Abstract
The antibody response elicited after immunization with vaccinia virus (VacV) is known to be sufficient to confer host protection against VacV or smallpox. In humans it has been shown that such anti-VacV antibody production can be sustained for decades. Nevertheless, little is known about the kinetics and the role in protection of the early antibody response after vaccination. In this study we identify VacV neutralizing IgM antibodies as early as four days after infection of C57BL/6 mice. Most of this IgM production is T cell dependent and predominantly independent of the germinal center reaction (SAP / SH2D1A independent). Importantly, the IgM neutralized both infectious forms of VacV: the intracellular mature virion (MV, IMV) and the extracellular enveloped virion (EV, EEV). Moreover, in mice primed with MHCII restricted peptides, an increase in the total VacV neutralizing antibody titers was seen, a large component of which was neutralizing IgM against the same protein from which the priming peptide was derived. To further demonstrate the biological relevance of this early neutralizing response, we examined anti-VacV antibodies in humans after vaccination. Human subjects could be divided into two groups early after immunization: IgGhi and IgGlo. VacV IgM neutralizing antibodies were detected in the IgGlo group. Taken together these results indicate that both in a small animal model and in humans an early neutralizing IgM response after VacV immunization is present and likely contributes to control of the infection prior to the development of a robust IgG response.
Keywords: Smallpox vaccine, Vaccinia virus, IgM, Neutralizing antibodies
Introduction
Immunization with the smallpox vaccine results in long term protection against VacV itself and other orthopoxviruses, most importantly variola (smallpox). The protective activity is mainly due to anti-VacV neutralizing antibodies [1], which are continuously produced for decades after vaccination [2-5]. Many studies have described roles of different innate and adaptive immune response cell types in the control of a primary VacV infection or vaccination [1, 6, 7]. However, while B cells and antibodies are crucial for the long term protection, the roles of B cells and antibodies in the primary response to the smallpox vaccine remain less clear [1, 8].
Mice deficient of B cells (μMT/μMT) have an increased susceptibility to VacV infection, and higher viral titers are found in μMT mice compared to wild-type (WT) controls [6], suggesting that the early antibody production has an important role in the control of the infection. Furthermore, antibodies are essential for control and clearance of the related poxvirus, ectromelia (mousepox) [9-11], while T cell responses are also critical for control of a primary ectromelia infection [9, 12]. VacV has two different infectious forms, the intracellular mature virion (IMV, MV) thought to be primarily responsible from host to host infection, and the extracellular enveloped virion (EEV, EV), responsible for much of the viral dissemination within the host [13]. Studies have demonstrated that antibodies directed against either virion form are protective in mice [14-17], and we have recently demonstrated that B5-specific anti-EV antibodies can efficiently clear virally infected cells, illustrating that the protective value of anti-VacV antibodies is not limited to free virions [18]. Moreover, in the rhesus macaque model it has been shown that B cells but not CD4+ nor CD8+ T cells are essential for protection against a lethal challenge with monkeypox virus [19]. All of these reports highlight the importance of antibodies in the control of VacV and related poxviruses.
While it is clear that neutralizing antibodies are a very potent mechanism for protection against VacV, monkeypox, and variola, the neutralizing IgG response is relatively slow to develop after a primary infection or immunization. In vaccinated humans, neutralizing IgG is reported at 2-3 weeks after vaccination [1, 20, 21], after the resolution of the primary infection and lesion. In mice, significant anti-VacV IgG titers have not been reported earlier than 8 days post-infection (i.p.), which is after the bulk of the infection has been cleared. Therefore, it appears that the neutralizing IgG response develops too late in humans and mice to be of significant value in controlling a primary VacV infection/immunization. Given that, is there any role for neutralizing antibodies in control of the primary VacV infection?
The study reported here demonstrates for the first time that there is an early induction of neutralizing IgM after VacV immunization of mice and humans, and implicates a protective role for IgM during the primary response.
Materials and Methods
Mouse Procedures
C57BL/6J (B6 or WT) and C57BL/6J Iab-Ea−/− (MHC class II−/−) mice were purchased from The Jackson Laboratory. SAP−/− mice [22] back-crossed into C57BL/6J [23] were bred in-house.
Mice were infected with the vaccinia virus Western Reserve strain (VacV) by bilateral intraperitoneal (i.p.) injection of 2×106 PFU total with standard purified VacV stocks. For peptide immunizations, 30 μg of peptide was emulsified in complete Freund adjuvant (CFA) and injected subcutaneously between the scapula, and 11–13 days after peptide immunization were infected with VacV. Serum was obtained by retro-orbital bleed at determined time points post-infection. To determine the kinetics of the appearance of neutralizing antibodies, two groups of WT mice were infected with VacV, one group was bled on odd days and the other was bled on even days. All mice were maintained in an accredited facility at LIAI, and all the experiments were conducted in accordance with approved animal protocols.
Human study subjects
A cohort of 14 normal healthy volunteer donors were immunized for the first time with the smallpox vaccine (Dryvax) and blood samples were obtained at the indicated time post-vaccination. The gender distribution of the cohort was□□~50:50, within an age range from 23-60 years.
Plasma and serum
Plasma samples from the human subjects and sera from VacV infected mice was stored as aliquots at −80°C. To eliminate IgM antibodies, aliquots were treated with an equal volume of 0.1M 2-mercaptoethanol (2-ME) in PBS for 1 h at room temperature, as described [24-26].
MV production
VacV MV stocks were produced as described in [27]. Briefly, MV were in grown on HeLa cells in D-10 (Dulbecco's modified Eagle medium [DMEM] plus 10% heat inactivated fetal calf serum [FCS] plus penicillin/streptomycin/glutamine) in T175 flasks (Falcon; Becton Dickinson), infecting at a multiplicity of infection (MOI) of 0.1 to 0.5. Cells were harvested at 2.5 to 3 days, and virus was isolated by rapidly freeze-thawing the cell pellet three times in a volume of 2.3 ml DMEM or RPMI supplemented with 1% heat inactivated FCS. Cell debris was removed by centrifugation (700×g, 8 min). Clarified supernatant was frozen at −80°C as virus stock. Titers of VacV stocks were determined with VeroE6 cells (~2×108 PFU/ml). Purified VacV stocks were made by sonication of the VacV stock (40 s) using a water sonicator (Branson Ultrasonics, CT) and layered over 36% sucrose in TM buffer (10 mM Tris-HCl [pH 7.4], 5 mM MgCl2). VacV was centrifuged (SW28 rotor) at 13,500 rpm (33,000×g) for 80 min at 4°C. The VacV pellet was resuspended in 1 ml TM buffer and then brought up to 10 ml with DMEM medium supplemented with 1% of heat inactivated FCS. Purified VacV was stored at −80°C.
EV production
VacV EV was prepared as described elsewhere [18]. Briefly, HeLa cells were cultured in D-10 in T75 flasks (Falcon, Becton Dickinson) at 90% confluence and infected with VacV at a MOI of 0.5. The medium containing EV was harvested at 2 days, and virus was isolated by centrifugating twice (450 × g, 8 min) to remove cells and debris. Clarified supernatant was used immediately or stored at 4 °C for a maximum of 3 weeks. EV VacV stocks were titrated on VeroE6 cells (~5 × 105 PFU/ml).
MV neutralization assay
Titration of VacV MV neutralizing antibodies, was performed according to Newman et al. [28]. Briefly, VeroE6 cells were seeded at 2×105 cells/well into 24-well Costar plates (Corning, Inc., Corning, NY) and used the following day (75 to 90% confluence). Total or IgM depleted plasma or serum was incubated overnight with 50 μl of freshly sonicated VacV (104 PFU/ml) at 37°C with 5% CO2. Plasma from non-vaccinated human individuals or sera from naive mice were treated under the same conditions and used as negative controls. Multiple wells of VacV-alone controls were always used. Medium was aspirated, and the samples were added and allowed to adsorb for 60 min at 37°C. Then the cells were rinsed with warm phosphate-buffered saline (PBS) and 1 ml of D-10 medium was added. Plates were incubated for 40 to 48 h and then fixed and stained with 0.1% crystal violet in 25% reagent alcohol (90% ethanol, 5% methanol, 5% isopropanol) to count viral plaques.
EV neutralization assay
VacV EV neutralization assay was described elsewhere [18]. Briefly, all the samples and FCS were heat inactivated (56°C, 30-60 min) prior to use to eliminate complement function. VeroE6 cells were prepared in 24-well Costar plates as described. The samples were incubated for 30 min at 37°C with an equal volume (50 μl) of EV stock (1:100 - 1:400 dilution) supplemented with 10% (final concentration) sterile baby rabbit complement (Cedarlane Laboratories, Ontario, Canada) and rabbit anti-L1 (1:25-1:100 final). Anti-L1 was used to neutralize the MV present in the EV stock [18, 29]. EV supplemented with anti-L1 antibody alone was regularly used in each assay +/− baby rabbit complement as negative controls. Medium from 24-well plate wells was aspirated and samples were added and allowed to adsorb for 45 minutes at 37°C. After this step the plates were treated as in the MV neutralization assay.
In both neutralization assays the PRNT50 was defined as the reciprocal of the last dilution of the plasma that reduced the average number of plaques by 50% compared to the mean number of VacV-alone plaques.
VacV proteome microarray
Production and use of protein microarrays is described elsewhere [17, 27, 30]. Briefly, VacV proteome arrays were probed with the sera or plasma samples and bound antibodies were detected with a Cy3-conjugated goat anti-mouse IgG (heavy and light chains) secondary antibody (Jackson ImmunoResearch). The arrays were examined in a GSI Lumonics ScanArray 4000 confocal glass slide scanner and intensities were quantified using QuantArray software. Results were quantified as relative fluorescent units (RU) over background. Signals from three negative control spots were averaged, and that background signal was subtracted from all spots on the array to give RU.
ELISA
For whole VacV enzyme-linked immunosorbent assay (ELISA), the antigen preparation was made by incubating unpurified VacV (108 PFU/ml) in 0.1% bovine serum albumin with 10 μg/ml trioxsalen-psoralen (4’ aminomethyl-trioxsalen HCl; Calbiochem) for 10 min at RT [31]. VacV was then UV inactivated with 2.25 J/cm2 (Stratalinker 1800; Stratagene, CA). This resulted in a >108-fold reduction in PFU. The UV-inactivated virus was then used at a 1:25 dilution in PBS to coat Nunc Polysorp flat-bottomed 96-well plates. Plates were washed and samples were added in PBS plus 0.05% Tween-20 plus 10% FCS (PBS-T+FCS). Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Caltag) or biotinylated anti-human IgG (BD-Pharmingen) followed by HRP-strepatvidin (Vector labs) diluted in PBS-T+FCS were used for detection of murine and human IgG respectively. For human IgM detection, biotinylated anti-human IgM (BD-Pharmingen) followed by HRP-strepatvidin diluted in PBS-T+FCS was used. The plates were developed using o-phenylenediamine and the optical density (OD) at 490 nm was read on a SpectraMax 250 (Molecular Devices).
For B5 ELISA, the NUNC Polysorp flat-bottomed 96-well plates were coated overnight with 50 μl of a 2 μg/ml solution of recombinant B5 protein in PBS and processed as described.
Flow cytometry
Surface staining of mouse splenocytes was done as previously described [23]. Intracellular staining was done as previously described [32, 33]. Briefly, DCs were generated by subcutaneous implantation of Flt3L-producing B16 cells and harvesting CD11c+ DCs from spleen at day 12 after implantation with CD11c paramagnetic beads (MACS Miltenyi). Splenocytes from B546-60-immunized mice (11-13 days after immunization) were incubated with peptide-pulsed CD11c+ DC for 1 hr prior to addition of brefeldin A. After 5 hr, cells were surface stained for CD62L and CD4, followed by intracellular staining for CD40L and IFN-γ or CD40L and IL-2. Cells expressing CD40L and producing IFN-γ or IL-2 were determined by gating lymphocytes on FSC/SSC and then gating on CD62Llo/CD4+ T cells. All antibodies for flow cytometry were purchased from eBiosciences or BD PharMingen.
Statistical analysis
Tests were performed using Prism 5.0 (GraphPad, San Diego, CA). Statistics were done using two-tailed, unpaired T test with 95% confidence bounds. Error bars are ± SEM.
Results
VacV infected mice produce IgM neutralizing antibodies before the production of IgG
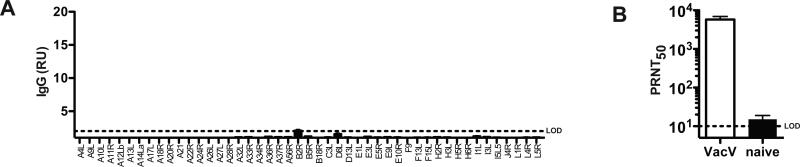
Intraperitoneal VacV infection of mice results in a potent adaptive immune response that leads to virus clearance from most tissues within seven days. Using VacV proteome microarrays, we were able to identify the first IgG specificities elicited by the virus infection [17, 33, 34]. While IgG responses were detected in most mice, a low percentage of mice exhibited very low or undetectable IgG titers at day 8 post-infection (Figure 1A), while all mice exhibited consistently high IgG titers by day 15 after infection. However, we observed that even day 8 serum samples from mice with very low anti-VacV IgG titers (Figure 1A) still consistently neutralized VacV in vitro (Figure 1B). This observation led us to hypothesize that a substantial early anti-VacV IgM neutralizing antibody response was generated after immunization with VacV, before an IgG response, and this early response contributed to host protection. To test our hypothesis, we then proceeded to determine the kinetics of the appearance of anti-VacV antibodies after infection and the effects of IgM depletion on VacV neutralization.
Figure 1. VacV neutralizing antibody titers are found early after infection.
(A) IgG titers to VacV proteins using a proteome array. Day 8 post-infection. The result is from an individual wild type (WT) mouse with a low IgG titer. RU = relative units. (B) Anti-VacV neutralizing antibody titers were quantified at day 8 post-infection in i.p. infected “VacV” and uninfected “naïve” mice (P = 0.0012). N = 6/group. Dotted line indicates limit of detection (LOD). Error bars represent SEM.
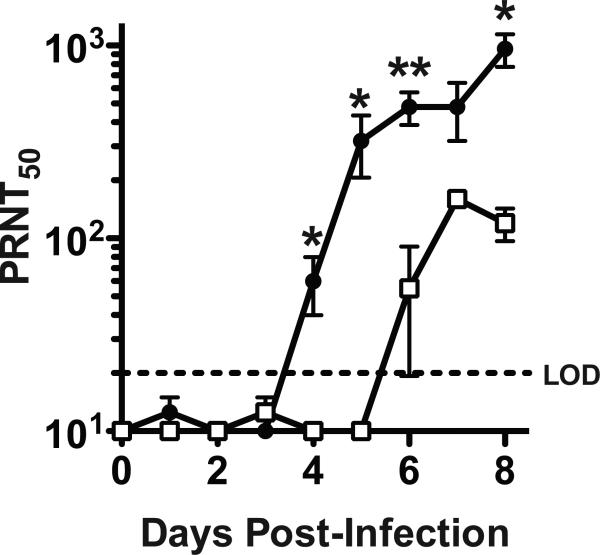
Two groups of B6 mice were infected i.p. with VacV and bled at different days to determine neutralizing antibodies in serum (Figure 2). Virus neutralization was first detected at day 4 post-infection; after that, the neutralizing antibody titers increased daily. IgM, but not IgG, can be fully inactivated by treatment with 0.1M 2-mercaptoethanol [24-26]. At days 4 and 5 post-infection all the VacV neutralization was due to IgM antibodies, as the elimination of IgM resulted in no detectable neutralizing activity (Day 4, P = 0.0153. Day 5, P = 0.0338. Figure 2). Day 6 was the earliest time point that VacV neutralizing IgG antibodies were detected (Figure 2). These results prove that the earliest anti-VacV neutralizing antibodies are of the IgM isotype.
Figure 2. Anti-VacV neutralizing antibody kinetics.
VacV neutralizing antibody titers (PRNT50) from intact serum sample (closed circles) and IgM depleted serum samples (open squares) were determined by testing daily serum samples after VacV infection. N = 4/group. Total vs IgM depleted samples at day 4 post-infection, P = 0.0153. **, P < 0.01. *, P < 0.05. Error bars represent SEM. Dotted line indicates LOD. Data is representative of 2 independent time course experiments.
Production of anti-VacV neutralizing IgM depends on CD4 T cell help but is independent of germinal centers
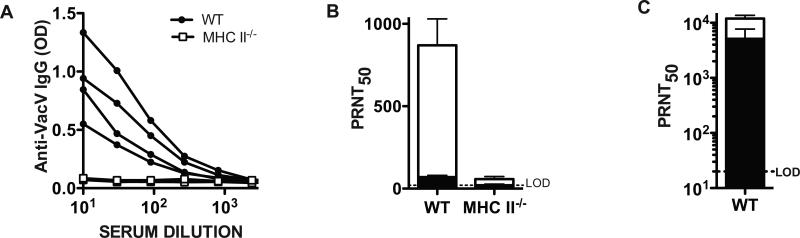
Previously we have shown that almost all of the anti-VacV IgG response is T cell help dependent (TD) [33]. Nevertheless, others have detected the presence of some T cell independent (TI) anti-VacV antibodies in the sera of infected mice [6, 26]. Therefore, we used CD4 T cell deficient mice (MHCII−/−) to evaluate the T cell help requirement for production of anti-VacV neutralizing IgM. No anti-VacV IgG was found in MHCII−/− mice (Figure 3A). In addition there was very low VacV neutralization capacity of sera from class II−/− mice compared to WT mice (Figure 3B). By depleting IgM we determined that 91% of the VacV neutralizing activity in the WT mice was due to IgM antibodies (P = 0.0039), similarly to what we observed before (Figure 2). The small amount of anti-VacV neutralizing antibody detected in the sera of MHCII−/− mice dropped below the limit of detection of the neutralization test after IgM depletion (Figure 3B), indicating that the few neutralizing antibodies generated in MHCII−/− mice were IgM.
Figure 3. Anti-VacV neutralizing IgM and IgG are primarily CD4 T cell help dependent.
(A) Titration curves of anti-VacV specific IgG at day 7 post-infection from WT (closed circles) and MHC II-deficient (MHC II−/−, open squares) mice. Graph shows curves of individual mice. n = 4/group. Data is representative of two independent experiments. (B) Anti-VacV neutralizing antibody titers were measured in WT and MHC II−/− mice at day 7 post-infection. Total (white bars) and IgM depleted (black bars) neutralizing antibody levels are shown. WT vs MHC II−/− untreated, P = 0.0032. WT untreated vs. WT IgM inactivated, P = 0.0039. MHC II−/− untreated vs. MHC II−/− IgM inactivated, P >> 0.05. n = 4/group. Data is representative of two independent experiments. (C) Anti-VacV neutralizing antibody titers measured in WT controls at day 64 post-infection. Neutralizing antibody levels from total (white bars) and IgM depleted sera (black bars) is shown. Error bars represent SEM. Dotted line indicates LOD. Data is representative of 2 independent experiments.
To confirm that the treatment of the sera with 2-ME only inactivated the IgM, leaving the IgG antibodies intact, we measured VacV neutralization in sera from WT mice 64 days after VacV infection, with or without IgM inactivation (2-ME treated). At memory time points, high levels of anti-VacV IgG are present and no anti-VacV IgM is detectable (data not shown). As expected, the IgM inactivation resulted in no significant reduction in neutralizing antibodies (Figure 3C. P >>0.05). Altogether, these results show that the majority of the early anti-VacV IgM neutralizing antibody production, and all of the IgG neutralizing antibody production, is the result of cognate T cell help to VacV-specific B lymphocytes.
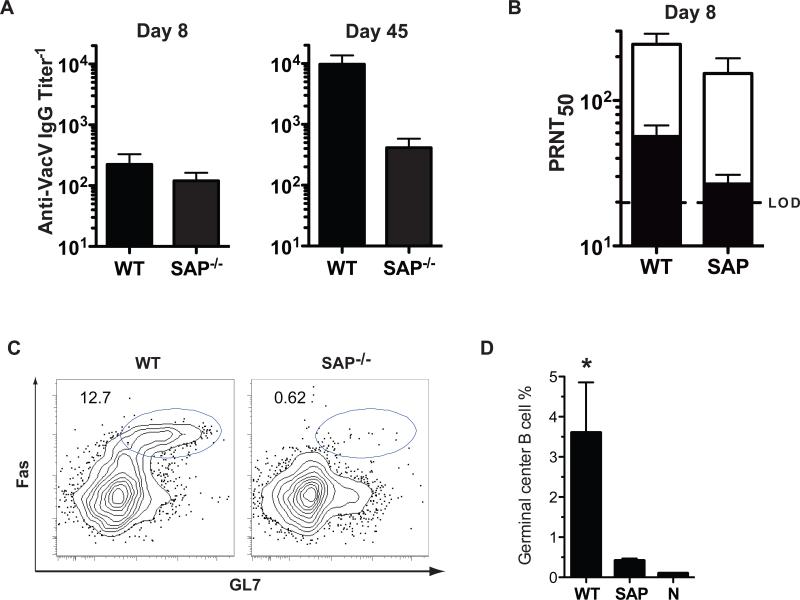
Once the B lymphocytes receive T cell help in vivo there are two possible outcomes. One is a rapid B lymphocyte differentiation to short lived plasma cells at an extrafollicular site, and the other is the initiation of a germinal center (GC) reaction [35-38]. Given the kinetics, it was unlikely that the anti-VacV IgM was GC-dependent. To formally dissect the nature of the early anti-VacV IgM response, we utilized SAP−/− (sh2d1a−/−) mice. SAP-deficient mice lack germinal center CD4 T cell help and no germinal center reaction occurs [23, 39, 40], but extrafollilcular T-dependent antibody responses can still occur [39]. When SAP−/− mice were infected with VacV, IgG antibodies were detected at day 8 post-infection. This IgG production faded with time (Figure 4A). In contrast, the anti-VacV IgG titers in the WT mice increased with time(Figure 4A). These results are in agreement with previous reports of SAP−/− mice infected with LCMV [39, 41], or immunized with SRBC [42]. There was no statistical difference in VacV neutralizing antibodies between WT and SAP−/− mice at day 8 (Figure 4B). IgM depletion resulted in a pronounced diminution of the VacV neutralizing titers in both cases (~70%, WT, P = 0.0178. SAP−/−, P = 0.0388, Figure 4B). SAP−/− mice make no germinal centers after infection with VacV, in contrast to the robust germinal center response observed in WT mice (Fig. 4C-D. WT vs. SAP−/−, P < 0.0001). These results demonstrate that the VacV-neutralizing IgM response is CD4+ T cell dependent but precedes the germinal center reaction.
Figure 4. T cell help but not a germinal center reaction is needed for the production of VacV neutralizing IgM.
(A) VacV specific IgG titers in WT and SAP−/− mice at days 8 (left) and 45 (right) post-infection (B) Anti-VacV neutralizing antibody titers in WT and SAP−/− mice at day 8 post-infection. Total (white bars) and IgM depleted sera (black bars) is shown. N = 6 for both groups. Error bars represent SEM. Dotted line indicates LOD. Data is representative of 2 independent experiments. (C) Flow cytometry of germinal center B cells from WT and SAP−/− mice at day 8 after VacV infection. B220+ gated cells are shown. Germinal center B cells are FashiGL7+. (D) Quantitation of germinal center B cells (B220+FashiGL7+), as a percentage of total splenic B cells. n = 4/group. Data are representative of three independent experiments.
Peptide immunization primes an increased VacV neutralizing IgM response
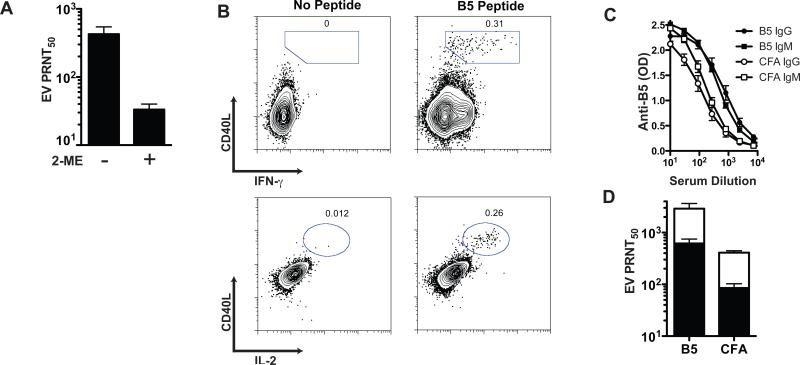
In addition to VacV neutralizing anti-MV IgG antibodies, immunized mice also make neutralizing anti-EV IgG antibodies. Having shown that mice make anti-MV VacV neutralizing IgM responses (Figures 2-4), we then tested whether immunized mice make anti-EV neutralizing IgM responses. Using a complement-dependent “physiological EV neutralization” assay [18], we detected a clear anti-EV VacV neutralizing antibody response at day 7 after VacV infection (Figure 5A), and the response was demonstrated to be predominantly IgM (Figure 5A).
Figure 5. Neutralizing anti-EV VacV IgM.
(A) PRNT50 [EV] from sera of WT mice at day 7 post-VacV infection, plus 2-ME (IgM depleted) or minus 2-ME (total antibodies). (B) Intracellular staining of CD4 T cells at day 12 after priming with a VacV class II restricted peptide (B546-60). Graphs are from gated CD4+ CD62Llo lymphocytes. Splenocytes stimulated with B546-60 peptide or without peptide (negative control) for each staining is shown, numbers in the plots indicate the frequency of the gated population in relation to total CD4+ T cells. (C) Anti-B5 IgM and IgG ELISA from sera of primed mice at day 7 post-VacV infection. IgM (squares) and IgG (circles) were measured from B546-60 primed mice (closed symbols) and no peptide control mice (open symbols). Anti-B5 IgM in B5 primed vs. CFA primed mice, P < 0.0008. Anti-B5 IgG in B5 primed vs. CFA primed mice, P < 0.0002. (D) PRNT50 [EV] from sera of B546-60 primed or control mice (n = 4). Total (white bars) and IgM depleted sera (black bars) is shown. Error bars represent SEM. Results are representative of 4 independent experiments.
Previously we have shown that VacV-specific CD4 T cells preferentially provide help to B cells of paired protein specificity [33]. Since the anti-VacV IgM response is dependent on T cell help, we wanted to determine if priming the CD4 T cell response induced an increased anti-VacV IgM response. Mice were immunized subcutaneously with the class II restricted peptide B546-60, derived from the EV-specific protein B5. After 12 days, we observed a population of B5 specific CD4 T cells expressing CD40L, IFN-γ, and IL-2 (Figure 5B). Groups of peptide primed or mock primed mice were then infected i.p. with VacV and bled at day 7 post-infection. Sera was used to determine anti-B5 IgM and IgG antibodies by ELISA and to measure VacV neutralizing activity. The physiological EV neutralization assay was utilized [18]. Mice with B546-60 specific CD4 T cells developed higher titers of anti-B5 IgG and IgM antibodies than the control group (Figure 5C, P < 0.0008 and P < 0.0002). Importantly, mice pre-immunized to develop B546-60 specific CD4 T cells showed a significant increase in VacV neutralizing antibody titers compared with the CFA only group (P = 0.0148, Figure 5D). This difference was maintained even after IgM inactivation (P = 0.0009, Figure 5D), demonstrating that both IgM and IgG neutralizing antibodies were boosted by preexisting VacV-specific CD4 T cells. The bulk of the EV neutralizing activity at day 8 was due to IgM antibodies, as IgM depletion resulted in >70% reduction in VacV neutralizing titers (B546-60 = 73%, CFA only = 74%). These results suggest that in the extrafollicular environment, as well as the germinal center, the virus-specific B cells preferentially interact with T cells of the exact same protein specificity.
Early neutralizing IgM in vaccinated humans
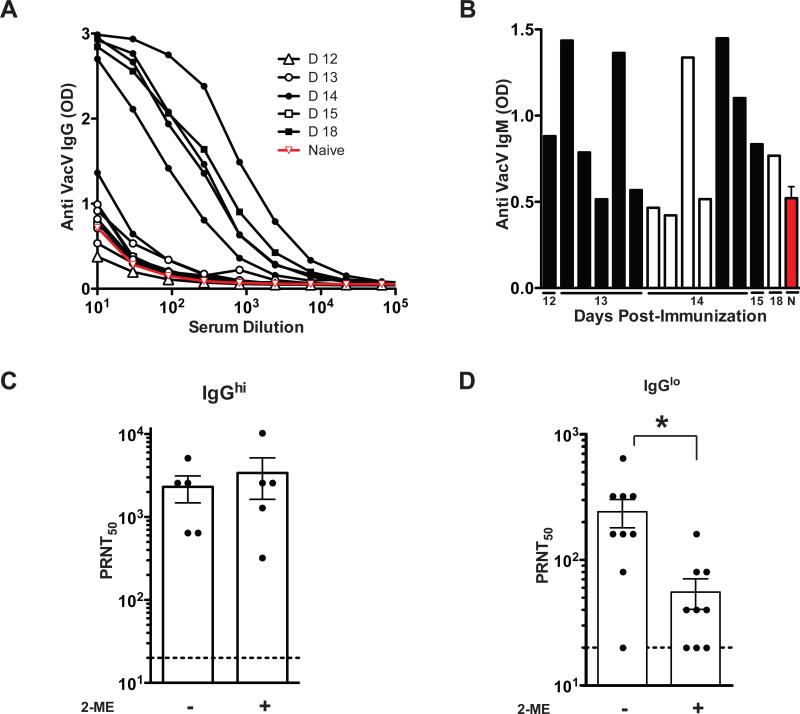
Is an early anti-VacV neutralizing IgM response produced in humans after vaccination? To determine the time needed for the appearance of VacV neutralizing antibodies, and the role of IgM antibodies in such neutralization, a group of 14 healthy human subjects were immunized with Dryvax, the licensed US smallpox vaccine. Blood samples were obtained between days 12 to 18 post-vaccination. Based on anti-VacV IgG titers, we were able to divide the vaccinees in two groups. One group presented an anti-VacV IgG response (IgGhi), and consisted of five vaccinees, 4 that were bled at day 14 post-vaccination and one bled at day 18 post-vaccination (Figure 6A). The other group showed a poor or absent anti-VacV IgG response (IgGlo), and consisted of nine individuals bled primarily at earlier time points: days 12 (n = 1), 13 (n = 5), 14 (n = 2), or 15 (n = 1) post-vaccination (Figure 6A). The IgGhi group had 2/5 vaccinees that presented IgM titers above background, meanwhile, the IgGlo group had 7/9 vaccinees with an IgM response above background (Figure 6B). When the samples were tested for neutralizing antibodies, both groups (IgGhi and IgGlo) showed significant VacV-neutralizing titers. Importantly, the IgGhi group did not show a significant reduction in VacV neutralization titers when the plasma was IgM depleted (P >> 0.05. Figure 6C). In contrast, when the plasma of the IgGlo group was depleted of IgM, a statistically significant 77% decrease in neutralizing antibody titer was observed (P = 0.0095, Figure 6D), showing that IgM was a substantial contributor to the VacV neutralization antibody titer.
Figure 6. Human anti-VacV neutralizing IgM is found before the appearance of IgG.
(A) Titration curves of anti-VacV specific IgG. The symbols indicate days after vaccination of individual subjects, the red line represents the average OD from the plasma of 5 naïve (unvaccinated) subjects. The samples were then divided in two groups according IgG titers: IgGhi (n = 5) and IgGlo (n = 9) (B) Anti-VacV IgM levels from individuals of the IgGhi group (white bars), the IgGlo group (black bars) and naïve group (red bar). (C) VacV neutralizing antibody titers from plasma of individuals from the IgGhi group, plus 2-ME (IgM depleted) or minus 2-ME (total antibodies). (D) VacV neutralizing antibody titers from plasma of individuals from the IgGlo group, plus 2-ME (IgM depleted) or minus 2-ME (total antibodies), *P = 0.0218. Error bars represent SEM. Data is representative of 3 independent assays.
Discussion
Most of the smallpox vaccine antibody research has been focused on IgG and particularly the long-lived IgG response, as these antibodies are responsible for the long-term protection against smallpox, VacV, and related orthopoxvirus infections [1]. While antibodies after vaccination or passive transfer are very effective at protecting mice [14, 17, 18, 43, 44], primates [19, 45], and humans [46-50] [51] against subsequent poxvirus infections, the role of antibodies in control and clearance of a primary VacV immunization is less clear. VacV infection in mice is well under control by the time IgG is present at day 8. A human subject immunized with VacV will not develop circulating anti-VacV IgG until day 14 or later in our study (Fig. 6), with a strong IgG response present by day 30 [1, 20, 52, 53]. This is well past the point when the primary lesion has begun to be controlled, and in many vaccinees the lesion is completely resolved by this time point. Therefore, do neutralizing antibodies play a role in control of a primary vaccinia infection or immunization?
This study demonstrates the presence of early IgM antibodies after vaccination, capable of neutralizing VacV. Importantly, the IgM response was capable of neutralizing both infectious forms of the VacV, MV and EV. Anti-VacV neutralizing IgM was found in the sera of immunized mice and human subjects. This neutralizing IgM response is detectable as early as day four post-vaccination in mice (Figure 2), and suggests an important role for the first wave of anti-VacV IgM antibodies in the control of the infection. The IgM EV neutralization activity was complement dependent, consistent with our previous EV neutralization studies using IgG [18]. Germinal center independence was consistent with a rapid T-cell dependent extrafollicular B cell differentiation to plasma cells (Figure 4). Extending our previous work [33], the linkage found in which CD4 T cells preferentially provide help to B cells of the same virion protein specificity is also seen in the extrafollicular IgM response (Figure 5).
IgM can be detected prior to IgG after monkeypox infection, as early as 5 days after rash formation (likely ~13 days after infection, based on known kinetics of vaccinia and smallpox infections in humans) [54]. There are three reports in the literature that implicate neutralizing IgM in the control of human poxvirus infections. There is one report of a severe smallpox infection in a patient with selective IgM deficiency [55]. Interestingly, this person had been previously vaccinated multiple times with VacV, and the only evidence of immundeficiency in the patient was the severe reduction in circulating IgM. Surprisingly, the patient was reported to have “adequate levels” of anti-VacV IgG. A second case involved generalized non-progressive vaccinia after vaccination of a child with IgM deficiency [56]. A third case involved a 16 year old female with disseminated molluscum contagiosum [57]. Again the only evident immunodeficiency in each case was IgM, all other serum immunoglobulin levels were normal. Human IgM deficiency is a rare and poorly understood condition without an identified genetic cause. As such, it is difficult to put IgM-deficient patients into a clear immunological context. Nevertheless, these reports of severe poxvirus infections in humans with IgM deficiency is consistent with our findings of very early neutralizing IgM to VacV in immunized mice and humans. We suggest that this neutralizing IgM response likely contributes to early control of viral spread.
ACKNOWLEDGEMENTS
This work was supported in part by NIH NIAID AI63107, NIH NIAID AI077953, NIH NIAID AI072543, a Pew Scholar Award, and a Cancer Research Institute Award to SC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amanna IJ, Slifka MK, Crotty S. Immunity and immunological memory following smallpox vaccination. Immunol Rev. 2006 Jun 1;211:320–37. doi: 10.1111/j.0105-2896.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 2.Pütz MM, Alberini I, Midgley CM, Manini I, Montomoli E, Smith GL. Prevalence of antibodies to Vaccinia virus after smallpox vaccination in Italy. J Gen Virol. 2005 Nov 1;86(Pt 11):2955–60. doi: 10.1099/vir.0.81265-0. [DOI] [PubMed] [Google Scholar]
- 3.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003 Nov 15;171(10):4969–73. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 4.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003 Sep;9(9):1131–7. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 5.Taub DD, Ershler WB, Janowski M, Artz A, Key ML, McKelvey J, et al. Immunity from smallpox vaccine persists for decades: a longitudinal study. Am J Med. 2008 Dec 1;121(12):1058–64. doi: 10.1016/j.amjmed.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol. 2004 May 15;172(10):6265–71. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]
- 7.Fischer MA, Norbury CC. Initiation of primary anti-vaccinia virus immunity in vivo. Immunol Res. 2007;37(2):113–33. doi: 10.1007/BF02685894. [DOI] [PubMed] [Google Scholar]
- 8.Panchanathan V, Chaudhri G, Karupiah G. Correlates of protective immunity in poxvirus infection: where does antibody stand? Immunol Cell Biol. 2008 Jan 1;86(1):80–6. doi: 10.1038/sj.icb.7100118. [DOI] [PubMed] [Google Scholar]
- 9.Fang M, Sigal LJ. Antibodies and CD8+ T cells are complementary and essential for natural resistance to a highly lethal cytopathic virus. J Immunol. 2005 Nov 15;175(10):6829–36. doi: 10.4049/jimmunol.175.10.6829. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhri G, Panchanathan V, Bluethmann H, Karupiah G. Obligatory requirement for antibody in recovery from a primary poxvirus infection. Journal of Virology. 2006 Jul 1;80(13):6339–44. doi: 10.1128/JVI.00116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panchanathan V, Chaudhri G, Karupiah G. Protective immunity against secondary poxvirus infection is dependent on antibody but not on CD4 or CD8 T-cell function. Journal of Virology. 2006 Jul 1;80(13):6333–8. doi: 10.1128/JVI.00115-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhri G, Panchanathan V, Buller RM, van den Eertwegh AJ, Claassen E, Zhou J, et al. Polarized type 1 cytokine response and cell-mediated immunity determine genetic resistance to mousepox. Proc Natl Acad Sci USA. 2004 Jun 15;101(24):9057–62. doi: 10.1073/pnas.0402949101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moss B. Poxvirus entry and membrane fusion. Virology. 2006 Jan 5;344(1):48–54. doi: 10.1016/j.virol.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 14.Lustig S, Fogg C, Whitbeck JC, Eisenberg RJ, Cohen GH, Moss B. Combinations of polyclonal or monoclonal antibodies to proteins of the outer membranes of the two infectious forms of vaccinia virus protect mice against a lethal respiratory challenge. Journal of Virology. 2005 Nov 1;79(21):13454–62. doi: 10.1128/JVI.79.21.13454-13462.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fogg CN, Americo JL, Earl PL, Resch W, Aldaz-Carroll L, Eisenberg RJ, et al. Disparity between levels of in vitro neutralization of vaccinia virus by antibody to the A27 protein and protection of mice against intranasal challenge. Journal of Virology. 2008 Aug 1;82(16):8022–9. doi: 10.1128/JVI.00568-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z, Earl P, Americo J, Damon I, Smith SK, Yu F, et al. Characterization of chimpanzee/human monoclonal antibodies to vaccinia virus A33 glycoprotein and its variola virus homolog in vitro and in a vaccinia virus mouse protection model. Journal of Virology. 2007 Sep 1;81(17):8989–95. doi: 10.1128/JVI.00906-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies DH, McCausland MM, Valdez C, Huynh D, Hernandez JE, Mu Y, et al. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. Journal of Virology. 2005 Sep 1;79(18):11724–33. doi: 10.1128/JVI.79.18.11724-11733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benhnia MR, McCausland MM, Moyron J, Laudenslager J, Granger S, Rickert S, et al. Vaccinia virus extracellular enveloped virion neutralization in vitro and protection in vivo depend on complement. Journal of Virology. 2009 Feb 1;83(3):1201–15. doi: 10.1128/JVI.01797-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edghill-Smith Y, Golding H, Manischewitz J, King LR, Scott D, Bray M, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005 Jul 1;11(7):740–7. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 20.Frey SE, Newman FK, Yan L, Lottenbach KR, Belshe RB. Response to smallpox vaccine in persons immunized in the distant past. JAMA. 2003 Jun 25;289(24):3295–9. doi: 10.1001/jama.289.24.3295. [DOI] [PubMed] [Google Scholar]
- 21.McClain DJ, Harrison S, Yeager CL, Cruz J, Ennis FA, Gibbs P, et al. Immunologic responses to vaccinia vaccines administered by different parenteral routes. J Infect Dis. 1997 Apr;175(4):756–63. doi: 10.1086/513968. [DOI] [PubMed] [Google Scholar]
- 22.Czar MJ, Kersh EN, Mijares LA, Lanier G, Lewis J, Yap G, et al. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc Natl Acad Sci U S A. 2001 Jun 19;98(13):7449–54. doi: 10.1073/pnas.131193098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCausland MM, Yusuf I, Tran H, Ono N, Yanagi Y, Crotty S. SAP regulation of follicular helper CD4 T cell development and humoral immunity is independent of SLAM and Fyn kinase. J Immunol. 2007 Jan 15;178(2):817–28. doi: 10.4049/jimmunol.178.2.817. [DOI] [PubMed] [Google Scholar]
- 24.Hosono M, Muramatsu S. Use of 2-mercaptoethanol for distinguishing between IgM and IgG antibody-producing cells of mice immunized with bovine globulin. J Immunol. 1972 Oct 1;109(4):857–63. [PubMed] [Google Scholar]
- 25.Okuno T, Kondelis N. Evaluation of dithiothreitol (DTT) for inactivation of IgM antibodies. J Clin Pathol. 1978 Dec 1;31(12):1152–5. doi: 10.1136/jcp.31.12.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ochsenbein AF, Pinschewer DD, Odermatt B, Carroll MC, Hengartner H, Zinkernagel RM. Protective T cell-independent antiviral antibody responses are dependent on complement. J Exp Med. 1999 Oct 18;190(8):1165–74. doi: 10.1084/jem.190.8.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benhnia MR, McCausland MM, Su HP, Singh K, Hoffmann J, Davies DH, et al. Redundancy and plasticity of neutralizing antibody responses are cornerstone attributes of the human immune response to the smallpox vaccine. Journal of Virology. 2008 Apr 1;82(7):3751–68. doi: 10.1128/JVI.02244-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newman FK, Frey SE, Blevins TP, Mandava M, Bonifacio A, Yan L, et al. Improved assay to detect neutralizing antibody following vaccination with diluted or undiluted vaccinia (Dryvax) vaccine. J Clin Microbiol. 2003 Jul 1;41(7):3154–7. doi: 10.1128/JCM.41.7.3154-3157.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lustig S, Fogg C, Whitbeck JC, Moss B. Synergistic neutralizing activities of antibodies to outer membrane proteins of the two infectious forms of vaccinia virus in the presence of complement. Virology. 2004 Oct 10;328(1):30–5. doi: 10.1016/j.virol.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 30.Davies DH, Molina D, Wrammert J, Miller J, Hirst S, Mu Y, et al. Proteome-wide analysis of the serological response to vaccinia and smallpox. Proteomics. 2007 May 1;7(10):1678–86. doi: 10.1002/pmic.200600926. [DOI] [PubMed] [Google Scholar]
- 31.Tsung K, Yim JH, Marti W, Buller RM, Norton JA. Gene expression and cytopathic effect of vaccinia virus inactivated by psoralen and long-wave UV light. J Virol. 1996 Jan;70(1):165–71. doi: 10.1128/jvi.70.1.165-171.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moutaftsi M, Bui H, Peters B, Sidney J, Salek-Ardakani S, Oseroff C, et al. Vaccinia virus-specific CD4+ T cell responses target a set of antigens largely distinct from those targeted by CD8+ T cell responses. J Immunol. 2007 Jun 1;178(11):6814–20. doi: 10.4049/jimmunol.178.11.6814. [DOI] [PubMed] [Google Scholar]
- 33.Sette A, Moutaftsi M, Moyron-Quiroz J, McCausland MM, Davies DH, Johnston RJ, et al. Selective CD4+ T cell help for antibody responses to a large viral pathogen: deterministic linkage of specificities. Immunity. 2008 Jun 1;28(6):847–58. doi: 10.1016/j.immuni.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies DH, Liang X, Hernandez JE, Randall A, Hirst S, Mu Y, et al. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc Natl Acad Sci USA. 2005 Jan 18;102(3):547–52. doi: 10.1073/pnas.0408782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Odegard JM, Marks BR, Diplacido LD, Poholek AC, Kono DH, Dong C, et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. The Journal of Experimental Medicine. 2008 Nov 3; doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okada T, Cyster JG. B cell migration and interactions in the early phase of antibody responses. Curr Opin Immunol. 2006 Jun 1;18(3):278–85. doi: 10.1016/j.coi.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Schoenberger SP, Crotty S. Immunologic Memory. Fundamental Immunology. 2009:862–97. [Google Scholar]
- 38.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–39. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 39.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003 Jan 16;421(6920):282–7. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 40.Schwartzberg PL, Mueller KL, Qi H, Cannons JL. SLAM receptors and SAP influence lymphocyte interactions, development and function. Nat Rev Immunol. 2009 Jan 1;9(1):39–46. doi: 10.1038/nri2456. [DOI] [PubMed] [Google Scholar]
- 41.Crotty S, McCausland MM, Aubert RD, Wherry EJ, Ahmed R. Hypogammaglobulinemia and exacerbated CD8 T-cell-mediated immunopathology in SAP-deficient mice with chronic LCMV infection mimics human XLP disease. Blood. 2006 Nov 1;108(9):3085–93. doi: 10.1182/blood-2006-04-018929. [DOI] [PubMed] [Google Scholar]
- 42.Cannons JL, Yu LJ, Jankovic D, Crotty S, Horai R, Kirby M, et al. SAP regulates T cell-mediated help for humoral immunity by a mechanism distinct from cytokine regulation. J Exp Med. 2006 Jun 12;203(6):1551–65. doi: 10.1084/jem.20052097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Law M, Pütz MM, Smith GL. An investigation of the therapeutic value of vaccinia-immune IgG in a mouse pneumonia model. J Gen Virol. 2005 Apr 1;86(Pt 4):991–1000. doi: 10.1099/vir.0.80660-0. [DOI] [PubMed] [Google Scholar]
- 44.Galmiche MC, Goenaga J, Wittek R, Rindisbacher L. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology. 1999 Feb 1;254(1):71–80. doi: 10.1006/viro.1998.9516. [DOI] [PubMed] [Google Scholar]
- 45.Fogg CN, Americo JL, Lustig S, Huggins JW, Smith SK, Damon I, et al. Adjuvant-enhanced antibody responses to recombinant proteins correlates with protection of mice and monkeys to orthopoxvirus challenges. Vaccine. 2007 Apr 12;25(15):2787–99. doi: 10.1016/j.vaccine.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fenner F, Henderson DA, Arita I, Jezek z, Ladnyi i. Smallpox and its Eradication. 2001 Oct 17;:4. [Google Scholar]
- 47.Hopkins RJ, Lane JM. Clinical efficacy of intramuscular vaccinia immune globulin: a literature review. Clin Infect Dis. 2004 Sep 15;39(6):819–26. doi: 10.1086/422999. [DOI] [PubMed] [Google Scholar]
- 48.Kempe CH, Berge TO, England B. Hyperimmune vaccinial gamma globulin. Pediatrics. 1956;18:177–88. [PubMed] [Google Scholar]
- 49.Kempe CH, Bowles C, Meiklejohn G, Berge TO, St. Vincent L, Sundara Babu BV, et al. The use of vaccinia hyperimmune gamma-globulin in the prophylaxis of smallpox. Bulletin of the World Health Organization. 1961;25:41–8. [PMC free article] [PubMed] [Google Scholar]
- 50.Marennikova SS. The use of hyperimmune antivaccinia gamma-globulin for the prevention and treatment of smallpox. Bull World Health Organ. 1962;27:325–30. [PMC free article] [PubMed] [Google Scholar]
- 51.Hobday TL. Antivaccinial gamma-globulin in the control of smallpox. Lancet. 1962 Apr 28;1:907–8. doi: 10.1016/s0140-6736(62)91935-9. [DOI] [PubMed] [Google Scholar]
- 52.Lawrence SJ, Lottenbach KR, Newman FK, Buller RM, Bellone CJ, Chen JJ, et al. Antibody responses to vaccinia membrane proteins after smallpox vaccination. J Infect Dis. 2007 Jul 15;196(2):220–9. doi: 10.1086/518793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pütz MM, Midgley CM, Law M, Smith GL. Quantification of antibody responses against multiple antigens of the two infectious forms of Vaccinia virus provides a benchmark for smallpox vaccination. Nat Med. 2006 Nov 1;12(11):1310–5. doi: 10.1038/nm1457. [DOI] [PubMed] [Google Scholar]
- 54.Karem KL, Reynolds M, Braden Z, Lou G, Bernard N, Patton J, et al. characterization of acute-phase humoral immunity to monkeypox: use of immunoglobulin M enzyme-linked immunosorbent assay for detection of monkeypox infection during the 2003 North American outbreak. Clin Diagn Lab Immunol. 2005 Jul;12(7):867–72. doi: 10.1128/CDLI.12.7.867-872.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brilliant LB, Nakano JH, Kitamura T, Hodakevic LN, Bharucha PB. Occupationally-acquired smallpox in an IgM-deficient health worker. Bull World Health Organ. 1981 Jan 1;59(1):99–106. [PMC free article] [PubMed] [Google Scholar]
- 56.Chandra RK, Kaveramma B, Soothill JF. Generalised non-progressive vaccinia associated with IgM deficiency. Lancet. 1969 Apr 5;1(7597):687–9. doi: 10.1016/s0140-6736(69)92643-9. [DOI] [PubMed] [Google Scholar]
- 57.Mayumi M, Yamaoka K, Tsutsui T, Mizue H, Doi A, Matsuyama M, et al. Selective immunoglobulin M deficiency associated with disseminated molluscum contagiosum. Eur J Pediatr. 1986 Apr 1;145(12):99–103. doi: 10.1007/BF00441866. [DOI] [PubMed] [Google Scholar]