Abstract
As a key part of the innate immune system, complement plays an important role not only in defending invading pathogens but also in many other biological processes. Inappropriate or excessive activation of complement has been linked to many autoimmune, inflammatory, and neurodegenerative diseases, as well as ischemia-reperfusion injury and cancer. A wide array of low molecular weight complement inhibitors has been developed to target various components of the complement cascade. Their efficacy has been demonstrated in numerous in vitro and in vivo experiments. Though none of these inhibitors has reached the market so far, some of them have entered clinical trials and displayed promising results. This review provides a brief overview of the currently developed low molecular weight complement inhibitors, including short peptides and synthetic small molecules, with an emphasis on those targeting components C1 and C3, and the anaphylatoxin receptors.
Keywords: complement inhibitors, compstatin, C5aR antagonist, C3aR antagonist, PMX53, PMX205, SB290157
1. Introduction
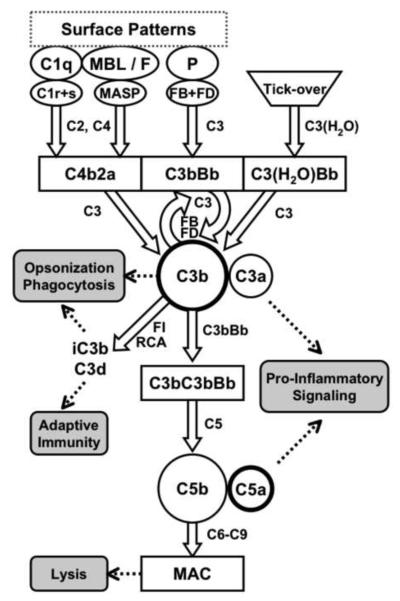
As a central component of the innate immune system, complement is a key player in the body's defense against invading microorganisms. In addition, it is also involved in the clearance of self-antigens and apoptotic cells (Flierman and Daha, 2007), forms a bridge to adaptive immunity, and plays an important role in inflammation (Barrington et al., 2001), tissue regeneration (DeAngelis et al., 2006), and tumor growth (Markiewski et al., 2008). For exercising these various functions, the complement cascade relies on a complex interplay of soluble and cell-surface-bound proteins. Traditionally, complement activation is described to be initiated through three major pathways (classical, lectin, and alternative pathways), which are all mainly based on the detection of surface structures by pattern recognition proteins (Fig. 1). The classical pathway (CP) is mainly activated when complement component C1q interacts with patches of surface-bound antibodies (IgM and IgG), but also with C-reactive protein, serum amyloid P, pentraxin 3, and other ligands on the surface of apoptotic or microbial cells (Gewurz et al., 1995; Marschang et al., 1997; Nauta et al., 2004; Paidassi et al., 2008; Steel and Whitehead, 1994; Warren et al., 2002). These interactions lead to sequential activation of the serine proteases C1r and C1s, which then cleave C4 and C2 to form the CP C3 convertase (C4bC2a). In the case of the lectin pathway (LP), the same C3 convertase is generated by mannose-binding lectin (MBL)-associated serine proteases (MASPs) in response to the binding of MBL or ficolins to a wide array of carbohydrate structures on microbial, apoptotic, or necrotic cell surfaces (Jensen et al., 2007; Kuraya et al., 2005). The MASPs that cleave C4 and C2 in the LP are structurally analogous to C1r and C1s in the CP (Gal et al., 2007).
Fig. 1.
Simplified representation of the complement cascade. Pattern-recognition receptors such as C1q, MBL, ficolins (F), or properdin (P) bind to surface patches on microbial or apoptotic cells and initiate the formation of C3 convertase complexes, which all cleave C3 into C3a and C3b. In addition, low levels of C3b are constantly produced via spontaneous hydrolysis of C3 (tick-over). Opsonization with C3b leads to the local generation of additional C3 convertases (self-amplification) and the C5 convertase. In the terminal pathway, this C5 convertase cleaves C5 into the potent anaphylatoxin C5a and into C5b, which forms the lytic membrane attack complex (MAC) pores. Regulators of complement activation (RCA) modulate convertase activity and enable factor I (FI) to degrade C3b into fragments iC3b and C3d, which participate in signaling and link to adaptive immune responses.
The alternative pathway (AP) not only represents an individual recognition pathway, but also serves as an amplification loop of the classical and lectin pathways. In vivo studies have shown that the AP alone can contribute to >80% of the total activation induced by either pathways (Harboe and Mollnes, 2008). There is a constant low-level activation of the alternative pathway as a result of the spontaneous hydrolysis of C3 to C3(H2O), which is called “tick-over”. This hydrolysis allows the formation of an initial AP C3 convertase (C3(H2O)Bb) in the presence of the two serine proteases factor B (FB) and factor D (FD). The C3 convertases from all pathways cleave native C3 into its two active fragments, the anaphylatoxin C3a and the opsonin C3b, the latter of which gets covalently attached to nearby surfaces. In the absence of complement regulators, surface-deposited C3b can be gradually amplified through the formation of the final AP C3 convertase (C3bBb) and cleavage of more C3. The AP can also be triggered directly by properdin, various proteins, lipids, and carbohydrate structures on foreign surfaces (Harboe et al., 2006; Kimura et al., 2008; Spitzer et al., 2007; Xu et al., 2008). C3b can be either degraded into fragments iC3b and C3d that cannot participate in convertase formation any longer but harbor important signaling functions, or it can initiate the formation of C5 convertases in both the CP/LP (C3bC4bC2a) or the AP (C3bC3bBb). These C5 convertases cleave C5 into the potent anaphylatoxin C5a and into C5b, thereby forming a membrane attack complex (MAC; C5b-9), which can lyse the target cells or foreign microorganisms (Fig. 1).
In addition to these three major activation mechanisms, there are several bypass routes that have been shown to directly trigger complement response at various stages. For example, extrinsic proteases such as thrombin or kallikrein are able to cleave and activate C3, which may indicate cross-talks with other systems such as the coagulation cascade (Markiewski et al., 2007; Ricklin and Lambris, 2007). IgM has been reported to be able to bind to MBL and directly activate the lectin pathway (McMullen et al., 2006; Zhang et al., 2006). Furthermore, C2/C4 can be bypassed in the case of complement activation induced by antibodies or oligosaccharides (Selander et al., 2006; Wagner et al., 1999). Similarly, C5 can be cleaved by thrombin, bypassing C3 (Ganter et al., 2007; Huber-Lang et al., 2006; Huber-Lang et al., 2002). Silica and asbestos fibers have been reported to be able to cleave C5 via mechanisms involving free radical generation and kallikrein activation (Governa et al., 2005; Governa et al., 2000; Governa et al., 2002). In addition, C3a and C5a can be generated directly from C3 and C5 by proteases found in the allergenic feces produced by dust mites (Maruo et al., 1997).
Complement is nonspecific in that it can attack both foreign invaders and host cells. Under normal conditions, host cells are protected from potential complement-mediated damage by various fluid-phase and membrane-bound complement regulatory proteins, including C1 inhibitor (C1-Inh), C4b-binding protein (C4BP), factor H (FH), complement receptor 1 (CR1; CD35), complement receptor Ig (CRIg), decay accelerating factor (DAF; CD55), membrane cofactor protein (MCP; CD46), and CD59 (Holers, 2008; Mollnes and Kirschfink, 2006). However, deficiencies of these protective components or excessive activation of complement in response to certain pathological conditions can overwhelm this protective mechanism. Such unbalanced activation has been associated with a growing number of diseases and pathological disorders (Mollnes and Kirschfink, 2006; Sjoholm et al., 2006). In addition, the anaphylatoxin C5a has been shown to contribute to tumor growth in mice (Markiewski et al., 2008). In these situations in which complement has deleterious effects, it is desirable to modulate its activation by using appropriate complement inhibitors (Ricklin and Lambris, 2007). The effectiveness of such therapeutic interventions has been demonstrated in numerous preclinical and clinical studies using both biopharmaceuticals (e.g. recombinant proteins and antibodies) and small complement inhibitors (Table 1). Furthermore, the U.S. Food and Drug Administration (FDA) recently approved human C1-Inh (Cinryze, ViroPharma) for hereditary angioedema (Cocchio and Marzella, 2009) and eculizumab (Soliris, Alexion Pharmaceuticals), a humanized anti-C5 monoclonal antibody for the treatment of paroxysmal nocturnal hemoglobinuria caused by an absence of the glycophosphatidylinositol-anchored complement regulatory proteins DAF/CD55 and CD59 (Inoue et al., 2003; Rother et al., 2007).
Table 1.
Examples of low molecular weight complement inhibitors used disease models
| Compound | Target | Conditions studied | Model |
|---|---|---|---|
| Compstatin | C3 | Transplantation | Human islet, in vitro (Tjernberg et al., 2008) Pig kidney, ex vivo (Fiane et al., 1999) |
| Bioincompatibility | Artificial surface-induced, in vitro (Lappegard et al., 2008; Lappegard et al., 2005; Nilsson et al., 1998; Schmidt et al., 2003) |
||
| Inflammation | E. coli-induced, in vitro (Mollnes et al., 2002) Heparin/protamine complex-induced, baboon (Soulika et al., 2000) |
||
| Age-related macular degeneration | Rabbit, monkey (Francois et al., 2009) | ||
| SB290157 | C3aR | Lung inflammation | Guinea pig, LPS-induced (Ames et al., 2001) |
| Arthritis | Rat, adjuvant-induced (Ames et al., 2001) | ||
| Acute respiratory distress syndrome (ARDS) |
Rat, cobra venom factor-induced (Proctor et al., 2006) |
||
| Allergic asthma | Mouse (Baelder et al., 2005) | ||
| Lupus nephritis | Mouse (Bao et al., 2005a) | ||
| I/R injury | Mouse, focal cerebral (Ducruet et al., 2008) Rat, intestinal (Proctor et al., 2004) |
||
| PMX53 | C5aR | Inflammatory bowel disease (IBD) | Rat, TNBS-induced (Woodruff et al., 2005) |
| ARDS | Rat, cobra venom factor-induced (Proctor et al., 2006) |
||
| Sepsis | Mouse, cecal ligation/puncture (Huber-Lang et al., 2002b) |
||
| Multiple organ injury | Rat, ruptured abdominal aortic aneurysm (Harkin et al., 2004) |
||
| Inflammatory pain | Rat, mouse (Ting et al., 2008) | ||
| Lupus nephritis | Mouse (Bao et al., 2005b) | ||
| Huntington's disease | Rat, 3-nitropropionic acid-induced (Woodruff et al., 2006) | ||
| Tumor growth | Mouse (Markiewski et al., 2008) | ||
| I/R injury | Rat, hepatic (Arumugam et al., 2004) Rat, renal (Arumugam et al., 2003) Rat, intestinal (Proctor et al., 2004) |
||
| PMX205 | C5aR | IBD | Rat, TNBS-induced (Woodruff et al., 2005) |
| Huntington's disease | Rat, 3-nitropropionic acid-induced (Woodruff et al., 2006) | ||
| Alzheimer's disease | Mouse (Fonseca et al., 2009) | ||
| C089 | C5aR | Allergic asthma | Rat (Abe et al., 2001) |
| Thrombotic glomerulonephritis | Rat (Kondo et al., 2001) | ||
| JPE1375 | C5aR | Renal allograft transplantation | Mouse (Gueler et al., 2008) |
| Tubulointerstitial fibrosis | Mouse (Boor et al., 2007) | ||
| C1s-INH-248 | C1s | I/R injury | Rabbit, myocardial (Buerke et al., 2001) |
Thus, complement inhibitors are not only needed for the treatment of complement-related disorders but also as invaluable tools for understanding the roles played by key complement components in disease models. Whereas all the complement-inhibiting drugs in clinical use and the majority of those in trials represent large biotherapeutics (Ricklin and Lambris, 2007), there is an urgent need for low molecular weight complement inhibitors that are therapeutically effective. Despite their large efficacy and many advantages, protein drugs generally have several drawbacks: They are often expensive to produce, difficult to formulate, potentially immunogenic, and their oral bioavailability and tissue penetration are often poor. Thus, to date, these drawbacks have limited the full potential of complement inhibitors. For example, the failure of the anti-C5 mAb pexelizumab (Alexion Pharmaceuticals) use for the treatment of acute myocardial infarction may have been partly caused by its poor tissue penetration (APEX AMI Investigators et al., 2007).
In contrast to protein inhibitors, low molecular weight drugs do not suffer from these disadvantages, and therefore they hold promise as candidates for the treatment of acute as well as chronic diseases associated with inappropriate or excessive complement activation. A large number of low molecular weight compounds have been reported to be capable of inhibiting complement; these early inhibitor candidates have been extensively reviewed in the past (Asghar, 1984; Lambris et al., 1993; Makrides, 1998). However, most of these inhibitors have proved to be plagued by a variety of problems, including poor selectivity, high toxicity, low potency, and short half-life, and will not be discussed here. Instead, this review will focus on the development of more recent low molecular weight (under 2 kDa) complement inhibitors, including small molecules, peptides, and peptidomimetics that target key complement proteins, proteases, and anaphylatoxin receptors.
2. Inhibitors targeting complement protein-protein interactions
Compared with many other pathways, the proper function of the complement cascade seems to rely on an exceptionally large number of protein-protein interactions. Despite some promising efforts, the inhibition of such protein-protein interactions using low molecular weight drugs is still a challenging endeavor (Wells and McClendon, 2007). The interaction interfaces are usually much larger compared to e.g. the pocket of enzymes, and amino acid residues involved in such interactions are often not contiguous. In addition, the contact surfaces are usually shallow and lack any grooves that would enable tight binding of small compounds. It is telling, therefore, that all the physiological complement regulators, including the protease inhibitor C1-Inh, are relatively large proteins. Despite this challenge, use of low molecular weight compounds is a valid and promising approach to regulate complement activation, as shown by the discovery of short peptides that can selectively inhibit the normal functions of C1q and C3.
2.1. C1q-selective inhibitors
The classical pathway has been identified as the major complement activation mechanism in pathological conditions such as hyperacute xenograft rejection (Platt, 1996). Inhibiting the hexameric pattern-recognition molecule C1q can effectively control classical pathway activation at its earliest stages, while leaving the lectin and alternative pathways intact to fight invading pathogens. Both small molecules and short peptides have been identified that can inhibit C1q-antibody interactions without activating the classical pathway. However, many of the small molecule inhibitors that have been reported thus far, such as derivatives of bisphenol disulfates (Bureeva et al., 2005), steroids and triterpenoids (Bureeva et al., 2007), have generally had a low potency against complement. In addition, there are concerns about their safety, pharmacokinetics, and selectivity (Assefa et al., 1999; Assefa et al., 2001; Dinkova-Kostova et al., 2005; Roos et al., 2002). Despite these obstacles, a novel class of peptide-based inhibitors has emerged in recent years and brought new momentum to this substance group.
The cyclic peptide 2J ([CEGPFGPRHDLTFC]W), selected from a phage-displayed library, was reported to bind to the globular head domains of C1q and inhibit its interaction with IgG in a dose-dependent fashion. The disulfide bond in peptide 2J has been shown to be necessary for this activity. Binding of peptide 2J did not lead to activation of C1q, probably because of the monomeric nature of this interaction, as opposed to the multimeric binding that is exhibited by surface-deposited antibody complexes. In the study, peptide 2J showed activity across a wide range of species, including humans, chimpanzees, monkeys, rats, and mice, with an IC50 of 2-6 μM (Roos et al., 2001). Its efficacy has been demonstrated by the fact that it can significantly reduce C3 and C4 deposition onto pig cells exposed to human serum. Peptide 2J was recently found to share homology (GXFGXXXDXXXC) with human beta-defensin 2 (LPGVFGGIGDPVTCL), which has also been reported to interact with C1q and inhibit complement activation via the classical pathway, but not the alternative pathway (Bhat et al., 2007). A molecular modeling study has shown that the proposed consensus binding site appears on the same side of the peptide 2J and human beta-defensin 2 helix displays. The binding of this consensus sequence probably involves a hydrophilic interaction between the aspartic acid residue in the peptides and an arginine residue in the C1q binding site (Comis and Easterbrook-Smith, 1985). Peptide 2J could be a promising inhibitor candidate considering its ability to bind to both murine and human C1q. However, follow-up studies with the inhibitor have not been reported so far.
2.2. C3-specific inhibitors
In many pathological situations in which complement plays a critical role, several complement pathways are activated at the same time (Harboe and Mollnes, 2008). In these cases, damage to the host tissue can be caused directly or indirectly by the generation of opsonins, the MAC, and anaphylatoxins, regardless of the initiator (Fig. 1) (Lucchesi and Tanhehco, 2000). Therefore, an intervention at the central level of C3 is an attractive strategy because this approach can effectively modulate the production of all the critical complement mediators. In fact, more than half of the naturally occurring complement regulatory proteins that have been identified (FH, CR1, CRIg, DAF, and MCP) inhibit at the C3 level. As a consequence, C3 inhibitors can offer great therapeutic benefits with regard to many diseases, including AMD, in which a large variety of complement components are involved in the disease pathogenesis (Gehrs et al., 2006; Ricklin and Lambris, 2008).
Compstatin, a 13-residue cyclic peptide (H-I[CVVQDWGHHRC]T-NH2) discovered by screening a phage peptide library, is able to selectively bind to primate C3 (and its C3b and C3c fragments) and inhibit the cleavage of C3 by both the AP and CP C3 convertases (Ricklin and Lambris, 2008; Sahu et al., 1996). The recently determined co-crystal structure of fragment C3c in complex with a compstatin derivative (Ac-I[CVWQDWGAHRC]T-NH2) has revealed that the binding site is located on a shallow groove between domains MG4 and MG5 of the β-chain of C3c and is far distant from the C3 convertase cleavage site (Janssen et al., 2007). This co-crystal structure is considered to closely resemble binding of the compstatin analogue to intact C3 because of a lack of conformational differences in the binding domains between C3 and C3c. As the MG4/5 domains are likely to be involved in the initial binding of C3 to the C3 convertase, it has been hypothesized that binding of compstatin may sterically hinder this essential interaction and thereby prevent the cleavage of C3 (Ricklin and Lambris, 2008; Rooijakkers et al., 2009). The fact that compstatin is more efficacious in inhibiting the alternative pathway than the classical pathway is in agreement with this proposed mechanism, since it binds to both C3 and C3b in the AP convertase C3bBb but cannot bind to the CP C3 convertase that involves C4b. Other inhibitors of the alternative pathway that bind to the same side on C3b, such as CRIg (Wiesmann et al., 2006) or the anti-C3b mAb S77 (Katschke et al., 2009) are likely to act in a similar way as compstatin.
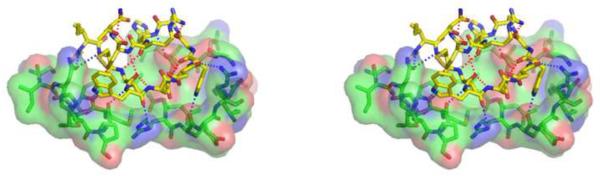
The structure-activity relationships (SAR) relating to compstatin have been discussed in great details elsewhere (Holland et al., 2004; Katragadda et al., 2006; Magotti et al., 2009; Mastellos et al., 2004; Ricklin and Lambris, 2008), and the present review will therefore focus on the most recent developments in this area. The bound conformation of the compstatin derivative that was revealed by co-crystallization proved to be clearly distinct from that seen for the average free conformation of compstatin in solution, as determined by NMR. The observed difference suggests a major conformational change upon peptide binding to C3c that involves a shift in the essential beta-turn (Janssen et al., 2007; Morikis et al., 1998). The bound conformation is stabilized by factors including a Cyc2-to-Cyc12 disulfide bond, a beta-turn encompassing Gly8 and Arg11, several intramolecular polar interactions (i.e. Arg11-Gly8, Val3-His10, Val3-Gln5/Cys12, Gln5-Try7), and a sulfur-aromatic interaction between Cys12 and Trp4. This conformation is also optimized for forming a tight intermolecular contact interface to the shallow pocket on the C3c surface via hydrophobic (side chains of residues 3, 4, 7, and 10) and polar (Acetyl, and residues 1, 2, 4, 5, 6, 7, and 10) interactions with C3c (Fig. 2).
Fig. 2.
Stereo representation of the polar interactions within the complex between C3c and a compstatin derivative. Red dotted lines indicate intramolecular contacts within the compstatin derivative and blue dotted lines represent intermolecular contacts between the compstatin derivative and C3c. Only C3c residues within 5 Å of the compstatin derivative are shown. The figure was prepared from PDB file 2QKI (Janssen et al., 2007) by using PyMOL (www.pymol.org).
The potency of compstatin was improved by 264-fold by using a combination of rational ligand-based design, molecular modeling, and biophysical studies even before the co-crystal structure had been obtained (Katragadda et al., 2006). The biggest improvement came from substitution of Val4 for Trp(Me)4. Val4 was identified earlier to be exchangeable in an alanine scan (Morikis et al., 1998). The Trp4 substitution established stronger hydrophobic interactions between the peptide and C3c, according to the crystal structure. Methylation of the Trp4 indole nitrogen further strengthened this hydrophobic interaction, as evidenced by the smaller entropy penalty (−TΔS=6.94 kcal/mol) and slower dissociation rate (koff=0.011 s−1) in the [Trp(Me)4]-Ac-compstatin analogue than in the [Trp4]-Ac-compstatin analogue (−TΔS=8.79 kcal/mol, koff=0.134 s−1) (Katragadda et al., 2006; Magotti et al., 2009). More recently, we have developed a novel compstatin analogue with a more than two-fold increase in potency over the [Trp(Me)4]-Ac-compstatin (manuscript in preparation). Thus, potent compstatin analogues are now available for research and clinical applications.
Compstatin derivatives have been shown to be safe and effective in a series of ex vivo and in vivo experiments (Table 1). Recently, a compstatin derivative successfully completed a phase I clinical trial under the name POT-4 (Potentia Pharmaceuticals, Inc.) for the treatment of AMD. Interestingly, compstatin analogues form a gel-like deposit in the eye after intravitreal injection, from which the active peptide is slowly released over time (Francois et al., 2009). This distinct pharmacokinetic behavior may prove highly advantageous for the treatment of chronic eye diseases like AMD and is expected to reduce the frequency of intravitreal injections. Based on other experimental data, compstatin derivatives also hold great promise for the treatment of acute phase disorders from biomaterial incompatibility and autoimmune diseases such as rheumatoid arthritis (Table 1) (Ricklin and Lambris, 2007; Ricklin and Lambris, 2008).
3. Inhibitors targeting serine proteases
The complement cascade contains a series of serine proteases, which are involved in pathway initiation (C1r, C1s, MASPs, C2), amplification (FB, FD) and regulation (FI) (Sim and Tsiftsoglou, 2004). From a pharmaceutical standpoint, proteases represent suitable targets for low molecular weight inhibitors because of the small size and distinct shape of their catalytic sites. Due to this high druggability and their central involvement in the complement cascade, serine proteases have been among the earliest targets for developing complement-specific therapeutics. However, the large number of structurally similar catalytic domains of serine proteases in complement and other systems makes it often difficult to discover inhibitors that show a sufficiently high selectivity for a particular complement protease. Futhan (FUT-175, Nafamostat) is a well-known example of such broad-spectrum, small molecule serine protease inhibitors, which inhibits many systems such as pancreatic or coagulation enzymes but also the classical and alternative pathways of complement activation (Schwertz et al., 2008). Although a number of low molecular weight complement protease inhibitors have been reported to be selective, none have been widely used for in vivo studies so far.
3.1. C1s inhibitors
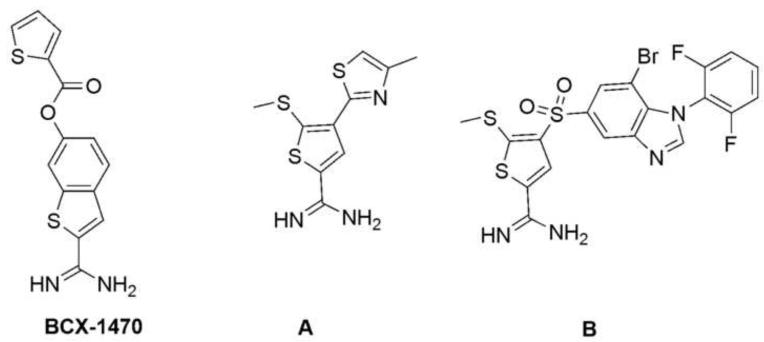
C1s is an 80-kDa serine protease that mediates the proteolytic activity of the C1 complex by cleaving both C2 and C4. BCX-1470, initially discovered in 1997 as a FD inhibitor with an IC50 of 96 nM, turned out to be a very potent C1s inhibitor, with an IC50 of 1.6 nM (Fig. 3) (Makrides, 1998; Szalai et al., 2000). BCX-1470 has also been tested for safety in clinical trials with healthy volunteers but the outcome of these trials have not officially been published (Morikis and Lambris, 2002; Pugsley et al., 2003). Whereas no further clinical trials have been reported, a new patent may indicate a future use of the compound in the treatment of AMD (Romano, 2008). More recently, a novel type of small molecule C1s inhibitor has been reported (Subasinghe et al., 2004; Subasinghe et al., 2006; Travins et al., 2008). A lead compound (Fig. 3; molecule A, Ki=3.5 μM) featuring a thiopheneamidine motif has been discovered by screening small molecule libraries (Subasinghe et al., 2004). The docking of this lead compound into a crystal structure of the catalytic domain of C1s indicated that the amidine moiety forms a salt bridge with Asp611 of C1s, and the thiophene moiety fully occupies the S1 binding pocket. The 5-methylthio substituent on the thiophene ring was found to be important for effective binding. Furthermore, a 4-arylsulfonyl group was found to confer better selectivity as a result of a hydrogen bond formed between the sulfonyl group and Lys614, which sits at the entrance to the C1s binding pocket. Over the past few years, the affinity of the lead compound has been significantly improved, from the micromolar range to the nanomolar range, by optimizing the 4-arylsulfonyl group. Compound B (Fig. 3, Ki=10 nM) is the most potent C1s-selective inhibitor reported thus far, with good selectivity over other proteases such as urokinase-type plasminogen activator, tissue plasminogen activator, Factor Xa, thrombin, and plasmin. However, in vivo data are still lacking, and there is concern about the possible in vivo toxicity of the thiophene moiety (Treiber et al., 1997).
Fig. 3.
Examples of small molecule C1s inhibitors. Whereas the potent inhibitor of C1s (and FD) BCX-1470 (left) has been tested in clinical trials, a novel class of compounds based on the lead structure A (middle) is being developed and produced an improved inhibitor (B; right) with specificity and affinity for C1s.
Development of a peptidomimetic C1s inhibitor, C1s-INH-248 (M.W. 520.5), was based on the thrombin inhibitor D-Phe-Pro-Arg. This inhibitor was reported to be very potent against human and rabbit C1s (IC50 of 2 nM and 0.7 nM, respectively) (Buerke et al., 2001). It showed over 1000-fold higher selectivity for C1s compared to C1r, MASP-1, thrombin, and other related serine proteases such as plasma kallikrein or factors XIa and XIIa, and it did not inhibit the lectin pathway. Its cardioprotective effects have been demonstrated in a rabbit model of ischemia-reperfusion injury (Buerke et al., 2006; Buerke et al., 2001). Despite these positive indicators, however, C1s-INH-248 may have a short half-life in vivo, and its structure has not been disclosed (Ricklin and Lambris, 2007).
3.2. Inhibitors of FB and C2
Despite its central involvement in both the AP C3 and C5 convertases, only little progress has been reported on designing small inhibitors of FB. Truncation of the complement C2 receptor inhibitor trispanning (CRIT), a regulator that is e.g. found in human hemopoietic cells and some parasites, derived a 11-residue peptide (CRIT-H17; HEVKIKHFSPY), which was reported to bind FB, inhibit its FD-mediated cleavage, and reduce complement activation in a hemolysis assay (Inal et al., 2005). Furthermore, a short peptide (Ac-SHLGLAR-H) was recently described to be able to bind to the catalytic domain of human FB and inhibit FB-mediated C3 cleavage reversibly in a dose-dependent manner, with an IC50 of 19 μM (Le et al., 2007). It also inhibited the enzymatic activity of the C3 convertase C3bBb when the general serine protease inhibitors leupeptin and phenylmethylsulfonyl fluoride did not. Its IC50 value for the alternative pathway was determined to be 15 μM. This peptide was designed on the basis of C3 sequence at the cleavage site of FB. The aldehyde group was designed to react with the catalytic serine side-chain hydroxyl group to form a tetrahedral intermediate, thus achieving competitive inhibition. A serine is preferred in position 1 over charged or hydrophobic residues. In addition, peptides shorter than seven residues were suggested to be poor substrates of FB and therefore less potent. Unfortunately, this peptide also inhibited trypsin (IC50=0.7 μM) and thrombin (IC50=24 μM). It may also have C3a-like activity because it shares the same sequence with the C-terminus of C3a (SHLGLAR).
Very recently, the same authors have published that the same peptide (Ac-SHLGLAR-H) also inhibits the enzymatic activity of complement component C2, which is structurally similar to FB, with an IC50 of 4.2 μM. They also reported a hexapeptide inhibitor (Ac-RLLLAR-H) with increased potency of C2 inhibition (IC50 = 0.33 μM) (Halili et al., 2009). Given the similarities with the FB-inhibiting peptide described above, this new C2 inhibitor may share similar potential drawbacks and nothing is reported on the specificity of the new hexapeptide so far.
4. Inhibitors targeting anaphylatoxin receptors
The complement system comprises three known anaphylatoxin receptors, i.e. C3aR, C5aR and C5L2, which all belong to the family of G protein-coupled receptors (GPCR) and represent potential targets for pharmaceutical intervention. Whereas the functions of the C3aR and C5aR have been extensively and conclusively described, it is still a matter of debate whether C5L2 is a decoy receptor or functional receptor (Lee et al., 2008). While C3a and C5a bind to C3aR and C5aR, respectively, with high affinity, they are quickly degraded to C3a-desArg and C5a-desArg that have greatly reduced potency. However, both C5a and C5a-desArg bind C5L2 with affinity in the nanomolar range (Cain and Monk, 2002). C3a and C5a are potent inflammatory mediators with distinct hemodynamic effects (Proctor et al., 2009). Targeting anaphylatoxin receptors can effectively regulate complement-associated downstream signaling stimulated by C3a and C5a while preserving other important early-stage complement functions, such as opsonization and MAC formation. While a selective low molecular weight inhibitor of C5L2 is yet to be discovered for receptor functional studies, a variety of small and promising antagonists for C3aR and C5aR have been developed and used in many disease models (Table 1).
4.1. Antagonists of the C3a receptor (C3aR)
Release of C3a can lead to a wide range of cellular responses, including contraction of smooth muscle, chemotaxis, increases in vascular permeability, and the activation of leukocytes, such as macrophages, mast cells, and eosinophils (Gerard and Gerard, 2002; Hawlisch et al., 2004). Data from in vivo experiments have indicated that C3aR plays an important role in inflammatory pulmonary diseases and ischemia/reperfusion injury of the brain (Gerard and Gerard, 2002; Hawlisch et al., 2004; Mocco et al., 2006). It should be noted, though, that novel functions have been reported for C3a in recent years, some of which are not necessarily mediated by C3aR (Honczarenko et al., 2005; Jinsmaa et al., 2000; Nordahl et al., 2004; Ohinata and Yoshikawa, 2008; Ratajczak et al., 2004). Furthermore, C3a, along with other cationic amphiphilic neuropeptides, has been shown to be able to activate G protein independent of the C3aR, via sialic acid residues displayed on the surface of the cell membrane (Emadi-Khiav et al., 1995).
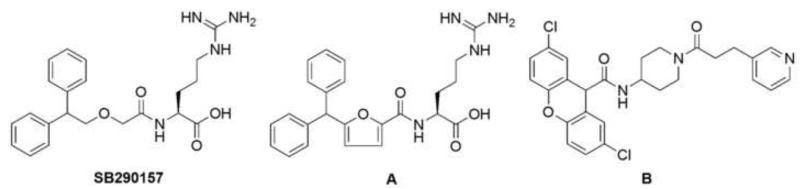
Currently available C3aR antagonists are built on two different types of scaffold: The first type has an arginine moiety (Fig. 4, SB290157, molecule A), while the second one features an amino-piperidine linker and a pyridine moiety (Fig. 4, molecule B). SB290157 (IC50 = 200 nM) was developed in 2001 by using library screening and chemical optimization (Ames et al., 2001). This compound was able to inhibit C3a-induced calcium mobilization in C3aR-expressing rat basophilic leukemia cells and human neutrophils (IC50 of 27.7 and 28 nM, respectively), C3a-induced receptor internalization in human neutrophils, C3a-mediated ATP release from guinea pig platelets, and C3a-induced potentiation of the contractile response to field stimulation of perfused rat caudal artery. Its in vivo therapeutic efficacy has been demonstrated in several disease models (Table 1). However, Mathieu and coworkers demonstrated in 2005 that SB290157 had potent agonist activities in a variety of assays involving different cell types that overexpress C3aR (Mathieu et al., 2005). The authors concluded that SB290157 was a partial agonist that displays antagonist activity only in systems with very low receptor densities. Compound A (Fig. 4, pIC50=7.2) is a modification of SB290157 (Denonne et al., 2007b); it has a higher affinity because of its more rigid linker. SAR studies have shown that the arginine residue and the ether oxygen are essential for strong binding. The aryl substitution has dramatic effects on affinity as well as functional selectivity. However, both antagonists have low bioavailability and a short in vivo half-life because of the presence of the arginine moiety. Therefore, further optimization is clearly warranted.
Fig. 4.
Structures of C3aR antagonists. SB290157 (left) and its optimized derivative (A; middle) represent antagonists that contain an arginine moiety, whereas molecule B (right) follows a different structural concept that includes a pyridine moiety and an amino-piperidine linker.
Compound B (Fig. 4, pIC50=5.8) was discovered by small molecule library screening (Denonne et al., 2007a). It was the first C3aR antagonist without an arginine moiety. However, attempts to obtain more potent analogues with antagonist activity have not been successful so far. Although modifications at the xanthene or pyridine groups have provided analogues with higher affinity, these compounds act as agonists of the C3aR. In addition, in vivo data have shown that compound B and its analogues have a very poor drug metabolism and pharmacokinetic profile, probably because of the amino-piperidine core.
4.2. Antagonists of the C5a receptor (C5aR)
C5aR, along with its native agonist C5a, modulates a wide variety of important cell-dependent activities, including phagocytosis, degranulation, chemotaxis, peroxide production, granule enzyme release, vasodilation, and cellular apoptosis (Lee et al., 2008). Numerous experiments have established the involvement of the C5aR in various inflammatory, autoimmune, and neurodegenerative disorders, in liver regeneration (DeAngelis et al., 2006), and in tumor growth (Markiewski et al., 2008; Monk et al., 2007). The development of low molecular weight C5aR antagonists was a hot topic even before the cloning of the C5aR in 1991 (Lee et al., 2008). Over the past decade, many small potent C5aR antagonists have been reported and used in various animal models as well as in humans (Table 1).
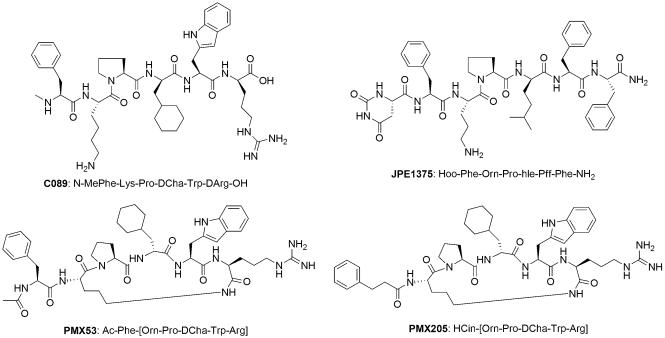
The most useful C5aR antagonists are peptidomimetics (Fig. 5), including cyclic PMX53 (Finch et al., 1999), PMX205 (March et al., 2004), and linear JPE1357 (Schnatbaum et al., 2006). All three peptides originated from C089 (Fig. 5), which was derived from the C-terminus of C5a and represented the first full C5aR antagonist (Konteatis et al., 1994). These antagonists all bind to the second binding site (effector site) of C5a at the transmembrane region of C5aR near the extracellular interface (Monk et al., 2007). Residues in positions 1, 4, and 6 are proposed to be important for binding, while position 5 is responsible for the antagonist activity. The sequence Pro-DXaa is critical for the preference for an important reverse turn structure, which is further stabilized by a lactam ring in the cyclic analogues PMX53 and PMX205 (March et al., 2004). It is interesting to note that the binding pocket can accommodate either a charged guanidinyl group or a hydrophobic phenyl group at the C-terminus of these peptide antagonists (Schnatbaum et al., 2006). N-terminal modification affects the antagonist activity to varying degrees, depending on the species (Woodruff et al., 2001). Both PMX53 and JPE1375 effectively inhibit the release of glucosaminidase from human polymorphonuclear neutrophils in vitro with similar IC50 values (31 nM and 41 nM, respectively). Both inhibitors are orally available and have good in vivo stability as a result of their peptidomimetic nature. Despite their functional similarities, the microsomal stability and receptor selectivity of JPE1375 are superior to those of PMX53. In addition, JPE1375 was 18 times more effective than PMX53 in blocking the chemotaxis of mouse J774A.1 cells (Schnatbaum et al., 2006).
Fig. 5.
Structures of the peptidomimetic C5aR antagonists PMX53, PMX205, JPE1375, and C089. Though all these antagonists were originally derived from a C-terminal peptide fragment of C5a, they have been optimized in different ways and are either linear (C089, JPE1379) or cyclic (PMX53, PMX205).
PMX53 has been shown to be safe and well tolerated in Phase I clinical trials for rheumatoid arthritis and psoriasis (Kohl, 2006). However, a Phase Ib trial of PMX53 in patients with active rheumatoid arthritis failed to show a reduction in synovial inflammation despite that a comparable serum level of PMX53 was shown to be able to block C5aR-mediated cell activation in vitro (Vergunst et al., 2007). Similarly, PMX53 administered intra-articularly or intravenously did not show measurable effects on rat acute synovitis induced by intra-articular antibody injection (Mizuno et al., 2000). Given the prevalence of AMD and the large involvement of complement in its etiology, both PMX53 and JPE1357 have been considered for the treatment of the disease. In the case of JPE1357, the recent closure of Jerini Ophthalmic (a subsidiary of Jerini targeting eye diseases) will likely affect the current development of the compound for the treatment of advanced stages of dry AMD. Whereas clinical trials of PMX53 for AMD have also been discontinued because of insufficient activity, ongoing pre-clinical studies of the compound for other indications will be continued according to Arana Therapeutics. PMX205 (Fig. 5), an analogue of PMX53 with a hydrocinamate moiety in the place of Ac-Phe1, demonstrated a much-improved pharmacokinetic profile and in vivo efficacy in rat inflammatory bowel disease (10- to 30-fold lower oral dose requirement) and neurodegeneration models (two-times better in crossing blood brain barrier), an improvement that was attributed to its resistance to intestinal metabolism and increased lipophilicity (Woodruff et al., 2006; Woodruff et al., 2005). Even though no clinical development of PMX205 has been announced so far, the compound showed beneficial effects concerning reduction of memory loss in a mouse model of Alzheimer's disease very recently (Fonseca et al., 2009).
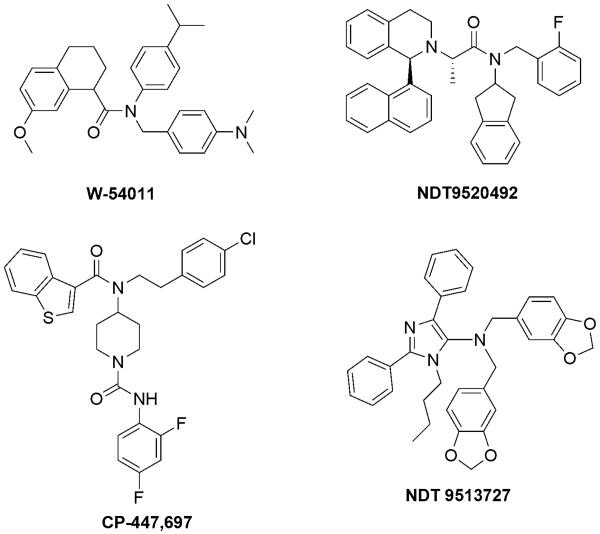
Many small molecule C5aR antagonists (Fig. 6) that are not based on peptides have been reported in the literature, including W-54011 (Sumichika et al., 2002), NDT9520492 (Waters et al., 2005), NGD 2000-1 (Lee et al., 2008), CP-447,697 (Blagg et al., 2008), and NDT 9513727 (Brodbeck et al., 2008). W-54011, a racemic compound discovered as a result of a high-capacity screening and chemical optimization, is the first potent competitive small molecule C5aR antagonist (IC50=2.2 nM) with good oral availability (Waters et al., 2005). It was shown to be effective in inhibiting C5a-induced calcium mobilization, chemotaxis, and generation of reactive oxygen species in human neutrophils. Pre-treatment of gerbils with W-54011 inhibited C5a-induced neutropenia. The presence of W-54011 almost completely abrogated the enhancement of platelet-neutrophil microaggregate formation and the redistribution of neutrophil P-selectin glycoprotein ligand-1 induced by haemodialysis membrane-treated plasma (Itoh et al., 2008). W-54011 also inhibited the production of cysteinyl-leukotrienes, which are important mediators involved in the pathophysiology of allergic asthma, in human lung tissue that was stimulated by C5a and human serum (Hama et al., 2007). However, preclinical studies have been complicated by the hydrophobicity of W-54011 and its narrow species specificity for primates and gerbils.
Fig. 6.
Structures of small molecule C5aR antagonists. In contrast to peptidomimetic antagonists, small molecules are often selected from compound libraries and further optimized, and therefore show a much larger variability concerning scaffolds.
Like W-54011, NDT9520492 is also species-specific for primates and gerbils (Waters et al., 2005). It was able to inhibit [35S]GTPγS binding to wild-type human C5aR with a Ki of 15.2 nM. NGD 2000-1 is structurally similar to NDT9520492 and did not show significant therapeutic effects in a Phase II asthma study (Monk et al., 2007). Although it showed slight improvement at a maximum dose of 100 mg in a Phase II trial for rheumatoid arthritis, it was also found to inhibit cytochrome P450 3A4. Therefore, further clinical trials were halted (Lee et al., 2008). Potent analogues based on CP-447,697 (IC50=31 nM) have recently been reported, but they have shown poor bioavailability and a short half-life (Blagg et al., 2008). NDT 9513727 is a novel competitive reverse agonist that was recently disclosed by Neurogen. In cell-based assays, it inhibits a wide range of C5a-induced functions, including [35S]GTPγS binding, calcium mobilization, chemotaxis, degranulation, oxidative burst, and CD11b cell-surface expression, with an IC50 of 1.1 to 9.2 nM. Studies in gerbils and cynomolgus macaques have demonstrated its ability to inhibit C5a-induced neutropenia, and it has proved to be highly selective for C5aR but not for C5L2 and more than 50 neurotransmitter and hormone receptors. In vivo experiments in rats and monkeys have also demonstrated that it is orally available, with a desirable pharmacokinetic profile. However, like other small molecule C5aR antagonists, it also shares the problem of narrow species selectivity and displayed only a rather moderate in vivo IC50 of 0.6 μM due to a very high plasma protein binding (>99%), likely as a result of its hydrophobicity (Brodbeck et al., 2008).
The species selectivity of peptide and small molecule C5aR antagonists is likely caused by the existence of differences in the binding sites and poor C5aR sequence homology among the various species (Waters et al., 2005). Results of receptor mutation studies have suggested that as compared to their peptide counterparts, small molecule antagonists bind more deeply within transmembrane domain V in gerbils, humans, and non-human primates, in which a Trp residue is conserved and critically involved in the binding. The lack of binding of small molecule C5aR antagonists in the case of mouse, rat, and dog is a result of the mutation of the Trp to Leu, Val, or Gly.
5. Perspectives
Complement has long been recognized as a valid and promising target for therapeutic intervention in a number of disease states. The confirmed involvement of complement in the etiology of prevalent diseases such as AMD, and the recent FDA approval of complement-targeting drugs (i.e. Soliris and Cinryze) have certainly fueled the efforts of developing complement drugs. Differential targeting of complement components is essential for the success of this endeavor, a challenge that is complicated by our limited understanding of the complement system and its complex functions and of the many disease mechanisms in which it is involved. In some situations, it is best to block anaphylatoxin receptors at a late stage of the complement cascade. In other situations, inhibition of a selective pathway at early stages, or at the C3 level, is required. Our increasing knowledge about the complement interaction network and the steady release of (co-)crystal structures of key complement components, such as FB (Janssen et al., 2009) or the C3 convertase (Rooijakkers et al., 2009), are likely to provide better tools for designing novel complement inhibitors.
Even though the quest for complement therapeutics began more than 25 years ago (Asghar, 1984), there is still a shortage of small complement inhibitors targeting different stages of the complement cascade. Peptides or peptidomimetics are among the most successful complement inhibitors because of their improved safety, selectivity, and accessibility. Their drawbacks, such as a short half-life, can be minimized through recent technological advancements in peptide optimization. Compstatin and C5aR antagonists are good examples of such efforts. Despite these achievements, however, more effort is clearly needed in terms of developing low molecular weight complement inhibitors that can selectively block the classical pathway, the alternative pathway, or C5L2 in vivo. The availability of a complete range of such inhibitors will greatly benefit our efforts to decipher the roles of complement in various complement-associated pathological conditions and the development of effective dugs for clinical applications.
ACKNOWLEDGEMENTS
We thank Deborah McClellan for editorial assistance. This work was supported by National Institutes of Health grants GM-069736, GM-62134, AI-30040, EB003968, CA112162 and AI-068730.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ames RS, Lee D, Foley JJ, Jurewicz AJ, Tornetta MA, Bautsch W, Settmacher B, Klos A, Erhard KF, Cousins RD, Sulpizio AC, Hieble JP, McCafferty G, Ward KW, Adams JL, Bondinell WE, Underwood DC, Osborn RR, Badger AM, Sarau HM. Identification of a selective nonpeptide antagonist of the anaphylatoxin C3a receptor that demonstrates antiinflammatory activity in animal models. J. Immunol. 2001;166:6341–6348. doi: 10.4049/jimmunol.166.10.6341. [DOI] [PubMed] [Google Scholar]
- APEX AMI Investigators. Armstrong PW, Granger CB, Adams PX, Hamm C, Holmes D, Jr., O'Neill WW, Todaro TG, Vahanian A, Van de Werf F. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. JAMA. 2007;297:43–51. doi: 10.1001/jama.297.1.43. [DOI] [PubMed] [Google Scholar]
- Asghar SS. Pharmacological manipulation of complement system. Pharmacol. Rev. 1984;36:223–244. [PubMed] [Google Scholar]
- Assefa H, Nimrod A, Walker L, Sindelar R. Synthesis and evaluation of potential complement inhibitory semisynthetic analogs of oleanolic acid. Bioorg. Med. Chem. Lett. 1999;9:1889–1894. doi: 10.1016/s0960-894x(99)00314-5. [DOI] [PubMed] [Google Scholar]
- Assefa H, Nimrod A, Walker L, Sindelar R. Enantioselective synthesis and complement inhibitory assay of A/B-ring partial analogues of oleanolic acid. Bioorg. Med. Chem. Lett. 2001;11:1619–1623. doi: 10.1016/s0960-894x(01)00210-4. [DOI] [PubMed] [Google Scholar]
- Barrington R, Zhang M, Fischer M, Carroll MC. The role of complement in inflammation and adaptive immunity. Immunol. Rev. 2001;180:5–15. doi: 10.1034/j.1600-065x.2001.1800101.x. [DOI] [PubMed] [Google Scholar]
- Bhat S, Song YH, Lawyer C, Milner SM. Modulation of the complement system by human beta-defensin 2. J. Burns Wounds. 2007;5:e10. [PMC free article] [PubMed] [Google Scholar]
- Blagg J, Mowbray C, Pryde DC, Salmon G, Schmid E, Fairman D, Beaumont K. Small, non-peptide C5a receptor antagonists: part 1. Bioorg. Med. Chem. Lett. 2008;18:5601–5604. doi: 10.1016/j.bmcl.2008.08.106. [DOI] [PubMed] [Google Scholar]
- Brodbeck RM, Cortright DN, Kieltyka AP, Yu J, Baltazar CO, Buck ME, Meade R, Maynard GD, Thurkauf A, Chien DS, Hutchison AJ, Krause JE. Identification and characterization of NDT 9513727 [N,N-bis(1,3-benzodioxol-5-ylmethyl)-1-butyl-2,4-diphenyl-1H-imidazole-5-methanam ine], a novel, orally bioavailable C5a receptor inverse agonist. J. Pharmacol. Exp. Ther. 2008;327:898–909. doi: 10.1124/jpet.108.141572. [DOI] [PubMed] [Google Scholar]
- Buerke M, Schwertz H, Langin T, Buerke U, Prondzinsky R, Platsch H, Richert J, Bomm S, Schmidt M, Hillen H, Lindemann S, Blaschke G, Muller-Werdan U, Werdan K. Proteome analysis of myocardial tissue following ischemia and reperfusion--effects of complement inhibition. Biochim. Biophys. Acta. 2006;1764:1536–1545. doi: 10.1016/j.bbapap.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Buerke M, Schwertz H, Seitz W, Meyer J, Darius H. Novel small molecule inhibitor of C1s exerts cardioprotective effects in ischemia-reperfusion injury in rabbits. J. Immunol. 2001;167:5375–5380. doi: 10.4049/jimmunol.167.9.5375. [DOI] [PubMed] [Google Scholar]
- Bureeva S, Andia-Pravdivy J, Petrov G, Igumnov M, Romanov S, Kolesnikova E, Kaplun A, Kozlov L. Inhibition of classical pathway of complement activation with negative charged derivatives of bisphenol A and bisphenol disulphates. Bioorg. Med. Chem. 2005;13:1045–52. doi: 10.1016/j.bmc.2004.11.056. [DOI] [PubMed] [Google Scholar]
- Bureeva S, Andia-Pravdivy J, Symon A, Bichucher A, Moskaleva V, Popenko V, Shpak A, Shvets V, Kozlov L, Kaplun A. Selective inhibition of the interaction of C1q with immunoglobulins and the classical pathway of complement activation by steroids and triterpenoids sulfates. Bioorg. Med. Chem. 2007;15:3489–98. doi: 10.1016/j.bmc.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Cain SA, Monk PN. The orphan receptor C5L2 has high affinity binding sites for complement fragments C5a and C5a des-Arg(74) J. Biol. Chem. 2002;277:7165–7169. doi: 10.1074/jbc.C100714200. [DOI] [PubMed] [Google Scholar]
- Cocchio C, Marzella N. Cinryze, a Human Plasma-Derived C1 Esterase Inhibitor for Prophylaxis Of Hereditary Angioedema. P T. 2009;34:293–328. [PMC free article] [PubMed] [Google Scholar]
- Comis A, Easterbrook-Smith SB. Evidence for arginine residues in the immunoglobulin-binding sites of human Clq. Biochim. Biophys. Acta. 1985;842:45–51. doi: 10.1016/0304-4165(85)90291-0. [DOI] [PubMed] [Google Scholar]
- DeAngelis RA, Markiewski MM, Lambris JD. Liver regeneration: a link to inflammation through complement. Adv. Exp. Med. Biol. 2006;586:17–34. doi: 10.1007/0-387-34134-X_2. [DOI] [PubMed] [Google Scholar]
- Denonne F, Binet S, Burton M, Collart P, Defays S, Dipesa A, Eckert M, Giannaras A, Kumar S, Levine B, Nicolas JM, Pasau P, Pegurier C, Preda D, Van houtvin N, Volosov A, Zou D. Discovery of new C3aR ligands. Part 2: amino-piperidine derivatives. Bioorg. Med. Chem. Lett. 2007a;17:3262–3265. doi: 10.1016/j.bmcl.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Denonne F, Binet S, Burton M, Collart P, Dipesa A, Ganguly T, Giannaras A, Kumar S, Lewis T, Maounis F, Nicolas JM, Mansley T, Pasau P, Preda D, Stebbins K, Volosov A, Zou D. Discovery of new C3aR ligands. Part 1: arginine derivatives. Bioorg. Med. Chem. Lett. 2007b;17:3258–3261. doi: 10.1016/j.bmcl.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Liby KT, Stephenson KK, Holtzclaw WD, Gao X, Suh N, Williams C, Risingsong R, Honda T, Gribble GW, Sporn MB, Talalay P. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc. Natl. Acad. Sci. U.S.A. 2005;102:4584–4589. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emadi-Khiav B, Mousli M, Bronner C, Landry Y. Human and rat cutaneous mast cells: involvement of a G protein in the response to peptidergic stimuli. Eur. J. Pharmacol. 1995;272:97–102. doi: 10.1016/0014-2999(94)00628-k. [DOI] [PubMed] [Google Scholar]
- Finch AM, Wong AK, Paczkowski NJ, Wadi SK, Craik DJ, Fairlie DP, Taylor SM. Low-molecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. J. Med. Chem. 1999;42:1965–1974. doi: 10.1021/jm9806594. [DOI] [PubMed] [Google Scholar]
- Flierman R, Daha MR. The clearance of apoptotic cells by complement. Immunobiology. 2007;212:363–70. doi: 10.1016/j.imbio.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Fonseca MI, Ager RR, Chu SH, Yazan O, Sanderson SD, LaFerla FM, Taylor SM, Woodruff TM, Tenner AJ. Treatment with a C5aR antagonist decreases pathology and enhances behavioral performance in murine models of Alzheimer's disease. J. Immunol. 2009;183:1375–1383. doi: 10.4049/jimmunol.0901005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois C, Deschatelets P, Olson P. Potentia Pharmaceuticals, Inc.; patent WO 2009046198 Sustained delivery of compstatin analogs from gels. 2009
- Gal P, Barna L, Kocsis A, Zavodszky P. Serine proteases of the classical and lectin pathways: similarities and differences. Immunobiology. 2007;212:267–277. doi: 10.1016/j.imbio.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Ganter MT, Brohi K, Cohen MJ, Shaffer LA, Walsh MC, Stahl GL, Pittet JF. Role of the alternative pathway in the early complement activation following major trauma. Shock. 2007;28:29–34. doi: 10.1097/shk.0b013e3180342439. [DOI] [PubMed] [Google Scholar]
- Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann. Med. 2006;38:450–71. doi: 10.1080/07853890600946724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard NP, Gerard C. Complement in allergy and asthma. Curr. Opin. Immunol. 2002;14:705–708. doi: 10.1016/s0952-7915(02)00410-7. [DOI] [PubMed] [Google Scholar]
- Gewurz H, Zhang XH, Lint TF. Structure and function of the pentraxins. Curr. Opin. Immunol. 1995;7:54–64. doi: 10.1016/0952-7915(95)80029-8. [DOI] [PubMed] [Google Scholar]
- Governa M, Amati M, Fenoglio I, Valentino M, Coloccini S, Bolognini L, Carlo Botta G, Emanuelli M, Pierella F, Volpe AR, Astolfi P, Carmignani M, Fubini B. Variability of biological effects of silicas: different degrees of activation of the fifth component of complement by amorphous silicas. Toxicol. Appl. Pharmacol. 2005;208:68–77. doi: 10.1016/j.taap.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Governa M, Amati M, Valentino M, Visona I, Fubini B, Botta GC, Volpe AR, Carmignani M. In vitro cleavage by asbestos fibers of the fifth component of human complement through free-radical generation and kallikrein activation. J. Toxicol. Environ. Health A. 2000;59:539–552. doi: 10.1080/009841000156664. [DOI] [PubMed] [Google Scholar]
- Governa M, Fenoglio I, Amati M, Valentino M, Bolognini L, Coloccini S, Volpe AR, Carmignani M, Fubini B. Cleavage of the fifth component of human complement and release of a split product with C5a-like activity by crystalline silica through free radical generation and kallikrein activation. Toxicol. Appl. Pharmacol. 2002;179:129–136. doi: 10.1006/taap.2002.9351. [DOI] [PubMed] [Google Scholar]
- Halili MA, Ruiz-Gomez G, Le GT, Abbenante G, Fairlie DP. Complement Component C2, Inhibiting a Latent Serine Protease in the Classical Pathway of Complement Activation. Biochemistry. 2009 doi: 10.1021/bi900679r. Epub, ahead of print. [DOI] [PubMed] [Google Scholar]
- Hama H, Ono N, Abe M. Study for an allergic inflammation model using human lungs and its pharmacological application. Yakugaku Zasshi. 2007;127:721–727. doi: 10.1248/yakushi.127.721. [DOI] [PubMed] [Google Scholar]
- Harboe M, Garred P, Borgen MS, Stahl GL, Roos A, Mollnes TE. Design of a complement mannose-binding lectin pathway-specific activation system applicable at low serum dilutions. Clin. Exp. Immunol. 2006;144:512–520. doi: 10.1111/j.1365-2249.2006.03072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M, Mollnes TE. The alternative complement pathway revisited. J. Cell. Mol. Med. 2008;12:1074–1084. doi: 10.1111/j.1582-4934.2008.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawlisch H, Wills-Karp M, Karp CL, Kohl J. The anaphylatoxins bridge innate and adaptive immune responses in allergic asthma. Mol. Immunol. 2004;41:123–131. doi: 10.1016/j.molimm.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Holers VM. The spectrum of complement alternative pathway-mediated diseases. Immunol. Rev. 2008;223:300–316. doi: 10.1111/j.1600-065X.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- Holland MC, Morikis D, Lambris JD. Synthetic small-molecule complement inhibitors. Curr. Opin. Investig. Drugs. 2004;5:1164–1173. [PubMed] [Google Scholar]
- Honczarenko M, Ratajczak MZ, Nicholson-Weller A, Silberstein LE. Complement C3a enhances CXCL12 (SDF-1)-mediated chemotaxis of bone marrow hematopoietic cells independently of C3a receptor. J. Immunol. 2005;175:3698–3706. doi: 10.4049/jimmunol.175.6.3698. [DOI] [PubMed] [Google Scholar]
- Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, Lambris JD, Warner RL, Flierl MA, Hoesel LM, Gebhard F, Younger JG, Drouin SM, Wetsel RA, Ward PA. Generation of C5a in the absence of C3: a new complement activation pathway. Nat. Med. 2006;12:682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- Huber-Lang M, Younkin EM, Sarma JV, Riedemann N, McGuire SR, Lu KT, Kunkel R, Younger JG, Zetoune FS, Ward PA. Generation of C5a by phagocytic cells. Am. J. Pathol. 2002;161:1849–1859. doi: 10.1016/S0002-9440(10)64461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inal JM, Hui KM, Miot S, Lange S, Ramirez MI, Schneider B, Krueger G, Schifferli JA. Complement C2 receptor inhibitor trispanning: a novel human complement inhibitory receptor. J. Immunol. 2005;174:356–66. doi: 10.4049/jimmunol.174.1.356. [DOI] [PubMed] [Google Scholar]
- Inoue N, Murakami Y, Kinoshita T. Molecular genetics of paroxysmal nocturnal hemoglobinuria. Int. J. Hematol. 2003;77:107–12. doi: 10.1007/BF02983208. [DOI] [PubMed] [Google Scholar]
- Itoh S, Takeshita K, Susuki C, Shige-Eda K, Tsuji T. Redistribution of P-selectin ligands on neutrophil cell membranes and the formation of platelet-neutrophil complex induced by hemodialysis membranes. Biomaterials. 2008;29:3084–3090. doi: 10.1016/j.biomaterials.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Janssen BJ, Gomes L, Koning RI, Svergun DI, Koster AJ, Fritzinger DC, Vogel CW, Gros P. Insights into complement convertase formation based on the structure of the factor B-cobra venom factor complex. EMBO J. 2009 doi: 10.1038/emboj.2009.184. Epub, ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen BJ, Halff EF, Lambris JD, Gros P. Structure of compstatin in complex with complement component C3c reveals a new mechanism of complement inhibition. J. Biol. Chem. 2007;282:29241–29247. doi: 10.1074/jbc.M704587200. [DOI] [PubMed] [Google Scholar]
- Jensen ML, Honore C, Hummelshoj T, Hansen BE, Madsen HO, Garred P. Ficolin-2 recognizes DNA and participates in the clearance of dying host cells. Mol. Immunol. 2007;44:856–865. doi: 10.1016/j.molimm.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Jinsmaa Y, Takahashi M, Yoshikawa M. Anti-analgesic and anti-amnesic effect of complement C3a. Life Sci. 2000;67:2137–2143. doi: 10.1016/s0024-3205(00)00800-6. [DOI] [PubMed] [Google Scholar]
- Katragadda M, Magotti P, Sfyroera G, Lambris JD. Hydrophobic effect and hydrogen bonds account for the improved activity of a complement inhibitor, compstatin. J. Med. Chem. 2006;49:4616–4622. doi: 10.1021/jm0603419. [DOI] [PubMed] [Google Scholar]
- Katschke KJ, Jr., Stawicki S, Yin J, Steffek M, Xi H, Sturgeon L, Hass PE, Loyet KM, Deforge L, Wu Y, van Lookeren Campagne M, Wiesmann C. Structural and functional analysis of a C3b-specific antibody that selectively inhibits the alternative pathway of complement. J. Biol. Chem. 2009;284:10473–10479. doi: 10.1074/jbc.M809106200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Miwa T, Zhou L, Song WC. Activator-specific requirement of properdin in the initiation and amplification of the alternative pathway complement. Blood. 2008;111:732–740. doi: 10.1182/blood-2007-05-089821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl J. Drug evaluation: the C5a receptor antagonist PMX-53. Curr. Opin. Mol. Ther. 2006;8:529–538. [PubMed] [Google Scholar]
- Konteatis ZD, Siciliano SJ, Van Riper G, Molineaux CJ, Pandya S, Fischer P, Rosen H, Mumford RA, Springer MS. Development of C5a receptor antagonists. Differential loss of functional responses. J. Immunol. 1994;153:4200–4205. [PubMed] [Google Scholar]
- Kuraya M, Ming Z, Liu X, Matsushita M, Fujita T. Specific binding of L-ficolin and H-ficolin to apoptotic cells leads to complement activation. Immunobiology. 2005;209:689–697. doi: 10.1016/j.imbio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Lambris JD, Becherer JD, Servis C, Alsenz J. In: Use of synthetic peptides in exploring and modifying complement reactivities. Activators and inhibitors of complement activation. Sim RB, editor. Kluwer Academic Publishers; Netherlands: 1993. pp. 201–232. [Google Scholar]
- Le GT, Abbenante G, Fairlie DP. Profiling the enzymatic properties and inhibition of human complement factor. B. J. Biol. Chem. 2007;282:34809–34816. doi: 10.1074/jbc.M705646200. [DOI] [PubMed] [Google Scholar]
- Lee H, Whitfeld PL, Mackay CR. Receptors for complement C5a. The importance of C5aR and the enigmatic role of C5L2. Immunol. Cell Biol. 2008;86:153–160. doi: 10.1038/sj.icb.7100166. [DOI] [PubMed] [Google Scholar]
- Lucchesi BR, Tanhehco EJ. Therapeutic potential of complement inhibitors in myocardial ischaemia. Expert Opin. Investig. Drugs. 2000;9:975–991. doi: 10.1517/13543784.9.5.975. [DOI] [PubMed] [Google Scholar]
- Magotti P, Ricklin D, Qu H, Wu Y, Lambris JD. Structure-kinetic relationship analysis of the therapeutic complement inhibitor compstatin. J. Mol. Recognit. 2009 doi: 10.1002/jmr.972. Epub, ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrides SC. Therapeutic inhibition of the complement system. Pharmacol. Rev. 1998;50:59–87. [PubMed] [Google Scholar]
- March DR, Proctor LM, Stoermer MJ, Sbaglia R, Abbenante G, Reid RC, Woodruff TM, Wadi K, Paczkowski N, Tyndall JD, Taylor SM, Fairlie DP. Potent cyclic antagonists of the complement C5a receptor on human polymorphonuclear leukocytes. Relationships between structures and activity. Mol. Pharmacol. 2004;65:868–879. doi: 10.1124/mol.65.4.868. [DOI] [PubMed] [Google Scholar]
- Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, Coukos G, Lambris JD. Modulation of the antitumor immune response by complement. Nat. Immunol. 2008;9:1225–1235. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007;28:184–192. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Marschang P, Kruger U, Ochsenbauer C, Gurtler L, Hittmair A, Bosch V, Patsch JR, Dierich MP. Complement activation by HIV-1-infected cells: the role of transmembrane glycoprotein gp41. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1997;14:102–109. doi: 10.1097/00042560-199702010-00002. [DOI] [PubMed] [Google Scholar]
- Maruo K, Akaike T, Ono T, Okamoto T, Maeda H. Generation of anaphylatoxins through proteolytic processing of C3 and C5 by house dust mite protease. J. Allergy Clin. Immunol. 1997;100:253–260. doi: 10.1016/s0091-6749(97)70233-1. [DOI] [PubMed] [Google Scholar]
- Mastellos D, Morikis D, Strey C, Holland MC, Lambris JD. From atoms to systems: a cross-disciplinary approach to complement-mediated functions. Mol. Immunol. 2004;41:153–164. doi: 10.1016/j.molimm.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Mathieu MC, Sawyer N, Greig GM, Hamel M, Kargman S, Ducharme Y, Lau CK, Friesen RW, O'Neill GP, Gervais FG, Therien AG. The C3a receptor antagonist SB 290157 has agonist activity. Immunol. Lett. 2005;100:139–145. doi: 10.1016/j.imlet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- McMullen ME, Hart ML, Walsh MC, Buras J, Takahashi K, Stahl GL. Mannose-binding lectin binds IgM to activate the lectin complement pathway in vitro and in vivo. Immunobiology. 2006;211:759–766. doi: 10.1016/j.imbio.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Nishikawa K, Morgan BP, Matsuo S. Comparison of the suppressive effects of soluble CR1 and C5a receptor antagonist in acute arthritis induced in rats by blocking of CD59. Clin. Exp. Immunol. 2000;119:368–375. doi: 10.1046/j.1365-2249.2000.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocco J, Mack WJ, Ducruet AF, Sosunov SA, Sughrue ME, Hassid BG, Nair MN, Laufer I, Komotar RJ, Claire M, Holland H, Pinsky DJ, Connolly ES., Jr. Complement component C3 mediates inflammatory injury following focal cerebral ischemia. Circul. Res. 2006;99:209–217. doi: 10.1161/01.RES.0000232544.90675.42. [DOI] [PubMed] [Google Scholar]
- Mollnes TE, Kirschfink M. Strategies of therapeutic complement inhibition. Mol. Immunol. 2006;43:107–121. doi: 10.1016/j.molimm.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Monk PN, Scola AM, Madala P, Fairlie DP. Function, structure and therapeutic potential of complement C5a receptors. Br. J. Pharmacol. 2007;152:429–448. doi: 10.1038/sj.bjp.0707332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikis D, Assa-Munt N, Sahu A, Lambris JD. Solution structure of Compstatin, a potent complement inhibitor. Protein Sci. 1998;7:619–627. doi: 10.1002/pro.5560070311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikis D, Lambris JD. Structural aspects and design of low-molecular-mass complement inhibitors. Biochem. Soc. Trans. 2002;30:1026–1036. doi: 10.1042/bst0301026. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Roos A, Daha MR. A regulatory role for complement in innate immunity and autoimmunity. Int. Arch. Allergy Immunol. 2004;134:310–323. doi: 10.1159/000079261. [DOI] [PubMed] [Google Scholar]
- Nordahl EA, Rydengard V, Nyberg P, Nitsche DP, Morgelin M, Malmsten M, Bjorck L, Schmidtchen A. Activation of the complement system generates antibacterial peptides. Proc. Natl. Acad. Sci. U.S.A. 2004;101:16879–84. doi: 10.1073/pnas.0406678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohinata K, Yoshikawa M. Food intake regulation by central complement system. Adv. Exp. Med. Biol. 2008;632:35–46. [PubMed] [Google Scholar]
- Paidassi H, Tacnet-Delorme P, Garlatti V, Darnault C, Ghebrehiwet B, Gaboriaud C, Arlaud GJ, Frachet P. C1q binds phosphatidylserine and likely acts as a multiligand-bridging molecule in apoptotic cell recognition. J. Immunol. 2008;180:2329–2338. doi: 10.4049/jimmunol.180.4.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt JL. The immunological barriers to xenotransplantation. Crit. Rev. Immunol. 1996;16:331–358. [PubMed] [Google Scholar]
- Proctor LM, Moore TA, Monk PN, Sanderson SD, Taylor SM, Woodruff TM. Complement factors C3a and C5a have distinct hemodynamic effects in the rat. Int. Immunopharmacol. 2009;9:800–806. doi: 10.1016/j.intimp.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Pugsley MK, Abramova M, Cole T, Yang X, Ammons WS. Inhibitors of the complement system currently in development for cardiovascular disease. Cardiovasc Toxicol. 2003;3:43–70. doi: 10.1385/ct:3:1:43. [DOI] [PubMed] [Google Scholar]
- Ratajczak J, Reca R, Kucia M, Majka M, Allendorf DJ, Baran JT, Janowska-Wieczorek A, Wetsel RA, Ross GD, Ratajczak MZ. Mobilization studies in mice deficient in either C3 or C3a receptor (C3aR) reveal a novel role for complement in retention of hematopoietic stem/progenitor cells in bone marrow. Blood. 2004;103:2071–2078. doi: 10.1182/blood-2003-06-2099. [DOI] [PubMed] [Google Scholar]
- Ricklin D, Lambris JD. Complement-targeted therapeutics. Nat. Biotechnol. 2007;25:1265–1275. doi: 10.1038/nbt1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D, Lambris JD. Compstatin: a complement inhibitor on its way to clinical application. Adv. Exp. Med. Biol. 2008;632:273–292. doi: 10.1007/978-0-387-78952-1_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C. Alcon Research, Ltd.; patent WO 2008137236 Treatment of age-related macular degeneration using inhibitors of complement factor D. 2008
- Rooijakkers SH, Wu J, Ruyken M, van Domselaar R, Planken KL, Tzekou A, Ricklin D, Lambris JD, Janssen BJ, van Strijp JA, Gros P. Structural and functional implications of the alternative complement pathway C3 convertase stabilized by a staphylococcal inhibitor. Nat. Immunol. 2009;10:721–727. doi: 10.1038/ni.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A, Nauta AJ, Broers D, Faber-Krol MC, Trouw LA, Drijfhout JW, Daha MR. Specific inhibition of the classical complement pathway by C1q-binding peptides. J. Immunol. 2001;167:7052–7059. doi: 10.4049/jimmunol.167.12.7052. [DOI] [PubMed] [Google Scholar]
- Roos A, Ramwadhdoebe TH, Nauta AJ, Hack CE, Daha MR. Therapeutic inhibition of the early phase of complement activation. Immunobiology. 2002;205:595–609. doi: 10.1078/0171-2985-00157. [DOI] [PubMed] [Google Scholar]
- Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat. Biotechnol. 2007;25:1256–1264. doi: 10.1038/nbt1344. [DOI] [PubMed] [Google Scholar]
- Sahu A, Kay BK, Lambris JD. Inhibition of human complement by a C3-binding peptide isolated from a phage-displayed random peptide library. J. Immunol. 1996;157:884–891. [PubMed] [Google Scholar]
- Schnatbaum K, Locardi E, Scharn D, Richter U, Hawlisch H, Knolle J, Polakowski T. Peptidomimetic C5a receptor antagonists with hydrophobic substitutions at the C-terminus: increased receptor specificity and in vivo activity. Bioorg. Med. Chem. Lett. 2006;16:5088–5092. doi: 10.1016/j.bmcl.2006.07.036. [DOI] [PubMed] [Google Scholar]
- Schwertz H, Carter JM, Russ M, Schubert S, Schlitt A, Buerke U, Schmidt M, Hillen H, Werdan K, Buerke M. Serine protease inhibitor nafamostat given before reperfusion reduces inflammatory myocardial injury by complement and neutrophil inhibition. J. Cardiovasc. Pharmacol. 2008;52:151–160. doi: 10.1097/FJC.0b013e318180188b. [DOI] [PubMed] [Google Scholar]
- Selander B, Martensson U, Weintraub A, Holmstrom E, Matsushita M, Thiel S, Jensenius JC, Truedsson L, Sjoholm AG. Mannan-binding lectin activates C3 and the alternative complement pathway without involvement of C2. J. Clin. Invest. 2006;116:1425–1434. doi: 10.1172/JCI25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim RB, Tsiftsoglou SA. Proteases of the complement system. Biochem. Soc. Trans. 2004;32:21–27. doi: 10.1042/bst0320021. [DOI] [PubMed] [Google Scholar]
- Sjoholm AG, Jonsson G, Braconier JH, Sturfelt G, Truedsson L. Complement deficiency and disease: an update. Mol. Immunol. 2006;43:78–85. doi: 10.1016/j.molimm.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Spitzer D, Mitchell LM, Atkinson JP, Hourcade DE. Properdin can initiate complement activation by binding specific target surfaces and providing a platform for de novo convertase assembly. J. Immunol. 2007;179:2600–2608. doi: 10.4049/jimmunol.179.4.2600. [DOI] [PubMed] [Google Scholar]
- Steel DM, Whitehead AS. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol. Today. 1994;15:81–88. doi: 10.1016/0167-5699(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Subasinghe NL, Ali F, Illig CR, Jonathan Rudolph M, Klein S, Khalil E, Soll RM, Bone RF, Spurlino JC, DesJarlais RL, Crysler CS, Cummings MD, Morris PE, Jr., Kilpatrick JM, Sudhakara Babu Y. A novel series of potent and selective small molecule inhibitors of the complement component C1s. Bioorg. Med. Chem. Lett. 2004;14:3043–3047. doi: 10.1016/j.bmcl.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Subasinghe NL, Travins JM, Ali F, Huang H, Ballentine SK, Marugan JJ, Khalil E, Hufnagel HR, Bone RF, DesJarlais RL, Crysler CS, Ninan N, Cummings MD, Molloy CJ, Tomczuk BE. A novel series of arylsulfonylthiophene-2-carboxamidine inhibitors of the complement component C1s. Bioorg. Med. Chem. Lett. 2006;16:2200–2204. doi: 10.1016/j.bmcl.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Sumichika H, Sakata K, Sato N, Takeshita S, Ishibuchi S, Nakamura M, Kamahori T, Ehara S, Itoh K, Ohtsuka T, Ohbora T, Mishina T, Komatsu H, Naka Y. Identification of a potent and orally active non-peptide C5a receptor antagonist. J. Biol. Chem. 2002;277:49403–49407. doi: 10.1074/jbc.M209672200. [DOI] [PubMed] [Google Scholar]
- Szalai AJ, Digerness SB, Agrawal A, Kearney JF, Bucy RP, Niwas S, Kilpatrick JM, Babu YS, Volanakis JE. The Arthus reaction in rodents: species-specific requirement of complement. J. Immunol. 2000;164:463–468. doi: 10.4049/jimmunol.164.1.463. [DOI] [PubMed] [Google Scholar]
- Travins JM, Ali F, Huang H, Ballentine SK, Khalil E, Hufnagel HR, Pan W, Gushue J, Leonard K, Bone RF, Soll RM, DesJarlais RL, Crysler CS, Ninan N, Kirkpatrick J, Cummings MD, Huebert N, Molloy CJ, Gaul M, Tomczuk BE, Subasinghe NL. Biphenylsulfonyl-thiophenecarboxamidine inhibitors of the complement component C1s. Bioorg. Med. Chem. Lett. 2008;18:1603–1606. doi: 10.1016/j.bmcl.2008.01.064. [DOI] [PubMed] [Google Scholar]
- Treiber A, Dansette PM, El Amri H, Girault J-P, Ginderow D, Mornon J-P, Mansuy D. Chemical and Biological Oxidation of Thiophene: Preparation and Complete Characterization of Thiophene S-Oxide Dimers and Evidence for Thiophene S-Oxide as an Intermediate in Thiophene Metabolism in Vivo and in Vitro. J. Am. Chem. Soc. 1997;119:1565–1571. [Google Scholar]
- Vergunst CE, Gerlag DM, Dinant H, Schulz L, Vinkenoog M, Smeets TJ, Sanders ME, Reedquist KA, Tak PP. Blocking the receptor for C5a in patients with rheumatoid arthritis does not reduce synovial inflammation. Rheumatology (Oxyford) 2007;46:1773–1778. doi: 10.1093/rheumatology/kem222. [DOI] [PubMed] [Google Scholar]
- Wagner E, Platt JL, Howell DN, Marsh HC, Jr., Frank MM. IgG and complement-mediated tissue damage in the absence of C2: evidence of a functionally active C2-bypass pathway in a guinea pig model. J. Immunol. 1999;163:3549–3558. [PubMed] [Google Scholar]
- Warren J, Mastroeni P, Dougan G, Noursadeghi M, Cohen J, Walport MJ, Botto M. Increased susceptibility of C1q-deficient mice to Salmonella enterica serovar Typhimurium infection. Infect. Immun. 2002;70:551–557. doi: 10.1128/iai.70.2.551-557.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters SM, Brodbeck RM, Steflik J, Yu J, Baltazar C, Peck AE, Severance D, Zhang LY, Currie K, Chenard BL, Hutchison AJ, Maynard G, Krause JE. Molecular characterization of the gerbil C5a receptor and identification of a transmembrane domain V amino acid that is crucial for small molecule antagonist interaction. J. Biol. Chem. 2005;280:40617–40623. doi: 10.1074/jbc.M509245200. [DOI] [PubMed] [Google Scholar]
- Wells JA, McClendon CL. Reaching for high-hanging fruit in drug discovery at protein-protein interfaces. Nature. 2007;450:1001–1009. doi: 10.1038/nature06526. [DOI] [PubMed] [Google Scholar]
- Wiesmann C, Katschke KJ, Yin J, Helmy KY, Steffek M, Fairbrother WJ, McCallum SA, Embuscado L, DeForge L, Hass PE, van Lookeren Campagne M. Structure of C3b in complex with CRIg gives insights into regulation of complement activation. Nature. 2006;444:217–220. doi: 10.1038/nature05263. [DOI] [PubMed] [Google Scholar]
- Woodruff TM, Crane JW, Proctor LM, Buller KM, Shek AB, de Vos K, Pollitt S, Williams HM, Shiels IA, Monk PN, Taylor SM. Therapeutic activity of C5a receptor antagonists in a rat model of neurodegeneration. FASEB J. 2006;20:1407–1417. doi: 10.1096/fj.05-5814com. [DOI] [PubMed] [Google Scholar]
- Woodruff TM, Pollitt S, Proctor LM, Stocks SZ, Manthey HD, Williams HM, Mahadevan IB, Shiels IA, Taylor SM. Increased potency of a novel complement factor 5a receptor antagonist in a rat model of inflammatory bowel disease. J. Pharmacol. Exp. Ther. 2005;314:811–817. doi: 10.1124/jpet.105.086835. [DOI] [PubMed] [Google Scholar]
- Woodruff TM, Strachan AJ, Sanderson SD, Monk PN, Wong AK, Fairlie DP, Taylor SM. Species dependence for binding of small molecule agonist and antagonists to the C5a receptor on polymorphonuclear leukocytes. Inflammation. 2001;25:171–177. doi: 10.1023/a:1011036414353. [DOI] [PubMed] [Google Scholar]
- Xu W, Berger SP, Trouw LA, de Boer HC, Schlagwein N, Mutsaers C, Daha MR, van Kooten C. Properdin binds to late apoptotic and necrotic cells independently of C3b and regulates alternative pathway complement activation. J. Immunol. 2008;180:7613–21. doi: 10.4049/jimmunol.180.11.7613. [DOI] [PubMed] [Google Scholar]
- Zhang M, Takahashi K, Alicot EM, Vorup-Jensen T, Kessler B, Thiel S, Jensenius JC, Ezekowitz RA, Moore FD, Carroll MC. Activation of the lectin pathway by natural IgM in a model of ischemia/reperfusion injury. J. Immunol. 2006;177:4727–4734. doi: 10.4049/jimmunol.177.7.4727. [DOI] [PubMed] [Google Scholar]