Abstract
The majority of HIV infections occur via sexual transmission across a mucosal barrier. In the case of male-to-female transmission, HIV-susceptible target cells are abundant in the ectocervix and vagina but are also present in the upper reproductive tract (endocervix and uterus). While the mechanisms of HIV transmission in the female reproductive tract are an active area of investigation, cell-mediated immune responses in reproductive tissues have not been thoroughly characterized. HIV-specific CD8+ T cells are present in reproductive tissues, to some extent mirroring populations present in the blood and gastrointestinal mucosa. Innate natural killer (NK) cells and regulatory T cells are also present in the genital tract. Furthermore, there is mounting evidence that the female reproductive tract may be uniquely susceptible to infection at specific times during the menstrual cycle, due to hormonal regulation of both innate and adaptive immune responses. This review provides an overview of recent findings on cell-mediated immunity to HIV in the female reproductive tract.
Keywords: Mucosa, CTL, HIV
Introduction: HIV infection of the female reproductive tract
Several mechanisms have been proposed to explain how HIV gains access to reproductive tract tissues [for a detailed review, see (Hladik and McElrath, 2008)]. These include direct infection of epithelial cells; transcytosis of viral particles across the epithelium; penetration of virus and/or infected cells through epithelial breaches; and binding to and/or direct infection of mucosal dendritic cells via receptors including C-type lectins. While each of these pathways has been substantiated in experimental models, there remains an active debate concerning their relevance to HIV transmission in vivo. Nevertheless, it is generally agreed that (a) the rate of HIV transmission per high-risk sexual contact is low (Wawer et al., 2005) and (b) once infection occurs at the mucosal surf ace, virus spreads rapidly to draining lymph nodes, from which it undergoes amplification and is disseminated throughout the body [reviewed in (Haase, 2005)]. Tissues of both lower (ectocervix and vagina) and upper (endocervix and uterus) female reproductive tract can serve as targets for HIV, yet antiviral immune defenses at these sites remain to be fully characterized.
Gender-based differences in immunity to HIV
Gender-based differences in the regulation of immunity, or sexual dimorphisms, have been documented in the settings of infectious disease and autoimmunity (Whitacre et al., 1999). Many autoimmune diseases are more prevalent in women than in men; these include Graves’ disease, systemic lupus erythematosis (SLE), and multiple sclerosis (MS). The underlying reasons for this bias remain poorly understood, but may include gender-based differences in immune responsiveness, sex-linked genetic factors, and the effects of sex steroid hormones.
Differences in susceptibility to viral infections based on gender have been documented in several murine infections, including vesicular stomatitis virus (Barna et al., 1996), herpes simplex virus (HSV-1) (Burgos et al., 2005) and lymphocytic choriomeningitis virus (LCMV) (Hildeman and Muller, 2000). Healthy women have higher absolute CD4+ T cell counts than men, and HIV-infected women maintain higher CD4+ T cell counts than HIV-infected men for several years after seroconversion (Prins et al., 1999). HIV-infected women also have lower plasma viral loads than men with similar CD4+ counts; nevertheless, both genders progress to AIDS at similar rates (Napravnik et al., 2002).
Sex hormones an d cell-mediated immunity
Estrogen receptor expression has been reported on a variety of cell types, including T and B cells, macrophages, and thymocytes (Phiel et al., 2005). Estrogen regulates the IFNγ promoter (Fox et al., 1991), and may affect chemokine receptor gene expression and chemotaxis in CD4+ T cells (Mo et al., 2005). Evidence from murine models supports a role for estrogen in promoting cell-mediated Th1 responses, perhaps by modulating expression of the transcription factor T-bet (Kawana et al., 2005); however, the effects of estrogen are also dose-dependent. For example, in the third trimester of pregnancy, high doses of estrogen and progesterone promote a “Th2 shift,” stimulating Th2 responses and antibody production (Doria et al., 2006). Progesterone alone may also promote a Th2-like environment. In males, testosterone is also a strong promoter of Th2-type responses (Dalal et al., 1997). Accordingly, the differential effects of these hormones on cell-mediated immune responses may contribute to the sexual dimorphisms observed in autoimmune and infectious diseases.
Sex hormones also drive the menstrual cycle, promoting changes in the uterine endometrium. Wira and colleagues have proposed that hormonal fluctuations during the menstrual cycle, whose biological purpose is to facilitate implantation and retention of the conceptus, may render the upper female reproductive tract uniquely vulnerable to HIV infection [reviewed in (Wira and Fahey, 2008)]. The expression of HIV receptors and coreceptors by CD4+ T cells and epithelial cells in the upper reproductive tract peaks at ovulation, providing an abundance of potential target cells for HIV. Furthermore, after ovulation, during the secretory phase, CTL and NK cell activity in the uterus are suppressed, potentially contributing to a permissive environment for HIV infection and dissemination. In contrast, in the lower reproductive tract, CTL activity persists throughout the menstrual cycle (Wira and Fahey, 2008).
Physical barriers and innate defenses relevant to HIV transmission
The female reproductive tract is equipped with a variety of physical barriers and innate defenses [reviewed in (Wira and Fahey, 2008)]. Physical defenses include a multi-layered squamous epithelium in the vagina and ectocervix, in contrast to a single, friable layer of columnar epithelium lining the endocervix. As an additional barrier, the endocervical canal is lined with mucus. Innate defenses include compounds such as secretory leukocyte protease inhibitor (SLPI) (Fahey and Wira, 2002), and lactoferrin, a milk protein (Berkhout et al., 2004), both of which are found in a variety of secretions and exhibit anti-HIV activity in vitro. Numerous cytokines, chemokines (including RANTES and MIP-1β), and antimicrobial peptides are also secreted by epithelial cells in reproductive tissues (Fahey et al., 2005). One study reported that highly-exposed yet HIV-uninfected sex workers had significantly higher levels of beta chemokines in cervical secretions as compared to low-risk women (Iqbal et al., 2005).
HIV, cell-mediated immunity, and the lower female reproductive tract
Cervical mucosa-associated lymphoid tissue (MALT)
The cervical transformation zone is a unique anatomic site, situated at the intersection between squamous epithelium of the lower genital tract and columnar epithelium of the upper tract. The transformation zone is constantly remodeled in response to hormonal influences and changes brought on by pregnancy and aging. This unique region of the female reproductive tract is rich in T-lymphocytes and antigen-presenting cells and serves as both an inductive and an effector site for cell-mediated immunity. In the past, it was generally accepted that the genital mucosa did not harbor mucosal inductive sites. However, recent studies have demonstrated that the cervical transformation zone is an inducible site of organized MALT in women with human papilloma virus (HPV)-induced cervical intraepithelial neoplasia (CIN) (Kobayashi et al., 2002). These dense aggregates resemble germinal centers. Aggregates were observed significantly more frequently in immunocompetent women as compared to HIV-positive women with low CD4+ T cell counts (Kobayashi et al., 2002), suggesting that the induction of cervical MALT may be impaired in women with progressive HIV disease.
Cervicovaginal antigen-presenting cells
Antigen-presenting cells in the reproductive tract, particularly dendritic cells, appear to play a major role HIV transmission and dissemination, as well as driving the early inflammatory response to infection (Hladik and McElrath, 2008). Langerhans cells in the cervicovaginal epithelium express CD1a and the C-type lectin receptor langerin (CD207). They also express CD4 and CCR5, but not CXCR4 or DC-SIGN. Some reports suggest that genital Langerhans cells harbor SIV virions within 24 h of experimental intravaginal inoculation (Hu et al., 2000), and Langerhans cells derived from human skin can be experimentally infected with HIV at a low level. However, whether cervicovaginal Langerhans cells can be productively infected in vivo, or whether they simply endocytose HIV and transport intact virions to CD4+ T cells, remains controversial. Dendritic cells located in the underlying cervicovaginal stroma express DC-SIGN as well as CD4 and CCR5; however, the role of stromal dendritic cells in HIV transmission is unclear (Hladik and McElrath, 2008).
Recent studies in the simian immunodeficiency virus (SIV) model system have revealed that CD123+ plasmacytoid dendritic cells play a unique role in triggering the early post-infection inflammatory response that facilitates viral dissemination (Li et al., 2009). As early as 1–3 days post-intravaginal infection, expression of the chemoattractant CCL20 (MIP-3α) is increased just below the endocervical epithelium. Plasmacytoid dendritic cells, arriving in response to CCL20, secrete CCL3 and CCL4 (MIP-1α and β, respectively), which are chemotactic for CCR5+ CD4+ T cells. This, in turn, creates a microenvironment rich in SIV-susceptible target cells. Plasmacytoid dendritic cells are also major producers of IFNα, a critical mediator of innate immunity whose expression may be pivotal in establishing immune hyperactivation during acute HIV/SIV infection (Mandl et al., 2008).
Cervicovaginal CTL in acute HIV infection
Once infection is established, HIV-specific cytotoxic T cells (CTL) are detected at several mucosal sites, including the reproductive tract and gastrointestinal tract. The kinetics of CTL induction in tissues are difficult to address in humans, but have been studied in rhesus macaques infected with simian immunodeficiency virus (SIVmac) (Reynolds et al., 2005). Following vaginal exposure of female macaques to SIVmac, virus was broadly disseminated in tissues by 10 to 14 days post-infection. SIV-specific CTL responses remained below the threshold of detection in the reproductive tract until several days after the peak in plasma viremia. Furthermore, CTL responses in distal tissues, such as the gastrointestinal tract, were not detected until more than 20 days post-infection. These findings led to the conclusion that the CTL response to HIV in mucosal tissues is "too little and too late" to contain viral replication and dissemination (Reynolds et al., 2005).
Intriguingly, HIV-specific CTL have also been identified in cervical cytobrush cells from women who are repeatedly exposed to HIV via sexual contact, yet remain seronegative and apparently uninfected; this finding has been interpreted to suggest a protective role for mucosal CTL in highly-exposed, seronegative individuals (Kaul et al., 2000).
Cervicovaginal CTL in chronic HIV infection
Throughout chronic infection, antigen-specific CD8+ T cells can be detected in cervical mucosa of HIV-infected women (Gumbi et al., 2008; Kaul et al., 2003; Musey et al., 2003; Musey et al., 1997). Due to the difficulties associated with obtaining fresh tissues, only limited studies have explored HIV-specific T cell populations in the reproductive tract and the extent to which these cells mirror CTL in blood. However, Musey and colleagues compared the T cell receptor sequence and clonality of HIV-specific CD8+ T cells from gastrointestinal mucosa, semen and cervix (Musey et al., 2003). They found that the majority of such clones were shared between mucosal sites and PBMC (Musey et al., 2003). Unexpectedly, these investigators also demonstrated that several CTL clones from cervical tissues were MHC class II-restricted, CD4+ T cells specific for HIV Env protein (Musey et al., 1997).
Recently, Gumbi et al. used cytokine flow cytometry to measure HIV Gag protein-specific CD8+ T cell function in cervical cytobrush cells from 51 HIV-infected and 24 HIV-negative women (Gumbi et al., 2008). In HIV-positive women, up to 10% of cervical CD8+ T cells responded to HIV Gag peptides. There was no correlation between blood and cervical responses, indicating that blood responses are not necessarily predictive of those in the reproductive tract. There was also no relationship between the cervical T cell responses and HIV viral load in cervical secretions. However, there was a positive association between cervical T cell responses and TNFα and IL-10 levels in cervical secretions. Furthermore, women who were shedding HIV in genital secretions had significantly higher levels of proinflammatory cytokines in secretions than non-shedding women (Gumbi et al., 2008). While these results could not establish a cause-effect relationship, they did suggest that local inflammation in the genital tract might promote HIV replication and shedding.
HIV, cell-mediated immunity, and the upper reproductive tract
Uterine endometrium
Uterine T cells express multiple HIV receptors and coreceptors, including CD4, CXCR4 and CCR5, and are readily accessible to virus in the endocervical and endometrial cavities (Wira and Fahey, 2008). The uterine endometrium also contains organized lymphoid aggregates, located in the stratum basalis layer. These structures, which consist of a B cell core surrounded by CD8+ T cells and macrophages, are hormonally regulated in pre-menopausal women, and are absent from post-menopausal women (Yeaman et al., 1997). Aggregate size is smallest during the proliferative phase of the menstrual cycle, and largest (2000–3000 cells) near ovulation and during the secretory phase. Whether these structures play an active role in antiviral defense is not yet known.
Within the endometrium, HIV may replicate in cells of the monocyte/macrophage lineage (Peuchmaur et al., 1989). Cells isolated from the uterus can be infected in vitro with HIV-1, and polarized human endometrial cell line, HEC-1, can bind and transcytose both CXCR4-tropic and CCR5-tropic HIV (Saidi et al., 2007). Reports describing HIV-specific T cell responses in the upper genital tract are rare; however, in two women with antiretroviral drug-treated progressive HIV infection, upper genital tract tissue lacked HIV-specific CTL activity (White et al., 2001). In contrast, tissue from a "long-term non-progressing" woman displayed robust HIV-specific CTL responses. While limited in scope, these studies reveal that uterine cells may play a role in HIV transmission and/or dissemination, and that HIV-specific T cells are also present in the upper reproductive tract.
Uterine natural killer (NK) cells: A unique defense
Human natural killer (NK) cells are distributed throughout the body and kill virally infected cells, primarily by exocytosis of CD107-expressing granules containing perforin and granzymes (Yokoyama, 2008). NK cells also release cytokines, such as IFNγ, and can mediate antibody-dependent cellular cytotoxicity (ADCC) via antibody Fc receptors (FcR). NK cells are a significant fraction of leukocytes in the uterine endometrium [reviewed in (Wira et al., 2005)]. These cells increase in number after ovulation and can comprise up to 70% of endometrial leukocytes prior to menstruation. The unique cell surface phenotype of uterine NK cells distinguishes them from blood NK cells: most uterine NK express CD56 but not CD57 or CD16.
Uterine NK cells appear to secrete a variety of cytokines and growth factors (including angiogenic growth factor and leukemia inhibitory factor) that serve to prepare the endometrium for embryo implantation. Accordingly, these cells may be critical not only in defending the endometrium from pathogens, but also in creating the microenvironment necessary for implantation. High uterine NK cell activity is also associated with recurrent miscarriage (Wira et al., 2005). Considerable effort has been devoted to characterizing the NK cell response to HIV in peripheral blood; however, the extent to which uterine NK cells respond to HIV infection is not known.
Regulatory T cells
During pregnancy, the maternal immune system must tolerate a semi-allogeneic fetus. The mechanisms contributing to this tolerance are incompletely understood, but both CD4+/CD25+ regulatory T (Treg) cells and uterine NK cells appear to participate in this process (Saito et al., 2007). A recent study of pregnant women provided evidence for migration of Foxp3+ Treg cells from maternal blood to the maternal-fetal interface (Tilburgs et al., 2008). Thus, analogous to the gastrointestinal mucosa, which must tolerate food antigens and commensal bacteria, the upper female reproductive tract must develop and maintain tolerance to fetal tissue using a variety of immunological mechanisms.
Treg cells have been proposed to play a dual role in HIV pathogenesis: by silencing HIV-specific T cell responses, they may contribute to viral dissemination; however, they may also limit potentially harmful immune activation. Increased frequencies of Treg cells have been documented in the gastrointestinal tract of macaques with progressive SIVmac infection, where their frequency is positively correlated with viral load (Boasso et al., 2007). However, it is not currently known whether Treg cells are more abundant in the reproductive tract of HIV-infected individuals as compared to healthy controls, or whether Treg cells modulate T cell responses and/or immune activation in reproductive tissues.
Immunological parallels between the reproductive tract and gastrointestinal tract
The mucosal tissues of the female reproductive tract and the gastrointestinal tract, while functionally distinct, share a number of immunological similarities (Table 1). From the perspective of HIV infection, both tissues serve as potential portals of entry during sexual contact. Both tissues are rich in activated CD4+ memory T cells, many of which express CCR5 and CXCR4 and are ideal target cells for HIV. Both the reproductive tract and the gut are equipped with a broad range of immunological defenses, including physical barriers and elements of innate and adaptive immunity. Epithelial cells at both sites produce cytokines, growth factors, chemokines, and antimicrobial peptides, and can present antigen. The gut contains organized lymphoid tissue (Peyer's Patches and lymphoid aggregates); the cervix contains inducible MALT aggregates and the endometrium contains large, cycle-dependent lymphoid aggregates consisting of CD8+ T cells and B cells. Although the responsiveness of the reproductive tract to sex steroids is well documented, certain cells of the gastrointestinal tract are also responsive to these hormones, and several gastrointestinal disorders exhibit sexual dimorphism.
Table 1.
Immunological parallels between the female reproductive tract and the gastrointestinal tract.
| Feature | Female Reproductive Tract | Gastrointestinal Tract |
|---|---|---|
| Abundant HIV target cells | Activated memory CD4+ T cells (CXCR4+, CCR5+) Dendritic cells Macrophages | Activated memory CD4+ T cells (CXCR4+, CCR5+) Dendritic cells Macrophages |
| Barrier epithelium | Stratified squamous epithelium (ecto-cx1, vagina); columnar epithelium (endo-cx1, uterus); mucus plug | Columnar epithelium; mucus layer |
| Commensal flora | Flora present in lower tract; some may also enter upper tract. | Rich, diverse flora throughout gastrointestinal tract. |
| Intraepithelial lymphocytes | CD8+ cytotoxic T cells | CD8+ cytotoxic T cells |
| Immune tolerance | Directed towards preservation of conceptus | Directed towards tolerance to flora and food antigens |
| Innate effector cells | Unique uterine NK cell phenotype; TCR-γδ T cells | Unique gut NK cell phenotype; TCR-γδ T cells |
| Innate effector molecules | SLP1, lactoferrin, defensins | Defensins |
| Organized inductive sites | Aggregates have been identified in cervical tissue and uterine endometrium. | Abundant throughout the GI tract (Peyer's patches and organized follicles) |
| Sex steroid responsiveness | Significant modulation of tissue architecture and function of individual cell types | Estrogen receptor ligands affect crypt/villus architecture, may play a role in inflammatory bowel diseases |
ecto-cx, ectocervix; endo -cx, endocervix; GI, gastrointestinal
Both the reproductive tract and gastrointestinal tract engage in immunological tolerance. In the reproductive tract, tolerance is related to embryo implantation; in the gastrointestinal tract, tolerance is maintained with respect to food antigens and commensal bacteria. Regulatory T cells and cytokines such as TGFβ appear to play an important role in tolerance at both sites.
Innate effector cells, including NK cells and gamma-delta T cells, are prevalent in both reproductive and gastrointestinal tissues. NK cells at both sites have properties that distinguish them from their counterparts in peripheral blood. Currently, little is known of the response of mucosal NK cells to HIV infection.
Both the reproductive mucosa and gastrointestinal tract harbor significant populations of antigen-specific memory CD8+ T cells. Our laboratory is currently comparing HIV-specific CD8+ T cell responses in blood, cervical and rectal mucosa of HIV-infected women. Our results suggest that CD8+ T cell responses to immunodominant epitopes in HIV Gag protein are broadly distributed throughout the body (D. Lemongello, A.L. Ferre, B.L. Shacklett, unpublished) (Figure 1). These findings corroborate earlier work by Ibarrondo and colleagues, who mapped HIV-specific CD8+ T cell responses in rectal mucosa and blood using pools of overlapping HIV peptides (Ibarrondo et al., 2005). These authors described similar response specificities and immunodominance hierarchies at both sites.
Figure 1.
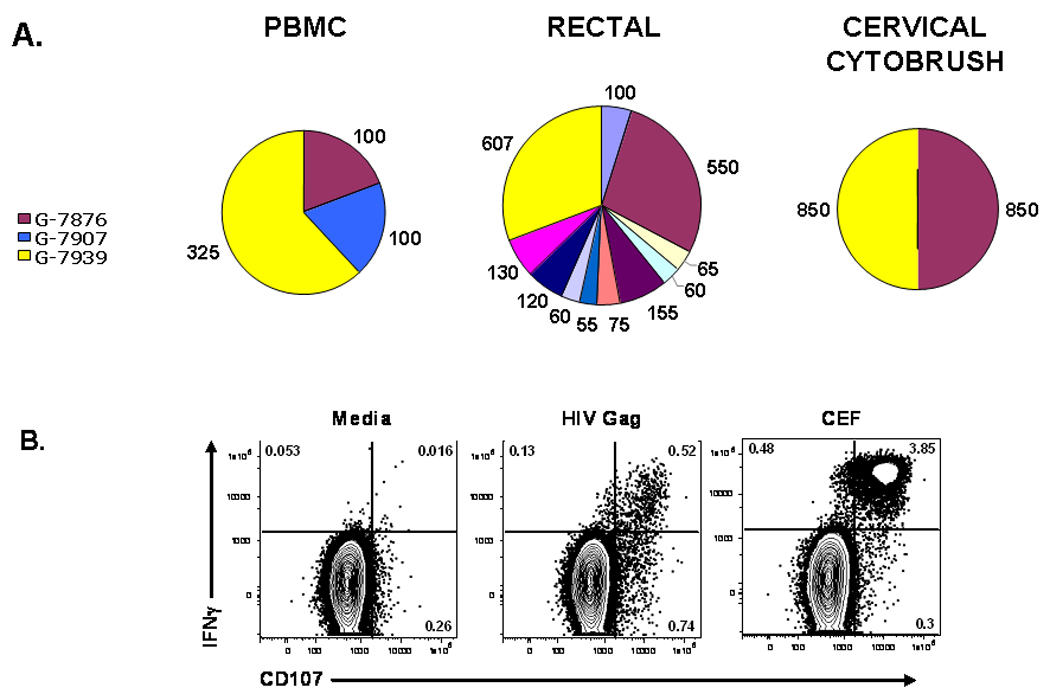
HIV Gag-specific CD8+ T cell responses in cervicovaginal cytobrush from two HIV-positive women. (A) Immunodominant Gag protein-specific responses are shared between PBMC, rectal mucosa and cervical mucosa. Pie charts summarize ELISPOT data from patient number 045. Polyclonally expanded cytobrush cells from PBMC, rectal mucosa, and cervical cytobrush of patient 045 were tested for responsiveness to HIV Gag peptides. Each peptide is indicated by a different color. The three peptides eliciting the strongest response from PBMC were peptides Gag-7876, Gag-7907, and Gag-7939, shown in maroon, blue, and yellow respectively. Numbers adjacent to each pie slice represent the number of IFNγ spot-forming cells per million. Strong responses to peptides 7876 and 7939 were detected at all three tissue sites. (B) Cervical CD8+ T cells express IFNγ and CD107 in response to HIV Gag stimulation. Flow cytometry plots summarize cervical CD8+ T cell responses in patient number 063, an HIV-positive woman not on antiretroviral therapy. Polyclonally expanded cervical cytobrush cells were stimulated with media alone (negative control, left panel); a pool of HIV Gag Clade B consensus 15-mer peptides (HIV Gag); or a pool of immunodominant peptides selected from cytomegalovirus, Epstein-Barr virus, and influenza A (CEF, positive control, right panels). Responses were measured in an intracellular cytokine assay to detect IFNγ and CD107. Numbers in each quadrant represent the percentage of CD8+ T cells producing the factors indicated. All studies were performed with informed consent under protocols approved by the UC Davis Institutional Review Board (IRB).
Summary and Conclusions
The female reproductive tract serves as a major portal for HIV infection. Although much attention has focused on the mechanisms by which HIV penetrates the mucosal barrier, little is known of cell-mediated immune defenses within the reproductive tract. This topic is potentially of high relevance to the development of an HIV vaccine. Furthermore, the comparison of immune responses in reproductive and gastrointestinal mucosal tissues may shed new light on the concept of the “common mucosal immune system”. There is currently no consensus concerning what constitutes a truly protective immune response to HIV, and studies of mucosal immunity in acutely-infected individuals, patients with progressive HIV disease, high-risk yet uninfected cohorts, and long-term nonprogressors may reveal important new insights in this area.
Acknowledgments
The author thanks Drs. Linda Giudice, Ruth Greenblatt, Warner C. Greene, Laura Napolitano, and Karen Smith-McCune, University of California at San Francisco, for helpful discussions. The author’s research is supported by the National Institutes of Health (NIH-NIAID R01 AI-057020 and P01 AI083050), the California HIV/AIDS Research Program (CHRP CH05-D-606), and the James B. Pendleton Charitable Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barna M, et al. Sex differences in susceptibility to viral infection of the central nervous system. J Neuroimmunol. 1996;67:31–39. doi: 10.1016/0165-5728(96)00022-7. [DOI] [PubMed] [Google Scholar]
- Berkhout B, et al. The antiviral activity of the milk protein lactoferrin against the human immunodeficiency virus type 1. Biometals. 2004;17:291–294. doi: 10.1023/b:biom.0000027707.82911.be. [DOI] [PubMed] [Google Scholar]
- Boasso A, et al. Regulatory T cell markers, indoleamine 2,3-dioxygenase, and virus levels in spleen and gut during progressive simian immunodeficiency virus infection. J Virol. 2007;81:11593–11603. doi: 10.1128/JVI.00760-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos JS, et al. Herpes simplex virus type 1 infection via the bloodstream with apolipoprotein E dependence in the gonads is influenced by gender. J Virol. 2005;79:1605–1612. doi: 10.1128/JVI.79.3.1605-1612.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal M, et al. Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen-specific T lymphocyte response. J Immunol. 1997;159:3–6. [PubMed] [Google Scholar]
- Doria A, et al. Th2 immune deviation induced by pregnancy: the two faces of autoimmune rheumatic diseases. Reprod Toxicol. 2006;22:234–241. doi: 10.1016/j.reprotox.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Fahey JV, et al. Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Hum Reprod. 2005;20:1439–1446. doi: 10.1093/humrep/deh806. [DOI] [PubMed] [Google Scholar]
- Fahey JV, Wira CR. Effect of menstrual status on antibacterial activity and secretory leukocyte protease inhibitor production by human uterine epithelial cells in culture. J Infect Dis. 2002;185:1606–1613. doi: 10.1086/340512. [DOI] [PubMed] [Google Scholar]
- Fox HS, et al. Estrogen regulates the IFN-gamma promoter. J Immunol. 1991;146:4362–4367. [PubMed] [Google Scholar]
- Gumbi PP, et al. Impact of mucosal inflammation on cervical HIV-1-specific CD8 T cell responses in the female genital tract during chronic HIV infection. J Virol. 2008;82:8529–8536. doi: 10.1128/JVI.00183-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol. 2005;5:783–792. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- Hildeman D, Muller D. Immunopathologic weight loss in intracranial LCMV infection initiated by the anorexigenic effects of IL-1beta. Viral Immunol. 2000;13:273–285. doi: 10.1089/08828240050144617. [DOI] [PubMed] [Google Scholar]
- Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nat Rev Immunol. 2008;8:447–457. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, et al. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol. 2000;74:6087–6095. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarrondo FJ, et al. Parallel human immunodeficiency virus type 1-specific CD8+ T-lymphocyte responses in blood and mucosa during chronic infection. J Virol. 2005;79:4289–4297. doi: 10.1128/JVI.79.7.4289-4297.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal SM, et al. Elevated T cell counts and RANTES expression in the genital mucosa of HIV-1-resistant Kenyan commercial sex workers. J Infect Dis. 2005;192:728–738. doi: 10.1086/432482. [DOI] [PubMed] [Google Scholar]
- Kaul R, et al. HIV-1-Specific Mucosal CD8+ Lymphocyte Responses in the Cervix of HIV-1- Resistant Prostitutes in Nairobi. J Immunol. 2000;164:1602–1611. doi: 10.4049/jimmunol.164.3.1602. [DOI] [PubMed] [Google Scholar]
- Kaul R, et al. Quantitative ex vivo analysis of functional virus-specific CD8 T lymphocytes in the blood and genital tract of HIV-infected women. AIDS. 2003;17:1139–1144. doi: 10.1097/00002030-200305230-00004. [DOI] [PubMed] [Google Scholar]
- Kawana K, et al. Female steroid hormones use signal transducers and activators of transcription protein-mediated pathways to modulate the expression of T-bet in epithelial cells: a mechanism for local immune regulation in the human reproductive tract. Mol Endocrinol. 2005;19:2047–2059. doi: 10.1210/me.2004-0489. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, et al. Lymphoid follicles are generated in high-grade cervical dysplasia and have differing characteristics depending on HIV status. Am J Pathol. 2002;160:151–164. doi: 10.1016/s0002-9440(10)64359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, et al. Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Science. 2009;323:1726–1729. doi: 10.1126/science.1168676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl JN, et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008;14:1077–1087. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- Mo R, et al. Estrogen regulates CCR gene expression and function in T lymphocytes. J Immunol. 2005;174:6023–6029. doi: 10.4049/jimmunol.174.10.6023. [DOI] [PubMed] [Google Scholar]
- Musey L, et al. Ontogeny and specificity of mucosal and blood human immunodeficiency virus-1 specific CD8+ cytotoxic T lymphocytes. J. Virol. 2003;77:291–300. doi: 10.1128/JVI.77.1.291-300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musey L, et al. HIV-1 induces cytotoxic T lymphocytes in the cervix of infected women. J Exp Med. 1997;185:293–303. doi: 10.1084/jem.185.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napravnik S, et al. Gender difference in HIV RNA levels: a meta-analysis of published studies. J Acquir Immune Defic Syndr. 2002;31:11–19. doi: 10.1097/00126334-200209010-00002. [DOI] [PubMed] [Google Scholar]
- Peuchmaur M, et al. HIV-associated endometritis. AIDS. 1989;3:239–241. doi: 10.1097/00002030-198904000-00008. [DOI] [PubMed] [Google Scholar]
- Phiel KL, et al. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett. 2005;97:107–113. doi: 10.1016/j.imlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Prins M, et al. Do gender differences in CD4 cell counts matter? AIDS. 1999;13:2361–2364. doi: 10.1097/00002030-199912030-00007. [DOI] [PubMed] [Google Scholar]
- Reynolds MR, et al. CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: too late and too little. J Virol. 2005;79:9228–9235. doi: 10.1128/JVI.79.14.9228-9235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidi H, et al. R5- and X4-HIV-1 use differentially the endometrial epithelial cells HEC-1A to ensure their own spread: implication for mechanisms of sexual transmission. Virology. 2007;358:55–68. doi: 10.1016/j.virol.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Saito S, et al. Regulatory T cells and regulatory natural killer (NK) cells play important roles in feto-maternal tolerance. Semin Immunopathol. 2007;29:115–122. doi: 10.1007/s00281-007-0067-2. [DOI] [PubMed] [Google Scholar]
- Tilburgs T, et al. Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J Immunol. 2008;180:5737–5745. doi: 10.4049/jimmunol.180.8.5737. [DOI] [PubMed] [Google Scholar]
- Wawer MJ, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- Whitacre CC, et al. A gender gap in autoimmunity. Science. 1999;283:1277–1278. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- White HD, et al. Human immunodeficiency virus-specific and CD3-redirected cytotoxic T lymphocyte activity in the human female reproductive tract: lack of correlation between mucosa and peripheral blood. J Infect Dis. 2001;183:977–983. doi: 10.1086/319253. [DOI] [PubMed] [Google Scholar]
- Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;22:1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira CR, et al. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev. 2005;206:306–335. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- Yeaman GR, et al. Unique CD8+ T cell-rich lymphoid aggregates in human uterine endometrium. J Leukoc Biol. 1997;61:427–435. [PubMed] [Google Scholar]
- Yokoyama WM. Mistaken notions about natural killer cells. Nat Immunol. 2008;9:481–485. doi: 10.1038/ni1583. [DOI] [PubMed] [Google Scholar]


