Abstract
Prolyl endopeptidase (PE), a protease that cleaves after proline residues in oligopeptides, is highly active in brain and degrades neuropeptides in vitro. We have recently demonstrated that PE, in concert with MMP's, can generate PGP (prolineglycine-proline), a novel, neutrophil chemoattractant, from collagen. In this study, we demonstrate that human peripheral blood neutrophils contain PE, which is constitutively active, and can generate PGP de novo from collagen after activation with LPS. This novel, pro-inflammatory role for PE raises the possibility of a self-sustaining pathway of neutrophilic inflammation and may provide biomarkers and therapeutic targets for diseases caused by chronic, neutrophilic inflammation.
Keywords: neutrophil, prolyl endopeptidase, PGP, collagen, inflammation
1. INTRODUCTION
Prolyl endopeptidase (PE) is an endopeptidase, which cleaves at the carboxyl side of proline residues in oligopeptides. PE is the only proline-specific endopeptidase currently known in mammals and belongs to a group of serine proteases, which also includes dipeptidyl peptidase IV, oligopeptidase B and acylaminoacyl peptidase (Polgar, 2002). These peptidases differ significantly from classical serine proteases, such as trypsin or subtilisin, in their structure and selectivity for small peptide substrates. In PE, the catalytic triad is covered by the central tunnel of a β propeller domain, which excludes peptides larger than 30-100 amino acids from the active site (Fulop et al., 1998).
Peptide bonds involving proline residues are seldom cleaved by classical serine proteases since they do not fit into the catalytic site. Many biologically active peptides contain prolines within their amino acid sequence and enzymes that cleave peptides at a proline may consequently have important biological effects (Cunningham and O'Connor, 1997; Mentlein, 1988). PE is highly active in brain tissue (Kalwant and Porter, 1991), degrades neuropeptides in vitro (Knisatschek and Bauer, 1979; Taylor and Dixon, 1980; Wilk et al., 1979) and may play a role in the pathogenesis of depression and Alzeheimer's disease (Maes et al., 1994; Rossner et al., 2005). PE inactivates bradykinin, a vasodilator, and converts angiotensin I and II to angiotensin (residues 1-7), which liberates vasopressin from the hypothalamus, and may play a role in hypertension (Welches et al., 1993). However, the physiologic function of PE remains obscure despite its ubiquitous presence in human tissues as well as serum (Goossens et al., 1996).
We have recently identified a novel pathway signaling neutrophil influx to the lung in which PE plays a major role. Chemical or enzymatic breakdown of collagen releases a tripeptide, proline-glycine-proline (PGP) that is chemotactic for neutrophils in vitro and in vivo (Weathington et al., 2006). The neutrophil chemotactic activity of PGP may be due to a marked structural relatedness to a receptor-binding domain of CXC chemokines, such as interleukin-8, which contain this collagen sequence or a close analog. PGP production from collagen is dependent on initial digestion of collagen by MMP-8 and MMP-9 with PE catalyzing the final reaction (Gaggar et al., 2008). PGP and PE are elevated in lung diseases characterized by chronic, neutrophilic, airway inflammation. Sputum from patients with chronic obstructive pulmonary disease (COPD) or cystic fibrosis (CF) contains increased amounts of PGP and generates PGP from collagen in a PE-dependent fashion (Gaggar et al., 2008; O'Reilly et al., 2009). PE activity is elevated in sputum from CF patients and bronchoalveolar lavage fluid from lung transplant patients with chronic allograft rejection (Gaggar et al., 2008; Hardison et al., 2009). To our knowledge, this is the first time PE has been implicated in inflammation or in disorders of the respiratory system.
Given the detection of PE in neutrophilic lung diseases, we hypothesized that neutrophils might be a source of PE. We demonstrate herein, using a variety of molecular and biochemical techniques, that human peripheral blood neutrophils contain constitutively active PE and can generate PGP from collagen. The presence in neutrophils of all the enzymes necessary for generation of PGP raises the possibility of a self-perpetuating cycle of neutrophilic inflammation.
2. MATERIALS AND METHODS
Materials
Neutrophils were isolated from peripheral blood of healthy volunteers as previously described (Hardison et al., 2009) by separation on a Ficoll gradient (Sigma-Aldrich, St. Louis, MO). Neutrophil lysate was obtained by three freeze-thaw cycles and brief sonication with aprotinin (Sigma-Aldrich), followed by centrifugation at 13000rpm. Research using human samples was approved by the Institutional Review Board at the University of Alabama at Birmingham. Informed consent of all participating subjects was obtained. A monospecific, polyclonal, anti-PE antibody was raised against a synthetic peptide representing residues 190–219 of mouse PE as described (Hardison et al., 2009). This antibody detects human PE whose sequence differs from mouse by one residue between residues 190 and 219. Recombinant human PE (rhPE) was cloned using a human PE cDNA, kindly provided by Drs. Anne Mudge and Michael Lumb (University College London). PE cDNA was cloned into the pTrcHisB vector (Invitrogen, Carlsbad, CA), which contains a His tag. PE expression in E. coli was induced with IPTG and purified on a nickel column.
PE activity assay
PE activity was determined as previously described (Gaggar et al., 2008; Hardison et al., 2009) by incubating samples with a PE-specific substrate, 2mM ZGP-pNA (benzylcarboxy-glycine-proline-p-nitroaniline, Chem Impex International, Wood Dale, IL) at 37°C and 5% CO2. Cleavage of p-nitroaniline by PE was detected using a spectrophotometer at 405nm.
Immunofluorescence microscopy
Cytospin preparations of neutrophils on glass slides were fixed with 4% paraformaldehyde in PBS and permeabilized with 0.1% Triton X-100. After blocking with 3% BSA in PBS, neutrophils were incubated with anti-PE antibody (45 μg/ml in PBS/1% BSA), pre-immune rabbit antibody or anti-PE antibody which had been pre-adsorbed with rhPE (200 μg/ml) for two hours at room temperature. After a second blocking step with 3% BSA, neutrophils were incubated with FITC-labeled goat anti-rabbit secondary antibody (1:12,000 in PBS/1% BSA, Southern Biotechnology, Birmingham, AL) for one hour. Nuclei were stained with Hoechst (1:2000, Sigma-Aldrich) and neutrophils examined by immunofluorescence microscopy.
Western blotting
Neutrophil lysate was separated by SDS-PAGE under reducing conditions and transferred onto nitrocellulose membranes. Membranes were blocked in 5% BSA in PBS for one hour and incubated with polyclonal, rabbit, anti-PE antibody (22.4μg/ml) for one hour at room temperature. After incubation, membranes were washed and incubated with goat anti-rabbit secondary antibody conjugated with horseradish peroxidase (Southern Biotechnology, Birmingham, AL) for one hour. Immunoblots were developed by chemiluminescence (Pierce, Rockford, IL).
RT-PCR
Total RNA from human peripheral blood neutrophils was isolated and first strand cDNA synthesized using a kit according to manufacturer's specifications (Superarray, Frederick, MD). PCR for human PE was performed using specific primers (Superarray) and continued for 45 cycles.
PGP generation assay
Human peripheral blood neutrophils (1× 106) were incubated with 15 μl of a 1 mg/ml solution of Type 1 collagen (Sigma-Aldrich) in PBS containing bestatin (Cayman Chemical, Ann Arbor, MI) with or without LPS (100μg/ml, Sigma-Aldrich) for 1.5 hours at 37°C and 5% CO2. The collagen was extensively dialyzed beforehand to remove PGP. After incubation, samples were 10kDa filtered, washed with 40 μl of 1N HCl, and analyzed by ESI- LC-MS/MS for levels of PGP and N-α-PGP.
ESI-LC-MS/MS protocol
PGP and N-α-PGP were measured simultaneously in samples as previously described (Hardison et al., 2009) using a MDS Sciex API-4000 spectrometer (Applied Biosystems, Foster City, CA) equipped with HPLC (Shimadzu, Kyoto, Japan). HPLC was performed using a 2.1 x 150mm Develosi C30 column (buffer A: 0.1% formic acid, buffer B: acetonitrile plus 0.1% formic acid; 80% buffer A/20% buffer B from 0 to 0.6 min, 0% buffer A/100% buffer B from 0.6 to 5 min). Background was removed by flushing with 100% isopropanol/0.1% formic acid. Positive electrospray mass transitions were at 270-70 and 270-116 (PGP) and 312-140 and 312-112 (N-α-PGP).
3. RESULTS
PE protein and activity are detected in human peripheral blood neutrophils
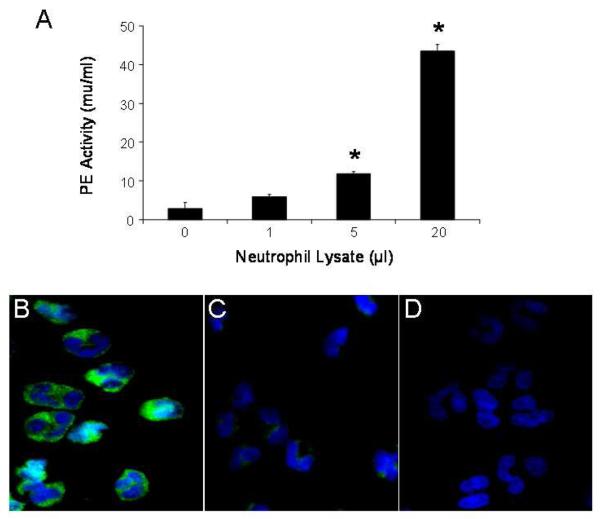
Cell lysates from un-stimulated, human, peripheral blood neutrophils were able to cleave the PE substrate, ZGP-pNA. Increased cleavage of ZGP-pNA was detected with increasing amount of neutrophil lysate (Fig. 1A). This suggested that human neutrophils contain a PE-like enzymatic activity, which is constitutively active.
Figure 1.
Indicated amounts of human neutrophil lysate (106 cells) were assayed for PE enzymatic activity (expressed as mU/ml). Results are presented as mean ± SEM (A) and compared using the two group t test. Increased PE activity was observed with greater amounts of neutrophil lysate (* p < 0.05 compared with 0μl lysate, n = 3 per group). Results are representative of several experiments. Cytospins of human peripheral blood neutrophils on glass slides were incubated with anti-PE antibody (B), anti-PE antibody pre-adsorbed with PE (C) or pre-immune rabbit antibody (D), followed by FITC-labeled goat anti-rabbit IgG, and examined by immunofluorescence microscopy. PE in human neutrophils was located in the cytoplasm in a granular pattern (B).
As there are additional enzymes with PE-like activity that can cleave ZGP-pNA and may be present in neutrophils (Shariat-Madar et al., 2002), we developed specific reagents to ensure we were detecting PE and not another enzyme. Using a monospecific, polyclonal anti-PE antibody, PE was clearly detected in human peripheral blood neutrophils by immunofluorescence microscopy (Fig. 1B). PE appeared to have a diffuse cytoplasmic distribution in neutrophils with increased concentration in granule-like structures.
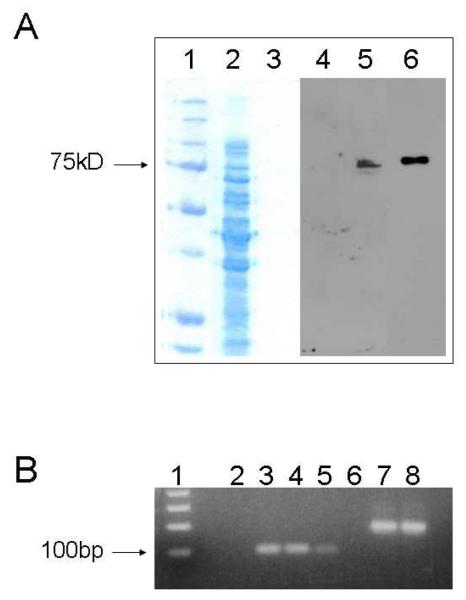
In further confirmation of these findings, we detected PE in human neutrophil lysates by Western blotting, using the same anti-PE antibody. PE migrated as a 75kDa monomer, similar to recombinant human PE (rhPE) used as a positive control (Fig. 2A). Total RNA was isolated from human peripheral blood neutrophils and examined for PE mRNA by RT-PCR. A PCR product of the appropriate size of 95bp was found in human neutrophils and in a pTrcHisB vector containing rhPE, used as a positive control (Fig. 2B).
Figure 2.
Human neutrophil lysate was separated by SDS-PAGE, transferred to nitrocellulose membranes and probed for PE (A). Lanes 1, 2 and 3 are a coomassie blue stained gel of MW markers, human PMN lysate (18 μg protein per lane) and rhPE respectively. Lanes 4, 5 and 6 are a chemiluminescence developed Western using our rabbit anti-PE antibody corresponding to Lanes 1, 2 and 3. PE in human neutrophils was a monomer and migrated at 75kDa, similar to rhPE. PCR for PE was performed on total RNA isolated from human peripheral blood neutrophils and the product run on a 2% agarose gel (B). Human PE primers should amplify a 95bp product. PCR for actin was performed concurrently as a positive control. Lanes: Invitrogen 1Kb+ ladder (1), negative control (2), 95bp PCR product from human PMN (3 and 4), 95bp PCR product from pTrcHisB vector containing rhPE (5), negative control (6), 200bp PCR product for actin (7 and 8).
Human neutrophils generate PGP de novo from collagen
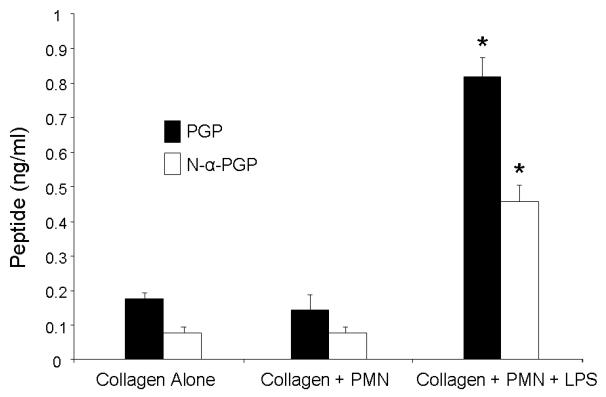
Since neutrophils are known to contain MMP-8 and MMP-9 and, having demonstrated that neutrophils contain PE, we hypothesized that they would contain all the enzymes necessary to generate PGP from intact collagen. We tested this idea by incubating human neutrophils with collagen and measuring PGP generation. When incubated alone with collagen, human neutrophils did not generate any PGP in excess of that found in collagen alone. However, when incubated with LPS and collagen, human neutrophils generated large amounts of PGP and N-acetyl-PGP (N-α-PGP, Fig. 3).
Figure 3.
Previously dialyzed Type 1 collagen was incubated with human peripheral blood neutrophils (1 × 106) with and without LPS (100μg/ml) for 1.5 hours. Supernatants were passed through a 10kDa filter and analyzed by ESI-LC-MS/MS for PGP and N-α-PGP. Levels of PGP and N-α-PGP generated are presented as mean ± SEM and compared using the two group t test. LPS-stimulated neutrophils generated significantly greater amounts of PGP and N-α-PGP (* p < 0.01 compared with collagen alone or collagen incubated with neutrophils without LPS, n = 6 per group).
4. DISCUSSION
These results demonstrate that PE is present and constitutively active in human neutrophils, where it had a cytoplasmic distribution and was a monomer of 75kDa in size. These results concur with previous studies in other human cell types and tissues, where PE was generally found in cytoplasm and was a monomer with a molecular mass between 65 and 80kDa (Goossens et al., 1995; Hasebe et al., 2001; Kalwant and Porter, 1991; Mizutani et al., 1984; Pratt et al., 1989), although nuclear and membrane-bound isoforms have also been described (Dresdner et al., 1982; Ishino et al., 1998; O'Leary and O'Connor, 1995). Although PE appears to be a single copy gene, different forms of PE, arising from post-translational modification and/or different gene products, may account for the variety of functions and locations of the enzyme (Kimura et al., 1999).
The action of PE along with MMP-8 (neutrophil collagenase) and/or MMP-9 (neutrophil gelatinase) is sufficient to generate the neutrophil chemoattractant, PGP, from collagen (Gaggar et al., 2008). In PGP generation, PE likely acts on oligopeptides generated by the prior digestion of collagen by MMP's. As human neutrophils are known to express MMP- 8 and MMP-9 (Gaggar et al., 2007; Lin et al., 2008) and are now shown to contain PE, we hypothesized that they would be capable, by themselves, of generating PGP from collagen. Consistent with this idea, LPS-stimulated human neutrophils, but not unstimulated neutrophils, generated PGP from collagen (Fig. 3). This provides further evidence that human neutrophils contain PE, which, to our knowledge, is the only protease capable of releasing PGP from the often-repeated PPGP sequence in collagen. PE appeared to be concentrated in granule-like structures in neutrophils (Fig. 1B) and may be released from neutrophils through degranulation in response to pro-inflammatory stimuli along with other neutrophil products, such as elastase, myeloperoxidase and MMP's. Human neutrophils also appear to contain an enzymatic activity, which acetylates PGP to N-α-PGP (Fig.3). N-α-PGP is a more potent neutrophil chemoattractant than PGP (Haddox et al., 1999) and, like PGP, is a biomarker for COPD and CF in sputum (Gaggar et al., 2008; O'Reilly et al., 2009). Precise localization of PE within neutrophils, the mechanism of its release and the stimuli that cause PE release are the subject of ongoing studies.
Although PE is widely distributed in tissues, its precise physiological role is unknown. It has been suggested to play a role in neuropeptide processing and secretion (Schulz et al., 2005) and in pathogenesis of hypertension, through effects on the renin-angiotensin system. A role for PE in inflammation has been suggested by a small number of studies. For example, higher amounts of PE were detected in the synovial fluid of patients with rheumatoid arthritis than of patients with osteoarthritis (Kamori et al., 1991). However, no specific mechanisms have been elicited for this pro-inflammatory role. Our data provide a novel mechanism where PE may contribute to neutrophilic inflammation by generating the neutrophil chemoattractant, PGP, from collagen. Generation of PGP by PE from neutrophils provides a pathway for neutrophilic inflammation to feed forward in a self-perpetuating manner after an initial pro-inflammatory insult and provides a link between neutrophilic inflammation and the matrix destruction and remodeling seen in a wide variety of chronic inflammatory diseases. Our previous studies have implicated PE in PGP generation in patients with COPD and CF (Gaggar et al., 2008; O'Reilly et al., 2009). As we have previously shown for PGP (van Houwelingen et al., 2008), PE may be a biomarker and therapeutic target for these and other chronic inflammatory disorders.
Acknowledgements
The authors would like to thank Dr. Douglas Weigent, Department of Physiology & Biophysics, University of Alabama at Birmingham for assistance with PCR. AG is funded through the Cystic Fibrosis Foundation (GAGGAR07A0). RJS is funded by the Wellcome Trust (082727/Z/07/Z). The UAB Mass Spectrometry Shared Facility is funded through the NIH (RR19231, P30CA13148, P50AT00477, U54CA100949, P30AR050948 and P30DK740380). This project was supported by grants R01 HL07783, R01 HL090999 and R01 HL087824 (JEB) from the National Heart Lung and Blood Institute (NHLBI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Cunningham DF, O'Connor B. Proline specific peptidases. Biochim Biophys Acta. 1997;1343:160–186. doi: 10.1016/s0167-4838(97)00134-9. [DOI] [PubMed] [Google Scholar]
- Dresdner K, Barker LA, Orlowski M, Wilk S. Subcellular distribution of prolyl endopeptidase and cation-sensitive neutral endopeptidase in rabbit brain. J Neurochem. 1982;38:1151–1154. doi: 10.1111/j.1471-4159.1982.tb05362.x. [DOI] [PubMed] [Google Scholar]
- Fulop V, Bocskei Z, Polgar L. Prolyl oligopeptidase: an unusual beta-propeller domain regulates proteolysis. Cell. 1998;94:161–170. doi: 10.1016/s0092-8674(00)81416-6. [DOI] [PubMed] [Google Scholar]
- Gaggar A, Jackson PL, Noerager BD, O'Reilly PJ, McQuaid DB, Rowe SM, Clancy JP, Blalock JE. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol. 2008;180:5662–5669. doi: 10.4049/jimmunol.180.8.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggar A, Li Y, Weathington N, Winkler M, Kong M, Jackson P, Blalock JE, Clancy JP. Matrix metalloprotease-9 dysregulation in lower airway secretions of cystic fibrosis patients. Am J Physiol Lung Cell Mol Physiol. 2007;293:L96–L104. doi: 10.1152/ajplung.00492.2006. [DOI] [PubMed] [Google Scholar]
- Goossens F, De Meester I, Vanhoof G, Hendriks D, Vriend G, Scharpe S. The purification, characterization and analysis of primary and secondary-structure of prolyl oligopeptidase from human lymphocytes. Evidence that the enzyme belongs to the alpha/beta hydrolase fold family. Eur J Biochem. 1995;233:432–441. doi: 10.1111/j.1432-1033.1995.432_2.x. [DOI] [PubMed] [Google Scholar]
- Goossens F, De Meester I, Vanhoof G, Scharpe S. Distribution of prolyl oligopeptidase in human peripheral tissues and body fluids. Eur J Clin Chem Clin Biochem. 1996;34:17–22. doi: 10.1515/cclm.1996.34.1.17. [DOI] [PubMed] [Google Scholar]
- Haddox JL, Pfister RR, Muccio DD, Villain M, Sommers CI, Chaddha M, Anantharamaiah GM, Brouillette WJ, DeLucas LJ. Bioactivity of peptide analogs of the neutrophil chemoattractant, N-acetyl-proline-glycineproline. Invest Ophthalmol Vis Sci. 1999;40:2427–2429. [PubMed] [Google Scholar]
- Hardison MT, Galin FS, Calderon CE, Djekic UV, Parker SB, Wille KM, Jackson PL, Oster RA, Young KR, Blalock JE, Gaggar A. The presence of a matrix-derived neutrophil chemoattractant in bronchiolitis obliterans syndrome after lung transplantation. J Immunol. 2009;182:4423–4431. doi: 10.4049/jimmunol.0802457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasebe T, Hua J, Someya A, Morain P, Checler F, Nagaoka I. Involvement of cytosolic prolyl endopeptidase in degradation of p40-phox splice variant protein in myeloid cells. J Leukoc Biol. 2001;69:963–968. [PubMed] [Google Scholar]
- Ishino T, Ohtsuki S, Homma K, Natori S. cDNA cloning of mouse prolyl endopeptidase and its involvement in DNA synthesis by Swiss 3T3 cells. J Biochem. 1998;123:540–545. doi: 10.1093/oxfordjournals.jbchem.a021970. [DOI] [PubMed] [Google Scholar]
- Kalwant S, Porter AG. Purification and characterization of human brain prolyl endopeptidase. Biochem J. 1991;276(Pt 1):237–244. doi: 10.1042/bj2760237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamori M, Hagihara M, Nagatsu T, Iwata H, Miura T. Activities of dipeptidyl peptidase II, dipeptidyl peptidase IV, prolyl endopeptidase, and collagenase-like peptidase in synovial membrane from patients with rheumatoid arthritis and osteoarthritis. Biochem Med Metab Biol. 1991;45:154–160. doi: 10.1016/0885-4505(91)90016-e. [DOI] [PubMed] [Google Scholar]
- Kimura A, Yoshida I, Takagi N, Takahashi T. Structure and localization of the mouse prolyl oligopeptidase gene. J Biol Chem. 1999;274:24047–24053. doi: 10.1074/jbc.274.34.24047. [DOI] [PubMed] [Google Scholar]
- Knisatschek H, Bauer K. Characterization of “thyroliberin-deamidating enzyme” as a post-proline-cleaving enzyme. Partial purification and enzyme-chemical analysis of the enzyme from anterior pituitary tissue. J Biol Chem. 1979;254:10936–10943. [PubMed] [Google Scholar]
- Lin M, Jackson P, Tester AM, Diaconu E, Overall CM, Blalock JE, Pearlman E. Matrix metalloproteinase-8 facilitates neutrophil migration through the corneal stromal matrix by collagen degradation and production of the chemotactic peptide Pro-Gly-Pro. Am J Pathol. 2008;173:144–153. doi: 10.2353/ajpath.2008.080081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Goossens F, Scharpe S, Meltzer HY, D'Hondt P, Cosyns P. Lower serum prolyl endopeptidase enzyme activity in major depression: further evidence that peptidases play a role in the pathophysiology of depression. Biol Psychiatry. 1994;35:545–552. doi: 10.1016/0006-3223(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Mentlein R. Proline residues in the maturation and degradation of peptide hormones and neuropeptides. FEBS Lett. 1988;234:251–256. doi: 10.1016/0014-5793(88)80092-9. [DOI] [PubMed] [Google Scholar]
- Mizutani S, Sumi S, Suzuki O, Narita O, Tomoda Y. Post-proline endopeptidase in human placenta. Biochim Biophys Acta. 1984;786:113–117. doi: 10.1016/0167-4838(84)90161-4. [DOI] [PubMed] [Google Scholar]
- O'Leary RM, O'Connor B. Identification and localisation of a synaptosomal membrane prolyl endopeptidase from bovine brain. Eur J Biochem. 1995;227:277–283. doi: 10.1111/j.1432-1033.1995.tb20385.x. [DOI] [PubMed] [Google Scholar]
- O'Reilly P, Jackson PL, Noerager B, Parker S, Dransfield M, Gaggar A, Blalock JE. N-alpha-PGP and PGP, potential biomarkers and therapeutic targets for COPD. Respir Res. 2009;10:38. doi: 10.1186/1465-9921-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar L. The prolyl oligopeptidase family. Cell Mol Life Sci. 2002;59:349–362. doi: 10.1007/s00018-002-8427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt G, Hough R, Rechsteiner M. Proteolysis in heat-stressed HeLa cells. Stabilization of ubiquitin correlates with the loss of proline endopeptidase. J Biol Chem. 1989;264:12526–12532. [PubMed] [Google Scholar]
- Rossner S, Schulz I, Zeitschel U, Schliebs R, Bigl V, Demuth HU. Brain prolyl endopeptidase expression in aging, APP transgenic mice and Alzheimer's disease. Neurochem Res. 2005;30:695–702. doi: 10.1007/s11064-005-6863-y. [DOI] [PubMed] [Google Scholar]
- Schulz I, Zeitschel U, Rudolph T, Ruiz-Carrillo D, Rahfeld JU, Gerhartz B, Bigl V, Demuth HU, Rossner S. Subcellular localization suggests novel functions for prolyl endopeptidase in protein secretion. J Neurochem. 2005;94:970–979. doi: 10.1111/j.1471-4159.2005.03237.x. [DOI] [PubMed] [Google Scholar]
- Shariat-Madar Z, Mahdi F, Schmaier AH. Identification and characterization of prolylcarboxypeptidase as an endothelial cell prekallikrein activator. J Biol Chem. 2002;277:17962–17969. doi: 10.1074/jbc.M106101200. [DOI] [PubMed] [Google Scholar]
- Taylor WL, Dixon JE. Catabolism of neuropeptides by a brain proline endopeptidase. Biochem Biophys Res Commun. 1980;94:9–15. doi: 10.1016/s0006-291x(80)80179-3. [DOI] [PubMed] [Google Scholar]
- van Houwelingen AH, Weathington NM, Verweij V, Blalock JE, Nijkamp FP, Folkerts G. Induction of lung emphysema is prevented by Larginine-threonine-arginine. FASEB J. 2008;22:3403–3408. doi: 10.1096/fj.07-096230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- Welches WR, Brosnihan KB, Ferrario CM. A comparison of the properties and enzymatic activities of three angiotensin processing enzymes: angiotensin converting enzyme, prolyl endopeptidase and neutral endopeptidase 24.11. Life Sci. 1993;52:1461–1480. doi: 10.1016/0024-3205(93)90108-f. [DOI] [PubMed] [Google Scholar]
- Wilk S, Benuck M, Orlowski M, Marks N. Degradation of luteinizing hormone-releasing hormone (LHRH) by brain prolyl endopeptidase with release of des-glycinamide LHRH and glycinamide. Neurosci Lett. 1979;14:275–279. doi: 10.1016/0304-3940(79)96161-5. [DOI] [PubMed] [Google Scholar]