Abstract
The stromal microenvironment regulates mammary gland branching morphogenesis. We have observed that mast cells are present in the mammary gland throughout its postnatal development and in particular, are found around the terminal end buds and ductal epithelium of the pubertal gland. Mast cells contribute to allergy, inflammatory diseases, and cancer development, but have not been implicated in normal development. Genetic and pharmacological disruption of mast cell function in the mammary gland revealed that mast cells are involved in rapid proliferation and normal duct branching during puberty, and this effect is independent of macrophage recruitment, which also regulates mammary gland development. For mast cells to exert their effects on normal morphogenesis required activation of their serine proteases and degranulation. Our observations reveal a novel role for mast cells during normal pubertal development in the mammary gland.
Keywords: Mammary gland, mast cell, branching morphogenesis, development, terminal end bud
Introduction
The developing mouse mammary gland is a good model for investigating the mechanisms that regulate ductal epithelial growth and invasion, in this case, into the mammary fat pad (Cunha et al., 2000; Richert et al., 2000; Wiseman and Werb, 2002). At birth, the mammary gland consists of a fat pad with a rudimentary ductal epithelial tree descending into it from the overlying nipple. These structures parallel the growth of the mouse until the start of puberty (around 3 weeks of age), when ovarian hormones stimulate the development of multilayered bulbous structures called terminal end buds (TEBs) at the tips of growing and branching ducts (Richert et al., 2000; Wiseman and Werb, 2002). Proliferation of TEB cells leads to the bifurcation and elongation of duct structures towards the outer edges of the fat pad by 10-12 weeks of age, when the TEBs regress and become terminal duct ends (Richert et al., 2000; Topper and Freeman, 1980). After the cycle of pregnancy, lactation, and involution, the mammary gland returns to a state similar to that prior to pregnancy.
Although hormones are responsible for the initiation and maintenance of TEB formation and duct elongation, a major influence in mammary epithelial development is the mammary stroma. Fibroblasts and adipocytes, blood vessels and bone marrow-derived resident macrophages and eosinophils and their products, growth and survival factors, extracellular matrix proteins and their degrading proteases all contribute to mammary ductal development (Cunha and Hom, 1996; Cunha et al., 2000; Gouon-Evans et al., 2000; Wiseman and Werb, 2002). Proper epithelial growth requires coordination of these diverse stromal components; therefore, the mammary fat pad is a dynamic structure whose resident cells participate actively in the process of mammary gland branching morphogenesis.
Mast cells are found in vascularized tissues, although they tend to reside around blood vessels, nerves, epithelial cells, and smooth muscle cells (Galli and Tsai, 2008; Kitamura, 1989; Metcalfe et al., 1997). Mast cells are derived from hematopoietic progenitors in bone marrow and circulate through the blood to tissues where they mature into cells with a specific granule phenotype; the content of their granules and/or phenotype differs depending on factors in their destination's environment (Galli, 2000; Galli et al., 2005; Kitamura, 1989; Metcalfe et al., 1997).
Long known as potent effectors of the innate immune response, mast cells ordinarily house and release factors capable of influencing their extracellular environment (Metcalfe et al., 1997). Best known for their role in releasing histamine, causing vasodilation and blood vessel leakiness, mast cells also produce a host of serine proteases, pro-inflammatory cytokines, pro-angiogenic factors such as vascular endothelial growth factor (VEGF) and chemokines that contribute to the metastatic potential of tumors [reviewed in (Coussens and Werb, 2002; Metcalfe et al., 1997)]. Mast cells may secrete different molecules selectively or in large amounts through the exocytic process of degranulation (Galli and Tsai, 2008).
In previous work we showed that connective tissue-type mast cells are present in the mammary gland and are functionally involved in mammary gland post-lactational involution through their binding of plasma kallikrein (Lilla et al., 2009). These studies raise the possibility that the presence and activity of mast cells may have numerous consequences on the normal development and maintenance of mammary gland. In this study, we show that mast cells must play a significant role in normal mammary gland development during puberty.
Materials and Methods
Experimental animal models
Care of animals and animal experiments were performed in accordance with protocols approved by the UCSF Institutional Animal Use and Care Committee (IACUC). DPPI-/- mice (Pham and Ley, 1999) were provided by L. Coussens (UCSF). Wild-type FVB mice were obtained from Charles River Laboratories. C57BL/6J-KitW-sh/W-sh (W-sash) mice (Duttlinger et al., 1993) obtained from the Jackson Laboratory and C57BL/6 mice obtained from Charles River Laboratories (Wilmington, MA) were crossed to generate Kit+/W-sh offspring, which were subsequently crossed to produce KitW-sh//W-sh, Kit+/W-sh, and Kit+/+ siblings.
To stabilize mast cells from degranulating, CD1 female mice (Charles River Laboratories) were injected intraperitoneally daily from 3 to 5 weeks of age with 50 mg/kg body weight sodium cromoglycate (Sigma, St. Louis, MO, USA) dissolved in saline as previously described (Jamieson et al., 2005).
Estrus stage was determined for every mouse by vaginal smear (Rugh, 1968) and only mice in similar estrus phases were compared at each time point.
Whole mount mammary gland preparation and morphometric analysis
For whole mount analysis, abdominal mammary glands were removed at 3, 5, 8, and 12 weeks of age, spread on a glass slide, and fixed overnight in 75% ethanol, 25% glacial acetic acid. They were then rehydrated and stained overnight at 4°C in Alum Carmine stain [2 g/l carmine dye (Sigma, St. Louis, MO, USA) and 5 g/l aluminum potassium sulfate in distilled water]. The glands were then dehydrated and immersed in Histoclear (Fisher Scientific) for lipid removal and storage. Images were acquired using Nikon ACT-1 software on a Leica dissecting microscope and analyzed using Adobe Photoshop and NIH Image (ImageJ) software. Duct lengths (in mm) were measured from the nipple base to the tips of the 3 longest ducts and averaged. The mammary gland invasion front was defined by epithelial structures that had extended past a bisecting line through the lymph node perpendicular to the long axis of the abdominal mammary fat pad. Numbers of terminal end buds and total numbers of primary and secondary duct ends in the invasion front were counted. Two-tailed Student's t-tests and one-way ANOVAs were used to determine statistical significance between groups (n = 5 to 8 mice/group). Statistical significance was considered when P≤0.05.
Histology, enzyme histochemistry and immunofluorescence
Contralateral abdominal mammary glands were removed at 3, 5, 8, and 12 weeks of age and immediately embedded in OCT (Sakura) medium on dry ice or were fixed in 10% neutral buffered formalin and processed for paraffin embedding. 5 μm frozen or paraffin sections were cut for use in histology, enzyme histochemistry, and immunohistochemistry. To visualize mast cells, frozen sections were air-dried for ≥30 minutes, post-fixed in ice-cold acetone for 10 minutes, rinsed in PBS, and then stained using an enzymatic reaction with naphthol AS-D chloroacetate esterase (Sigma) to detect chymase activity (Moloney et al., 1960), then counterstained with Gill's hematoxylin or 4,6-diamidino-2-phenylindole (DAPI, Vector Laboratories, Burlingame, CA). Alternatively, paraffin sections were rehydrated and stained with Toluidine blue [a metachromatic dye for mast cells, made up as 0.1% Toluidine blue O (Sigma) in 1% sodium chloride, pH 2.3]. To examine collagen deposition and overall morphology of the glands, frozen sections were dried and fixed with 10% neutral buffered formalin, then stained with 0.1% sirius red in saturated picric acid (picro-sirius red) (Junqueira et al., 1979) and observed under polarized light. Lipid content was determined by Oil Red O staining. Frozen sections were fixed for 1-2 days in 4% parafomaldehyde at 4°C, rinsed in distilled water, air-dried for ≥90 minutes, then stained in working Oil Red [3 parts saturated Oil Red O (Sigma, 0.5% in isopropanol); 2 parts dextrin solution (1% type III corn dextrin (Sigma) in distilled water)] for 20 minutes and counterstained with Gill's hematoxylin (Sigma) and stored in PBS.
To identify macrophages and eosinophils in mammary glands, frozen sections were stained for a macrophage cell surface glycoprotein (Malorny et al., 1986) with rat anti-mouse F4/80 mAb (Caltag Laboratories, Invitrogen, Carlsbad, CA), which also is found on eosinophils (McGarry and Stewart, 1991). Slides were blocked in 4% goat serum and 2% BSA (Sigma) in PBS, then incubated overnight with 1:200 rat anti-mouse F4/80 at 4°C followed by an Alexa 488-conjugated goat anti-rat IgG (Molecular Probes, Invitrogen) with a DAPI counterstain (Vector Laboratories, Burlingame, CA). For controls, tissue sections were treated with an isotype-matched primary antibody or by omission of the primary antibody. To identify mitotic cells, frozen sections were blocked in 4% goat serum and 2% BSA (Sigma) in PBS, then incubated overnight with a 1:500 dilution of polyclonal anti-human phospho-histone H3 antibody (Ser10; Upstate, Millipore, Billerica, MA) at 4°C. Phospho-histone H3+ cells were detected using an Alexa 594-conjugated goat anti-rabbit IgG (Molecular Probes, Invitrogen) with a DAPI counterstain (Vector Laboratories, Burlingame, CA). Controls were performed as described above. Immunofluorescence images were acquired at 200× using a Leica DMR microscope and Leica FireCam with accompanying software. Quantification of percentages of mitotic cells in mammary ducts and TEBs was performed by excising duct or TEB structures from images using Adobe Photoshop 7, counting the number of DAPI+ pixels (blue), counting the number of phospho-histone H3+ pixels (red), and expressing the ratio as a percentage of phospho-histone H3+ nuclei per structure. A minimum of 10 images of ducts and TEBs apiece were analyzed per W-sash phenotype at both 5 and 8 weeks of age. One-way ANOVAs were used to determine statistical significance, which was considered when P≤0.05.
Results
Mast cells are present in the stromal microenvironment of the mouse mammary gland
Mast cells are present in pregnant, lactating, and involuting mammary glands (Lilla et al., 2009; Russell et al., 2007; Szewczyk et al., 2000). We first analyzed the localization of mast cells during mammary ductal morphogenesis. We identified a population of histamine- and chymase-positive mast cells present in the developing mammary gland. At 1 through 3 weeks of age, the prepubertal mammary gland consists of a rudimentary epithelial tree near the nipple at one end of a predominantly fatty stroma, consisting of unilocular adipocytes, fibroblasts, blood vessels, nerves and a lymph node, all surrounded by loose areolar connective tissue. In the prepubertal gland mast cells were scattered throughout the stromal tissue, although in relatively small numbers, and did not appear to localize to any particular structures (data not shown). By 5 weeks of age, when the mammary gland begins to proliferate and grow in response to ovarian hormones, the advancing ductal front is characterized by the presence of TEBs. At 5 weeks (Fig. 1A,B) and 8 weeks (data not shown), mast cells were frequently observed in the stroma immediately surrounding TEBs, adjacent to the periductal stroma or just ahead of the advancing TEB. Mast cells were still scattered throughout the mammary stroma, but more were found around the more mature ducts (Fig. 1C,D) and blood vessels (Fig. 1E). At all ages, mast cells also were observed at the periphery of the mammary lymph node (Fig. 1F). The presence of mast cells throughout mammary gland development, and their increased number and proximity to the growing ducts, led us to hypothesize that mast cells play a role in normal mammary epithelial development.
Figure 1.

Mast cells are present in the mammary stroma during postnatal development. A-F: Formalin fixed, paraffin embedded 5 μm sections of 5-week-old #4 mammary glands stained with Toluidine blue, a metachromatic stain for mast cell granules. Scale bar = 50 μm. Red arrowheads indicate mast cells; black arrowheads specify mast cells shown in insets. Insets are magnified from original image 3.2 times. A) and B) Terminal end buds (TEB) with proximal mast cells. C) and D) Both stable (C) and degranulating (D) mast cells surrounded more mature sections of the developing mammary ducts. E) Mast cells were frequently observed adjacent to blood vessels (BV) in the mammary stroma. F) Varying numbers of mast cells were also located at the periphery of the resident lymph node (LN).
Mast cells are required for normal pubertal mammary gland development
To test the hypothesis that mast cells are involved in pubertal mammary gland development, we took a genetic approach using the W-sash mast cell-deficient mouse line (KitW-sh). This hypomorphic mutation is the result of a spontaneous inversion mutation in the regulatory region of the c-kit gene (Nagle et al., 1995), thus causing a profound deficiency of mast cells in all tissues and significant deficits in melanocytes without affecting other c-kit-dependent cell types (Duttlinger et al., 1993; Grimbaldeston et al., 2005). In most tissues, heterozygous Kit+/W-sh mice also have significantly fewer mast cells than wild-type littermates (Grimbaldeston et al., 2005), providing a useful tool for examining the potential dosage effect of mast cells under physiological conditions. By histology and enzyme histochemistry, we verified that mast cells were completely absent from the mammary glands of KitW-sh/W-sh mice, but were present in both Kit+/W-sh and wild-type littermates throughout development (Fig. 2A and data not shown). The localization of mast cells in Kit+/W-sh and wild-type mice did not differ.
Figure 2.
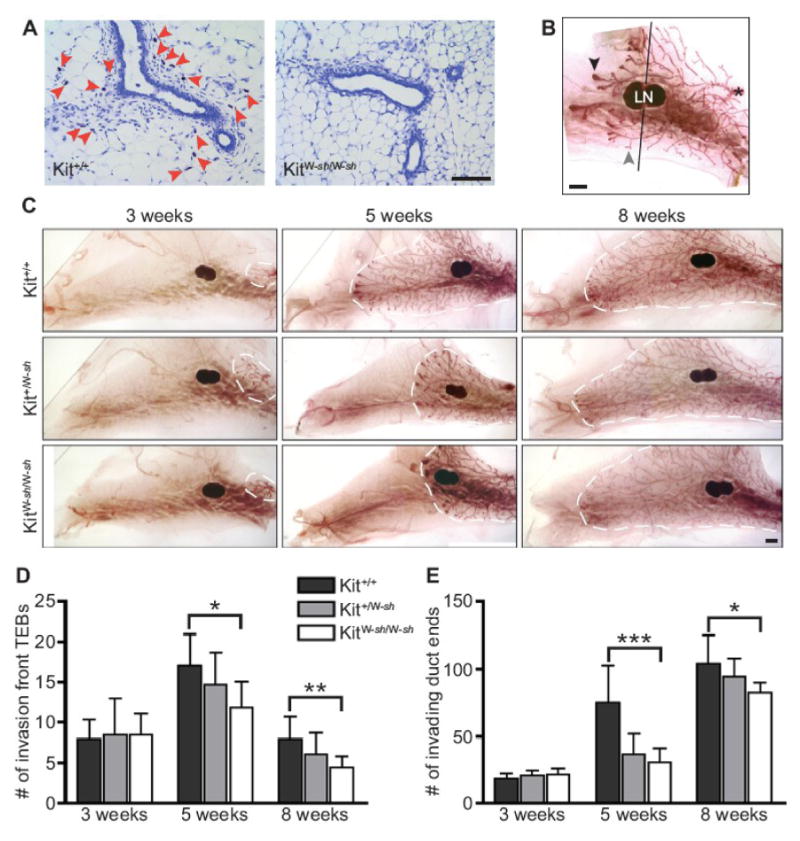
Mice lacking mast cells have defective mammary gland branching. A) Mast cells are present in wild-type mammary gland but absent in W-sash mutant mice. Frozen 5 μm sections of abdominal mammary glands from 5-week-old littermates stained with Toluidine blue. Red arrowheads indicate mast cells. Scale bar = 100 μm. B) Analysis of carmine-stained mammary gland whole mounts required counting of TEBs (black arrowhead) and duct ends (gray arrowhead) past the mid-lymph node (denoted by line, LN=lymph node). The nipple is indicated by an asterisk. Scale bar = 1 mm. C) Comparison of carmine-stained whole mounts of left abdominal mammary glands from W-sash wild-type, heterozygote, and mutant mice at 3, 5 and 8 weeks of age. Scale bar = 1 mm. The edge of mammary gland growth is outlined by a white dotted line. D) Counts of TEBs past the mid-lymph node from whole mount analysis reveal that mast cell-deficient mice have fewer TEBs in the pubertal mammary gland (n=8 mice/genotype/group; ANOVA values: *5 weeks, P=0.038; **8 weeks, P=0.024). E) Counts of duct ends (primary and secondary branching) past the mid-lymph node from whole mount analysis reveal that mast cell-deficient mice have fewer duct ends in the pubertal mammary gland (n=8 mice/genotype/group; ANOVA values: ***5 weeks, P=0.0003; *8 weeks, P=0.0325). All error bars represent standard deviation.
We analyzed mammary gland morphogenesis in KitW-sh/W-sh, Kit+/W-sh and Kit+/+ mice at 3, 5 and 8 weeks of age by comparing whole-mounts of abdominal mammary glands for ductal outgrowth (Fig. 2B-C). At 3 weeks, just before the onset of pubertal development, the total number of primary and secondary duct ends and TEBs did not differ by genotype. However, by 5 weeks of age, mast-cell deficient KitW-sh/W-sh mice had significantly fewer duct ends in the invasion front as well as fewer TEBs than wild-type controls, while Kit+/W-sh had an intermediate phenotype (Fig. 2D-E). The fewer number of TEBs directly paralleled the reduced number of ducts. This effect on duct end and TEB number persisted until 8 weeks of age, when the advancing ducts terminated near the boundaries of the mammary fat pad (Fig. 2D-E). Additionally, at both 5 and 8 weeks of age, duct lengths were reduced in KitW-sh/W-sh mice when compared to wild-type, and Kit+/W-sh had intermediate mean lengths, though these observations did not reach statistical significance (data not shown). Observations of littermates at 12 weeks of age (n=2/genotype) indicate that the developmental defects persisted in adult virgin W-sash mutants and heterozygotes, with reduced duct lengths and numbers of duct ends. However, as KitW-sh/W-sh and Kit+/W-sh are capable of normal lactation and supporting average-sized litters, these deficits did not significantly impair lactational mammary gland function (data not shown).
These mast cell-dependent differences were not due to an overall reduction in body growth or relative mammary fat pad sizes related to mast cell deficiency. When we monitored mouse body weights as well as the ratio of mammary gland weights to body weight, we found no significant differences between the different W-sash genotypes at any of the ages analyzed (data not shown). Therefore, these data indicate that the impairment in TEB formation and fewer ducts observed in W-sash mutant and heterozygous as compared to wild-type mice are due to mast cell deficiency and reduced mast cell numbers, respectively, and point to a role for mast cells in normal postnatal mammary gland development.
Mast cells in the mammary gland do not regulate macrophage recruitment or collagen deposition around TEBs
Mast cells have been identified as key recruiters of leukocytes in vivo (Gaboury et al., 1995; Kubes and Kanwar, 1994; Metz et al., 2007; Walsh et al., 1991). Thus, a possible mechanism for the function of mast cells in mammary development might be to effect macrophage recruitment (Chen et al., 2001). Moreover, macrophages regulate mammary gland development, as macrophage-deficient mammary glands have delayed and reduced duct outgrowth (Gouon-Evans et al., 2002; Gouon-Evans et al., 2000; Ingman et al., 2006). Macrophages around TEBs during mammary development also play a role in organizing the collagen-rich matrix surrounding the epithelial structures (Ingman et al., 2006). We found that mast cell deficiency in KitW-sh mutant mammary glands at 5 and 8 weeks of age did not alter the presence of F4/80+ macrophage and eosinophils around TEBs (Fig. 3A), as staining was similar in distribution and intensity in mammary glands of heterozygote and Kit wild-type genotypes. These results suggest that the W-sash mutant mammary developmental defect was not due to a secondary loss of macrophages and/or eosinophils subsequent to mast cell deficiency. We then used picro-sirius red staining to observe birefringent fibrillar collagen under polarized light (Junqueira et al., 1979). At both 5 and 8 weeks of age, we noticed no difference in collagen deposition by picro-sirius red staining (Fig. 3B), or overall morphology of the TEBs and surrounding stroma. These results suggest that the mast cell-dependent mammary development phenotype is independent of macrophage-mediated effects.
Figure 3.
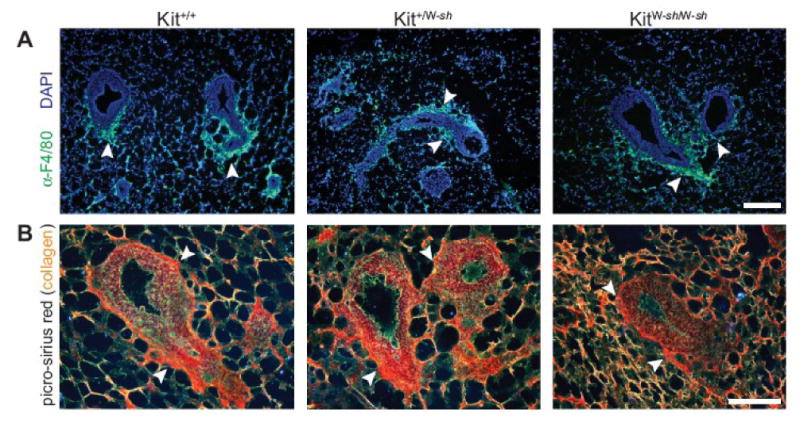
Mast cell-deficient mammary glands have normal macrophage recruitment and collagen deposition. A) Immunofluorescence on KitW-sh mutant, heterozygote, and Kit wild-type mammary glands at 5 weeks of age using anti-F4/80 antibody with Alexa 488-conjugated secondary antibody (green), used to localize macrophages and eosinophils, DAPI counterstain (blue), scale bar = 100 μm. Arrowheads indicate areas of F4/80+ cells. B) Picro-sirius red staining of mammary glands of mast cell-deficient or mast cell-reduced mice at 5 weeks of age to demonstrate by birefringence under polarized light the difference between thick (orange-yellow) and thin (green) collagen fibrils around TEBs. Scale bar = 100 μm. Arrowheads indicate areas of collagen staining around the necks of TEBs.
Mast cells are required for normal levels of proliferation in ducts and TEBs in developing mammary glands
Mast cells are a rich source of growth factors. As mast cell deficiency results in fewer duct ends and TEBs, we next determined whether the mechanism underlying this effect was due to an altered proliferative capacity of epithelial cells in the developing mammary gland. We performed immunofluorescence with an antibody against the phosphorylated serine 10 residue of histone H3, a specific marker for cells entering mitosis (Hendzel et al., 1997), to indicate proliferation in KitW-sh/W-sh, Kit+/W-sh and Kit+/+ mice. We found fewer mitotic cells in both ducts and TEBs of KitW-sh/W-sh mice than in wild-type in 5- and 8-week-old mammary glands (Fig. 4A-C). Kit+/W-sh mice had an intermediate percentage of mitotic duct and TEB cells, consistent with their observed deficiency in TEB and duct ends intermediate to that of the mast cell-deficient mice and wild-type controls. This decrease in proliferation was not accompanied by any alteration in cell death, nor in the relative numbers of luminal and myoepithelial cells (data not shown). Thus, these data indicate that mast cells regulate TEB and duct cell proliferation during normal pubertal mammary gland development.
Figure 4.
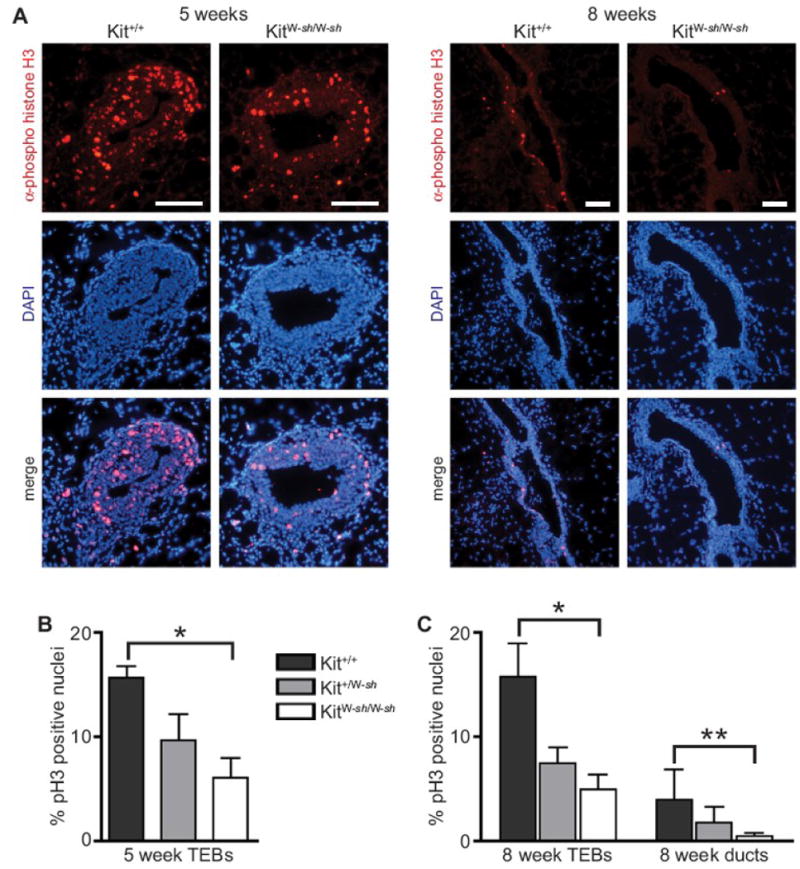
Epithelial cell proliferation is reduced in TEBs and ducts in mast cell-deficient mice. A) Immunofluorescence was performed on frozen sections of KitW-sh mutant, heterozygote, and Kit wild-type mammary glands at 5 and 8 weeks of age using an antibody against phospho-histone H3, a mitotic marker, and counterstained with DAPI. Shown are a representative 5 week old TEB and 8 week old duct from Kit wild-type and KitW-sh/W-sh. Scale bar for each = 100 μm. Phospho-histone H3+ nuclei were counted and expressed as a percentage of mitotic cells per TEB or duct, respectively (n=8 mice/group). B) Quantification of mitotic epithelial cells in mast cell-deficient mouse TEBs at 5 weeks (*ANOVA: P<0.0001). C) Quantification of mitotic epithelial cells in mast cell-deficient mouse TEBs and ducts at 8 weeks (ANOVA values: *5 weeks, P=0.0025; **8 weeks, P=0.0013). All error bars represent standard deviation.
Mast cell degranulation is necessary for normal mammary gland development
Mast cell function generally is mediated by factors secreted from mast cell granules. Conventionally activated mast cells either degranulate and release a host of factors or they secrete specific factors more selectively (Galli and Tsai, 2008; Metcalfe et al., 1997). Therefore, we asked whether the requirement for mast cells in mammary gland development involves their degranulation to exert their effects on ductal growth. We used sodium cromoglycate (cromolyn sodium), which is thought to stabilize mast cells and prevent degranulation by blocking the calcium ion influx required to trigger granule release from the cell (McIntyre et al., 1981).
We treated female CD1 outbred mice with cromolyn sodium during the first two weeks of pubertal development, from 3 to 5 weeks of age, when TEBs first are formed and the mammary ductal tree elongates from its prepubertal rudiment and begins to fill the mammary fat pad. Cromolyn sodium treatment significantly inhibited TEB formation and mammary duct end number (Fig. 5A-C). These data indicate that degranulation is required for mast cells to exert their effect on mammary duct growth. This suggests that during mammary gland development, mast cells release granules that contain some TEB growth-promoting mediator(s).
Figure 5.
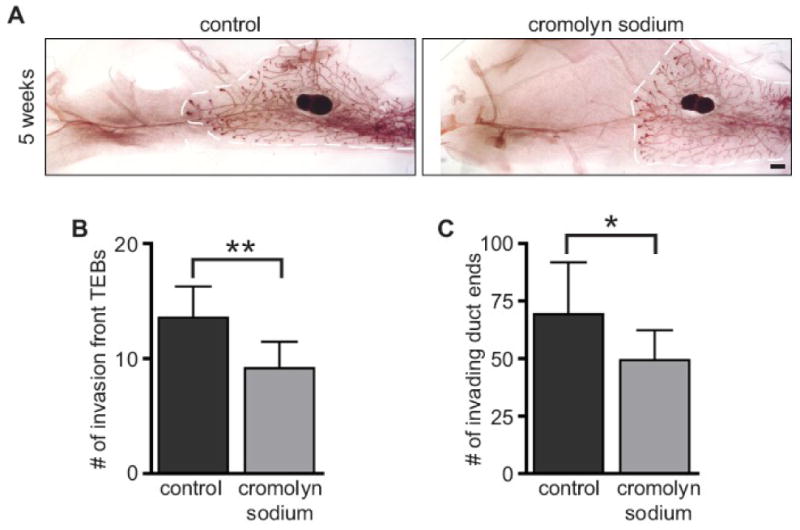
Inhibition of mast cell degranulation blocked normal mammary gland branching. Mice were treated with cromolyn sodium, a mast cell degranulation inhibitor, or control from weeks 3 to 5 of age (n=7 mice/group). A) Carmine-stained whole mounts of saline- and cromolyn sodium-treated mammary glands at 5 weeks. Scale bar = 1 mm. The edge of mammary gland growth is outlined in white. B) Cromolyn sodium-treated mammary glands have fewer TEBs after two weeks of treatment (Student's t-test: **P=0.0047). C) Cromolyn sodium-treated mammary glands have fewer invading duct ends after two weeks of treatment (Student's t-test: *P=0.049). All error bars represent standard deviation.
Serine protease activation is necessary for normal mammary gland development
Proteases play an important role in mammary gland morphogenesis (Wiseman and Werb, 2002). Leukocytes are rich in precursors of serine proteases, many of which are activated by dipeptidyl peptidase I (DPPI), also known as cathepsin C. Mast cells have a number of serine proteases concentrated in their granules. DPPI is the in vivo activator of most mast cell granule serine proteases including chymases, and also acts as a regulator of the amount of active tryptase (Wolters et al., 2001). These mast cell proteases mediate many of the functions ascribed to mast cells in disease processes (Reed and Kita, 2004; Reid et al., 2007). To identify whether such serine proteases influence mammary gland development, we used a genetic model that allowed us to determine whether the activity of mast cell serine proteases is involved. Mice deficient for DPPI are healthy and fertile, yet have many inactivated proteases in granulocytic leukocytes (Pham and Ley, 1999). We observed significantly inhibited TEB and duct end formation in mammary glands of mice that lacked DPPI compared to controls at both 5 and 8 weeks of age (Fig. 6A-C). These data suggest a need for DPPI-dependent activation of mast cell granule proteases in mammary development and indicate that these enzymes play a role in mammary development. However, as DPPI also activates serine proteases in other classes of leukocytes, the function of DPPI in mammary gland development may not only involve mast cells.
Figure 6.

Mammary gland development is defective in mice lacking normal mast cell protease activation. Mammary glands of DPPI-/- mice and wild-type controls at 5 and 8 weeks of age were examined in whole mount preparations (n=6 mice/group). A) Carmine-stained whole mounts of wild-type and DPPI-/- mammary glands at 5 and 8 weeks. Scale bar = 1 mm. The edge of mammary gland growth is outlined in white. B) Mammary glands of DPPI-/- mice have fewer TEBs than wild-type controls at both 5 and 8 weeks of age (Student's t-test: *5 weeks, P<0.0001; **8 weeks, P=0.0013). C) Mammary glands of DPPI-/- mice have fewer duct ends than wild-type controls as both 5 and 8 weeks of age (Student's t-test: *5 weeks, P=0.0015; **8 weeks, P=0.0006). All error bars represent standard deviation.
Discussion
The stromal microenvironment is an essential partner in the development of the mammary epithelial network during pubertal development. In this study we demonstrate a novel role for tissue mast cells in the normal developmental process of branching morphogenesis in the pubertal mammary gland. W-sash mice, which have alterations in the c-kit promoter leading to profound loss of mast cells in all tissues, provided us with a genetic model for mast cell ablation in the mammary gland and revealed that mast cells are necessary for proper TEB and duct formation. The numbers of mast cells necessary for mammary development were critical, as W-sash heterozygote glands, which have fewer mast cells than wild-type, showed an impairment in development intermediate to that of the mast cell-deficient and wild-type glands. Furthermore, we found that mammary gland mast cells must degranulate normally and have a normal complement of active granule proteases. Previous work has established that macrophages and eosinophils are necessary for pubertal development of the mammary gland ductal epithelium. Our study indicates that, along with macrophages and eosinophils (Gouon-Evans et al., 2002; Gouon-Evans et al., 2000), mast cells should be included in the host of stromal cells that are necessary for pubertal development of the mammary gland ductal epithelium (Fig. 7).
Figure 7.
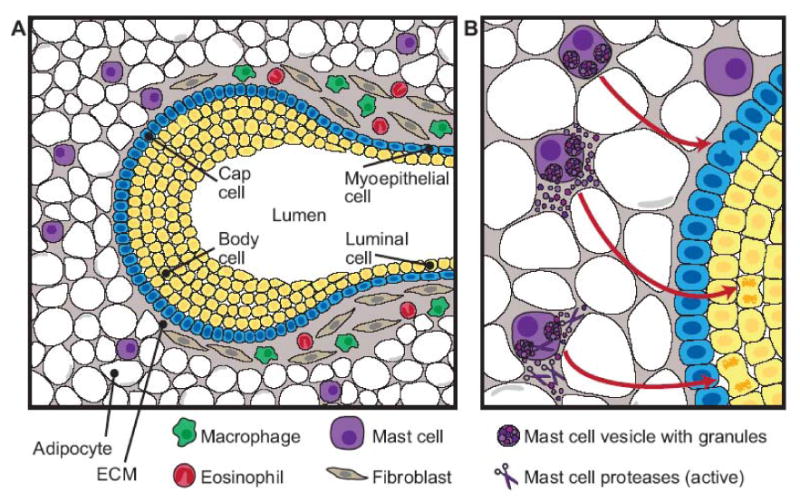
Diagram of innate immune cells that contribute to the stromal effects on mammary gland branching morphogenesis. A) The diagram depicts the stromal locations of eosinophils, macrophages, and mast cells relative to a terminal end bud (TEB). The TEB consists of a single layer of cap cells and multiple layers of body cells, which will eventually make up the luminal cells of the mature duct surrounded by a myoepithelial layer. The area surrounding the neck of the TEB consists of a collagen-rich stroma including fibroblasts, macrophages, and eosinophils. At the invading edge of the TEB, a thin basement membrane separates the advancing TEB from the adipocyte-rich stroma. Mast cells are found around the TEB in the stromal compartment, often just ahead of the advancing epithelium. B) Depiction of the effect of mast cell activation on mammary gland development. Mast cell degranulation and subsequent release of active proteases stimulate epithelial cell proliferation, promoting TEB formation and duct branching in the pubertal gland.
TEBs are the functional unit of mammary gland duct elongation and branching, and regulation of their cell proliferation and differentiation, shape, and extracellular matrix is essential for proper TEB formation and duct invasion into the mammary stroma. Reduced TEB number and duct branching have been reported in many mouse models, implicating hormones, cytokines, extracellular matrix proteins and their receptors and proteases, and growth and transcription factors in this morphogenetic process [for review, see (Howlin et al., 2006)]. Coordination of cell proliferation, differentiation, and apoptosis in the TEBs allows them to invade the adipocyte- and fibroblast-rich stroma, resulting in duct elongation, as well as allows the TEB to bifurcate, resulting in ductal branching (Richert et al., 2000). While mast cells are found throughout the mammary stroma, they are also concentrated around the invading TEBs ahead of and beside the advancing end buds. The proximity of mast cells near TEBs during pubertal development, taken together with our observations that mammary glands without or with fewer mast cells had a lower percentage of proliferating cells in both TEBs and ducts during this period, suggests that mast cells contribute to the complex regulation of cell proliferation in the growing mammary gland.
How might mast cells mediate this regulation of proliferation? First, mast cells might recruit other cells known to affect mammary development; second, mast cells might alter the stromal ECM immediately surrounding the invading ducts where they reside; or third, mast cell-derived mediators could regulate mitogens that affect mammary epithelial cells. Although mast cells can recruit leukocytes to tissues in vivo (Chen et al., 2001; Gaboury et al., 1995; Kubes and Kanwar, 1994; Walsh et al., 1991), we ruled out the possibility that the effects of mast cells were solely due to their recruitment of macrophages and/or eosinophils, which also regulate mammary gland development (Gouon-Evans et al., 2000), to the developing mammary gland. Moreover, we observed that mast cell loss had no effect on collagen deposition around TEBs, as has been reported for macrophage loss (Ingman et al., 2006). However, our results do not exclude other relationships between inflammatory cells or stromal matrix proteins and mast cells and their products in mammary gland development.
Resident mast cells in the mammary gland may arise from precursors recruited to the mammary stroma proper where they mature locally, or may migrate as mature cells to the mammary gland from the surrounding subdermal connective tissue. Given their role in leukocyte recruitment, it is not clear whether the mast cells localized to the mammary lymph nodes have the same function as the mast cells found throughout the rest of the mammary fat pad, especially those that are found around the developing gland. Interestingly, a genetic model for eosinophil overabundance through transgenic overexpression of interleukin-5 also results in reduced TEB and duct formation (Sferruzzi-Perri et al., 2003), suggesting that leukocyte recruitment and regulation must be finely controlled to promote mammary duct development.
The W-sash model revealed a role for stromal mast cells during mammary gland development. We further determined that mammary mast cells must be able to degranulate, and the granules must have active and normal levels of proteases within those granules to exert their effects on mammary gland development. As mast cell granules contain histamine, proteoglycans, growth factors, and many proteases including serine proteases such as chymases and tryptases (Galli and Tsai, 2008; Metcalfe et al., 1997), there are many granule components that potentially could contribute to regulation of mammary gland development. Of these, mast cell proteases are candidates for the mechanism by which mast cells affect the developing mammary gland.
Ideally the reconstitution of mast cells into the W-sash mice should revert the mast cell deficiency phenotype. Unfortunately, mast cells derived from bone marrow culture injected directly into the glands require at least 8 weeks post-injection to fully engraft (J. Lilla, unpublished observations). Since the onset of mammary pubertal development occurs at 3 weeks of age, with the greatest difference in phenoype observed at 5 weeks of age, we were not able to rescue the W-sash phenotype by engraftment of C57BL/6 Kit+/+ β-actin-CFP bone marrow cells into KitW-sh/W-sh animals due to the long lag period before mast cell reconstitution in the mammary gland, as has been observed in other tissues previously (Grimbaldeston et al., 2005).
Given the technical challenges in achieving a timely engraftment of mast cells to the pubertal mammary gland, we analyzed other, non-kit-dependent mast cell altered animals to confirm our findings. Further evidence supporting specific roles for mast cell granule components was obtained with cromolyn sodium treatment, showing that mast cell degranulation was necessary for the manifestation of mast cell function in mammary development.
Tissue-type mast cell proteases have been implicated in angiogenesis (Blair et al., 1997; Coussens et al., 1999) and extracellular matrix protein degradation either directly (Vartio et al., 1981; Wolters et al., 2000) or by activation of other ECM proteases (Johnson et al., 1998). Our data show an impairment in the mammary gland development of mice with reduced mast cell protease activity (DPPI-/- mice), implicating these proteases in mammary development. Aside from its function in normal tryptase expression and chymase activation in mast cells (Wolters et al., 2001), DPPI/cathepsin C has proteolytic activity towards ECM proteins such as fibronectin and collagens I and IV (Wolters et al., 2000). Thus, DPPI activity could account for the mammary gland phenotype reported here, either directly or through its activation of tryptase and chymase, which in turn might act directly or as activators of yet other proteases such as matrix metalloproteinases. Interestingly, our preliminary analysis of mMCP4 (mouse mast cell protease 4/mouse chymase) deficient mice at 5 weeks of age did not suggest a role for chymase in mammary development (data not shown). It is important to note that DPPI also is required for normal processing and activation of neutrophil serine proteases (Adkison et al., 2002), so the DPPI-/- mammary phenotype may not reflect solely a requirement for normal mast cell protease activity.
Our study implicates mast cell proteases as likely effectors of mast cell activity on the developing mammary gland; however, other mast cell mediators could also be involved in a protease-dependent pathway. The major effect of mast cells was to regulate mammary epithelial proliferation. Thus, mast cell-derived growth factors, chemokines, cytokines or lipid-derived mediators could also contribute to the effects on mammary gland development. Mast cells regulate angiogenesis through multiple pathways, including by direct release of vascular endothelial growth factor (VEGF) (Heissig et al., 2005) and indirectly, by releasing ECM proteases that remodel the stroma to encourage angiogenesis (Levi-Schaffer and Pe'er, 2001). Although VEGF and VEGF receptor expression is high in the stroma of pubertal mammary glands (Hovey et al., 2001), our preliminary observations of the vasculature of W-sash mammary glands at 5 weeks of age did not reveal any discernable differences between mast cell-deficient, reduced, or wild-type animals (data not shown).
Expression of histamine and some of its receptors changes significantly during the estrus cycle and is highly expressed by mammary alveolar epithelium during pregnancy and lactation (Maslinski et al., 1993; Wagner et al., 2003). Mice null for histidine decarboxylase, the major enzyme responsible for histamine synthesis, are fertile, yet have reduced numbers of tissue mast cells, which also have reduced granular content (Ohtsu et al., 2001). Whether the null mammary glands have a mammary gland developmental phenotype remains to be studied. Two other intriguing candidates for the effect of mast cells on mammary gland development are transforming growth factor-β (TGF-β) and prostaglandin D2. However, as TGF-β and prostaglandin D2 inhibit mammary epithelial proliferation (Silberstein et al., 1992; Yee et al., 2003), it is unlikely that loss of these mast cell mediators would account for the negative effects on mammary development we observed in our study.
Taken together, our results indicate that mast cells function in a developmental process, in addition to their well-studied functions in innate immune responses. Mast cells must be present, be capable of normal degranulation, and have activated granule-associated proteases to facilitate normal mammary gland pubertal development in the mouse. Their location near the advancing ducts throughout pubertal morphogenesis places them in an ideal position to promote cell proliferation during TEB formation and duct branching in the growing mammary gland.
Acknowledgments
We thank Pengfei Lu and Bryan Welm for helpful discussions and Ying Yu for assistance with mouse maintenance. This study was supported by grants from the National Cancer Institute and the National Institute of Environmental Health Sciences (CA057621 and ES012801) and a predoctoral fellowship from the DOD-CMDRP Breast Cancer Research Program (W81XWH-05-1-0272) to JNL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkison AM, Raptis SZ, Kelley DG, Pham CT. Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J Clin Invest. 2002;109:363–71. doi: 10.1172/JCI13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ, Meng H, Marchese MJ, Ren S, Schwartz LB, Tonnesen MG, Gruber BL. Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. J Clin Invest. 1997;99:2691–700. doi: 10.1172/JCI119458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Ning G, Zhao ML, Fleming MG, Diaz LA, Werb Z, Liu Z. Mast cells play a key role in neutrophil recruitment in experimental bullous pemphigoid. J Clin Invest. 2001;108:1151–8. doi: 10.1172/JCI11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–97. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Hom YK. Role of mesenchymal-epithelial interactions in mammary gland development. J Mammary Gland Biol Neoplasia. 1996;1:21–35. doi: 10.1007/BF02096300. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Wiesen JF, Werb Z, Young P, Hom YK, Cooke PS, Lubahn DB. Paracrine mechanisms of mouse mammary ductal growth. Adv Exp Med Biol. 2000;480:93–7. doi: 10.1007/0-306-46832-8_11. [DOI] [PubMed] [Google Scholar]
- Duttlinger R, Manova K, Chu TY, Gyssler C, Zelenetz AD, Bachvarova RF, Besmer P. W-sash affects positive and negative elements controlling c-kit expression: ectopic c-kit expression at sites of kit-ligand expression affects melanogenesis. Development. 1993;118:705–17. doi: 10.1242/dev.118.3.705. [DOI] [PubMed] [Google Scholar]
- Gaboury JP, Johnston B, Niu XF, Kubes P. Mechanisms underlying acute mast cell-induced leukocyte rolling and adhesion in vivo. J Immunol. 1995;154:804–13. [PubMed] [Google Scholar]
- Galli SJ. Mast cells and basophils. Curr Opin Hematol. 2000;7:32–9. doi: 10.1097/00062752-200001000-00007. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–86. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Tsai M. Mast cells: versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. J Dermatol Sci. 2008;49:7–19. doi: 10.1016/j.jdermsci.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouon-Evans V, Lin EY, Pollard JW. Requirement of macrophages and eosinophils and their cytokines/chemokines for mammary gland development. Breast Cancer Res. 2002;4:155–64. doi: 10.1186/bcr441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127:2269–82. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–48. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissig B, Rafii S, Akiyama H, Ohki Y, Sato Y, Rafael T, Zhu Z, Hicklin DJ, Okumura K, Ogawa H, Werb Z, Hattori K. Low-dose irradiation promotes tissue revascularization through VEGF release from mast cells and MMP-9-mediated progenitor cell mobilization. J Exp Med. 2005;202:739–50. doi: 10.1084/jem.20050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–60. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- Hovey RC, Goldhar AS, Baffi J, Vonderhaar BK. Transcriptional regulation of vascular endothelial growth factor expression in epithelial and stromal cells during mouse mammary gland development. Mol Endocrinol. 2001;15:819–31. doi: 10.1210/mend.15.5.0635. [DOI] [PubMed] [Google Scholar]
- Howlin J, McBryan J, Martin F. Pubertal mammary gland development: insights from mouse models. J Mammary Gland Biol Neoplasia. 2006;11:283–97. doi: 10.1007/s10911-006-9024-2. [DOI] [PubMed] [Google Scholar]
- Ingman WV, Wyckoff J, Gouon-Evans V, Condeelis J, Pollard JW. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev Dyn. 2006;235:3222–9. doi: 10.1002/dvdy.20972. [DOI] [PubMed] [Google Scholar]
- Jamieson T, Cook DN, Nibbs RJ, Rot A, Nixon C, McLean P, Alcami A, Lira SA, Wiekowski M, Graham GJ. The chemokine receptor D6 limits the inflammatory response in vivo. Nat Immunol. 2005;6:403–11. doi: 10.1038/ni1182. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Jackson CL, Angelini GD, George SJ. Activation of matrix-degrading metalloproteinases by mast cell proteases in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 1998;18:1707–15. doi: 10.1161/01.atv.18.11.1707. [DOI] [PubMed] [Google Scholar]
- Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–55. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- Kitamura Y. Heterogeneity of mast cells and phenotypic change between subpopulations. Annu Rev Immunol. 1989;7:59–76. doi: 10.1146/annurev.iy.07.040189.000423. [DOI] [PubMed] [Google Scholar]
- Kubes P, Kanwar S. Histamine induces leukocyte rolling in post-capillary venules. A P-selectin-mediated event. J Immunol. 1994;152:3570–7. [PubMed] [Google Scholar]
- Levi-Schaffer F, Pe'er J. Mast cells and angiogenesis. Clin Exp Allergy. 2001;31:521–4. doi: 10.1046/j.1365-2222.2001.01041.x. [DOI] [PubMed] [Google Scholar]
- Lilla JN, Joshi RV, Craik CS, Werb Z. Active plasma kallikrein localizes to mast cells and regulates epithelial cell apoptosis, adipocyte differentiation, and stromal remodeling during mammary gland involution. J Biol Chem. 2009;284:13792–803. doi: 10.1074/jbc.M900508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malorny U, Michels E, Sorg C. A monoclonal antibody against an antigen present on mouse macrophages and absent from monocytes. Cell Tissue Res. 1986;243:421–8. doi: 10.1007/BF00251059. [DOI] [PubMed] [Google Scholar]
- Maslinski C, Kierska D, Fogel WA, Kinnunen A, Panula P. Histamine: its metabolism and localization in mammary gland. Comp Biochem Physiol C. 1993;105:269–73. doi: 10.1016/0742-8413(93)90206-z. [DOI] [PubMed] [Google Scholar]
- McGarry MP, Stewart CC. Murine eosinophil granulocytes bind the murine macrophage-monocyte specific monoclonal antibody F4/80. J Leukoc Biol. 1991;50:471–8. doi: 10.1002/jlb.50.5.471. [DOI] [PubMed] [Google Scholar]
- McIntyre JA, Neerunjun ED, Faulk WP, Papamichail M. Inhibition of in vitro allogeneic reactions with disodium cromoglycate. Int Arch Allergy Appl Immunol. 1981;66:244–50. doi: 10.1159/000232827. [DOI] [PubMed] [Google Scholar]
- Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–79. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- Metz M, Grimbaldeston MA, Nakae S, Piliponsky AM, Tsai M, Galli SJ. Mast cells in the promotion and limitation of chronic inflammation. Immunol Rev. 2007;217:304–28. doi: 10.1111/j.1600-065X.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- Moloney WC, McPherson K, Fliegelman L. Esterase activity in leukocytes demonstrated by the use of naphthol AS-D chloroacetate substrate. J Histochem Cytochem. 1960;8:200–7. doi: 10.1177/8.3.200. [DOI] [PubMed] [Google Scholar]
- Nagle DL, Kozak CA, Mano H, Chapman VM, Bucan M. Physical mapping of the Tec and Gabrb1 loci reveals that the Wsh mutation on mouse chromosome 5 is associated with an inversion. Hum Mol Genet. 1995;4:2073–9. doi: 10.1093/hmg/4.11.2073. [DOI] [PubMed] [Google Scholar]
- Ohtsu H, Tanaka S, Terui T, Hori Y, Makabe-Kobayashi Y, Pejler G, Tchougounova E, Hellman L, Gertsenstein M, Hirasawa N, Sakurai E, Buzas E, Kovacs P, Csaba G, Kittel A, Okada M, Hara M, Mar L, Numayama-Tsuruta K, Ishigaki-Suzuki S, Ohuchi K, Ichikawa A, Falus A, Watanabe T, Nagy A. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett. 2001;502:53–6. doi: 10.1016/s0014-5793(01)02663-1. [DOI] [PubMed] [Google Scholar]
- Pham CT, Ley TJ. Dipeptidyl peptidase I is required for the processing and activation of granzymes A and B in vivo. Proc Natl Acad Sci U S A. 1999;96:8627–32. doi: 10.1073/pnas.96.15.8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed CE, Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J Allergy Clin Immunol. 2004;114:997–1008. doi: 10.1016/j.jaci.2004.07.060. [DOI] [PubMed] [Google Scholar]
- Reid AC, Silver RB, Levi R. Renin: at the heart of the mast cell. Immunol Rev. 2007;217:123–40. doi: 10.1111/j.1600-065X.2007.00514.x. [DOI] [PubMed] [Google Scholar]
- Richert MM, Schwertfeger KL, Ryder JW, Anderson SM. An atlas of mouse mammary gland development. J Mammary Gland Biol Neoplasia. 2000;5:227–41. doi: 10.1023/a:1026499523505. [DOI] [PubMed] [Google Scholar]
- Rugh R. The mouse; its reproduction and development. Minneapolis: Burgess Pub. Co; 1968. [Google Scholar]
- Russell JS, McGee SO, Ip MM, Kuhlmann D, Masso-Welch PA. Conjugated linoleic acid induces mast cell recruitment during mouse mammary gland stromal remodeling. J Nutr. 2007;137:1200–7. doi: 10.1093/jn/137.5.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sferruzzi-Perri AN, Robertson SA, Dent LA. Interleukin-5 transgene expression and eosinophilia are associated with retarded mammary gland development in mice. Biol Reprod. 2003;69:224–33. doi: 10.1095/biolreprod.102.010611. [DOI] [PubMed] [Google Scholar]
- Silberstein GB, Flanders KC, Roberts AB, Daniel CW. Regulation of mammary morphogenesis: evidence for extracellular matrix-mediated inhibition of ductal budding by transforming growth factor-beta 1. Dev Biol. 1992;152:354–62. doi: 10.1016/0012-1606(92)90142-4. [DOI] [PubMed] [Google Scholar]
- Szewczyk G, Szukiewicz D, Zaczek R, Maslinska D. Mast cells in the mouse mammary gland--correlation with the development of lactiferous structures. Folia Biol (Krakow) 2000;48:13–7. [PubMed] [Google Scholar]
- Topper YJ, Freeman CS. Multiple hormone interactions in the developmental biology of the mammary gland. Physiol Rev. 1980;60:1049–106. doi: 10.1152/physrev.1980.60.4.1049. [DOI] [PubMed] [Google Scholar]
- Vartio T, Seppa H, Vaheri A. Susceptibility of soluble and matrix fibronectins to degradation by tissue proteinases, mast cell chymase and cathepsin. G J Biol Chem. 1981;256:471–7. [PubMed] [Google Scholar]
- Wagner W, Ichikawa A, Tanaka S, Panula P, Fogel WA. Mouse mammary epithelial histamine system. J Physiol Pharmacol. 2003;54:211–23. [PubMed] [Google Scholar]
- Walsh LJ, Trinchieri G, Waldorf HA, Whitaker D, Murphy GF. Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci U S A. 1991;88:4220–4. doi: 10.1073/pnas.88.10.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296:1046–9. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters PJ, Laig-Webster M, Caughey GH. Dipeptidyl peptidase I cleaves matrix-associated proteins and is expressed mainly by mast cells in normal dog airways. Am J Respir Cell Mol Biol. 2000;22:183–90. doi: 10.1165/ajrcmb.22.2.3767. [DOI] [PubMed] [Google Scholar]
- Wolters PJ, Pham CT, Muilenburg DJ, Ley TJ, Caughey GH. Dipeptidyl peptidase I is essential for activation of mast cell chymases, but not tryptases, in mice. J Biol Chem. 2001;276:18551–6. doi: 10.1074/jbc.M100223200. [DOI] [PubMed] [Google Scholar]
- Yee LD, Guo Y, Bradbury J, Suster S, Clinton SK, Seewaldt VL. The antiproliferative effects of PPARgamma ligands in normal human mammary epithelial cells. Breast Cancer Res Treat. 2003;78:179–92. doi: 10.1023/a:1022978608125. [DOI] [PubMed] [Google Scholar]


