Abstract
Silk-elastinlike protein polymers (SELP’s) are block copolymers of silk-like and elastin-like tandem repeats. With appropriate sequence and composition SELPs can be liquid at room temperature and form hydrogels at body temperature. The influence of polymer structure and concentration on biodegradation in vitro and controlled delivery of adenoviruses carrying reporter genes to head and neck tumors in vivo was evaluated using hydrogels made from three SELP analogs. SELP-815K, with eight silk and fifteen elastin units and a lysine (K) modified elastin, was compared to SELP-415K and SELP-47K. Hydrogels with higher silk content and concentration degraded at a slower rate compared to other analogs. Intratumoral injection of adenoviruses with SELPs enhanced gene expression in tumor tissue up to 10 fold compared to viral injection without polymer. Viruses delivered with SELP-815K at 4 wt% polymer concentration showed the highest level of gene expression. Tumor to liver ratio of expression was up to 55 fold higher for SELP-mediated systems. This study demonstrates the influence of exquisite control over polymer structure using recombinant techniques on spatial and temporal control over adenoviral delivery and establishes the utility of SELP matrices as biodegradable systems for gene therapy of head and neck cancer.
Keywords: Protein Polymers, Gene Delivery, Hydrogels, Head and Neck Cancer
1. Introduction
Viral mediated anticancer gene therapy is a promising approach for treatment of Head and Neck Squamous Cell Carcinoma (HNSCC), which caused an estimated 7,590 deaths among American patients in 2008 alone [1]. Gene therapy can potentially reduce the morbidity associated with traditional surgical and radiation therapies which quite often leaves the patients with unsightly and socially debilitating scars and deformities [2]. However, rapid clearance of adenoviruses from the body ultimately limits the number of cells which can be infected by the therapeutic virus [3–6]. In addition, dissemination of viral vector carrying its therapeutic gene beyond tumor boundaries decreases the treatment efficacy and results in unwanted side effects in normal organs. Localized matrix-mediated delivery of adenoviruses to solid tumors can prolong gene expression and limit dissemination to non-target organs.
Materials for localized delivery of bioactive agents can be synthetic, natural, or a hybrid of both. Chemically synthesized polymers generally have a random sequence with limited control over molecular weight and molecular weight distribution. As a consequence it is difficult to make a precise correlation between polymer structure and properties such as gelation, controlled release and biodegradation. In the case of naturally occurring biomaterials such as collagen there is limited ability to chemically modify the polymers for the desired functions. Recombinant polymers on the other hand can be synthesized such that different natural motifs from nature exist in one polymer chain with precise control over sequence and length of the copolymer. In the context of gene delivery this exquisite control over structure can lead to the design and development of new polymers to prolong gene expression and increase transfection efficiency.
Over the past few years we have focused on the design and development of silk-elastinlike protein polymers (SELPs) for matrix-mediated gene delivery to solid tumors (reviewed in [7]). SELPs are produced by recombinant DNA technology using relevant genes of silkworm and human elastin in E. Coli to produce peptide repeats of silk (GAGAGS) and elastin-like (GVGVP) units [8]. These copolymers provide the biocompatibility and elasticity of human elastin along with mechanical and tensile strength of silk in a structure that does not coexist naturally in one polymer chain. SELP’s containing two or more silk units in the polymer backbone can be liquid at room temperature and form hydrogels at body temperature. This property enables co-injection of genetic materials with liquid SELPs to solid tumors for matrix-mediated delivery. We have shown that gene expression is prolonged and localized when adenoviruses are delivered intratumorally with these hydrogels to solid tumors of head and neck [9, 10]. For successful application of these matrices for gene therapy of head and neck cancer it is desirable to maximize duration and extent of gene expression in solid tumors, mimimize expression in non-target organs such as liver and evaluate the biodegradation of these systems.
Recently we evaluated the influence of polymer sequence on mechanical properties and pore structures of hydrogels made from three SELP analogs, namely SELP-47K, SELP-415K and SELP-815K (Figure 1) [11]. The cross-linking densities in these hydrogels followed the order SELP-47K > SELP-815K > SELP-415K alluding to the importance of the length of elastin blocks in governing the spacing of the cross-linked hydrogel network and that of silk in governing the stiffness of their 3-dimensional structures. This study examines the influence of SELP structure and concentration among these three analogs on spatial and temporal control over gene expression in a murine model of head and neck tumor xenograft. In addition, the structure-dependent changes in degradation of SELP hydrogels in the presence and absence of elastase are examined in vitro.
Figure 1.

Single amino acid representations of three SELP analogs.
2. Materials and Methods
2.1. Materials
SELP-47K was obtained from Protein Polymer Technologies, Inc. (San Diego, CA, USA). Linear SELP-415k [12] and -815K [11] were biosynthesized and characterized as described previously. Replication defective human adenoviruses (Ad) Type 5 with E1/E3 deletion, under the control of the CMV promoter, encoding for either β-galactosidase (β-gal) or firefly luciferase (Luc) reporter genes were purchased from Vector Biolabs (Philadelphia, PA, USA). JHU-022 oral cavity cancer cell line was a kind gift from Professor David Sidransky of Johns Hopkins University (Baltimore, MD, USA). Human Leukocyte Elastase was purchased from Elastin Products Company (Owensville, MO) and solubilized in Phosphate Buffered Saline (Sigma Aldrich, St. Louis, MO). Bicinchoninic acid protein assay was purchased from Thermo Scientific (Waltham, MA, USA) and the optical density (OD) measurements were performed using the SpectraMax M2 micro plate reader from Molecular Devices (Sunny Vale, CA, USA) coupled with Softmax analytic software package. For bioluminescent imaging studies, luciferin was obtained from Gold Biotech (St. Louis, MO, USA). Animals were evaluated for luciferase expression using a Xenogen IVIS100 bioluminescent imaging system from Caliper Life Sciences (Hopkinton, MA, USA) coupled with analytical software, Igor PRO v.2.2.0, Wavemetrics Inc. (Lake Oswego, OR, USA). For establishing xenografts JHU-022 cells were cultured in Advanced Roswell Park Memorial Institute (RPMI 1640) medium containing 2mM L-Glutamine and 10% fetal calf serum (Gibco, Carlsbad, CA, USA). Chlorophenol Red-β-D-Galactopyranoside (CPRG) β-gal quantitative kit was purchased from Imgenex (San Diego, CA, USA). β-Galactosidase Reporter Gene Staining Kit was purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA ). Luciferase assay system was purchased from Promega (Madison, WI, USA). Six week old female athymic (nu/nu) mice were purchased from Charles River Laboratories (Davis, CA, USA) and were used for the animal studies in accordance with the Institutional Animal Care and Use Committee (IACUC) protocol of the University of Utah.
2.2. Quantitative evaluation of viral gene expression in vivo
Head and neck cancer xenografts were established by subcutaneously injecting 2×106 JHU-022 cells suspended in 200 ul Phosphate Buffer Saline (PBS) bilaterally in the flank of athymic nude (nu/nu) mice. Tumors were allowed to grow for two weeks to reach an average diameter of 7 mm. A dose of 5×108 PFU of Ad-CMV-LacZ was administered to mice withSELP-415K (10.8 wt%), SELP-47K (8 wt% and 10.8 wt%), SELP-815K (4 wt%, 8 wt%, and 10.8 wt%) or physiological saline. Virus-polymer solutions were prepared by thawing SELP and virus stocks and mixing them gently with physiological saline. Mice were anesthetized using 4 wt% isoflurane mixed with oxygen, and intratumorally injected with 25μl of the polymer-virus solutions using a Hamilton syringe. On day 7, 14 and 21 mice were euthanized and tumor and liver tissue were isolated. CPRG β-gal colorimetric assay was performed as described previously [6]. Briefly, fresh tissue samples from the tumor and liver of animals were collected, snap-frozen in liquid nitrogen, ground with mortar and pestle, re- suspended in 1 mL lysis buffer (50 mM HEPES, 5 mM CHAPS, pH 7.4) followed by sonication on ice in 10–15 second bursts for a total of one minute. Recovered tissue lysates were then centrifuged for 45 minutes at 14,000 rpm and subsequently upper aqueous layers were used for β-gal quantification. Colorimetric assays were then performed as outlined in the protocol provided with the kit. The optical density (OD) dynamic study was performed to assay the enzyme substrate kinetics, and time point of measurements were set at 40 minutes for tumor β-gal expression and 110 minutes for liver expression which represent the time points of peak interactions respectively.
2.3. Tumor histology
For spatial distribution of β-gal in tumors, tissues were cryo-sectioned at 5 μm and stained with a β-gal reporter gene staining kit. Kit manufacturer instructions were modified as follows: tissue sections were fixed for 3 minutes in fixation buffer, and then rinsed twice with PBS. Fixed sections were then stained with staining solution for 24 hours at 37° C, rinsed with PBS and observed under light microscope. X-Gal stains β-galactosidase expressing cells and tissue areas dark blue.
2.4. Bioluminescence imaging of gene expression in vivo
Bioluminescent imaging was used as a secondary method of assessment of spatial control over gene expression in JHU-022 tumor-bearing athymic nu/nu mice as established above. Tumors were then injected with 25 μl of SELP-415K (10.8 wt%), SELP-47K (4 wt%, 8 wt% and 10.8 wt%, SELP-815K (4 wt%, 8 wt%, and 10.8 wt%) or physiological saline containing 5×108 PFU of Ad.CMV.Luc. Imaging was performed on days 4, 7, 10, 14, and 21 by injection with 200 μl of 15 mg/ml luciferin intraperitoneally. Luminescence was imaged after 30 minutes, using Xenogen IVIS100, and images were analyzed using IgorPRO v.2.2.0.
2.5. In vitro assessment of hydrogel degradation
50μl SELP hydrogels were made by drawing 250μl of 12 wt% SELP-47K, SELP-415K, and SELP-815K, and 8 wt% SELP-815K solutions into separate 1 ml syringes. Syringes were placed in a 37°C incubator and allowed to gel for 24 hours. After incubation, the end was cut off of each syringe and 50 μl gel disks were cut from the 250 μl cylinder. These gels were placed in the inner wells of a 48-well plate containing 800 μl of PBS + elastase, with a 1:6 dilution curve of elastase starting at 1000 ng/ml. The empty wells of the plate were filled with 1.5 ml PBS to control evaporation, and then the plates were sealed with self-adhesive sealing foil and agitated at 120 RPM. At each time point (1, 3, 7, 10, 14, 17, 21, 24, and 28 days) the full sample volume of 800 μl was removed from each well, placed in a separate 1.5 ml microcentrifuge tube, and stored at −80°C. In order to assess the degradation of the SELPs, the micro BCA protein assay was used according to manufacturer’s instructions to measure the soluble protein for each sample, followed by measuring absorbance at 561 nm.
2.6. Statistical analysis
The results of the quantitative evaluation of viral gene expression in vivo and comparative degradation rate in vitro are expressed as the mean ± SE. Student’s T test was used to analyze the significance of difference in reporter gene expression in different SELP treatment groups compared to plain Ad-LacZ treatment, and to analyze the significance of SELP structure’s influence on degradation rate. P values of < 0.05 were indicative of statistically significant differences.
3. Results and Discussions
3.1 Spatio-temporal control of gene expression as a function of polymer structure
Selective delivery of sufficient number of therapeutic gene copies to cancer cells remains a challenge in gene therapy. Current data from clinical trials using adenoviral vectors for gene delivery show that acute liver toxicity and immunogenicity are the major adverse effects limiting the dose of adenovirus that can be given to the patients [13, 14]. These challenges indicate the need for tailor-making polymeric systems that can limit delivery of adenoviruses to solid tumors, prolong gene expression and are degraded and eliminated from the body over a specified period of time. In this study, we compared the duration and extent of gene expression in head and neck tumors when adenoviruses were delivered from hydrogels made from three SELP analogs. These polymers (Figure 1) were produced by recombinant techniques such that molecular weight of each linear chain remained constant while the sequence of silk and elastin repeats within each chain varied. SELP-47K has 8 elastin units less in each monomer repeat than SELP-415K (Figure 1). As the temperature is raised silk units hydrogen bond and elastin units self-assemble forming hydrogels. At equivalent polymer concentrations SELP-47K forms more robust hydrogels than SELP-415K as evidenced previously by a lower degree of swelling and higher mechanical strength [11]. SELP-815K has a higher number of silk units per monomer repeat compared to SELP-415K (Figure 1). At equivalent polymer concentrations, we had previously observed that hydrogels made from SELP-815K have intermediate degrees of swelling and storage moduli between SELP-47K and SELP-415K [11]. This is despite the fact that SELP-815K has a similar ratio of silk to elastin units compared to SELP-47K, providing evidence that in addition to the ratio of silk and elastin units, the sequence of these units in the polymer chain influences the physicochemical properties of the resulting hydrogels. We had also observed previously that polymer concentration influences the network properties of SELP hydrogels where a lower polymer concentration resulted in increased degree of swelling and release of bioactive agents [12, 15–18]. Based on these observations we hypothesized that by controlling both polymer structure and concentration, the location and duration of gene expression can be controlled when adenoviruses are delivered by SELPs to head and neck tumors.
Lac Z gene expression after co-injection of adenoviral vectors with each SELP structure and as a function of polymer concentration was evaluated in tumor and liver tissues of mice. Strong dependence of structure and concentration effect on gene expression was evident in terms of transfection levels and duration of gene expression (Figure 2). The results of the enzymatic assay in tumor lysates show that SELP-815K at 4 wt% exhibits the highest transfection levels at all time points, with a greater than 10-fold increase of transfection over free virus after one week and 6-fold increase at two weeks. As the concentration of SELP-815K increased from 4 wt% to 8wt% and 10.8 wt%, transfection levels decreased for the duration of study. The increased concentration leads to increased crosslinking density of hydrogel network resulting in decreased release of the viruses and transfection. Matrix-mediated adenoviral delivery from SELP-47K hydrogels resulted in a lower transfection efficiency than delivery with SELP-815K polymers at equivalent polymer concentrations. Compared to SELP-815K, SELP-47 K has shorter elastin segments between the silk crosslink points leading to lower pore size and virus release.
Figure 2.
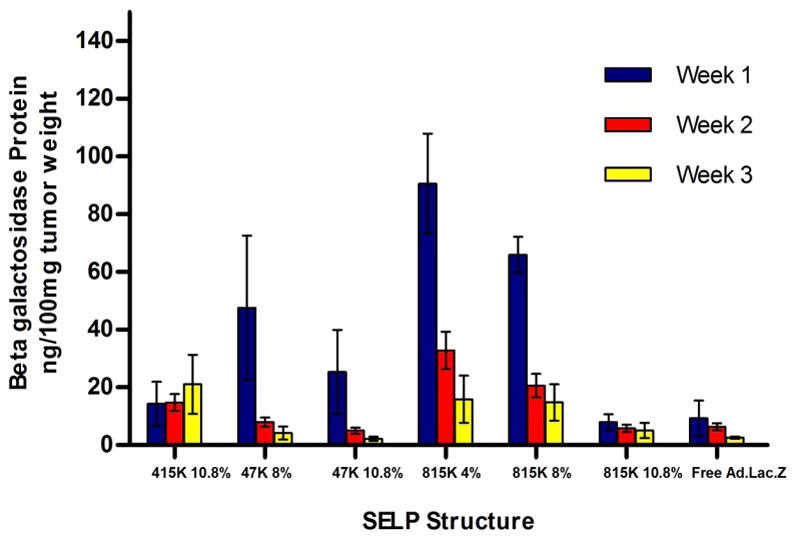
β-galactosidase expression in xenograft tumors injected with SELP+Ad.LacZ or saline +Ad.LacZ (free Ad.LacZ).
Due to longer elastin units the loose network of SELP-415K can only form stable hydrogels at the higher concentration of 10.8 wt% [11, 12]. At this concentration, higher transfection levels were observed compared to virus only group demonstrating that some virus was trapped in the hydrogel network that influenced release. However lower transfection was observed compared to other polymeric analogs demonstrating that increased silk units in more robust gels made from SELP-47K and -815K prolong release of adenoviruses in vivo. In the case of plain Ad-LacZ injection, burst-release out of the tumor tissue is the most plausible explanation in which the bulk of the virus was released over the first week following injection, and was cleared before significant transfection could take place at week one and beyond. The utility of SELP matrices in limiting virus distribution into the liver was evaluated at various polymer structures and concentrations. No significant differences in liver expression were observed for SELP polymers compared to free virus (Figure 3). However when considering the tumor/liver ratio of gene expression, SELP-815K at 4 wt% exhibited 55 fold higher tumor expression than liver (Figure 4). In the case of plain viral injection, the highest tumor/liver expression ratio was only 5 fold. Clearly SELP hydrogels localize gene expression to tumors and can augment the dose of adenoviruses that can be delivered up to 11 times for the same liver distribution as that of free viral injection. The spatial and temporal patterns of β-galactosidase expression in SELP-415K, -815K, and plain virus treatment groups were evaluated by staining with X-gal of both tumor and liver tissues (Figure 5). The observed patterns of gene expression in these tissues were in good agreement with the transfection data. In the SELP-415K group (Figure 5A), expression was apparent throughout the tissue, although in a lighter intensity than in the more localized and intense SELP-815K group (Figure 5C). The virus only group also had a high area of expression with a less dense staining (Figure 5E). Expression patterns in the SELP-415K and SELP-815K tumors showed prolonged expression through week 3 (Figures 5B and 5D), whereas expression in the virus only group was undetectable by week 3 (Figure 5F).
Figure 3.

β-galactosidase expression in the livers of xenograft tumor-bearing mice injected with SELP+Ad.LacZ or free Ad.LacZ
Figure 4.

Ratio of tumor: liver expression of β-galactosidase for head and neck cancer xenograft-bearing mice injected with either SELP+Ad.LacZ or free Ad.LacZ.
Figure 5.

Spatial and temporal control of adenoviral gene expression in xenograft tumors injected with SELP+Ad.LacZ vs saline+Ad.LacZ. Tissue sections were taken at 5μm and stained for β-galactosidase expression.
3.2 Bioluminescent imaging of SELP-mediated gene expression
Bioluminescent imaging of live tumor-bearing animals has the advantage that gene expression can be monitored in the same animal over the study period without the need to sacrifice the mice to recover tumor and liver tissue for measurement of expression. On the other hand issues with light attenuation, scattering, and reflection reduce the value of this technique for quantitative measurement. In this study both methods were used to provide a more comprehensive assessment of gene expression by SELP-mediated delivery of adenoviruses to head and neck tumors. Representative images are shown in Figure 6. Panels A–C show post-injection 4, 14, and 21 days respectively, of the SELP-815K 4 wt%+Ad.CMV.Luc group. Each animal expresses luciferase in the tumor at day 4, and that expression continues for all animals up to Day 14. The two patches of expression anterior to the tumor in panels B and C are due to liver expression, which appeared in only one animal in each group over the course of the study. At day 21 (Panel C), there is still tumor expression in all but one animal, illustrating the ability of SELP to prolong gene expression compared to free virus injection at the same time point, which shows only one animal with appreciable tumor expression starting on day 14 (panel E). The Figure 6 bioluminescent images of gene expression correlate with the data acquired through enzyme assay and confirm the influence of SELP on controlling gene expression, which would likely improve the efficacy of treatment of head and neck cancer with a virus/prodrug system. Additionally, based on these results, SELP could improve the safety of this treatment, as SELP causes lower cumulative transfection in the liver, the primary organ affected by adenovirus toxicity [13, 14].
Figure 6.

Bioluminescent images of mice injected with Ad.CMV.Luc + SELP815K at 4% (A–C) and free virus (Ad.CMV.Luc, D–F). Images are at days 4, 10, and 21 (left to right).
3.3 In vitro enzymatic degradation of SELP hydrogels
To successfully develop SELPs for matrix-mediated delivery, the biodegradation of these polymers needs to be studied. An ideal matrix is one that prolongs gene expression and degrades to amino acids for elimination from the body after gene delivery. The structure of the linear SELPs influences their degradation rate. We evaluated in vitro the influence of elastase concentration, polymer structure and polymer concentration on degradation of the hydrogels. Degradation was measured as percent protein loss from the hydrogels. Protein loss is a function of release of soluble proteins that do not participate in the polymer network. An increase in degradation results in increased protein release. Figure 7A shows the time-dependent protein release of SELP hydrogels in a solution of human leukocyte elastase. Figure 7B shows a concentration-dependence of degradation with degradation increasing with increasing elastase concentration. After one month of incubation, SELP-47K 12wt% and SELP-415K 12wt% each lost more than 10% of their total weight, while SELP-815K at 12 wt% lost 6.1% of its weight and SELP815K 8 wt% lost 7.5% of its weight in 1 μg/ml elastase. In 285 ng/ml elastase, SELP47K 12wt% lost only 3.6%, SELP-415K 12 wt% lost 3.2%, and SELP-815 12% and 8wt % lost 2.6% and 3.5%, respectively. These results show that, in general, SELPs with lower numbers of silk units degrade faster than SELPs with higher numbers of silk units. This is likely due to a combination of two factors: presence of less elastin units causes less degradation, and hydrogels with more silk units have a higher crosslinking density leading to potentially less diffusion of the enzyme in the matrix and subsequent degradation. Pore size of the hydrogels is likely to influence the diffusion of elastase molecules which are around 25kDa. The differences in protein mass loss are due to differences in the three dimensional network of the hydrogels. The longer silk units potentially form a more extensive network which can contribute to steric hindrance and hence physically shield the more degradation-susceptible elastin units, causing SELP-815K to degrade more slowly than both SELP-47K and SELP-415K. Similarly the higher crosslinking of SELP-815K at 12wt% results in a lower degradation rate.
Figure 7.

Results of SELP biodegradation studies. Figure 7a shows the loss of protein from each SELP structure over time at equivalent elastase concentration of 1μg/ml, starting from day 3. SELP47K 12% (●), SELP415K 12% (■), SELP815K 12% (▲), SELP815K 8% (△). Figure 7b shows the total protein loss of each SELP hydrogel after one month as a function of elastase concentration.
In assessing the elastase-driven degradation of SELP hydrogels another important parameter is the concentration of the enzyme in the media. An increase in concentration of elastase led to increased degradation of the hydrogels. This effect was more pronounced for hydrogels SELP-47K and SELP-415K where at the lower concentrations of 285 ng/ml protein loss was minimal and similar or equal to the base line loss of soluble fraction of the hydrogels. The differences in degradation and protein release from SELP hydrogels play an important role in the selection of appropriate structures for matrix-mediated gene delivery. The next logical step is to investigate the degradation of SELP hydrogels in vivo to help further understand the rate of SELP degradation in solid tumors and correlate polymer structure with in vivo degradation, gene release, transfection efficiency and therapeutic efficacy.
Conclusions
This study demonstrates the utility of silk-elastinlike protein polymers in matrix-mediated gene delivery to head and neck solid tumors. By using recombinant techniques it is possible to tailor-make the structure of the hydrogels to control gel formation, mechanical properties, release, in vivo transfection and degradation. In the series studied, viruses released from SELP-815K at 4wt% polymer concentration showed the highest transfection efficiency and prolonged gene expression up to day 21. These hydrogels also minimized dissemination of the viruses to the liver. At equivalent concentrations of polymers and elastase, degradation rate of SELP-815K was lower than SELP-415K and SELP-47K. Future steps in this research are to evaluate the influence of polymer structure on delivery of therapeutic adenoviruses and evaluate the degradation of these systems in vivo.
Acknowledgments
The authors thank Jennifer Gifford, Bart Claeys and Stephanie Scharff for their contributions to this research. This work was supported by a National Institutes of Health grant (R01-CA107621) and the Utah Science Technology and Research (USTAR) Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.AmericanCancerSociety, Cancer Facts and Figures (2008).
- 2.O’Malley BW., Jr Adenovirus-mediated gene therapy for human head and neck squamous cell cancer in a nude mouse model. Cancer Research. 1995;55(5):1080–1085. [PubMed] [Google Scholar]
- 3.Dai Y, Schwarz EM, Gu D, Zhang WW, Sarvetnick N, Verma IM. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci U S A. 1995;92(5):1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verma IM, Somia N. Gene therapy -- promises, problems and prospects. Nature. 1997;389(6648):239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y, Ertl HC, Wilson JM. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1(5):433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Li Q, Ertl HC, Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69(4):2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dandu R, Ghandehari H. Delivery of bioactive agents from recombinant polymers. Progress in Polymer Science. 2007;32(8–9):1008–1030. [Google Scholar]
- 8.Cappello J, Crissman J, Dorman M, Mikolajczak M, Textor G, Marquet M, Ferrari F. Genetic engineering of structural protein polymers. Biotechnol Prog. 1990;6(3):198–202. doi: 10.1021/bp00003a006. [DOI] [PubMed] [Google Scholar]
- 9.Hatefi A, Cappello J, Ghandehari H. Adenoviral gene delivery to solid tumors by recombinant silk-elastinlike protein polymers. Pharm Res. 2007;24(4):773–779. doi: 10.1007/s11095-006-9200-5. [DOI] [PubMed] [Google Scholar]
- 10.Cresce AV, Dandu R, Burger A, Cappello J, Ghandehari H. Characterization and real-time imaging of gene expression of adenovirus embedded silk-elastinlike protein polymer hydrogels. Mol Pharm. 2008;5:891–897. doi: 10.1021/mp800054w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dandu R, Cresce AV, Briber R, Dowell P, Cappello J, Ghandehari H. Silk–elastinlike protein polymer hydrogels: Influence of monomer sequence on physicochemical properties. Polymer. 2009;50:366–374. [Google Scholar]
- 12.Haider M, Leung V, Ferrari F, Crissman J, Powell J, Cappello J, Ghandehari H. Molecular engineering of silk-elastinlike polymers for matrix-mediated gene delivery: biosynthesis and characterization. Mol Pharm. 2005;2(2):139–150. doi: 10.1021/mp049906s. [DOI] [PubMed] [Google Scholar]
- 13.Mahasreshti PJ, Kataram M, Wang MH, Stockard CR, Grizzle WE, Carey D, Siegal GP, Haisma HJ, Alvarez RD, Curiel DT. Intravenous delivery of adenovirus-mediated soluble FLT-1 results in liver toxicity. Clin Cancer Res. 2003;9(7):2701–2710. [PubMed] [Google Scholar]
- 14.Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol. 2005;79(12):7478–7491. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinerman AA, Cappello J, Ghandehari H, Hoag SW. Swelling behavior of a genetically engineered silk-elastinlike protein polymer hydrogel. Biomaterials. 2002;23(21):4203–4210. doi: 10.1016/s0142-9612(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 16.Dinerman AA, Cappello J, Ghandehari H, Hoag SW. Solute diffusion in genetically engineered silk–elastinlike protein polymer hydrogels. Journal of Controlled Release. 2002;82(2–3):277–287. doi: 10.1016/s0168-3659(02)00134-7. [DOI] [PubMed] [Google Scholar]
- 17.Megeed Z, Cappello J, Ghandehari H. Controlled release of plasmid DNA from a genetically engineered silk-elastinlike hydrogel. Pharm Res. 2002;19(7):954–959. doi: 10.1023/a:1016406120288. [DOI] [PubMed] [Google Scholar]
- 18.Dandu R, Ghandehari H, Cappello J. Characterization of structurally related adenovirus-laden silk-elastinlike hydrogels. Journal of Bioactive and Compatible Polymers. 2008;23(1):5–19. [Google Scholar]


