Abstract
Much of what we know about the neurosciences is the direct result of studying psychoactive natural products. Unfortunately, there are many gaps in our understanding of the basic biological processes that contribute to the etiology of many CNS disorders. The investigation of psychoactive natural products offers an excellent approach to identify novel agents to treat CNS disorders and to find new chemical tools to better elucidate their biological mechanisms. This review will detail recent progress in a program directed towards investigating psychoactive natural products with the goal of treating drug abuse by targeting κ opioid receptors.
Introduction
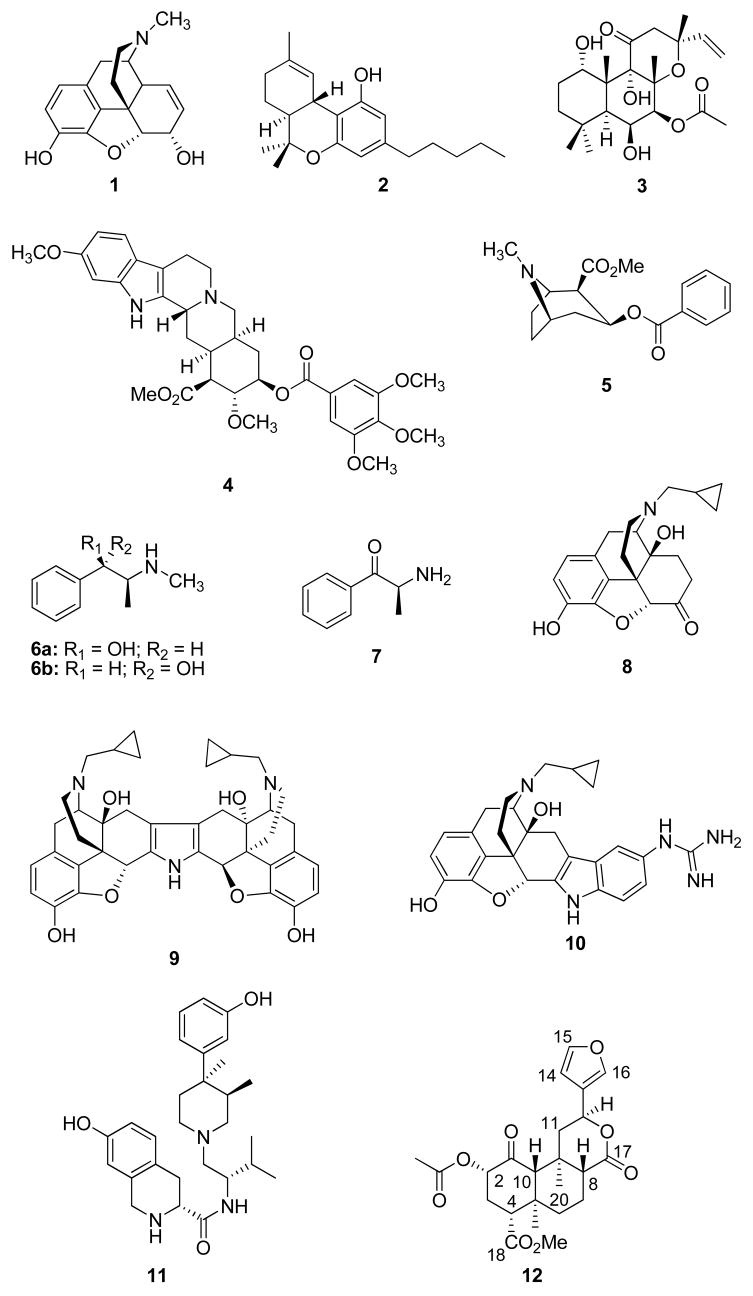
The use of psychoactive substances has a long and rich tradition and, except for a few cultures, is almost universal.1 Historically, psychoactive natural products have been used for their medicinal value and/or their ability to produce altered consciousness. Much of what modern science knows about the neurochemistry of the brain and the functions of the central nervous system can be traced directly to the study of psychoactive natural products.2 For example, intensive study of the chemistry and pharmacology of the alkaloid morphine (1) from Papaver somniferum L. (Papaveraceae) led to the identification of opioid receptors and the endogenous opioid system and has given much insight into the mechanisms of nociception.3–5 Similarly, studies focused on better understanding the effects of Δ9-tetrahydrocannabinol (2) and other cannabinoids, the active principles of marijuana (Cannabis sativa L. Cannabaceae), led to the identification of cannabinoid receptors and the endocannabinoid system.6 Forskolin (3), a diterpene isolated from Coleus forskohlii Briq. (Lamiaceae), is a potent and specific activator of adenylate cyclase and has been used extensively to investigate the function of CNS receptors.7,8 Furthermore, the study of alkaloids from Rauvolfia serpentina Benth. (Apocynaceae), such as reserpine (4), greatly expanded our understanding of the neurotransmitters, serotonin, norepinephrine, and dopamine, as well as the etiology of depression.9
Unfortunately, the abuse of psychoactive natural products leading to addiction has also had a negative effect on public health, by behavioral and neuropsychiatric morbidity, as well as by facilitating the spread of HIV-1, hepatitis B and C, and drug-resistant tuberculosis.10,11 Government estimates put the annual demand for the alkaloid cocaine (5) from Erythroxylum coca Lamarck (Erythroxylaceae) in the United States as being approximately 300 metric tons or about 50 percent of the world illict production.12 Further estimates put the number of hard-core cocaine users each year between 3.3 million to 3.6 million.12 In addition to the problems associated with cocaine abuse, a rise in the abuse of methamphetamine has been noted in West Coast cities of the U.S., in particular.13 Methamphetamine may be derived from the alkaloids, ephedrine (6a) and pseudoephedrine (6b), constituents specific of the genus Ephedra of the family Ephedraceae. In less than 10 years, methamphetamine has grown from a problem limited to the southwestern and midwestern U.S. to one of nationwide concern.13,14 Furthermore, the World Health Organization estimated that 10 million people worldwide chew khat, Catha edulis Forsk. (Celastraceae). This use is commonly found in the southwestern part of the Arabian Peninsula and in East Africa, where it has been used for centuries as part of an established cultural tradition. The major pharmacologically active constituent in khat is cathinone (7), an amphetamine-like alkaloid.15 Its current use among particular migrant communities in the United States has raised concern but there are no reliable estimates of its prevalence.
Stimulant dependence is a chronic relapsing disease that results from the prolonged effects of drugs on the brain.16 At present, there are no FDA-approved therapeutic agents available for the treatment of stimulant abuse or for the prevention of its relapse. However, various types of medications are currently being pursued based on the dopamine hypothesis.17–21 This hypothesis speculates that chronic exposure to stimulants causes excessive stimulation of dopamine neurons that, over time, produces dopamine depletion in critical reward circuits in the brain.22 The drug-induced depletion is then responsible for anhedonia, withdrawal, and relapse.22,23 To date, some promising clinical results for the dopamine hypothesis have been achieved with dextroamphetamine24 and modafinil.25 However, other neurochemical mechanisms appear to be involved and additional therapeutic approaches need to be explored.26,27 One approach to developing novel medications is to target κ opioid (KOP) receptors.28
κ Opioid Receptors and Drug Abuse
Opioid receptors are members of the superfamily of seven transmembrane-spanning (7TM) G-protein coupled receptors (GPCRs) and are divided into three types, μ (MOP), δ (DOP), and κ (KOP).5,29 The existence of additional opioid receptor subtypes has been suggested through multiple pharmacological studies.30–32 Each opioid receptor type plays a role in antinociception, as well as other biological responses.33 Interestingly, a growing body of evidence suggests that KOP receptors modulate the effects of psychostimulants.34–36 The KOP receptor system has been found to be critical for the development and relapse to drug seeking36 and have a role in the modulation of dopamine levels.37–44 More importantly, administration of KOP agonists: (1) decreases self-administration of cocaine; (2) inhibits cocaine place preference and locomotor sensitization; and (3) decreases cocaine-induced reinstatement.45–50 Collectively, these findings suggest that KOP receptor agonists could potentially treat cocaine abuse.28,51
Disruption of the KOP receptor also has potential utility in treating drug abuse. A central problem in treating drug addiction is the vulnerability to relapse during abstinence.52 Behavioral studies have shown that presentation of drug-associated cues, drug priming, and acute footshock stress each increased drug self-adminstration.53–55 It is believed that release of dynorphins, the endogenous agonists for KOP receptors, may mediate a component of stress-induced drug craving in reinstatement models (models of drug relapse).52 Studies have shown that interference of the KOP system by pretreatment with antagonists or gene disruption of KOP receptor attenuates the reinstatement of extinguished drug-taking behavior.52,56,57 Interference of the KOP system has also been shown to produce effects in animal models often used to study psychiatric illness. KOP receptor antagonists significantly decrease immobility and increase swimming time in the forced swim stress test similar to the antidepressant desipramine in rats.56 Furthermore, KOP antagonists have anxiolytic-like effects in models of unlearned and learned fear in rats.58 This suggests that KOP antagonists may have utility in treating depression and anxiety, as well as drug relapse.
Development of KOP Antagonists
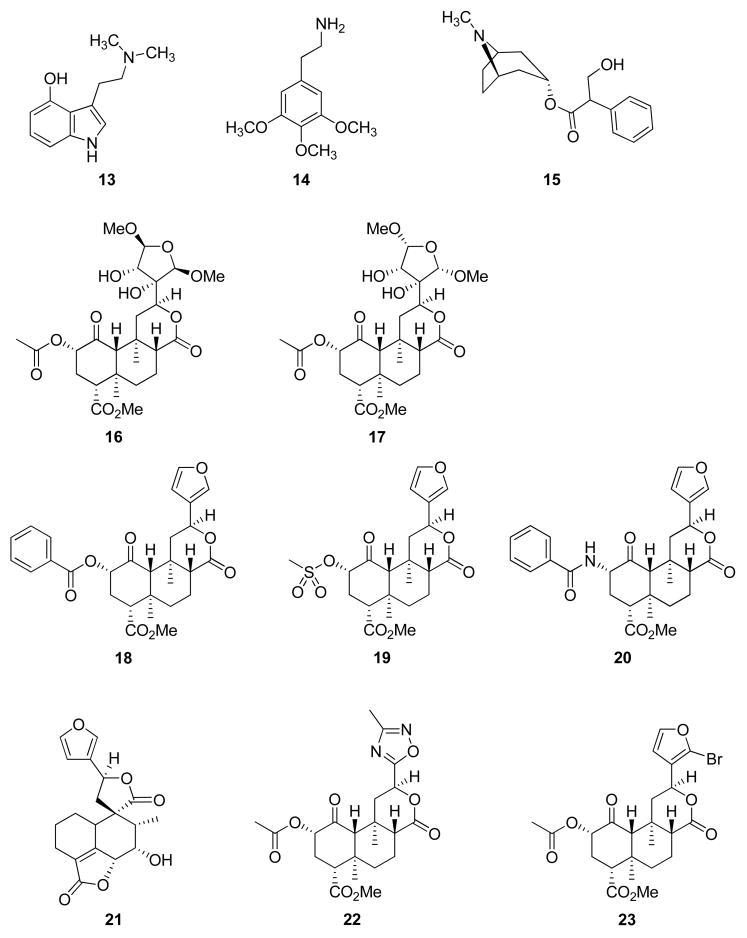
Among the first nonpeptide KOP antagonists identified were those derived from the morphine derivative naltrexone (8) such as nor-BNI (9) and GNTI (10).59,60 While 9 has been extensively used to study KOP receptors, its pharmacological properties are not optimal. It exhibits a much longer than expected half-life in vivo.61 Further study of its structure-activity relationships identified 10 as having increased potency in vivo as a KOP antagonist but also had an extended duration of action.62–68
More recently, several novel classes of KOP antagonists have been discovered.69–73 In particular, JDTic (11) was identified as a KOP antagonist more potent than 9.70 Additional pharmacological studies have shown that 11 blocks KOP agonist induced antinociception in mice and squirrel monkey, antagonizes KOP agonist induced diuresis in rats,74 decreases withdrawal signs in rodents,75 significantly reduced foot-shock-induced reinstatement of cocaine responding in rats,56 and has anxiolytic-like effects in rats.58
For the reasons stated above, targeting KOP receptors is an excellent pharmacological approach to treating stimulant abuse and its pendent pathology. However, currently available KOP receptor ligands suffer from several therapeutic limitations. First, KOP agonists have been shown to potentiate cocaine reward and produce psychotomimesis, sedation, and nausea.34,76–78 Second, almost all currently available KOP antagonists have a slow onset of action and are extremely long in duration of action and disrupt KOP signaling by activating c-Jun N-terminal kinase (JNK).79,80 Thus, there is a pressing need to identify new molecules that modulate KOP receptors devoid of these limitations.
Most KOP receptor ligands are derivatives of morphine and are likely to suffer from the same therapeutic limitations. One approach to circumvent the problems of therapeutic limitations seen with traditional KOP ligands is to identify novel structural scaffolds for chemical development through investigation of psychoactive natural products, such as Salvia divinorum Epling & Játiva (Lamiaceae).2
Salvia divinorum
Salvia divinorum is a sage native to the southern Mexican State of Oaxaca, Mexico. The genus Salvia is one of the most widespread taxa of the Lamiaceae family and is featured prominently in the pharmacopeias of many countries throughout the world.81 Mazatec Indians living in Oaxaca utilize the leaves of S. divinorum as a divinatory or pyschotomimetic agent.82,83 An infusion prepared from four or five pairs of fresh or dried leaves is also used by the Mazatecs to stop diarrhea and to relieve headache and rheumatism.84 The active ingredient in S. divinorum is the neoclerodane diterpene, salvinorin A (12), which was identified nearly simultaneously by two groups.85–88 A smoked dose of approximately 200 to 500 μg produces profound hallucinations lasting up to one hour.87,89 The molecular target for the hallucinatory actions of 12 was not clear given its lack of activity at the targets of other known hallucinogens, namely, the serotonin 5-HT2A and NMDA receptors.87 Remarkably, 12 was identified as a potent and selective KOP agonist in vitro.90
Chemical studies of S. divinorum have yielded the structurally related neoclerodanes salvinorins B – I, 89,91–94 divinatorins A – F,92,93,95 and salvidivins A – D.93 An extract of S. divinorum was found to have no effect on intestinal motility under physiological conditions but did inhibit motility in mice with inflammation.96,97 However, an extract of S. divinorum was shown to inhibit enteric cholinergic transmission in guinea pig ileum.98 Recently, the biosynthetic route of 12 was found to be consistent with the deoxyxylulose phosphate pathway using several different labeling experiments.99 Furthermore, the total synthesis of 12 by two different synthetic routes has been described.100,101
Additional pharmacological studies have provided further evidence that 12 acts as a KOP agonist. Diterpene 12 produces a discriminative effect similar to other KOP agonists in both nonhuman primates102 and rats103 and has been shown to produce antinociception in mice that is blocked a KOP antagonist.104,105 It also produces an aversive response in the conditioned place preference assay,106 decreases dopamine levels in the caudate putamen of mice,106 dose dependently increases immobility in the forced swim test,107 disrupts climbing behavior on an inverted screen task,108 blocks the locomotor-stimulant effects of cocaine,109 and does not exert DOM-like effects in nonhuman primates.110 Furthermore, 12 decreases mesostriatal neurotransmission by affecting DA release and not DA uptake.111 In contrast to other KOP agonists, 12 does not cause diuresis in rats, likely due to its short duration of action.112 However, differences were seen in the interaction of 12 and other KOP agonists with respect to the behavioral responses to cocaine.111
Additional work in nonhuman primates found that 12 acts as a high-efficacy KOP agonist in a translationally viable neuroendocrine biomarker assay and produces facial relaxation and ptosis that can be detected within 1 – 2 min of injection.113,114 Previous studies indicated that the half-life of 12 in nonhuman primates was found to be approximately 30 min.115,116 Recent pharmacokinetic studies in baboons using carbon-11 labeled 12 were highly consistent with this observation and suggest that less than 10 μg in the human brain account for the psychoactive effects of 12.117
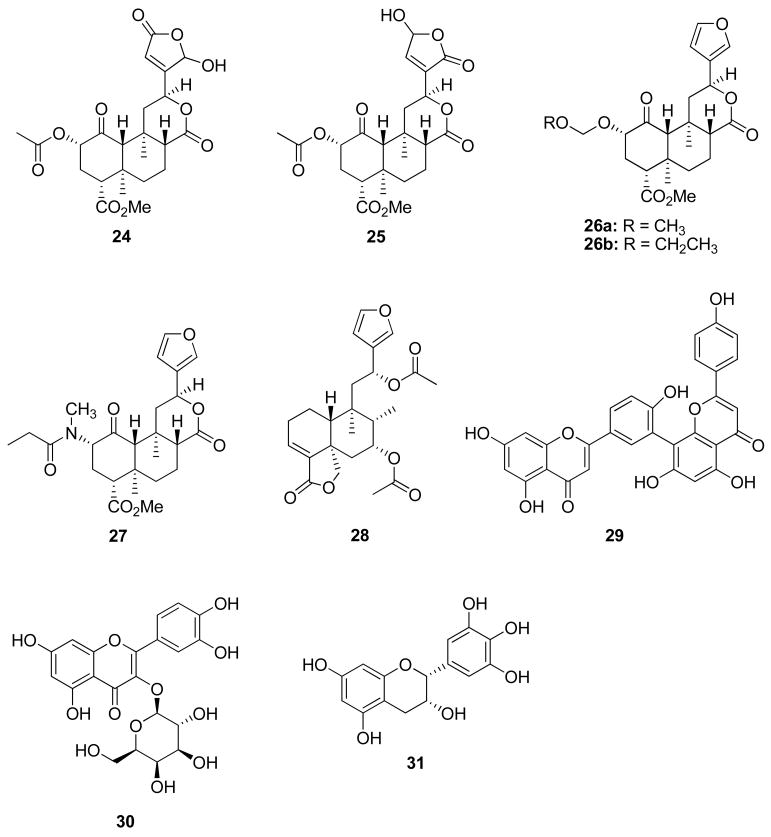
As a neoclerodane diterpenoid, 12 is a very interesting psychoactive natural product. First, it is structurally unique as an opiomimetic. Until the discovery of 12, it had been assumed that an alkaloid or a compound bearing a basic nitrogen was required in order to interact with opioid receptors.118 Given the lack of basic nitrogen in 12, it would appear this interaction is not an absolute requirement. Second, it is structurally unique among known hallucinogens. Diterpene 12 bears little resemblance to other hallucinogenic natural products such as Δ9-THC (2), psilocin (13), mescaline (14), and L-hyoscyamine (15), the presumed active component responsible for the hallucinogenic effects of jimson weed (Datura stramonium L.; Solanaceae). Moreover, 12 is the first report of an exogenous natural product interacting with KOP receptors to produce hallucinations. Given its unique structure and interesting pharmacological properties, we initiated a program to develop 12 as a potential stimulant abuse medication.
As a first step in this program, we sought to better understand the chemistry and pharmacology associated with S. divinorum. One early investigation was to isolate and identify other psychoactive compounds that might be present in the same species. This work led to the isolation of two new neoclerodane diterpenes, salvinicins A (16) and B (17), from the dried leaves of Salvia divinorum.119 The structures of 16 and 17 were elucidated by spectroscopic techniques, including 1H and 13C, NOESY, HMQC, and HMBC NMR. The absolute stereochemistry of these compounds was assigned on the basis of single crystal X-ray crystallographic analysis of 16 and a 3,4-dichlorobenzoate derivative of salvinorin B, the desacetyl derivative of 12. Neoclerodanes 16 and 17 possess a rare 3,4-dihydroxy-2,5-dimethoxytetrahydrofuran ring. Pharmacological evaluation of these compounds at opioid receptors was then conducted and indicated that 16 and 17 showed activity at κ and μ opioid receptors, respectively. Further work indicated that 16 exhibited partial KOP agonist activity with an EC50 value of 4.1 ± 0.6 μM [Emax = 80% relative to (−)-U50,488H, a standard KOP agonist]. Interestingly, 17 exhibited antagonist activity at μ receptors with a Ki of > 1.9 μM. This was the first report of a neoclerodane with opioid antagonist activity. Given the unique pharmacological properties of 16 and 17 and the small amounts isolated, our laboratory developed a practical method for the synthesis of 16 and 17 from 12 isolated from S. divinorum.120 This methodology proved useful in the further elucidation of the structure-activity relationships of 16 and 17 at opioid receptors.121,122
In addition to our isolation efforts, we and others initiated studies to better understand the high affinity and activity of 12 as an opioid receptor ligand.29 Our initial structural modification transformations of 12 resulted in the synthesis of several neoclerodane diterpenes with opioid receptor affinity and activity.123 In our structure-activity relationship studies, we identified herkinorin (18) as a MOP agonist and mesylate 19 as a likely more metabolically stable KOP agonist roughly equipotent to 12. The discovery of 18 represented the first report of a non-nitrogenous MOP agonist and identified neoclerodanes as a novel structural class of MOP receptor ligands. To further explore the structure-affinity relationships of this interesting class of compounds, we synthesized additional neoclerodanes from 12.124,125 Our efforts showed that chain lengthening in the C-2 position generally decreases affinity for KOPs and increases affinity to MOPs and C-2 esters appear to bind in a different manner than do C-2 sulfonates at both KOPs and MOPs. Furthermore, we found benzamide 20 to be the most potent neoclerodane MOP agonist described to date.125
GPCR desensitization and trafficking are important regulators of opioid receptor signaling that can modulate drug responsiveness in vivo. For example, morphine binding produces a MOP receptor with low affinity for β-arrestin proteins and limited receptor internalization, whereas DAMGO, a peptide selective for MOP receptors, promotes robust trafficking of β-arrestins and receptor internalization. Given its unique structure relative to other MOP agonists, we evaluated the effects of 18 on MOP receptor trafficking and internalization.126 We found that 18, unlike other MOP receptor ligands, does not promote the recruitment of β-arrestin-2 to MOP receptors and does not lead to receptor internalization under any of the conditions tested. Studies in mice have shown that β-arrestin-2 plays an important role in the development of morphine-induced tolerance, constipation, and respiratory depression.127–130 Considering the important role MOP receptor regulation plays in determining physiological responsiveness to opioid narcotics, other MOP preferring natural products may offer a unique template for the development of functionally selective MOP receptor ligands with the ability to produce analgesia while limiting adverse side effects.125
Other studies in our laboratory have focused on gaining a better understanding the role of the furan ring present in 12. These studies were initiated based on our discovery of 16 and 17, as well as the desirability of reducing the potential for hepatotoxicity by 12. Previous studies have shown that teucrin A (21), a neoclerodane present in germander (Teucrium chamaedrys L.; Lamiaceae), has the ability to form a reactive metabolite resulting from bioactivation of the furan ring by cytochrome P450 enzymes (CYP450s).131–134 By analogy, as a furan containing neoclerodane, 12 has the potential to also form reactive metabolites resulting from bioactivation by CYP450s. Our approach to overcoming this pitfall was to explore structural modifications of the furan ring. Generally, we found that modification of the furan ring of 12 resulted in neoclerodanes with reduced efficacy at opioid receptors.121,122 Interestingly, we identified oxadiazole 22 as the first neoclerodane with KOP-antagonist activity and bromo analogue 23 was identified as a potent KOP agonist. In addition, we reported the synthesis of salvidivins A (24) and B (25), two natural products formed in commercially available S. divinorum leaves from 12.93 Collectively, these results indicate that additional structural modifications of 12 may lead to analogues with higher potency and utility as drug abuse medications.
Recently, the methoxymethyl ether of salvinorin B (26a) was identified as a more potent and longer lasting in vivo analogue of 12.135,136 Similarly, the N-methylacetamide analogue of 12 (27) was found to have similar in vitro potency and selectivity compared to 12 but also has improved stability and longer lasting actions in vivo.137 Additional structure-activity relationship studies have identified the ethoxymethyl ether of salvinorin B (26b) as the most potent neoclerodane KOP agonist described to date.138
Other Natural Products Leads
As an opiomimetic, 12 has a unique molecular scaffold for development and opens many interesting questions regarding molecular recognition. However, the neoclerodane nucleus of 12 is readily found in nature and many similar compounds have been identified.139,140 Recently, we have begun to explore other naturally occurring and semisynthetic neoclerodanes for their ability to interact with opioid receptors. Several structural congeners of 12 isolated from Salvia splendens Ker Gawl. (Lamiaceae) together with a series of semisynthetic derivatives, were tested for affinity at human opioid receptors.141 None of these compounds showed high affinity binding to these receptors but 28 showed modest affinity for KOP receptors. However, this indicates that other naturally occurring neoclerodanes may indeed possess opioid affinity and activity. Furthermore, this suggests the likelihood of identifying other non-nitrogenous opioids from natural sources. To further explore this possibility, we have begun to study additional psychoactive plants in search of structurally unique opioids.
Extracts of St. John’s Wort (Hypericum perforatum L.; Clusiaceae) have been shown to attenuate alcohol self-administration in different strains of alcohol-preferring rats.142,143 The endogenous opioid system plays a key role in the rewarding properties of alcohol and opioid receptor antagonists are used clinically to treat alcohol abuse.144,145 Interestingly, H. perforatum extracts have also been shown to act synergistically with opioid receptor antagonists to attenuate ethanol intake in rats and inhibit the binding of [3H]naloxone and [3H]deltorphin to opioid receptors.146–148 Furthermore, amentoflavone (29), a biflavone present in extracts of H. perforatum, was found to bind to opioid receptors.149 Efforts in our laboratory found that 29 is a KOP receptor antagonist more than 10-fold selective over the DOP receptor.150 This was the first report of a flavonoid with antagonist activity and opens a new structural scaffold for the development of opioid antagonists. Additional structure-activity relationship studies found that hyperoside (30), another flavonoid present in extracts of H. perforatum, and (−)-epigallocatechin (31), a catechin found in green tea, also have KOP antagonist activity in vitro.150 Collectively, these findings provide evidence that additional investigation of natural sources will identify new leads for opioid receptors and potentially other CNS targets.
Summary and Perspective
As well described elsewhere, natural products have played an important role in the development of medications for a number of diseases.151–153 However, the search for natural products with utility in the neurosciences is an area much less developed than the search for anticancer agents.154 Investigation of psychoactive natural products, such as 12, provides an opportunity to identify novel scaffolds and selective agents to better characterize known receptor types and study their role in various disorders. As highlighted above, these investigations have identified novel agents to potentially treat drug abuse. However, they also have the potential to identify novel agents to other complex CNS disorders such as anxiety, chronic pain, depression, and schizophrenia.
The application of natural products chemistry to neuroscience drug discovery is not without problems or limitations. In contrast to the search for anticancer drugs, there are few, if any, simple, relevant prescreen assays such as the brine shrimp test.155 Historically, radioligand binding assays have been used to drive bioguided fractionation but this method is not optimal as it generates large amounts of radioactive waste. Recent developments in cell-based assays may provide more user-friendly approaches.
Many of the most interesting targets in the neurosciences, such as GPCRs, ion channels, and transporters, trigger Ca2+ mobilization upon activation.156 Currently used cell-based assays are focused on the detection of intracellular Ca2+ and use various fluorescence probes to measure levels of increased concentrations of calcium called FLIPR.157,158 Another available method uses aequorin-derived luminescence to monitor intracellular Ca2+ levels.159 Aequorin is a photoprotein isolated from the jelly fish Aequorea victoria that has been used as a calcium indicator for more than three decades.160,161 The active protein is formed in the presence of molecular oxygen from apoaequorin and its cofactor, coelenterazine.162 Upon calcium binding, aequorin oxidizes coelenterazine into coelenteramide with production of CO2 and emission of light which is a reliable tool for measurement of intracellular Ca2+ flux.163 This method is amendable for HTS assays where many samples can be tested. Furthermore, a Tango assay to monitor protein interactions in a cell with a high degree of selectivity and sensitivity has been recently developed.164 In this assay, a transcription factor is tethered to a membrane-bound receptor with a linker that contains a cleavage site for a specific protease. Activation of the receptor of interest recruits a signaling protein fused to the protease that then cleaves and releases the transcription factor to activate reporter genes in the nucleus which can then be observed. Unfortunately, few reports have used these cell-based assays to identify novel CNS active natural products. This is likely due to their expense and the fact that these approaches are not widely employed.
Over the past decade, we have witnessed unparalleled advances in our understanding of the basic biological processes that contribute to many human disorders, although a detailed understanding of the etiology of complex CNS disorders remains elusive.165 However, this lack of detailed understanding offers a unique opportunity for natural products chemists working in collaboration with pharmacologists. Expanding the exploration of natural sources with a focus on the neurosciences is likely to identify novel active agents that may serve as drug leads and new chemical tools to better understand the etiology of complex CNS disorders.
Acknowledgments
The author thanks all former and current group members who have contributed to this research program and Drs. R. B. Rothman, E. R. Butelman, L. M. Bohn, H. A. Navarro, and K. G. Holden for fruitful collaborations. Financial support from the National Institute on Drug Abuse (R01 DA18151) and the Universities of Iowa and Kansas is gratefully acknowledged.
Footnotes
Dedicated to Dr. David G. I. Kingston of Virginia Polytechnic Institute and State University for his pioneering work on bioactive natural products. Adapted from a Matt Suffness (Young Investigator) Award address, 7th Joint Meeting of AFERP, ASP, GA, PSE & SIF and 49th Annual Meeting of the American Society for Pharmacognosy, Athens, Greece, August 3 – 8, 2008.
References and Notes
- 1.Spinella M. The Psychopharmacology of Herbal Medicine: Plant Drugs that Alter Mind, Brain, and Behavior. MIT Press; Cambridge, MA: 2001. [Google Scholar]
- 2.McKenna DJ. Behav Brain Res. 1996;73:109–116. doi: 10.1016/0166-4328(96)00079-4. [DOI] [PubMed] [Google Scholar]
- 3.Calixto JB, Scheidt C, Otuki M, Santos AR. Expert Opin Emerg Drugs. 2001;6:261–279. doi: 10.1517/14728214.6.2.261. [DOI] [PubMed] [Google Scholar]
- 4.Aldrich JV, Vigil-Cruz SC. In: Burger’s Medicinal Chemistry and Drug Discovery. 6. Abraham DA, editor. Vol. 6. John Wiley; New York: 2003. pp. 329–441. [Google Scholar]
- 5.Waldhoer M, Bartlett SE, Whistler JL. Annu Rev Biochem. 2004;73:953–990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- 6.Di Marzo V. Rev Physiol Biochem Pharmacol. 2008;160:1–24. doi: 10.1007/112_0505. [DOI] [PubMed] [Google Scholar]
- 7.Seamon KB, Padgett W, Daly JW. Proc Natl Acad Sci USA. 1981;78:3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Insel PA, Ostrom RS. Cell Mol Neurobiol. 2003;23:305–314. doi: 10.1023/A:1023684503883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slattery DA, Hudson AL, Nutt DJ. Fundam Clin Pharmacol. 2004;18:1–21. doi: 10.1111/j.1472-8206.2004.00195.x. [DOI] [PubMed] [Google Scholar]
- 10.McCoy CB, Inciardi JA. Sex, Drugs, and the Continuing Spread of AIDS. Roxbury; Los Angeles: 1995. [Google Scholar]
- 11.Mitscher LA, Baker W. Med Res Rev. 1998;18:363–374. doi: 10.1002/(sici)1098-1128(199811)18:6<363::aid-med1>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 12.National Drug Intelligence Center. National Drug Threat Assessment 2004. U.S. Department of Justice; Johnstown, PA: 2004. Report 2004-Q0317-002. [Google Scholar]
- 13.Rawson RA, Anglin MD, Ling W. J Addict Dis. 2002;21:5–19. doi: 10.1300/j069v21n01_02. [DOI] [PubMed] [Google Scholar]
- 14.Anglin MD, Burke C, Perrochet B, Stamper E, Dawud-Noursi S. J Psychoact Drugs. 2000;32:137–141. doi: 10.1080/02791072.2000.10400221. [DOI] [PubMed] [Google Scholar]
- 15.Al-Motarreb A, Baker K, Broadley KJ. Phytother Res. 2002;16:403–413. doi: 10.1002/ptr.1106. [DOI] [PubMed] [Google Scholar]
- 16.Leshner AI. Science. 1997;278:45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- 17.Carroll FI, Howell LL, Kuhar MJ. J Med Chem. 1999;42:2721–2736. doi: 10.1021/jm9706729. [DOI] [PubMed] [Google Scholar]
- 18.Kulkarni SS, Newman AH, Houlihan WJ. J Med Chem. 2002;45:4119–4127. doi: 10.1021/jm0102093. [DOI] [PubMed] [Google Scholar]
- 19.Kreek MJ, LaForge KS, Butelman E. Nat Rev Drug Discov. 2002;1:710–726. doi: 10.1038/nrd897. [DOI] [PubMed] [Google Scholar]
- 20.Prisinzano T, Rice KC, Baumann MH, Rothman RB. Curr Med Chem CNS Agents. 2004;4:47–59. [Google Scholar]
- 21.Rothman RB, Baumann MH, Prisinzano TE, Newman AH. Biochem Pharmacol. 2008;75:2–16. doi: 10.1016/j.bcp.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dackis CA, Gold MS. Neurosci Biobehav Rev. 1985;9:469–477. doi: 10.1016/0149-7634(85)90022-3. [DOI] [PubMed] [Google Scholar]
- 23.Gawin FH. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- 24.Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG. J Clin Psychopharmacol. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Neuropsychopharmacology. 2007;33:761–768. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- 26.Vocci FJ, Acri J, Elkashef A. Am J Psychiatry. 2005;162:1432–1440. doi: 10.1176/appi.ajp.162.8.1432. [DOI] [PubMed] [Google Scholar]
- 27.Preti A. Addict Biol. 2007;12:133–151. doi: 10.1111/j.1369-1600.2007.00061.x. [DOI] [PubMed] [Google Scholar]
- 28.Prisinzano TE, Tidgewell K, Harding WW. AAPS J. 2005;7:E592–E599. doi: 10.1208/aapsj070361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prisinzano TE, Rothman RB. Chem Rev. 2008;108:1732–1743. doi: 10.1021/cr0782269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothman RB. Analgesia. 1994;1:27–49. [Google Scholar]
- 31.Zaki PA, Bilsky EJ, Vanderah TW, Lai J, Evans CJ, Porreca F. Annu Rev Pharmacol Toxicol. 1996;36:379–401. doi: 10.1146/annurev.pa.36.040196.002115. [DOI] [PubMed] [Google Scholar]
- 32.Pasternak GW. Neuropharmacology. 2004;47(Suppl 1):312–323. doi: 10.1016/j.neuropharm.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Kieffer BL, Gaveriaux-Ruff C. Prog Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 34.Mello NK, Negus SS. Ann NY Acad Sci. 2000;909:104–132. doi: 10.1111/j.1749-6632.2000.tb06678.x. [DOI] [PubMed] [Google Scholar]
- 35.Shippenberg TS, Chefer VI, Zapata A, Heidbreder CA. Ann NY Acad Sci. 2001;937:50–73. doi: 10.1111/j.1749-6632.2001.tb03558.x. [DOI] [PubMed] [Google Scholar]
- 36.Shippenberg TS, Zapata A, Chefer VI. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Werling L, Frattali A, Portoghese P, Takemori A, Cox B. J Pharmacol Exp Ther. 1988;246:282–286. [PubMed] [Google Scholar]
- 38.Di Chiara G, Imperato A. J Pharmacol Exp Ther. 1988;244:1067–1080. [PubMed] [Google Scholar]
- 39.Di Chiara G, Imperato A. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spanagel R, Herz A, Shippenberg TS. J Neurochem. 1990;55:1734–1740. doi: 10.1111/j.1471-4159.1990.tb04963.x. [DOI] [PubMed] [Google Scholar]
- 41.Spanagel R, Herz A, Shippenberg T. Proc Natl Acad Sci USA. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackisch R, Hotz H, Hertting G. Naunyn-Schmiedebergs Arch Pharmacol. 1993;348:234–241. doi: 10.1007/BF00169150. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki T, Kishimoto Y, Ozaki S, Narita M. Eur J Pain. 2001;5:63–65. doi: 10.1053/eujp.2001.0282. [DOI] [PubMed] [Google Scholar]
- 44.Margolis EB, Hjelmstad GO, Bonci A, Fields HL. J Neurosci. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glick SD, Maisonneuve IM, Raucci J, Archer S. Brain Res. 1995;681:147–152. doi: 10.1016/0006-8993(95)00306-b. [DOI] [PubMed] [Google Scholar]
- 46.Crawford CA, McDougall SA, Bolanos CA, Hall S, Berger SP. Psychopharmacology. 1995;120:392–399. doi: 10.1007/BF02245810. [DOI] [PubMed] [Google Scholar]
- 47.Mello NK, Negus SS. J Pharmacol Exp Ther. 1998;286:812–824. [PubMed] [Google Scholar]
- 48.Negus SS, Mello NK, Portoghese PS, Lin CE. J Pharmacol Exp Ther. 1997;282:44–55. [PubMed] [Google Scholar]
- 49.Schenk S, Partridge B, Shippenberg TS. Psychopharmacology. 1999;144:339–346. doi: 10.1007/s002130051016. [DOI] [PubMed] [Google Scholar]
- 50.Schenk S, Partridge B, Shippenberg TS. Psychopharmacology. 2000;151:85–90. doi: 10.1007/s002130000476. [DOI] [PubMed] [Google Scholar]
- 51.Hasebe K, Kawai K, Suzuki T, Kawamura K, Tanaka T, Narita M, Nagase H, Suzuki T. Ann NY Acad Sci. 2004;1025:404–413. doi: 10.1196/annals.1316.050. [DOI] [PubMed] [Google Scholar]
- 52.Redila V, Chavkin C. Psychopharmacology. 2008;200:59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 54.Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 55.Epstein D, Preston K, Stewart J, Shaham Y. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beardsley PM, Howard JL, Shelton KL, Carroll FI. Psychopharmacology. 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- 57.Carey AN, Borozny K, Aldrich JV, McLaughlin JP. Eur J Pharmacol. 2007;569:84–89. doi: 10.1016/j.ejphar.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr J Pharmacol Exp Ther. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- 59.Portoghese PS, Lipkowski AW, Takemori AE. Life Sci. 1987;40:1287–1292. doi: 10.1016/0024-3205(87)90585-6. [DOI] [PubMed] [Google Scholar]
- 60.Portoghese PS, Lipkowski AW, Takemori AE. J Med Chem. 1987;30:238–239. doi: 10.1021/jm00385a002. [DOI] [PubMed] [Google Scholar]
- 61.Ko MCH, Johnson MD, Butelman ER, Willmont KJ, Mosberg HI, Woods JH. J Pharmacol Exp Ther. 1999;291:1113–1120. [PMC free article] [PubMed] [Google Scholar]
- 62.Portoghese PS, Nagase H, Lipkowski AW, Larson DL, Takemori AE. J Med Chem. 1988;31:836–841. doi: 10.1021/jm00399a026. [DOI] [PubMed] [Google Scholar]
- 63.Portoghese PS, Nagase H, Takemori AE. J Med Chem. 1988;31:1344–1347. doi: 10.1021/jm00402a015. [DOI] [PubMed] [Google Scholar]
- 64.Portoghese PS, Garzon-Aburbeh A, Nagase H, Lin CE, Takemori AE. J Med Chem. 1991;34:1292–1296. doi: 10.1021/jm00108a008. [DOI] [PubMed] [Google Scholar]
- 65.Olmsted SL, Takemori AE, Portoghese PS. J Med Chem. 1993;36:179–180. doi: 10.1021/jm00053a025. [DOI] [PubMed] [Google Scholar]
- 66.Portoghese PS, Lin CE, Farouz-Grant F, Takemori AE. J Med Chem. 1994;37:1495–500. doi: 10.1021/jm00036a015. [DOI] [PubMed] [Google Scholar]
- 67.Jones RM, Portoghese PS. Eur J Pharmacol. 2000;396:49–52. doi: 10.1016/s0014-2999(00)00208-9. [DOI] [PubMed] [Google Scholar]
- 68.Stevens WC, Jr, Jones RM, Subramanian G, Metzger TG, Ferguson DM, Portoghese PS. J Med Chem. 2000;43:2759–2769. doi: 10.1021/jm0000665. [DOI] [PubMed] [Google Scholar]
- 69.Thomas JB, Fall MJ, Cooper JB, Rothman RB, Mascarella SW, Xu H, Partilla JS, Dersch CM, McCullough KB, Cantrell BE, Zimmerman DM, Carroll FI. J Med Chem. 1998;41:5188–5197. doi: 10.1021/jm980511k. [DOI] [PubMed] [Google Scholar]
- 70.Thomas JB, Atkinson RN, Rothman RB, Fix SE, Mascarella SW, Vinson NA, Xu H, Dersch CM, Lu Y, Cantrell BE, Zimmerman DM, Carroll FI. J Med Chem. 2001;44:2687–2690. doi: 10.1021/jm015521r. [DOI] [PubMed] [Google Scholar]
- 71.Thomas JB, Atkinson RN, Namdev N, Rothman RB, Gigstad KM, Fix SE, Mascarella SW, Burgess JP, Vinson NA, Xu H, Dersch CM, Cantrell BE, Zimmerman DM, Carroll FI. J Med Chem. 2002;45:3524–3530. doi: 10.1021/jm020084h. [DOI] [PubMed] [Google Scholar]
- 72.Thomas JB, Atkinson RN, Vinson NA, Catanzaro JL, Perretta CL, Fix SE, Mascarella SW, Rothman RB, Xu H, Dersch CM, Cantrell BE, Zimmerman DM, Carroll FI. J Med Chem. 2003;46:3127–3137. doi: 10.1021/jm030094y. [DOI] [PubMed] [Google Scholar]
- 73.Cai TB, Zou Z, Thomas JB, Brieaddy L, Navarro HA, Carroll FI. J Med Chem. 2008;51:1849–1860. doi: 10.1021/jm701344b. [DOI] [PubMed] [Google Scholar]
- 74.Carroll I, Thomas JB, Dykstra LA, Granger AL, Allen RM, Howard JL, Pollard GT, Aceto MD, Harris LS. Eur J Pharmacol. 2004;501:111–119. doi: 10.1016/j.ejphar.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 75.Carroll FI, Harris LS, Aceto MD. Eur J Pharmacol. 2005;524:89–94. doi: 10.1016/j.ejphar.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 76.Pfeiffer A, Brantl V, Herz A, Emrich HM. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- 77.Negus SS. Psychopharmacology. 2004;176:204–213. doi: 10.1007/s00213-004-1878-7. [DOI] [PubMed] [Google Scholar]
- 78.McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Neuropsychopharmacology. 2005;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Metcalf MD, Coop A. AAPS J. 2005;7:E704–E722. doi: 10.1208/aapsj070371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bruchas MR, Yang T, Schreiber S, Defino M, Kwan SC, Li S, Chavkin C. J Biol Chem. 2007;282:29803–29811. doi: 10.1074/jbc.M705540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kintzios SE. Sage: The Genus Salvia. Harwood Academic Publishers; Amsterdam: 2000. [Google Scholar]
- 82.Epling C, Jativa-M CD. Bot Museum Leaflets, Harvard Univ. 1962;20:75–76. [Google Scholar]
- 83.Tyler VE. Lloydia. 1966;29:275–292. [Google Scholar]
- 84.Valdes LJ., III . Ph.D. Thesis. University of Michigan; Ann Arbor, MI: 1983. The Pharmacognosy of Salvia Divinorum (Epling and Jativa-M): An Investigation of Ska Maria Pastora. [Google Scholar]
- 85.Ortega A, Blount JF, Manchand PS. J Chem Soc, Perkin Trans. 1;1982:2505–2508. [Google Scholar]
- 86.Valdes LJ, III, Butler WM, Hatfield GM, Paul AG, Koreeda M. J Org Chem. 1984;49:4716–4720. [Google Scholar]
- 87.Siebert DJ. J Ethnopharmacol. 1994;43:53–56. doi: 10.1016/0378-8741(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 88.Valdes LJ., III J Psychoact Drugs. 1994;26:277–283. doi: 10.1080/02791072.1994.10472441. [DOI] [PubMed] [Google Scholar]
- 89.Valdes LJ, III, Chang HM, Visger DC, Koreeda M. Org Lett. 2001;3:3935–3937. doi: 10.1021/ol016820d. [DOI] [PubMed] [Google Scholar]
- 90.Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB. Proc Natl Acad Sci USA. 2002;99:11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Munro TA, Rizzacasa MA. J Nat Prod. 2003;66:703–705. doi: 10.1021/np0205699. [DOI] [PubMed] [Google Scholar]
- 92.Lee DY, Ma Z, Liu-Chen LY, Wang Y, Chen Y, Carlezon WA, Jr, Cohen B. Bioorg Med Chem. 2005;13:5635–5639. doi: 10.1016/j.bmc.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 93.Shirota O, Nagamatsu K, Sekita S. J Nat Prod. 2006;69:1782–1786. doi: 10.1021/np060456f. [DOI] [PubMed] [Google Scholar]
- 94.Ma Z, Lee DYW. Tetrahedron Lett. 2007;48:5461–5464. doi: 10.1016/j.tetlet.2007.05.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bigham AK, Munro TA, Rizzacasa MA, Robins-Browne RM. J Nat Prod. 2003;66:1242–1244. doi: 10.1021/np030313i. [DOI] [PubMed] [Google Scholar]
- 96.Capasso R, Borrelli F, Zjawiony J, Kutrzeba L, Aviello G, Sarnelli G, Capasso F, Izzo AA. Neurogastroenterol Motil. 2008;20:142–148. doi: 10.1111/j.1365-2982.2007.00994.x. [DOI] [PubMed] [Google Scholar]
- 97.Capasso R, Borrelli F, Cascio MG, Aviello G, Huben K, Zjawiony JK, Marini P, Romano B, Di Marzo V, Capasso F, Izzo AA. Br J Pharmacol. 2008;155:681–689. doi: 10.1038/bjp.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Capasso R, Borrelli F, Capasso F, Siebert DJ, Stewart DJ, Zjawiony JK, Izzo AA. Neurogastroenterol Motil. 2006;18:69–75. doi: 10.1111/j.1365-2982.2005.00725.x. [DOI] [PubMed] [Google Scholar]
- 99.Kutrzeba L, Dayan FE, Howell JL, Feng J, Giner JL, Zjawiony JK. Phytochemistry. 2007;68:1872–1881. doi: 10.1016/j.phytochem.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Scheerer JR, Lawrence JF, Wang GC, Evans DA. J Am Chem Soc. 2007;129:8968–8969. doi: 10.1021/ja073590a. [DOI] [PubMed] [Google Scholar]
- 101.Nozawa M, Suka Y, Hoshi T, Suzuki T, Hagiwara H. Org Lett. 2008;10:1365–1368. doi: 10.1021/ol800101v. [DOI] [PubMed] [Google Scholar]
- 102.Butelman ER, Harris TJ, Kreek MJ. Psychopharmacology. 2004;172:220–224. doi: 10.1007/s00213-003-1638-0. [DOI] [PubMed] [Google Scholar]
- 103.Willmore-Fordham CB, Krall DM, McCurdy CR, Kinder DH. Neuropharmacology. 2007;53:481–486. doi: 10.1016/j.neuropharm.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 104.John TF, French LG, Erlichman JS. Eur J Pharmacol. 2006;545:129–133. doi: 10.1016/j.ejphar.2006.06.077. [DOI] [PubMed] [Google Scholar]
- 105.McCurdy CR, Sufka KJ, Smith GH, Warnick JE, Nieto MJ. Pharmacol Biochem Behav. 2006;83:109–113. doi: 10.1016/j.pbb.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Psychopharmacology. 2005;179:551–558. doi: 10.1007/s00213-004-2087-0. [DOI] [PubMed] [Google Scholar]
- 107.Carlezon WA, Jr, Beguin C, Dinieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- 108.Fantegrossi WE, Kugle KM, Valdes LJ, 3rd, Koreeda M, Woods JH. Behav Pharmacol. 2005;16:627–633. doi: 10.1097/00008877-200512000-00005. [DOI] [PubMed] [Google Scholar]
- 109.Chartoff EH, Potter D, Damez-Werno D, Cohen BM, Carlezon WA., Jr Neuropsychopharmacology. 2008;33:2676–2687. doi: 10.1038/sj.npp.1301659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li JX, Rice KC, France CP. J Pharmacol Exp Ther. 2008;324:827–833. doi: 10.1124/jpet.107.130625. [DOI] [PubMed] [Google Scholar]
- 111.Gehrke B, Chefer V, Shippenberg T. Psychopharmacology. 2008;197:509–517. doi: 10.1007/s00213-007-1067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Inan S, Lee DY, Liu-Chen LY, Cowan A. Naunyn-Schmiedebergs Arch Pharmacol. 2008 doi: 10.1007/s00210-008-0358-8. [DOI] [PubMed] [Google Scholar]
- 113.Butelman ER, Mandau M, Tidgewell K, Prisinzano TE, Yuferov V, Kreek MJ. J Pharmacol Exp Ther. 2007;320:300–306. doi: 10.1124/jpet.106.112417. [DOI] [PubMed] [Google Scholar]
- 114.Butelman ER, Prisinzano TE, Deng H, Rus S, Kreek MJ. J Pharmacol Exp Ther. 2008 doi: 10.1124/jpet.108.145342. jpet.108.145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schmidt MD, Schmidt MS, Butelman ER, Harding WW, Tidgewell K, Murry DJ, Kreek MJ, Prisinzano TE. Synapse. 2005;58:208–210. doi: 10.1002/syn.20191. [DOI] [PubMed] [Google Scholar]
- 116.Schmidt MS, Prisinzano TE, Tidgewell K, Harding WW, Butelman ER, Kreek MJ, Murry DJ. J Chromatogr B. 2005;818:221–225. doi: 10.1016/j.jchromb.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 117.Hooker JM, Xu Y, Schiffer W, Shea C, Carter P, Fowler JS. NeuroImage. 2008;41:1044–1050. doi: 10.1016/j.neuroimage.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rees DC, Hunter JC. In: Comprehensive Medicinal Chemistry. Emmet JC, editor. Pergamon; New York: 1990. pp. 805–846. [Google Scholar]
- 119.Harding WW, Tidgewell K, Schmidt M, Shah K, Dersch CM, Snyder J, Parrish D, Deschamps JR, Rothman RB, Prisinzano TE. Org Lett. 2005;7:3017–3020. doi: 10.1021/ol0510522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Harding WW, Schmidt M, Tidgewell K, Kannan P, Holden KG, Gilmour B, Navarro H, Rothman RB, Prisinzano TE. J Nat Prod. 2006;69:107–112. doi: 10.1021/np050398i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Harding WW, Schmidt M, Tidgewell K, Kannan P, Holden KG, Dersch CM, Rothman RB, Prisinzano TE. Bioorg Med Chem Lett. 2006;16:3170–3174. doi: 10.1016/j.bmcl.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 122.Simpson DS, Katavic PL, Lozama A, Harding WW, Parrish D, Deschamps JR, Dersch CM, Partilla JS, Rothman RB, Navarro H, Prisinzano TE. J Med Chem. 2007;50:3596–3603. doi: 10.1021/jm070393d. [DOI] [PubMed] [Google Scholar]
- 123.Harding WW, Tidgewell K, Byrd N, Cobb H, Dersch CM, Butelman ER, Rothman RB, Prisinzano TE. J Med Chem. 2005;48:4765–4771. doi: 10.1021/jm048963m. [DOI] [PubMed] [Google Scholar]
- 124.Tidgewell K, Harding WW, Lozama A, Cobb H, Shah K, Kannan P, Dersch CM, Parrish D, Deschamps JR, Rothman RB, Prisinzano TE. J Nat Prod. 2006;69:914–918. doi: 10.1021/np060094b. [DOI] [PubMed] [Google Scholar]
- 125.Tidgewell K, Groer CE, Harding WW, Lozama A, Schmidt M, Marquam A, Hiemstra J, Partilla JS, Dersch CM, Rothman RB, Bohn LM, Prisinzano TE. J Med Chem. 2008;51:2421–2431. doi: 10.1021/jm701162g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, Bohn LM. Mol Pharmacol. 2007;71:549–557. doi: 10.1124/mol.106.028258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 128.Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- 129.Bohn LM, Lefkowitz RJ, Caron MG. J Neurosci. 2002;22:10494–10500. doi: 10.1523/JNEUROSCI.22-23-10494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Raehal KM, Walker JKL, Bohn LM. J Pharmacol Exp Ther. 2005;314:1195–1201. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- 131.Kouzi SA, McMurtry RJ, Nelson SD. Chem Res Toxicol. 1994;7:850–856. doi: 10.1021/tx00042a020. [DOI] [PubMed] [Google Scholar]
- 132.Dalvie DK, Kalgutkar AS, Khojasteh-Bakht SC, Obach RS, O’Donnell JP. Chem Res Toxicol. 2002;15:269–299. doi: 10.1021/tx015574b. [DOI] [PubMed] [Google Scholar]
- 133.Druckova A, Marnett LJ. Chem Res Toxicol. 2006;19:1330–1340. doi: 10.1021/tx060143k. [DOI] [PubMed] [Google Scholar]
- 134.Druckova A, Mernaugh RL, Ham AJ, Marnett LJ. Chem Res Toxicol. 2007;20:1393–1408. doi: 10.1021/tx7001405. [DOI] [PubMed] [Google Scholar]
- 135.Lee DYW, Karnati VVR, He M, Liu-Chen LY, Kondaveti L, Ma Z, Wang Y, Chen Y, Beguin C. Bioorg Med Chem Lett. 2005;15:3744–3747. doi: 10.1016/j.bmcl.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 136.Wang Y, Chen Y, Xu W, Lee DYW, Ma Z, Rawls SM, Cowan A, Liu-Chen LY. J Pharmacol Exp Ther. 2008;324:1073–1083. doi: 10.1124/jpet.107.132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Beguin C, Potter DN, DiNieri JA, Munro TA, Richards MR, Paine TA, Berry L, Zhao Z, Roth BL, Xu W, Liu-Chen L-Y, Carlezon WA, Jr, Cohen BM. J Pharmacol Exp Ther. 2008;324:188–195. doi: 10.1124/jpet.107.129023. [DOI] [PubMed] [Google Scholar]
- 138.Munro TA, Duncan KK, Xu W, Wang Y, Liu-Chen L-Y, Carlezon WA, Jr, Cohen BM, Beguin C. Bioorg Med Chem. 2008;16:1279–1286. doi: 10.1016/j.bmc.2007.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hanson JR. Nat Prod Rep. 2006;23:875–885. doi: 10.1039/b516326a. [DOI] [PubMed] [Google Scholar]
- 140.Hanson JR. Nat Prod Rep. 2007;24:1332–1341. doi: 10.1039/b705951p. [DOI] [PubMed] [Google Scholar]
- 141.Fontana G, Savona G, Rodríguez B, Dersch CM, Rothman RB, Prisinzano TE. Tetrahedron. 2008;64:10041–10048. doi: 10.1016/j.tet.2008.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rezvani AH, Overstreet DH, Perfumi M, Massi M. Pharmacol Biochem Behav. 2003;75:593–606. doi: 10.1016/s0091-3057(03)00124-2. [DOI] [PubMed] [Google Scholar]
- 143.Overstreet DH, Keung WM, Rezvani AH, Massi M, Lee DY. Alcohol Clin Exp Res. 2003;27:177–185. doi: 10.1097/01.ALC.0000051022.26489.CF. [DOI] [PubMed] [Google Scholar]
- 144.Herz A. Psychopharmacology. 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- 145.Oswald LM, Wand GS. Physiol Behav. 2004;81:339–358. doi: 10.1016/j.physbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 146.Simmen U, Schweitzer C, Burkard W, Schaffner W, Lundstrom K. Pharm Acta Helv. 1998;73:53–56. doi: 10.1016/s0031-6865(97)00049-6. [DOI] [PubMed] [Google Scholar]
- 147.Simmen U, Burkard W, Berger K, Schaffner W, Lundstrom K. J Recept Signal Transduct Res. 1999;19:59–74. doi: 10.3109/10799899909036637. [DOI] [PubMed] [Google Scholar]
- 148.Perfumi M, Santoni M, Cippitelli A, Ciccocioppo R, Froldi R, Massi M. Alcohol Clin Exp Res. 2003;27:1554–1562. doi: 10.1097/01.ALC.0000092062.60924.56. [DOI] [PubMed] [Google Scholar]
- 149.Butterweck V, Nahrstedt A, Evans J, Hufeisen S, Rauser L, Savage J, Popadak B, Ernsberger P, Roth BL. Psychopharmacology. 2002;162:193–202. doi: 10.1007/s00213-002-1073-7. [DOI] [PubMed] [Google Scholar]
- 150.Katavic PL, Lamb K, Navarro H, Prisinzano TE. J Nat Prod. 2007;70:1278–1282. doi: 10.1021/np070194x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Butler MS. Nat Prod Rep. 2005;22:162–195. doi: 10.1039/b402985m. [DOI] [PubMed] [Google Scholar]
- 152.Wilkinson B, Micklefield J. Nat Chem Biol. 2007;3:379–386. doi: 10.1038/nchembio.2007.7. [DOI] [PubMed] [Google Scholar]
- 153.Newman DJ, Cragg GM. J Nat Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 154.Clement JA, Yoder BJ, Kingston DGI. Mini Rev Org Chem. 2004;1:183–208. [Google Scholar]
- 155.Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Planta Med. 1982;45:31–34. [PubMed] [Google Scholar]
- 156.Rink TJ. FEBS Lett. 1990;268:381–385. doi: 10.1016/0014-5793(90)81290-5. [DOI] [PubMed] [Google Scholar]
- 157.Bovolenta S, Foti M, Lohmer S, Corazza S. J Biomol Screen. 2007;12:694–704. doi: 10.1177/1087057107301497. [DOI] [PubMed] [Google Scholar]
- 158.Xin H, Wang Y, Todd MJ, Qi J, Minor LK. J Biomol Screen. 2007;12:705–714. doi: 10.1177/1087057107301522. [DOI] [PubMed] [Google Scholar]
- 159.Fichna J, Gach K, Piestrzeniewicz M, Burgeon E, Poels J, Broeck JV, Janecka A. J Pharmacol Exp Ther. 2006;317:1150–1154. doi: 10.1124/jpet.105.099986. [DOI] [PubMed] [Google Scholar]
- 160.Shimomura O, Johnson FH, Saiga Y. J Cell Comp Physiol. 1962;59:223–239. doi: 10.1002/jcp.1030590302. [DOI] [PubMed] [Google Scholar]
- 161.Poul EL, Hisada S, Mizuguchi Y, Dupriez VJ, Burgeon E, Detheux M. J Biomol Screen. 2002;7:57–65. doi: 10.1177/108705710200700108. [DOI] [PubMed] [Google Scholar]
- 162.Shimomura O, Johnson FH. Nature. 1975;256:236–238. doi: 10.1038/256236a0. [DOI] [PubMed] [Google Scholar]
- 163.Brini M, Marsault R, Bastianutto C, Alvarez J, Pozzan T, Rizzuto R. J Biol Chem. 1995;270:9896–9903. doi: 10.1074/jbc.270.17.9896. [DOI] [PubMed] [Google Scholar]
- 164.Barnea G, Strapps W, Herrada G, Berman Y, Ong J, Kloss B, Axel R, Lee KJ. Proc Natl Acad Sci USA. 2008;105:64–69. doi: 10.1073/pnas.0710487105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Conn PJ, Roth BL. Neuropsychopharmacology. 2008;33:2048–2060. doi: 10.1038/sj.npp.1301638. [DOI] [PubMed] [Google Scholar]