Abstract
Previous in vitro studies in our laboratory have shown that lymphocytes can influence macrophage adhesion and fusion on biomaterial surfaces. However, few studies have evaluated how material adherent macrophages can influence lymphocyte behavior, specifically T cells. In this study, we cultured human peripheral blood mononuclear cells from healthy donors on three synthetic non-biodegradable biomedical polymers: Elasthane 80A (PEU), Silicone rubber (SR), or polyethylene terephthalate (PET) and tissue culture polystyrene (TCPS). Upregulation of T cell surface activation markers (CD69 and CD25), lymphocyte proliferation, and interleukin-2 (IL-2) and interferon-γ (IFNγ) concentrations were evaluated by flow cytometry, carboxy-fluorescein diacetate, succinimydyl ester (CFSE) incorporation, and multiplex cytokine immunoassay, respectively, to assess T cell activation. Following 3 and 7 days of culture, CD4+ helper T cells from cultures of any of the material groups did not express the activation markers CD69 and CD25 and lymphocyte proliferation was not present. IL-2 and IFNγ levels were produced, but dependent on donor. These data indicate that T cells are not activated in response to clinically relevant synthetic biomaterials. The data also suggest that lymphocyte subsets exclusive of T cells are the source of the lymphokines, IL-2 and IFN-γ, in certain donors.
Keywords: Lymphocyte activation, biomaterials, interferonγ, Interleukin-2
Introduction
Lymphocytes may affect macrophage adhesion and fusion on biomaterial surfaces via both juxtacrine (cell-cell) and paracrine (cytokine-cell) interactions. We have previously shown that macrophage adhesion and fusion was significantly increased when monocytes and lymphocytes were cultured simultaneously in comparison to monocytes cultured alone. Separation of the monocyte population from the lymphocyte population using a transwell insert also resulted in increased levels of macrophage adhesion and fusion indicating indirect (paracrine) cell-cell interactions, such as cytokine production, are playing a prominent role in these effects1, 2. The inverse relationship, in which biomaterial adherent monocytes/macrophages affect lymphocytes, has not been studied extensively.
There is evidence that T lymphocytes can be activated in response to biomaterials. T lymphocytes cultured in the presence of polyurethane particles from the flexible diaphragms left ventricular assist devices (LVAD) resulted in intracellular calcium flux, CD40 ligand expression, and nuclear translocation of nuclear factor of activated T-cells (NFAT). NFAT translocation was reduced by a calcineurin inhibitor and CD40 ligand expression was reduced by both a calcineurin inhibitor and CD25 blockade indicating IL-2 dependent activation pathways3, 4.
T lymphocytes in response to polyurethane particles exhibited classic activation indicators, i.e. calcium flux, translocation of transcription factors, upregulation of activation cell surface markers, and proliferation. T cell activation occurs after a series of well orchestrated events following appropriate stimulation. T cell receptor (TCR) engagement with specific peptides presented in the context of MHC and adequate positive co-stimulation leads to signal transduction pathways necessary for new protein transcription5. Following TCR crosslinking and phosphorylation and the activation of several protein kinases and adaptor proteins, phosphatidylinositol 4,5 bisphosphate (PIP2) is cleaved in the membrane to produce the second messengers inositol tri-phosphate (IP3) and diacylglycerol (DAG). IP3 mediates pathways necessary for the release of calcium from intracellular stores and entrance of extracellular calcium into the cell.
Increased intracellular calcium levels activate calmodulin which in turn activates calcineurin. Calcineurin dephosphorylates the transcription factor NFAT, which can now translocate into the nucleus and participate in gene activation6, 7. Other signaling pathways initiated by DAG result in the activation of the transcription factors nuclear factor kB (NFkB) and activation protein 1 (AP-1). The promoter of the IL-2 gene has binding sites for the transcription factors, NFkB, NFAT, and AP-1. The promoter of the IL-2 receptor alpha chain (CD25) also has binding sites for activation induced transcription factors such as NFkB. IL-2 was originally described as a T cell growth factor. The IL-2/IL-2 receptor system is involved in regulating clonal expansion, i.e. antigen specific T cell proliferation8, 9.
Synthetic biomaterials are not considered to be antigens therefore T lymphocyte activation in response to these materials via MHC/peptide/TCR induced signaling pathways is not expected. However there are alternative non-cognate pathways that can lead to activation, such as mitogen induced activation. Mitogens can induce lymphocyte activation by crosslinking glycoproteins on the plasma membrane surface. There is evidence that some polymers can act as mitogens. A polymer synthesized to act as a mitogen contains phenylboronic acid, which is a moiety capable of binding glycoproteins10. Coating boronate containing polymers on a surface also induced lymphocyte proliferation in proportion to the concentration of phenyl boronic acid incorporated11. These studies indicate that if functional groups present on the polymer surface are capable of binding glycoproteins on the lymphocyte plasma membrane surface, the polymer may act as a mitogen.
In this study we investigated in vitro T cell activation to three commonly used clinical synthetic biomaterials using human peripheral blood mononuclear cells. Tissue culture polystyrene was used as the control material surface. T cell activation was assayed by analyzing the upregulation of surface activation markers (i.e.CD69 and CD25), proliferation, and cytokine production (i.e. IL-2 and IFNγ).
Materials and Methods
Biomaterial Preparation
Elasthane 80A, a polyether urethane (PEU), was synthesized by Polymer Technology Group (Berkeley, CA, USA) and extruded by Medtronic (Minneapolis, MN, USA). Polyethylene terephthalate (PET) (Toray Co., Japan) and a silicate resin filled, cross-linked polydimethylsiloxane (SR) (Dow Corning, Midland, MI) were also used.
Polymer surfaces were punched into 1.5 cm diameter disks, rinsed in 100% ethanol, and sterilized with ethylene oxide by sterilization services at University Hospitals of Cleveland. Silicone rings were sectioned from tubing (Cole-Parmer, Vernon Hills, IL), sonicated in 100% ethanol, and autoclaved. Polymer disks were secured in 24 well tissue culture plates with sterile silicone rings. Silicone rings of equal size were also placed in the tissue culture polystyrene (TCPS) wells in order to maintain the same surface area.
In vitro Cell Culture
Human peripheral mononuclear cells were isolated from whole, venous blood of three healthy donors using a density gradient centrifugation method using Ficoll-Paque (GE Health Biosciences, Sweden). Peripheral blood was mixed 1:1 with PBSE and layered over the Ficoll-Paque column and centrifuged for 30min at 1700rpm. The interface containing mononuclear cells was removed and washed 2 times with PBSE. Viability was assayed by a trypan blue exclusion test. A portion of these cells were stained for flow cytometry using the following directly conjugated mouse anti-human monoclonal antibodies: CD3-APC, CD8-APC, CD4-APC, CD25-APC-Cy7 (clone M-A251), CD69-APC-Cy7 (Clone FN50) and appropriate isotype controls (BD Pharmingen, Franklin Lakes, USA).
Mononuclear cells were labeled with carboxy-fluorescein diacetate, succinimydyl ester (CFSE) prior to plating (Invitrogen). 50µM CFSE solution was prepared by diluting the stock 5mM CFSE solution 1:100 with PBS. 110µl of this solution was added per ml of cells. Cells were at a concentration of 12×106 cells/ml suspended in PBS containing 5% fetal bovine serum (FBS). To ensure uniform labeling, the cell suspension was added to the bottom of a plastic tube and held almost horizontally. The CFSE solution was then added to a non-wetted portion of the plastic at the top of the tube. The tube is then capped while still in the nearly horizontal position, and rapidly inverted several times. CFSE solution and cells were mixed for 5 minutes at room temperature and then washed three times with 10X volume of PBS containing 5% FBS. Cells were washed in serum free media (SFM) (Gibco, Grand Island, NY) before plating. CFSE labeled mononuclear cells were cultured in 1ml of SFM with 20% autologous serum (AS) at a concentration of 2×106 cells/ml under sterile conditions. All cultures were incubated at 37°C with a 5% CO2 environment. CFSE labeled mononuclear cells were cultured in duplicate on unaltered TCPS, PEU, SR, and PET surfaces for 3 and 7 days. Positive control cultures were stimulated with 2% phytohemagglutinin M-form (PHA-M) (Invitrogen, Carlsbad, CA). A portion of the CFSE labeled mononuclear cells were treated with 50µg/ml of mitomycin C (Sigma, St. Louis, MO) in order to arrest these cells at the parent generation. After treatment with mitomycin C, cells were washed and fixed with 4% paraformaldehyde (BD Pharmingen).
Flow Cytometry
At days 3 and 7, non adherent cells were collected via pipetting. Cells were centrifuged at 300g and supernatants were aliquoted and stored at −80°C. Cells were then resuspended in stain buffer (BD Pharmingen, Franklin Lakes, USA) and stained with the following mouse anti-human directly conjugated monoclonal antibodies: CD3-APC, CD8-APC, CD4-APC, CD25-APC-Cy7 (clone M-A251), CD69-APC-Cy7 (Clone FN50) and appropriate isotype controls (BD Pharmingen, Franklin Lakes, USA). Samples were incubated with the antibodies for 1hr on ice and subsequently washed twice with stain buffer (BD Biosciences). Samples were run on a Becton Dickinson LSRII flow cytometer within two hours. Fluorescence positivity of a particular antibody was determined by comparing to the appropriate isotype control. Proliferation was determined by comparing CFSE fluorescent peaks to the mitotically inhibited control from the respective donor.
Cytokine Analysis
A multiplex cytokine immunoassay containing a human cytokine panel (IFNγ, IL-2) was purchased from Lincoplex (Millipore, Billerica, MA). Due to the multiplex technology, the cytokines in the human panel were measured simultaneously in each sample. Samples collected from days 3 and 7 cell culture supernatants were run in duplicate. The multiplex immunoassay was run in accordance with manufacturer’s instructions. Samples were incubated overnight with antibody immobilized beads. The plate was run on a Luminex® 200 Instrument using Bio-plex manager 4.1 standard software (Bio-Rad Laboratories, Hercules, CA). Raw fluorescence data was analyzed by the software using a 5 parameter logistic method. Minimum detection concentrations were 0.55 pg/ml and 0.38 pg/ml, for IFNγ and IL-2 respectively. To account for serum cytokine levels in autologous serum, concentrations of cytokines in media controls were subtracted from supernatant cytokine concentrations.
Adherent Cell Density Analysis
At days 3 and 7, adherent cells were washed twice with warm PBS++ (37°C) and fixed with 100% methanol for 5 minutes. May Grunwald reagent was added to surfaces for 5min. Surfaces were rinsed in PBS twice and Giemsa reagent added for 15min immediately. Surfaces were rinsed with distilled water twice and allowed to air dry. Adherent cell densities were determined by counting the number of nuclei from 5 representative 20× fields for each sample and expressed as cells/mm2. Percent fusion was determined by dividing the number of nuclei within foreign body giant cells (containing 3 or more nuclei as identified histologically) by the total number of nuclei in the field. Cell densities and percent fusion were averaged from the five fields per sample from 3 donors (n=3).
Statistics
All results were presented as an average ± the standard error of the mean (SEM) or standard deviation (SD) as indicated (n = 3). Statistical analysis was performed utilizing Minitab statistical software (Minitab Inc., State College, PA) and statistically significant differences were determined by ANOVA and the Tukey post hoc test.
Results
T Lymphocyte Cell Surface Activation Markers and Proliferation
On average, isolated cell suspensions contained 82±4% lymphocytes and 18±3% monocytes. The majority of the lymphocyte population (81±2%) was T lymphocytes. Of the T lymphocyte population, 70±3% were CD4+ and 31±4% were CD8+. Prior to plating mononuclear cells on different biomaterial surfaces, donor CD4+ T cells did not express CD69. However there was an average of 3±1% CD25 expression on donor peripheral CD4+ T cells. Mononuclear cells were isolated 3 and 7 days following culture on biomaterial surfaces and CD69 expression was not present on CD4+ T cells. CD25 expression did not exceed levels prior to plating (Table 1). Positive control cultures containing PHA-M did have CD69 and CD25 expression on CD4+ T cells as expected and no material trends were noted (Table 1).
Table 1.
CD69 and CD25 Expression on CD4+ T Lymphocytes at Days 3 and 7*
| Day 3 | |||||
|---|---|---|---|---|---|
| TCPS | PEU | SR | PET | ||
| CD69 | Experimental | 0% | 0% | 0% | 0% |
| PHA Control | 17% ± 4 | 18% ± 5 | 19% ± 4 | 16% ± 3 | |
| CD25 | Experimental | 2% ± 1 | 3% ± 2 | 3% ± 1 | 3% ± 1 |
| PHA Control | 66% ± 6 | 71% ± 8 | 68% ± 5 | 70% ± 8 | |
| Day 7 | |||||
|---|---|---|---|---|---|
| TCPS | PEU | SR | PET | ||
| CD69 | Experimental | 0% | 0% | 0% | 0% |
| PHA Control | 15% ± 6 | 17% ± 3 | 12% ± 2 | 15% ± 1 | |
| CD25 | Experimental | 3% ± 1 | 3% ± 2 | 4% ± 1 | 3% ± 1 |
| PHA Control | 50% ± 5 | 48% ± 4 | 49% ± 6 | 49% ± 2 | |
Data represent average ± standard deviation (n=3).
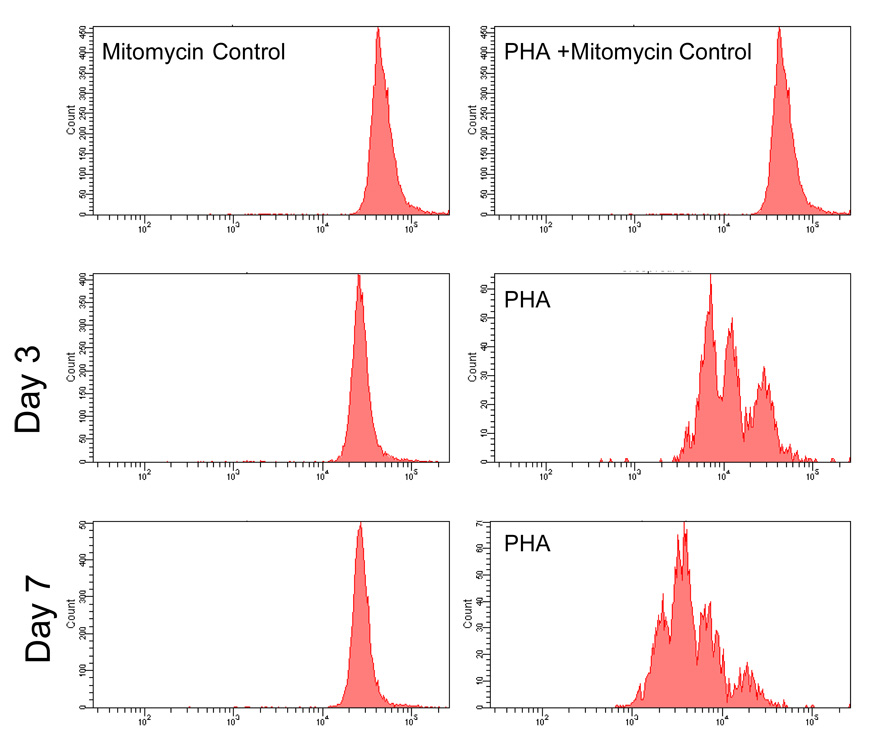
When mononuclear cells were cultured on the three different biomaterial surfaces, no lymphocytes proliferated as determined by comparison to the mitotically inhibited control at days 3 and 7 (Figure 1). Lymphocytes cultured on biomaterial surfaces in the presence of the mitogen PHA-M did proliferate and proliferation increased from day 3 to day 7 in the positive control cultures. No differences were seen between different material surfaces (Figure 1).
Figure 1.
Flow cytometry CFSE histograms of lymphocytes cultured on PEU, SR, PET, and TCPS(Control). The histograms from the PEU,SR, PET, and TCPS groups were identical at each respective time and treatment. The left column shows no lymphocyte proliferation on any of the surfaces when compared to the right column which shows no proliferation with PHA + mitomycin stimulation and proliferation with PHA alone at days 3 and 7.
Lymphocyte Cytokines: IL-2 and IFNγ
IL-2 and IFNγ was not detectable in donor serum and in supernatants from mononuclear cells cultured on biomaterial surfaces in 2 out of 3 donors. One donor did have detectable levels of IL-2 and IFNγ. IL-2 levels were below 16pg/ml. IFNγ levels ranged from 12pg/ml to 411pg/ml. No material or time dependent trends were seen (Table 2). IL-2 and IFNγ were present in PHA stimulated positive control cultures. IL-2 concentrations significantly decreased from day 3 to day7 whereas IFNγ concentrations were comparable at these two time points (Table 2).
Table 2.
IL-2 and IFNγ concentrations (pg/ml) from cell culture supernatants*
| Day 3 | |||||
|---|---|---|---|---|---|
| TCPS | PEU | SR | PET | ||
| IL-2 (pg/mL) | Experimental | 11 | 16 | 6 | 5 |
| PHA Control | 195 ± 100 | 291 ± 120 | 326 ± 150 | 334 ± 127 | |
| IFNγ (pg/mL) | Experimental | 49 | 18 | 191 | 168 |
| PHA Control | 6610 ± 2430 | 7260 ± 2160 | 6680 ± 2640 | 7590 ± 2740 | |
| Day 7 | |||||
|---|---|---|---|---|---|
| TCPS | PEU | SR | PET | ||
| IL-2 (pg/mL) | Experimental | 1 | 3 | 4 | 1 |
| PHA Control | 3.8 ± 0.4 | 7.1 ± 0.7 | 6.6 ± 5.0 | 8.8 ± 2.8 | |
| IFNγ (pg/mL) | Experimental | 12 | 411 | 148 | 77 |
| PHA Control | 8100 ± 2470 | 8700 ± 3000 | 8430 ± 2620 | 8570 ± 2850 | |
Data from experimental group represent values from one donor (concentrations of IL-2 and IFNγ were not detectable in all other donors). Data from PHA controls represent data from cultures stimulated with 2% PHA from 3 donors.
Adherent Cell Density Analysis
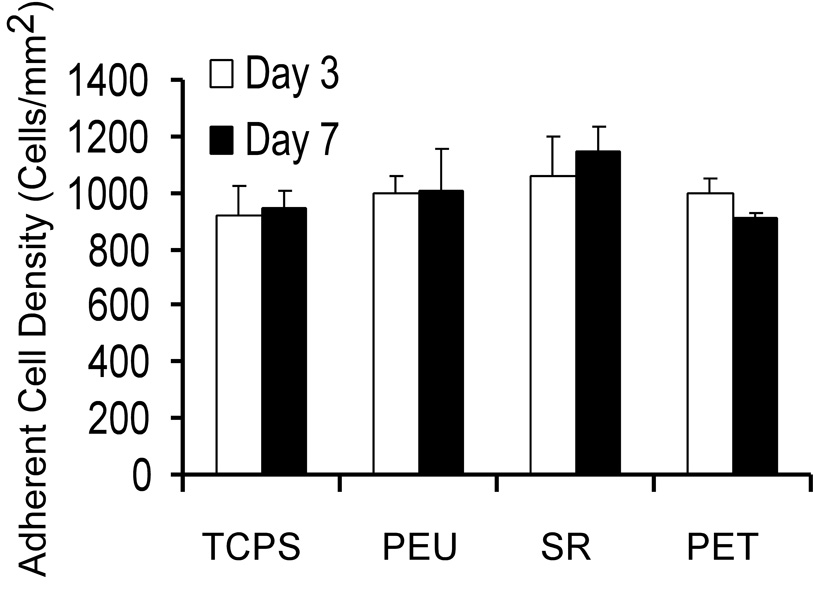
Adherent cell density was comparable between material groups and time points (Figure 2). Macrophage fusion and FBGC formation were not found on PEU and SR surfaces. FBGCs were present in only 5 fields and 8 fields on TCPS at days 3 and 7, respectively. However, percent fusion was not calculated for TCPS because it was below 1%. Percent fusion was on average 5% on PET surfaces at days 3 and 7. FBGCs on PET surfaces were small with the largest FBGC counted containing 10 nuclei.
Figure 2.
Discussion
Our goal was to determine if T lymphocytes are activated in response to synthetic biomaterials in vitro. Previous studies in our laboratory have found that when lymphocytes were co-cultured with monocytes on biomaterials surfaces, lymphocytes proliferated using a tetrazolium salt assay1. However, this assay may reflect a different state of cell function other than DNA synthesis12–14. Therefore in this study we used a CFSE proliferation assay along with other parameters of T lymphocyte activation, namely, the upregulation of activation markers and cytokine secretion.
One of the methods to analyze T lymphocyte activation included measuring the upregulation of cell surface activation markers CD69 and CD25. After mononuclear cells were cultured with biomaterial surfaces for 3 or 7 days, there was no CD69 expression on CD4+ T lymphocytes (Table 1). CD69 is a very early activation marker and appears as early as 2–3 hours after activation. CD69 expression reaches maximum expression between 18 and 24 hours of stimulation15. The function of CD69 has not been thoroughly elucidated with discrepancies between in vitro and in vivo models, and its ligand is not known16. CD69 is induced on CD4+ T cells when mouse splenocytes are cultured with the biodegradable polymers chitosan and alginate. CD69 expression was induced but proliferation was not17. In this study we used non-biodegradable polymers and CD69 lymphocyte expression was not induced at days 3 and 7 of culture. Despite CD69 being an early activation marker, upregulation of this marker was seen in the PHA-stimulated cultures even on day 7 (Table 1).
We also studied the upregulation of the lymphocyte high-affinity IL-2 receptor CD25. Schuster et al. were able to show that T cell activation in response to polyurethane particles was inhibited by CD25 blockade. Prior to plating mononuclear cells, there was CD25 expression on approximately 3% of CD4+ isolated from peripheral blood. These data can be explained by the fact that there is constitutive expression of CD25 on a portion of peripheral blood T cells18. Following culture periods of 3 and 7 days on biomaterial surfaces, CD25 expression was not upregulated on CD4+ T cells (Table 1). In vivo, we have identified CD4+/CD25+ T lymphocytes at biomaterial implant sites but it is not known if they are non-specifically recruited or are activated in response to the biomaterial19. Previous studies that investigated the inflammatory response to silicone breast implants identified activated T cells at the biomaterial site20. This study indicates that the in vivo environment may be necessary for activation of lymphocytes in response to synthetic biomaterials.
Proliferation, another parameter of T cell activation, was assayed by using CFSE incorporation. Comparison of CFSE histograms to a mitotically inhibited control showed that lymphocytes did not proliferate after culturing on biomaterial surfaces at both time points (Figure 1). Lymphocytes from PHA-stimulated cultures did undergo multiple cell divisions and proliferation increased from day 3 to day 7 (Figure 1). As another measure of T cell activation, we investigated IL-2 and IFNγ concentrations in cell culture supernatants. IL-2 and IFNγ concentrations were not detected in 2 out of 3 donors. One donor did have detectable levels of IL-2 and IFNγ at day 3 and 7. IFNγ levels were much higher than IL-2 levels in this donor. IL-2 levels ranged from 1 to 16 pg/ml, but the sensitivity of the assay is 0.38pg/ml. In contrast IFNγ levels ranged from 12 to 411 pg/ml (Table 2). The donor variability in this study may be due to differences in human leukocyte antigen (HLA) gene inheritance and therefore major histocompatibility complex (MHC) diversity. MHC loci are among the most genetically variable genetic loci in humans. The MHC class II proteins (DP, DQ, DR) are found on antigen presenting cells. Diversity in MHCII proteins results in individual variability in antigen presentation and in turn immune responses. Due to this diversity individuals mount immune responses to different epitopes of pathogens. LVAD recipients that are predisposed to develop B-cell hyperreactivity have HLA-DR3 expression indicating that lymphocyte responses to biomaterials are variable and dependent on the individual’s genetic profile21. It is possible that only individuals with certain MHCII receptors can interact with biomaterials in a mechanism that results in a lymphocyte response.
The donor that exhibited IL-2 and IFNγ production did not demonstrate CD69 or CD25 upregulation on T lymphocytes indicating that other lymphocytes are likely to be the source of these cytokines. NK and NKT cells are also capable of producing IFNγ as part of the innate immune response. Soluble and contact dependent stimuli from antigen presenting cells (APCs) activate NK cells. Most notably the cytokine IL-12 can stimulate the secretion of IFNγ from NK cells. Unstimulated NKT cells transcribe genes encoding for the cytokines IFNγ and IL-4. IL-12 also induces the production of IFNγ from NKT cells22. IFNγ is an immunoregulatory cytokine and has a variety of functions which include increasing the expression of MHC II on APCs, promoting macrophage activation, and upregulating the production of reactive oxygen species in phagocytes23. These functions could potentially be critical in the foreign body response, but IFNγ has not yet been quantified at biomaterial implant sites. Previously, Chang et al. reported IL-2 and IFNγ were not detected when lymphocytes and monocytes were cultured on biomaterials surfaces using a protein array24. However the protein array system is not as sensitive as the multiplex immunoassay or ELISA methodology. Subsequent studies by Chang et al. utilizing ELISA methodology demonstrate IFNγ is produced in lymphocyte-monocyte co-cultures in response to synthetic biomaterials in vitro. IL-2 was not found to be produced. IFNγ was also produced in indirect co-cultures indicating that direct lymphocyte-monocyte contact is not necessary for IFNγ production. The lymphocyte subtype/s that produce IFNγ in response to synthetic biomaterials is not known (Chang et al. submitted). Chang et al. did not have donor variability in IFNγ production. It is likely that this is due to the utilization of surfaces that are more activating whereas the materials used in this study are relatively passive.
In conclusion, we have shown that T lymphocytes are not activated in response to synthetic non-biodegradable biomaterials. We did have donor variability in the production of the cytokines IL-2 and IFNγ indicating the cytokines were produced by lymphocyte subsets exclusive of T lymphocytes, which did not show activation. We have previously shown that the FBR can occur in a murine model without thymus matured T lymphocytes25. Therefore investigation of the participation of other lymphocyte subsets such as NK cells, NKT cells, and γ/δ T cells is warranted.
Acknowledgments
Contract Grant Sponsor: National Institutes of Health (NIH)
Contract Grant Number: EB-000282, T32-GM007250
References
- 1.Brodbeck WG, Macewan M, Colton E, Meyerson H, Anderson JM. Lymphocytes and the foreign body response: lymphocyte enhancement of macrophage adhesion and fusion. J Biomed Mater Res A. 2005;74(2):222–229. doi: 10.1002/jbm.a.30313. [DOI] [PubMed] [Google Scholar]
- 2.MacEwan MR, Brodbeck WG, Matsuda T, Anderson JM. Student Research Award in the Undergraduate Degree Candidate category, 30th Annual Meeting of the Society for Biomaterials, Memphis, Tennessee, April 27–30, 2005. Monocyte/lymphocyte interactions and the foreign body response: in vitro effects of biomaterial surface chemistry. J Biomed Mater Res A. 2005;74(3):285–293. doi: 10.1002/jbm.a.30316. [DOI] [PubMed] [Google Scholar]
- 3.Schuster M, Kocher A, Lietz K, Ankersmit J, John R, Edwards N, Oz M, Itescu S. Induction of CD40 Ligand expression in human T cells by biomaterials derived from left ventricular assist device surfaces. Transplantation Proceedings. 2001;33:1960–1961. doi: 10.1016/s0041-1345(00)02754-8. [DOI] [PubMed] [Google Scholar]
- 4.Schuster M, Kocher A, John R, Hoffman M, Ankersmit J, Lietz K, Edwards N, Oz M, Itescu S. B-cell activation and allosensitization after left ventricular assist device implantation is due to T-cell activation and CD40 ligand expression. Hum Immunol. 2002;63(3):211–220. doi: 10.1016/s0198-8859(01)00380-9. [DOI] [PubMed] [Google Scholar]
- 5.Aringer M. T lymphocyte activation–an inside overview. Acta Med Austriaca. 2002;29(1):7–13. doi: 10.1046/j.1563-2571.2002.01044.x. [DOI] [PubMed] [Google Scholar]
- 6.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5(6):472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 7.Im SH, Rao A. Activation and deactivation of gene expression by Ca2+/calcineurin-NFAT-mediated signaling. Mol Cells. 2004;18(1):1–9. [PubMed] [Google Scholar]
- 8.Garrity PA, Chen D, Rothenberg EV, Wold BJ. Interleukin-2 transcription is regulated in vivo at the level of coordinated binding of both constitutive and regulated factors. Mol Cell Biol. 1994;14(3):2159–2169. doi: 10.1128/mcb.14.3.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HP, Imbert J, Leonard WJ. Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor Rev. 2006;17(5):349–366. doi: 10.1016/j.cytogfr.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Miyazaki H, Kikuchi A, Koyama Y, Okano T, Sakurai Y, Kataoka K. Boronate-containing polymer as novel mitogen for lymphocytes. Biochem Biophys Res Commun. 1993;195(2):829–836. doi: 10.1006/bbrc.1993.2120. [DOI] [PubMed] [Google Scholar]
- 11.Otsuka H, Ikeya T, Okano T, Kataoka K. Activation of lymphocyte proliferation by boronate-containing polymer immobilised on substrate: the effect of boron content on lymphocyte proliferation. Eur Cell Mater. 2006;12:36–43. doi: 10.22203/ecm.v012a04. discussion 36–43. [DOI] [PubMed] [Google Scholar]
- 12.Gerlier D, Thomasset N. Use of MTT colorimetric assay to measure cell activation. Journal of Immunological Methods. 1986;94:57–63. doi: 10.1016/0022-1759(86)90215-2. [DOI] [PubMed] [Google Scholar]
- 13.Rollino C, Borsa S, Bellone G, Piccoli G, Emanuelli G. False positive results with MTT assay. Journal of Immunological Methods. 1995;185:141–143. doi: 10.1016/0022-1759(95)00171-6. [DOI] [PubMed] [Google Scholar]
- 14.Chen CH, Campbell PA, Newman LS. MTT colorimetric assay detects mitogen responses of spleen but not blood lymphocytes. Int Arch Allergy Immunol. 1990;93:249–255. doi: 10.1159/000235309. [DOI] [PubMed] [Google Scholar]
- 15.Reddy M, Eirikis E, Davis C, Davis HM, Prabhakar U. Comparative analysis of lymphocyte activation marker expression and cytokine secretion profile in stimulated human peripheral blood mononuclear cell cultures: an in vitro model to monitor cellular immune function. J Immunol Methods. 2004;293(1–2):127–142. doi: 10.1016/j.jim.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Sancho D, Gomez M, Sanchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26(3):136–140. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Borges O, Borchard G, de Sousa A, Junginger HE, Cordeiro-da-Silva A. Induction of lymphocytes activated marker CD69 following exposure to chitosan and alginate biopolymers. Int J Pharm. 2007;337(1–2):254–264. doi: 10.1016/j.ijpharm.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Jackson AL, Matsumoto H, Janszen M, Maino V, Blidy A, Shye S. Restricted expression of p55 interleukin 2 receptor (CD25) on normal T cells. Clin Immunol Immunopathol. 1990;54(1):126–133. doi: 10.1016/0090-1229(90)90012-f. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez A, Voskerician G, Meyerson H, MacEwan SR, Anderson JM. T cell subset distributions following primary and secondary implantation at subcutaneous biomaterial implant sites. J Biomed Mater Res A. 2008;85(2):556–565. doi: 10.1002/jbm.a.31562. [DOI] [PubMed] [Google Scholar]
- 20.Katzin WE, Feng LJ, Abbuhl M, Klein MA. Phenotype of lymphocytes associated with the inflammatory reaction to silicone gel breast implants. Clin Diagn Lab Immunol. 1996;3(2):156–161. doi: 10.1128/cdli.3.2.156-161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itescu S, Schuster M, Burke E, Ankersmit J, Kocher A, Deng M, John R, Lietz K. Immunobiologic consequences of assist devices. Cardiol Clin. 2003;21(1):119–133. ix–x. doi: 10.1016/s0733-8651(02)00135-2. [DOI] [PubMed] [Google Scholar]
- 22.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 23.Gattoni A, Parlato A, Vangieri B, Bresciani M, Derna R. Interferon-gamma: biologic functions and HCV therapy (type I/II) (1 of 2 parts) Clin Ter. 2006;157(4):377–386. [PubMed] [Google Scholar]
- 24.Chang DT, Jones JA, Meyerson H, Colton E, Kwon IK, Matsuda T, Anderson JM. Lymphocyte/Macrophage interactions: biomaterial surface dependent cytokine, chemokine, and matrix protein production. J Biomed Mater Res A. 2007 doi: 10.1002/jbm.a.31630. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez A, Macewan SR, Meyerson H, Kirk J, Anderson JM. The foreign body reaction in T cell deficient mice. Journal of Biomedical Materials Research. 2008 doi: 10.1002/jbm.a.32050. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]