Abstract
We have reported previously that moderate intensity aerobic exercise training attenuates airway inflammation in a murine asthma model. Recent studies implicate regulatory T (Treg) cells in decreasing asthma-related airway inflammation; as such, the current study examined the effect of exercise on Treg cell function in a murine asthma model. Mice were sensitized with ovalbumin (OVA) prior to the start of exercise training at a moderate intensity 3× / week for 4 wks; exercise was performed as treadmill running (13.5 m/min, 0% grade). Mice were OVA challenged repeatedly throughout the exercise protocol. At protocol completion, mice were analyzed for changes in the number and suppressive function of CD4+CD25+Foxp3+ cells isolated from lungs, mediastinal lymph nodes, and spleens. Results show that exercise increased significantly the number of Foxp3+ cells within the lungs and mediastinal lymph nodes, but not the spleens, of OVA-treated mice as compared with sedentary controls. Exercise also enhanced the suppression function of CD4+CD25+Foxp3+ Treg cells derived from OVA-treated mice as compared with sedentary controls. Specifically, Treg cells from exercised, OVA-treated mice more effectively suppressed CD4+CD25− cell proliferation and Th2 cytokine production in vitro. Enhanced suppression was associated with increased protein levels of TGF-β and lesser amounts of IL-10 and IL-17; however, blocking TGF-β had no effect on suppressive functions. These data demonstrate that exercise-mediated increases in Treg cell function may play a role in the attenuation of airway inflammation. Further, these results indicate that moderate intensity aerobic exercise training may alter the Treg cell function within the asthmatic airway.
Keywords: Treg, asthma, aerobic exercise, regulatory T cells
1. Introduction
Asthma is identified by the presence of wheezing, chest tightness, dyspnea and cough, and by the presence of reversible airway narrowing and/or airway hyperresponsiveness (AHR) to a variety of bronchoconstrictor stimuli. Several clinical studies suggest that continued aerobic exercise training improves the overall physical fitness and health of asthmatics and reduces their disease-related hospital admissions (Lucas and Platts-Mills, 2005, 2006; Satta, 2000). We have reported previously that moderate intensity aerobic exercise training reduces lung inflammatory responses and airway hyperresponsiveness in an ovalbumin (OVA)-driven mouse model of asthma (Pastva et al., 2004, 2005; Hewitt, et al. 2009b).
Increasing evidence demonstrates that regulatory T lymphocytes, including CD4+CD25+Foxp3+regulatory T (Treg) cells, play a central role in suppressing asthma pathogenesis. Specifically, CD4+CD25+Foxp3+ Treg cells have been shown to inhibit Th2 responses, airway eosinophilia, and allergen-induced AHR (Umetsu et al., 2003; Umetsu and DeKruyff, 2006). The effect of aerobic exercise training on T-cell function, in general, has also been reported. Woods and co-workers have shown that, following repeated bouts of exercise, the ratio of CD4:CD8 T cells and the ratio naïve:memory T cells is decreased in the periphery of a rodent model (Woods et al., 2003). Likewise, Lancaster and colleagues demonstrated that acute exercise markedly affects the distribution of both Th1 and Th2 cells (Lancaster et al., 2004). A recent study by Yeh et al (2008) indicates that exercise training increases the T cell expression of Foxp3, a Treg-specific transcription factor, in the periphery of type-2 diabetic patients. Despite these reports, the effect of exercise on Treg cell responses in asthmatic subjects is not known.
In the present study, we hypothesized that aerobic exercise at a moderate intensity would increase the number and function of Treg cells in OVA-treated mice. To test this hypothesis, mice were sensitized and challenged with OVA or control saline and exercised on a motorized treadmill repetitively at a moderate intensity. At the conclusion of the protocol, changes in the number and suppressive function of CD4+CD25+Foxp3+ Treg cells were determined. Results presented herein demonstrate that repeated exercise bouts increase the number and suppressive function of CD4+CD25+Foxp3+ Treg cells in OVA-treated mice. Together, these results suggest that aerobic exercise training at a moderate intensity may alter the function of Treg cells within the asthmatic airway.
2. Methods
2.1 Animals
Five to six week-old female C.Cg-Foxp3tm2Tch/J reporter (Foxp3+ reporter) mice bred on a Balb/cj background (Jackson Laboratories) were used for all experiments described herein. These mice co-express Foxp3 and the reporter EGFP (enhanced green fluorescence protein) under the control of an endogenous promoter located on the X chromosome. All mice were maintained in autoclaved Microisolator cages (Lab Products) and provided with food (Teklad) and water ad libitum.
2.2 OVA-sensitization protocol
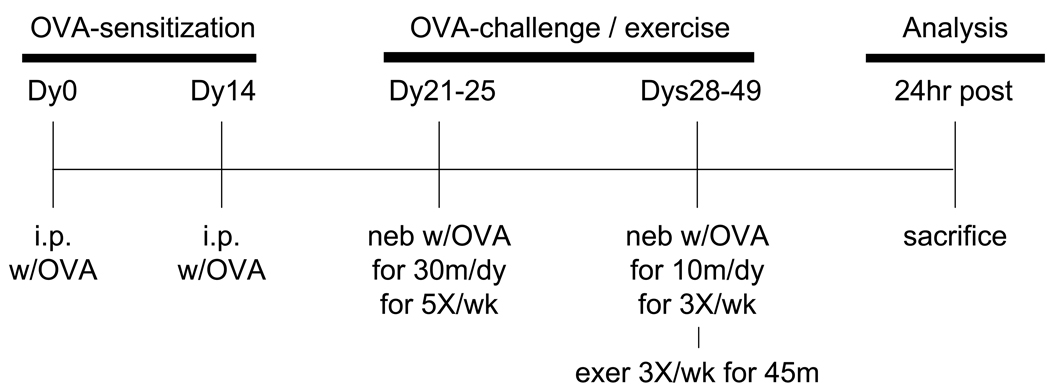
Mice were assigned randomly to four experimental groups: sedentary non-sensitized (S); sedentary OVA-sensitized (SO); exercised non-sensitized (E); and exercised OVA-sensitized (EO). Mice were sensitized and challenged with OVA or saline as described (Pastva et al., 2004) (see Fig. 1). Briefly, OVA-sensitized mice (SO, EO) were injected intraperitoneally (i.p.) (200µl/mouse) on days 0 and 14 with 50 µg of alum-precipitated, chicken egg ovalbumin (OVA; Imject Alum; Grade VII OVA, ≥ 98% pure, Sigma Chemical). Beginning on Day 21, mice were exposed to aerosolized OVA at a concentration of 5 mg/ml (in 0.85% NaCl solution, 30min/d) for 5 consecutive days (Days 21–25). Aerosolized OVA (same concentration) was administered (10min/d) 3d/wk for the remainder of the study. It should be noted that we have demonstrated previously that this method of OVA-immunization induces an allergic response as indicated by increases in serum OVA-specific IgE levels (Pastva et al., 2004). Mice assigned to non-sensitized control groups (S, E groups) were injected and aerosolized with saline.
Figure 1.
Time-line of OVA treatment and exercise training. As described in the Methods section, mice were injected with OVA twice on dys 0 and 14 followed OVA aerosolizations on dys 21 – 25. Mice were then challenged repeatedly with OVA via aerosolization during exercise training on dys 28 – 49. 24hr post the last OVA/exercise bout, mice were sacrificed and analyzed as indicated.
2.3 Exercise training protocol
Exercised mice (E, EO) underwent bouts of aerobic exercise on a motorized treadmill (Exer 6M, Columbus Instruments) 1h post-aerosolization as described (Pastva et al., 2004); sedentary mice (S, SO groups) continued spontaneous activity in their cages. During the first week of the exercise regimen, treadmill speed and exercise duration were progressively increased from 10.0 m/min for 30 min to 13.5 m/min for 45 min in order to acclimate to and train mice on the treadmill. In the remainder of the protocol (weeks 2 – 4), mice exercised for 45 min at 13.5 m/ min (0% grade) (Pastva et al., 2004). Exercise bouts were performed 3d/wk for a total of 10 bouts (see Fig. 1). Mice were euthanized 24h after the final exercise bout via i.p. injection of ketamine (8.7 mg/100g body wt)/xylazine (1.3 mg/100g body wt) and prepared for analyses.
2.4 Bronchioalveolar lavage cell analyses
Mice were prepared for bronchoalveolar lavage (BAL) as described (Pastva et al., 2004). Cell viability was determined via trypan blue exclusion and cell types were differentiated on cytospin preps using Wright-Giemsa stain. Cell differentials were analyzed in a blinded fashion and determined from at least 500 leukocytes using standard hematological criteria (n = 4 – 6 per experimental group).
2.5 Flow cytometry
Lungs, mediastinal lymph nodes (MLN), and spleens were disassociated and red blood cells were lysed. Cells were then fixed in buffered Formalin (Sigma Chemical) and stored at 4°C until analysis using a Becton Dickinson LSR Benchtop Flow Cytometer with a 488nm argon-ion laser. Analysis for Foxp3 was performed by gating on the Foxp3EGFP+ population. A minimum of 30,000 gated events were collected for each sample (n = 7 – 14 per experimental group). The autofluorescence gate was set using an unstained, non-EGFP sample. Compensation for each antibody was set with the auto-compensation feature on the LSR by gating on singly-labeled samples. All autofluorescent samples fell within the first log, with stained samples falling between the first and third log.
2.6 In vitro suppression and cytokine analyses
Spleens and lungs were disassociated and red blood cells were lysed. CD4+CD25+ Treg cells and CD4+CD25− effector cells were isolated using a MACS isolation kit according to the manufacturer’s instructions (Miltenyi Biotech). In order to compare suppression function levels between treatment groups, CD4+CD25− effector cells for all suppression assays were derived from OVA-treated, sedentary (SO) mice. CD4+CD25+ cells were co-cultured in complete RPMI 1640 (supplemented with glutamine/10% FBS/1% Pen-Strep/1% Pyruvate/50µM 2-Mercaptoethanol) containing 2µg/mL anti-CD3 (eBioscience) and 2µg/mL anti-CD28 (eBioscience) at increasing titers with CD4+CD25− effector cells, which remained constant at 20,000 cells per well (OVA-treated mice: 16 – 22 mice per experimental group; saline-treated mice: 3 – 4 samples per experimental group with each sample containing cells pooled from 3 – 4 mice). For anti-TGFβ blocking studies, cultures also included either anti-TGFβ or an isotype-matched antibody control (10µg/ml, eBioscience; 3 – 5 samples per experimental group with each sample containing cells pooled from 3 – 4 mice) (Levings et al., 2002; You et al., 2005). Cells were cultured for 72hr (37°C, 5% CO2); tritiated thymidine (3H, 0.5µCi/well; Amersham) was then added for an additional 18hr. Cultures were analyzed for 3H incorporation via scintillation counting (PHD Harvester, BRANDEL). For cytokine analyses, supernatants were harvested from parallel cultures (non-3H-treated) and examined for differences in TGF-β, IL-4, IL-5, IL-10, and IL-17 protein levels via specific enzyme-linked immunosorbent (ELISA, R&D Systems) or multiplex (Millipore) assay systems as indicated (3 – 6 samples per experimental group with each sample containing cells pooled from 3 – 4 mice).
2.7 Statistics
Data were analyzed using two-way ANOVA analysis between treatments as indicated (GraphPad Prism 4). A Tukey’s-HSD post-hoc analysis determined which group means differed significantly. To analyze the effects of exercise on sedentary mice directly, an unpaired Student’s T-test was performed. Results are reported as group means ±SD with significance set at a level of p≤0.05.
3. Results
3.1 Aerobic exercise at a moderate intensity attenuates the number of immune cells within the lungs of Foxp3 reporter mice
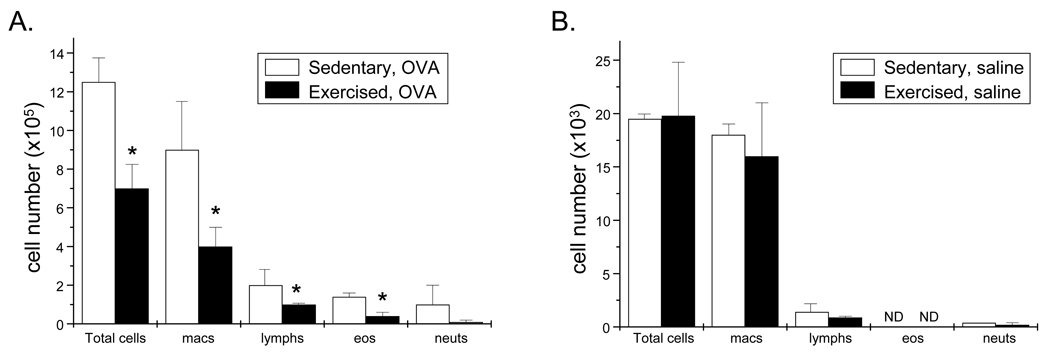
Foxp3 is a transcription factor expressed specifically in CD4+CD25+ Treg cells and is required for the development and function of these cells (Ziegler, 2006). To assess the effects of aerobic exercise training at a moderate intensity on Treg cell responses, we employed the Foxp3EGFP reporter mouse. In order to validate the use of this mouse model in our exercise studies, mice were bronchioalveolar lavaged and resulting lavagates were assessed for differences in immune cell number at 24hr post conclusion of the OVA-treatment and exercise protocol (Fig. 1). Results demonstrate that, in OVA-treated Foxp3EGFP reporter mice, exercise decreased total immune cell numbers, including the numbers of macrophages, eosinophils, and lymphocytes, between 50% – 80% as compared with sedentary controls (Fig. 2A); saline-treated sedentary and exercised mice exhibited a similar trend (Fig. 2B). These results support our previous findings and, thereby, indicate that the Foxp3 reporter gene does not alter the OVA-and/or exercise-related phenotype we have described previously (Pastva et al., 2004, 2005). Further, these findings also validate the use of the Foxp3EGFP reporter mice in the analyses described below.
Figure 2.
Exercise decreases cellular infiltration in the lungs of OVA-treated, Foxp3 reporter mice. 24 hr post protocol completion, BAL was performed and resulting lavagates were analyzed for changes in total number of cells, macrophages, lymphocytes, eosinophils, and neutrophils. Results are reported as A) cell number × 105 (OVA-treated) and B) cell number × 103 (saline-treated) (*p < 0.03 as compared with OVA-treated, sedentary mice via unpaired Student’s T-test; n = 3 – 6 mice per group; ND – none detected). Two-way ANOVA indicated that OVA-treatment significantly increased cell numbers in all groups (p = 0.001, F = 26). OVA groups differed from saline groups as determined via Tukey’s post hoc analysis (p < 0.05).
3.2 Exercise increases the Foxp3+ Treg cell population in lung and mediastinal lymph nodes
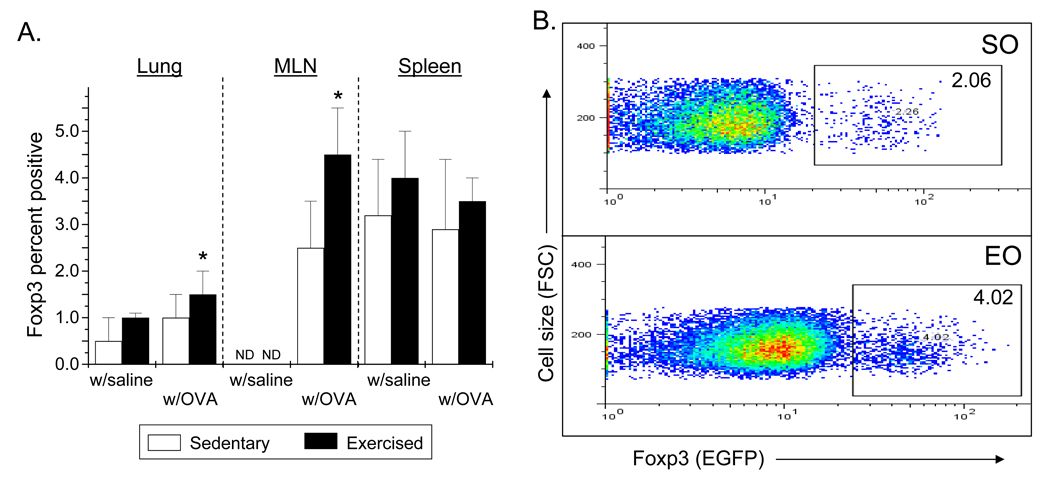
To determine the effect of moderate intensity aerobic exercise training on the numbers of Foxp3+ cells, lung, mediastinal lymph nodes, and spleens were harvested and disassociated; cells were analyzed for Foxp3EGFP expression via flow cytometry. Mediastinal lymph nodes (MLN) are regarded as the draining lymph nodes for the lung. OVA-treated and exercised mice displayed a significantly greater number of Foxp3EGFP positive cells in the lung and MLN as compared with sedentary controls (Fig. 3A, 3B); however, no differences in Foxp3EGFP detection were observed in spleen (Fig. 3A). Tissues harvested from saline-treated control (S, E) mice exhibited a similar trend of Foxp3+ expression as compared with OVA-treated, sedentary controls (Fig. 3A).
Figure 3.
Exercise increases the number of CD4+Foxp3+ cells in the lungs and mediastinal lymph nodes (MLNs) of OVA-treated mice. Foxp3+ reporter mice were OVA-treated and exercised as described in Methods. 24hr post protocol completion, cells were isolated from lungs, MLN, and spleens and analyzed via flow cytometry. A) Results are presented as percent Foxp3+ cells (*p = 0.01 as compared with SO mice via unpaired Student’s T-test; n = 3 – 14 per group; ND – none detected). Two-way ANOVA analysis indicated that exercise significantly increased the number of Foxp3+ cells in the in lung and spleen (p ≤ 0.02, F ≥ 5). For the lung and spleen samples, exercise groups differed from sedentary groups as determined via Tukey’s post hoc (p < 0.05). B) Representative histograms from the analyses of MLN (SO – sedentary, OVA-treated; EO – exercised, OVA-treated).
3.3 Exercise training significantly enhances CD4+CD25+Foxp3+ suppression function
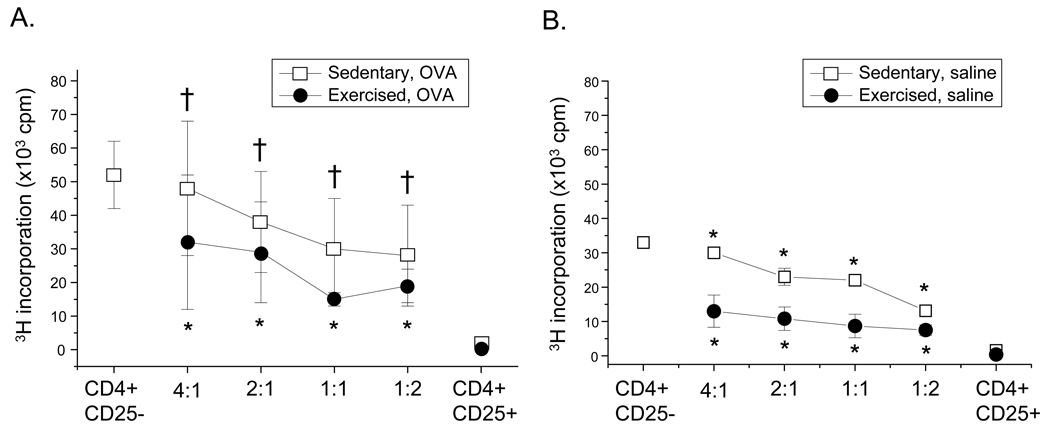
Suppression of effector T cell proliferation is a hallmark of Treg cell function. To delineate the effect of exercise training on Treg cell suppression function, CD4+CD25+Foxp3+ cells were isolated and co-cultured with CD4+CD25− effector cells derived from OVA-treated, sedentary mice; changes in proliferation were measured via 3H-thymidine incorporation. As shown in Figure 4, CD4+CD25+Foxp3+ cells isolated from spleens (Fig. 4A) of OVA-treated, exercised (EO) mice suppressed effector cell proliferation more effectively at all titers than did CD4+CD25+Foxp3+ cells derived from their sedentary counterparts (SO). Splenic CD4+CD25+Foxp3+ cells harvested from saline-treated, exercised (E) mice also displayed a greater level of suppression function at each titer as compared with saline-treated, sedentary (S) controls (Fig. 4B). Importantly, CD4+CD25+Foxp3+ cells isolated from the lungs and MLN tissues of OVA-treated SO and EO mice exhibited a similar trend as splenic-derived cells (data not shown).
Figure 4.
Exercise increases the suppressive function of CD4+CD25+Foxp3+ cells. Foxp3+ reporter mice were OVA-treated and exercised as described in Methods. 24 hr post-protocol completion, CD4+CD25+Foxp3+ cells were isolated from A) spleens of OVA-treated mice (n = 16 – 22 mice per group) as well as B) spleens of saline-treated mice (n = 3 – 4 samples per group) via MACS separation. Cells were co-cultured at increasing concentrations (effector:suppressor) with CD4+CD25− cells derived from sedentary, OVA-treated (SO) mice in the presence of anti-CD3 and anti-CD28 for 72 hrs. Co-cultures were pulsed for an additional 18h with [3H]thymidine and analyzed for suppression. Results are presented as 3H incorporation cpm. Two-way ANOVA analysis indicated that increased suppressive function is the result of exercise and not OVA treatment or cell ratio (exercise p<0.0001; F ≥ 10). An unpaired Student’s T test showed *p < 0.01 as compared with SO mice and †p < 0.01 as compared with S mice. Exercise groups differed from sedentary groups as determined via Tukey’s post hoc analysis (p < 0.0001).
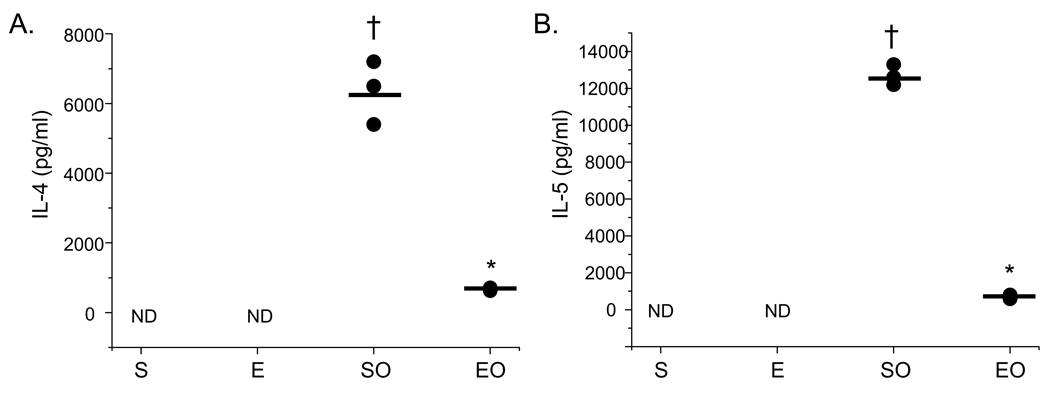
To determine whether CD4+CD25+Foxp3+ cells from exercised mice also suppressed Th2 function more effectively than cells derived from SO mice, cytokine analyses were performed. For these analyses, the supernatants of splenic suppression cultures at the effector:suppressor ratio of 1:1 were harvested analyzed for differences in the levels of the Th2-derived cytokines IL-4 and IL-5. Because the greatest difference in CD4+CD25+Foxp3+-mediated suppression was observed at an effector:suppressor ratio of 1:1 (Fig. 4A), cytokine analyses were performed on the supernatants of these cultures. Findings presented in Figure 5 show that the production of IL-4 and IL-5 was decreased significantly in cultures that contained CD4+CD25+Foxp3+ cells derived from EO mice as compared with SO controls. In cultures that contained CD4+CD25+Foxp3+ cells derived from saline-treated controls (S, E), neither IL-4 nor IL-5 was detected (Fig. 5).
Figure 5.
Exercise decreases the protein levels of IL-4 and IL5 in CD4+CD25+Foxp3+ Treg cell suppression cultures. Supernatants were harvested from parallel cultures described in Figure 4 and analyzed for A) IL-4 and B) IL-5 protein content via ELISA (S – sedentary; E – exercised; SO – sedentary, OVA-treated; EO – exercised, OVA-treated). Results are presented as pg/ml (*p < 0.04 as compared with SO mice, †p < 0.04 as compared with S mice as determined via unpaired Student’s T-test; n = 3 – 6 samples per group; ND – none detected).
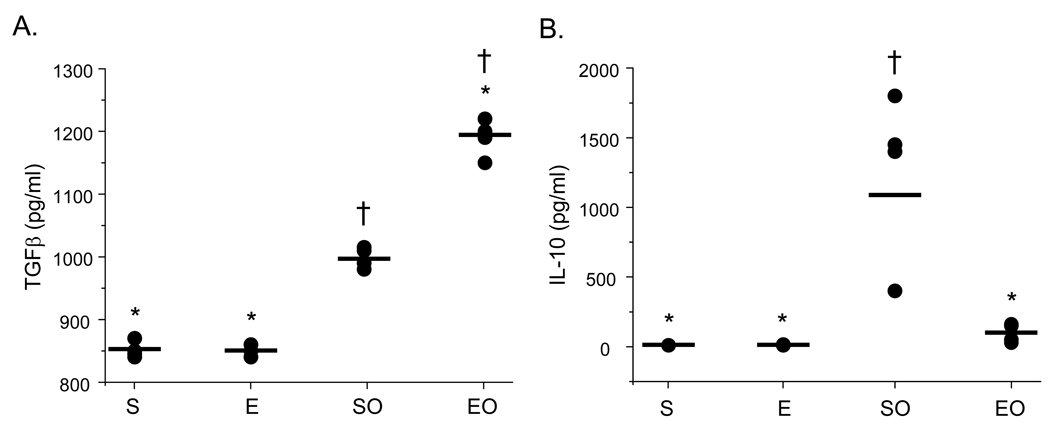
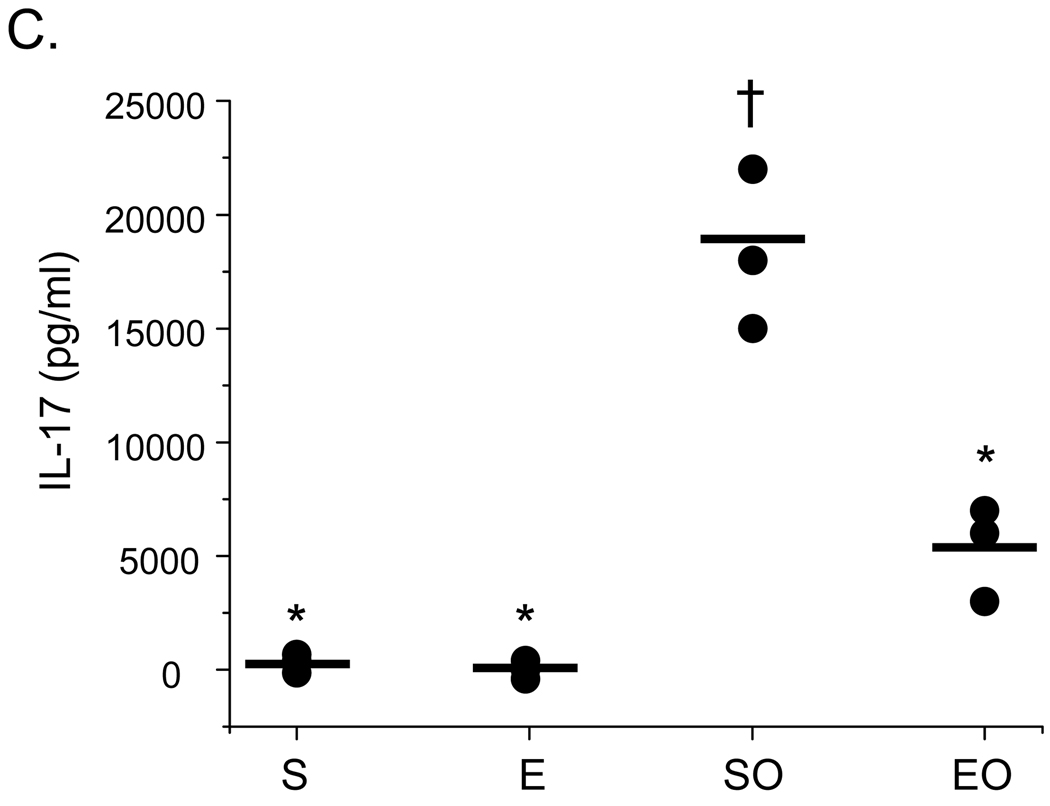
3.4 Exercise increases TGF-β yet decreases IL-10 and IL-17 production in OVA-sensitized mice
The cytokines TGF-β, IL-10, and IL-17 have been implicated in Treg cell-mediated suppression (Beriou et al., 2009; Fontenot and Rudensky, 2005; Sakaguchi, 2004; Oida et al., 2006). To determine whether TGF-β, IL-10, and/or IL-17 may play a role in exercise-mediated increases in CD4+CD25+Foxp3+ suppressor function, supernatants from parallel splenic effector:suppressor cell co-cultures described above (Fig. 4B) were collected and assessed for protein levels of TGF-β, IL-10, and IL-17 protein via ELISA. As noted above, the greatest difference in CD4+CD25+Foxp3+-mediated suppression was observed at a effector:suppressor ratio of 1:1; as such, cytokine analyses were performed on the supernatants of these cultures. Results in Figure 6 demonstrate that TGF-β production was increased significantly in cultures that contained CD4+CD25+Foxp3+ cells derived from OVA-treated, exercised (EO) mice as compared with controls. In contrast, IL-10 and IL-17 protein levels were decreased in these cultures (Fig. 6B, 6C). In cultures that contained CD4+CD25+Foxp3+ cells derived from saline-treated controls (S, E), significantly lower amounts of TGF-β and IL-10 were observed as compared with those of OVA-treated mice (Fig. 6A, 6C); however, levels of IL-10 were equivalent to EO samples (Fig. 6B).
Figure 6.
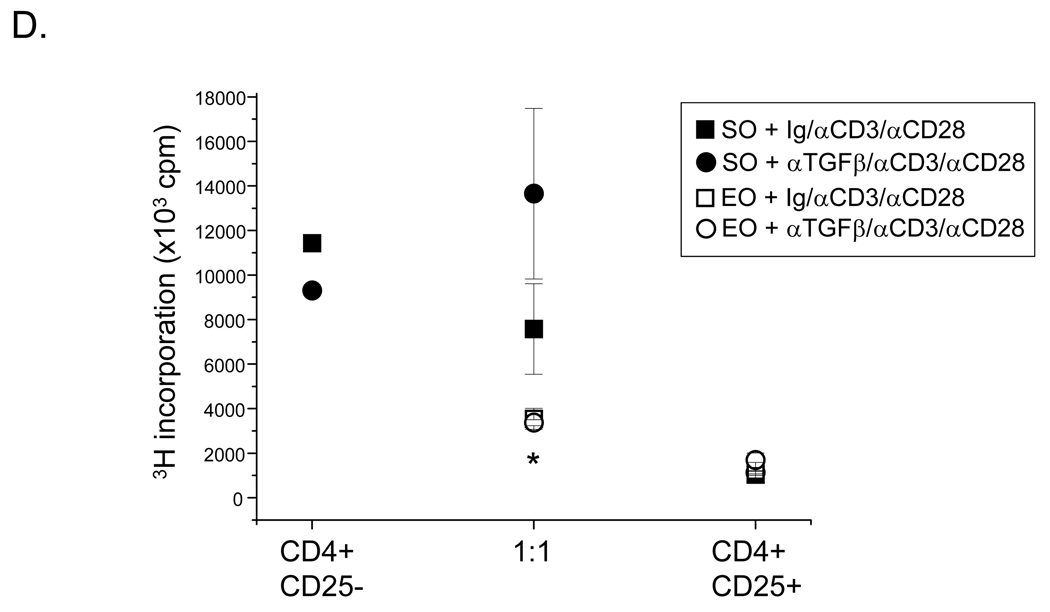
Exercise increases the protein levels of TGF-β, but not IL-10 or IL-17, in CD4+CD25+Foxp3+ Treg cell suppression cultures. Supernatants were harvested from parallel cultures described in Figure 4 and analyzed for A) TGF-β, B) IL-10, and C) IL-17 protein content via ELISA or multiplex assay (S – sedentary; E – exercised; SO – sedentary, OVA-treated; EO – exercised, OVA-treated). Results are presented as pg/ml (*p < 0.05 as compared with SO mice, †p < 0.05 as compared with S mice determined via unpaired Student’s T-test; n = 3 – 6 samples per group; each sample contained cells pooled from 3 – 4 mice). For TGFβ, two-way ANOVA analysis revealed significance of exercise at p = 0.03 and significance of OVA at p = 0.0001 (F = 69). Exercise groups differed from sedentary groups as determined via Tukey’s post hoc analysis (p ≤ 0.05). D) For anti-TGFβ blocking studies, an anti-TGFβ blocking antibody or isotype-matched antibody control was added to suppression assays, and analyzed as described in Figure 4. Results are presented as 3H incorporation (cpm; *p < 0.01 as compared with SO mice determined via unpaired Student’s T-test; n = 3 – 5 samples per group).
To determine whether increased levels of TGF-β observed in the cultures of CD4+CD25+Foxp3+ cells derived from EO mice played a role their enhanced suppressive function, an anti-TGF-β blocking antibody or isotype-matched Ig control was added to suppression assays in parallel. Results shown in Figure 6D demonstrate that the anti-TGF-β antibody had no effect on the suppressive function of CD4+CD25+Foxp3+ cells derived from EO mice. In contrast, the suppressive function of CD4+CD25+Foxp3+ cells derived from SO mice was blocked (Fig. 6D). The isotype-matched Ig control had no effect on suppressive responses of cells derived from either EO or SO mice (Fig. 6D).
4. Discussion
The findings detailed herein demonstrate for the first time that aerobic exercise training significantly increases the number and function of the CD4+CD25+Foxp3+ Treg cell population in a murine asthma model. In particular, these findings show that aerobic exercise at a moderate intensity increases the Foxp3+ Treg cell population within the lung and draining lymph nodes, but not spleens, of OVA-treated mice as compared with sedentary controls. Because the lungs of OVA-treated mice were challenged directly via aerosolization, these findings suggest that the effect of exercise on CD4+CD25+Foxp3+ Treg cell numbers was localized to the pulmonary compartment. Moreover, these findings support the work of Kearley et al (2005) who reported that the transfer of CD4+CD25+ Treg cells into OVA-treated mice migrate to the airways and draining lymph nodes following allergen exposure.
We have reported previously that, in OVA-treated mice, exercise training attenuates asthma-related airway inflammation and hyperresponsiveness (Pastva et al., 2004, 2005; Hewitt et al., 2009b). Recent studies suggest that an imbalance between Th2 and CD4+CD25+Foxp3+ Treg cell functions may underlie the development and progression of asthmatic responses (Heijink and Van Oosterhout, 2006; Jaffar et al., 2004; McGee and Agrawal, 2006; Umetsu and DeKruyff, 2006; Wing et al., 2005). To this end, several groups have shown that transfer of CD4+CD25+Foxp3+ Treg cells into OVA-treated mice prior to allergen challenge suppresses airway inflammation and AHR (Kearley et al., 2005; Van Oosterhout and Bloksma, 2005; Zuany-Amorim et al., 2002). In light of our previous work, we conclude from the current study that exercise-mediated increases in CD4+CD25+Foxp3+ Treg cell numbers within the airways may be the mechanism by which exercise attenuates asthmatic responses in vivo.
Our results also show that aerobic exercise at a moderate intensity enhances the capacity of CD4+CD25+Foxp3+ cells to suppress CD4+CD25− effector T cell proliferation and Th2-related cytokine production in vitro. The significant decrease in the number of lymphocytes observed within the BAL fluid of EO mice in vivo as compared with SO controls, further support these findings. Although the mechanisms that underlie the suppressive actions of the CD4+CD25+Foxp3+ Treg population are not fully understood, recent studies indicate that these cells may exert their suppressive effects through release of soluble factors, including TGF-β and IL-10 (Fontenot and Rudensky, 2005; Joetham et al., 2007; Sakaguchi, 2004; Vignali et al., 2008). Both TGF-β and IL-10 are considered inhibitory cytokines. TGF-β has also been reported to promote the expression of Foxp3+ in human and mouse Treg cells (Vignali et al., 2008). Despite these observations, the defined contribution of TGF-β and IL-10 to Treg cell suppression is a matter of debate (Vignali et al., 2008). In the current study, we observed that TGF-β protein levels were increased significantly in effector:suppressor cultures that contained CD4+CD25+Foxp3+ Treg cells derived from OVA-treated, exercised mice as compared with sedentary controls. The addition of an anti-TGFβ blocking antibody to these cultures, however, had no effect on the enhanced suppressive actions of these cells (Fig. 6D). In contrast, the anti-TGFβ antibody blocked the suppressive function of CD4+CD25+Foxp3+ Treg cells derived from OVA-treated, sedentary mice. These results suggest that TGFβ does not play a role in exercise-mediated increases in suppression function. Further, we observed that cultures containing CD4+CD25+Foxp3+ Treg cells-derived from OVA-treated, exercised mice contained significantly less IL-10 than their sedentary counterparts. These results are consistent with a report by Kearley et al (2005), which showed that IL-10 production by the Treg cells themselves is not required for suppression. Together, these findings indicate that aerobic exercise at a moderate intensity may alter the cytokine production profiles of CD4+CD25+Foxp3+ Treg cells and thereby, promote plasticity in Treg cell phenotypes. Moreover, these findings indicate that differing mechanisms underlie the suppression function of CD4+CD25+Foxp3+ Treg cells derived from exercised versus sedentary mice.
In addition to decreased amounts of IL-10, we observed that cultures containing CD4+CD25+Foxp3+ Treg cells from exercised, OVA-treated mice had lesser amounts of IL-17. IL-17 is expressed by Th17 cells, which have been defined as a unique population of pro-inflammatory helper T cells. Recent studies demonstrate that CD4+CD25+Foxp3+ Treg cells can be induced to express IL-17; however, the role of IL-17 in Treg cell-mediated suppression is currently under debate (Beriou et al., 2009; Voo et al., 2009). Voo and co-workers (2009) have reported that CD4+CD25+Foxp3+ Treg cells express IL-17 and maintain their ability to suppress CD4+CD25− effector cell proliferation in the presence of anti-CD3 and anti-CD28 stimulation. In contrast, Beriou et al (2009) has demonstrated that, while CD4+CD25+ Treg cells can both express IL-17 and suppress, this type of Treg cell transiently loses suppressive activity when secreting IL-17. This group also demonstrated that the strength of TcR signal in the presence of antigen presenting cells correlates positively with Treg cell-derived IL-17 production. In light of these reported findings, our data suggest that exercise may dampen Treg cell IL-17 production and, thereby, enhance suppression.
In summary, data presented herein show for the first time that aerobic exercise training at a moderate intensity significantly increases CD4+CD25+Foxp3+ Treg cell responses in a murine asthma model. Future studies will elucidate the mechanisms that underlie the effects of exercise training on these responses. One potential mechanism involves exercise-mediated increases in endogenous glucocorticoids. Exercise stimulates the hypothalamic-pituitary-adrenal axis to upregulate its release of endogenous GC into the circulation. A growing body of evidence suggests that endogenous GC is an important regulator of allergic disease progression. Circulating inflammatory cell number and lung function both vary with the diurnal production of endogenous GC (Schleimer, 2000). We have shown previously that exercise-mediated attenuation of airway inflammatory responses in OVA-treated mice is dependent upon increases in endogenous glucocorticoids (Pastva et al., 2005). In addition, Chen and co-workers have reported that glucocorticoid-mediated asthma therapy upregulates the CD4+CD25+ Treg cell population (2006).
Future studies will also determine the effects of moderate intensity aerobic exercise applied at different times following OVA exposure and at different numbers of bouts on Treg cell responses. For example, we have reported previously that one bout of moderate intensity aerobic exercise attenuated airway inflammatory responses, including cell infiltration, but not airway hyperresponsiveness (Hewitt et al 2009a). Because Treg cells have been implicated in attenuating both airway inflammation and hyperresponsiveness, it is unclear from our current data whether a single bout of exercise would likewise enhance Treg cell responses; therefore, additional experiments and analyses are needed. Collectively, such analyses will provide insight into the translation of this work into the clinical setting where exercise could be used to enhance the Treg cell responses of asthmatic patients in vivo.
Acknowledgements
The authors would like to thank Drs. Marcas Bamman, Scott Barnum, Casey Weaver, and Jillian Wohler for helpful discussions and Enid Keyser and the UAB Imaging Core Facility for technical assistance. This work was supported by 1F32HL092726 (to TL), 5T32HL007553-25 (to KD), 1R01HL075465 (to LMS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: All authors declare that there are no conflicts of interest
References
- Beriou G, Costantino C, Ashley C, Yang L, Kuchroo V, Baecher-Allan C, et al. IL-17 producing human peripheral regulatory T cells retain suppressive function. Blood. 2009 doi: 10.1182/blood-2008-10-183251. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Oppenheim J, Winkler-Pickett R, Ortaldo J, Howard O. Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3+CD4+CD25+ T regulatory cells in vivo and enhances their capacity to suppress EAE. Eur J Immunol. 2006;36:2139–2149. doi: 10.1002/eji.200635873. [DOI] [PubMed] [Google Scholar]
- Fontenot J, Rudensky A. A well adapted regulatory contrivance: regulatory T cell development and the foxhead family transciption factor Foxp3. Nat.Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- Heijink I, Van Oosterhout A. Strategies for targeting T-cells in allergic disease and asthma. Pharmacol Ther. 2006;112:489–500. doi: 10.1016/j.pharmthera.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Hewitt M, Creel A, Estell K, Davis I, Schwiebert L. Acute exercise decreases airway inflammation, but not responsiveness, in an allergic asthma model. Am J Respir Cell Mol Biol. 2009;40:83–89. doi: 10.1165/rcmb.2008-0172OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt M, Estell K, Davis I, Schwiebert L. Repeated bouts of moderate intensity aerobic exercise reduce airway reactivity in a murine asthma model. Am J Respir Cell Mol Biol. 2009 doi: 10.1165/rcmb.2009-0038OC. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffar Z, Sivakuru T, Roberts K. CD4+CD25+ T cells regulate airway eosinophilic inflammation by modulating the Th2 cell phenotype. J Immunol. 2004;172:3842–3849. doi: 10.4049/jimmunol.172.6.3842. [DOI] [PubMed] [Google Scholar]
- Joetham A, Takeda K, Taube C, Miyahara N, Matsubara S, Koya T, et al. Naturally occurring lung CD4(+)CD25(+) T cell regulation of airway allergic responses depends on IL-10 induction of TGF-beta. J.Immunol. 2007;178:1433–1442. doi: 10.4049/jimmunol.178.3.1433. [DOI] [PubMed] [Google Scholar]
- Kearley J, Barker J, Robinson D, Lloyd C. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 1 dependent. J Exp Med. 2005;202:1539–1547. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster G, Halson S, Khan Q, Drysdale P, Wallace F, Jeukendrup A, et al. Effects of acute exhaustive exercise and chronic exercise training on type 1 and type 2 T lymphocytes. Exerc Immunol Rev. 2004;10:91–106. [PubMed] [Google Scholar]
- Levings MK, Sangregorio R, Sartirana C, Moschin AL, Battaglia M, Orban PC, et al. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor b, but not interleukin 10, and are distinct from type 1 T regulatory cells. J Exp Med. 2002;196:1335–1346. doi: 10.1084/jem.20021139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas S, Platts-Mills TA. Physical activity and exercise with asthma: Relevance to etiology and treatment. J All Clin Immunol. 2005;115:928–934. doi: 10.1016/j.jaci.2005.01.033. [DOI] [PubMed] [Google Scholar]
- Lucas S, Platts-Mills TA. Paediatric asthma and obesity. Paed Respir Rev. 2006;7:233–238. doi: 10.1016/j.prrv.2006.08.001. [DOI] [PubMed] [Google Scholar]
- McGee H, Agrawal D. TH2 cells in the pathogenesis of airway remodeling: regulatory T cells a plausible panacea of asthma. Immunol Res. 2006;35:219–232. doi: 10.1385/IR:35:3:219. [DOI] [PubMed] [Google Scholar]
- Oida T, Xu L, Weiner H, Kitani A, Strober W. TGF--Mediated Suppression by CD4+CD25+ T Cells Is Facilitated by CTLA-4 Signaling. J Immunol. 2006;177:2331–2339. doi: 10.4049/jimmunol.177.4.2331. [DOI] [PubMed] [Google Scholar]
- Pastva A, Estell K, Schoeb TR, Atkinson TP, Schwiebert LM. Aerobic exercise attenuates airway inflammation in a mouse model of atopic asthma. J Immunol. 2004;172:4520–4526. doi: 10.4049/jimmunol.172.7.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastva A, Estell K, Schoeb TR, Schwiebert L. RU486 blocks the anti-inflammatory effects of exercise in a murine model of allergen-induced pulmonary inflammation. Brain Beh Immun 2005. 2005;19:415–422. doi: 10.1016/j.bbi.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Ann.Rev.Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- Satta A. Exercise training in asthma. J Sports Med Phys Fitness. 2000;40:277–283. [PubMed] [Google Scholar]
- Schleimer RP. Interactions between the hypothalamic-pituitary-adrenal axis and allergic inflammation. J.All.Clin.Immunol. 2000;106:S270–S274. doi: 10.1067/mai.2000.110162. [DOI] [PubMed] [Google Scholar]
- Umetsu DT, Akbari O, DeKruyff RH. Regulatory T cells control the development of allergic disease and asthma. J Allergy Clin Immunol. 2003;112:480–487. [PubMed] [Google Scholar]
- Umetsu DT, DeKruyff RH. Immune dysregulation in asthma. Curr Opin Immunol. 2006;18:727–732. doi: 10.1016/j.coi.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Van Oosterhout A, Bloksma N. Regulatory T lymphocytes in asthma. Eur Respir J. 2005;26:918–932. doi: 10.1183/09031936.05.00011205. [DOI] [PubMed] [Google Scholar]
- Vignali D, Collison L, Workman C. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voo K, Wang Y, Santori F, Boggiano C, Wang Y, Arima K, et al. Proc Natl Acad Sci USA. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing K, Suri-Payer E, Rudin A. CD4+CD25+ -regulatory T cells from mouse to man. Scand J Immunol. 2005;62:1–15. doi: 10.1111/j.1365-3083.2005.01634.x. [DOI] [PubMed] [Google Scholar]
- Woods J, Ceddia MA, Zack M, Lowder T, Lu Q. Exercise training increases the naive to memory T cell ratio in old mice. Brain Beh Immun. 2003;17:384–392. doi: 10.1016/s0889-1591(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Yeh S, Chuang H, Lin L, Hsiao C, Wang P, Liu R, et al. Regular Tai Chi Chuan exercise improves T cell helper function of type 2 DM patients with an increase in T-bet transcription factor and IL-12 production. Br J Sports Med epub. 2008 doi: 10.1136/bjsm.2007.043562. [DOI] [PubMed] [Google Scholar]
- You S, Belgith M, Cobbold S, Alyanakian MA, Gouarin C, Barriot S, Garcia C, et al. Autoimmune diabetes onset results from qualitative rather than quantitative age-dependent changes in pathogenic T-cells. Diabetes. 2005;54:1415–1422. doi: 10.2337/diabetes.54.5.1415. [DOI] [PubMed] [Google Scholar]
- Ziegler S. Foxp3: Of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- Zuany-Amorim C, Sawicka E, Manlius C, Le Moine A, Brunet L, Kemeny D, et al. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T cells. Nat Med. 2002;8:625–629. doi: 10.1038/nm0602-625. [DOI] [PubMed] [Google Scholar]