Abstract
3D cell-matrix cultures provide a useful model to analyze and dissect the structural, functional, and mechanical aspects of cell-matrix interactions and motile behavior important for cell and tissue morphogenesis. In the current studies we tested the effects of serum and physiological growth factors on the morphogenetic behavior of human fibroblasts cultured on the surfaces of 3D collagen matrices. Fibroblasts in medium containing serum contracted into clusters, whereas cells in medium containing platelet derived growth factor (PDGF) were observed to migrate as individuals. The clustering activity of serum appeared to depend on lysophosphatidic acid, required cell contraction based on inhibition by blocking Rho kinase or myosin II, and was reversed upon switching to PDGF. Oncogenic Ras transformed human fibroblasts did not exhibit serum-stimulated cell clustering. Our findings emphasize the importance of cell specific promigratory and procontractile growth factor environments in the differential regulation of cell motile function and cell morphogenesis.
Keywords: Platelet-derived growth factor, lysophosphatidic acid, 3D collagen matrix, cell motility, cell contraction
INTRODUCTION
3D cell-matrix cultures provide useful models to analyze and dissect the structural, functional, and mechanical aspects of cell-matrix interactions and motile behavior important for cell and tissue morphogenesis [1–5]. Unlike conventional 2D cell cultures (e.g., plastic or glass), cells can penetrate into 3D matrices, cell-matrix adhesive interactions are localized to matrix elements, and matrix fibrils can be reorganized mechanically. Mechanical remodeling of the extracellular matrix has been implicated in diverse aspects of normal tissue physiology and pathology including wound repair [6–9], fibrosis [10, 11], tumorigenesis [12–14], and aging [15]. Matrix remodeling also has become an important design consideration for tissue engineering [16–18].
Compared to cell migration on 2D surfaces [19, 20], tissue cells interacting with 3D matrices exhibit greater plasticity and multiple mechanisms of migration [21–25]. Local matrix remodeling can produce collagen fibril alignment and formation of tension lines along which cell migration occurs [26–31]. If the matrix is stiff enough to resist cell tractional force, then the cells move. If the matrix cannot resist cell tractional force, then the matrix moves [32].
Fibroblast migration frequently has been analyzed in serum-containing medium, and platelet-derived growth factor (PDGF) has been reported to be the major promigratory factor for fibroblasts in serum [33, 34]. However, in experiments using 3D collagen matrix assays of fibroblast migration and contraction, we observed that unlike PDGF, serum stimulation established a procontractile rather than promigratory environment [35]. If human fibroblasts were transformed by oncogenic Ras, then serum became as promigratory as PDGF [36].
Fibroblasts and endothelial cells cultured in serum-containing medium on polyacrylamide surfaces contract into cell clusters if the surface is sufficiently compliant [37, 38]. In light of our previous observations, we speculated that cells cultured on the surfaces of collagen matrices might exhibit differential responses to serum and PDGF growth factor environments. The current work was carried out to test this possibility. We found that fibroblasts in serum medium formed clusters on 1.5 mg/ml collagen matrices, exhibited less clustering on 4 mg/ml matrices, and did not cluster on collagen-coated coverslips. Cells in PDGF medium, on the other hand, migrated as individuals and did not form clusters. Individual cell migration and cell clustering were completely reversible upon switching from serum to PDGF. The clustering effect of serum could be attributed to lysophosphatidic acid and was not observed using oncogenic Ras transformed human fibroblasts. Our findings emphasize the importance of cell specific promigratory and procontractile growth factor environments in the differential regulation of cell motile function and cell morphogenesis.
MATERIALS AND METHODS
Materials
Type I collagen (high concentration, rat tail) was purchased from BD bioscience (Bedford, MA). Dulbecco’s modified Eagle medium (DMEM), CO2-independent DMEM and 0.25% trypsin/EDTA solution were purchased from Invitrogen (Gaithersburg, MD). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Lawrenceville, GA). Rho kinase inhibitor Y-27632 was obtained from Calbiochem Corp. (La Jolla, CA). Blebbistatin was obtained from Toronto Research Chemicals Inc. (Toronto, Canada). Platelet-derived growth factor BB isotype (PDGF) was obtained from Upstate Biotechnology, Inc. (Lake Placid, NY). Fatty acid-free bovine serum albumin (BSA), lysophosphatidic acid (LPA), sphingosine-1-phosphate (S1P), anti-vinculin antibody, and activated charcoal were obtained from Sigma (St. Louis, MO). Alexa Fluor 488 phalloidin and propidium iodine (PI) were obtained from Molecular Probes, Inc. (Eugene, OR). RNase (DNase free) was purchased from Roche (Indianapolis, IN). Fluoromount G was obtained from Southern Biotechnology Associates (Birmingham, AL)
Cell culture and preparation of collagen matrices
Use of de-identified human foreskin fibroblasts was approved by the University Institutional Review Board (Exemption #4). BR-5 cells (hTERT immortalized early passage human foreskin fibroblasts) and oncogenic Ras transformed BR-5 cells [36] were cultured in DMEM supplemented with 10% fetal bovine serum at 37°C in a 5% CO2 humidified incubator. Cell culture and experimental incubations were carried out at 37 in a 5% CO2 incubator. Collagen matrices were prepared at the concentrations indicated according to manufacturer’s instructions. Experimental incubation media was DMEM with 10% FBS (serum medium) or DMEM with 5 mg/ml BSA and 50 ng/ml PDGF (PDGF medium) and other reagents added as indicated in the figure legends. Cells (1–5×104/matrix) were seeded on top of polymerized collagen matrices or incubated on glass coverslips that had been coated 15 min at 37o C with 50 μg/ml collagen in DMEM.
Immunofluorescence microscopy
Preparation of samples for actin staining with Alexa Fluor 488-conjugated phalloidin and propidium iodine (PI) was carried out as previously described [39]. Images for morphometric analysis were collected at 22°C with a Nikon Elipse 400 fluorescent microscope using 10X/0.45, 20X/0.75, and 40X/0.75 Nikon Plan Apo infinity corrected objectives, Photometrics SenSys CCD camera and MetaVue acquisition and imaging software (Molecular Devices). Morphometric measurements were made using MetaVue. Dendritic cell index -- perimeter2/4π area (= 1.0 for a round cell) -- was measured with the Integrated Morphometry Analysis function. For each condition, data shown are the average of region measurements made on 20 cells. Image processing was carried out using Adobe Photoshop.
Time-lapse microscopy
For time-lapse microscopic analyses, cells were incubated on collagen matrices polymerized in 24 well dishes within a 37oC environmental chamber using CO2-independent DMEM in place of DMEM. Images were collected at 10–15 min intervals using an Axiovert 200M inverted microscope (Zeiss, Thornwood, NY) with 10X/0.25 Achroplan objective (Zeiss), DXM1200F camera (Nikon), and Metamorph acquisition software.
RESULTS
Cell spreading in PDGF and serum media on 1.5 and 4 mg/ml collagen matrices and collagen-coated coverslips
Initial studies were carried out to compare effects of PDGF and serum on cell spreading on collagen matrices. Preliminary findings indicated that the pattern of spreading varied markedly depending on the collagen concentration of the matrix. For instance, Figure 1 shows fibroblasts in PDGF medium after 4 hr on 1.5 mg/ml and 4 mg/ml collagen matrices. On 1.5 mg/ml matrices, fibroblasts exhibited dendritic morphology and contained few stress fibers or vinculin-staining focal adhesions. However, on 4 mg/ml matrices, fibroblasts appeared lamellar with prominent stress fibers and focal adhesions. Therefore increasing the collagen matrix density from 1.5 to 4 mg/ml permits cells to acquire high cell-matrix tension state [40].
Figure 1. Fibroblast spreading in PDGF medium on 1.5 mg/ml and 4 mg/ml collagen matrices.
Fibroblasts were cultured 4 hr on 1.5 mg/ml and 4 mg/ml collagen matrices in PDGF medium. At the end of incubations, samples were fixed and stained for actin and vinculin. Judging from appearance of stress fibers and focal adhesions, cells on 4 mg/ml collagen matrices developed high cell-matrix tension state. Scale bar, 50 μm (insert; 20 μm)
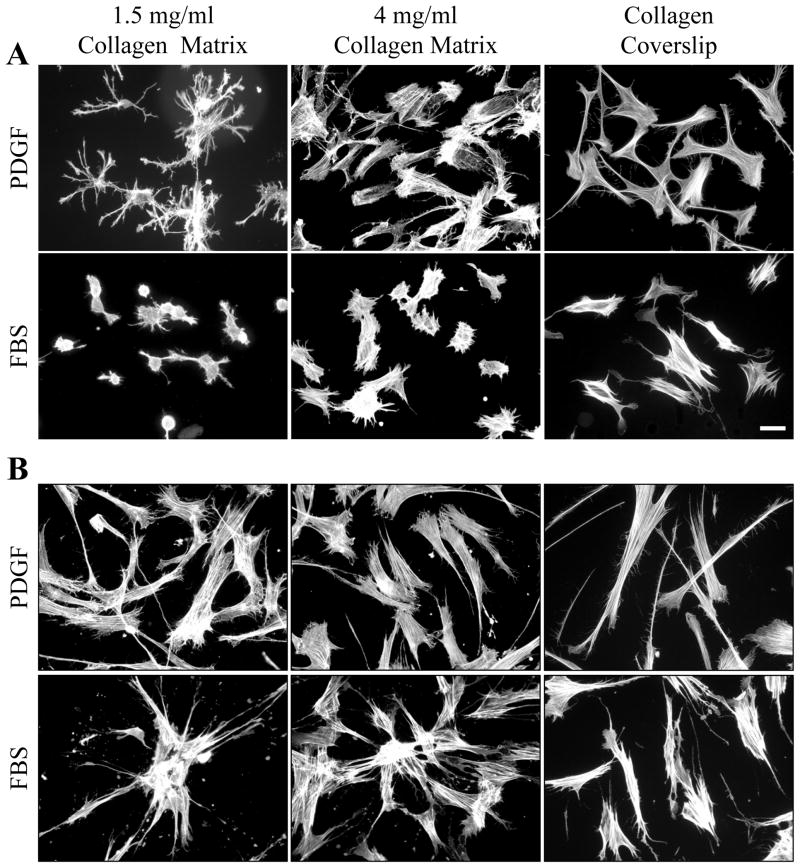
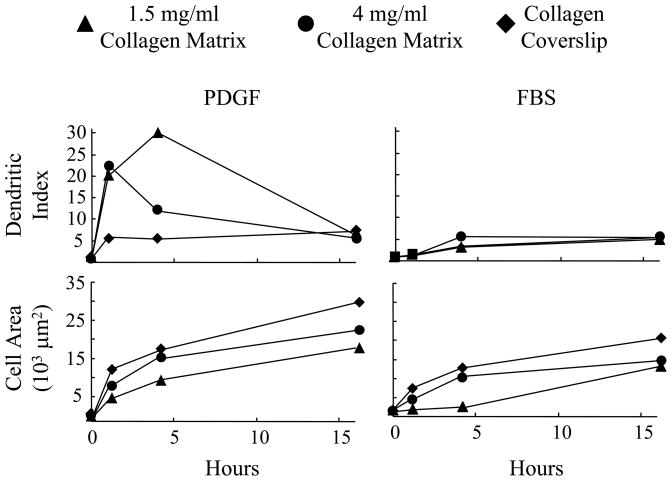
Figure 2 (A, 4 hr; B, 16 hr) and Figure 3 (morphometric measurements at 1, 4 and 16 hr) compare fibroblast spreading in PDGF and serum media on 1.5 and 4 mg/ml collagen matrices and collagen-coated coverslips. Initially the biggest differences in cell behavior concerned cell shape, and these differences were most evident with cells on 1.5 mg/ml collagen matrices. In PDGF medium, the cellular dendritic index was 20–25 after 1 hr and increased further by 4 hr by which time the area of cell spreading was ~7500 μm2. In serum medium, the cellular dendritic index was ~1 (round) after 1 hr and increased only slightly by 4 hr at which time the area of cell spreading was ~500 μm2, i.e., little spreading had occurred.
Figure 2. Fibroblast spreading in PDGF and serum (FBS) media on 1.5 mg/ml and 4 mg/ml collagen matrices and collagen-coated coverslips.
Fibroblasts were cultured 4 hr (A) and 16 hr (B) on 1.5 mg/ml and 4 mg/ml collagen matrices and collagen-coated coverslips in PDGF and serum media. At the end of incubations, samples were fixed and stained for actin. On 1.5 mg/ml collagen matrices, PDGF medium favored formation of dendritic extensions. Serum medium delayed cell spreading. After 16 hr, cells in PDGF medium were dispersed. Cells in serum medium were clustered. Formation of dendritic extensions, delayed cell, and cell clustering did not occur on collagen-coated coverslips. Scale bar, 50 μm.
Figure 3. Morphometric analysis of fibroblast spreading in PDGF and serum media on 1.5 mg/ml and 4 mg/ml collagen matrices and collagen-coated coverslips.
Fibroblasts were cultured 1 hr, 4 hr and 16 hr on 1.5 mg/ml and 4 mg/ml collagen matrices and collagen-coated coverslips in PDGF and serum media. At the end of incubations, cells were fixed and stained for actin and evaluated by morphometric analysis. Marked differences between PDGF and serum media in formation of dendritic extensions and time of cell spreading occurred for cells on 1.5 mg/ml collagen matrices. These differences were less evident with 4 mg/ml collagen matrices and not detected with collagen-coated coverslips.
The dendritic index of cells in PDGF medium decreased between 1–4 hr on 4 mg/ml matrices and was low at all times on collagen-coated coverslips where the fibroblasts became lamellar more rapidly and the area of cell spreading increased fastest. In serum medium, cells on 4 mg/ml matrices and collagen coverslips also tended to be round, but spreading was less delayed compared to 1.5 mg/ml matrices and could be observed even after 1 hr.
By 16 hr, the biggest difference in cell behavior in PDGF and serum media concerned cell distribution rather than shape. Here also, the differences were most evident with cells on 1.5 mg/ml collagen matrices. In PDGF medium, fibroblasts appeared to be dispersed. In serum medium, the cells had formed clusters. Clusters were less evident on 4 mg/ml matrices and did not occur on coverslips.
Differential effects of PDGF and serum media on cell migration and contraction-dependent cell clustering
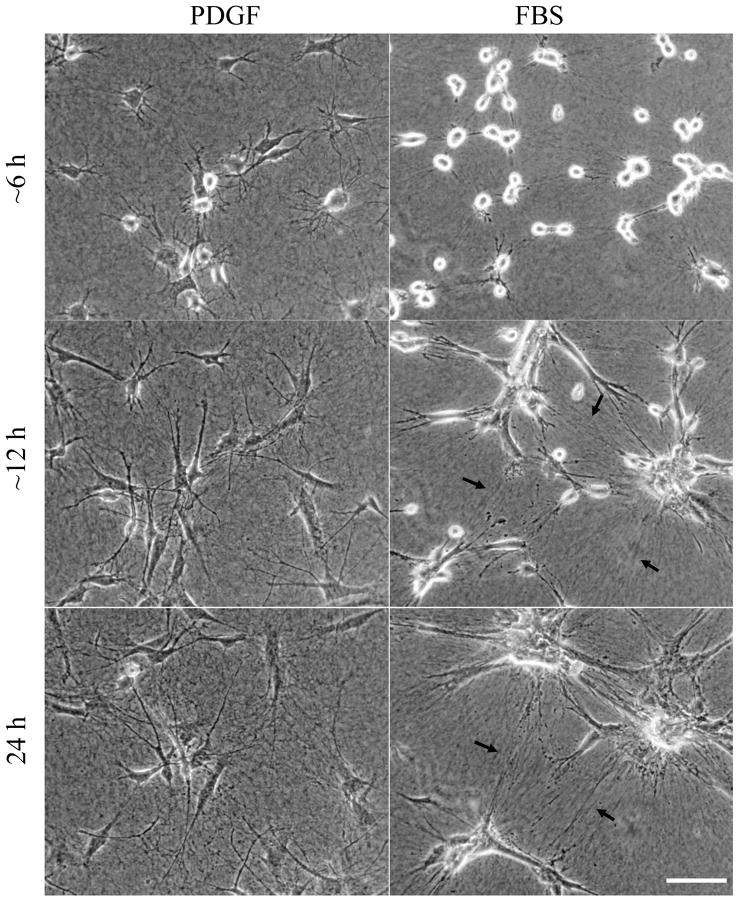
To learn more about the differences in cell morphogenesis and redistribution that occurred over 16 hr, we carried out time-lapse video microscopy. Figure 4 presents phase-contrast images from representative videos of cells interacting with 1.5 mg/ml collagen matrices in PDGF medium (Supplemental Video 1) and serum (FBS) medium (Supplemental Video 2). In PDGF medium, cell spreading initially was dendritic as described above. Over time, dendritic extensions reorganized into a more bipolar organization and cell migration began. In serum medium, spreading was delayed. Cell protrusions formed and retracted. The combination of cell extension, migration and contraction resulted in formation of large cell clusters. Tension lines between clusters became increasingly apparent in the collagen matrix (e.g., arrows). Occasionally, cells began to migrate away from the clusters, but cell-cell interactions remained sufficiently strong to keep the clusters together.
Figure 4. Cell migration in PDGF medium vs. clustering in serum medium.
Phase-contrast images from videos of fibroblasts interacting with 1.5 mg/ml collagen matrices in PDGF (Supplemental Video 1) and serum media (Supplemental Video 2) with the same field shown after ~6hr, ~12 hr, and 24 hr. Alignment of collagen fibrils between cells (arrows) becomes increasingly apparent in serum but not PDGF medium. Cells moved as individuals in PDGF medium but contracted into clusters in serum medium. Scale bar, 100 μm.
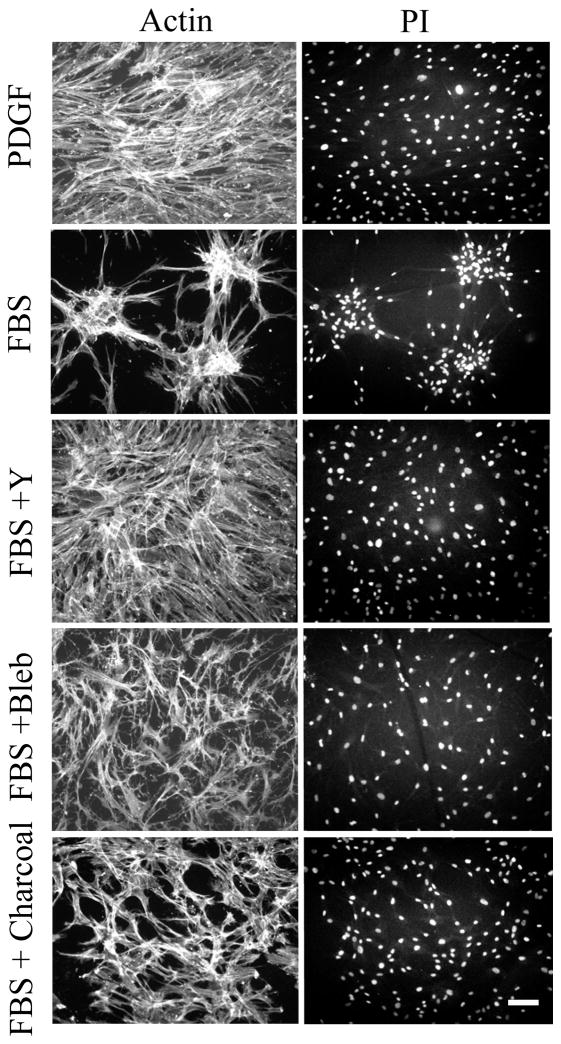
To gain a more quantitative view of cell clustering, fibroblasts were visualized by staining for nuclear distribution and actin cytoskeleton. Figure 5 (PDGF) shows that even with cells in close proximity, the nuclear distribution remained dispersed indicating that little tendency for cell clustering occurred in PDGF medium. In serum medium (Figure 5, FBS), clusters containing 25–50 cells formed under similar conditions. Serum stimulation of fibroblasts activates the small G-protein Rho [41], and Rho activation causes cell contraction [42, 43]. Blocking the Rho effector Rho kinase with the pharmacological inhibitor Y27632 (Figure 5, FBS+Y) or blocking myosin II activity with blebbistatin (Figure 5, FBS+Bleb) completely prevented clustering.
Figure 5. Role of Rho kinase and myosin II in cell clustering.
Fibroblasts were cultured 16 hr on 1.0 mg/ml collagen matrices in PDGF or serum (FBS) media as indicated. Other additions were 10 μM Y27632 (Y) (Rho kinase inhibitor) or 20 μM blebbistatin (Bleb) (myosin II inhibitor). For FBS + charcoal, serum was treated with two rounds of activated charcoal. At the end of incubations, samples were fixed and stained for actin and with propidium iodide. Fibroblasts remained dispersed in PDGF medium, in medium containing charcoal-treated serum, and in serum medium with Rho kinase or myosin II inhibitors added. Scale bar, 100 μm.
Additional experiments were carried out to learn about the serum factor responsible for cell clustering. Serum lysophospholipids originally were identified as the Rho activators in serum based on use of activated charcoal to remove lipid agonists [41]. Figure 5 (FBS + charcoal) shows an experiment in which cells were incubated with serum that had been subjected to two rounds of treatment with activated charcoal. The serum activity responsible for cell clustering was lost as a result of this procedure. Protein profiles of control and charcoal-treated serum were almost identical (not shown).
Serum contains two lysophospholipid activators of Rho, LPA and S1P [42, 44–46]. Previously, we observed that LPA and S1P both stimulate fibroblast contraction in collagen matrices [35]. Figure 6 compares cell clustering in LPA and S1P at two different cell concentrations. With LPA, cell clustering occurred similarly as observed in serum. S1P, on the other hand, was unable to stimulate formation of large cell clusters. Rather, clusters that formed in the presence of S1P were much smaller than in LPA or serum. This finding was consistent with our observation that S1P inhibits protrusion of dendritic extensions and cell migration [35].
Figure 6. Effects of LPA and S1P on cell clustering.
Fibroblasts at the cell numbers indicated were cultured 16 hr on 1.0 mg/ml collagen matrices in 10 μM LPA or 1 μM S1P as shown. At the end of incubations, samples were fixed and stained for actin and with propidium iodide. LPA caused cell clustering similar to serum. With S1P, clusters were smaller and limited to nearby cells. Scale bar, 100 μm.
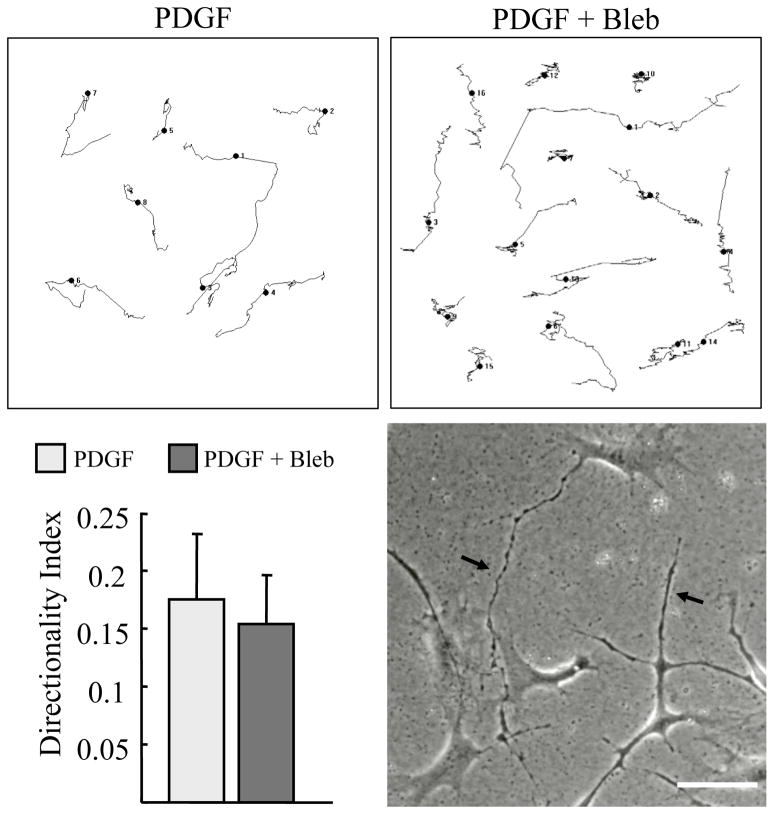
Cell migration generally is thought to occur through the combination of cell protrusion and contraction [19, 20]. However, for cells migrating in 3D matrices, the requirement for actomyosin contractility under some conditions is low [23] or absent [22]. Since PDGF did not stimulate contraction sufficient for cell clustering in our studies, and fibroblasts in collagen matrices exert little contractile force in response to PDGF [47], we tested if PDGF-stimulated migration required myosin II activity. Figure 7 shows migration tracks of individual cells in PDGF medium observed by time-lapse microscopy and analyzed by morphometric analysis. At blebbistatin concentrations that completely inhibited serum-stimulated cell clustering (Figure 5) and caused formation of long dendritic extensions (phase contrast, arrows), blebbistatin had only a minimal effect on cell migration (Supplemental Video 3). These findings indicate that PDGF-stimulated cell migration on the surface of 1.5 mg/ml collagen matrices requires little if any myosin II-dependent contractile activity.
Figure 7. PDGF stimulated migration is not inhibited by blocking myosin II.
Fibroblasts were cultured 16 hr on 1.5 mg/ml collagen matrices in PDGF media with 20 μM blebbistatin added as indicated. Cell migration was recorded by time-lapse microscopy. Randomly selected individual cell migration tracks were copied and combined into a single figure. Persistence of directional migration was determined morphometrically by measuring D/T ratios (direct distance from start to end point [D] divided by the total track distance [T]). Addition of blebbistatin caused cells to exhibit abnormally long dendritic extensions (arrows) but had little effect on overall cell migration in PDGF medium (Supplemental Video 3). Scale bar, 100 μm.
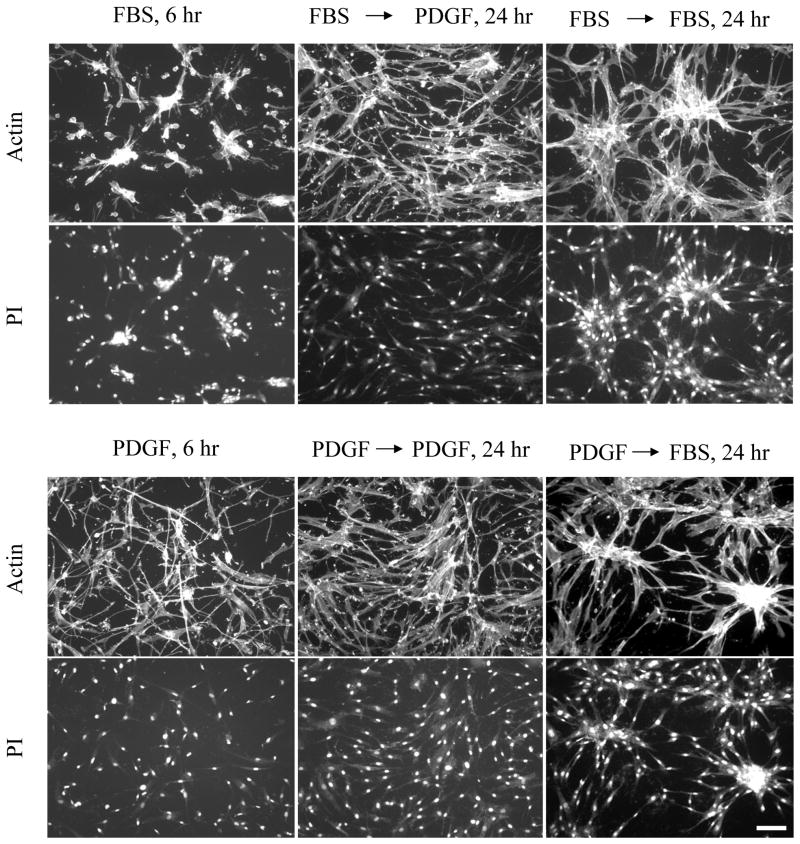
We also tested the reversibility of promigratory and procontractile environments. Figure 8 shows that the effects of PDGF and serum on cell dispersion and clustering were completely reversible. Cells preclustered for 6 hr in serum (or LPA) medium, dispersed upon switching to PDGF medium. Cells initially dispersed in PDGF medium, clustered upon switching to serum (or LPA) medium. Even large clusters that formed after 24 hr exhibited extensive dispersion when switched to PDGF medium (Supplemental Video 4).
Figure 8. Reversibility of cell clustering patterns.
Fibroblasts were cultured for an initial period of 6 hr on 1.0 mg/ml collagen matrices in PDGF and serum media. Subsequently, the samples were rinsed and placed into PDGF and serum media as indicated for an additional 18 hr. At the end of the initial 6 hr period and after 24 hr, samples were fixed and stained for actin and with propidium iodide (PI). Morphogenetic cell patterns in PDGF and serum media were completely reversible. Scale bar, 100 μm.
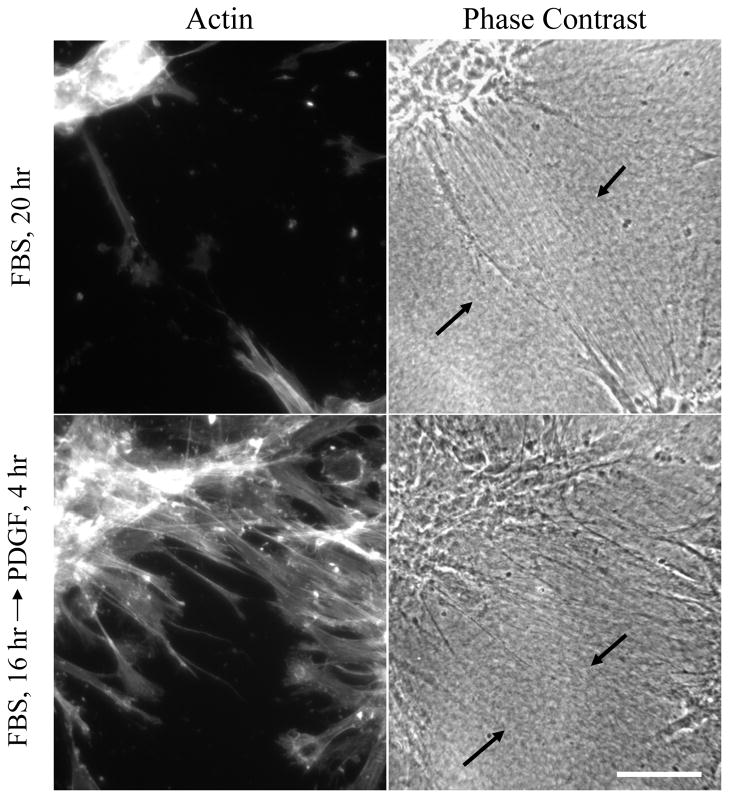
Two hr after addition of PDGF, little change could be detected in the collagen matrix tension lines between cell clusters. However, as illustrated by the typical example in Figure 9, tension lines were less apparent after 4–6 hr in areas where fibroblasts began to migrate out of the clusters. Therefore, changes in the growth factor environment can have a rapid and profound effect on collagen organization as well as cell distribution.
Figure 9. Reversibility of morphogenetic patterns.
Fibroblasts were cultured for an initial period of 16 hr on 1.0 mg/ml collagen matrices in serum (FBS) media. Subsequently, half the samples were rinsed and placed into PDGF media. At the end of an additional 4 hr period, samples were fixed and stained for actin and also observed by phase contrast microscopy. Alignment of collagen fibrils between cell clusters was evident in serum (arrows). In PDGF region, where cells were migrating out of clusters, the alignment of collagen fibrils became less obvious (arrows) Scale bar, 100 μm.
Oncogenic Ras transformed fibroblasts lose the cell clustering response to serum
Since the differential response of fibroblasts to PDGF and serum/LPA media reflected promigratory and procontractile environments, we predicted that oncogenic Ras transformed human fibroblasts would no longer show this difference. Oncogenic Ras transformed cells migrate in serum or LPA almost as well as PDGF [36]. Figure 10 presents phase-contrast images from videos of control and oncogenic Ras transformed cells interacting with 1.5 mg/ml collagen matrices in serum medium. Control cells in serum medium formed clusters as already described. In parallel experiments, oncogenic Ras transformed cells did not (Supplemental Video 5).
Figure 10. Migration of oncogenic Ras transformed cells in serum media.
Phase-contrast images from videos of control (BR5) and oncogenic Ras transformed BR5 fibroblasts interacting with 1.5 mg/ml collagen matrices in serum medium. Unlike control cells that clustered, oncogenic Ras transformed cells moved as individuals in serum medium (Supplemental Video 4). Scale bar, 100 μm.
DISCUSSION
Previously, we demonstrated the importance of promigratory and procontractile growth factor environments in the differential regulation of cell motile function [35]. In the current studies we extend our previous work and demonstrate that promigratory and procontractile growth factor environments can differentially regulate the balance between individual cell migration and cell clustering. Cells under promigratory conditions (PDGF medium) migrate as individuals, whereas cells under procontractile conditions (serum medium) form clusters. In serum medium, as adjacent cells attempt to spread, they contract and align the matrix in between and pull closer to each other. Local matrix alignment establishes preferential paths of further cell protrusion because of contact guidance [48, 49]. A positive feedback loop is established as more and more cells contract and align the collagen in a given region resulting in further clustering.
Since collagen did not accumulate in the cell clusters, cell-cell interactions that formed during clustering must have been stronger than and possibly even caused the release of cell-collagen interactions. The mechanisms responsible for stabilizing cell-cell interactions in serum-stimulated cell clusters requires further investigation. Adherens junctions may play a role [50] and are believed to be responsible for the synchronized contractile activity of interconnected wound fibroblasts [51]. Alternatively, fibronectin receptors interacting with cell surface fibronectin matrix has been implicated in establishing cell-cell interactions required to stabilize cell spheroids [52, 53].
Fibroblasts and endothelial cells cultured in serum-containing medium on polyacrylamide surfaces also have been reported to contract into clusters if the polyacrylamide surface is sufficiently compliant [37, 38]. Similarly, we found that cell clustering was more apparent on 1.5 mg/ml collagen matrices compared to 4 mg/ml matrices and was not observed on collagen-coated coverslips.
Serum activates cell contraction through the small G protein Rho [41, 44], and clustering was inhibited by blocking the Rho effector Rho kinase and downstream target myosin II. Additional experiments were carried out to learn about the serum factor responsible for cell clustering. Serum lysophospholipids originally were identified as the Rho activators in serum based on use of activated charcoal to remove lipid agonists [41], and cluster-promoting activity was lost when lipid growth factors were removed from serum. Serum contains two lysophospholipid activators of Rho, LPA and S1P [42, 44–46]. Previously, we observed that LPA and S1P both stimulate fibroblast contraction in collagen matrices. However, S1P inhibits protrusion of dendritic extensions and cell migration [35]. In the current experiments, we observed that cell clustering occurred similarly with LPA and serum. S1P, on the other hand, was unable to stimulate formation of large cell clusters. Rather, clusters that formed in the presence of S1P were much smaller than in LPA. Therefore, we suggest that LPA is the growth primarily responsible for serum-stimulated fibroblast clustering activity
Cell clustering was completely reversible by switching from serum to PDGF medium, in which case fibroblasts exhibited individual cell migration. Rather than activation of the small G-protein Rho, the initial response of fibroblasts to PDGF stimulation is activation of the small G-protein Rac [41, 54], and fibroblasts in collagen matrices exert much less contractile force in response to PDGF stimulation compared to serum or LPA [47]. Previous analyses of collagen tension lines formed between cell explants in collagen matrices showed that destruction of the cells or their actin cytoskeleton resulted in partial reorganization of collagen and loss of tension lines [28, 55]. Similarly, we found that when clustered fibroblasts were switched from serum to PDGF, tension lines in the collagen became less visible in regions where cells were migrating out of the clusters.
Cell clustering on soft polyacrylamide surfaces and the possibly related feature known as durotaxis – cell migration from softer to stiffer polyacrylamide surfaces but not the reverse [56, 57] -- are both characteristics of motile behavior that are unique to the serum medium, i.e., a procontractile growth factor environment. As shown in the current work, a promigratory growth factor environment permits individual cell migration instead of clustering. Similarly, a promigratory growth factor environment permits cells to move in the opposite direction of durotaxis. For instance, in PDGF but not serum or LPA medium, fibroblasts in nested collagen matrices can migrate out of a ~15 mg/ml collagen inner matrix (stiffness ~600 Pa) into a 1.5 mg/ml collagen outer matrix (stiffness ~6 Pa) [35, 58] (stiffness measurements, M. Miron-Mendoza, unpublished). The foregoing differences emphasize the importance of carrying out studies on cell motile function in both promigratory and procontractile growth factor environments to gain a comprehensive account.
Whether serum creates a a promigratory or procontractile environment likely will vary depending on cell type. The serum lysophospholipids LPA and S1P interact with a family of G-protein coupled receptors that are expressed differentially by different cell types [42, 45, 46]. Depending on receptor expression, LPA and S1P potentially can activate cell contraction or cell migration signaling pathways. S1P1 receptor links to Rac activation and cell migration, whereas S1P2 receptor links to Rho activation, Rac inhibition, and inhibition of cell migration [59–62]. With oncogenic Ras transformed human fibroblasts, PDGF, LPA, and serum are all promigratory [36], and serum stimulated cell clustering was not observed.
Analysis of tissue cells interacting with 2D surfaces led to the idea that actomyosin-generated cellular contractile force is required for cells to migrate [19, 20]. The situation with cells in 3D matrices is more complex. Under some conditions the requirement for actomyosin contractility can be low [23] or absent [22]. Since blocking myosin II with blebbistatin inhibited serum-dependent fibroblast clustering but not PDGF-dependent cell migration, our studies provide another example of cell migration that depends on little if any myosin II-dependent contractile activity. In contrast to the current findings for cell migration on the surfaces of collagen matrices, we showed previously that blebbistatin blocks PDGF-dependent cell migration within nested collagen matrices [58]. The difference between myosin II-dependence of fibroblast migration on the surface vs. within collagen matrices parallels the situation of leukocytes exhibiting myosin II-independent or dependent migration depending upon the absence or presence of steric hindrance in the collagen matrices through which the cells are moving [22]. Without resistance from strong adhesive forces or steric hindrance, a myosin II-independent mechanism such as actin polymerization and depolymerization [63, 64] may be sufficient to provide the motor for cell migration.
It has become increasingly clear that matrix mechanics plays a critical role in cell and tissue morphogenesis [1–5]. We suggest that whether the growth factor environment is promigratory or procontractile also will play an important role. Differential cell migration vs. clustering depending on growth factor environment has the potential to contribute to tissue morphogenetic responses such as mesenchymal condensation [65, 66]. In addition, although connective tissue fibroblasts do not form clusters, interconnected cell-cell networks have been shown to play important roles in coordinated cell mechanical activity [51, 67]. Individual cell migration requires disruption of these networks.
Supplementary Material
Supplemental Video 1. Cell migration in PDGF medium on 1.5 mg/ml collagen matrices.
See text Figure 4. Time of observation was 0–24 hr. Frames were collected every 10 min, and display rate is 10 frames/sec.
Supplemental Video 2. Cell clustering in serum medium on 1.5 mg/ml collagen matrices.
See text Figure 4. Time of observation was 0–24 hr. Frames were collected every 10 min, and display rate is 10 frames/sec.
Supplemental Video 3. Cell migration in PDGF medium on 1.5 mg/ml collagen matrices in the presence of blebbistatin.
See text Figure 7. Time of observation was 0–24 hr. Frames were collected every 10 min, and display rate is 10 frames/sec.
Supplemental Video 4. Reversibility of cell clusters formed on 1.5 mg/ml collagen matrices by switching from serum to PDGF medium.
Time of observation was 24–48 hr. During the first 24 hr, cells were allowed to cluster in serum medium. Then the samples were switched to PDGF medium. After switching, frames were collected every 10 min, and display rate is 10 frames/sec.
Supplemental Video 5. Migration of oncogenic Ras transformed cells in serum medium on 1.5 mg/ml collagen matrices.
Corresponds to text Figure 10. Time of observation was 0–24 hr. Frames were collected every 15 min, and display rate is 7 frames/sec.
Acknowledgments
We are indebted to Drs. Matt Petroll and William Snell for their advice and suggestions. This research was supported by grants from the National Institutes of Health GM031321.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–24. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 3.Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–10. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Rhee S, Grinnell F. Fibroblast mechanics in 3D collagen matrices. Adv Drug Deliv Rev. 2007 doi: 10.1016/j.addr.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol. 2009;10:34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401–4. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silver FH, Siperko LM, Seehra GP. Mechanobiology of force transduction in dermal tissue. Skin Research and Technology. 2002;8:1–21. doi: 10.1034/j.1600-0846.2003.00358.x. [DOI] [PubMed] [Google Scholar]
- 8.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 9.Petroll WM, Cavanagh HD, Jester JV. Assessment of stress fiber orientation during healing of radial keratotomy wounds using confocal microscopy. Scanning. 1998;20:74–82. doi: 10.1002/sca.1998.4950200202. [DOI] [PubMed] [Google Scholar]
- 10.Abraham DJ, Eckes B, Rajkumar V, Krieg T. New developments in fibroblast and myofibroblast biology: implications for fibrosis and scleroderma. Curr Rheumatol Rep. 2007;9:136–43. doi: 10.1007/s11926-007-0008-z. [DOI] [PubMed] [Google Scholar]
- 11.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–16. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 13.Weigelt B, Bissell MJ. Unraveling the microenvironmental influences on the normal mammary gland and breast cancer. Semin Cancer Biol. 2008;18:311–21. doi: 10.1016/j.semcancer.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–22. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol. 2008;144:666–72. doi: 10.1001/archderm.144.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark RA, Ghosh K, Tonnesen MG. Tissue engineering for cutaneous wounds. J Invest Dermatol. 2007;127:1018–29. doi: 10.1038/sj.jid.5700715. [DOI] [PubMed] [Google Scholar]
- 17.Brown RA, Wiseman M, Chuo CB, Cheema U, Nazhat SN. Ultrarapid engineering of biomimetic materials and tissues: Fabrication of nano- and microstructures by plastic compression. Advanced Functional Materials. 2005;15:1762–1770. [Google Scholar]
- 18.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 19.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–69. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 20.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 21.Friedl P, Brocker EB. The biology of cell locomotion within three-dimensional extracellular matrix. Cell Mol Life Sci. 2000;57:41–64. doi: 10.1007/s000180050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, Sixt M. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–5. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 23.Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, Sahai E, Marshall CJ. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–23. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 24.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 25.Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS, Sahai E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr Biol. 2006;16:1515–23. doi: 10.1016/j.cub.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 26.Stopak D, Harris AK. Connective tissue morphogenesis by fibroblast traction. I. Tissue culture observations. Dev Biol. 1982;90:383–98. doi: 10.1016/0012-1606(82)90388-8. [DOI] [PubMed] [Google Scholar]
- 27.Guido S, Tranquillo RT. A methodology for the systematic and quantitative study of cell contact guidance in oriented collagen gels. Correlation of fibroblast orientation and gel birefringence. J Cell Sci. 1993;105( Pt 2):317–31. doi: 10.1242/jcs.105.2.317. [DOI] [PubMed] [Google Scholar]
- 28.Sawhney RK, Howard J. Slow local movements of collagen fibers by fibroblasts drive the rapid global self-organization of collagen gels. J Cell Biol. 2002;157:1083–91. doi: 10.1083/jcb.200203069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raeber GP, Lutolf MP, Hubbell JA. Part II: Fibroblasts preferentially migrate in the direction of principal strain. Biomech Model Mechanobiol. 2008;7:215–25. doi: 10.1007/s10237-007-0090-1. [DOI] [PubMed] [Google Scholar]
- 31.Beloussov LV, Louchinskaia NN, Stein AA. Tension-dependent collective cell movements in the early gastrula ectoderm of Xenopus laevis embryos. Dev Genes Evol. 2000;210:92–104. doi: 10.1007/s004270050015. [DOI] [PubMed] [Google Scholar]
- 32.Miron-Mendoza M, Seemann J, Grinnell F. Collagen Fibril Flow and Tissue Translocation Coupled to Fibroblast Migration in 3D Collagen Matrices. Mol Biol Cell. 2008;19:2051–2058. doi: 10.1091/mbc.E07-09-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao Z, Sasaoka T, Fujimori T, Oya T, Ishii Y, Sabit H, Kawaguchi M, Kurotaki Y, Naito M, Wada T, Ishizawa S, Kobayashi M, Nabeshima Y, Sasahara M. Deletion of the PDGFR-beta gene affects key fibroblast functions important for wound healing. J Biol Chem. 2005;280:9375–89. doi: 10.1074/jbc.M413081200. [DOI] [PubMed] [Google Scholar]
- 34.Li W, Fan J, Chen M, Guan S, Sawcer D, Bokoch GM, Woodley DT. Mechanism of human dermal fibroblast migration driven by type I collagen and platelet-derived growth factor-BB. Mol Biol Cell. 2004;15:294–309. doi: 10.1091/mbc.E03-05-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang H, Rhee S, Ho CH, Grinnell F. Distinguishing fibroblast promigratory and procontractile growth factor environments in 3D collagen matrices. FASEB J. 2008;22:2151–2160. doi: 10.1096/fj.07-097014. [DOI] [PubMed] [Google Scholar]
- 36.Menezes GC, Miron-Mendoza M, Ho CH, Jiang H, Grinnell F. Oncogenic Ras-transformed human fibroblasts exhibit differential changes in contraction and migration in 3D collagen matrices. Exp Cell Res. 2008;314:3081–91. doi: 10.1016/j.yexcr.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo WH, Frey MT, Burnham NA, Wang YL. Substrate rigidity regulates the formation and maintenance of tissues. Biophys J. 2006;90:2213–20. doi: 10.1529/biophysj.105.070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reinhart-King CA, Dembo M, Hammer DA. Cell-cell mechanical communication through compliant substrates. Biophys J. 2008;95:6044–51. doi: 10.1529/biophysj.107.127662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhee S, Grinnell F. P21-activated kinase 1: convergence point in PDGF- and LPA-stimulated collagen matrix contraction by human fibroblasts. J Cell Biol. 2006;172:423–32. doi: 10.1083/jcb.200505175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rhee S, Jiang H, Ho CH, Grinnell F. Microtubule function in fibroblast spreading is modulated according to the tension state of cell-matrix interactions. Proc Natl Acad Sci U S A. 2007;104:5425–30. doi: 10.1073/pnas.0608030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–99. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 42.Goetzl EJ, An S. Diversity of cellular receptors and functions for the lysophospholipid growth factors lysophosphatidic acid and sphingosine 1-phosphate. Faseb J. 1998;12:1589–98. [PubMed] [Google Scholar]
- 43.Jalink K, van Corven EJ, Hengeveld T, Morii N, Narumiya S, Moolenaar WH. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol. 1994;126:801–10. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moolenaar WH. Lysophosphatidic acid signalling. Curr Opin Cell Biol. 1995;7:203–10. doi: 10.1016/0955-0674(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 45.Gardell SE, Dubin AE, Chun J. Emerging medicinal roles for lysophospholipid signaling. Trends Mol Med. 2006;12:65–75. doi: 10.1016/j.molmed.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 46.Watterson KR, Lanning DA, Diegelmann RF, Spiegel S. Regulation of fibroblast functions by lysophospholipid mediators: potential roles in wound healing. Wound Repair Regen. 2007;15:607–16. doi: 10.1111/j.1524-475X.2007.00292.x. [DOI] [PubMed] [Google Scholar]
- 47.Kolodney MS, Elson EL. Correlation of myosin light chain phosphorylation with isometric contraction of fibroblasts. J Biol Chem. 1993;268:23850–5. [PubMed] [Google Scholar]
- 48.Friedl P, Maaser K, Klein CE, Niggemann B, Krohne G, Zanker KS. Migration of highly aggressive MV3 melanoma cells in 3-dimensional collagen lattices results in local matrix reorganization and shedding of alpha2 and beta1 integrins and CD44. Cancer Res. 1997;57:2061–70. [PubMed] [Google Scholar]
- 49.Dickinson RB, Guido S, Tranquillo RT. Biased cell migration of fibroblasts exhibiting contact guidance in oriented collagen gels. Ann Biomed Eng. 1994;22:342–56. doi: 10.1007/BF02368241. [DOI] [PubMed] [Google Scholar]
- 50.El Sayegh TY, Kapus A, McCulloch CA. Beyond the epithelium: cadherin function in fibrous connective tissues. FEBS Lett. 2007;581:167–74. doi: 10.1016/j.febslet.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 51.Hinz B, Gabbiani G. Cell-matrix and cell-cell contacts of myofibroblasts: role in connective tissue remodeling. Thromb Haemost. 2003;90:993–1002. doi: 10.1160/TH03-05-0328. [DOI] [PubMed] [Google Scholar]
- 52.Robinson EE, Zazzali KM, Corbett SA, Foty RA. Alpha5beta1 integrin mediates strong tissue cohesion. J Cell Sci. 2003;116:377–86. doi: 10.1242/jcs.00231. [DOI] [PubMed] [Google Scholar]
- 53.Robinson EE, Foty RA, Corbett SA. Fibronectin matrix assembly regulates alpha5beta1-mediated cell cohesion. Mol Biol Cell. 2004;15:973–81. doi: 10.1091/mbc.E03-07-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–10. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 55.Sawhney RK, Howard J. Molecular dissection of the fibroblast-traction machinery. Cell Motil Cytoskeleton. 2004;58:175–85. doi: 10.1002/cm.20004. [DOI] [PubMed] [Google Scholar]
- 56.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–52. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang HB, Dembo M, Hanks SK, Wang Y. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc Natl Acad Sci U S A. 2001;98:11295–300. doi: 10.1073/pnas.201201198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grinnell F, Rocha LB, Iucu C, Rhee S, Jiang H. Nested collagen matrices: a new model to study migration of human fibroblast populations in three dimensions. Exp Cell Res. 2006;312:86–94. doi: 10.1016/j.yexcr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Sugimoto N, Takuwa N, Okamoto H, Sakurada S, Takuwa Y. Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine-1-phosphate receptor isoform. Mol Cell Biol. 2003;23:1534–45. doi: 10.1128/MCB.23.5.1534-1545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goparaju SK, Jolly PS, Watterson KR, Bektas M, Alvarez S, Sarkar S, Mel L, Ishii I, Chun J, Milstien S, Spiegel S. The S1P2 receptor negatively regulates platelet-derived growth factor-induced motility and proliferation. Mol Cell Biol. 2005;25:4237–49. doi: 10.1128/MCB.25.10.4237-4249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 62.Peters SL, Alewijnse AE. Sphingosine-1-phosphate signaling in the cardiovascular system. Curr Opin Pharmacol. 2007;7:186–92. doi: 10.1016/j.coph.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 63.Bottino D, Mogilner A, Roberts T, Stewart M, Oster G. How nematode sperm crawl. J Cell Sci. 2002;115:367–84. doi: 10.1242/jcs.115.2.367. [DOI] [PubMed] [Google Scholar]
- 64.Mogilner A, Oster G. Polymer motors: pushing out the front and pulling up the back. Curr Biol. 2003;13:R721–33. doi: 10.1016/j.cub.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 65.Newman SA, Bhat R. Activator-inhibitor dynamics of vertebrate limb pattern formation. Birth Defects Res C Embryo Today. 2007;81:305–19. doi: 10.1002/bdrc.20112. [DOI] [PubMed] [Google Scholar]
- 66.Widelitz RB, Chuong CM. Early events in skin appendage formation: induction of epithelial placodes and condensation of dermal mesenchyme. J Investig Dermatol Symp Proc. 1999;4:302–6. doi: 10.1038/sj.jidsp.5640234. [DOI] [PubMed] [Google Scholar]
- 67.Goldsmith EC, Hoffman A, Morales MO, Potts JD, Price RL, McFadden A, Rice M, Borg TK. Organization of fibroblasts in the heart. Dev Dyn. 2004;230:787–94. doi: 10.1002/dvdy.20095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video 1. Cell migration in PDGF medium on 1.5 mg/ml collagen matrices.
See text Figure 4. Time of observation was 0–24 hr. Frames were collected every 10 min, and display rate is 10 frames/sec.
Supplemental Video 2. Cell clustering in serum medium on 1.5 mg/ml collagen matrices.
See text Figure 4. Time of observation was 0–24 hr. Frames were collected every 10 min, and display rate is 10 frames/sec.
Supplemental Video 3. Cell migration in PDGF medium on 1.5 mg/ml collagen matrices in the presence of blebbistatin.
See text Figure 7. Time of observation was 0–24 hr. Frames were collected every 10 min, and display rate is 10 frames/sec.
Supplemental Video 4. Reversibility of cell clusters formed on 1.5 mg/ml collagen matrices by switching from serum to PDGF medium.
Time of observation was 24–48 hr. During the first 24 hr, cells were allowed to cluster in serum medium. Then the samples were switched to PDGF medium. After switching, frames were collected every 10 min, and display rate is 10 frames/sec.
Supplemental Video 5. Migration of oncogenic Ras transformed cells in serum medium on 1.5 mg/ml collagen matrices.
Corresponds to text Figure 10. Time of observation was 0–24 hr. Frames were collected every 15 min, and display rate is 7 frames/sec.