Abstract
Background
Persons with heart failure (HF) have significantly lower sleep quantity and quality than persons without HF. The purpose of this article is to propose a conceptual model describing how poor sleep may contribute to inadequate self-care and untoward outcomes in persons with HF.
Aims
Our overarching hypothesis is that sleep affects self-care and outcomes through its effects on cognition. Building on the work of others, we outline a conceptual model that illustrates that even transient sleep disruption prevents sleep-related restorative processes and contributes to cognitive dysfunction—especially in the 25–50% of HF patients with existing cognitive impairment. Poor sleep may be sufficient to impair cognition to a level that interferes with higher order functions involved in effective HF self-care practices. Through these mechanisms, inadequate sleep may contribute to poor outcomes such as low health-related quality of life and greater risk of unplanned hospitalization.
Conclusion
The proposed model (1) bridges physical, neuropsychological and behavioral phenomena, (2) suggests a mechanism by which poor sleep affects daytime behavior, and (3) is empirically testable. Exploring factors that interfere with sleep may improve self-care and outcomes in persons with HF.
Keywords: self-care, self-management, patient compliance, cognition disorders, sleep disorders, aging, theory
1. Introduction
It is well-established that a significant segment of the heart failure (HF) population experiences frequent episodes of congestion, recurring unplanned hospitalizations, and poor health-related quality of life (HRQL) [1, 2]. Others have proposed that poor sleep and daytime sleepiness may contribute to these outcomes [3–6]. A recent Institute of Medicine report calls attention to the devastating effects of excessive daytime sleepiness, including potential problems with treatment adherence—a key component of self-care [7]. Mechanisms explaining how excessive daytime sleepiness may impede self-care and influence health outcome in the HF population have neither been proposed nor tested.
Accordingly, the purpose of this article is to describe a conceptual model of how poor sleep may contribute to self-care and outcomes in persons with HF. We suggest that poor sleep may impair cognition sufficiently to interfere with self-care [8]. In particular, it may accentuate the often subtle cognitive problems known to exist in persons with HF [9]. In turn, poor self-care may impair HRQL and contribute to symptom burden and decreased functional capacity. Frequent and persistent symptoms and impaired functioning then increase the risk of unplanned hospitalizations. Assuredly, the progressive nature of HF and aging are major contributors to poor outcomes, but in this article we suggest that efforts to improve sleep may be one way to increase patients’ abilities to concentrate. Better cognition may promote HF self-care, especially in HF patients with preexisting cognitive impairment. Better HF self-care may decrease symptoms, improve functioning and HRQL, and decrease unplanned hospitalizations.
The proposed conceptual model is theoretical at this point. Our hope is that by sharing our depiction of possible relationships, we may stimulate others to think about these issues and contribute to the growing science of sleep and HF self-care.
2. Definitions
Sleep is a state of perceptual or conscious unresponsiveness, lessened movement of the skeletal muscles, and slowed metabolism. Many people experience excessive daytime sleepiness when sleep is inadequate in quantity or quality. Excessive daytime sleepiness is manifested in a subjective and/or objective manner. Subjective daytime sleepiness refers to the feeling of drowsiness with a tendency to nap [10]. Objective sleepiness is defined as an impaired ability to sustain attention and respond quickly to stimuli [11]. Fatigue, despite being a common symptom of HF, is only weakly associated with daytime sleepiness [12]. Fatigue refers to a temporary feeling of weariness, tiredness, or lack of energy that usually resolves with rest [13] while excessive daytime sleepiness typically reflects chronic sleep loss.
Self-care refers to the behaviors that people use to maintain their health (self-care maintenance) and the decisions they make about symptoms when they occur (self-care management) [14]. Treatment adherence alone is not sufficient to capture the complexity involved in the decisions that patients make during self-care. Self-care does not just involve following directions. Self-care decisions require cognitive or mental processes used to gain knowledge as well as comprehension, including thinking, knowing, remembering, judging, and problem solving. The set of abilities that control and regulate other abilities and behaviors (e.g., planning, decision-making, and thinking) are referred to as higher order executive functions.
The outcomes most problematic in persons with HF include escalating symptoms, severe functional limitations, acute hospitalization for episodes of congestion, impaired HRQL, and early death. HRQL is defined as the perception of the impact of an illness and its treatment on physical, emotional, social, and economic aspects of daily life [15].
3. What is Known about Sleep?
Sleep is a dynamic state during which brain activity is coordinated by subcortical structures and mediated by neurotransmitters [16]. Humans sleep in a consolidated bout, which if disturbed, results in problems during waking hours. While all functions of sleep are not as yet fully understood, sleep appears to (1) restore energy and well-being [17], (2) promote learning and consolidation of memory [16], and (3) bolster immune function [18]. Time asleep is less important than the quality of sleep obtained. Poor sleep quality adversely influences well-being by negatively affecting psychosocial, physical and occupational functioning. Overall, poor sleep quality causes mood disturbance, cognitive inefficiency, motor impairment, social discomfort, nonspecific physical ailments, reduced productivity, and poor HRQL [5, 6, 19–21].
Numerous studies have demonstrated the important cognitive functions of sleep: memory encoding, memory consolidation, brain plasticity, and memory reconsolidation [16]. Memory encoding involves receiving or registering stimuli through the senses. In memory consolidation, short-term memories are converted into long-term memory. Brain plasticity refers to the capacity of the brain to modify the organization of neuronal networks in response to particular experiences. Reconsolidation refers to a process of restabilizing an established memory [22]. Through these processes, short-term memories are converted into long-term memories for later retrieval and use. Interestingly, different types of memories are reconsolidated during different sleep states. For example, physical skills or motor learning occurs after a night of sleep following skills training. Declarative memory for events and facts are reconsolidated during early nocturnal sleep. Emotional memory or the positive or negative affect associated with specific stimuli is reconsolidated during late sleep when REM (rapid eye movement) sleep predominates [17].
4. Conceptual Model
Conceptual models describe a system of relationships between and among concepts. These relationships are often based on assumptions about interrelationships and system boundaries. As such, conceptual models provide a mental image of a process. Convincing conceptual models provide a written and graphic representation of the ordering of the concepts representing a specific phenomenon that can be used by others to think about and test the phenomenon.
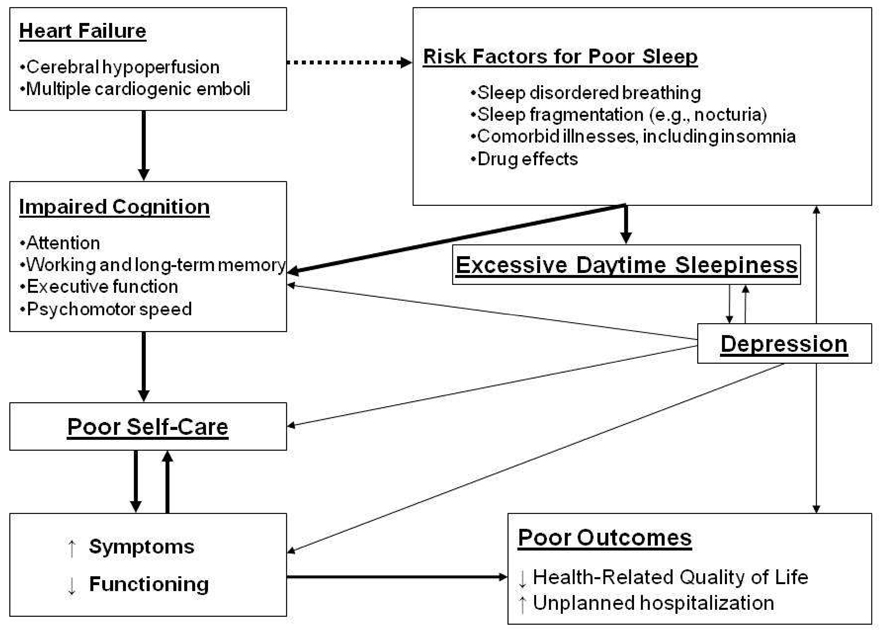
Our model (Figure 1) represents the relationships among HF, poor sleep, excessive daytime sleepiness, cognition, self-care and health outcomes. Our model extends previous work by Trupp and Corwin [23] and Beebe and Gozal [24] by moving the focus beyond sleep disordered breathing (SDB) to address the multitude of other factors that may contribute to poor sleep in persons with HF. Trupp and Corwin [23] described how the repetitive cycles of hypoxia-reoxygenation that occur with SDB activate the sympathetic nervous system and interfere with sleep. They linked poor sleep with fatigue, cognitive impairment, and treatment adherence. The strength of their model was the inclusion of treatment adherence. However, their focus on SDB is limiting.
Figure 1.
Conceptual Model of How Poor Sleep Influences Self-Care and Outcomes in Persons with Heart Failure
Two known effects of chronic heart failure are disturbed sleep and impaired cognition. Many other factors accentuate the sleep problems experienced by the majority of this patient population. These factors further impair cognitive functioning and can cause excessive daytime sleepiness. Early evidence suggests that heart failure patients with impaired cognition find self-care difficult. Poor self-care is associated with an increase in symptoms and impaired functioning, which may predispose patients to poor long-term outcomes and independently contribute to impaired cognition. Depression, common in persons with heart failure, independently contributes to many of the concepts in this model.
In the conceptual model by Beebe and Gozal [24], the sleep disruption and blood gas abnormalities associated with obstructive sleep apnea were proposed to induce central nervous system cellular injury and cortical dysfunction, resulting in maladaptive daytime behaviors. Strengths of their model included a description of the mechanisms by which prefrontal cortical dysfunction occurs and a detailed account of the specific effects on cognitive functioning. The behavioral effects specified focus primarily on those comprising executive function.
Our model builds on this prior work by (1) focusing on the factors other than SDB that may impair sleep in persons with HF, (2) emphasizing the contribution of sleep to daily functioning, including self-care, (3) including both short-term and long-term outcomes that are important to patients, clinicians, and society, and (4) separating out the unique contribution of depression to many of the components in the model.
5. Heart Failure as a Cause of Impaired Cognition
Impaired cognition has been documented in 25–50% of persons with HF [9]. The dimensions of cognition impaired in persons with HF and in those experiencing sleep deprivation overlap: attention; working memory; long-term memory; executive functioning; and psychomotor speed [25, 26]. In HF, the reasons for cognitive impairment are not yet understood, but the major hypotheses are that cerebral hypoperfusion and multiple cardiogenic emboli affect regions of the brain involved in higher order functioning [27]. Chronic hypotension [28] and low cardiac output can be understood as potential contributors to impaired cognition because of inadequate brain perfusion [29]. Multiple cardiogenic emboli may be associated with dysrhythmic events and with low cardiac output [27].
6. Risk Factors for Poor Sleep
Excessive daytime sleepiness can usually be attributed to one of four causes (Figure 2): (1) qualitative or quantitative sleep deficiencies resulting from sleep loss or fragmentation; (2) drug effects; (3) central nervous system diseases; and (4) circadian rhythm disruption [30]. All of these factors, except perhaps jet lag, routinely contribute to poor sleep in persons with HF. Those causes most amenable to intervention (e.g., sleep deficiencies and drug effects) are the focus of this conceptual model.
Figure 2.
Causes of Excessive Daytime Sleepiness
6.1 Sleep Disordered Breathing
Persons with HF commonly have SDB [31], defined as an apnea-hypopnea index (number of apnea and hypopnea events/hour of sleep) ≥5 [32]. Obstructive sleep apnea is found in up to 40% of adults with HF [33]. Central sleep apnea is thought to be even more common in the HF population [31]. A recent study conducted in HF patients confirmed to be treated with evidence-based therapies found that 61% of the sample had SBD; about half of these 61% had central and half had obstructive SDB [34]. Many more HF patients with SDB may be undiagnosed. Persons with HF and SDB have shorter total sleep times, markedly reduced sleep efficiency, significantly longer sleep onset latency and wake time after sleep onset, and significantly shorter sleep time compared with those without HF [35]. Sleep efficiency is the ratio of time spent asleep to the amount of time spent in bed. Sleep onset latency is the length of time that it takes to go from full wakefulness to sleep. A sleep onset latency of less than five minutes suggests sleep deprivation.
Obstructive sleep apnea is the result of occlusion of the upper airway caused by relaxation of the oropharyngeal muscles. Aging and obesity are two major risk factors for obstructive sleep apnea. Central sleep apnea, technically a central nervous system disease, is characterized as a temporary failure in breathing rhythm resulting in the loss of ventilatory effort lasting at least 10 seconds. Failure to breath causes retention of carbon dioxide and acidosis, which stimulates abnormally deep and rapid breathing, alkalosis, and hypocapnia, which causes another bout of apnea and the cycle repeats [36].
6.2 Sleep Fragmentation
Numerous factors interrupt sleep in the HF population, but interrupting sleep to void is one of the most common causes of disrupted sleep in elders [37]. In HF, nocturia is commonly caused by diuretics.
Nocturnal dyspnea is another cause of sleep fragmentation [38]. When someone with HF assumes a supine position, within a brief period, airway resistance increases [37], and vascular engorgement and cardiac enlargement expand the chest wall, causing dyspnea and increased work of breathing. Those who experience paroxysmal nocturnal dyspnea (PND) report feeling unable to breath and rise to open the window for fresh air, which further interrupts sleep.
Other contributors to sleep fragmentation are, age-related changes in sleep efficiency, comorbid illnesses, excess body weight, poor exercise patterns, alcohol use, chronic insomnia, and stress [39]. Of note, when somatic and mental health are taken into account, the impact of age on sleep is almost eliminated [40].
6.3 Comorbid Illnesses
Comorbid illnesses contribute to sleep loss and fragmentation in persons with HF [41]. Several illnesses that are common comorbid diagnoses in those with HF are associated with poor sleep including diabetes, chronic obstructive pulmonary disease, nasal problem, thyroid disease, stroke, and arthritis [42]. Depression is common and impaired sleep is a primary diagnostic indicator of depression [43]. Most HF patients are elderly, and insomnia is common in older people [44]. In a survey of elders, pain at night at least three times per week and wheezing or whistling from chest at night were associated with excessive daytime sleepiness [45].
6.4 Drug Effects
One common but frequently overlooked contributor to poor sleep is the medication regimen including drugs commonly prescribed for HF [46, 47]. Beta-blockers, for example, are associated with impaired sleep, perhaps by causing a decrease in nocturnal production of melatonin [17]. Polypharmacy, common in HF, increases the likelihood of taking drugs that cause excessive daytime sleepiness as a side-effect.
7. Excessive Daytime Sleepiness
In the general population, excessive daytime sleepiness affects as many as 37% of adults [48]. Interestingly, though, persons with HF do not report subjective daytime sleepiness as frequently as community dwelling adults without HF, even though the majority of persons with HF have SDB [31]. For example, in one study, 155 consecutive HF patients and 1139 adults randomly chosen from the community were assessed for obstructive sleep apnea [35]. For any given severity of obstructive sleep apnea (none, mild, or moderate to severe), patients with HF had less daytime sleepiness (all p ≤ .01). Further, the HF sample reported less daytime sleepiness despite sleeping significantly less than the community sample (all p < .001).
Subjective complaints of excessive daytime sleepiness are typically the basis for referral for SDB. Thus, the observation that in HF poor sleep is not necessarily associated with the subjective perception of excessive daytime sleepiness is particularly interesting. Yet, others have found that despite the lack of subjective reports of daytime sleepiness, HF patients have evidence of objective sleepiness [49]. This finding of objective evidence without the subjective complaint of sleepiness supports the need for further study of sleep in persons with HF.
8. Cognitive Effects of Excessive Daytime Sleep
Working memory and executive function, higher order cognitive functions that include decision-making, are particularly vulnerable to sleep loss and fragmentation [50]. When sleepy, the performance of cognitive tasks such as logical reasoning slows and memory problems occur. When deprived of sleep, the ability to sustain attention decreases and the number and duration of lapses in attention increases [11]. Accuracy in performing tasks can be maintained only when tasks are performed more slowly. The need to perform more slowly when sleep deprived reflects what is now recognized as very brief uncontrollable naps or “microsleeps” [11]. Even after people adapt to feeling subjectively sleepy, chronic partial sleep deprivation, which closely replicates sleep loss in society, causes cognitive deficits that accumulate over time [51].
In the general population, sleep deprivation is associated with deficits in sustained attention, memory, executive function, and psychomotor speed [51]. In HF, the studies of sleep deprivation and cognition focus on SDB, where the cessation of breathing during sleep is known to impair cognitive functioning [52].
9. Effect of Impaired Cognition on Self-Care
Impaired ability to concentrate sufficiently to perform self-care has been suggested by some authors as a mechanism by which impaired sleep could affect HF outcomes [3, 53]. Some evidence of this mechanism was found in a mixed methods study describing how expertise in HF self-care develops [53]. Patients poor in HF self-care were found to have more complaints of excessive daytime sleepiness, worse cognition and higher depression scores. Experts in HF self-care had less daytime sleepiness than patients who were judged qualitatively to be only adequate in self-care.
The element of self-care most directly linked with sleep is decision-making. When HF patients have chronic sleep loss or fragmentation they may have problems mentally manipulating information, planning, organizing, making decisions, and maintaining the attention required for self-care. Patients with impaired memory may forget to take medicines. Those unable to concentrate may have difficulty learning how to choose low sodium foods. With impaired executive functioning, they may have difficulty interpreting symptoms, solving problems, applying learned skills in their daily lives, or coping when the routine is disrupted or the environment is altered. The decision-making required to interpret and respond to changes in symptoms may be impaired [8]. In these ways, poor sleep may be sufficient to cause poor self-care.
10. Failed Self-Care Exacerbates Symptoms and Decreases Functioning
As many as one third of persons with HF admit to skipping medication doses [54], and this may be an underestimation [55]. When patients skip a medication such as an angiotensin converting enzyme inhibitor [ACE-I], therapeutic neurohormonal antagonism is low [56]. The imbalance of hormones leads to vasoconstriction, fluid and sodium retention and myocardial stretch, thus exacerbating HF symptoms [57].
Most HF patients have difficulty following a low sodium diet. In one HF sample, mean daily sodium intake ranged from 1,398 mg to 5,807 mg; 58% had a sodium intake above the daily recommended level of 2000 mg [58]. When foods high in sodium are eaten, fluid retention increases circulating volume, diastolic filling pressure and afterload, all which augment myocardial stretch. These effects stimulate the release of tissue angiotensin II and growth factors, both of which allow progressive ventricular remodeling [59]. Fluid retention also attenuates the effectiveness of pharmaceutical therapy [60], making a delay in or reversal of remodeling relatively less likely. These seemingly inconsequential lapses in self-care—forgetting a medication dose, a hot dog at a ball game—cause a cascade of physiologic events that worsen fluid retention, increase symptoms and decrease functional capacity.
11. Contribution of Symptoms, Functioning on Heart Failure Outcomes
Heart failure (HF) is the most common and expensive chronic illness among older people in developed countries [1]. Hospitalization rates for HF have doubled in the last two decades [61]. Twenty-seven percent of patients are readmitted within 90 days for recurrent HF, 29% of these are readmitted more than once, and 6-month readmission rates are 44%-47% [62, 63]. HF is notable for its strikingly negative effect on patients’ HRQL [2, 64–67]. Heart failure has more influence on HRQL than arthritis, chronic lung disease [68] and other cardiac illnesses [69].
Poor HRQL can be traced to symptom burden and functional limitations. In one study [70], an average of 7.2 symptoms was identified on HF hospital admission; six weeks after discharge these same patients still had 4.2 symptoms, on average. Initially, shortness of breath was most prevalent, but by six weeks after discharge, fatigue became the primary symptom. In another sample of HF patients, higher dyspnea scores were associated with poorer HRQL [71]. These symptoms cause significant physical limitations that decrease mobility and usual activities [72]. Persons with HF report levels of vigor lower than other patient populations and similar to those with cancer [70]. Worse HRQL is associated with hospitalization and death in persons with HF [73].
12. Depression
Depression is extremely common in persons with HF. In a recent study, as many as 25% of HF outpatients and 51% of hospitalized HF patients scored above the cut-point for depression [74]. Conversely, in the general adult population, only 9.5% have a depressive disorder [75]. Depression complicates the causal paths in the proposed conceptual model by directly and indirectly influencing the major variables. For example, sleep disturbances and depression coexist in a significant proportion of persons with HF; in one study, 40% of those with both HF and depression had severe sleep disturbance [76]. In persons with HF but without depression, only 18% had a significant sleep disturbance.
Depression is associated with impaired cognition in the general population [77], where depression is known to cause prefrontal cortical dysfunction with impaired concentration and decisional capacity [78]. In HF, some [79, 80] but not all [81, 82] studies have demonstrated that depression is associated with impaired cognition. Depression is also a strong predictor of treatment nonadherence, a component of self-care, with a linearly incremental influence on adherence across the full range of symptom severity [83]. Depressed HF patients have more HF symptoms, decreased HRQL, and an increased risk of premature death [84].
13. Conclusions
To date, initiatives to improve HF self-care have had limited success, suggesting that important operative factors have not been identified. The proposed conceptual model builds most directly on the work of Beebe and Gozal [24] by expanding their description of how sleep influences cognition and daytime activities. Here we have woven together research conducted with other populations to build a case for the possibility that the effects of sleep on cognition have an important impact on the ability of persons with HF to perform self-care. If we can demonstrate through our on-going research that factors other than SDB cause excessive daytime sleepiness, that even minimal impairments in cognition are sufficient to impair patients’ abilities to perform self-care, and that these effects are associated with important outcomes, the implications for clinical practice will be significant.
Acknowledgement
The authors gratefully acknowledge the revision suggestions of Dr. Harleah Buck.
This work was funded by a grant from the National Heart, Lung & Blood Institute (RO1HL084394-01A1).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Barbara Riegel, University of Pennsylvania School of Nursing.
Terri E. Weaver, University of Pennsylvania School of Nursing.
REFERENCES
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart Disease and Stroke Statistics--2009 Update. A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008 doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Heo S, Moser DK, Lennie TA, et al. A comparison of health-related quality of life between older adults with heart failure and healthy older adults. Heart Lung. 2007;36:16–24. doi: 10.1016/j.hrtlng.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Brostrom A, Johansson P, Stromberg A, et al. Obstructive sleep apnoea syndrome--patients' perceptions of their sleep and its effects on their life situation. J Adv Nurs. 2007;57:318–327. doi: 10.1111/j.1365-2648.2006.04110.x. [DOI] [PubMed] [Google Scholar]
- 4.Naughton MT. The link between obstructive sleep apnea and heart failure: underappreciated opportunity for treatment. Curr Heart Fail Rep. 2006;3:183–188. doi: 10.1007/s11897-006-0020-z. [DOI] [PubMed] [Google Scholar]
- 5.Johansson P, Dahlstrom U, Brostrom A. Factors and interventions influencing health-related quality of life in patients with heart failure: a review of the literature. Eur J Cardiovasc Nurs. 2006;5:5–15. doi: 10.1016/j.ejcnurse.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Redeker NS, Hilkert R. Sleep and quality of life in stable heart failure. J Card Fail. 2005;11:700–704. doi: 10.1016/j.cardfail.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Institute of Medicine. 2006 [PubMed] [Google Scholar]
- 8.Dickson VV, Tkacs N, Riegel B. Cognitive influences on self-care decision making in persons with heart failure. Am Heart J. 2007;154:424–431. doi: 10.1016/j.ahj.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 9.Pressler SJ. Cognitive functioning and chronic heart failure: a review of the literature (2002-July 2007) J Cardiovasc Nurs. 2008;23:239–249. doi: 10.1097/01.JCN.0000305096.09710.ec. [DOI] [PubMed] [Google Scholar]
- 10.Laffont F, Mallet A, Mayer G, et al. Study of a patient population investigated for excessive daytime sleepiness (EDS) Neurophysiol Clin. 2002;32:343–351. doi: 10.1016/s0987-7053(02)00312-x. [DOI] [PubMed] [Google Scholar]
- 11.Dinges DF. Probing the limits of functional capacity: The effects of sleep loss on short-duration tasks. In: Broughton RJ, Ogilvie RD, editors. Sleep, Arousal, and Performance. 1992. [Google Scholar]
- 12.Hossain JL, Ahmad P, Reinish LW, et al. Subjective fatigue and subjective sleepiness: two independent consequences of sleep disorders? J Sleep Res. 2005;14:245–253. doi: 10.1111/j.1365-2869.2005.00466.x. [DOI] [PubMed] [Google Scholar]
- 13.Drexler H, Coats AJ. Explaining fatigue in congestive heart failure. Annu Rev Med. 1996;47:241–256. doi: 10.1146/annurev.med.47.1.241. [DOI] [PubMed] [Google Scholar]
- 14.Riegel B, Carlson B, Moser DK, et al. Psychometric testing of the self-care of heart failure index. J Card Fail. 2004;10:350–360. doi: 10.1016/j.cardfail.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Vaishnava P, Lewis EF. Assessment of quality of life in severe heart failure. Curr Heart Fail Rep. 2007;4:170–177. doi: 10.1007/s11897-007-0037-y. [DOI] [PubMed] [Google Scholar]
- 16.Kalia M. Neurobiology of sleep. Metabolism. 2006;55:S2–S6. doi: 10.1016/j.metabol.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Zisapel N. Sleep and sleep disturbances: biological basis and clinical implications. Cell Mol Life Sci. 2007;64:1174–1186. doi: 10.1007/s00018-007-6529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapsimalis F, Richardson G, Opp MR, Kryger M. Cytokines and normal sleep. Curr Opin Pulm Med. 2005;11:481–484. doi: 10.1097/01.mcp.0000183062.98665.6b. [DOI] [PubMed] [Google Scholar]
- 19.Brostrom A, Stromberg A, Dahlstrom U, Fridlund B. Sleep difficulties, daytime sleepiness, and health-related quality of life in patients with chronic heart failure. J Cardiovasc Nurs. 2004;19:234–242. doi: 10.1097/00005082-200407000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Principe-Rodriguez K, Strohl KP, Hadziefendic S, Pina IL. Sleep symptoms and clinical markers of illness in patients with heart failure. Sleep Breath. 2005;9:127–133. doi: 10.1007/s11325-005-0023-0. [DOI] [PubMed] [Google Scholar]
- 21.Gary R, Lee SY. Physical function and quality of life in older women with diastolic heart failure: effects of a progressive walking program on sleep patterns. Prog Cardiovasc Nurs. 2007;22:72–80. doi: 10.1111/j.0889-7204.2007.05375.x. [DOI] [PubMed] [Google Scholar]
- 22.Alberini CM, Milekic MH, Tronel S. Mechanisms of memory stabilization and de-stabilization. Cell Mol Life Sci. 2006;63:999–1008. doi: 10.1007/s00018-006-6025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trupp RJ, Corwin EJ. Sleep-disordered breathing, cognitive functioning, and adherence in heart failure: linked through pathology? Prog Cardiovasc Nurs. 2008;23:32–36. doi: 10.1111/j.1751-7117.2008.08000.x. [DOI] [PubMed] [Google Scholar]
- 24.Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11:1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- 25.Michalsen A, Konig G, Thimme W. Preventable causative factors leading to hospital admission with decompensated heart failure. Heart. 1998;80:437–441. doi: 10.1136/hrt.80.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinges D, Kribbs N. Performing while sleepy: effects of experimentally induced sleepiness. In: Monk T, editor. Sleep, Sleepiness, and Performance. New York: John Wiley; 1991. pp. 97–128. [Google Scholar]
- 27.Vogels RL, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: a systematic review of the literature. Eur J Heart Fail. 2007;9:440–449. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Stanek KM, Gunstad J, Paul RH, et al. Longitudinal Cognitive Performance in Older Adults with Cardiovascular Disease: Evidence for Improvement in Heart Failure. J Cardiovasc Nurs. doi: 10.1097/JCN.0b013e31819b54de. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hossmann KA. The hypoxic brain Insights from ischemia research. Adv Exp Med Biol. 1999;474:155–169. [PubMed] [Google Scholar]
- 30.Roth T, Roehrs TA. Etiologies and sequelae of excessive daytime sleepiness. Clin Ther. 1996;18:562–576. doi: 10.1016/s0149-2918(96)80207-4. discussion 561. [DOI] [PubMed] [Google Scholar]
- 31.Somers VK, White DP, Amin R, et al. Sleep Apnea and Cardiovascular Disease. An American Heart Association/American College of Cardiology Foundation Scientific Statement From the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing Council. Circulation. 2008 doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163:685–689. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 33.Cormican LJ, Williams A. Sleep disordered breathing and its treatment in congestive heart failure. Heart. 2005;91:1265–1270. doi: 10.1136/hrt.2004.048314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacDonald M, Fang J, Pittman SD, et al. The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med. 2008;4:38–42. [PMC free article] [PubMed] [Google Scholar]
- 35.Arzt M, Young T, Finn L, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166:1716–1722. doi: 10.1001/archinte.166.16.1716. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Touchard A, Somers VK, Olson LJ, Caples SM. Central sleep apnea: implications for congestive heart failure. Chest. 2008;133:1495–1504. doi: 10.1378/chest.07-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gehlbach BK, Geppert E. The pulmonary manifestations of left heart failure. Chest. 2004;125:669–682. doi: 10.1378/chest.125.2.669. [DOI] [PubMed] [Google Scholar]
- 38.Asplund R. Nocturia in relation to sleep, health, and medical treatment in the elderly. BJU Int. 2005;96 Suppl 1:15–21. doi: 10.1111/j.1464-410X.2005.05653.x. [DOI] [PubMed] [Google Scholar]
- 39.Middelkoop HA, Smilde-van den Doel DA Neven AK, et al. Subjective sleep characteristics of 1,485 males and females aged 50–93: effects of sex and age, and factors related to self-evaluated quality of sleep. J Gerontol Series A-Biolog Sci & Med Sci. 1996;51:M108–M115. doi: 10.1093/gerona/51a.3.m108. [DOI] [PubMed] [Google Scholar]
- 40.Asplund R. Sleep and hypnotic use in relation to perceived somatic and mental health among the elderly. Arch Gerontol Geriatr. 2000;31:199–205. doi: 10.1016/s0167-4943(00)00075-3. [DOI] [PubMed] [Google Scholar]
- 41.Roth T, Drake C. Evolution of insomnia: current status and future direction. Sleep Med. 2004;5 Suppl 1:23–30. doi: 10.1016/s1389-9457(04)90004-4. [DOI] [PubMed] [Google Scholar]
- 42.Gooneratne NS, Gehrman PR, Nkwuo JE, et al. Consequences of comorbid insomnia symptoms and sleep-related breathing disorder in elderly subjects. Arch Intern Med. 2006;166:1732–1738. doi: 10.1001/archinte.166.16.1732. [DOI] [PubMed] [Google Scholar]
- 43.American Psychological Association. Diagnostic and Statistical Manual of Mental Disorders, Primary Care Version (DSM-IV-PC) 4th Edition ed. Washington, DC: American Psychiatric Association Press; 2000. [Google Scholar]
- 44.Ancoli-Israel S, Cooke JR. Prevalence and comorbidity of insomnia and effect on functioning in elderly populations. J Am Geriatr Soc. 2005;53:S264–S271. doi: 10.1111/j.1532-5415.2005.53392.x. [DOI] [PubMed] [Google Scholar]
- 45.Pack AI, Dinges DF, Gehrman PR, et al. Risk factors for excessive sleepiness in older adults. Ann Neurol. 2006;59:893–904. doi: 10.1002/ana.20863. [DOI] [PubMed] [Google Scholar]
- 46.Qureshi A, Lee-Chiong TJ. Medications and their effects on sleep. Medical Clinics of North America. 2004;88:751–766. doi: 10.1016/j.mcna.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Ko DT, Hebert PR, Coffey CS, et al. Beta-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction.[see comment] JAMA. 2002;288:351–357. doi: 10.1001/jama.288.3.351. [DOI] [PubMed] [Google Scholar]
- 48.National Sleep Foundation. 2002 Sleep in America Poll, Executive Summary. National Sleep Foundation. 2002:44. [Google Scholar]
- 49.Hastings PC, Vazir A, O'Driscoll DM, et al. Symptom burden of sleep-disordered breathing in mild-to-moderate congestive heart failure patients. Eur Respir J. 2006;27:748–755. doi: 10.1183/09031936.06.00063005. [DOI] [PubMed] [Google Scholar]
- 50.Killgore WD, Balkin TJ, Wesensten NJ. Impaired decision making following 49 h of sleep deprivation. J Sleep Res. 2006;15:7–13. doi: 10.1111/j.1365-2869.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- 51.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 52.Banno K, Kryger MH. Sleep apnea: clinical investigations in humans. Sleep Med. 2007;8:400–426. doi: 10.1016/j.sleep.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Riegel B, Dickson VV, Goldberg LR, Deatrick JA. Factors associated with the development of expertise in heart failure self-care. Nurs Res. 2007;56:235–243. doi: 10.1097/01.NNR.0000280615.75447.f7. [DOI] [PubMed] [Google Scholar]
- 54.Gonzalez B, Lupon J, Parajon T, et al. Nurse evaluation of patients in a new multidisciplinary Heart Failure Unit in Spain. Eur J Cardiovasc Nurs. 2004;3:61–69. doi: 10.1016/j.ejcnurse.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Tu W, Morris AB, Li J, et al. Association between adherence measurements of metoprolol and health care utilization in older patients with heart failure. Clin Pharmacol Ther. 2005;77:189–201. doi: 10.1016/j.clpt.2004.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frigerio M, Roubina E. Drugs for left ventricular remodeling in heart failure. Am J Cardiol. 2005;96:10L–18L. doi: 10.1016/j.amjcard.2005.09.060. [DOI] [PubMed] [Google Scholar]
- 57.Yamazaki T, Komuro I, Yazaki Y. Molecular mechanism of cardiac cellular hypertrophy by mechanical stress. J Mol Cell Cardiol. 1995;27:133–140. doi: 10.1016/s0022-2828(08)80013-2. [DOI] [PubMed] [Google Scholar]
- 58.Lennie TA, Moser DK, Habash D, Trupp R. Nutritional adequacy of low sodium diets in patients with heart failure. Circulation. 2003 [Google Scholar]
- 59.Katz AM. Heart failure: a hemodynamic disorder complicated by maladaptive proliferative responses. J Cell Mol Med. 2003;7:1–10. doi: 10.1111/j.1582-4934.2003.tb00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 61.Haldeman GA, Croft JB, Giles WH, Rashidee A. Hospitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995. Am Heart J. 1999;137:352–360. doi: 10.1053/hj.1999.v137.95495. [DOI] [PubMed] [Google Scholar]
- 62.Miller LW, Missov ED. Epidemiology of heart failure. Cardiol Clinics. 2001;19:547–555. doi: 10.1016/s0733-8651(05)70242-3. [DOI] [PubMed] [Google Scholar]
- 63.Starling RC. The heart failure pandemic: changing patterns, costs, and treatment strategies. Cleve Clin J Med. 1998;65:351–358. doi: 10.3949/ccjm.65.7.351. [DOI] [PubMed] [Google Scholar]
- 64.Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–1146. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garty M, Shotan A, Gottlieb S, et al. The management, early and one year outcome in hospitalized patients with heart failure: a national Heart Failure Survey in Israel--HFSIS 2003. Isr Med Assoc J. 2007;9:227–233. [PubMed] [Google Scholar]
- 66.Azevedo A, Bettencourt P, Alvelos M, et al. Health-related quality of life and stages of heart failure. Int J Cardiol. 2007 doi: 10.1016/j.ijcard.2007.07.091. [DOI] [PubMed] [Google Scholar]
- 67.Lewis EF, Lamas GA, O'Meara E, et al. Characterization of health-related quality of life in heart failure patients with preserved versus low ejection fraction in CHARM. Eur J Heart Fail. 2007;9:83–91. doi: 10.1016/j.ejheart.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 68.Alonso J, Ferrer M, Gandek B, et al. Health-related quality of life associated with chronic conditions in eight countries: results from the International Quality of Life Assessment (IQOLA) Project. Qual Life Res. 2004;13:283–298. doi: 10.1023/b:qure.0000018472.46236.05. [DOI] [PubMed] [Google Scholar]
- 69.Dixon L, Goldberg R, Lehman A, McNary S. The impact of health status on work, symptoms, and functional outcomes in severe mental illness. J Nerv Mental Dis. 2001;189:17–23. doi: 10.1097/00005053-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 70.Friedman M. Older adults’ symptoms and their duration before hospitalization for heart failure. Heart Lung. 1997;26:169–176. doi: 10.1016/s0147-9563(97)90053-4. [DOI] [PubMed] [Google Scholar]
- 71.Lewis EF, Johnson PA, Johnson W, et al. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant. 2001;20:1016–1024. doi: 10.1016/s1053-2498(01)00298-4. [DOI] [PubMed] [Google Scholar]
- 72.Calvert MJ, Freemantle N, Cleland JG. The impact of chronic heart failure on health-related quality of life data acquired in the baseline phase of the CARE-HF study. Eur J Heart Fail. 2005;7:243–251. doi: 10.1016/j.ejheart.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 73.Rodriguez-Artalejo F, Guallar-Castillon P, Pascual CR, et al. Health-related quality of life as a predictor of hospital readmission and death among patients with heart failure. Arch Intern Med. 2005;165:1274–1279. doi: 10.1001/archinte.165.11.1274. [DOI] [PubMed] [Google Scholar]
- 74.Freedland KE, Rich MW, Skala JA, et al. Prevalence of depression in hospitalized patients with congestive heart failure. Psychosom Med. 2003;65:119–128. doi: 10.1097/01.psy.0000038938.67401.85. [DOI] [PubMed] [Google Scholar]
- 75.Katz DA, McHorney CA. Clinical correlates of insomnia in patients with chronic illness. Arch Int Med. 1998;158:1099–1107. doi: 10.1001/archinte.158.10.1099. [DOI] [PubMed] [Google Scholar]
- 76.Szuba MP. The psychobiology of sleep and major depression. Dep Anxiety. 2001;14:1–2. doi: 10.1002/da.1040. [DOI] [PubMed] [Google Scholar]
- 77.Lake AE, 3rd, Rains JC, Penzien DB, Lipchik GL. Headache and psychiatric comorbidity: historical context, clinical implications, and research relevance. Headache. 2005;45:493–506. doi: 10.1111/j.1526-4610.2005.05101.x. [DOI] [PubMed] [Google Scholar]
- 78.Merriam EP, Thase ME, Haas GL, et al. Prefrontal cortical dysfunction in depression determined by Wisconsin Card Sorting Test performance. Am J Psychiat. 1999;156:780–782. doi: 10.1176/ajp.156.5.780. [DOI] [PubMed] [Google Scholar]
- 79.Trojano L, Antonelli Incalzi R, Acanfora D, et al. Cognitive impairment: a key feature of congestive heart failure in the elderly. J Neurol. 2003;250:1456–1463. doi: 10.1007/s00415-003-0249-3. [DOI] [PubMed] [Google Scholar]
- 80.Bornstein RA, Starling RC, Myerowitz PD, Haas GJ. Neuropsychological function in patients with end-stage heart failure before and after cardiac transplantation. Acta Neurol Scand. 1995;91:260–265. doi: 10.1111/j.1600-0404.1995.tb07001.x. [DOI] [PubMed] [Google Scholar]
- 81.Grubb NR, Simpson C, Fox KA. Memory function in patients with stable, moderate to severe cardiac failure. Am Heart J. 2000;140:1–5. doi: 10.1067/mhj.2000.106647. [DOI] [PubMed] [Google Scholar]
- 82.Putzke JD, Williams MA, Daniel JF, et al. Neuropsychological functioning among heart transplant candidates: a case control study. J Clin Exp Neuropsychol. 2000;22:95–103. doi: 10.1076/1380-3395(200002)22:1;1-8;FT095. [DOI] [PubMed] [Google Scholar]
- 83.Glazer KM, Emery CF, Frid DJ, Banyasz RE. Psychological predictors of adherence and outcomes among patients in cardiac rehabilitation. J Cardiopulm Rehab. 2002;22:40–46. doi: 10.1097/00008483-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 84.Johansson P, Dahlstrom U, Brostrom A. Consequences and predictors of depression in patients with chronic heart failure: implications for nursing care and future research. Prog Cardiovasc Nurs. 2006;21:202–211. doi: 10.1111/j.0889-7204.2006.05415.x. [DOI] [PubMed] [Google Scholar]