior findings (Irwin et al., 2006). The fixed effect for time was significant for the cells expressing TNF-α, IL-6, co-expressing TNF-α and IL-6, and the aggregate measure (F (4,184)= 4.3, P < 0.01; F (4,184)= 18.7, P < 0.001; F (4,184)= 21.1, P < 0.001; F (4,184)= 11.5, P < 0.001). In addition, there were condition effects for cells expressing TNF-α, IL-6, co-expressing TNF-α and IL-6 (F (4,184)= 8.2, P < 0.01; F (4,184)= 15.7, P < 0.001; F (4,184)= 6.8, P < 0.01) but not for the aggregate measure (F (1,185)= 0.8, P = 0.37).
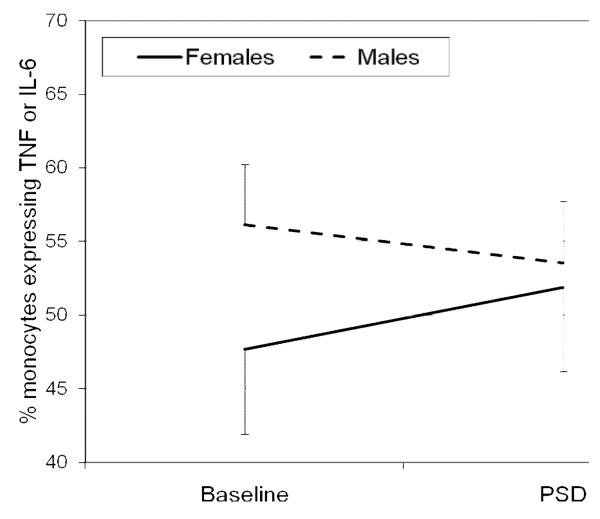
Figure 1.
Sex differences in aggregate expression of interleukin (IL) 6 and tumor necrosis factor- α (TNF) in lipopolysaccharide-stimulated CD14+ cells between baseline and partial sleep deprivation PSD conditions. A significant condition × sex interaction in the aggregate expression of TNF-α and IL-6 was found in which females showed relative increases in aggregate expression of TNF-α and IL-6 as compared to decreases in men (F (1,184)= 13.7, P > 0.001). Data are represented as mean±SEM.
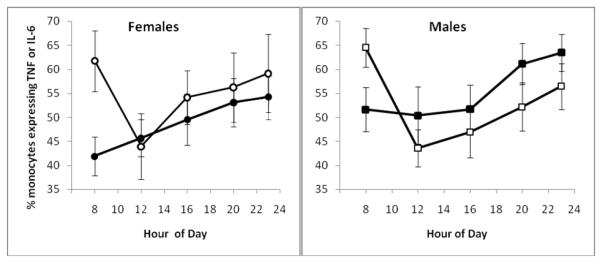
There were sex differences in the capacity of monocytes to express proinflammatory cytokines following PSD, with signficant condition × sex interactions for cells expressing TNF-α, co-expressing TNF-α and IL-6, and the aggregate measure (Figure 1) (F (1,184)= 3.8, P < 0.06; F (1,184)= 14.4, P < 0.001; F (1,184)= 13.7, P > 0.001) but not for cells expressing IL-6 (F (1,184)= 0.3, P = 0.84). Additional analyses examined differential responses at individual timepoints and found condition × sex interactions for cells co-expressing TNF-α and IL-6 and the aggregate measure at two timepoints: 20:00 (F (1,19.0)= 9.4, P < 0.01; F (1,19.0)= 6.0, P < 0.05) and 23:00 (F (1,19.0)= 4.7, P < 0.05; F (1,19.9)= 7.4, P < 0.05) in which females showed increases in the co-and aggregate expression of TNF-α and IL-6 as compared to decreases in men. Figure 2 displays the results for the aggregate measure of TNF-α and IL-6 expression across the individual timepoints. Similar responses between the sexes were found at 8:00, 12:00, and 16:00 (all P > 0.1) and for cells expressing TNF-α or IL-6 at all timepoints (all P > 0.1). No main effect for sex found for cells expressing TNF-α, IL-6, co-expressing TNF-α and IL-6, and the aggregate (all P > 0.1).
Figure 2.
Sex differences in aggregate expression of interleukin (IL) 6 and tumor necrosis factor- α (TNF) in lipopolysaccharide-stimulated CD14+ cells between baseline (•—•) and partial sleep deprivation (o—o)PSD conditions. Significant condition × sex interactions in the aggregate expression of TNF-α and IL-6 was found at two timepoints: 20:00 (F (1,19.0)= 6.0, P < 0.05) and 23:00 (F (1,19.9)= 7.4, P < 0.05) in which females showed increases in aggregate expression of TNF-α and IL-6 as compared to decreases in men. Data are represented as mean±SEM.
Discussion
Here we present evidence that acute sleep loss induces a differential alteration in a functional cellular innate immune response. In the morning after a night of sleep loss, LPS ligation of TLR4 or CD14 triggered significantly greater production of IL-6 and TNF-α in peripheral blood monocyte populations relative to morning levels following uninterrupted sleep. Whereas both females and males showed a similar increase in morning levels of IL-6 and TNF-α, the two sexes differed in cellular immune activation during the subsequent daytime period. As compared to responses in males, females showed increases in the cellular expression of these proinflammatory cytokines during the early- and late evening hours. Consistent with these observations, Suarez (2008) found that poor sleep quality and prolonged sleep latency were associated with increases of circulating levels of CRP and IL-6 in females, but not in males. Moreover, our prior findings demonstrate sex differences in nuclear translocation of NF-kB following sleep loss (Irwin et al., 2006), in which females show elevations of NF-kB in the morning following sleep loss. Although prior work did not evaluate nocturnal levels of NF-kB following PSD, it is possible that this transcription factor is similarly activated in both sexes during the night, which in turn contributes to similar increases in morning levels of IL-6 and TNF-α. To account for sex differences in the daytime expression of IL-6 and TNF-α, we hypothesize that such activation of NF-kB persists into the morning in females, but not in males, and drives subsequent elevations of proinflammatory cytokines during the latter part of the day in females. Together, these data further demonstrate that sleep loss enhances inflammatory biology in females as compared to changes in males, with implications for understanding the increased risk profile for inflammatory disorders in females.
Transient changes in monocyte production of proinflammatory cytokines, even when extended across the course of the day, might not translate into increased risk for disease. However, it is known that aberrant increases of TLR activity are found in association with rheumatoid arthritis (Andreakos et al., 2004), Crohn’s disease (Andreakos et al., 2004), and heart failure (Satoh et al., 2005). Furthermore, increases in monocyte responses to LPS stimulation correlate with increases in circulating inflammatory markers (Collado-Hidalgo et al., 2006), and small elevations in circulating inflammatory mediators, for example, are associated with the syndrome of insulin resistance and type II diabetes mellitus, independent of adiposity (Festa et al., 2000).
PSD induces marked increases in cardiovascular responses (Irwin and Ziegler, 2005) as well as sympathoadrenal activity upon awakening (Irwin et al., 1999; Irwin and Ziegler, 2005). In turn, adrenergic output is known to facilitate in vivo release of inflammatory mediators into circulating blood (Friedman and Irwin, 1997; Johnson et al., 2005). Although catecholamines in vitro are reported to suppress proinflammatory cytokine production (Cole et al., 1998; Wahle et al., 2005), physiological concentrations of norepinephrine are reported to be sufficient to result in a significant dose dependent increase of NF-kB-binding activity in vitro (Bierhaus et al., 2003), which might drive increases in the monocyte expression of proinflammatory cytokines. To our knowledge, no prior study has examined whether sex differences alter adrenergic outflow following sleep loss, although in women, but not men, sympathovagal balance as indexed by heart rate variability has been found to be negatively associated with monocyte IL-6 expression (O’Connor M et al., 2007). Moreover in women, but not in men, vagal tone is positively associated with production of this cytokine (O’Connor M et al., 2007). In turn, circadian variation in sympathetic output might contribute to the circadian variation in stimulated monocyte production of proinflammatory cytokines, which we have previously demonstrated (O’Connor et al., 2007; Irwin et al., 2006). Finally, it is possible that differences in reproductive hormones including changes in levels of such hormones across the menstrual phase contributed to these differences, although these findings were demonstrated even after levels of progesterone and estradiol were statistically adjusted.
Loss of sleep during only part of the night is one of the most common complaints of persons who experience environmental or psychological stress (Akerstedt et al., 1990; McDermott et al., 1997). In this study, PSD was used as an experimental model of difficulties falling asleep. Our results show that a modest amount of sleep loss differentially activates cellular markers of inflammation in females as compared to responses in males. Given these data, further investigations are needed to define the effects of sleep loss on inflammatory mechanisms in females and males, with implications for understanding increased risk of chronic inflammatory disorders in females.
Acknowledgments
The authors have no financial gain related to the outcome of this research, and there are no potential conflicts of interest. This work was supported in part by grants T32-MH19925, HL 079955, AG 026364, CA 10014152, CA116778, RR00827, P30-AG028748, General Clinical Research Centers Program, the UCLA Cousins Center at the Semel Institute for Neurosciences, and the UCLA Older Americans Independence Center Inflammatory Biology Core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akerstedt T, Arnetz BB, Anderzen I. Physicians during and following night call duty--41 hour ambulatory recording of sleep. Electroencephalogr Clin Neurophysiol. 1990;76:193–196. doi: 10.1016/0013-4694(90)90217-8. [DOI] [PubMed] [Google Scholar]
- Andreakos E, Foxwell B, Feldmann M. Is targeting Toll-like receptors and their signaling pathway a useful therapeutic approach to modulating cytokine-driven inflammation? Immunol Rev. 2004;202:250–265. doi: 10.1111/j.0105-2896.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, Stranges S, Kandala NB, Miller MA, Taggart FM, Kumari M, Ferrie JE, Shipley MJ, Brunner EJ, Marmot MG. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50:693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Korin YD, Fahey JL, Zack JA. Norepinephrine accelerates HIV replication via protein kinase A-dependent effects on cytokine production. J Immunol. 1998;161:610–616. [PubMed] [Google Scholar]
- Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12:2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004;5:975–979. doi: 10.1038/ni1116. [DOI] [PubMed] [Google Scholar]
- Dew MA, Hoch CC, Buysse DJ, Monk TH, Begely AE, Houck PR, Hall M, Kupper DJ, Reynold CF. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- Festa A, D’Agostino R, Jr., Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- Friedman EM, Irwin M. Modulation of immune cell function by the autonomic nervous system. Pharm Ther. 1997;74:27–38. doi: 10.1016/s0163-7258(96)00200-8. [DOI] [PubMed] [Google Scholar]
- Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Opler MG, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Sleep duration associated with mortality in elderly, but not middle-aged, adults in a large US sample. Sleep. 2008;31:1087–1096. [PMC free article] [PubMed] [Google Scholar]
- Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30:1667–1673. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M, Rinetti G, Redwine L, Motivala S, Dang J, Ehlers C. Nocturnal proinflammatory cytokine-associated sleep disturbances in abstinent African American alcoholics. Brain Beh Immun. 2004;18:349–360. doi: 10.1016/j.bbi.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Irwin M, Thompson J, Miller C, Gillin JC, Ziegler M. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J Clin Endocrin Metab. 1999;84:1979–1985. doi: 10.1210/jcem.84.6.5788. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, Breen EC, Martinez-Maza O, Cole S. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–540. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Ziegler M. Sleep deprivation potentiates activation of cardiovascular and catecholamine responses in abstinent alcoholics. Hypertension. 2005;45:252–257. doi: 10.1161/01.HYP.0000153517.44295.07. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neurosci. 2005;135:1295–1307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- Karin M. Inflammation and cancer: the long reach of Ras. Nature Med. 2005;11:20–21. doi: 10.1038/nm0105-20. [DOI] [PubMed] [Google Scholar]
- McDermott OD, Prigerson HG, Reynolds CFI, Houck PR, Dew MA, Hall M, Mazumdar S, Buysse DJ, Hoch CC, Kupfer DJ. Sleep in the wake of complicated grief symptoms: An exploratory study. Biol Psychiatry. 1997;41:710–716. doi: 10.1016/S0006-3223(96)00118-7. [DOI] [PubMed] [Google Scholar]
- Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- Newman AB, Spiekerman CF, Enright P, Lefkowitz D, Manolio T, Reynolds CF, Robbins J. Daytime sleepiness predicts mortality and cardiovascular disease in older adults. The Cardiovascular Health Study Research Group. J Am Ger Soc. 2000;48:115–123. doi: 10.1111/j.1532-5415.2000.tb03901.x. [DOI] [PubMed] [Google Scholar]
- O’Connor M,F, Motivala SJ, Valladares EM, Olmstead R, Irwin MR. Sex differences in monocyte expression of IL-6: role of autonomic mechanisms. Am J Physiol Regul Integr Comp Physiol. 2007;293:R145–151. doi: 10.1152/ajpregu.00752.2006. [DOI] [PubMed] [Google Scholar]
- Pascual G, Glass CK. Nuclear receptors versus inflammation: mechanisms of transrepression. Trends in endocrinology and metabolism: TEM. 2006;17:321–327. doi: 10.1016/j.tem.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Satoh M, Shimoda Y, Maesawa C, Akatsu T, Ishikawa Y, Minami Y, Hiramori K, Nakamura M. Activated toll-like receptor 4 in monocytes is associated with heart failure after acute myocardial infarction. Int J Cardiol. 2005 doi: 10.1016/j.ijcard.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Shankar A, Koh WP, Yuan JM, Lee HP, Yu MC. Sleep duration and coronary heart disease mortality among Chinese adults in Singapore: a population-based cohort study. Am J Epidem. 2008;168:1367–1373. doi: 10.1093/aje/kwn281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee BN, Smith EO, Szuba MP, Van Dongen HP, Dinges DF. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165–170. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: evidence for gender disparity. Brain Behav Immun. 2008;22:960–968. doi: 10.1016/j.bbi.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpato S, Guralnik JM, Ferrucci L, Balfour J, Chaves P, Fried LP, Harris TB. Cardiovascular disease, interleukin-6, and risk of mortality in older women: the women’s health and aging study. Circulation. 2001;103:947–953. doi: 10.1161/01.cir.103.7.947. [DOI] [PubMed] [Google Scholar]
- Wahle M, Neumann RP, Moritz F, Krause A, Buttgereit F, Baerwald CG. Beta2-adrenergic receptors mediate the differential effects of catecholamines on cytokine production of PBMC. J Interferon Cytokine Res. 2005;25:384–394. doi: 10.1089/jir.2005.25.384. [DOI] [PubMed] [Google Scholar]
- Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]