Abstract
Most patients with paraneoplastic encephalomyelitis/sensory neuronopathy PEM/SN have small-cell lung cancer (SCLC) and develop antibodies against neuronal-specific Hu proteins, which are abnormally expressed in the tumor. Anti-Hu reactivity is present in ~16% of SCLC patients without PEM/SN. Here we test the hypothesis that engineered SCLC-prone mice may exhibit anti-Hu reactivity. We show that tumors from SCLC-prone mice misexpress Hu proteins, and 14% of mice harbor anti-Hu antibodies. Mice appear to show reactivity prior to clinical diagnosis of SCLC. This mouse model system will be useful to study SCLC-associated autoimmunity, its diagnostic value, and the potential protective role of oncoantigen-directed autoantibodies.
Keywords: autoantigen, autoantibody, small-cell lung cancer
1. Introduction
Small-cell lung cancer (SCLC) accounts for up to 15% of all newly diagnosed lung cancers (Ries et al., 2007). Initially, SCLC patients respond well to chemotherapy, but they inevitably relapse (Sandler, 2003). Only 5% of patients are alive after five years (Worden and Kalemkerian, 2000), making SCLC the most aggressive lung cancer subtype. There are currently no effective early detection methods for this disease.
Paraneoplastic encephalomyelitis/sensory neuronopathy (PEM/SN) is one of several rare autoimmune diseases associated with SCLC (Henson and Urich, 1982, Horwich et al., 1977) and occurs in less than 1% of SCLC patients (Anderson et al., 1987). SCLC patients with PEM/SN harbor high titers of antibodies that react against neuronal Hu proteins (Dalmau et al., 1990, Dalmau et al., 1991, Graus et al., 1986, Graus et al., 2001). Hu proteins are a family of four RNA-binding proteins, three of which are normally expressed in the nervous system (Good, 1995). In SCLC, however, they are abnormally expressed in all tumors and act as onconeuronal antigens. Through an unknown mechanism, the immune system identifies them as foreign, generating anti-Hu autoantibodies. It is thought that these antibodies may be the result of a complex immune response that may react with Hu proteins in the healthy nervous system, leading to PEM/SN (Graus et al., 1985, Posner and Dalmau, 1997). There is evidence to suggest that PEM/SN is mediated by a cytotoxic T-cell response against Hu proteins (Dalmau and Posner, 1994, Voltz et al., 1998); however, the mechanism coupling the immune response (humoral, T-cell mediated, or both) to the pathogenesis of the autoimmune disease remains in question.
Approximately 16% of SCLC patients without PEM/SN have detectable levels of anti-Hu antibody in their blood, albeit at much lower titers than PEM/SN patients (Dalmau et al., 1990, Graus et al., 1997). It has been reported that the presence of even low levels of anti-Hu autoantibodies correlates with more indolent tumor growth (Dalmau et al., 1992, Graus et al., 1997), suggesting that these antibodies might be protective. Interestingly, symptoms of PEM/SN often antedate tumor detection (Darnell and Posner, 2003). If the antibody response were to arise when the cancer is still very small, it might be of use for SCLC early detection.
Studies of the origin and timing of anti-Hu response in SCLC patients are important, but the rapid progression of SCLC and relatively uncommon anti-Hu response make such analyses in human patients difficult. A mouse model for SCLC and associated autoimmune diseases would therefore be very valuable. A unique SCLC mouse model system has recently been established based on the high frequency inactivation of tumor suppressor genes p53 and Rb in human SCLC (Meuwissen et al., 2003). In this mouse model, both copies of p53 and Rb are homozygously floxed and conditionally inactivated in the lungs of 6–8 week old animals by intratracheal instillation of Adenovirus carrying Cre-recombinase (Adeno-Cre). SCLC tumors in these mice become detectable after 6–12 months (Meuwissen et al., 2003). The anti-Hu response has not been investigated in this model system.
Here we demonstrate that the murine tumors express neuronal Hu proteins. Even more importantly, we find that a fraction of these SCLC-prone mice develop anti-Hu antibodies, mimicking what happens in human SCLC patients. Interestingly, anti-Hu reactivity appears to arise prior to clinical evidence of cancer in these mice, suggesting that anti-Hu and other SCLC-related autoantibodies may offer methods for early detection of SCLC.
2. Materials and Methods
SCLC Mouse Model
The mouse model for SCLC has been previously described (Meuwissen et al., 2003). With the exception of those mice that were arbitrarily sacrificed for experimental purposes (Table 1), all tumor-bearing mice carried a Cre-recombination-dependent luciferase reporter allele, allowing imaging of Cre-dependent tumorigenesis (Lyons et al., 2003). Time points for arbitrarily sacrificed mice were defined in advance, and these mice were sacrificed prior to evidence of SCLC. Tissues from these mice (specifically lungs, liver, kidney and adrenal glands) were processed for histology, and blood was collected.
Table 1.
Occurrence of anti-Hu reactivity in mice.
| Genotype | Total # of mice | # of mice with SCLC tumors | # of mice with positive anti-Hu reactivity (%)* | # of mice with positive anti-Hu reactivity above background (%)** | Lesion latency (days) |
|---|---|---|---|---|---|
|
Wildtype FVB |
41 | NA | 4 (10) | 0 (0) | NA |
|
SCLC-prone LucR;P53F;RbF |
71 | 55a | 19b (27) | 10c (14) | 141–520 |
Positive Western Blot signal at 1:250 dilution of plasma or serum
Positive Western Blot signal at fold dilution > 1:5000 of plasma or serum
Sixteen mice had no SCLC lesions: fourteen mice were arbitrarily sacrificed for experimental purposes before SCLC became apparent; one mouse spontaneously died; one mouse showing obvious discomfort was euthanized eleven months post Adeno-Cre infection.
Includes four mice without SCLC lesions (three arbitrarily sacrificed and one sick)
Includes two mice without SCLC lesions (one arbitrarily sacrificed and one sick)
NA= not applicable
Tumors were detected by in vivo non-invasive bioluminescence imaging (once every two weeks beginning four months after tumor induction (Lyons et al., 2003)) and CT scanning (beginning five months after tumor induction, once every four weeks or when the mice showed symptoms of sickness, such as weight loss, reduced activity, breathing difficulties, kyphosis, and disturbed coat). Bioluminescence was considered positive when the signal in the lung area was above the background luciferase signal seen in control mice.
Analysis of Hu mRNA Expression and Immunohistochemistry
Total RNA was isolated from snap-frozen tissue samples using Trizol. After DNAse I treatment and purification on RNeasy columns (Qiagen, Valencia, CA), RNA was subjected to reverse transcription using SSRTII (Invitrogen, Carlsbad, CA) and random hexamers, according to manufacturer’s instructions. Single-stranded cDNA was then assayed by quantitative real-time PCR on an ABI Prism 7000 sequence detection system in duplicate on two different plates. TaqMan Universal PCR Master Mix and TaqMan probes (Applied Biosystems, Foster City, CA) were used according to the manufacturer’s instructions. The following gene expression assays were used: HuR (Elavl1): Mm00516011_m1; HuB (Elavl2): Mm00516015_m1; HuC (Elavl3): Mm00809661_s1; HuD (Elavl4): Mm00516018_m1; Hprt: Mm00446968_m1.
To assess Hu protein expression, tissues were fixed in 4% formalin in phosphate buffered saline solution and embedded in paraffin. 4 μm sections were prepared and rehydrated before staining. Haematoxylin and eosin stainings were performed according to standard procedures. Immunohistochemistry was performed after heat-mediated antigen retrieval using citrate buffer (pH 6). Sections were incubated with affinity-purified rabbit anti-HuD antibody (see below) overnight at 4°C. Biotinylated goat anti-rabbit secondary antibody (Dako, Glostrup, Denmark) and HRP-sABC system (Dakocytomation) were used according to manufacturer’s instructions, and immunocomplexes were revealed using diaminobenzidine. Tissue sections were counterstained with haematoxylin, dehydrated and mounted in permanent medium. Images were captured using a Zeiss Axioskop2 Plus microscope (Carl Zeiss Microscopy, Hertfordshire, UK) equipped with a Zeiss AxioCam HRc digital camera and processed using AxioVision 4 software (Carl Zeiss Vision, San Diego, CA).
HuD rabbit antiserum was obtained by immunizing rabbits with a fragment of recombinant human HuD encompassing amino acids 3-224, which includes RNA recognition motifs (RRMs) 1 and 2. The antibodies were affinity purified as follows. N-terminally biotinylated hexahistidine-tagged HuD (amino acids 3-224, generated as described (Park-Lee et al., 2003) using a HuD coding region carrying a sequence encoding a 14-amino acid biotinylatable tag at its 5′ end) was used to generate an affinity column using Immobilized NeutrAvidin™ Protein, Disposable Plastic Columns, and Zeba™ Desalt Spin Columns according to the manufacturer’s instructions (Thermo Scientific/Pierce Biotechnology, Rockford, IL).
Mouse Plasma and Serum Samples
Plasma was obtained from mice at regular intervals using heparin-coated glass capillaries for tail vein bleeds. At sacrifice, 0.5 mL of serum was collected by heart puncture. All samples were stored at −80°C. Plasma and sera from 41 control mice and 71 SCLC-prone mice were analyzed. Control mice were age and sex-matched FVB/N mice that did not receive intratrachael Adeno-Cre virus. Control mice were bled just before sacrifice at ages ranging from 20–60 weeks, comparable to when the SCLC-prone mice develop tumors.
Determination of Anti-Hu reactivity
Anti-Hu reactivity in mouse plasma and serum was determined by Western Blot analysis using recombinant human Hu proteins (HuB, HuC, HuD, and HuR, deletion constructs), prepared as described (Park et al., 2000), with the following modifications. Sonication buffer consisted of 10 mM Tris (pH 8.0), 150 mM NaCl, and 0.5% Triton X-100. Proteins were eluted from Ni2+ beads using sonication buffer containing 10% glycerol and 50–500mM imidazole. Protein concentration was determined by the Bradford assay (Bio-Rad Laboratories, Hercules, CA). Protein (0.1 ug in 28 ul total volume) was resolved on 12% SDS and transferred to PVDF filters as described (Towbin et al., 1979), with the following modifications. Transfer was performed for 60 minutes at 100 V while chilling the buffer with a cold pack. The filters were blocked with 5% milk in Tris-buffered saline, Tween-20 (TBST: 10 mM Tris/HCl pH 8.0, 150 mM NaCl, 0.05% Tween-20) for at least one hour at room temperature. Blots were incubated overnight at 4°C with 1:250, 1:500, 1:1000, 1:2500, 1:5000, 1:10,000, 1:20,000, 1:40,000, and 1:80,000 dilutions of plasma/serum in 5% milk/TBST. Blots were washed three times for 10 minutes with TBST at room temperature. Secondary antibody, goat anti-mouse IgG HRP conjugate (Bio-Rad, Hercules, CA) was diluted 1:2000 in TBST and added for 1 hour. After washing three times for 10 minutes in TBST, the blots were submerged for three minutes in Millipore Immobilon Western Chemiluminescent HRP Substrate, prepared according to the manufacturer’s instructions (Millipore, Billerica, MA). The blots were imaged using Bio-Rad Fluor-S™ MultiImager, and images were taken every five seconds during a five-minute exposure time. Only plasma/serum samples yielding a band of the correct size at 1:250 dilution of plasma/serum were scored as showing positive reactivity against HuD (~42 kDa). Reactivity against HuB (~39kDa), HuC (~39kDa), and HuR (~36kDa), as well as HuD deletion constructs was examined in those samples that showed positive reactivity to HuD. Reactivity of rabbit anti-HuD serum was used as a positive control to confirm the identity of the correct bands.
Survival Analysis
In order to determine whether anti-Hu reactivity was related to survival-time among SCLC-prone mice, we conducted survival analysis using the product-limit method of estimation in relationship to time until death from SCLC or censorship. Of the 71 SCLC-prone mice, fourteen mice were arbitrarily sacrificed, and two mice died spontaneously. These mice were censored in the analysis. Of the 71 mice, nine showed background level anti-Hu antibodies, while ten mice were highly anti-Hu responsive (titers ≥1:20,000). Thus, we examined survival of three groups: mice showing no detectable antibodies (n=52), mice showing low titer antibodies similar to levels seen in control animals (n=9) and mice showing high titer antibodies (n=10). Survival analyses were conducted with SAS version 9.2, using the LIFETEST procedure (Statistical Analysis System (SAS) version 9.2. 2008. Cary, NC). See supplemental data.
3. Results
The SCLC-prone mouse model system recapitulates anti-Hu response seen in human SCLC patients
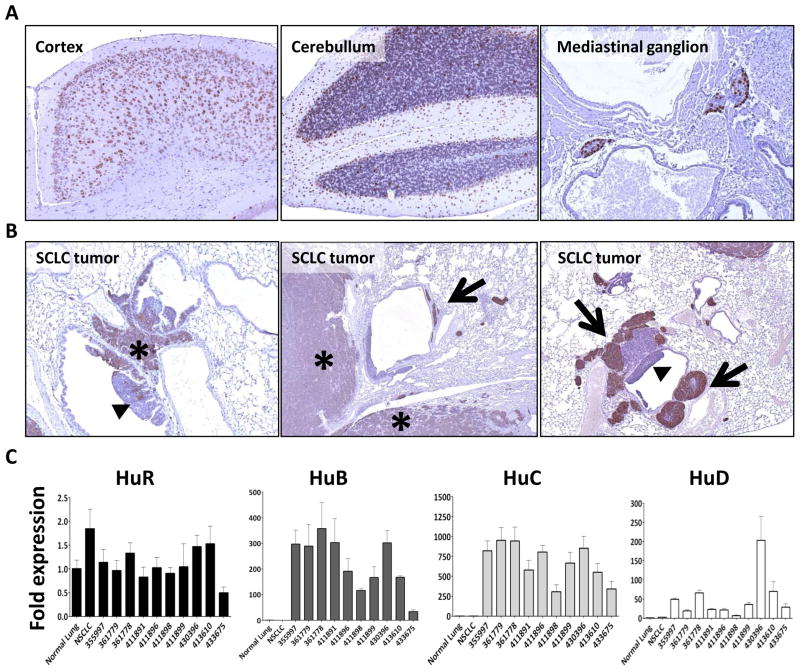
To determine whether the SCLC mouse model could be used to study anti-Hu response, we first examined Hu protein expression in mouse SCLC tumors. Using affinity-purified rabbit anti-HuD antibody, which cross-reacts strongly with the highly homologous neuronal Hu proteins HuB, HuC, HuD ((Manley et al., 1995), our unpublished data), we performed immunohistochemistry on murine SCLC tumors and in neuronal tissue as a positive control. Both tissue types expressed Hu proteins (Fig. 1A and B). To explore the relative expression of the neuronal Hu proteins and the ubiquitous HuR, we compared mRNA expression levels between SCLC samples from the mice, lung tissue from a cancer-free littermate, and a non-SCLC mouse (derived from a K-ras lung cancer model mouse (Meuwissen et al., 2001)) (Fig. 1C). While HuR was equally expressed in normal lung, NSCLC, and SCLC tumors, neuronal Hu protein expression was several fold increased compared to normal lung in all SCLC samples. As is seen in human SCLC (King, 1997), varying levels of the neuronal Hu proteins were present in each cancer.
Fig. 1.
Expression of Hu protein in mice. Panels A and B: Expression of Hu protein detected by immunohistochemistry on mouse tissue. Panel A: (from left to right) Hu staining in cortex, cerebullum, and mediastinal ganglion. Panel B: Hu staining in tumors of SCLC-prone mice. SCLC lesions (star) stain positive for Hu. Negative or weak staining is detected in intraluminal lesions (triangles). Weak staining is also observed in tumors invading the lung parenchyma (stars). Strong staining is observed in tumor cells growing in or along blood and lymph vessels (arrows). All sections shown at 10x magnification. Panel C: Expression levels of Hu family genes in normal lung and lung tumors by quantitative Reverse Transcriptase PCR using TaqMan probes. Results were normalized using the expression measurement of the HPRT gene, analyzed by the ΔΔCt method, and are shown as fold difference in expression relative to normal lung. While the expression of the ubiquitous protein HuR is similar in both normal lung and tumor samples, the expression of the neuron-specific HuB, HuC, and HuD is substantially increased in SCLC tumors compared to normal lung and NSCLC tumor. Results shown are the average of four replicates (two independent tests, two replicates in each test). Error bars represent the standard deviation. Mouse identification numbers appear on the x-axis. Please note that the scale of the Y-axis differs in each panel.
We subsequently examined whether an anti-Hu immune response was detectable. Blood samples were collected from SCLC-prone mice at 1–17 months post Adeno-Cre instillation (n=71; Table 1). Additionally, one bleed per mouse from age- and sex- matched control animals (n=41) was collected. The blood samples were used to prepare plasma and/or serum. Anti-Hu antibodies have been found in both the plasma and serum of human SCLC patients (Dalmau et al., 1990, Graus et al., 1997, Monstad et al., 2004, Tsou et al., 2009, Verschuuren et al., 1999). We examined the titer in both types of fluids and found no indication of differences. The anti-Hu immune reactivity in blood samples from the SCLC-prone and control mice was assessed using Western blots to ensure reactivity against the correct protein. The use of recombinant Hu protein in Western blot analysis is considered the most sensitive and specific method for detection of anti-Hu activity (Graus et al., 1997, Moll et al., 1995). Recombinant human HuD (98% identical to the mouse protein (Abe et al., 1994)) was subjected to SDS-PAGE and transferred to blots which were probed with the collected samples (1:250 dilution). Amongst the 71 SCLC-prone mice that were infected with Adeno-Cre virus, 19 showed detectable anti-HuD reactivity (27%) at a dilution of 1:250 (Table 1, Fig. 2A). Four out of 41 control mice (10%) showed reactivity as well. Next, the titers were measured based on the highest fold dilution at which a band remained detectable on the immunoblot (Fig. 2B). Using the highest titer at which the four control mice remained positive (1:5000) as a cutoff value for background reactivity (Fig. 2A, dashed line), ten SCLC-prone mice (14%) were considered highly positive (all of these showed titers ≥1:20,000).
Fig. 2.
Anti-Hu titer of control mice and Adeno-Cre infected SCLC-prone mice by Western blot analysis. A) Ten SCLC-prone mice have higher titers of anti-Hu reactivity than untreated controls as determined by the highest fold dilution of plasma/serum in which there was a positive Western blot signal against HuD (human anti-Hu sera react with HuB, HuC, and HuD, which are highly conserved). The medians of the two groups were significantly different (p=0.0239). The dashed line shows the highest titer in the control mice (positive in 1:5000 dilution). B) Representative examples (cropped images) of titer analysis in four SCLC-prone that showed anti-Hu reactivity at a plasma/serum dilution of (from top row to bottom row) 1:2500, 1:5000, 1:20,000 or 1:80,000.
Pattern of anti-Hu reactivity against Hu protein family and HuD deletion constructs
Human anti-Hu sera react with HuB, HuC, and HuD, which are highly conserved. Of these three, HuD is the most commonly expressed neuronal Hu protein in human SCLC tumors (Manley et al., 1995). In those mice showing positive anti-HuD reactivity, we examined the reactivity profile against the Hu protein family. All anti-HuD positive samples from control mice and SCLC-prone mice showed reactivity against HuB and HuC, with variable responses to the three proteins (Supplementary Fig. S1). No reactivity was observed against the ubiquitously expressed HuR.
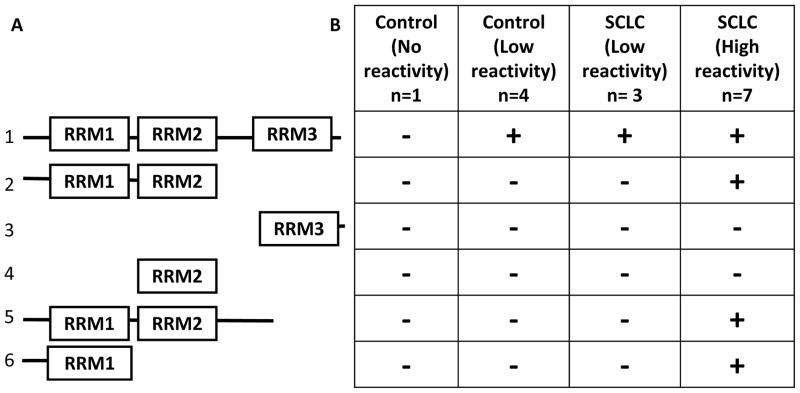
To further characterize our model in relation to previous studies, we examined the pattern of reactivity against HuD deletion constructs in SCLC-prone and control mice (Fig. 3). Hu proteins are composed of three RNA recognition motifs: RRM1, 2, and 3 (Fig. 3A, line 1). A presumably unstructured N-terminal region precedes RRM1, and RRM2 and 3 are separated by a hinge region. We studied five control mice, including the four mice with background anti-Hu reactivity, and ten anti-Hu positive SCLC-prone mice, seven of which were highly positive (titer ≥1:20,000). The highly positive SCLC-prone mice showed consistent reactivity against constructs containing the RRM1 region (Fig. 3B), whereas none of the SCLC-prone mice exhibiting low reactivity or control mice showed reactivity against the HuD deletion constructs.
Fig. 3.
Reactivity against HuD fragments. A) Diagram of fragments used, indicating the RNA recognition motif (RRM) domains. Full-length HuD (line 1) served as the positive control. B) Blots of recombinant human HuD deletion constructs were probed with 1:500 dilutions of mouse plasma/serum, a titer at which the positive plasma/serum reacted with the full length protein. Consistent reactivity against RRM1, RRM1+2, and RRM1+2+hinge were observed in SCLC-prone mice with high reactivity (titer ≥ 20,000). No control mice or SCLC-prone with low reactivity showed a response against these domains, even when they reacted against full-length HuD.
Elevated anti-Hu reactivity appears to precede clinical detection of SCLC
To gain an understanding of the timing with which anti-Hu reactivity develops in relationship to the cancer, the anti-Hu titer of bleeds obtained at different times from highly positive SCLC-prone mice was measured. We observed that once a mouse exhibited positive reactivity at one bleed, all subsequent bleeds remained positive, although some variability in titers was seen (Fig. 4).
Fig. 4.
Timing of anti-Hu reactivity relative to SCLC detection. Anti-HuD reactivity was detected 40–100 days prior to clinical detection (radiological diagnosis or signs of sickness) of SCLC. Graphs from the six highly positive mice for which multiple bleeds were available are shown. The dotted line represents the date of first clinical detection of tumor by CT scan and/or luminescence detection. The titer was determined by the highest fold dilution of plasma or serum in which there was a positive Western blot signal against HuD. The time (in days) between the measurement of a titer above background and clinical detection is indicated by a bar. SCLC tumor was not detected in mouse 489556, though the mouse became very sick. Mouse 509631 was sacrificed at the time of high titer detection, and showed no signs of illness. The lungs of this animal showed neuroendocrine lesions in situ, but cancer was not detected. Crosses indicate dates of sacrifice. Titer values are in thousands.
We next asked whether anti-Hu reactivity was present prior to clinical detection of SCLC by comparing the date anti-Hu reactivity was first detected to the date of clinical tumor detection. It is worth noting that the sensitivity of detection of SCLC by bioluminescence is limited due to the central localization of the tumors. However, this is to date the most sensitive high-throughput non-invasive detection method available to detect tumors smaller than 1 cm3, and allowed us to detect tumors 1–5 weeks before the clinical symptoms became evident. Anti-Hu reactivity became detectable 40–100 days prior to the first clinical diagnosis of lung cancer (Fig. 4, dotted line). Examples of images taken before and after cancer detection in mouse 484305 are shown in Fig. 5A and B, and average radiance values from the bioluminescence detection were calculated (Fig. 5C). Changes in weight were also noted (Fig. 5D). This mouse became highly anti-Hu positive (titer ≥20,000) at 180 days after Adeno-Cre infection. Cancer was detected 76 days later. One highly anti-Hu positive mouse that showed obvious discomfort (weight loss and difficulty breathing) was euthanized, but autopsy did not reveal any underlying lung cancer (Fig. 4, mouse 509631, see Table 1). At the time of death, the titer of this mouse appeared to have decreased, but the last bleed before sacrifice (day 300) was consistently positive for all dilutions tested (1:5000, 1:10,000, 1:20,000, 1:40,000 and 1:80,000). Another mouse (509631) that developed a high titer anti-Hu response but showed no other symptoms was sacrificed, and its lungs were analyzed. Hyperplasia was observed, but no overt cancer. Mice were not examined for evidence of neurological disease in this study.
Fig. 5.
Image-based screening of SCLC-prone mice. LucR; p53F/F; RbF/F mice were infected with adenovirus carrying the Cre recombinase gene via intratracheal instillation. After induction, mice were monitored for tumor formation and disease, using bioluminescence detection (A, B, left panels, and C), X-ray based CT-scan (A and B, right panels), and clinical symptoms like weight loss (D), altered coat, or kyphosis. The figure shows the follow up of mouse 484305 (Fig. 4) which showed anti-Hu reactivity with a titer of 80,000 at 180 days (arrows) after Adeno-Cre infection. At that time-point, tumor was not detectable by imaging techniques (images taken 203 and 256 days after Adeno-Cre infection) (A). Tumor became detectable 256 days after Adeno-Cre infection (B). Acute weight loss was observed 286 days after Adeno-Cre infection (D). Open circles, heart. Arrowheads, tumor mass.
4. Discussion
We have provided data showing that, like human SCLC, the cancers induced in the SCLC mouse model express Hu proteins (Fig. 1). Using background reactivity to set a threshold above which serum/plasma can be considered highly anti-Hu positive, 15–22% of human SCLC patients show true anti-Hu reactivity (Dalmau et al., 1990, Graus et al., 1997, Monstad et al., 2004, Tsou et al., 2009, Verschuuren et al., 1999). This is similar to the 14% of SCLC mice that show above background anti-Hu response in our analysis (Fig. 2A, Table 1). The pattern of reactivity against the Hu protein family (Supplementary Fig. S1) is similar to that seen in human SCLC ((Manley et al., 1995), unpublished data). Our data are also in agreement with previous studies (Manley et al., 1995, Sillevis Smitt et al., 1996, Sodeyama et al., 1999) indicating that the N-terminal part of the protein may play a key role in triggering anti-Hu reactivity (Fig. 3). Because individual RRM domains have highly conserved features, it is possible that the response seen in the control animals and in weakly positive SCLC-prone mice is due to combined weak reactivity against more than one RRM. This (very weak) positive reaction may be lost when fewer RRMs are presented in the context of deletion mutants. In contrast, reactivity in the strongly positive SCLC-prone mice appears to be localized to the N-terminal part of the protein, containing the RRM1 domain. Thus, the SCLC-prone mouse model system not only simulates the molecular features of human SCLC, but also recapitulates the immune response. The striking similarity suggests that a similar mechanism underlies anti-Hu response in both cases and supports the utility of this mouse model to study SCLC-related autoimmunity.
Our data suggest that an immune response to Hu proteins may arise when the cancer is small, asymptomatic, and not yet readily detectable by imaging modalities (Figs. 4 and 5). If anti-Hu antibodies are indeed present at early stages of the cancer, this might provide an opportunity for early detection. Among lung cancer histological subtypes, SCLC is the most lethal. For such highly malignant tumors, the question of early detection appears particularly daunting. If SCLCs are small at metastasis, there may be little opportunity to detect them early. One potential advantage of antibody-based detection is that even small tumors may trigger an immune response, and hence, anti-tumor antibodies might be detectable at a stage prior to rapid disease progression. A limitation of using anti-Hu response as an early detection test for SCLC would be its low sensitivity; anti-Hu antibodies are present in less than one quarter of human SCLC patients. However, SCLC is associated with a number of different autoimmune responses, such as those against Nova1 and voltage-gated calcium channels (Darnell and Posner, 2003). The SCLC mouse model can be used to determine whether other SCLC-associated antibodies are present in these animals and when they appear relative to clinically detectable cancer (such experiments should include a large number of mice, with detailed imaging followed by euthanasia and pathological examination when antibodies first become detectable). Examination of these autoantigens may allow development of an antigen panel with much higher sensitivity than could be achieved with one autoantigen. If such a panel were developed, much further work would be required. Among others, large population-based studies would be needed in at-risk individuals, such as heavy smokers, to determine the appropriate time to conduct early detection tests.
The presence of anti-Hu and other SCLC-associated autoantibodies in patients has been correlated with more indolent tumor growth, complete response to therapy, and longer survival in some studies (Tsou et al., 2009, Aguirre-Cruz et al., 2005, Dalmau et al., 1995, Dalmau et al., 1992, Graus et al., 1997, Sillevis Smitt et al., 2002, Maddison and Lang, 2008). One possibility is that the immune response limits tumor growth. The most extreme example is provided by those PEM/SN patients who have very high titers of antibodies but small or virtually undetectable SCLC lesions that are only identifiable at autopsy (Dalmau et al., 1992). Thus, it is conceivable that even a modest anti-Hu response might be protective. We did not see a survival benefit in our exploratory analysis of survival of SCLC mice (Supplemental Fig. S2). However, our sample size was very small. Power analyses indicate that over 180 mice would be needed to observe a significant difference in survival in relation to anti-Hu response. Thus, further studies with larger numbers of mice will be needed.
In our analysis, one mouse appeared sick and showed high anti-Hu reactivity, but no underlying SCLC was observed (Fig. 4, mouse 489556). It is possible that this particular mouse could have been suffering from PEM/SN with a very small, undetectable tumor. We did not incorporate any neurological tests into the evaluation of the SCLC-prone mice. Considering that in humans, less than one percent of SCLC patients develop PEM/SN (Anderson et al., 1987), a larger number of animals will be needed determine if some mice develop PEM/SN.
Our studies demonstrate the applicability of a SCLC mouse model system to the study of immunological events that occur during SCLC development and that are likely to drive the development of paraneoplastic disease. Future studies using this model promise to provide insight into the prognostic value of antibodies against Hu proteins and other SCLC-related autoantigens, the possible use of SCLC-related autoantibodies as tools for early SCLC detection, and perhaps even for SCLC immunotherapy strategies.
Supplementary Material
Representative examples (cropped images) of reactivity against Hu proteins in control and SCLC-prone mice with variable anti-HuD positivity. Blots of recombinant human Hu proteins were probed with a 1:250 dilution of mouse plasma/serum. The titer was determined by the highest fold dilution of plasma or serum in which there was a positive Western blot signal against HuD in a five minute exposure time. Reactivity is only detectable against neuronal Hu proteins (HuB, HuC, and HuD). Different exposure times with similar signal intensity are shown. Note that exposure times for plasma from the control mice (3 minutes 45 seconds; 2 minutes 30 seconds) are much longer than for anti-Hu positive SCLC-prone mice (1 minute 15 seconds; 25 seconds). The titer was determined by the highest fold dilution of plasma or serum in which there was a positive Western blot signal against HuD. Coomassie gel shows equal loading of proteins. NA= not applicable.
Although the number of SCLC-prone mice in the current study was too low to provide statistically significant data, preliminary analysis of survival probability distributions was performed for three groups of Adeno-Cre infected mice: no detectable anti-Hu reactivity (n=52); intermediate reactivity (1:250–1:5000; n=9); and high reactivity (>1:5000, n=10). Mice that displayed no anti-Hu reactivity had the shortest median survival time of 274.5 days, while mice at intermediate anti-Hu reactivity had a median survival of 319.0 days. Mice with high levels of anti-Hu reactivity had the longest median survival (328.0 days). While these results were suggestive, they were not significant; testing for equality over the three strata of anti-Hu reactivity yielded an overall log-rank p-value of 0.59.
Acknowledgments
The authors would like to thank the members of the Laird and Laird-Offringa labs for helpful discussions and Paul Anglim for reviewing the manuscript. We acknowledge John Zevenhoven for technical assistance with immunohistochemistry, and Ji-Ying Song for pathology advice. This work was supported by a generous donation by Eri and Mary Lou Mettler to the ILO laboratory and NCI CCSG grant number 5 P30 CA014089. Part of this work was supported by Dutch Cancer Society (J.C.). None of the funding persons or agencies had any influence on the experimental design, analysis, or decision to publish. None of the authors have a conflict of interest related to the current study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ABE R, UYENO Y, YAMAMOTO K, SAKAMOTO H. Tissue-specific expression of the gene encoding a mouse RNA binding protein homologous to human HuD antigen. DNA Res. 1994;1:175–80. doi: 10.1093/dnares/1.4.175. [DOI] [PubMed] [Google Scholar]
- AGUIRRE-CRUZ L, CHARUEL JL, CARPENTIER AF, BENYAHIA B, DELATTRE JY, MUSSET L. Clinical relevance of non-neuronal auto-antibodies in patients with anti-Hu or anti-Yo paraneoplastic diseases. J Neurooncol. 2005;71:39–41. doi: 10.1007/s11060-004-4536-3. [DOI] [PubMed] [Google Scholar]
- ANDERSON NE, CUNNINGHAM JM, POSNER JB. Autoimmune pathogenesis of paraneoplastic neurological syndromes. Crit Rev Neurobiol. 1987;3:245–99. [PubMed] [Google Scholar]
- DALMAU J, FURNEAUX HM, GRALLA RJ, KRIS MG, POSNER JB. Detection of the anti-Hu antibody in the serum of patients with small cell lung cancer--a quantitative western blot analysis. Ann Neurol. 1990;27:544–52. doi: 10.1002/ana.410270515. [DOI] [PubMed] [Google Scholar]
- DALMAU J, FURNEAUX HM, ROSENBLUM MK, GRAUS F, POSNER JB. Detection of the anti-Hu antibody in specific regions of the nervous system and tumor from patients with paraneoplastic encephalomyelitis/sensory neuronopathy. Neurology. 1991;41:1757–64. doi: 10.1212/wnl.41.11.1757. [DOI] [PubMed] [Google Scholar]
- DALMAU J, GRAUS F, CHEUNG NK, ROSENBLUM MK, HO A, CANETE A, DELATTRE JY, THOMPSON SJ, POSNER JB. Major histocompatibility proteins, anti-Hu antibodies, and paraneoplastic encephalomyelitis in neuroblastoma and small cell lung cancer. Cancer. 1995;75:99–109. doi: 10.1002/1097-0142(19950101)75:1<99::aid-cncr2820750117>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- DALMAU J, GRAUS F, ROSENBLUM MK, POSNER JB. Anti-Hu--associated paraneoplastic encephalomyelitis/sensory neuronopathy. A clinical study of 71 patients. Medicine (Baltimore) 1992;71:59–72. doi: 10.1097/00005792-199203000-00001. [DOI] [PubMed] [Google Scholar]
- DALMAU J, POSNER JB. Neurologic paraneoplastic antibodies (anti-Yo; anti-Hu; anti-Ri): the case for a nomenclature based on antibody and antigen specificity. Neurology. 1994;44:2241–6. doi: 10.1212/wnl.44.12.2241. [DOI] [PubMed] [Google Scholar]
- DARNELL RB, POSNER JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349:1543–54. doi: 10.1056/NEJMra023009. [DOI] [PubMed] [Google Scholar]
- GOOD PJ. A conserved family of elav-like genes in vertebrates. Proc Natl Acad Sci U S A. 1995;92:4557–61. doi: 10.1073/pnas.92.10.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAUS F, CORDON-CARDO C, POSNER JB. Neuronal antinuclear antibody in sensory neuronopathy from lung cancer. Neurology. 1985;35:538–43. doi: 10.1212/wnl.35.4.538. [DOI] [PubMed] [Google Scholar]
- GRAUS F, DALMOU J, RENE R, TORA M, MALATS N, VERSCHUUREN JJ, CARDENAL F, VINOLAS N, GARCIA DEL MURO J, VADELL C, MASON WP, ROSELL R, POSNER JB, REAL FX. Anti-Hu antibodies in patients with small-cell lung cancer: association with complete response to therapy and improved survival. J Clin Oncol. 1997;15:2866–72. doi: 10.1200/JCO.1997.15.8.2866. [DOI] [PubMed] [Google Scholar]
- GRAUS F, ELKON KB, CORDON-CARDO C, POSNER JB. Sensory neuronopathy and small cell lung cancer. Antineuronal antibody that also reacts with the tumor. Am J Med. 1986;80:45–52. doi: 10.1016/0002-9343(86)90047-1. [DOI] [PubMed] [Google Scholar]
- GRAUS F, KEIME-GUIBERT F, RENE R, BENYAHIA B, RIBALTA T, ASCASO C, ESCARAMIS G, DELATTRE JY. Anti-Hu-associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain. 2001;124:1138–48. doi: 10.1093/brain/124.6.1138. [DOI] [PubMed] [Google Scholar]
- HENSON RA, URICH H. Cancer in the nervous system. Oxford, England: Blackwell Scientific; 1982. [Google Scholar]
- HORWICH MS, CHO L, PORRO RS, POSNER JB. Subacute sensory neuropathy: a remote effect of carcinoma. Ann Neurol. 1977;2:7–19. doi: 10.1002/ana.410020103. [DOI] [PubMed] [Google Scholar]
- KING PH. Differential expression of the neuroendocrine genes Hel-N1 and HuD in small-cell lung carcinoma: evidence for down-regulation of HuD in the variant phenotype. Int J Cancer. 1997;74:378–82. doi: 10.1002/(sici)1097-0215(19970822)74:4<378::aid-ijc3>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- LYONS SK, MEUWISSEN R, KRIMPENFORT P, BERNS A. The generation of a conditional reporter that enables bioluminescence imaging of Cre/loxP-dependent tumorigenesis in mice. Cancer Res. 2003;63:7042–6. [PubMed] [Google Scholar]
- MADDISON P, LANG B. Paraneoplastic neurological autoimmunity and survival in small-cell lung cancer. J Neuroimmunol. 2008;201–202:159–62. doi: 10.1016/j.jneuroim.2008.05.024. [DOI] [PubMed] [Google Scholar]
- MANLEY GT, SMITT PS, DALMAU J, POSNER JB. Hu antigens: reactivity with Hu antibodies, tumor expression, and major immunogenic sites. Ann Neurol. 1995;38:102–10. doi: 10.1002/ana.410380117. [DOI] [PubMed] [Google Scholar]
- MEUWISSEN R, LINN SC, LINNOILA RI, ZEVENHOVEN J, MOOI WJ, BERNS A. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell. 2003;4:181–9. doi: 10.1016/s1535-6108(03)00220-4. [DOI] [PubMed] [Google Scholar]
- MEUWISSEN R, LINN SC, VAN DER VALK M, MOOI WJ, BERNS A. Mouse model for lung tumorigenesis through Cre/lox controlled sporadic activation of the K-Ras oncogene. Oncogene. 2001;20:6551–8. doi: 10.1038/sj.onc.1204837. [DOI] [PubMed] [Google Scholar]
- MOLL JW, ANTOINE JC, BRASHEAR HR, DELATTRE J, DRLICEK M, DROPCHO EJ, GIOMETTO B, GRAUS F, GREENLEE J, HONNORAT J, et al. Guidelines on the detection of paraneoplastic anti-neuronal-specific antibodies: report from the Workshop to the Fourth Meeting of the International Society of Neuro-Immunology on paraneoplastic neurological disease, held October 22–23, 1994, in Rotterdam, The Netherlands. Neurology. 1995;45:1937–41. doi: 10.1212/wnl.45.10.1937. [DOI] [PubMed] [Google Scholar]
- MONSTAD SE, DRIVSHOLM L, STORSTEIN A, AARSETH JH, HAUGEN M, LANG B, VINCENT A, VEDELER CA. Hu and voltage-gated calcium channel (VGCC) antibodies related to the prognosis of small-cell lung cancer. J Clin Oncol. 2004;22:795–800. doi: 10.1200/JCO.2004.01.028. [DOI] [PubMed] [Google Scholar]
- PARK-LEE S, KIM S, LAIRD-OFFRINGA IA. Characterization of the interaction between neuronal RNA-binding protein HuD and AU-rich RNA. J Biol Chem. 2003;278:39801–8. doi: 10.1074/jbc.M307105200. [DOI] [PubMed] [Google Scholar]
- PARK S, MYSZKA DG, YU M, LITTLER SJ, LAIRD-OFFRINGA IA. HuD RNA recognition motifs play distinct roles in the formation of a stable complex with AU-rich RNA. Mol Cell Biol. 2000;20:4765–72. doi: 10.1128/mcb.20.13.4765-4772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSNER JB, DALMAU J. Paraneoplastic syndromes. Curr Opin Immunol. 1997;9:723–9. doi: 10.1016/s0952-7915(97)80055-6. [DOI] [PubMed] [Google Scholar]
- RIES LAG, MELBERT D, KRAPCHO M. SEER Cancer Statistics Review, 1975–2004. National Cancer Institute; 2007. [Google Scholar]
- SANDLER AB. Chemotherapy for small cell lung cancer. Semin Oncol. 2003;30:9–25. doi: 10.1053/sonc.2003.50012. [DOI] [PubMed] [Google Scholar]
- SILLEVIS SMITT P, GREFKENS J, DE LEEUW B, VAN DEN BENT M, VAN PUTTEN W, HOOIJKAAS H, VECHT C. Survival and outcome in 73 anti-Hu positive patients with paraneoplastic encephalomyelitis/sensory neuronopathy. J Neurol. 2002;249:745–53. doi: 10.1007/s00415-002-0706-4. [DOI] [PubMed] [Google Scholar]
- SILLEVIS SMITT P, MANLEY G, DALMAU J, POSNER J. The HuD paraneoplastic protein shares immunogenic regions between PEM/PSN patients and several strains and species of experimental animals. J Neuroimmunol. 1996;71:199–206. doi: 10.1016/s0165-5728(96)00153-1. [DOI] [PubMed] [Google Scholar]
- SODEYAMA N, ISHIDA K, JAECKLE KA, ZHANG L, AZUMA A, YAMADA M, MIZUSAWA H, WADA Y. Pattern of epitopic reactivity of the anti-Hu antibody on HuD with and without paraneoplastic syndrome. J Neurol Neurosurg Psychiatry. 1999;66:97–9. doi: 10.1136/jnnp.66.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOWBIN H, STAEHELIN T, GORDON J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSOU JA, KAZARIAN M, PATEL A, GALLER JS, LAIRD-OFFRINGA IA, CARPENTER CL, LONDON SJ. Low level anti-Hu reactivity: A risk marker for small cell lung cancer? Cancer Detect Prev. 2009;32:292–9. doi: 10.1016/j.cdp.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERSCHUUREN JJ, PERQUIN M, TEN VELDE G, DE BAETS M, VRIESMAN PB, TWIJNSTRA A. Anti-Hu antibody titre and brain metastases before and after treatment for small cell lung cancer. J Neurol Neurosurg Psychiatry. 1999;67:353–7. doi: 10.1136/jnnp.67.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLTZ R, DALMAU J, POSNER JB, ROSENFELD MR. T-cell receptor analysis in anti-Hu associated paraneoplastic encephalomyelitis. Neurology. 1998;51:1146–50. doi: 10.1212/wnl.51.4.1146. [DOI] [PubMed] [Google Scholar]
- WORDEN FP, KALEMKERIAN GP. Therapeutic advances in small cell lung cancer. Expert Opin Investig Drugs. 2000;9:565–79. doi: 10.1517/13543784.9.3.565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative examples (cropped images) of reactivity against Hu proteins in control and SCLC-prone mice with variable anti-HuD positivity. Blots of recombinant human Hu proteins were probed with a 1:250 dilution of mouse plasma/serum. The titer was determined by the highest fold dilution of plasma or serum in which there was a positive Western blot signal against HuD in a five minute exposure time. Reactivity is only detectable against neuronal Hu proteins (HuB, HuC, and HuD). Different exposure times with similar signal intensity are shown. Note that exposure times for plasma from the control mice (3 minutes 45 seconds; 2 minutes 30 seconds) are much longer than for anti-Hu positive SCLC-prone mice (1 minute 15 seconds; 25 seconds). The titer was determined by the highest fold dilution of plasma or serum in which there was a positive Western blot signal against HuD. Coomassie gel shows equal loading of proteins. NA= not applicable.
Although the number of SCLC-prone mice in the current study was too low to provide statistically significant data, preliminary analysis of survival probability distributions was performed for three groups of Adeno-Cre infected mice: no detectable anti-Hu reactivity (n=52); intermediate reactivity (1:250–1:5000; n=9); and high reactivity (>1:5000, n=10). Mice that displayed no anti-Hu reactivity had the shortest median survival time of 274.5 days, while mice at intermediate anti-Hu reactivity had a median survival of 319.0 days. Mice with high levels of anti-Hu reactivity had the longest median survival (328.0 days). While these results were suggestive, they were not significant; testing for equality over the three strata of anti-Hu reactivity yielded an overall log-rank p-value of 0.59.