Abstract
Objective
To assess the effect of HIV care (including HAART if eligible) on neurodevelopment.
Design
Prospective cohort study
Methods
Motor and mental development of 35 HIV-infected children (age 18-71 months) was assessed at entry into care, and after 6 and 12 months using age-appropriate tools. Developmental trajectory was compared to 35 HIV-uninfected, affected and 90 control children using linear mixed effects models. Effects of age (≤ or >29 months) and timing of entry into care (before or after HAART eligibility) were explored in secondary analyses.
Results
At baseline, HIV-infected children had the lowest, control children the highest, and HIV-uninfected affected children intermediate mean developmental scores. After one year of care, HIV-infected children achieved mean motor and cognitive scores that were similar to HIV uninfected, affected children although lower compared to control children. Overall, HIV-infected children experienced accelerated motor development but similar gains in cognitive development compared to control children. Exploratory analyses suggest that younger children and those presenting early may experience accelerated greater gains in development.
Conclusions
HIV-infected children accessing care experience improved motor development, and may, if care is initiated at a young age or an early stage of the disease, also experience gains in cognitive development.
Keywords: HIV, children, Africa, neurodevelopment, HAART
INTRODUCTION
Human Immunodeficiency Virus (HIV)-associated neurodevelopmental delay is a consequence of the central nervous system (CNS) invasion of the virus early after infection and may present as early as the first year of life.1-3 HIV-associated CNS manifestations can include global neurodevelopmental delay 4, overall impaired cognitive functioning, selective delays in executive function 5, 6, language 7, 8, or visual-spatial tasks9, and subtle differences in specific areas such as processing speed 10. This broad spectrum of HIV-associated CNS manifestations reflects the multiple factors that influence the impact of the virus in an individual child, including timing of infection 11, 12, age at neurodevelopmental assessment 2, 4, 13, and the quality of the child’s environment14.
Prior to the availability of antiretroviral treatment, HIV—associated encephalopathy was a common manifestation of vertically acquired pediatric HIV infection, with reported prevalence rates of HIV encephalopathy, the most severe CNS manifestation, as high as 50%.15, 16 The effect of antiretroviral drugs on pediatric CNS manifestations was first demonstrated by Pizzo and Brouwers, who documented a reversal of pediatric HIV encephalopathy in response to continuous intravenous zidovudine mono-therapy.17, 18 Raskino et al. found that combination therapy with ZDV and didanosine was more effective against HIV—associated CNS manifestations than either drug in mono-therapy.19 On a population level, access to HAART has substantially decreased the incidence of pediatric HIV encephalopathy in the Unites States. A retrospective cohort study observed a decrease in the prevalence of HIV encephalopathy from 40.7% to 18.2% in children born before and after 1996, respectively.20 A prospective study of vertically infected children (60% on HAART) found a low (1.6%) prevalence of active progressive HIV encephalopathy, but a relatively high rate (10%) of arrested HIV encephalopathy.21 Even though more than 80% of new HIV infections in children occur in the African continent, few studies have documented the developmental course of the cognitive and motor development of HIV-infected children in Africa, and no data have been published on the neurodevelopment in HIV-infected African children who gained access to HIV care and treatment including HAART.
To characterize cognitive and motor development in HIV-infected African children we performed a longitudinal, prospective, observational study of HAART-naïve HIV-infected children and compared their neurodevelopment following initiation of HIV care to that of HIV uninfected children affected by AIDS and healthy control children
METHODS
Study participants
HIV-infected children, HIV-uninfected, affected children, and control children aged 18-71 months at the time of the first visit were enrolled between November 2004 and September 2005. The age range 18 to 71 months was selected to focus on preschool children in whom HIV diagnosis was possible using ELISA based methods. HIV-infected children were HAART naïve children identified at time of presentation to a pediatric HIV care and treatment program. HIV-affected children, defined as maternal AIDS orphans or children living with a mother with symptomatic AIDS, were recruited through an organization for social support to HIV-infected women and AIDS orphans. Control children were children born to HIV-uninfected healthy mothers and healthy fathers, with equal numbers of girls and boys for every 6 month age group between 18 and 71 months
Measurement of Neurodevelopment
Age-appropriate tests were administered to assess the cognitive and motor development at enrollment and approximately 6 and 12 months later. Individual children were assessed using the same instrument at each of the three study visits. For children aged 18-29 months, the Bayley Scales of Infant Development, 2nd edition, (BSID-II) were used to assess cognitive (mental) and motor development. Children 30-71 months were assessed using the Peabody Developmental Motor Scales (2nd edition) for motor development, and the Snijders-Oomen Nonverbal (SON) Intelligence Test 2½-7 for cognitive development. The abbreviated version of the SON test was used, in which the puzzles and analogies subtest were eliminated, leaving situations, mosaics, categories and patterns. Test results were recorded as raw scores and subsequently transformed to age-adjusted standardized scores using the normative tables provided in the manual.
Interventions
HIV infected children received primary HIV care and HAART if eligible based on the World Health Organization (WHO) clinical and immunological criteria for antiretroviral therapy in resource-limited settings.22 Parents and caregivers of children identified as having mild or moderate neurodevelopmental delay, the parents were given simple tips on how they could interact with their child to stimulate the child’s development. Medical care and nutritional supplements were not provided by the study.
Data Analysis Strategies and Methods
To characterize the longitudinal patterns of cognitive and motor development, we employed linear mixed-effects models fitted by maximum-likelihood methods.23 For the primary analysis, the model and methods accounted for the occasional left-censoring (scores ≤ 49) of the two standardized outcome measures (cognitive score, motor score) by correct specification of the likelihood function. The analysis included all subjects with one or more assessments and ignored any mis-timing of the two follow-up visits.24 The model coped with missing values via correct specification of the likelihood function and via an iterative algorithm for maximization of the likelihood function. The model was fitted separately for each of the two scores (cognitive and motor) and was conditional on two explanatory variables: visit and cohort. The parameter estimates obtained from the fitted models were used to test a priori hypotheses and to graphically represent the cohort-specific growth curves defined by expected score as a function of time. For each score (cognitive, motor), comparing the three cohorts in terms of these curves relied on a hierarchical testing procedure which began with an F-test of the overall null hypothesis of “no differences among the three cohort-specific curves.” If and only if this identicaltrajectories hypothesis was rejected at level α = 0.05, then component sub-hypotheses regarding the shapes and locations of the curves were tested. Thus, cohorts were deemed to have different rates of development only if the overall hypothesis and the common-shape sub-hypothesis were each rejected at level α = 0.05. Similarly, detection of cohort differences at baseline required rejection of the overall hypothesis and rejection of the common-baseline sub-hypothesis. By way of this hierarchical testing strategy, the rate of type I errors was controlled separately for each of the two scores (cognitive, motor).
Two secondary, post-hoc exploratory analyses were performed, one to investigate the effects of delay in presentation for treatment and one to assess the effects of age group, using the same methods and procedures that were used for the primary analyses. To assess the effect of delay in presentation, the cohort of HIV-infected children was partitioned into two cohorts. Those who presented prior to eligibility to HAART (i.e. at clinical HIV stage 1 or 2, or prior to the severe immuno-suppression as defined by the 2006 WHO classification) were assigned to the “early presentation to care” cohort. The remaining HIV-infected children were assigned to the “late presentation to care” cohort as they were eligible for HAART at time of presentation (i.e. clinical stage 3 or 4, or severe immunosuppression as indicated by low CD4 percentage or CD4 count). To assess the effect of age, each cohort was stratified by age group: younger (18 ≤ age ≤ 29 months), older (29 < age ≤ 71 months)
All statistical computations were performed using SAS 9.1.3 (SAS Institute, Cary, NC).
Ethics approval
The study was approved by the institutional review boards of the Schools of Public Health of the University of North Carolina at Chapel Hill and the University of Kinshasa. Informed consent for participation of children was obtained from parents or guardians.
RESULTS
A total of 160 children aged 18-71 months at the time of the first visit were enrolled: 35 HIV-infected children, 35 HIV-uninfected, affected children, and 90 healthy control children. Overall, half of all children were male and the median age was 44.8 months. At baseline, malnutrition and stunting occurred in all three groups but was more frequent among HIV infected and affected children (Table 1). Almost all (86%) HIV infected children were in clinical WHO stage 3 or 4, median CD4 percentage was 14% (range 0 to 28%). During the one year study period, 71% (25/35) children initiated HAART after a median time of 13 days (range −6 to 206 days). All children initiated a first line regimen consisting of stavudine, lamuvidine and nevirapine in children weighing 15 kilograms or more, or zidovudine, lamuvidine and nevirapine on children under 15 kilograms.
Table 1.
Anthropometric and demographic indicators HIV-infected children (cohort 1), HIV-uninfected affected children (cohort 2), and control children (cohort 3).
| Cohort | P values | ||||||
|---|---|---|---|---|---|---|---|
| All children (n=160) |
Cohort 1 HIV- infected (n=35) |
Cohort 2 HIV- affected (n=35) |
Cohort 3 Control (n=90) |
Cohort 1 vs. 3 |
Cohort 1 vs. 2 |
Cohort 2 vs. 3 |
|
|
Age, median (months) |
44.8 | 45.7 | 33.4 | 45.6 | 0.8971 | 0.6826 | 0.2186 |
|
Gender Male n (%) |
80 (50.0) | 15 (42.9) | 20 (57.1) | 45 (50.0) | 0.4729a | 0.2320a | 0.4729a |
|
Maternal orphans |
13 (8.1) | 10 (28.6) | 3 (8.6) | - | - | 0.0624a | - |
| WAZ < -2 SD | 49 (30.6) | 20 (57.1) | 14 (40.0) | 15 (16.7) | <0.0001a | 0.1513a | 0.0055a |
| HAZ < -2 SD | 39 (24.4) | 19 (54.3) | 14 (40.0) | 6 (6.7) | <0.0001a | 0.2312a | <0.0001a |
| WHZ < -2 SD | 27 (16.9) | 12 (34.3) | 5 (14.3) | 10 (11.1) | 0.0023a | 0.0510a | 0.7597b |
Based on Chi-squared test
Based on Fisher’s exact test
Among HIV infected children, 26/35 (74%) were assessed at all visits, 1 (3%) at two visits, and 8 (23%) at baseline only. Most (88%) missed visits occurred in 7 children who died during the study. Among HIV uninfected, affected children, 29/35 (83%) were assessed at all visits, 4 (11%) at two visits, and 2 (6%) at baseline only. Missed visits were due to child death (n=1), illness at time of scheduled visit (n=2), pregnancy in mother (n=1), and loss to follow up (n=2). Among healthy control children, 39/90 (43%) were assessed at all visits, 34 (38%) at two visits and 17 (19%) at baseline only. Most missed visits occurred due to political unrest and prolonged health care strike. The proportion of children with missed visits was higher among the younger (18 ≤ age ≤ 29 months) compared to the older (29 < age ≤ 71 months) children, a difference that was especially prominent in the HIV-infected cohort where only 45% (5/11) of the younger children returned for visit 2 and/or 3, while 92% (22/24) of the older children returned.
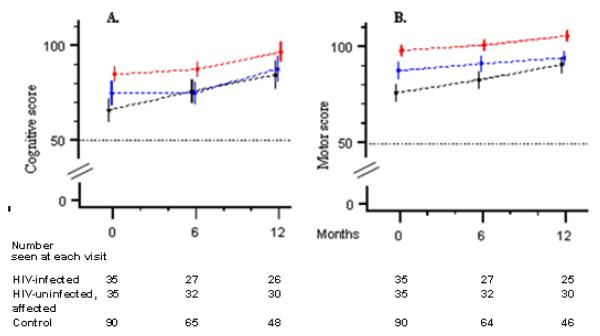
Results of the baseline neurodevelopmental evaluation have been described previously.25 Mean values for the standardized cognitive score and motor score, estimated by the linear mixed-effects model, are presented in Table 2. Figure 1 graphically displays the corresponding neurodevelopmental trajectories (growth curves) defined by these means. The cognitive score and motor score were moderately correlated ( Pearson r = 0.48 at baseline) and had the following in common for both cognitive and motor scores (table 2): (a) at baseline, mean scores were lowest for HIV-infected children, intermediate for HIV uninfected, affected children and highest for healthy control children , (b) after one year (visit 3), the mean scores for the HIV infected children was lower than that of control children (p<0.01) but similar to those of HIV uninfected, affected children; (c) all three groups exhibited improvement in mean score from visit 1 to visit 3; (d) the group that experienced the largest improvements in mean scores were the HIV-infected children, this difference achieved statistical significance for motor development but not for cognitive development.
Table 2.
Mean cognitive and motor development scores of HIV-infected children (cohort 1), HIV-uninfected affected children (cohort 2), and control children (cohort 3).
| Mean scores* | P-values** for Cohort Differences | ||||||
|---|---|---|---|---|---|---|---|
| Cohort 1 HIV infected N = 35 |
Cohort 2 HIV Affected N = 35 |
Cohort 3 Controls N = 90 |
Cohort 1 vs.2 vs.3 |
Cohort 1 vs. 3 |
Cohort 2 vs. 3 |
Cohort 1 vs. 2 |
|
| Cognitive development | |||||||
| Visit 1 (baseline) | 65.8 ± 6.0 | 74.8 ± 6.0 | 84.6 ± 3.8 | < 0.0001 | < 0.0001 | 0.0060 | 0.0350 |
| Visit 2 (6 month) | 75.8 ± 6.0 | 74.9 ± 5.6 | 87.3 ± 3.8 | 0.0002 | 0.0016 | 0.0004 | 0.8391 |
| Visit 3 (12 month) | 84.3 ± 7.2 | 87.6 ± 6.6 | 96.5 ± 5.0 | 0.0102 | 0.0056 | 0.0345 | 0.4945 |
| Mean change from visit 1 to 2 (Δ2-1) |
10.0 ± 5.7 | 0.1 ± 5.2 | 2.6 ± 3.6 | ns | ns | ns | ns |
| Mean change from visit 1 to 3 (Δ3-1) |
18.5 ± 7.7 | 12.8 ± 7.2 | 11.8 ± 5.2 | ns | ns | ns | ns |
| Motor development | |||||||
| Visit 1 (baseline) | 75.7 ± 4.2 | 87.2 ± 4.2 | 97.8 ± 2.6 | < 0.0001 | < 0.0001 | < 0.0001 | 0.0002 |
| Visit 2 (6 month) | 82.4 ± 4.0 | 91.0 ± 3.8 | 101.0 ± 2.6 | < 0.0001 | < 0.0001 | < 0.0001 | 0.0026 |
| Visit 3 (12 month) | 90.4 ± 3.8 | 94.0 ± 3.6 | 105.0 ± 2.6 | < 0.0001 | < 0.0001 | < 0.0001 | 0.1783 |
| Mean change from visit 1 to 2 (Δ2-1) |
6.7 ± 3.8 | 3.8 ± 3.6 | 2.7 ± 2.4 | 0.2134 | ns | ns | ns |
| Mean change from visit 1 to 3 (Δ3-1) |
14.6 ± 4.2 | 6.8 ± 3.8 | 7.6 ± 2.8 | 0.0090 | 0.0055 | 0.7323 | 0.0063 |
Statistical estimate of the mean obtained from the linear mixed-effects model and 95% CI
Model-based p-value for F-test. Type I error controlled by hierarchical testing. P-values significant at 0.05 level.
“ns” indicates a non-significant result due to a preceding non-significant test in the hierarchical sequence of hypothesis testing; e.g., cohorts 1 and 3 (sub-hypothesis) cannot be different if it is true that cohorts 1, 2 and 3 (overall hypothesis) are all the same.
Figure 1.
Growth curves (mean score point estimates and 95% confidence intervals) for cognitive (A) and motor (B) development in HIV-infected children (black lines), HIV-uninfected, affected children (blue lines) and control children (red lines).
When comparing the rate of development of HIV infected children to that of healthy control children, the null hypothesis “same growth curve” was rejected for both motor and cognitive development (table 3). The null hypothesis “same shape of growth curve” was also rejected for motor development (p = 0.0187) but not for cognitive development, indicating that the observed difference in cognitive growth may be due to differences in baseline values followed by similar rate of development in the subsequent 12 months.
Table 3.
Differences in mean cognitive and motor development growth curves of HIV-infected children (cohort 1), HIV-uninfected affected children (cohort 2), and control children (cohort 3).
| P-values* for Cohort Differences | ||||
|---|---|---|---|---|
| Cohort 1 vs.2 vs.3 |
Cohort 1 vs. 3 |
Cohort 2 vs. 3 |
Cohort 1 vs. 2 |
|
| Cognitive development | ||||
| Hypothesis: Same growth curve | < 0.0001 | < 0.0010 | 0.0034 | 0.0383 |
| Hypothesis: Same shape of growth curve | 0.0969 | ns # | ns | Ns |
| Motor development | ||||
| Hypothesis: Same growth curve | < 0.0001 | < 0.0001 | < 0.0001 | 0.0014 |
| Hypothesis: Same shape of growth curve | 0.0370 | 0.0187 | 0.7067 | 0.0223 |
Model-based p-value for the F-test of the indicated null hypothesis. Type I error controlled by hierarchical testing. P-values significant at 0.05 level.
“ns” indicates a non-significant result due to a preceding non-significant test in the hierarchical sequence of hypothesis testing
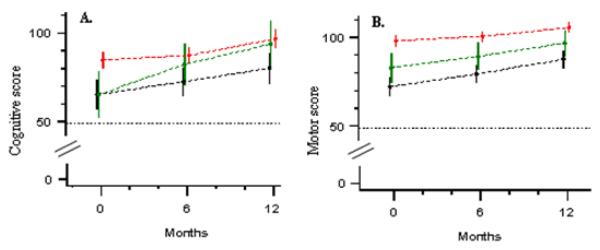
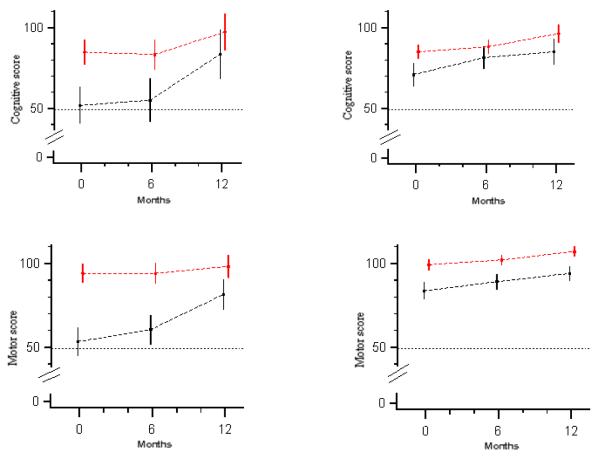
In the first hypothesis generating analysis, we investigated the effects of early versus late presentation for care of HIV-infected children. Less than one in three (10/35 or 28.6%) HIV-infected children presented early for care (i.e. prior to eligibility for HAART) and 25 (71.4%) presented late. Among those presenting late, 21 (84%) started HAART after a median time of ten days following enrollment; 4 (40 %) early presenters initiated HAART after a median time of 101 days. Regarding cognitive development, mean score at baseline was similar for those presenting early and late, but the increase in the mean cognitive score 12 months after entry into care was higher among those children presenting early for care (28.6 vs. 14.8; p = 0.1094). Even though figure 2 suggests an accelerated growth in cognitive development among children presenting early, the shape of the cognitive development growth curve for this group was not statistically significantly different (p=0.2176) from that of the children presenting late. Regarding motor development, HIV-infected children presenting late performed worse compared to those presenting early at baseline (71.6 vs. 80.9, p=0.0487) and at both follow up visits (p=0.0400 and p=0.0428). The two groups however experienced similar gains in motor development scores over time, with similar shapes of the motor growth curves (p=0.938). In a second exploratory analysis, we investigated the effects of age at time of presentation for care. Among HIV infected children, 11 children were age ≤ 29 months and 24 were older; among control children, 20 were age ≤ 29 months and 70 were older. Compared to older children, mean cognitive and motor development scores at baseline were lower among younger HIV-infected children (Figure 3).25 Compared to control children of the same age group, we observed an accelerated motor and cognitive development in the younger HIV infected children but not the older children. Age thus had a significant impact on the shape of the cognitive and motor development growth curves (p=0.0031 for cognitive and p=0.0025 for motor).
Figure 2.
Growth curves (mean score point estimates and 95% confidence intervals) for cognitive (A) and motor (B) development in HIV-infected children presenting early (green lines) and late (black lines) for care, and control children (red lines).
Figure 3.
Growth curves (mean score point estimates and 95% confidence intervals) for cognitive and motor development in younger (left) and older (right) HIV-infected children (black lines) and control children (red lines). Younger children are age 18 to 29 months at enrollment, older children 30 to 72 months.
We did not detect association between age group and early presentation for care among HIV infected children: 2/11 (18%, C.I. = [0%, 42%] ) of the younger children and 8/24 (33%, C.I. = [13%, 53% ] ) of the older children presented early for care.
DISCUSSION
In this prospective study, HIV-infected children accessing care at a pediatric HIV care and treatment facility in Kinshasa, capital of the Democratic Republic of Congo, demonstrated a lower mean level of both motor and cognitive development at entry into care compared to healthy control children. Analysis of the neurodevelopmental trajectories over time demonstrated that the HIV-infected children experienced accelerated motor development during the first year of access to care. This accelerated motor development was substantial (increase of one standard deviation in mean motor score) and can most likely be ascribed to the improved general condition of HIV-infected children upon initiation of prophylaxis for opportunistic infections, access to nutritional programs, and HAART for those eligible. In contrast to the findings for motor development, our results did not provide evidence of a difference in the rate of cognitive development between HIV-infected and control children. After one year of care, HIV infected children reached scores similar to those of HIV uninfected, affected children but were not able to attain scores similar to those of healthy control children, suggesting that the gain in neurodevelopment following access to care may remain restricted due to the negative living environment (including poverty and food insecurity, illness in parent) of these children.
Based on our exploratory analyses, we hypothesize that HIV-infected children who present either early in their disease process (prior to HAART eligibility) or at a younger age may experience greater gain in mean cognitive scores. We conjecture that slower gains in cognitive development in those presenting late in their HIV disease process or at an older age may be due to pervasive damage which is more resistant to improvements at time of access to care. Specifically designed studies will be needed to assess if there is an independent effect of age and degree of immunosupression on neurodevelopmental response to care and treatment, and to assess the extent to which delay in cognitive development is (1) attributable to permanent HIV-related damage to brain structures, (2) can be reversed by primary HIV care or developmental stimulation by the caregivers, and (3) is due to effects that can be reversed following initiation of HAART.
Our study illustrates the importance of the inclusion of relevant comparison groups when performing neurodevelopmental assessment. First, the presence of HIV is often accompanied with other stressors that hold back neurodevelopment, including extreme poverty and illness in the mother resulting in family stress and reduced opportunities for stimulation of early development. The inclusion of HIV uninfected children affected by HIV (symptomatic AIDS in mother or AIDS orphans) allowed us to explore these effects. Second, repeated assessments are necessary to assess trajectories patterns of neurodevelopment but can introduce a practice effect. In the primary analysis the observed gain in cognitive developmental scores among HIV infected children may have been attributed to provision of care had we not collected data in healthy control children. The practice effect (and/or the effect of provision of developmental tips to parents of children with delay) in this study may have been larger than that observed in US studies26 because of the low level of exposure to educational toys, books and multimedia in areas struggling with severe poverty and low maternal literacy.
Comparison with other studies is difficult. Reports from industrialized settings often differ in terms of the neurodevelopmental assessment tools used, the prior access to antiretroviral treatment of the participants, and high prevalence of exposure to drug and alcohol abuse in HIV-infected and exposed children.27 A large US based clinical trial observed the largest effects of HAART on neurodevelopment during the first 6 months of treatment, in children younger than 30 months of age, and in children with severe delay prior to start of ART.19 In contrast, Lindsey et al. observed only limited improvements in neurodevelopmental functioning upon HAART initiation in young children 26, and McCoig et al observed a decline in neurological abnormalities throughout the course of antiretroviral treatment.28 A longitudinal study of Ugandan infants age 6 to 24 months receiving care in the pre-HAART era.2 Similar to our findings, HIV-infected children were found to have lower cognitive and motor development at baseline, and static delay in cognitive development. In contrast to the HIV-infected children in our study, who experienced accelerated motor development over time, HIV infected children in the Ugandan pre-HAART study demonstrated deceleration in their rate of motor development. The most informative comparison is with a study performed in South Africa, in which 39 children (mean age 64 +/− 46 months) were followed up for 6 months following HAART initiation.29 Similar to findings in our study, the neurocognitive and motor deficits are frequent in HIV-infected children, but in contrast to our findings, the prevalence and extent of deficits did not change significantly in response to short-term HAART.
Findings from a study in the US support our hypothesis of the importance for the HIV infected children’s neurodevelopment of entry into care at an early age and at an early stage of HIV infection. In that study, children experiencing an early AIDS defining illness were at increased risk of encephalopathy during their preschool and early school years, whereas children with HIV infection but without a history of an AIDS defining illness performed similar to children not infected with HIV, indicating that delayed initiation of HAART is an important risk factor for the poor cognitive development.27 Early entry into care is not only important for the neurodevelopment of children, but also crucial for reduced infant mortality and disease progression, as recently demonstrated by the CHER trial. 30
Several limitations to this study should be noted. First, the sample size of our study was small precluding multivariate analysis to assess the impact of potential confounders such as baseline level of malnutrition, food insecurity, and socio-economic status.31 To avoid loss of statistical power due to missed visits, we used linear mixed-effects model analysis which allowed us to use all data instead of restricting the analysis to children who completed all three visits, and avoided the need for imputation of missing data. The relatively high death rate among the HIV infected children compared to the other two groups may have resulted in an overestimation of the accelerated developmental among HIV-infected children.
Second, participating children spanned a wide age range and presented over the entire clinical spectrum, from very advanced HIV disease (stage IV disease, CD4 of 3%) to asymptomatic (stage I) HIV infection. Children started HAART throughout the study period, which precluded an assessment of the effect of HAART, separate from the effect of access to care, and did not allow the evaluation of the effect of timing of HAART. Similarly, we were not able to differentiate the effect of care from the effect of the provision of developmental tips. Because this study was performed in one of the poorest countries of the world, we could not gain access to neuro-imaging techniques. As pediatric HAART only became available to children in the DRC at the time of the start of the study, participating children were “survivors” and thus not representative of all children living with HIV.
In conclusion, vertical transmission of HIV and its neurodevelopmental consequences remain an important public health problem in the developing world, but research is only beginning to evaluate the effects of HIV care and HAART on the neurodevelopmental trajectory of these children. Access to care leads to a catch-up in motor development, most likely as a consequence of improved general health status. Access to HIV care and treatment at a young age and at early stage of disease progression may also lead to improvements in cognitive development. Cognitive development is undeniably an important predictor of success throughout life, as children with low levels of cognitive development at preschool age have been shown to have lower school achievement and earl lower wages as adults.32 As access to treatment improves and HIV-infected children live longer, it will thus be important to raise awareness of neurodevelopmental difficulties in these children, to promote early entry into care, and to design therapeutic interventions to improve or preserve the neurodevelopment of these children. Ideally, all children infected with HIV should have access to HAART and neurodevelopmental interventions early in life, but this may be difficult to achieve as the early diagnosis of HIV remains complex, and the care for these children faces the additional barriers of stigma and secrecy 33.
ACKNOWLEDGEMENTS
We thank Paul De Cock and Jenifer Jelsma for the training of the study team in neurodevelopmental assessment. We also thank other study team members (Nadine Nossa, Iam Zephyrin, Nene Kilese, and Kashamuka Mwandagalirwa) for their efforts, and the patients and their families for their participation in the study. This research was supported by a grant from the National Institutes of Health, Fogarty International Center and National Institute of Mental Health (grant R21 MH071214)
Sources of support: This research was supported by a grant from the National Institutes of Health, Fogarty International Center and National Institute of Mental Health (grant R21 MH071214)
References
- 1.Tardieu M, Le Chenadec J, Persoz A, Meyer L, Blanche S, Mayaux MJ. HIV-1-related encephalopathy in infants compared with children and adults. French Pediatric HIV Infection Study and the SEROCO Group. Neurology. 2000 Mar 14;54(5):1089–1095. doi: 10.1212/wnl.54.5.1089. [DOI] [PubMed] [Google Scholar]
- 2.Drotar D, Olness K, Wiznitzer M, et al. Neurodevelopmental outcomes of Ugandan infants with HIV infection: an application of growth curve analysis. Health Psychol. 1999 Mar;18(2):114–121. doi: 10.1037//0278-6133.18.2.114. [DOI] [PubMed] [Google Scholar]
- 3.Gabuzda DH, Hirsch MS. Neurologic manifestations of infection with human immunodeficiency virus. Clinical features and pathogenesis. Ann Intern Med. 1987 Sep;107(3):383–391. doi: 10.7326/0003-4819-107-2-383. [DOI] [PubMed] [Google Scholar]
- 4.Msellati P, Lepage P, Hitimana DG, Van Goethem C, Van de Perre P, Dabis F. Neurodevelopmental testing of children born to human immunodeficiency virus type 1 seropositive and seronegative mothers: a prospective cohort study in Kigali, Rwanda. Pediatrics. 1993 Dec;92(6):843–848. [PubMed] [Google Scholar]
- 5.Koekkoek S, de Sonneville LM, Wolfs TF, Licht R, Geelen SP. Neurocognitive function profile in HIV-infected school-age children. Eur J Paediatr Neurol. 2007 Oct 17; doi: 10.1016/j.ejpn.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Martin SC, Wolters PL, Toledo-Tamula MA, Zeichner SL, Hazra R, Civitello L. Cognitive functioning in school-aged children with vertically acquired HIV infection being treated with highly active antiretroviral therapy (HAART) Dev Neuropsychol. 2006;30(2):633–657. doi: 10.1207/s15326942dn3002_1. [DOI] [PubMed] [Google Scholar]
- 7.Wolters PL, Brouwers P, Civitello L, Moss HA. Receptive and expressive language function of children with symptomatic HIV infection and relationship with disease parameters: a longitudinal 24-month follow-up study. Aids. 1997 Jul 15;11(9):1135–1144. doi: 10.1097/00002030-199709000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Coplan J, Contello KA, Cunningham CK, et al. Early language development in children exposed to or infected with human immunodeficiency virus. Pediatrics. 1998 Jul;102(1):e8. doi: 10.1542/peds.102.1.e8. [DOI] [PubMed] [Google Scholar]
- 9.Tardieu M, Mayaux MJ, Seibel N, et al. Cognitive assessment of school-age children infected with maternally transmitted human immunodeficiency virus type 1. J Pediatr. 1995 Mar;126(3):375–379. doi: 10.1016/s0022-3476(95)70451-5. [DOI] [PubMed] [Google Scholar]
- 10.Blanchette N, Smith ML, King S, Fernandes-Penney A, Read S. Cognitive development in school-age children with vertically transmitted HIV infection. Dev Neuropsychol. 2002;21(3):223–241. doi: 10.1207/S15326942DN2103_1. [DOI] [PubMed] [Google Scholar]
- 11.Smith R, Malee K, Charurat M, et al. Timing of perinatal human immunodeficiency virus type 1 infection and rate of neurodevelopment. The Women and Infant Transmission Study Group. Pediatr Infect Dis J. 2000 Sep;19(9):862–871. doi: 10.1097/00006454-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 12.McGrath N, Fawzi WW, Bellinger D, et al. The timing of mother-to-child transmission of human immunodeficiency virus infection and the neurodevelopment of children in Tanzania. Pediatr Infect Dis J. 2006 Jan;25(1):47–52. doi: 10.1097/01.inf.0000195638.80578.e0. [DOI] [PubMed] [Google Scholar]
- 13.Bagenda D, Nassali A, Kalyesubula I, et al. Health, neurologic, and cognitive status of HIV-infected, long-surviving, and antiretroviral-naive Ugandan children. Pediatrics. 2006 Mar;117(3):729–740. doi: 10.1542/peds.2004-2699. [DOI] [PubMed] [Google Scholar]
- 14.Coscia JM, Christensen BK, Henry RR, Wallston K, Radcliffe J, Rutstein R. Effects of home environment, socioeconomic status, and health status on cognitive functioning in children with HIV-1 infection. J Pediatr Psychol. 2001 Sep;26(6):321–329. doi: 10.1093/jpepsy/26.6.321. [DOI] [PubMed] [Google Scholar]
- 15.Belman AL, Diamond G, Dickson D, et al. Pediatric acquired immunodeficiency syndrome. Neurologic syndromes. Am J Dis Child. 1988 Jan;142(1):29–35. doi: 10.1001/archpedi.1988.02150010039017. [DOI] [PubMed] [Google Scholar]
- 16.Epstein LG, Sharer LR, Oleske JM, et al. Neurologic manifestations of human immunodeficiency virus infection in children. Pediatrics. 1986 Oct;78(4):678–687. [PubMed] [Google Scholar]
- 17.Pizzo PA, Eddy J, Falloon J, et al. Effect of continuous intravenous infusion of zidovudine (AZT) in children with symptomatic HIV infection. N Engl J Med. 1988 Oct 6;319(14):889–896. doi: 10.1056/NEJM198810063191401. [DOI] [PubMed] [Google Scholar]
- 18.Brouwers P, Moss H, Wolters P, et al. Effect of continuous-infusion zidovudine therapy on neuropsychologic functioning in children with symptomatic human immunodeficiency virus infection. J Pediatr. 1990 Dec;117(6):980–985. doi: 10.1016/s0022-3476(05)80150-7. [DOI] [PubMed] [Google Scholar]
- 19.Raskino C, Pearson DA, Baker CJ, et al. Neurologic, neurocognitive, and brain growth outcomes in human immunodeficiency virus-infected children receiving different nucleoside antiretroviral regimens. Pediatric AIDS Clinical Trials Group 152 Study Team. Pediatrics. 1999 Sep;104(3):e32. doi: 10.1542/peds.104.3.e32. [DOI] [PubMed] [Google Scholar]
- 20.Shanbhag MC, Rutstein RM, Zaoutis T, Zhao H, Chao D, Radcliffe J. Neurocognitive functioning in pediatric human immunodeficiency virus infection: effects of combined therapy. Arch Pediatr Adolesc Med. 2005 Jul;159(7):651–656. doi: 10.1001/archpedi.159.7.651. [DOI] [PubMed] [Google Scholar]
- 21.Chiriboga CA, Fleishman S, Champion S, Gaye-Robinson L, Abrams EJ. Incidence and prevalence of HIV encephalopathy in children with HIV infection receiving highly active anti-retroviral therapy (HAART) J Pediatr. 2005 Mar;146(3):402–407. doi: 10.1016/j.jpeds.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 22.WHO Scaling up Antiretroviral Therapy in Resource Limited Settings: treatment guidelines for a public health approach. 2004. [PubMed]
- 23.Muller KE, Stewart PW. Linear Model Theory: Univariate, Multivariate, and Mixed Models. Wiley Interscience; N.Y: 2006. [Google Scholar]
- 24.Little RJ, Rubin DB. Statistical Analysis with Missing Data. 2nd ed Wiley Interscience; N.Y: 2002. [Google Scholar]
- 25.Van Rie A, Mupuala A, Dow A. Impact of the HIV/AIDS epidemic on the neurodevelopment of preschool-aged children in Kinshasa, Democratic Republic of the Congo. Pediatrics. 2008 Jul;122(1):e123–128. doi: 10.1542/peds.2007-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindsey JC, Malee KM, Brouwers P, Hughes MD. Neurodevelopmental functioning in HIV-infected infants and young children before and after the introduction of protease inhibitor-based highly active antiretroviral therapy. Pediatrics. 2007 Mar;119(3):e681–693. doi: 10.1542/peds.2006-1145. [DOI] [PubMed] [Google Scholar]
- 27.Smith R, Malee K, Leighty R, et al. Effects of perinatal HIV infection and associated risk factors on cognitive development among young children. Pediatrics. 2006 Mar;117(3):851–862. doi: 10.1542/peds.2005-0804. [DOI] [PubMed] [Google Scholar]
- 28.McCoig C, Castrejon MM, Castano E, et al. Effect of combination antiretroviral therapy on cerebrospinal fluid HIV RNA, HIV resistance, and clinical manifestations of encephalopathy. J Pediatr. 2002 Jul;141(1):36–44. doi: 10.1067/mpd.2002.125007. [DOI] [PubMed] [Google Scholar]
- 29.Smith L, Adnams C, Eley B. Neurological and neurocognitive function of HIV-infected children commenced on antiretroviral therapy. South African Journal of Child Health. 2008;2(3) [Google Scholar]
- 30.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008 Nov 20;359(21):2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker SP, Wachs T, Gardner J. Meeks, Lozoff B, Wasserman G, Pollit E, Carter J. Child development: risk factors for adverse outcomes in developing countries. Lancet. 2007;369(9556):145–157. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- 32.Curie J, Thomas D. Early test scores, socioeconomic status and future outcomes. Cambridge, MA: 1999. NBER working paper No. 6943. [Google Scholar]
- 33.Willen EJ. Neurocognitive outcomes in pediatric HIV. Ment Retard Dev Disabil Res Rev. 2006;12(3):223–228. doi: 10.1002/mrdd.20112. [DOI] [PubMed] [Google Scholar]