Abstract
Background
Social anxiety disorder (SAD) is characterized by distorted negative self-beliefs (NSBs) which are thought to enhance emotional reactivity, interfere with emotion regulation, and undermine social functioning. Cognitive reappraisal is a type of emotion regulation used to alter NSBs, with the goal of modulating emotional reactivity. Despite its relevance, little is known about the neural bases and temporal features of cognitive reappraisal in patients with SAD.
Methods
Twenty-seven patients with SAD and 27 healthy controls (HC) were trained to react and to implement cognitive reappraisal in order to down-regulate negative emotional reactivity to NSBs while undergoing functional magnetic resonance imaging and providing ratings of negative emotion experience.
Results
Behaviorally, compared with HC, patients with SAD reported greater negative emotion both while reacting to and reappraising NSBs. However, when cued, participants in both groups were able to use cognitive reappraisal to decrease emotion. Neurally, reacting to NSBs resulted in early amygdala response in both groups. Reappraising NSBs resulted in greater early cognitive control, language, and visual processing in HC, but greater late cognitive control, visceral, and visual processing in patients with SAD. Functional connectivity analysis during reappraisal identified more regulatory regions inversely related to left amygdala in HCs than in patients with SAD. Reappraisal-related brain regions that differentiated patients and controls were associated with negative emotion ratings and cognitive reappraisal self-efficacy.
Conclusions
Findings regarding cognitive reappraisal suggest neural timing, connectivity, and brain-behavioral associations specific to patients with SAD, and elucidate neural mechanisms that might serve as biomarkers of interventions for SAD.
Keywords: brain, emotion, emotion regulation, social anxiety, fMRI, neuroimaging
Introduction
Social anxiety disorder (SAD) is a chronic psychiatric condition characterized by fear and avoidance of social situations (1). It has a high prevalence rate (up to 12.1% of the US adult population) (2), and its early onset (3) may predispose individuals to subsequent development of other anxiety, depressive (4), and substance use disorders (5). SAD is linked to significant emotional distress and functional impairment in work and social domains and typically persists until properly diagnosed and treated (5–10).
Negative Self-Beliefs in SAD
Cognitive models of social anxiety (11–13) suggest that in social situations patients with SAD generate distorted beliefs about themselves and about how others evaluate them. These negative self-referential beliefs (NSBs) are thought to induce exaggerated negative emotional reactivity (e.g., fear, anxiety), maladaptive behaviors (e.g., social avoidance) and affective dysregulation (14) which, in turn, maintain anxiety.
NSBs are conceptualized as self-representations that actively filter and misconstrue new information (15). Patients with SAD have more NSBs than healthy controls (12; 16–18). Furthermore, NSBs mediate the effects of trait social anxiety on state anxiety and heart rate variability during negative anticipation (17), speech-related anxiety (19), negative bias in memories of social performance (19), and reduction in social anxiety symptoms during cognitive-behavioral group therapy (20; 21). Because NSBs serve an important role both in the onset and treatment of SAD, understanding the neural bases of emotional reactivity and cognitive reappraisal of NSBs may yield a better understanding of brain mechanisms underlying SAD.
Neural Bases of Emotional Reactivity in SAD
Emotional reactivity in SAD is linked to brain activity in limbic/paralimbic regions, including the amygdala (22–27), anterior cingulate cortex (ACC) (28), and insular cortex (24; 29) in response to social cues (e.g., harsh facial expressions, praise, criticism) and anticipation and delivery of a speech (30–32). Greater medial prefrontal cortex (PFC) and amygdala activity have been observed in response to experimenter-selected NSBs statements in SAD (32). Amygdala response has sometimes been associated with SAD symptom severity (27; 33; 34). Treatment-induced changes in amygdala response have been shown to predict SAD symptoms after one year (31).
In addition to examining the magnitude of brain responses to negative emotional stimuli, the temporal dynamics of emotion-related neural responses can reveal patterns of emotional reactivity that differentiate healthy adults and mood disordered patients (27; 35). The single investigation of neural timing in SAD found that, compared to healthy controls, patients with SAD had delayed amygdala responses to fearful, angry, and happy facial expressions, but there were no group differences for PFC and fusiform face area responses (36).
Neural Bases of Emotion Regulation in SAD
Little is known about the neural mechanisms of emotion regulation in SAD, despite a general recognition of emotion dysregulation in patients with SAD (37). The single imaging study that investigated emotion regulation in SAD showed that during cognitive reappraisal of harsh facial expressions, compared to healthy controls, patients with SAD were less likely to recruit brain regions implicated in cognitive reappraisal (dorsolateral PFC, dorsal ACC) and attention modulation (medial cuneus, posterior cingulate, bilateral dorsal parietal) (38).
One limitation of that study was its focus on facial expressions, rather than the NSBs hypothesized to play a crucial role in SAD. A second limitation is that it - like most prior studies - did not examine the timing of the neural correlates of cognitive reappraisal. In healthy adults, cognitive reappraisal has been shown to engage regulatory PFC regions within the first 3s and then diminish (27). The timing of neural responses related to cognitive reappraisal has not been investigated in patients with SAD. Furthermore, although functional connectivity analyses have found an inverse association between PFC and amygdala in healthy adults, suggesting top-down cognitive reappraisal modulation of emotional reactivity (39), this brain systems interaction approach has not been applied in the context of emotion regulation in patients with SAD.
The Present Study
The present study examined differential magnitude and timing of neural responses during cognitive reappraisal of NSBs within the context of personally salient autobiographical social anxiety scripts in patients with SAD compared with demographically-matched nonpsychiatric controls. Temporal analysis investigated early (0–3s) and late (6–9s) peak BOLD responses during 9s trials of reacting to NSBs and reappraising NSBs. We compared early versus late responses to test our hypothesis of differential timing in patients and controls in brain regions involved in emotional reactivity and cognitive reappraisal. For emotional reactivity, we expected larger and delayed emotion-related limbic responses in patients compared to controls. For cognitive reappraisal, we expected delayed onset of reappraisal-related medial and dorsolateral PFC engagement in patients compared to controls.
Methods and Materials
Participants
Participants were 27 (12 female) adults who met DSM-IV (40) criteria for primary generalized SAD and 27 (12 female) demographically-matched healthy controls with no history of DSM-IV psychiatric disorders. Patients and controls did not differ in handedness, age, education, or ethnicity (Table 1). Among patients, current Axis-I co-morbidity included 3 with generalized anxiety disorder and 3 with specific phobia. Past Axis-I co-morbidity included 1 with past major depression, 1 with past dysthymia, and 1 with past substance abuse. Ten patients reported past (i.e., ended more than 1 year ago) non-cognitive-behavioral psychotherapy, and 8 reported past pharmacotherapy. All participants provided informed consent.
Table 1.
Demographic and Clinical Variables
| SAD Mean ± SD |
HC Mean ± SD |
t-value | Partial eta2 |
|
|---|---|---|---|---|
| Gender | 12 female | 12 females | ||
| Age (years) | 32.1 ± 9.2 | 32.2 ± 9.5 | 0 | |
| Education (years) | 16.8 ± 1.8 | 17.1 ± 1.5 | 1.2 | |
| Edinburgh | 9.9 ± 0.3 | 9.8 ± 0.4 | 0.3 | |
| Handedness Inventory | ||||
| Ethnicity | ||||
| - Caucasian | 16 | 16 | ||
| - Asian | 8 | 8 | ||
| - Latino | 1 | 2 | ||
| - Native American | 1 | 1 | ||
| - Native Hawaiian | 1 | 0 | ||
| LSAS-SR | 80.1 ± 16.8 | 15.7 ± 8.7 | 16.7 ** | .85 |
| BFNE | 49.0 ± 5.6 | 26.7 ± 4.9 | 15.4 ** | .82 |
| BDI-II | 6.3 ± 6.1 | 1.7 ± 1.9 | 4.2 ** | .26 |
| PANAS-Neg | 22.0 ± 6.9 | 14.8 ± 5.7 | 4.3 ** | .27 |
| PANAS-Pos | 28.7 ± 5.7 | 36.0 ± 5.3 | 4.5 ** | .28 |
| ERQ Reappraisal | ||||
| - Frequency | 34.5 ± 9.4 | 40.0 ± 5.5 | 2.6 * | .12 |
| - Self-Efficacy | 27.3 ± 10.5 | 42.3 ± 7.2 | 6.1** | .42 |
P<.005,
P<.001
Note: LSAS-SR=Liebowitz Social Anxiety Scale–Self-Report; BFNE=Brief Fear of Negative Evaluation Scale; BDI-II=Beck Depression Inventory–II; PANAS=Positive and Negative Affect Schedule; ERQ =Emotion Regulation Questionnaire.
Exclusion Criteria
Participants passed an MRI safety screen, and were excluded for current pharmacotherapy or psychotherapy, past cognitive-behavioral therapy, history of medical disorders or head trauma, and current psychiatric disorders other than generalized anxiety disorder, agoraphobia without a history of panic attacks, or specific phobia.
Clinical and Individual Difference Assessment
Clinical assessments were conducted by two PhD-level clinical psychologists (PG, KW) and one graduate student (TM) using the Anxiety Disorders Interview Schedule for DSM-IV (41). Patients met diagnostic criteria for generalized SAD (defined as greater than moderate anxiety/fear for 5 or more distinct social situations) and healthy controls had no history of DSM-IV disorders.
Participants completed self-report measures of clinical symptoms and individual differences. As shown in Table 1, compared with controls, patients reported greater social anxiety symptoms (Liebowitz Social Anxiety Scale-Self-Report (42; 43)), fear of negative evaluation (Brief Fear of Negative Evaluation Scale (44)), depressive symptoms (Beck Depression Inventory-II (45)), negative affect, and lesser positive affect (Positive and Negative Affect Schedule (46)) and reappraisal frequency and self-efficacy (Emotion Regulation Questionnaire (47; 48)).
Procedure
Participants provided information about four distinct autobiographical social situations. At the scanning session, participants were trained in reappraisal methods developed by Gross and Ochsner(49; 50) with two experimenter-composed social anxiety situations and instructed to either “REACT” by considering how the NSB reflected something true about themselves, or “REFRAME” by reinterpreting the NSB to down-regulate negative emotional reactions. Participants were instructed to “actively reframe the belief by thinking in a way that re-interprets the content of the belief and thereby make the belief less negative and toxic for you. For example, if the belief is “NO ONE LIKES ME”, REFRAMING may be telling yourself “That is not always true,” “Some people like me”, or “This is only a thought, not a fact.” How else might you dispute this BELIEF?”
During scanning, participants read their autobiographical social situations one sentence at a time, and after each NSB, provided a negative emotion rating using a button box and E-Prime software (Psychology Software Tools, Inc, Pittsburgh, Pennsylvania) by responding to “How negative do you feel?” (1=not at all to 5=very much).
Experimental Task
The task consisted of five situations. The first was an experimenter-composed neutral situation about cleaning a car that was used to obtain baseline emotion ratings and fMRI BOLD signals for reading neutral statements. Then four participant-generated autobiographical social anxiety situations characterized by social anxiety, humiliation, and embarrassment were presented. For each situation, participants composed a paragraph describing the events, thoughts, and feelings and five NSBs. Experimenters modified the NSBs so that there was a set of 5 self-only (e.g., I am incompetent) and 5 self-plus-other (e.g., Others think I am not normal) (Table S1 in Supplement 1).
Participants indicated their age at the time of each situation and provided ratings, on a scale of 1 (not at all) to 9 (very much), quantifying the vividness of the memory, the experience of shame at the time of the situation, as well as current shame, disturbance, avoidance, and frequency of talking about the situation.
Three situations were presented in a first run lasting 9 minutes, 21 seconds, followed by two situations in a second run of 6 minutes, 24 seconds. The sequence of 5 situations was fixed: Neutral, React NSB, Reappraise NSB, React NSB, and Reappraise NSB.
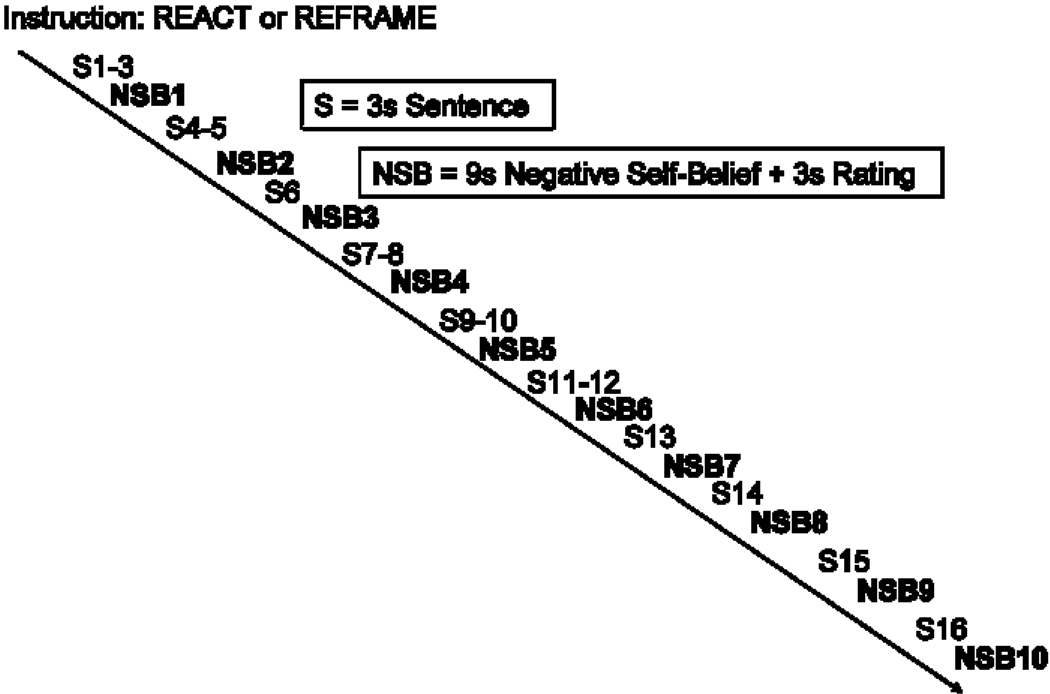
Each situation consisted of (a) an instruction to react or reappraise (6s), (b) 16 sentences (3 seconds each) in white font against a black background describing the situation, (c) 10 NSBs (9 seconds each) embedded in the unfolding story in uppercase letters that flashed 9 times (850 milliseconds on + 150 milliseconds off), and (d) a negative emotion rating after each NSB (3 seconds) (Figure 1). NSBs were flashed to maintain attention, and appeared in white font for Neutral and React trials and green for Reappraisal trials.
Figure 1.
Components and structure of one autobiographical social situation trial.
Image Acquisition
Imaging was performed on a GE 3-T Signa magnet with a T2*-weighted gradient echo spiral-in/out pulse sequence (51) and a custom-built quadrature “dome” elliptical bird-cage head-coil (GE Healthcare, Wisconsin). 630 functional volumes were obtained from 22 axial slices (repetition time=1500 milliseconds, echo time=30 milliseconds, flip angle=60°, field of view=22 cm, matrix=64×64, resolution=3.438 mm2 × 4.5 mm). High-resolution anatomical scans were acquired using fast spin-echo SPGR (.85942 × 1.5 mm; field of view=22 cm, frequency encoding=256).
fMRI Data Preprocessing
Using AFNI (52) software, preprocessing included volume registration, motion correction, 4 mm3 isotropic gaussian spatial-smoothing, high-pass filtering (.011 Hz), and linear detrending. No volumes demonstrated motion in excess of ±0.6 mm. There was no evidence of stimulus-correlated motion between condition-specific reference functions and x, y, z motion parameters (all Ps>.55).
fMRI Statistical Analysis
Using 3dDeconvolve, multiple-regression included parameters to remove mean, linear and quadratic trends, and motion-related variance in the blood oxygen level-dependent (BOLD) signal. Regressors (convolved with the gamma variate model (53) of the hemodynamic response function) were used to examine early (first 2 time points; 0–3s) and late (last 2 time points; 6–9s) BOLD responses for each of the 3 conditions (Neutral, React, Reappraise). Linear contrasts compared early versus late responses to test the hypothesis of linear decrease of emotional reactivity and increases of regulatory responses during the 9s trials. This method of investigating linear change in BOLD response over time within a trial has been used successfully in prior studies of emotion reactivity and reappraisal (27) and cognitive appraisal (54). BOLD signal intensity was computed as percentage of signal change, an effect size measure [(MR signal per voxel per time point / mean MR signal in that voxel for the entire functional run) × 100]. BOLD signal time series are relative to the neutral condition.
Individual brain maps were resampled to 3.438 mm3 and converted to Talairach atlas space (55) and second-level group statistical maps were produced according to a random-effects model. To correct for multiple comparisons, AlphaSim, a Monte Carlo simulation bootstrapping program, was used to protect against false positives (56). This method uses a voxel-wise and cluster volume joint-probability threshold to establish a cluster-wise false positive cluster detection level. Statistical thresholds consisted of a voxel-wise P<.005 and cluster volume >162 mm3 (4 voxels × 3.438 mm3) to protect against false-positive cluster detection at P<.01 for between-group contrasts, and voxel-wise P<.001 and cluster volume higher than 162 mm3 to protect against false-positive cluster detection at P<.005 for within-group early versus late contrasts.
Functional connectivity (FC) analysis was seeded to a group-level left dorsal amygdala activation common to patients and controls during React NSBs. The group-level left amygdala functional region of interest was transformed from Talairach atlas space to the native brain space of each participant. Participant-specific left amygdala time series were used as a regressor of whole-brain BOLD response in a multiple-regression model that included parameters to remove variance related to mean, linear and quadratic trends, head movement, and whole-brain average signal intensity at each time point. Within-group t-tests examined FC patterns during Reappraise NSBs. The resultant t-maps were thresholded using a joint-probability method consisting of voxel-wise P<.001 and cluster volume>162 mm3 to protect against false positive cluster detection at cluster-wise P<.005.
Results
Autobiographical Social Situations
Patients and controls reported similar time elapsed since the situation occurred, vividness of memory, age, and experience of shame during the situation. Compared to controls, patients reported greater current shame, avoidance, disturbance, and lesser current talk about the situation (Table S2 in Supplement 1).
Emotional Reactivity: Behavioral Responses
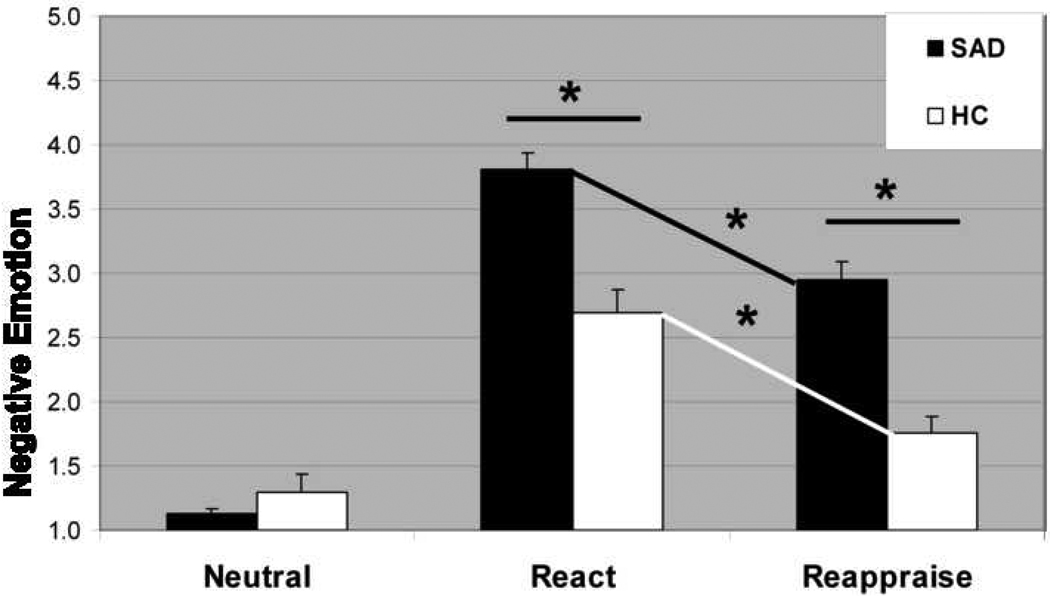
A 2 (group: SAD patients, healthy controls) × 2 (condition: Neutral, React NSB) repeated-measures analysis of variance of negative emotion ratings yielded an interaction of group × condition, F2,52=27.89, P<.001, partial eta2 (ηp2)=.35 (Figure 2). Between-group t-tests showed that, compared to controls, patients reported greater negative emotion during React NSB, t52=4.96, P<.001, ηp2=.32, but no difference during Neutral trials, t52=1.12, P>.26, ηp2=.02. There was no association of social anxiety symptom severity (LSAS) and negative emotion ratings during React trials.
Figure 2.
Negative emotion intensity ratings for Neutral Statements, React Negative Self-Beliefs, and Reappraise Negative Self-Beliefs in patients with social anxiety disorder and healthy controls. Negative emotion ratings after the offset of each stimulus were provided by participants in response to “How negative do you feel?” (1=not at all, 2=slightly, 3=moderately, and 4=very much, 5=extreme). * P<.001; error bars = standard error of the mean.
Emotional Reactivity: Neural Responses
Common responses
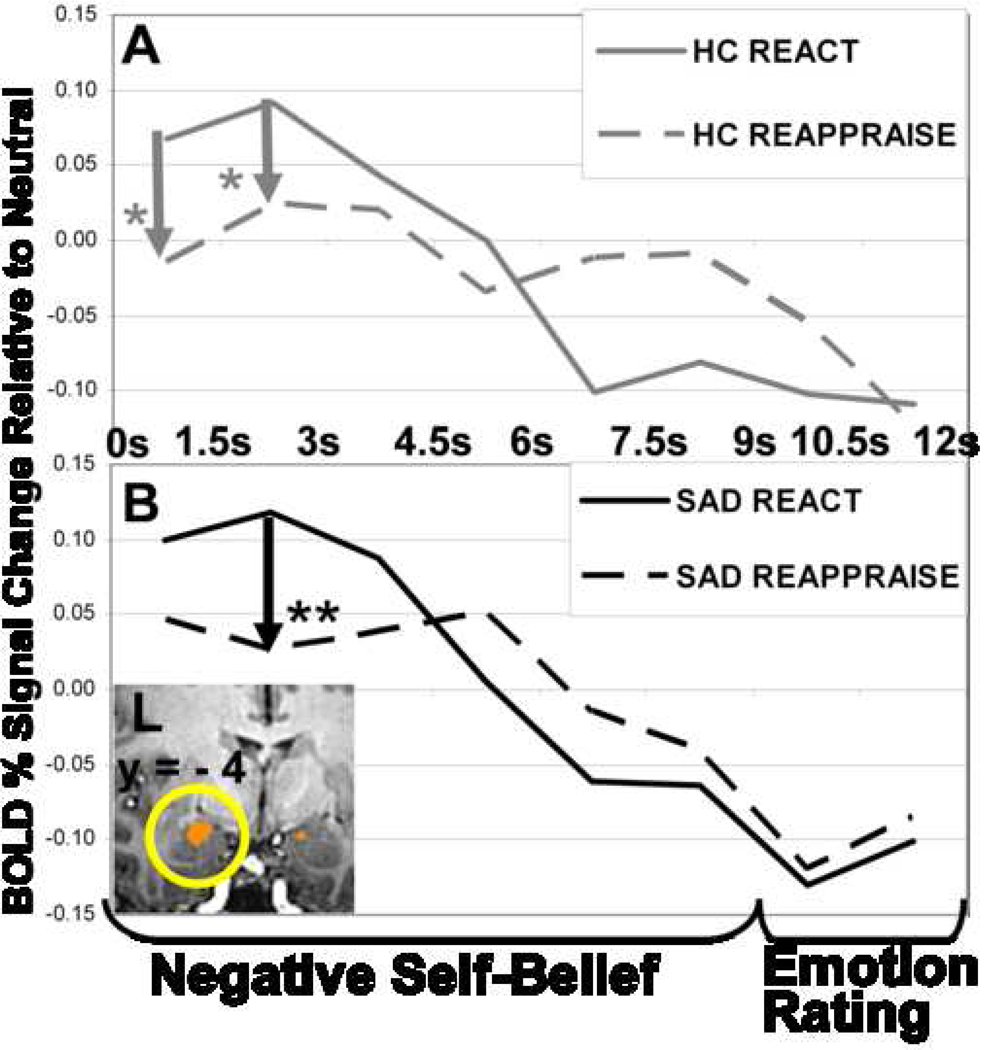
Whole-brain t-tests for the React NSB condition revealed early (0–3s) responses implicated in emotion, language, self-referential, and visual processing, and late (6–9s) responses involved in cognitive control and visceral experience in both groups. Both groups had a peak left amygdala response at 1.5–3s post-NSB onset (Figure 3A).
Figure 3.
Left dorsal amygdala BOLD signal time series for (A) healthy controls during reactivity and reappraisal and (B) patients with SAD during reactivity and reappraisal. Left dorsal amygdala was thresholded at voxel-wise P<.001 and cluster volume>162 mm3 to protect against false positive cluster detection at cluster-wise P<.005. Left dorsal amygdala early versus late contrast in HC (peak BOLD signal=.25; Talairach coordinates= −18, −3, −10; cluster volume= 733 mm3) and in SAD (peak BOLD signal=.20; Talairach coordinates=−17 −5, −10; 570 mm3). The overlapping ROI mask included both peaks and had a volume of 529 mm3. * P<.05, ** P<.01
Differential responses
A between-group t-test showed that controls had greater early responses in bilateral dorsolateral prefrontal cortex (PFC), left superior temporal gyrus (STG), and right supramarginal gyrus (Figure S1 in Supplement 1; Table 2), while patients had lesser early responses in right dorsolateral PFC, left STG, right supramarginal gyrus, left posterior cingulate, and greater late responses in bilateral inferior parietal lobule.
Table 2.
React to Negative Self-Beliefs Early versus Late BOLD Responses in Patients with Social Anxiety Disorder versus Healthy Controls
| Brain Region | BA | Peak x y z |
Vol mm3 |
t-value |
|---|---|---|---|---|
| Early > Late | ||||
| HC > SAD | ||||
| Frontal Lobe | ||||
| L Dorsolateral PFC | 9 | −41 7 36 | 244 | 3.41 |
| R Dorsolateral PFC | 6 | 41 1 36 | 244 | 3.44 |
| R Dorsolateral PFC | 9 | 52 18 36 | 163 | 3.28 |
| Temporal Lobe | ||||
| L Superior Temporal Gyrus | 42, 22 | −69 −13 8 | 285 | 3.96 |
| L Superior Temporal Gyrus | 21, 22 | −65 −6 1 | 244 | 2.97 |
| Parietal Lobe | ||||
| L Posterior Cingulate Cortex | 23 | −7 −20 29 | 203 | 3.74 |
| R Supramarginal Gyrus | 39, 40 | 58 −51 32 | 163 | 3.67 |
| Late > Early | ||||
| HC > SAD | ||||
| L Supplemental Motor Area | 6 | −14 −10 50 | 448 | 4.53 |
| SAD > HC | ||||
| L Inferior Parietal Lobule | 40 | −58 −34 36 | 163 | 3.42 |
| R Inferior Parietal Lobule | 40 | 48 −51 50 | 163 | 3.04 |
Note. Between-group t-test, t>2.93, voxel P<.005, cluster volume>162mm3, cluster P<.01. BA=Brodmann Areas, HC=healthy controls, L=left, PFC=prefrontal cortex, R=right, SAD=patients with social anxiety disorder.
Cognitive Reappraisal: Behavioral Responses
A 2 (group: SAD patients, healthy controls) × 2 (condition: React, Reappraisal) repeated-measures analysis of variance of negative emotion ratings did not result in an interaction of group × condition, F2,52=0.21, P=.65, ηp2=.00 (Figure 2). Reappraisal reduced negative emotion in patients, t26=6.24, P<.001, ηp2=.60, and controls, t26=7.23, P<.001, ηp2=.67. Pearson-product correlation analyses demonstrated that lesser down-regulation (react minus reappraise) of negative emotion was associated with greater social anxiety symptom severity (LSAS-SR) in patients, r(27)=−.44, P<.05, but not in controls, r(27)=−.05, P>.82 (zdiff=1.46, p>.14), and more recent social anxiety situations for patients, r(27)=−.47, P<.05, but not for controls, r(27)=−.01, P>.94 (zdiff=1.73, p>.08).
Cognitive Reappraisal: Neural Responses
Common responses
Whole-brain t-tests for Reappraise NSB demonstrated early (0–3s) brain responses implicated in emotion (left dorsal amygdala), cognitive reappraisal (dorsomedial PFC, left dorsolateral PFC), language (left ventrolateral PFC, posterior STG, middle temporal gyrus, supramarginal gyrus), and visual attention (cuneus, precuneus) in both groups. Both groups also showed late (6–9s) responses in brain regions involved in cognitive control (bilateral rostral middle frontal gyrus, dorsal anterior cingulate cortex (dACC)), and visceral processing (bilateral anterior insula). Thus both groups demonstrated evidence of early amygdala reactivity and co-occurring recruitment of a cognitive-linguistic-attention network supporting reappraisal efforts. Reappraisal resulted in significant reduction in left dorsal amygdala activity in both groups at 1.5–3s post-NSB onset (Figure 3).
Differential responses
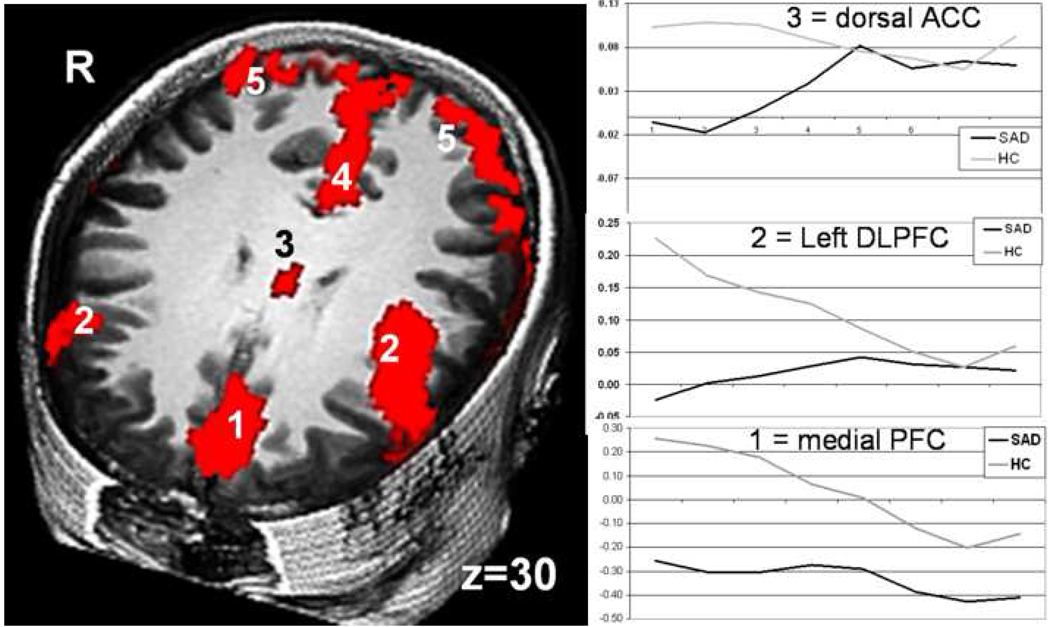
A between-group t-test of Reappraise NSB demonstrated two distinct patterns of brain responses: greater early linearly decreasing responses in controls and greater linearly increasing late responses in patients. Compared to patients, controls had greater early brain responses implicated in reappraisal (dACC, medial, dorsomedial, bilateral dorsolateral, and ventrolateral PFC), language (left inferior frontal gyrus), and visual processing (medial precuneus, bilateral inferior parietal lobule) (Figure 4, Table 3). Reappraisal self-efficacy was associated with greater early dACC response during reappraisal in controls (r=.50, P<.01), but not patients (r=−.11; zdiff=2.29, P<.05). Negative emotion experience following reappraisal was related to lesser early response in right dorsolateral PFC in patients (r=−.45, P<.05), but not in controls (r=.17; zdiff=2.27, P<.05). In patients, social anxiety symptoms were associated with greater early reappraise activity in right inferior frontal gyrus (r=.44, P <.05; LSAS), left thalamus (r=.44, P<.05; BFNE), and left inferior parietal lobule (r=.39, P =.05; BFNE). Compared to controls, patients had greater late responses in brain regions related to reappraisal (dorsolateral and ventrolateral PFC), visceral sensation (bilateral insular cortex), and visual processing (inferior parietal lobule, precuneus).
Figure 4.
Early cognitive reappraisal-related brain responses in patients with SAD versus healthy controls. RED = HC > SAD Early cognitive reappraisal of negative self-beliefs. 1=medial PFC, 2=dorsolateral PFC, 3=dorsal anterior cingulate cortex, 4=precuneus, 5=inferior parietal lobule. Between-group t-test, t>2.93, voxel P<.005, cluster volume>162; cluster P<.01
Table 3.
Reappraise Negative Self-Beliefs Early versus Late BOLD Responses in Patients with Social Anxiety Disorder versus Healthy Controls froi 40
| Brain Region | BA | Peak x y z |
Vol mm3 |
t-value |
|---|---|---|---|---|
| Early > Late | ||||
| HC > SAD | ||||
| Frontal Cortex | ||||
| R Inferior Frontal Gyrus | 45, 47 | 55 21 −2 | 5860 | 3.74 |
| Dorsomedial PFC | 6, 8 | 0 21 63 | 4640 | 3.12 |
| L Ventrolateral PFC | 10 | −34 62 −9 | 4396 | 2.99 |
| Dorsal Cingulate Gyrus | 24, 23 | −3 −10 32 | 100 | 4.17 |
| Dorsal Anterior Cingulate Cortex | 24 | 3 14 29 | 4070 | 3.98 |
| L Inferior Frontal Gyrus | 47 | −34 28 −2 | 1221 | 4.39 |
| L Dorsolateral PFC | 9 | −48 7 36 | 1221 | 2.97 |
| R Dorsolateral PFC | 9 | 48 18 36 | 7733 | 3.45 |
| Medial Anterior PFC | 10, 9 | −3 62 36 | 407 | 3.24 |
| Medial Anterior PFC | 10 | 14 62 19 | 285 | 3.36 |
| R Dorsolateral PFC | 45, 46 | 52 25 22 | 285 | 3.22 |
| Temporal Lobe | ||||
| L Superior Temporal Gyrus | 22, 21 | −69 −13 8 | 488 | 3.52 |
| L Posterior Middle Temporal Gyrus | 19 | −31 −75 22 | 285 | 4.13 |
| R Inferior Temporal Gyrus | 20 | 55 −34 −16 | 244 | 3.84 |
| Parietal Cortex | ||||
| R Supramarginal Gyrus, | 39, 40 | 55 −58 32 | 2564 | 3.51 |
| R Inferior Parietal Lobule | ||||
| Medial Precuneus | 7 | −3 −68 43 | 1302 | 3.01 |
| L Superior Parietal Lobule, | 7 | −38 −65 53 | 814 | 3.50 |
| L Inferior Parietal Lobule | ||||
| R Inferior Parietal Lobule | 40 | 48 −58 46 | 488 | 4.34 |
| Subcortical | ||||
| L Thalamus | −7 −10 −2 | 529 | 3.37 | |
| L Thalamus | −14 −20 −2 | 285 | 3.59 | |
| Late > Early | ||||
| SAD > HC | ||||
| Frontal Cortex | ||||
| R Dorsolateral PFC | 8, 9, 6 | 48 11 39 | 1262 | 3.18 |
| R Ventrolateral PFC | 10 | 28 69 12 | 4233 | 4.62 |
| L Dorsolateral PFC | 46, 10 | −41 38 26 | 692 | 3.64 |
| R Dorsolateral PFC | 6 | 17 28 56 | 570 | 2.97 |
| R Dorsolateral PFC | 9 | 41 38 32 | 488 | 3.16 |
| L Insula | 13 | −41 7 8 | 611 | 3.51 |
| L Anterior Insula | 13 | −31 18 8 | 163 | 3.58 |
| R Insula | 13 | 38 4 −2 | 570 | 3.49 |
| R Posterior Insula | 13 | 34 −17 19 | 529 | 3.23 |
| L Precentral Gyrus | 4 | −65 1 22 | 163 | 3.25 |
| Parietal Lobe | ||||
| L Inferior Parietal Lobule | 40 | −58 −31 32 | 1180 | 2.94 |
| L Inferior Parietal Lobule | 40 | −58 −48 39 | 163 | 3.20 |
| R Precuneus | 7 | 10 −58 36 | 977 | 4.15 |
| L Precuneus | 7 | −10 −37 50 | 448 | 3.62 |
| Temporal Lobe | ||||
| R Superior Temporal Gyrus | 22 | 55 −6 5 | 326 | 3.53 |
| Subcortical | ||||
| R Thalamus | 7 −10 12 | 1913 | 3.54 | |
| L Thalamus | −10 −13 12 | 326 | 3.39 | |
| R Lentiform Nucleus, Putamen | 17 4 −5 | 285 | 3.09 |
Note. Between-group t-test, t>2.932, voxel P<.005, cluster volume>162mm3, cluster P<.01 BA=Brodmann Areas, HC=healthy controls, L=left, PFC=prefrontal cortex, R=right, SAD=patients with social anxiety disorder.
Functional Connectivity Analysis
To further investigate the effect of reappraisal on brain system interactions during the 9s trials, the left dorsal amygdala time series within each group during React NSB was used as the seed for a functional connectivity (FC) analysis of BOLD signal during Reappraisal NSB as both groups showed a significant reduction in activity in this area. In both groups, left amygdala activity was inversely associated with bilateral dorsolateral PFC activation. However, we observed more PFC cognitive control regions that were inversely related to left amygdala activation in controls, including three regions in the left dorsolateral PFC and two regions in the right ventrolateral PFC, as well as attention regulation regions (inferior parietal lobule). Both groups had positive left amygdala-seeded FC with right amygdala, thalamus, putamen, bilateral temporal gyri, and bilateral parahippocampal gyri (Tables S3 and S4 in Supplement 1).
Discussion
This study investigated the neural mechanisms of cognitive reappraisal of NSBs embedded in social anxiety autobiographical scripts in adults diagnosed with SAD versus healthy controls. The primary finding was differential temporal onset of cognitive reappraisal-related neural responses, with early activation of cognitive control, linguistic and visual processes in controls, and late cognitive, attention and somatosensory brain responses in patients.
Emotional Reactivity
Autobiographical scripts represent a robust method of inducing salient emotions in the context of neuroimaging studies (57). While this method has been used extensively in the context of PTSD (58), it has not previously been implemented in neuroimaging studies of SAD. Presenting NSBs within social anxiety autobiographical scripts provides the context from which they arise. This method stands in contrast to use of static faces (22) and scenes (38) or reading single sentences (32) as emotional probes in previous fMRI studies of SAD.
Compared with controls, participants with SAD reported greater negative emotion when reacting to NSBs, confirming a pattern of exaggerated reactivity to anxiety probes observed in fMRI studies of SAD (22; 32; 38). Neurally, both groups had similar early brain responses implicated in emotion (57), self-referential (59), and language (60) processing, and late brain responses associated with cognitive control (61) and visceral experience (62). While some fMRI studies of emotional reactivity in patients with SAD compared to controls have observed increased (22) or delayed (36) amygdala response to harsh faces, other fMRI investigations of reactivity to physical and social threat have found no group differences (63). Our first hypothesis of between-group difference in the intensity or duration of emotion-related limbic brain regions was not confirmed. However, a between-group analysis revealed differential temporal onset of neural responses related to cognitive reappraisal (greater early dorsolateral PFC responses in controls than in patients) and attention (greater late inferior parietal lobule responses in patients than in controls).
Although both groups showed immediate amygdala response, the pattern of differential neural responses is consistent with divergent uninstructed emotion regulation (64). Specifically, controls may be engaging in early automatic cognitive control (dorsolateral PFC), whereas patients initially may be engaging in avoidance of the NSB, followed by later re-engagement of attention (inferior parietal lobule). This pattern of attentional processing suggests that patients with SAD may be attending to both the external stimulus (NSB text) and internal cues (cognitive and emotional reactivity to the NSB) (65). These differential neural responses and timing may reflect distinct habitual patterns of emotional responding in anxious and nonanxious samples and may be related to exaggerated negative emotion experience in patients with SAD.
Cognitive Reappraisal
Both groups reported a similar amount of reduction of negative emotion with cognitive reappraisal. Greater social anxiety symptom severity and more recent social anxiety situations, however, were associated with less down-regulation of negative emotion in patients suggesting that intensity of SAD may contribute to emotion dysregulation.
Neurally, both groups showed significant reduction of initial amygdala response, indicating down-regulation of emotion reactivity to NSBs that parallels reductions in negative emotion. Between-group analysis demonstrated differential timing of reappraisal-related brain systems that may reflect a discrepancy in the implementation of emotion regulation strategies. Controls had early brain responses implicated in cognitive reappraisal, as well as linguistic and visual processing. This neural temporal pattern has been observed in fMRI studies of cognitive reappraisal of negative emotion in healthy adults (27). The anxiety caused by NSBs did not interfere with the immediate recruitment of regulatory brain circuitry in controls. Patients with SAD, however, had later (and fewer) brain responses related to reappraisal.
This pattern of decreased recruitment of brain systems implicated in cognitive and attentional regulation in patients with SAD has been reported during cognitive regulation of social threat (63). This suggests that patients with SAD may require additional time to overcome the initial anxiety induced by the NSB and to implement cognitive reappraisal. Additionally, highly self-relevant stimuli may also require more and longer recruitment and implementation of emotion regulation strategies in SAD.
To further examine group differences in temporal coupling of neural responses during cognitive reappraisal, a left dorsal amygdala seeded FC analysis demonstrated that controls had five more PFC regions implicated in cognitive reappraisal of emotion (66) and one more attention regulation region (67) that co-varied inversely with amygdala activity than did patients. Patients and controls may be using different types of regulation strategies as evidenced by the differential patterns of top-down PFC regulatory influence on limbic reactivity.
Brain-behavioral associations highlighted two PFC regions that are core neural components of reappraisal (66): greater early dACC associated with greater reappraisal self-efficacy in controls, and lesser early right DLPFC activation associated with greater negative emotion in patients. The dACC is implicated in attention and executive cognitive functions (67; 68), has extensive connectivity with PFC regions (69), and is often coactivated with the dorsolateral PFC in cognitive tasks (70). The dACC is thought to recruit dorsolateral PFC to select and implement regulatory strategies, direct attentional control, and reduce cognitive conflict (68). Impaired early recruitment of the dACC and dorsolateral PFC during reappraisal may underlie problems with emotion regulation in patients with SAD. Diminished activation of cognitive and attention regulation brain networks in prefrontal and parietal cortex have been observed in individuals with high trait anxiety (71) and patients suffering from panic disorder, PTSD, and phobias (72).
Implications for Psychopathology and Treatment
Results from this study suggest that patients with SAD would benefit from understanding that (1) emotional reactivity to NSBs, one of the most common features of all psychological disorders, is rapid, transient, and modifiable, (2) reappraisal is a trainable skill that might be effectively applied early in the emotion generation process.
Clinical treatments that train different types of emotion regulation strategies (both antecedent and subsequent to emotion generation) may help patients with SAD work skillfully with NSBs, diminish experiential avoidance and emotional suppression, and increase interpersonal engagement.
Limitations
This study used participant-generated negative self-beliefs to enhance personal salience and intensity of the emotional stimuli. Due to this methodological choice, it is likely that the NSBs were more strongly self-relevant and emotionally evocative for the patients than for the controls.
This study is limited to inferences about cognitive reappraisal in relation to one type of stimulus (NSB). Direct comparison of reappraisal to other emotion regulation strategies (e.g., attention deployment, expressive suppression, decentering (73)) might help identify specific versus general deficits in emotion regulation in individuals with SAD. Also, inferences about the temporal dynamics of reappraisal neural responses are constrained to working with NSBs for only 9 seconds. An examination of neural timing of emotional reactivity and reappraisal for longer durations might reveal different patterns.
Participants were provided with minimal training prior to MR scanning to ensure that participants fully understood the task. We did not want to extensively train participants because this might have obscured naturally occurring differences of interest between the two groups. Thus, we cannot infer about the effects of extensive training in cognitive restructuring and graded exposure provided during cognitive-behavioral therapy for SAD. Investigations are needed to assess the differential effects of clinical interventions with distinct mechanisms (e.g., cognitive change, mindful awareness) on magnitude and timing of the responses of brain systems implicated in emotional reactivity and regulation.
Supplementary Material
Acknowledgments
We wish to thank Eileen Sisk and Elena Wright for their contribution to data collection and management, and Gary Glover, Ph.D. for his technical assistance with magnetic resonance imaging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stein MB, Stein DJ. Social anxiety disorder. Lancet. 2008;371:1115–1125. doi: 10.1016/S0140-6736(08)60488-2. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 3.Otto MW, Pollack MH, Maki KM, et al. Childhood history of anxiety disorders among adults with social phobia: Rates, correlates, and comparisons with patients with panic disorder. Depression and Anxiety. 2001;14:209–213. doi: 10.1002/da.1068. [DOI] [PubMed] [Google Scholar]
- 4.Beesdo K, Bittner A, Pine DS, et al. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Archives of General Psychiatry. 2007;64:903–912. doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- 5.Ruscio AM, Brown TA, Chiu WT, Sareen J, Stein MB, Kessler RC. Social fears and social phobia in the USA: results from the National Comorbidity Survey Replication. Psychological Medicine. 2008;38:15–28. doi: 10.1017/S0033291707001699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneier FR, Heckelman LR, Garfinkel R, et al. Functional impairment in social phobia. Journal of Clinical Psychiatry. 1994;55:322–331. [PubMed] [Google Scholar]
- 7.Lochner C, Mogotsi M, du Toit PL, Kaminer D, Niehaus DJ, Stein DJ. Quality of life in anxiety disorders: a comparison of obsessive-compulsive disorder, social anxiety disorder, and panic disorder. Psychopathology. 2003;36:255–262. doi: 10.1159/000073451. [DOI] [PubMed] [Google Scholar]
- 8.Clark DM, Wells A. A cognitive model of social phobia. New York, NY: Guilford Press; 1995. [Google Scholar]
- 9.Rapee RM. Descriptive psychopathology of social phobia. New York: Guilford Press; 1995. [Google Scholar]
- 10.Stein MB, Kean YM. Disability and quality of life in social phobia: Epidemiologic findings. American Journal of Psychiatry. 2000;157:1606–1613. doi: 10.1176/appi.ajp.157.10.1606. [DOI] [PubMed] [Google Scholar]
- 11.Hofmann SG. Cognitive factors that maintain social anxiety disorder: a comprehensive model and its treatment implications. Cognitive Behavior and Therapy. 2007;36:193–209. doi: 10.1080/16506070701421313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark DM, Wells A. A cognitive model of social phobia. Social phobia: Diagnosis, assessment, and treatment. 1995:69–93. [Google Scholar]
- 13.Rapee RM, Heimberg RG. A cognitive-behavioral model of anxiety in social phobia. Behavioral Research and Therapy. 1997;35:741–756. doi: 10.1016/s0005-7967(97)00022-3. [DOI] [PubMed] [Google Scholar]
- 14.Campbell-Sills L, Barlow DH. Incorporating emotion regulation into conceptualizations and treatments of anxiety and mood disorders. In: Gross JJ, editor. Handbook of emotion regulation. New York: Guilford; 2007. pp. 542–559. [Google Scholar]
- 15.Neisser U. Cognition and reality: principles and implications of cognitive psychology. San Francisco, CA: 1976. [Google Scholar]
- 16.Hackmann A, Clark DM, McManus F. Recurrent images and early memories in social phobia. Behavioral Research and Therapy. 2000;38:601–610. doi: 10.1016/s0005-7967(99)00161-8. [DOI] [PubMed] [Google Scholar]
- 17.Schulz SM, Alpers GW, Hofmann SG. Negative self-focused cognitions mediate the effect of trait social anxiety on state anxiety. Behavioral Research and Therapy. 2008 doi: 10.1016/j.brat.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stopa L, Clark DM. Social phobia and interpretation of social events. Behavioral Research and Therapy. 2000;38:273–283. doi: 10.1016/s0005-7967(99)00043-1. [DOI] [PubMed] [Google Scholar]
- 19.Rapee RM, Abbott MJ. Modelling relationships between cognitive variables during and following public speaking in participants with social phobia. Behavioral Research and Therapy. 2007;45:2977–2989. doi: 10.1016/j.brat.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann SG, Moscovitch DA, Kim HJ, Taylor AN. Changes in self-perception during treatment of social phobia. Journal of Consulting and Clinical Psychology. 2004;72:588–596. doi: 10.1037/0022-006X.72.4.588. [DOI] [PubMed] [Google Scholar]
- 21.Abbott MJ, Rapee RM. Post-event rumination and negative self-appraisal in social phobia before and after treatment. Journal of Abnormal Psychology. 2004;113:136–144. doi: 10.1037/0021-843X.113.1.136. [DOI] [PubMed] [Google Scholar]
- 22.Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Archives of General Psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- 23.Yoon KL, Fitzgerald DA, Angstadt M, McCarron RA, Phan KL. Amygdala reactivity to emotional faces at high and low intensity in generalized social phobia: a 4-Tesla functional MRI study. Psychiatry Research. 2007;154:93–98. doi: 10.1016/j.pscychresns.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Straube T, Kolassa IT, Glauer M, Mentzel HJ, Miltner WH. Effect of task conditions on brain responses to threatening faces in social phobics: An event-related functional magnetic resonance imaging study. Biological Psychiatry. 2004;56:921–930. doi: 10.1016/j.biopsych.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 25.McClure EB, Monk CS, Nelson EE, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Archives of General Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- 26.Killgore WD, Yurgelun-Todd DA. Social anxiety predicts amygdala activation in adolescents viewing fearful faces. Neuroreport. 2005;16:1671–1675. doi: 10.1097/01.wnr.0000180143.99267.bd. [DOI] [PubMed] [Google Scholar]
- 27.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amir N, Klumpp H, Elias J, Bedwell JS, Yanasak N, Miller LS. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biological Psychiatry. 2005;57:975–981. doi: 10.1016/j.biopsych.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 29.Straube T, Mentzel HJ, Miltner WH. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology. 2005;52:163–168. doi: 10.1159/000087987. [DOI] [PubMed] [Google Scholar]
- 30.Tillfors M, Furmark T, Marteinsdottir I, et al. Cerebral blood flow in subjects with social phobia during stressful speaking tasks: A PET study. American Journal of Psychiatry. 2001;158:1220–1226. doi: 10.1176/appi.ajp.158.8.1220. [DOI] [PubMed] [Google Scholar]
- 31.Furmark T, Tillfors M, Marteinsdottir I, et al. Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Archives of General Psychiatry. 2002;59:425–433. doi: 10.1001/archpsyc.59.5.425. [DOI] [PubMed] [Google Scholar]
- 32.Blair K, Geraci M, Devido J, et al. Neural response to self- and other referential praise and criticism in generalized social phobia. Archives of General Psychiatry. 2008;65:1176–1184. doi: 10.1001/archpsyc.65.10.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between Amygdala Hyperactivity to Harsh Faces and Severity of Social Anxiety in Generalized Social Phobia. Biological Psychiatry. 2006;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Evans KC, Wright CI, Wedig MM, Gold AL, Pollack MH, Rauch SL. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depression and Anxiety. 2007 doi: 10.1002/da.20347. [DOI] [PubMed] [Google Scholar]
- 35.Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biological Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- 36.Campbell DW, Sareen J, Paulus MP, Goldin PR, Stein MB, Reiss JP. Time-varying amygdala response to emotional faces in generalized social phobia. Biological Psychiatry. 2007;62:455–463. doi: 10.1016/j.biopsych.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 37.McClure EB, Pine DS. Social anxiety and emotion regulation: A model for developmental psychopathology perspectives on anxiety disorders. In: Cicchetti DEC, Donald J, editors. Developmental psychopathology, theory and method. 2nd ed. New Jersey: John Wiley & Sons; 2006. [Google Scholar]
- 38.Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Archives of General Psychiatry. 2009;66:170–180. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 41.DiNardo PA, Brown TA, Barlow DH. New York, NY: Oxford University Press; 1994. Anxiety Disorders Interview Schedule for DSM-IV: Lifetime version (ADIS-IV-L) [Google Scholar]
- 42.Liebowitz MR. Social phobia. Modern Problems in Pharmacopsychiatry. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- 43.Fresco DM, Coles ME, Heimberg RG, et al. The Liebowitz Social Anxiety Scale: a comparison of the psychometric properties of self-report and clinician-administered formats. Psychological Medicine. 2001;31:1025–1035. doi: 10.1017/s0033291701004056. [DOI] [PubMed] [Google Scholar]
- 44.Leary MR. A brief version of the Fear of Negative Evaluation Scale. Personality and Social Psychology Bulletin. 1983;9:371–375. [Google Scholar]
- 45.Beck AT, Steer RA, Brown GK. Beck Depression Inventory - Second Edition Manual. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 46.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 47.Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- 48.John OP, Catterson D, Eng J, Gross JJ. Emotion regulation self-efficacy. 2009 in preparation. [Google Scholar]
- 49.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: An FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 50.Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 51.Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance in Medicine. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- 52.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 53.Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- 54.Kalisch R, Wiech K, Critchley HD, Dolan RJ. Levels of appraisal: a medial prefrontal role in high-level appraisal of emotional material. Neuroimage. 2006;30:1458–1466. doi: 10.1016/j.neuroimage.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 55.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- 56.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 57.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 58.Pitman RK, Orr SP, Forgue DF, de Jong JB, Claiborn JM. Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Archives of General Psychiatry. 1987;44:970–975. doi: 10.1001/archpsyc.1987.01800230050009. [DOI] [PubMed] [Google Scholar]
- 59.Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain-A meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 60.Iacoboni M, Wilson SM. Beyond a single area: motor control and language within a neural architecture encompassing Broca's area. Cortex. 2006;42:503–506. doi: 10.1016/s0010-9452(08)70387-3. [DOI] [PubMed] [Google Scholar]
- 61.Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends in Cognitive Science. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 62.Craig AD. Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 63.Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Archives of General Psychiatry. 2009;66:170–180. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Drabant EM, McRae K, Manuck SB, Hariri AR, Gross JJ. Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biological Psychiatry. doi: 10.1016/j.biopsych.2008.09.007. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schultz LT, Heimberg RG. Attentional focus in social anxiety disorder: potential for interactive processes. Clinical Psychology Review. 2008;28:1206–1221. doi: 10.1016/j.cpr.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 66.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Science. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 67.Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 68.Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 69.Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. Journal of Comparative Neurology. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- 70.Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nature Review Neuroscience. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- 71.Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience. 2009;12:92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- 72.Berkowitz RL, Coplan JD, Reddy DP, Gorman JM. The human dimension: how the prefrontal cortex modulates the subcortical fear response. Reviews in the Neurosciences. 2007;18:191–207. doi: 10.1515/revneuro.2007.18.3-4.191. [DOI] [PubMed] [Google Scholar]
- 73.Gross JJ, Thompson RA. Emotion regulation: Conceptual foundations. In: Gross JJ, editor. Handbook of emotion regulation. New York: Guilford; 2007. pp. 3–24. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.