Summary
The obligate intracellular protozoan, Leishmania infantum chagasi (Lic) undergoes receptor-mediated phagocytosis by macrophages, followed by a transient delay in phagolysosome maturation. We found differences in the pathway through which virulent Lic metacyclic promastigotes or avirulent logarithmic promastigotes are phagocytosed by human monocyte-derived macrophages (MDMs). Both logarithmic and metacyclic promastigotes entered MDMs through a compartment lined by the third complement receptor (CR3). In contrast, many logarithmic promastigotes entered through vacuoles lined by mannose receptors (MR) whereas most metacyclic promastigotes did not (p < 0.005). CR3 positive vacuoles containing metacyclic promastigotes stained for caveolin-1 protein, suggesting CR3 localizes in caveolae during phagocytosis. Following entry, the kinetics of phagolysosomal maturation and intracellular survival also differed. Vacuoles containing metacyclic parasites did not accumulate lysosome-associated membrane protein-1 (LAMP-1) at early times after phagocytosis, whereas vacuoles with logarithmic promastigotes did. MDMs phagocytosed greater numbers of logarithmic than metacyclic promastigotes, yet metacyclics ultimately replicated intracellularly with greater efficiency. These data suggest that virulent metacyclic Leishmania promastigotes fail to ligate macrophage MR, and enter through a path that ultimately enhances intracellular survival. The relatively quiescent entry of virulent Leishmania spp. into macrophages may be accounted for by the ability of metacyclic promastigotes to selectively bypass deleterious entry pathways.
Introduction
Leishmania infantum chagasi (Lic), the causative agent of New World visceral leishmaniasis, is an obligate intracellular parasite in its mammalian hosts. The flagellated promastigote form of the parasite is inoculated through the bite of a sand fly into mammalian tissue, where it becomes internalized by host macrophages and converts into the highly replicative amastigote form (Alexander et al., 1999). Disease is largely mediated by the parasite's ability to evade microbicidal machinery, a process that contributes to its intracellular survival and proliferation in an otherwise hostile environment (Duclos and Desjardins, 2000).
Prior to inoculation by the sand fly, avirulent Leishmania spp. procyclic promastigotes develop into the highly virulent metacyclic promastigotes within the gut of the insect vector (Sacks and Perkins, 1985; Sacks and Perkins, 1984). This process can be mimicked in vitro during growth in culture, and metacyclic forms can be purified by buoyant density from a heterogeneous stationary phase population (Yao et al., 2008; Spath and Beverley, 2001). We previously reported that metacyclics are more infectious for mice than unpurified parasites from stationary or mid-logarithmic phase cultures (Yao et al., 2008). The present study constitutes a careful comparison of the macrophage entry and intracellular trafficking pathways undertaken by metacyclic compared to logarithmic promastigotes. The results led us to hypothesize that the difference in virulence between the two parasite forms reflects distinct events occurring at the initial step of entry into mammalian macrophages. These early events may guide both the intracellular routing and the ultimate fate of the parasite.
Several receptors have been implicated in Leishmania recognition by macrophages. Among the best characterized are the third complement receptor (CR3) and the mannose receptor (MR) (Wilson and Pearson, 1986; Blackwell et al., 1985; Mosser and Edelson, 1985). There is evidence that individual ligation of these receptors does not trigger classical macrophage activation, leading to consequent “safe uptake” of microorganisms through these pathways. CR3, which recognizes inactivated complement protein 3b (C3bi), does not stimulate the release of reactive oxygen intermediates upon its individual ligation (Aderem and Underhill, 1999). MR is a C-type lectin pattern recognition receptor that exhibits anti-inflammatory effects while clearing lysosomal hydrolases and myeloperoxidase during wound healing (Akilov et al., 2007; Allavena et al., 2004; Linehan et al., 2000a). Lic has been reported to enter macrophages via caveolae, cholesterol-rich membrane microdomains containing the proteins caveolin-1 or -2, and to delay early lysosomal fusion (Rodriguez et al., 2006). We speculated that CR3 and MR might localize in the caveolae that mediate parasite attachment, and questioned whether all forms of the parasite use the same receptor-mediated pathway for macrophage entry.
There are conflicting reports regarding the necessity of MR for Leishmania spp. promastigote to enter macrophages. Attachment of a mixed stationary population of L. donovani promastigotes to human monocyte derived macrophages (MDMs) was partially inhibited by the exogenous addition of receptor ligands or monoclonal antibodies to MR (Wilson and Pearson, 1988), suggesting MR contributes to but does not entirely account for parasite phagocytosis. In contrast, phagocytosis of metacyclic L. major or L. donovani by either wild type or MR-deficient murine bone-marrow macrophages (BMMs) occurred with equal efficiency (Akilov et al., 2007), leading to the conclusion that MR ligation is not necessary for internalization to occur. The current study is based upon the hypothesis that metacyclic parasites utilize different macrophage entry pathways than less virulent forms. We investigated (1) whether different growth stage forms of Lic promastigotes ligate CR3 or MR, (2) whether these receptors are localized in caveolae during phagocytosis and (3) whether differential uptake correlates with changes in phagolysosomal maturation and parasite killing. Supporting our hypothesis, the data showed differences in receptor utilization, lysosomal fusion kinetics and rates of intracellular survival between logarithmic and metacyclic forms of Lic.
Results
CR3 co-localizes with metacyclic and logarithmic phase Lic promastigotes
The binding of L. donovani promastigotes to macrophage CR3 has been documented using both immunofluorescence and antibody/ligand blockade of CR3 function (Wilson and Pearson, 1988; Mosser and Edelson, 1985). We chose a confocal approach to document parasite co-localization with CR3 or MR, and a gradient centrifugation method to purify metacyclic promastigotes from mixed stationary phase cultures (Yao et al., 2008; Spath and Beverley, 2001). Adherent MDMs were synchronously inoculated with serum-opsonized metacyclic or logarithmic Lic promastigotes. After 2 to 100 minutes, the distribution of CR3 and the invading parasites was assessed by confocal microscopy.
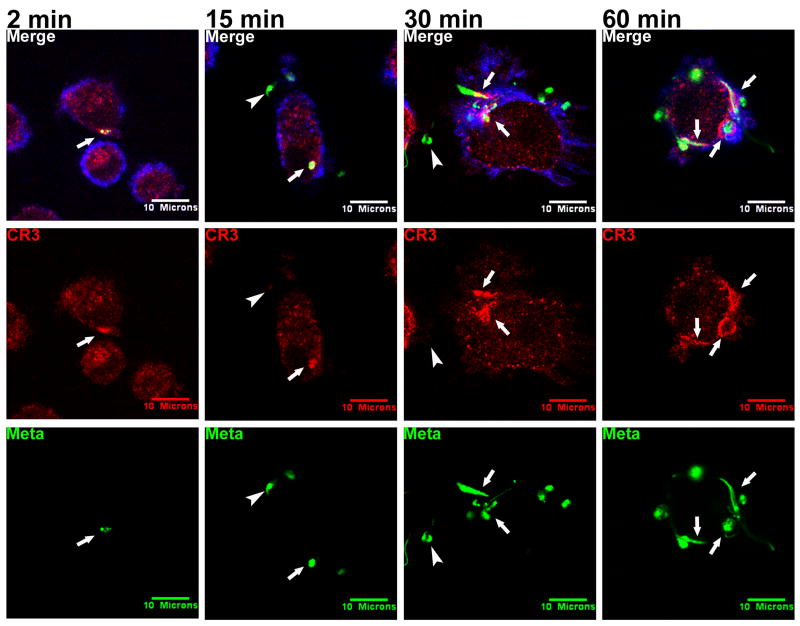
Metacyclic promastigotes strongly co-localized with CR3 throughout the first hour after phagocytosis (Figure 1, arrows). CR3 co-localization was apparent as early as 2 minutes after beginning the synchronized phagocytosis assay, at which time the promastigotes associated with the host cell but were not yet internalized. As the assay progressed, CR3 accumulated around the point of entry (15 and 30 min). Parasites were completely intracellular at 60 and 100 (not shown) minutes, at which point they remained co-localized with CR3. It is of interest that the arrangement of CR3 at 60 minutes and later time points formed a distinct “rim” around the parasites. It is possible that this represents phagosome enlargement such that the parasitophorous vacuole (PV) membrane is more distant from the parasite surface (Figure 1; Video S1 and unpublished data, Hsiao, Ueno, and Wilson). Consistently, metacyclic parasites remained near the periphery of the macrophage and did not proceed deep into the cytoplasm.
Figure 1.
Metacyclic Lic promastigotes (Meta) co-localize with CR3 during infection of MDMs. Purified metacyclic promastigotes were labeled with CFSE (green) and used in a synchronized phagocytosis assay with adherent MDMs at an MOI of 10:1. Coverslips were fixed after 2, 15, 30, 60, or 100 (not shown) minutes. CR3 was labeled with anti-human CR3 primary and Alexa Fluor 568 anti-mouse IgG secondary antibodies (red). Cellular actin was labeled with Alexa Fluor 647 phalloidin (blue). Arrows: examples of parasitophorous vacuoles that accumulated CR3. Arrowheads: extracellular parasites that remained unstained by antibody to CR3. Shown are representative photomicrographs from more than 12 experiments.
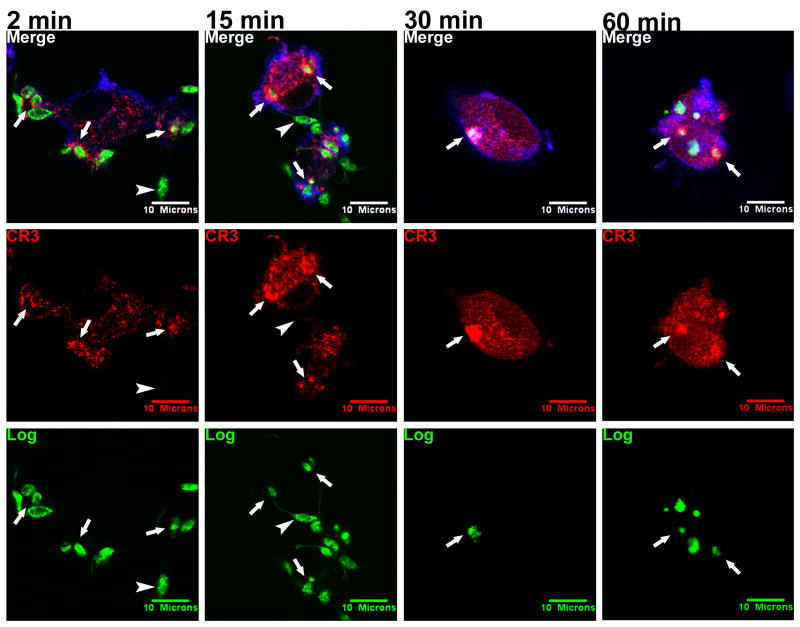
CR3 also co-localized with logarithmic Lic promastigotes studied in parallel with metacyclics (Figure 2, arrows). CR3 accumulated along the points of contact between the parasites and the host cell (2 min), and intense CR3 labeling remained localized with the intracellular parasites at later time points (15, 30, and 60 minutes). However, logarithmic parasites penetrated deeper into the macrophage cytoplasm than metacyclic promastigotes at equivalent time points, and did not become surrounded by a distinct circle of CR3 staining over time after internalization.
Figure 2.
Logarithmic Lic promastigotes (Log) co-localize with CR3 during internalization by MDMs. A mixed population of promastigotes in exponential growth was labeled with CFSE (green) and added to adherent MDMs at an MOI of 10:1. Coverslips were fixed after 2, 15, 30, 60, or 100 (not shown) minutes. CR3 was labeled with anti-human CR3 primary and Alexa Fluor 568 anti-mouse IgG secondary antibodies (red). Cellular actin was labeled with Alexa Fluor 647 phalloidin (blue). Arrows: examples of PVs that accumulated CR3. Arrowheads: extracellular parasites that remained unstained by antibody to CR3. Photomicrographs are representative of more than 5 experiments.
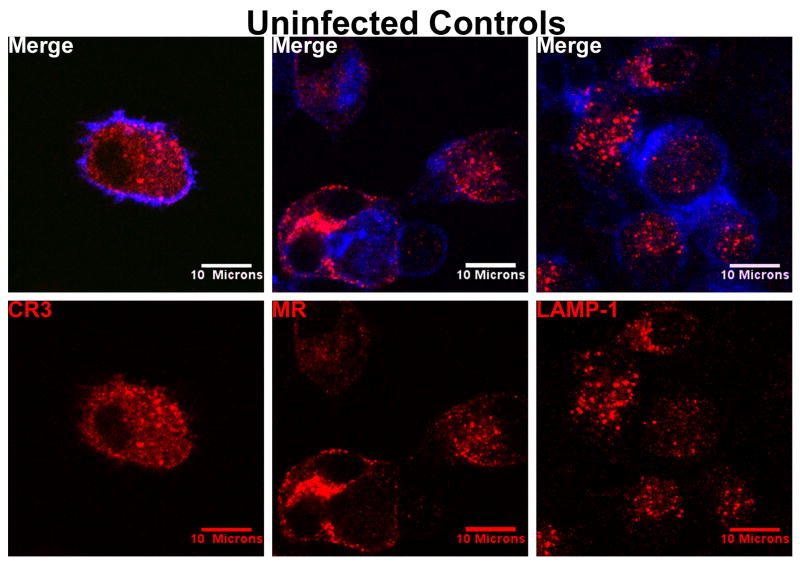
Unlike MDMs exposed to metacyclic or logarithmic parasite forms, uninfected MDMs show that CR3 is dispersed evenly throughout the cytoplasmic space and the periphery, without focal aggregation (Figure 3 left panels). The antibody to CR3 did not cross-react with extracellular promastigotes (see Figure 1, arrowheads, 15 and 30 minute panels; Figure 2, arrowheads, 2 and 15 minute panels). Fluorochrome-conjugated secondary antibodies alone did not recognize either parasites or MDMs (data not shown).
Figure 3.
Control micrographs show that CR3 and MR are evenly distributed throughout uninfected MDMs. LAMP-1 in uninfected MDMs is somewhat enriched in the perinuclear region as expected. Uninfected MDMs were fixed in parallel with infected MDMs at all the times described in Figures 1 and 2. Shown are controls for the 2 minute time point (CR3, MR) and 2 hour time point (LAMP-1). CR3, MR, or LAMP-1 was labeled with their corresponding primary monoclonal antibodies followed by Alexa Fluor 568 anti-mouse IgG secondary antibodies (red). Cellular actin was labeled with Alexa Fluor 647 phalloidin (blue).
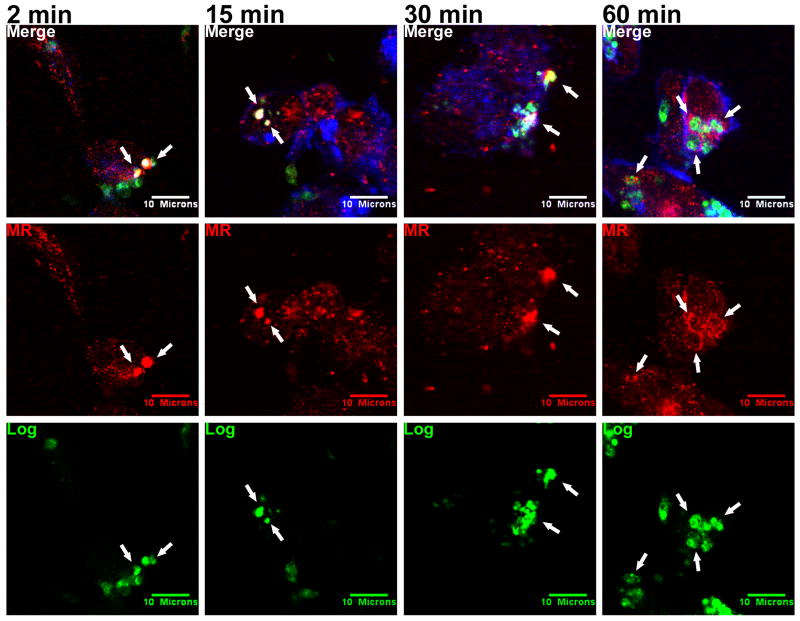
MR co-localizes with logarithmic phase but not metacyclic Lic promastigotes
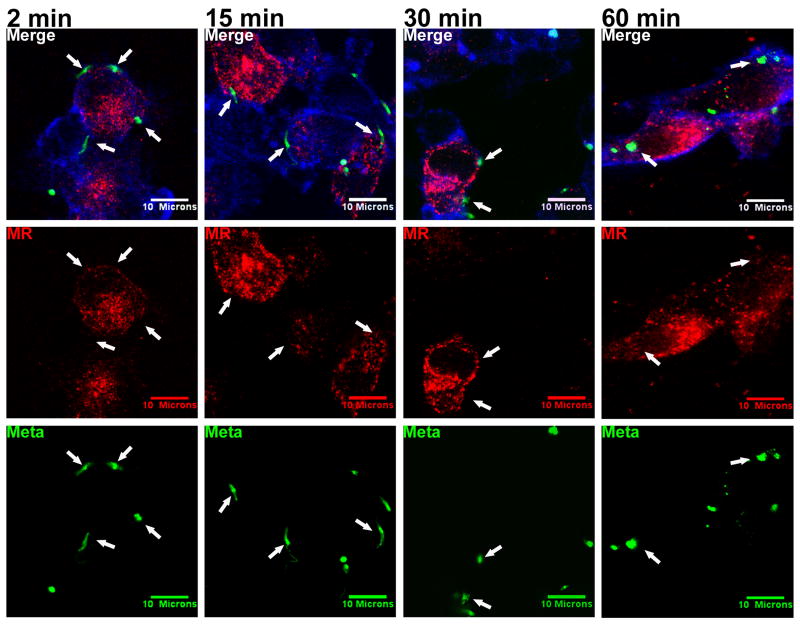
Conflicting reports regarding the importance of MR for attachment of L. donovani promastigotes to macrophages (Akilov et al., 2007; Wilson and Pearson, 1988; Wilson and Pearson, 1986) led us to hypothesize that the utilization of macrophage MR may vary with parasite developmental stage. We tested this hypothesis using confocal microscopy to examine MR distribution in adherent MDMs. These experiments revealed a striking difference between the parasite stages. Unlike the pattern with CR3, MR did not accumulate around the invading metacyclic promastigotes at any of the observed time points (Figure 4, arrows).
Figure 4.
Metacyclic Lic promastigotes (Meta) do not co-localize with MR during phagocytosis by MDMs. Purified metacyclic promastigotes were labeled with CFSE (green) and incubated with adherent MDMs at an MOI of 10:1 in a synchronized phagocytosis assay. Infected macrophages were fixed after 2, 15, 30, 60, or 100 (not shown) minutes. MR was labeled with anti-human MR primary and Alexa Fluor 568 anti-mouse IgG secondary antibodies (red). Cellular actin was labeled with Alexa Fluor 647 phalloidin (blue). Arrows: examples of parasites that failed to ligate MR. Photomicrographs are representative of more than 15 experiments.
In contrast to metacyclics, MR co-localized consistently with logarithmic promastigotes during phagocytosis (Figure 5, arrows). Similar to the co-localization of CR3 with metacyclics, MR concentrated focally over parasite bodies between 2 and 30 minutes after synchronous phagocytosis, but by 60 minutes a rim of MR surrounded the intracellular parasites. The pattern of MR fluorescence in Figures 3, 4 and 5 indicated staining throughout only some cells in the monolayers. Most MR was present in intracellular vesicles with only a small subset in the plasma membrane (Kang et al., 2005). Similar to Figures 1 and 2, metacyclic promastigotes in Figure 4 remained at the periphery of the MDM, whereas logarithmic parasites penetrated deep within the cell in Figure 5. This again suggests different rates of progression through the endocytic pathways following phagocytosis.
Figure 5.
Logarithmic Lic promastigotes (Log) co-localize with MR during phagocytosis by MDMs. A mixed population of promastigotes in exponential growth was labeled with CFSE (green) and added to adherent MDMs at an MOI of 10:1 in a synchronized phagocytosis assay. Infections were fixed after 2, 15, 30, 60, or 100 (not shown) minutes. MR was labeled with anti-human MR primary and Alexa Fluor 568 anti-mouse IgG secondary antibodies (red). Cellular actin was labeled with Alexa Fluor 647 phalloidin (blue). Arrows: examples of PVs that accumulated MR. Parasites remained surrounded by MR after 60 minutes. Photomicrographs are representative of more than 12 experiments.
Quantitative analysis of CR3 and MR co-localization
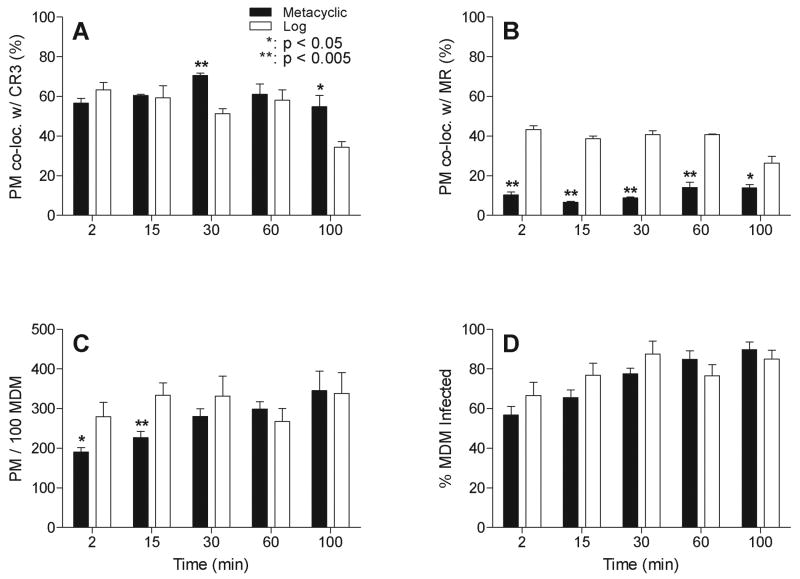
We quantified the numbers of metacyclic or logarithmic promastigotes that co-localized with CR3 or MR. More than 200 parasites were assessed for each condition at each time point by observers who were blinded to the condition. Receptor staining that either co-localized with or encircled the parasite was considered positive. The difference between co-localization of metacyclic versus logarithmic promastigotes with CR3 was not statistically significant immediately after inoculation (Figure 6A). Differences at later time points would be expected to reflect both the rate of phagocytosis and the rate of intracellular killing. Considering all time points, approximately 61% of infecting metacyclic promastigotes and 53% of logarithmic promastigotes scored positive for CR3 accumulation.
Figure 6.
Quantitative analysis of receptor co-localization with promastigotes. (A and B) Co-localization was scored and quantified for more than 200 phagocytosed parasites at each time point for each parasite type as described in the Experimental Procedures. The percent of metacyclic or logarithmic promastigotes co-localizing with CR3 (A) or MR (B) was calculated. Black bars: mean ± SE of 4 representative assays with metacyclic promastigotes. White bars: mean ± SE of 3 representative assays with logarithmic promastigotes. (C and D) The parasite load after phagocytosis of metacyclic or logarithmic stage promastigotes is shown as parasites per 100 MDMs (C) and the percent of MDMs with associated parasites over total MDMs (% MDM Infected) (D). Black bars: mean ± SE of 8 representative assays with metacyclic promastigotes. White bars: mean ± SE of 6 representative assays with logarithmic stage promastigotes. *: p < 0.05, **: p < 0.005. Statistical analyses utilized the one-tailed t test with 95% confidence.
In contrast to CR3, there was nearly a 4-fold difference between the numbers of metacyclic versus logarithmic promastigotes that co-localized with MR (Figure 6B). Overall, 11% or 38% of metacyclic or logarithmic promastigotes co-localized with MR, respectively.
Unexpectedly, fewer purified metacyclic than logarithmic promastigotes were observed attached to MDMs at 30 minutes or earlier time points after synchronized phagocytosis (Figure 6C). These early time points would most likely reflect differences in the rates of attachment to MDM surface receptors. The percent of MDMs infected with metacyclic versus logarithmic promastigotes remained relatively similar (Figure 6D). Taken together, the data show a number of differences between the entry pathways of metacyclic versus logarithmic Lic promastigotes. Metacyclic promastigotes were internalized by 60 minutes, co-localized with CR3 throughout the first 100 minutes of phagocytosis, and remained at the periphery of MDMs. However, logarithmic promastigotes were rapidly ingested (15 minutes), co-localized with both MR and CR3, and trafficked deeply into the MDM cell by 60 minutes.
Metacyclic Lic promastigotes co-localized with both caveolin and CR3 but not MR
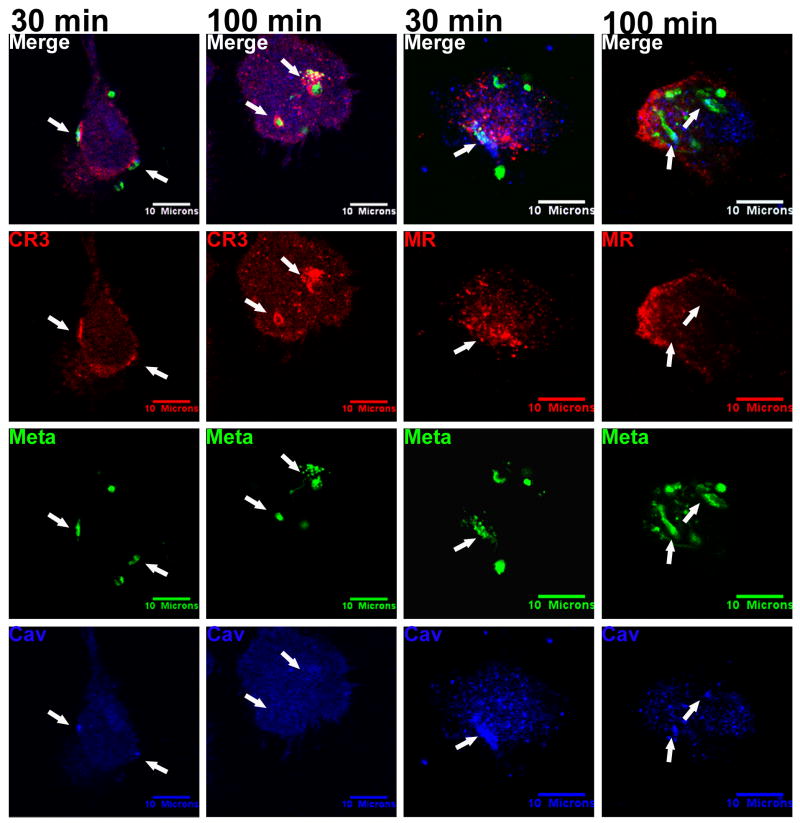
We previously reported that phagocytosis of unfractionated stationary phase Lic promastigotes occurs through caveolae, similar to several bacterial and viral pathogens. This route of macrophage entry leads to trafficking into an endocytic compartment that excludes or delays fusion of vacuoles containing Lic and other microbes with lysosomal markers (Nitsche-Schmitz et al., 2007; Rodriguez et al., 2006; Pelkmans et al., 2001). Several receptors including CR3 and receptors for mannose-binding lectins have been found in caveolin-containing microdomains in other cell types (Harris et al., 2002; Shin and Abraham, 2001). We therefore investigated whether macrophage CR3 was located in caveolae during phagocytosis of metacyclic Lic promastigotes.
Confocal photomicrographs of metacyclic promastigotes are shown during entry into MDMs (30 min) and after internalization (100 min) (Figure 7, arrows). As expected, metacyclics co-localized with CR3 at the periphery of the MDM at early time points and CR3 accumulated in the PV surrounding the parasite at 100 minutes, a time point when some parasites had trafficked further into the cell (Figure 7 column 2nd to the left). Caveolin-1 localized in the vicinity of parasites and CR3 at 30 minutes (Figure 7 far left column; Video S2), suggesting CR3 may be present in caveolae membrane domains. However after 100 minutes, caveolin-1 was conspicuously absent from CR3-positive PV membranes. This suggests that CR3 may separate from caveolae at this time, perhaps, due to phagosome remodeling. In contrast, there was no apparent co-localization of metacyclic promastigotes with MR, although co-localization of parasites and caveolin-1 was evident (Figure 7 right 2 columns). These data suggest that metacyclic promastigotes are phagocytosed through caveolae that contain CR3 but not MR.
Figure 7.
Metacyclic promastigotes co-localize with caveolin-1 and with CR3, but not MR. Purified metacyclic promastigotes were labeled with CFSE (green) and incubated with adherent MDMs at an MOI of 10:1. Coverslips were fixed at 2, 15, 30, 60 or 100 minutes after synchronized phagocytosis. Shown are the 30 and the 100 minute time points. CR3 or MR was labeled with anti-human CR3 or anti-human MR primary antibodies respectively, and Alexa Fluor 568 anti-mouse IgG secondary antibodies (red). Caveolin-1 was labeled with polyclonal anti-human caveolin primary and Alexa Fluor 647 anti-rabbit IgG secondary antibodies (blue). Parasites that clustered with caveolin-1 also accumulated CR3, but not MR (arrows, 30 min). Internalized parasites lost most of their caveolin staining (arrows) by 100 min after phagocytosis. Photomicrographs are representative of more than 5 experiments.
Acquisition of lysosomal markers LAMP-1 and cathepsin D is delayed during phagocytosis of metacyclic but not logarithmic Lic promastigotes by MDMs
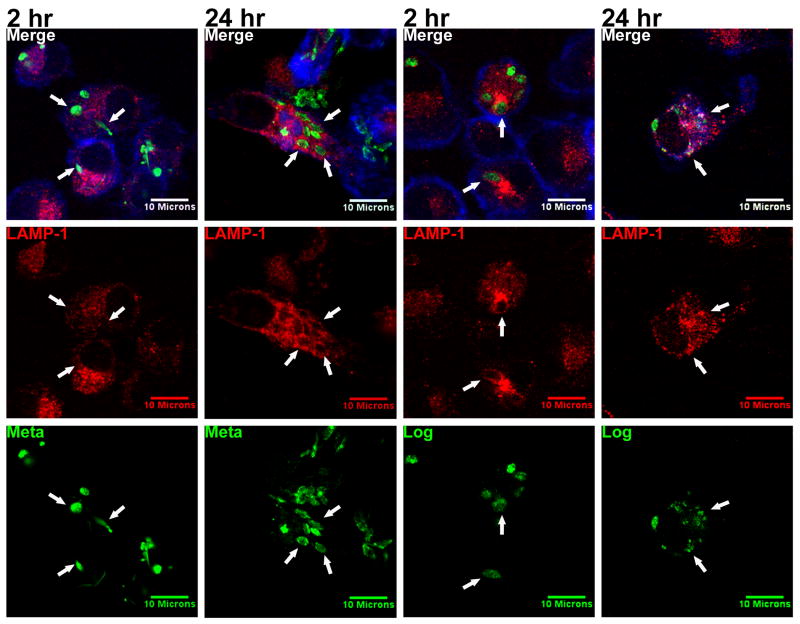
Following phagocytosis of Leishmania spp., the PV acquires markers of early endosomes but the acquisition of lysosomal markers is delayed (Dermine et al., 2000; Duclos and Desjardins, 2000). In order to examine whether the differential macrophage entry by purified metacyclic or logarithmic promastigotes led to differences in these downstream events, we evaluated the recruitment of the late endosome / lysosome marker, lysosome associated membrane protein 1 (LAMP-1), to the PV.
Metacyclic promastigotes were completely internalized by 2 hours after initiating the synchronized phagocytosis assay, and they were observed deeper in the MDM cytoplasm than at earlier time points. Very few PVs containing metacyclics accumulated LAMP-1 at this time point (Figure 8, far left column). In contrast, after 24 hours LAMP-1 was clearly enriched in the membranes of PVs infected with metacyclic promastigotes. This signifies that the recruitment of late endosomes or lysosomes to the phagosome occurs several hours following internalization (Figure 8 column 2nd to the left). Control uninfected MDMs showed that LAMP-1 was dispersed throughout the cytoplasm, showing slight perinuclear enrichment in the absence of parasites, as expected (Figure 3 right column).
Figure 8.
Logarithmic promastigotes recruit LAMP-1 more rapidly than metacyclic promastigotes. Purified metacyclic promastigotes or a mixed population of logarithmic promastigotes were labeled with CFSE (green) and added to adherent MDMs at an MOI of 10:1. Coverslips containing MDMs were fixed after 2 and 24 hours. LAMP-1 was labeled with anti-human LAMP-1 primary and Alexa Fluor 568 anti-mouse IgG secondary antibodies (red). Cellular actin was labeled with Alexa Fluor 647 phalloidin (blue). Metacyclic promastigotes accumulated LAMP-1 after 24 hours, whereas logarithmic promastigotes became encircled by LAMP-1 within 2 hours (arrows). Photomicrographs are representative of more than 4 experiments.
In contrast to metacyclics, LAMP-1 was observed to co-localize with the PV membranes in MDMs exposed to logarithmic promastigotes as early as 2 hours after synchronized phagocytosis (Figure 8 column 2nd to the right). The parasites became surrounded by intense LAMP-1 staining, and retained the stain for up to 24 hours. After this point, some of these avirulent promastigotes showed signs of lysis, suggested by the diffusion of CFSE fluorescence into the macrophage cytoplasm (Figure 8 far right column).
The delay in PV-lysosome fusion during phagocytosis of metacyclic but not logarithmic promastigotes was confirmed by staining for cathepsin D (Cat-D), a lysosomal protease (Figure S1, arrows). Similar to the LAMP-1 pattern, Cat-D entered the PV membrane and co-localized with the PV 2 hours after phagocytosis of logarithmic promastigotes, whereas phagosomes containing metacyclic promastigotes excluded Cat-D until 24 hours after phagocytosis. As with LAMP-1, intracellular logarithmic promastigotes could be seen retaining Cat-D and undergoing noticeable lysis during the 24 hours after phagocytosis. Cat-D localization in uninfected MDMs was similar to that of LAMP-1 (Figure S2).
Quantitative analysis of the 2 and 24 hour time points showed that LAMP-1 was recruited to approximately 2-fold more PVs containing logarithmic than metacyclic promastigotes (Figure 9A). The difference was pronounced at 2 hours, when only 22% of metacyclics versus 44% of logarithmic promastigotes co-localized with LAMP-1 (p < 0.005). LAMP-1 co-localization with metacyclics increased by 24 hours (39%), but still lagged behind PVs containing logarithmic promastigotes (56%) (p < 0.05). In summary, metacyclic Lic promastigotes, which are taken up more slowly and bypass MDM MRs, are capable of delaying the recruitment of harmful lysosomal machinery to the PV. Logarithmic promastigotes, which are taken up more vigorously, are less able to block phagosome-lysosome fusion, and therefore, more susceptible to intracellular killing.
Figure 9.
Quantitative analysis of LAMP-1 co-localization with promastigotes and long-term intracellular survival. (A) The percent of intracellular metacyclic or logarithmic promastigotes co-localizing with LAMP-1 was quantified microscopically as described in Experimental Procedures. Black bars: mean ± SE of 4 representative assays with metacyclic promastigotes. White bars: mean ± SE of 3 representative assays with logarithmic promastigotes. (B and C) The intracellular survival of metacyclic or logarithmic promastigotes in MDMs was calculated as the number of parasites per 100 MDMs (B) and the percent of MDMs with internalized parasites over total MDMs (% MDM Infected) (C). At least 400 metacyclic and 400 logarithmic parasites were scored for each time point. One internalized parasite was defined as one intracellular parasite within a host macrophage. Black bars: mean ± SE of 3 assays with metacyclic promastigotes. White bars: mean ± SE of 4 assays with logarithmic stage promastigotes. *: p < 0.05, **: p < 0.005. Statistical analyses utilized the one-tailed t test with 95% confidence.
Phagocytosis of metacyclic Lic promastigotes by MDMs is less efficient than logarithmic promastigotes, but intracellular survival is enhanced
The differential entry and intracellular trafficking of metacyclic versus logarithmic promastigotes after phagocytosis by MDMs led us to investigate whether these observations correlated with differential killing within the macrophage. Adherent MDM monolayers were incubated with purified metacyclic or logarithmic promastigotes, and then stained with Wright-Giemsa dye at 1, 24, 48, 72, and 96 hours after synchronized phagocytosis. Intracellular parasites were quantified microscopically at each time point in order to determine the differences in long-term survival between the two promastigote forms.
Figure 9B shows that significantly fewer metacyclic promastigotes were initially phagocytosed by MDMs than logarithmic promastigotes (49 versus 105 parasites per 100 MDMs, respectively at 1 hour post-phagocytosis). However, by 48 hours the parasite burden had risen in MDMs incubated with metacyclic promastigotes, and by 72 hours this burden exceeded that of MDMs incubated with logarithmic promastigotes (p < 0.005 for 1, 72, and 96 hour time points). In contrast, logarithmic promastigotes were readily taken up early (1 hr), but failed to replicate. By 72 hours, the parasite burden had begun to decline, and by 96 hours it was significantly lower than the initial infection level (p < 0.05). These differences were evident when the number of intracellular parasites was quantified either as parasites per 100 macrophages or the percent of total macrophages that contained intracellular microorganisms (Figure 9C).
Discussion
The ability of Leishmania to survive intracellularly in host cells likely is a critical determinant of its success or failure during mammalian infection. Leishmania spp. promastigotes are taken up by mammalian macrophages through receptor-mediated phagocytosis. Macrophage surface receptors implicated in Leishmania recognition include CR1, CR3, MR, TLR2 and 9, the fibronectin receptor, and the receptor for advanced glycoconjugates (Martinez-Salazar et al., 2008; Da Silva et al., 1989; Mosser et al., 1987; Wilson and Pearson, 1986; Blackwell et al., 1985; Mosser and Edelson, 1985; Wyler et al., 1985). During the current study we examined mechanisms that would explain recent reports that present conflicting data challenging the importance of MR in Leishmania phagocytosis (Akilov et al., 2007).
The Leishmania spp. parasites are found in different biological stages as they transit through their insect (Phlebotomine sand fly) and mammalian hosts. A critical change in virulence occurs during development of the promastigote to the highly infectious metacyclic form in the gut of the sand fly (Pimenta et al., 1994; Pimenta et al., 1992; Sacks and Perkins, 1985). Promastigotes also become more virulent during growth in vitro to stationary phase, and recent developments have allowed purification of Lic infectious metacyclic forms from these stationary phase cultures (Yao et al., 2008; Spath and Beverley, 2001). This advance allowed us to reevaluate the interactions between promastigotes and host cells using parasite forms that approximate those occurring naturally in the parasite's life cycle. There has previously been no evaluation of differential receptor utilization by virulent metacyclic versus avirulent logarithmic promastigote forms. Based upon the hypothesis that the mechanisms utilized for MDM entry by each parasite form would lead to its survival or killing, we investigated whether metacyclic or logarithmic promastigotes utilize different surface receptors for their entry into MDMs.
Data presented in this report demonstrated that (1) both metacyclic and logarithmic promastigotes entered MDMs via CR3, but (2) only the logarithmic forms ligated MR. (3) CR3 co-localized with caveolin-1 early after phagocytosis, suggesting these receptors were located in these lipid-rich membrane domains during the initial moments after phagocytosis. (4) Metacyclic promastigotes were taken up at a lower rate than logarithmic parasites and remained near the periphery of the macrophage, whereas logarithmic promastigotes were rapidly internalized and guided deep into the host cell. (5) Despite the lower rate of phagocytosis, metacyclics survived and replicated within MDMs, whereas logarithmic parasites were killed. (6) Fusion of the PV surrounding metacyclic but not logarithmic promastigotes with the lysosomal markers LAMP-1 and Cat-D was delayed.
These results provide an explanation for the prior conflicting reports on the importance of MR during phagocytosis of L. donovani promastigotes (Akilov et al., 2007). Early studies used mixed cultures of virulent (metacyclic) and avirulent promastigotes leading to co-ligation of CR3 and MR by subsets of promastigotes, whereas later studies were performed after methods for purification of infectious metacyclics were developed, yielding a population of parasites incapable of binding or utilizing MR. Our data showed that metacyclic promastigotes were taken up through membrane domains enriched in both CR3 and caveolin-1. This is consistent with our previous observation that promastigotes internalized through caveolae are guided toward a pathway that delays the recruitment of microbicidal machinery (Rodriguez et al., 2006). Our findings suggest that the phagocytosis of Lic metacyclic promastigotes through ligation of CR3 in macrophage caveolae contributes to their ultimate intracellular survival.
During entry into host tissue, Lic promastigotes are necessarily exposed to serum and interstitial tissue constituents. Promastigotes become efficiently opsonized with the third complement protein, which can be cleaved to the CR3 ligand C3bi (Brittingham et al., 1995; Sacks, 1992; Mosser and Edelson, 1987). We hypothesize that both avirulent and metacyclic promastigotes are able to ligate CR3, facilitating their uptake by macrophages. Entry via CR3 ligation is highly advantageous for Lic, since CR3 does not trigger NADPH oxidase assembly and activation at the phagosome membrane (Puentes et al., 1990; Da Silva et al., 1989; Wright and Silverstein, 1983). Thus, signals leading to local and systemic inflammation are likely kept to a minimum (Rodriguez et al., 2006; Aderem and Underhill, 1999).
MR is a pattern recognition receptor that binds terminal sugar residues such as high mannose oligosaccharides on microbial membranes. The outcome of MR ligation seems to vary depending upon the activation stage of the phagocyte and the ligand engaging the receptor. MR expression is up-regulated in alternatively activated macrophages, an activation pattern associated with the clearance of inflammatory enzymes during wound healing and tissue repair (Akilov et al., 2007; Allavena et al., 2004; Mosser, 2003). As an example of the anti-inflammatory effects of MR, virulent Mycobacterium tuberculosis engages MR through its mannose-capped lipoarabinomannan, leading to evasion of phagosome-lysosome fusion and to down-modulation of the pro-inflammatory cytokines TNF-α and IL-12 (Kang et al., 2005; Nigou et al., 2001). In contrast, other reports suggest MR ligation promotes selected inflammatory responses such as TNF-α production (Linehan et al., 2000b; Linehan et al., 2000a; Garner et al., 1994). It was recently documented that dermal dendritic cells fall into two distinct phenotypes: immature CDs lacking MR but expressing CD208 (DC-LAMP) and CD11c, and “dendritic appearing macrophages” expressing CD206 (MR) as well as CD209 (DC-SIGN), CD163 and CD68 (Ochoa et al., 2008). The observation that cutaneous lesions of tuberculoid leprosy also contain distinct populations of dendritic cells expressing either macrophage markers or dendritic cell markers suggests that such phenotypes could also occur during dermal infections, and apply at sites where metacyclic promastigotes are delivered by sand flies (Krutzik et al., 2005). The effect of MR and CR3 co-ligation, such as observed in our study, is unknown. Our observations suggest that metacyclic Lic evade MR ligation, so that at least in this case the MR is not critical for evasion of microbidical responses.
Both the high mannose glycosylated surface protease MSP (also called GP63) and the surface glycolipid lipophosphyglycan (LPG) have been implicated in macrophage attachment, complement opsonization, and evasion of complement-mediated killing (Joshi et al., 2002; Sacks, 1992; Russell, 1987). We cannot precisely conclude which parasite surface molecules are responsible for ligating macrophage receptors during entry of each parasite stage, although both of these molecules are candidates and both increase during promastigote metacyclogenesis. Whether ligation of specific receptors leads to unique PV membrane remodeling is also unknown. Furthermore, different species of Leishmania display different arrays of abundant surface molecules. It is therefore possible that macrophage receptor ligation is similarly variable between the Leishmania spp. Finally, our data were obtained through assaying parasite phagocytosis in an in vitro situation. In a true in vivo infection environment, the recruitment of inflammatory cells such as neutrophils and dendritic cells would also influence parasite fate, in addition to selective receptor usage and development into infectious promastigote stages. Nonetheless, our current data lead us to hypothesize that differences in receptor ligation and intracellular localization may reflect different rates of progression of metacyclic versus logarithmic promastigotes through the endocytic system. Precocious fusion with lysosomes is likely a disadvantage for promastigotes, which are susceptible to killing by oxidants (Miller et al., 2000). It follows that the transient delay in PV-lysosome fusion offers a survival advantage to metacyclic promastigotes (Alexander et al., 1999; Desjardins and Descoteaux, 1998; Desjardins, 1995; Alexander and Russell, 1992).
The diminished recruitment of LAMP-1 and Cat-D to the Leishmania PV during the early stages of infection has previously been observed (Russell and Wright, 1988). Promastigote surface LPG has been shown critical for the fusion delay, possibly by its ability to dissolve the organization of GM-1 ganglioside microdomains on the PV membrane and interfere with phagosome biogenesis (Dermine et al., 2005). Promastigotes express LPG whereas amastigotes do not, and one would expect that these evasion strategies would operate until the point of conversion to amastigotes, a life stage that is relatively resistant to oxidant-mediated microbicidal proteins (Dermine et al., 2005; Desjardins and Descoteaux, 1998; Desjardins, 1995).
Data in the present report document the utilization of different receptors by metacyclic versus logarithmic Lic promastigotes, and different downstream events leading to survival or killing of the two parasite forms, respectively. The combined effects of delaying lysosomal fusion and suppressing classical macrophage activation would be expected to enhance survival of metacyclic promastigotes, to the point of conversion to the amastigote stage. We propose that discrete routes of entry may account for survival or killing of parasites within phagocytes. Such studies could lead us to novel therapeutic applications aimed at channeling all promastigotes toward a microbicidal pathway in host macrophages.
Experimental Procedures
Parasites
The Brazilian strain of L. chagasi (MHOM/BR/00/1669) used in this study was originally isolated from a patient with visceral leishmaniasis and serially passed in male Syrian hamsters to maintain virulence. All experiments were performed using parasites that were within 3 weeks of isolation from the animal. Promastigotes were cultured at 26 °C in hemoflagellate-modified minimal essential medium (HOMEM), supplemented with 10% heat-inactivated fetal calf serum (Gibco, Carlsbad, CA), pH 7.4 (Hsiao et al., 2008; Berens et al., 1976).
Lic parasite cultures were initiated at 106/ml and grown for 7-9 days to stationary phase. Metacyclic promastigotes were isolated from stationary cultures on a modified Ficoll gradient as reported (Yao et al., 2008; Spath and Beverley, 2001). Briefly, promastigotes were overlaid on a step gradient of 10% Ficoll-Hypaque (Sigma Chemical Co., St. Louis, MO) in M199 medium (Amersham, Piscataway, NJ) and 40% Ficoll in phosphate buffered saline (PBS). After centrifugation at 365 × g at room temperature (10 min), metacyclic promastigotes were collected from the interface between the 0% and 10% Ficoll layers (Pinto-da-Silva et al., 2002; Nieves and Pimenta, 2000; Bandyopadhyay et al., 1991; Howard et al., 1987). A mixed population of exponentially growing logarithmic phase Lic promastigotes was collected after culturing for 4 days in HOMEM at 26 °C as above.
Human monocyte-derived macrophages
Peripheral blood was collected from healthy human donors during routine plateletpheresis at the DeGowin Blood Center at the University of Iowa. White blood cells were retained in sterile leukoreduction system chambers, which were generously provided to us for research purposes. Leukocytes were rinsed from the chambers using PBS within 2 hours of collection (Dietz et al., 2006). Peripheral blood mononuclear cells were isolated by density centrifugation on Ficoll and suspended in RPMI 1640, supplemented with 10% fetal calf serum and 50 ug streptomycin/ml and 100 U penicillin/ml (RP-10; reagents from Gibco) (Chang et al., 2007). Differentiation into MDMs was modified from published protocols (Schulert and Allen, 2006; Wilson and Pearson, 1986): Monocytes were separated by adherence to 100 mm × 20 mm Petri dishes (Corning, Lowell, MA). After incubation for 4-5 days at 37 °C, adherent MDMs were lifted off with 0.25% Trypsin-EDTA (Gibco) and gentle scraping, transferred to 12 mm coverslips in 24 well tissue culture plates, and cultured for an additional 24 hours at 37 °C to allow recovery from trypsinization.
Phagocytosis assay
Metacyclic or logarithmic promastigotes were fluorescently labeled by incubation in 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE, Molecular Probes, Carlsbad, CA) for 10 min at 37 °C as described (Chang et al., 2007). Labeled parasites were opsonized in 2.5% fresh C5-deficient human serum (Gibco) for 30 minutes at 37 °C. MDMs on coverslips were rinsed and incubated with either metacyclic or logarithmic promastigotes at a multiplicity of infection (MOI) of 10:1. Phagocytosis was synchronized by low-speed centrifugation (3min, 330 g, 4 °C), and incubated in 37 °C, 5% CO2 for 2, 15, 30, 60, or 100 minutes (Rodriguez et al., 2006). All media were replaced with fresh RP-10 at 60 minutes after infection to remove extracellular organisms (Schulert and Allen, 2006). Samples were fixed with 2% paraformaldehyde (EMS, Hatfield, PA), and processed for confocal microscopy as described below.
Confocal microscopy
Following fixation, MDMs were permeabilized with 0.2% Triton X-100 and blocked in 5% normal goat serum. Using a modified staining protocol (Allen, 2007), coverslips were incubated for 1 hour at room temperature in one of the following monoclonal antibodies: 1:20 affinity purified anti-human CD11b (clone H5A4-c, Developmental Studies Hybridoma Bank (DSHB), University of Iowa), 1:10 anti-human CD206 (MCA2155, Serotec, Oxford, UK), 1:100 affinity purified anti-human LAMP-1 (H4A3-c, DSHB at the U. of Iowa), or 1:100 anti-human cathepsin D (sc-13148, Santa Cruz Biotechnology Inc., Santa Cruz, CA). Samples were then incubated in 1:200 Alexafluor 568 goat anti-mouse IgG (Molecular Probes), followed by 1:40 Alexafluor 647 phalloidin (Molecular Probes) to stain macrophage actin filaments. Some coverslips were stained with 1:100 anti-human caveolin-1 polyclonal antibody (sc-894, Santa Cruz Biotechnology Inc.) followed by Alexafluor 647 goat anti-rabbit IgG (Molecular Probes). Coverslips were incubated in Vectashield H-1000 (Vector Labs, Burlingame, CA) and examined within 48 hours on a Bio-Rad 1024 Confocal Microscope, University of Iowa Central Microscopy Research Facility. ImageJ 1.4 software was used to prepare figures.
Light microscopy
MDMs on coverslips used for light microscopy were infected with 10:1 unlabeled metacyclic or logarithmic promastigotes, and incubated for 1, 24, 48, 72, or 96 hours, 37%C. Coverslips were fixed with methanol, air-dried, and stained with Protocol Hema 3 solutions 1 and 2 (Fisher Scientific, Kalamazoo, MI). The coverslips were mounted on slides with Permount (Fisher Scientific) and quantitated on an Olympus BX41 light microscope.
Quantification and Statistical Analysis
For confocal microscopy, co-localization and parasite burdens were quantified from the fluorescent photomicrographs in a blinded fashion by two independent observers. More than 200 parasites were scored for every time point and condition. Co-localization was defined as either direct overlay of pixels denoting parasite fluorescence and receptor fluorescence, or noticeable enrichment of receptor fluorescence surrounding the intracellular parasite body. Parasites that had made contact with a host cell were scored positive for parasite burden..
For basic light microscopy, parasite loads were quantified by scoring more than 500 macrophages for every time point and condition. An infecting parasite was defined as complete internalization of one parasite by a host cell.
For both studies, one-tailed t tests were calculated using GraphPad Prism 5.
Supplementary Materials
Figure S1. Logarithmic promastigotes fuse with Cat-D more rapidly than metacyclic promastigotes. Purified metacyclic promastigotes or a mixed population of logarithmic promastigotes were labeled with CFSE (green) and added to adherent MDMs at an MOI of 10:1 during a synchronized phagocytosis assay. Coverslips containing MDMs were fixed after 2 or 24 hours. Cat-D was labeled with anti-human Cat-D primary and Alexa Fluor 568 anti-mouse IgG secondary antibodies (red). Cellular actin was labeled with Alexa Fluor 647 phalloidin (blue). Metacyclic promastigotes did not accumulate Cat-D until 24 hours after initiation of phagocytosis. However, logarithmic promastigotes co-localized with Cat-D after 2 hours (arrows). Photomicrographs are representative of more than 4 experiments.
Figure S2. Control micrographs show that Cat-D is evenly distributed throughout uninfected MDMs, and slightly enriched in the perinuclear region. Uninfected MDMs were fixed in parallel with parasite-exposed MDMs as the times described in Figures 1 and 2. Shown are examples of the 2 hour time point. Cat-D was labeled with anti-human Cat-D primary and Alexa Fluor 568 anti-mouse IgG secondary antibodies (red). Cellular actin was labeled with Alexa Fluor 647 phalloidin (blue).
Video S1. Metacyclic promastigotes remain at the MDM periphery and are encircled by CR3. Phagocytosis assay and preparation for confocal microscopy were as described in Figure 1. 1 μm Z-sections were stacked and interpolated to create a 3-D projection of the 60 minute merge panel from Figure 1.
Video S2. Metacyclic promastigotes that co-localize with CR3 also cluster with caveolin-1 at the MDM surface. The phagocytosis assay and preparation for confocal microscopy were as described in Figure 7. 1 μm Z-sections were stacked and interpolated to create a 3-D projection of the 30 minute merge panel from Figure 7.
Acknowledgments
We thank Chantal Allamargot for help with confocal microscopy. The authors are grateful to Lee-Ann Allen, Ph.D. and Mark Stamnes, Ph.D. for careful review of this manuscript.
This work was supported by NIH grants AI045540, AI067874 and AI059451, and a Merit Review grant (MEW) and a Persian Gulf RFP (MEW) from the Department of Veterans' Affairs.
Abbreviations used
- Cat-D
cathepsin-D
- CR
complement receptor
- LAMP-1
lysosome-associated membrane protein-1
- Lic
Leishmania infantum chagasi
- MDM
monocyte-derived macrophage
- MOI
multiplicity of infection
- MR
mannose receptor
- PV
parasitophorous vacuole
References
- Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- Akilov OE, Kasuboski RE, Carter CR, McDowell MA. The role of mannose receptor during experimental leishmaniasis. J Leukoc Biol. 2007;81:1188–1196. doi: 10.1189/jlb.0706439. [DOI] [PubMed] [Google Scholar]
- Alexander J, Russell DG. The interaction of Leishmania species with macrophages. Adv Parasitol. 1992;31:175–254. doi: 10.1016/s0065-308x(08)60022-6. [DOI] [PubMed] [Google Scholar]
- Alexander J, Satoskar AR, Russell DG. Leishmania species: models of intracellular parasitism. J Cell Sci. 1999;112(Pt 18):2993–3002. doi: 10.1242/jcs.112.18.2993. [DOI] [PubMed] [Google Scholar]
- Allavena P, Chieppa M, Monti P, Piemonti L. From pattern recognition receptor to regulator of homeostasis: the double-faced macrophage mannose receptor. Crit Rev Immunol. 2004;24:179–192. doi: 10.1615/critrevimmunol.v24.i3.20. [DOI] [PubMed] [Google Scholar]
- Allen LA. Immunofluorescence and confocal microscopy of neutrophils. Methods Mol Biol. 2007;412:273–287. doi: 10.1007/978-1-59745-467-4_18. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay P, Ghosh DK, De A, Ghosh KN, Chaudhuri PP, Das P, Bhattacharya A. Metacyclogenesis of Leishmania spp: species-specific in vitro transformation, complement resistance, and cell surface carbohydrate and protein profiles. J Parasitol. 1991;77:411–416. [PubMed] [Google Scholar]
- Berens RL, Brun R, Krassner SM. A simple monophasic medium for axenic culture of hemoflagellates. J Parasitol. 1976;62:360–365. [PubMed] [Google Scholar]
- Blackwell JM, Ezekowitz RA, Roberts MB, Channon JY, Sim RB, Gordon S. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J Exp Med. 1985;162:324–331. doi: 10.1084/jem.162.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittingham A, Morrison CJ, McMaster WR, McGwire BS, Chang KP, Mosser DM. Role of the Leishmania surface protease gp63 in complement fixation, cell adhesion, and resistance to complement-mediated lysis. J Immunol. 1995;155:3102–3111. [PubMed] [Google Scholar]
- Chang HK, Thalhofer C, Duerkop BA, Mehling JS, Verma S, Gollob KJ, et al. Oxidant generation by single infected monocytes after short-term fluorescence labeling of a protozoan parasite. Infect Immun. 2007;75:1017–1024. doi: 10.1128/IAI.00914-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva RP, Hall BF, Joiner KA, Sacks DL. CR1, the C3b receptor, mediates binding of infective Leishmania major metacyclic promastigotes to human macrophages. J Immunol. 1989;143:617–622. [PubMed] [Google Scholar]
- Dermine JF, Scianimanico S, Prive C, Descoteaux A, Desjardins M. Leishmania promastigotes require lipophosphoglycan to actively modulate the fusion properties of phagosomes at an early step of phagocytosis. Cell Microbiol. 2000;2:115–126. doi: 10.1046/j.1462-5822.2000.00037.x. [DOI] [PubMed] [Google Scholar]
- Dermine JF, Goyette G, Houde M, Turco SJ, Desjardins M. Leishmania donovani lipophosphoglycan disrupts phagosome microdomains in J774 macrophages. Cell Microbiol. 2005;7:1263–1270. doi: 10.1111/j.1462-5822.2005.00550.x. [DOI] [PubMed] [Google Scholar]
- Desjardins M. Biogenesis of phagolysosomes: the ‘kiss and run’ hypothesis. Trends Cell Biol. 1995;5:183–186. doi: 10.1016/s0962-8924(00)88989-8. [DOI] [PubMed] [Google Scholar]
- Desjardins M, Descoteaux A. Survival strategies of Leishmania donovani in mammalian host macrophages. Res Immunol. 1998;149:689–692. doi: 10.1016/s0923-2494(99)80040-6. [DOI] [PubMed] [Google Scholar]
- Dietz AB, Bulur PA, Emery RL, Winters JL, Epps DE, Zubair AC, Vuk-Pavlovic S. A novel source of viable peripheral blood mononuclear cells from leukoreduction system chambers. Transfusion. 2006;46:2083–2089. doi: 10.1111/j.1537-2995.2006.01033.x. [DOI] [PubMed] [Google Scholar]
- Duclos S, Desjardins M. Subversion of a young phagosome: the survival strategies of intracellular pathogens. Cell Microbiol. 2000;2:365–377. doi: 10.1046/j.1462-5822.2000.00066.x. [DOI] [PubMed] [Google Scholar]
- Garner RE, Rubanowice K, Sawyer RT, Hudson JA. Secretion of TNF-alpha by alveolar macrophages in response to Candida albicans mannan. J Leukoc Biol. 1994;55:161–168. doi: 10.1002/jlb.55.2.161. [DOI] [PubMed] [Google Scholar]
- Harris J, Werling D, Hope JC, Taylor G, Howard CJ. Caveolae and caveolin in immune cells: distribution and functions. Trends Immunol. 2002;23:158–164. doi: 10.1016/s1471-4906(01)02161-5. [DOI] [PubMed] [Google Scholar]
- Howard MK, Sayers G, Miles MA. Leishmania donovani metacyclic promastigotes: transformation in vitro, lectin agglutination, complement resistance, and infectivity. Exp Parasitol. 1987;64:147–156. doi: 10.1016/0014-4894(87)90138-x. [DOI] [PubMed] [Google Scholar]
- Hsiao CH, Yao C, Storlie P, Donelson JE, Wilson ME. The major surface protease (MSP or GP63) in the intracellular amastigote stage of Leishmania chagasi. Mol Biochem Parasitol. 2008;157:148–159. doi: 10.1016/j.molbiopara.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi PB, Kelly BL, Kamhawi S, Sacks DL, McMaster WR. Targeted gene deletion in Leishmania major identifies leishmanolysin (GP63) as a virulence factor. Mol Biochem Parasitol. 2002;120:33–40. doi: 10.1016/s0166-6851(01)00432-7. [DOI] [PubMed] [Google Scholar]
- Kang PB, Azad AK, Torrelles JB, Kaufman TM, Beharka A, Tibesar E, et al. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med. 2005;202:987–999. doi: 10.1084/jem.20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutzik SR, Tan B, Li H, Ochoa MT, Liu PT, Sharfstein SE, et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med. 2005;11:653–660. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan SA, Martinez-Pomares L, Gordon S. Macrophage lectins in host defence. Microbes Infect. 2000a;2:279–288. doi: 10.1016/s1286-4579(00)00300-2. [DOI] [PubMed] [Google Scholar]
- Linehan SA, Martinez-Pomares L, Gordon S. Mannose receptor and scavenger receptor: two macrophage pattern recognition receptors with diverse functions in tissue homeostasis and host defense. Adv Exp Med Biol. 2000b;479:1–14. doi: 10.1007/0-306-46831-X_1. [DOI] [PubMed] [Google Scholar]
- Martinez-Salazar B, Berzunza-Cruz M, Becker I. Leishmania mexicana DNA activates murine macrophages and increases their TLR9 expression. Gac Med Mex. 2008;144:99–104. [PubMed] [Google Scholar]
- Miller MA, McGowan SE, Gantt KR, Champion M, Novick SL, Andersen KA, et al. Inducible resistance to oxidant stress in the protozoan Leishmania chagasi. J Biol Chem. 2000;275:33883–33889. doi: 10.1074/jbc.M003671200. [DOI] [PubMed] [Google Scholar]
- Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edelson PJ. The mouse macrophage receptor for C3bi (CR3) is a major mechanism in the phagocytosis of Leishmania promastigotes. J Immunol. 1985;135:2785–2789. [PubMed] [Google Scholar]
- Mosser DM, Edelson PJ. The third component of complement (C3) is responsible for the intracellular survival of Leishmania major. Nature. 1987;327:329–331. doi: 10.1038/327329b0. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Vlassara H, Edelson PJ, Cerami A. Leishmania promastigotes are recognized by the macrophage receptor for advanced glycosylation endproducts. J Exp Med. 1987;165:140–145. doi: 10.1084/jem.165.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieves E, Pimenta PF. Development of Leishmania (Viannia) braziliensis and Leishmania (Leishmania) amazonensis in the sand fly Lutzomyia migonei (Diptera: Psychodidae) J Med Entomol. 2000;37:134–140. doi: 10.1603/0022-2585-37.1.134. [DOI] [PubMed] [Google Scholar]
- Nigou J, Zelle-Rieser C, Gilleron M, Thurnher M, Puzo G. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J Immunol. 2001;166:7477–7485. doi: 10.4049/jimmunol.166.12.7477. [DOI] [PubMed] [Google Scholar]
- Nitsche-Schmitz DP, Rohde M, Chhatwal GS. Invasion mechanisms of Gram-positive pathogenic cocci. Thromb Haemost. 2007;98:488–496. [PubMed] [Google Scholar]
- Ochoa MT, Loncaric A, Krutzik SR, Becker TC, Modlin RL. “Dermal dendritic cells” comprise two distinct populations: CD1+ dendritic cells and CD209+ macrophages. J Invest Dermatol. 2008;128:2225–2231. doi: 10.1038/jid.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkmans L, Kartenbeck J, Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat Cell Biol. 2001;3:473–483. doi: 10.1038/35074539. [DOI] [PubMed] [Google Scholar]
- Pimenta PF, Turco SJ, McConville MJ, Lawyer PG, Perkins PV, Sacks DL. Stage-specific adhesion of Leishmania promastigotes to the sandfly midgut. Science. 1992;256:1812–1815. doi: 10.1126/science.1615326. [DOI] [PubMed] [Google Scholar]
- Pimenta PF, Saraiva EM, Rowton E, Modi GB, Garraway LA, Beverley SM, et al. Evidence that the vectorial competence of phlebotomine sand flies for different species of Leishmania is controlled by structural polymorphisms in the surface lipophosphoglycan. Proc Natl Acad Sci U S A. 1994;91:9155–9159. doi: 10.1073/pnas.91.19.9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto-da-Silva LH, Camurate M, Costa KA, Oliveira SM, da Cunha-e-Silva NL, Saraiva EM. Leishmania (Viannia) braziliensis metacyclic promastigotes purified using Bauhinia purpurea lectin are complement resistant and highly infective for macrophages in vitro and hamsters in vivo. Int J Parasitol. 2002;32:1371–1377. doi: 10.1016/s0020-7519(02)00137-6. [DOI] [PubMed] [Google Scholar]
- Puentes SM, Da Silva RP, Sacks DL, Hammer CH, Joiner KA. Serum resistance of metacyclic stage Leishmania major promastigotes is due to release of C5b-9. J Immunol. 1990;145:4311–4316. [PubMed] [Google Scholar]
- Rodriguez NE, Gaur U, Wilson ME. Role of caveolae in Leishmania chagasi phagocytosis and intracellular survival in macrophages. Cell Microbiol. 2006;8:1106–1120. doi: 10.1111/j.1462-5822.2006.00695.x. [DOI] [PubMed] [Google Scholar]
- Russell DG. The macrophage-attachment glycoprotein gp63 is the predominant C3-acceptor site on Leishmania mexicana promastigotes. Eur J Biochem. 1987;164:213–221. doi: 10.1111/j.1432-1033.1987.tb11013.x. [DOI] [PubMed] [Google Scholar]
- Russell DG, Wright SD. Complement receptor type 3 (CR3) binds to an Arg-Gly-Asp-containing region of the major surface glycoprotein, gp63, of Leishmania promastigotes. J Exp Med. 1988;168:279–292. doi: 10.1084/jem.168.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks DL. The structure and function of the surface lipophosphoglycan on different developmental stages of Leishmania promastigotes. Infect Agents Dis. 1992;1:200–206. [PubMed] [Google Scholar]
- Sacks DL, Perkins PV. Identification of an infective stage of Leishmania promastigotes. Science. 1984;223:1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- Sacks DL, Perkins PV. Development of infective stage Leishmania promastigotes within phlebotomine sand flies. Am J Trop Med Hyg. 1985;34:456–459. doi: 10.4269/ajtmh.1985.34.456. [DOI] [PubMed] [Google Scholar]
- Schulert GS, Allen LA. Differential infection of mononuclear phagocytes by Francisella tularensis: role of the macrophage mannose receptor. J Leukoc Biol. 2006;80:563–571. doi: 10.1189/jlb.0306219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JS, Abraham SN. Caveolae as portals of entry for microbes. Microbes Infect. 2001;3:755–761. doi: 10.1016/s1286-4579(01)01423-x. [DOI] [PubMed] [Google Scholar]
- Spath GF, Beverley SM. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp Parasitol. 2001;99:97–103. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Pearson RD. Evidence that Leishmania donovani utilizes a mannose receptor on human mononuclear phagocytes to establish intracellular parasitism. J Immunol. 1986;136:4681–4688. [PubMed] [Google Scholar]
- Wilson ME, Pearson RD. Roles of CR3 and mannose receptors in the attachment and ingestion of Leishmania donovani by human mononuclear phagocytes. Infect Immun. 1988;56:363–369. doi: 10.1128/iai.56.2.363-369.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SD, Silverstein SC. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983;158:2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyler DJ, Sypek JP, McDonald JA. In vitro parasite-monocyte interactions in human leishmaniasis: possible role of fibronectin in parasite attachment. Infect Immun. 1985;49:305–311. doi: 10.1128/iai.49.2.305-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Chen Y, Sudan B, Donelson JE, Wilson ME. Leishmania chagasi: homogenous metacyclic promastigotes isolated by buoyant density are highly virulent in a mouse model. Exp Parasitol. 2008;118:129–133. doi: 10.1016/j.exppara.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Logarithmic promastigotes fuse with Cat-D more rapidly than metacyclic promastigotes. Purified metacyclic promastigotes or a mixed population of logarithmic promastigotes were labeled with CFSE (green) and added to adherent MDMs at an MOI of 10:1 during a synchronized phagocytosis assay. Coverslips containing MDMs were fixed after 2 or 24 hours. Cat-D was labeled with anti-human Cat-D primary and Alexa Fluor 568 anti-mouse IgG secondary antibodies (red). Cellular actin was labeled with Alexa Fluor 647 phalloidin (blue). Metacyclic promastigotes did not accumulate Cat-D until 24 hours after initiation of phagocytosis. However, logarithmic promastigotes co-localized with Cat-D after 2 hours (arrows). Photomicrographs are representative of more than 4 experiments.
Figure S2. Control micrographs show that Cat-D is evenly distributed throughout uninfected MDMs, and slightly enriched in the perinuclear region. Uninfected MDMs were fixed in parallel with parasite-exposed MDMs as the times described in Figures 1 and 2. Shown are examples of the 2 hour time point. Cat-D was labeled with anti-human Cat-D primary and Alexa Fluor 568 anti-mouse IgG secondary antibodies (red). Cellular actin was labeled with Alexa Fluor 647 phalloidin (blue).
Video S1. Metacyclic promastigotes remain at the MDM periphery and are encircled by CR3. Phagocytosis assay and preparation for confocal microscopy were as described in Figure 1. 1 μm Z-sections were stacked and interpolated to create a 3-D projection of the 60 minute merge panel from Figure 1.
Video S2. Metacyclic promastigotes that co-localize with CR3 also cluster with caveolin-1 at the MDM surface. The phagocytosis assay and preparation for confocal microscopy were as described in Figure 7. 1 μm Z-sections were stacked and interpolated to create a 3-D projection of the 30 minute merge panel from Figure 7.