Abstract
Myoclonus is often observed in epilepsy. It is characterized by sudden involuntary shock-like movements of the body (myoclonic jerks, MJs). This study examined whether epileptic myoclonus was under genetic control. Inbred strains of mice were administered eight daily flurothyl exposures, a 28-day rest period, and a final flurothyl retest. For all trials, the latency to the first MJ (threshold) and the number of MJs (MJ#) were recorded. The inbred strains that we examined exhibited significant variability in initial myoclonic response, and myoclonus across the eight flurothyl exposures. C57BL/6J and DBA/2J mice displayed significantly different initial latencies to a MJ, MJ# preceding a generalized seizure (GS), and changes in MJ threshold and MJ# across the eight seizure trials. [C57BL/6JxDBA/2J] F1-hybrid mice showed an initial MJ threshold and decreases in MJ threshold over the eight trials, which were similar to C57BL/6J; however, F1-hybrids had an initial MJ# and trend in MJ# over the eight trials that were similar to DBA/2J. Decreases in MJ threshold and MJ# following multiple seizure trials, observed in C57BL/6J mice, were dependent on the expression of GSs and not on MJ occurrence. Our study is the first to document the potential for genetic heterogeneity of myoclonus in mice; we show that significant alterations in myoclonic behavior occur after GSs. These results indicate that multiple GSs affect MJ thresholds. An understanding of the genetics of myoclonus will be important for determination of the brain areas responsible for myoclonus as well as for identification of candidate genes.
Keywords: myoclonus, myoclonic jerk, seizure, inbred, mice, flurothyl, generalized seizure threshold
1. Introduction
Myoclonus is a condition characterized by sudden involuntary jerk/shock-like movements (myoclonic jerks) involving the face, trunk, and limbs (Tassinari et al., 1998; Caviness, 2003). Myoclonus is commonly seen in individuals with epilepsy and has been documented in both human (Duron et al., 2005; Jansen et al., 2008) and animal models (Takeda et al., 2004; Uzbay et al., 2007). Myoclonus is presumed to originate in cortical, subcortical, or spinal regions (Tassinari et al., 1998) and can be focal, segmental, multifocal, or generalized (Cassim and Houdayer, 2006). However, unlike the extensive knowledge gained in understanding the mechanisms involved in partial or generalized seizure induction, the underlying processes involved in myoclonus induction remain relatively unexplored.
Myoclonus has been studied with various seizure-inducing compounds (Lewin and Bleck, 1985; Mathis and Ungerer, 1992). Myoclonus has also been observed in mice following exposure to flurothyl (Samoriski and Applegate, 1997). Although myoclonus has been widely observed in animal models of disease (Joho et al., 2006; Tagliabracci et al., 2008), no studies have examined either the effect of genetic background on epileptic myoclonus in animal models, or the progression of the response following multiple seizures, in different strains of mice.
Accordingly, we examined the expression of epileptic myoclonus in various inbred strains of mice in the repeated flurothyl model (Samoriski et al., 1997; Ferland and Applegate, 1999). In this model, mice are subjected to one flurothyl-induced seizure per day for eight consecutive days (induction phase), followed by a 28-day rest period (incubation phase) in which no seizures are induced with flurothyl. Following the incubation phase, animals undergo another flurothyl-induced seizure trial (retest). Here, we assessed the latency to a myoclonic jerk (a measure of myoclonic jerk threshold) as well as the number of subsequent myoclonic jerks preceding the onset of a generalized seizure, across eight flurothyl trials and a post-incubation retest in multiple inbred strains of mice.
2. Materials and methods
2.1 Animals
Male DBA/2J, C57BL/6J, [C57BL/6J × DBA/2J] F1 hybrid, C57BLKS/J, 129S1/SvImJ, A/J, BALB/cJ, and C3H/HeJ (n≥10 per strain) adult mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). These mouse strains were chosen for analysis since they have significant interstrain genetic diversity (i.e., these strains are from separate arms of the mouse phylogenetic tree) providing a diverse genetic background for examination of myoclonus (Petkov et al., 2004). Female mice were not used in the current study given the known effects of hormonal fluctuations on seizure threshold (Woolley and Schwartzkroin, 1998; Edwards et al., 1999).
All mice were acclimated to the housing facilities for 1 week prior to seizure testing (testing began when the mice reached at least 7 weeks of age). Mice were maintained on a normal 12-h light-dark cycle with lights on at 6:00 AM, with unlimited access to food and water. All testing occurred between 7:00 AM and noon. Animals were tested under the same chemical rated fume hood in an AAALAC accredited vivarium. The environment was maintained at a consistent temperature and humidity that did not differ significantly from day to day. All procedures were performed under approval from the Institutional Animal Care and Use Committees of both Rensselaer Polytechnic Institute and the Wadsworth Center (New York State Department of Health), in accordance with The National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
2.2 Flurothyl Testing
Mice were acclimated to the procedure room for 30 min before testing. Mice were placed into a closed 2.4 liter Plexiglas chamber with an inner diameter of 149 mm, an outer diameter of 171 mm, and an overall height of 206 mm (cat. #24988-233, VWR, West Chester, PA). A 10% flurothyl solution (bis(2,2,2-trifluoroethyl) ether; Sigma Aldrich, St. Louis, MO) made up in 95% ethanol was infused at a rate of 100µl/min and dripped onto a dry gauze pad that was suspended from the top of the chamber. The flurothyl quickly evaporates, exposing the animal to the flurothyl vapors. Once the animal had been placed in the chamber and the infusion of flurothyl had begun, the latency to the first myoclonic jerk and the number of myoclonic jerks expressed before the onset of a generalized seizure were recorded. Behaviorally, myoclonic jerks were brief, but severe, contractions of the neck and body musculature, all occurring while maintaining postural control (Applegate et al., 1997; Samoriski and Applegate, 1997). One mouse at a time was tested in the flurothyl chamber using a new gauze pad each time.
Upon continued exposure to flurothyl, animals can undergo two types of seizures: 1) clonic-forebrain seizures and 2) brainstem seizures. Clonic-forebrain seizures are characterized by clonus of the face and limbs, rearing, and falling. Brainstem seizures are characterized by intense running and hopping, tonic extension of the limbs, and a loss of righting ability. Clonic-forebrain seizures can immediately progress into brainstem seizures as observed following the flurothyl induction, incubation phase, and flurothyl retest (referred to as a forebrain→brainstem seizure). At the start of the generalized seizure, the top of the chamber was removed, exposing the mouse to room air. Once the animal regained its posture and stopped displaying clonus of the face and/or limbs, it was placed into a cage separate from its untested littermates. After recovery, all animals were returned to their home cages and placed back in the colony. This procedure was repeated over eight consecutive days (induction phase). Mice were then given a 28-day rest period (incubation phase), during which they remained in their home cages with no flurothyl exposures. At the end of the incubation phase, mice were re-exposed to flurothyl (retest), and myoclonic jerk behavior was again recorded.
To examine the effects of generalized seizure expression on myoclonic jerk threshold (latency), we subjected an additional group of C57BL/6J mice (n=8) to one myoclonic jerk per day for 8 consecutive days. For administration of a single myoclonic jerk, we exposed each animal to flurothyl as described above; however, the top of the chamber was removed immediately after the mouse expressed a myoclonic jerk. Mice were then given a 28-day rest period during which they remained in their home cages with no flurothyl exposure. On the 28th day, there was a final exposure to flurothyl.
2.3 Statistical analysis
One-way analyses of variance (ANOVA), followed by Tukey Honest Significant Difference (HSD) post hoc comparisons, were used to assess differences in myoclonic jerk latencies and myoclonic jerk numbers on flurothyl trial 1. Repeated measures ANOVA, followed by Tukey HSD post hoc comparisons, were used to assess changes in myoclonic jerk latencies and myoclonic jerk numbers observed during the flurothyl induction period (across the eight trials). Student’s T-tests for dependent samples were employed to examine differences in seizure characteristics between trial 8 of the induction phase and the retest that followed the 28 day rest. All statistical analyses were conducted with the Statistica software package (StatSoft, Tulsa, OK).
3. Results
3.1 Inbred Strain Variability in Initial Flurothyl-induced (Trial 1) Myoclonic Jerk Threshold and Myoclonic Jerk Number
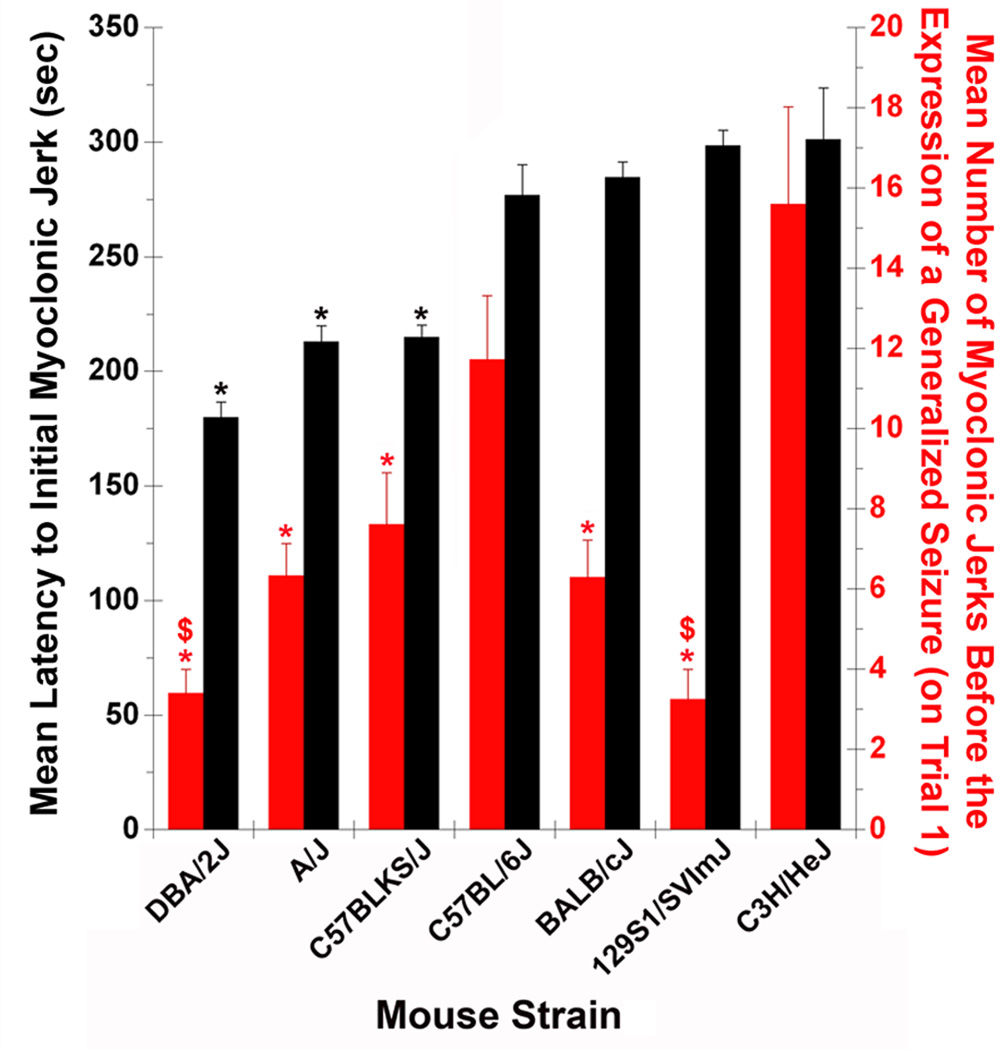
The latency to the first myoclonic jerk (threshold) following flurothyl exposure was measured in seven commonly used inbred strains of mice: C57BL/6J, C57BLKS/J, A/J, 129S1/SvImJ, C3H/HeJ, BALB/cJ, and DBA/2J (Figure 1). Among the inbred strains of mice examined, there were significant differences in the latencies to the first myoclonic jerk upon the first exposure to flurothyl (Figure 1 (black bars): F6,100 = 20.07, P < 0.000001). More specifically, C3H/HeJ, 129S1/SvImJ, BALB/cJ, and C57BL/6J mice required longer exposures to flurothyl to elicit a myoclonic jerk than did DBA/2J, C57BLKS/J, and A/J mice (P < 0.006; Tukey HSD)(Figure 1). There were no significant differences in the myoclonic jerk threshold among C3H/HeJ, 129S1/SvImJ, BALB/cJ, and C57BL/6J strains. Similarly, there were no significant differences in the myoclonic jerk threshold among DBA/2J, C57BLKS/J, and A/J strains.
Figure 1.
The genetic contributions to the expression of myoclonus in 7 inbred strains of mice. The mean latency to the first myoclonic jerk (black bars) and the mean number of myoclonic jerks preceding a generalized seizure (red bars) following one exposure to flurothyl in DBA/2J, C57BL/6J, C57BLKS/J, 129S1/SVlmJ, A/J, BALB/cJ, and C3H/HeJ mice. * denotes significantly different from C3H/HeJ mice (P < 0.009); $ denotes significantly different from C57BL/6J mice (P < 0.05).
The number of myoclonic jerks that occurred before the first flurothyl-induced generalized seizure was also recorded. There were significant differences in the number of myoclonic jerks expressed (Figure 1 (red bars): F6,100 = 10.60, P < 0.000001) among the inbred strains of mice tested. C3H/HeJ and C57BL/6J mice had the most myoclonic jerks among all of the strains (P < 0.009; Tukey HSD)(Figure 1). DBA/2J and 129S1/SvImJ mice had the fewest myoclonic jerks, and BALB/cJ, A/J, and C57BLKS/J mice had intermediate numbers of myoclonic jerks (Figure 1).
3.2 Correlational Analysis of Initial Flurothyl-induced (Trial 1) Myoclonic Jerk Threshold, Myoclonic Jerk Number, and Generalized Seizure Threshold
Correlational analysis of initial myoclonic jerk threshold and myoclonic jerk number did not suggest a significant effect (r = 0.58, not significant; data not shown). However, comparisons of this initial myoclonus data with previously published results on initial flurothyl-induced generalized seizure threshold (GST) in these strains (Papandrea et al., 2009; unpublished observations) demonstrated a significant correlation of initial GST with initial myoclonic jerk threshold (r = 0.853, P < 0.02), but not with initial myoclonic jerk number (r = 0.624, not significant).
3.3 Inbred Strain Variability in Myoclonic Jerk Threshold Across Eight Flurothyl Trials, Followed by a 28-Day Incubation Period and a Retest with Flurothyl
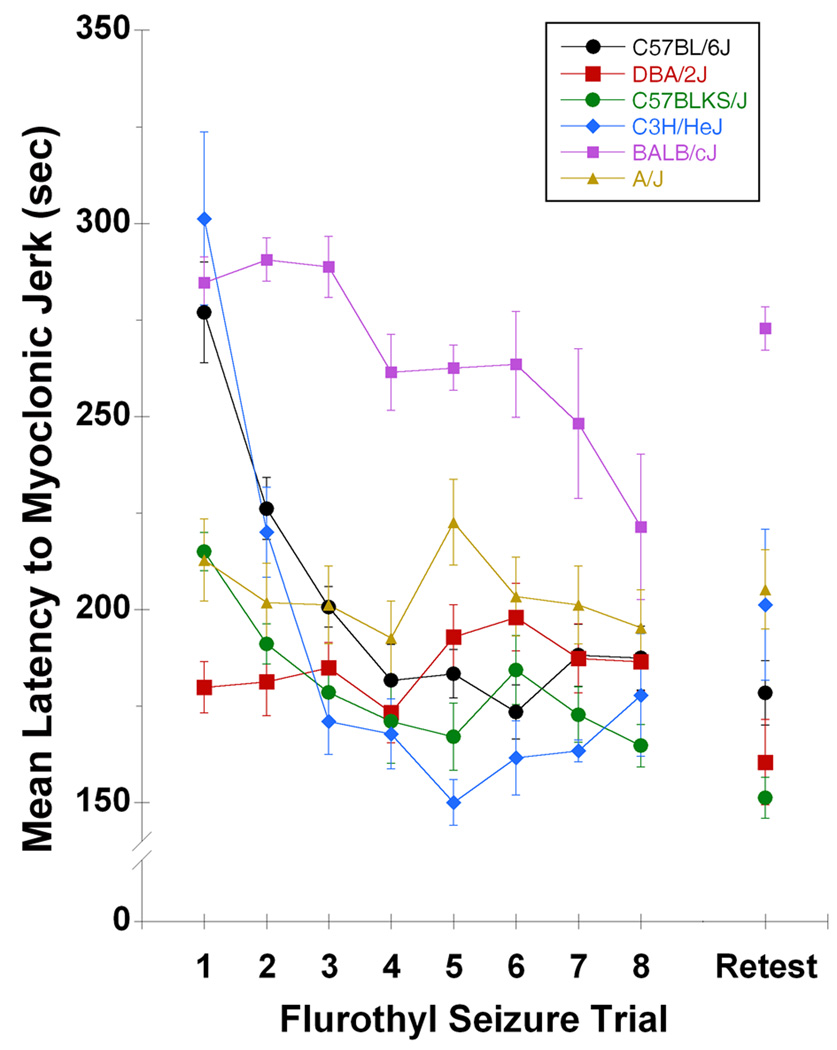
The myoclonic jerk thresholds across repeated flurothyl trials were recorded. DBA/2J and A/J strains displayed similar trends in myoclonic jerk threshold across trials, having low myoclonic jerk thresholds; the latencies remained consistent across eight flurothyl trials (DBA/2J (red squares): F7,168 = 1.29, not significant; A/J (gold triangles): F7,77 = 1.17, not significant; Figure 2). In contrast, C57BL/6J, C3H/HeJ, and BALB/cJ strains initially had high myoclonic jerk thresholds, while the C57BLKS/J strain had an intermediate myoclonic jerk threshold, but all of these strains had significantly decreasing myoclonic jerk thresholds across multiple flurothyl trials (C57BL/6J (black circles): F7,168 = 16.44, P < 0.0001; C57BLKS/J (green circles): F7,84 = 4.87, P < 0.0002; C3H/HeJ (blue diamonds): F7,28 = 11.55, P < 0.0001; BALB/cJ (purple squares): F7,56 = 3.91, P < 0.002; Figure 2). 129S1/SvlmJ mice showed a trend of decrease in myoclonic jerk threshold across flurothyl trials (data not shown), but since this strain expressed lethal forebrain→brainstem seizures during the 8 flurothyl trials, it’s myoclonic jerk profile throughout the entire repeated flurothyl model could not be examined. With the exception of the aforementioned trial 1 data, there were no significant associations of myoclonic jerk threshold with GST on trials 2–8 of the repeated flurothyl model.
Figure 2.
The genetic contributions to myoclonic jerk threshold across repeated flurothyl exposures. The mean latency to the first myoclonic jerk per flurothyl trial throughout the repeated flurothyl model for DBA/2J (red squares), C57BL/6J (black circles), C57BLKS/J (green circles), A/J (orange triangles), BALB/cJ (pink squares), and C3H/HeJ (blue diamonds) mouse strains.
When C57BL/6J, C57BLKS/J, DBA/2J, C3H/HeJ, and A/J mice were retested with flurothyl 28-days after their 8th flurothyl trial, the latencies to the first myoclonic jerk in the retest were not significantly different from those displayed in trial 8 (Figure 2). In contrast, BALB/cJ mice did not maintain their myoclonic jerk threshold following flurothyl retest (t8 = 2.84, P < 0.03), but actually reverted to myoclonic jerk latencies that were similar to those that characterized these animals’ initial exposure to flurothyl (Figure 2).
Since the mice tested in the repeated flurothyl model all displayed myoclonic jerks preceding the expression of generalized clonic seizures, we wished to assess whether the decreases in myoclonic jerk thresholds across flurothyl trials were due to either multiple myoclonic seizures or generalized clonic seizures. To test this, additional C57BL/6J mice were allowed to undergo only one myoclonic jerk per flurothyl trial (flurothyl exposure was terminated following the expression of the first myoclonic jerk; hence, no generalized seizures). There were no significant decreases in myoclonic jerk threshold across the eight flurothyl trials (F7,28 = 0.82, not significant; data not shown). Upon flurothyl retest after the 28-day rest, the myoclonic jerk threshold remained high, comparable to the threshold for the 8th flurothyl trial (t5 = 0.17, not significant; data not shown).
3.4 Inbred Strain Variability in Myoclonic Jerk Number Across Eight Flurothyl Trials, and a 28-Day Incubation Period and Retest with Flurothyl
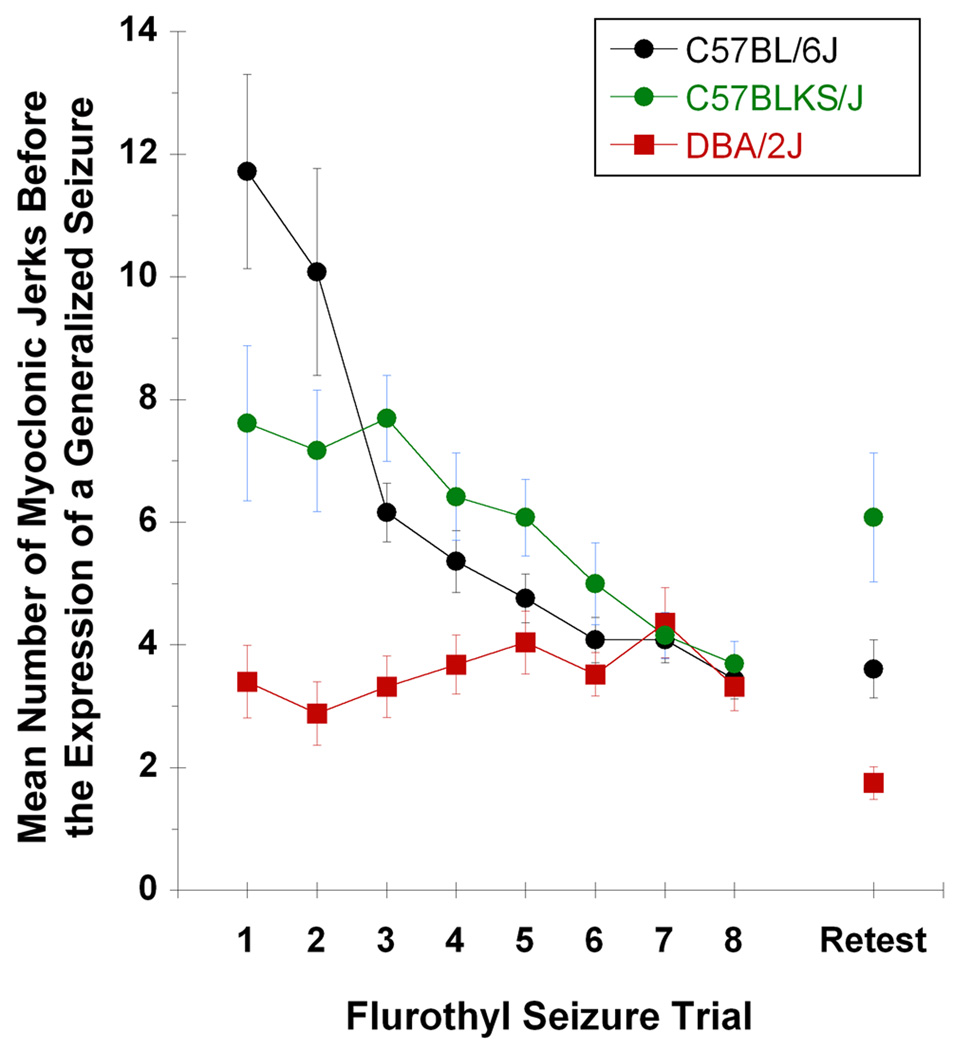
Since the C57BL/6J, C57BLKS/J, and DBA/2J strains did not show forebrain→brainstem seizures on induction phase trials, the number of myoclonic jerks before the expression of a generalized clonic-forebrain seizure could be compared across these three strains. The myoclonic jerk number in C57BL/6J mice significantly decreased (F7,168 = 12.26, P < 0.00001) and then plateaued across the eight flurothyl trials (Figure 3), whereas in DBA/2J, the number of myoclonic jerks remained consistently low throughout the induction-phase (F7,168 = 0.95, not significant; Figure 3). For C57BLKS/J mice, the number of myoclonic jerks remained constant across the first three trials and then decreased for each subsequent trial (F7,70 = 4.42, P < 0.0005; Figure 3). When the three strains were retested with flurothyl after the 28-day rest, the numbers of myoclonic jerks were unchanged in C57BL/6J mice, were significantly increased in C57BLKS/J mice (t12 = 2.23, P < 0.05), and were significantly decreased in DBA/2J mice (t39 = 2.97, P < 0.006) relative to the trial 8 values (Figure 3). Because of notable differences in both myoclonic jerk threshold and myoclonic jerk number between the C57BL/6J and DBA/2J strains, we chose to analyze flurothyl-induced myoclonus in [C57BL/6J × DBA/2J] F1 hybrid mice.
Figure 3.
The genetic contributions to myoclonic jerk number across repeated flurothyl exposures. The mean total number of myoclonic jerks preceding a generalized seizure per flurothyl trial throughout the repeated flurothyl model, for C57BL/6J (black circles), C57BLKS/J (green circles), and DBA/2J (red squares) mouse strains. Results are not presented for BALB/cJ, C3H/HeJ, A/J, and 129S1/SvImJ strains, since these strains can display forebrain→brainstem seizures on induction phase trials, and such seizures inherently confound the counting of myoclonic jerks (see Results section).
3.5 The Traits of Myoclonic Jerk Threshold and Myoclonic Jerk Number across Flurothyl Trials Segregate Independently in [C57BL/6J × DBA/2J] F1 Hybrid Mice
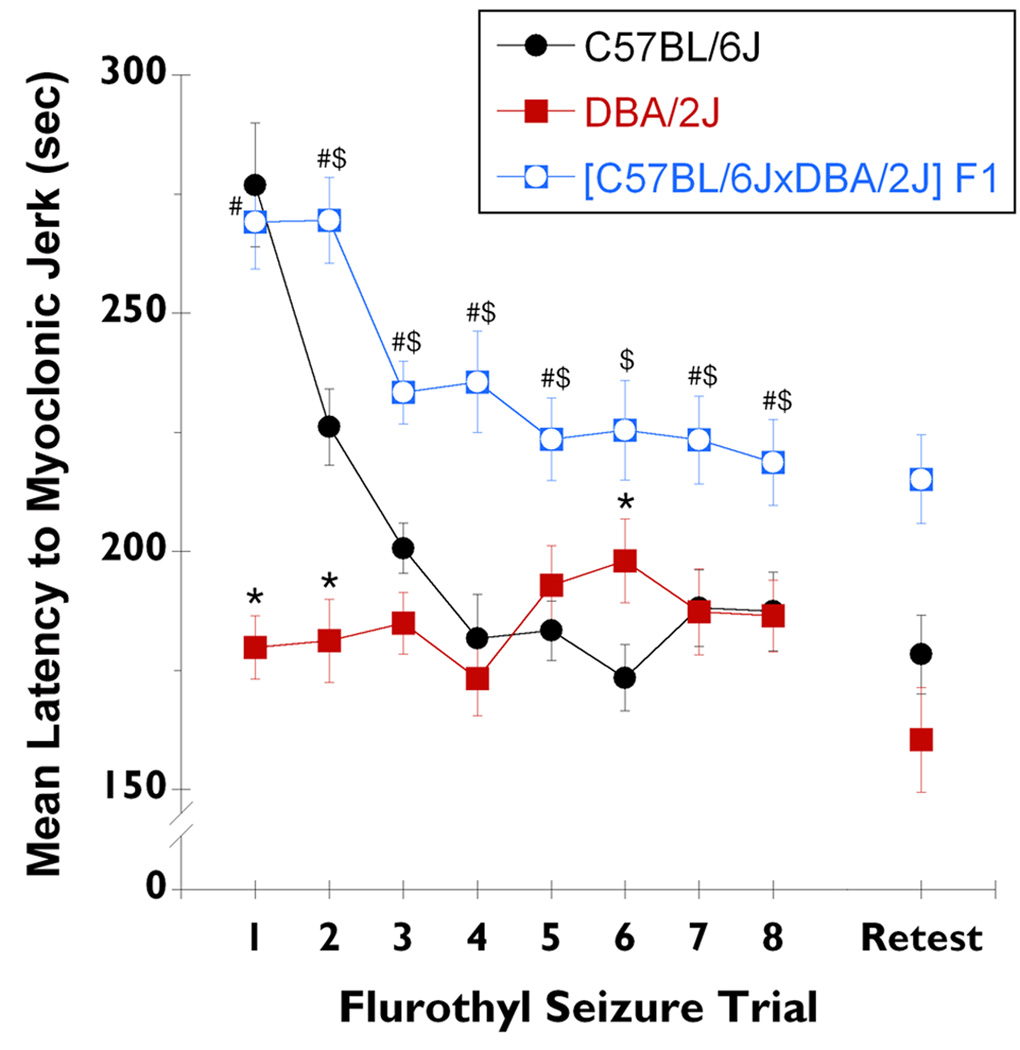
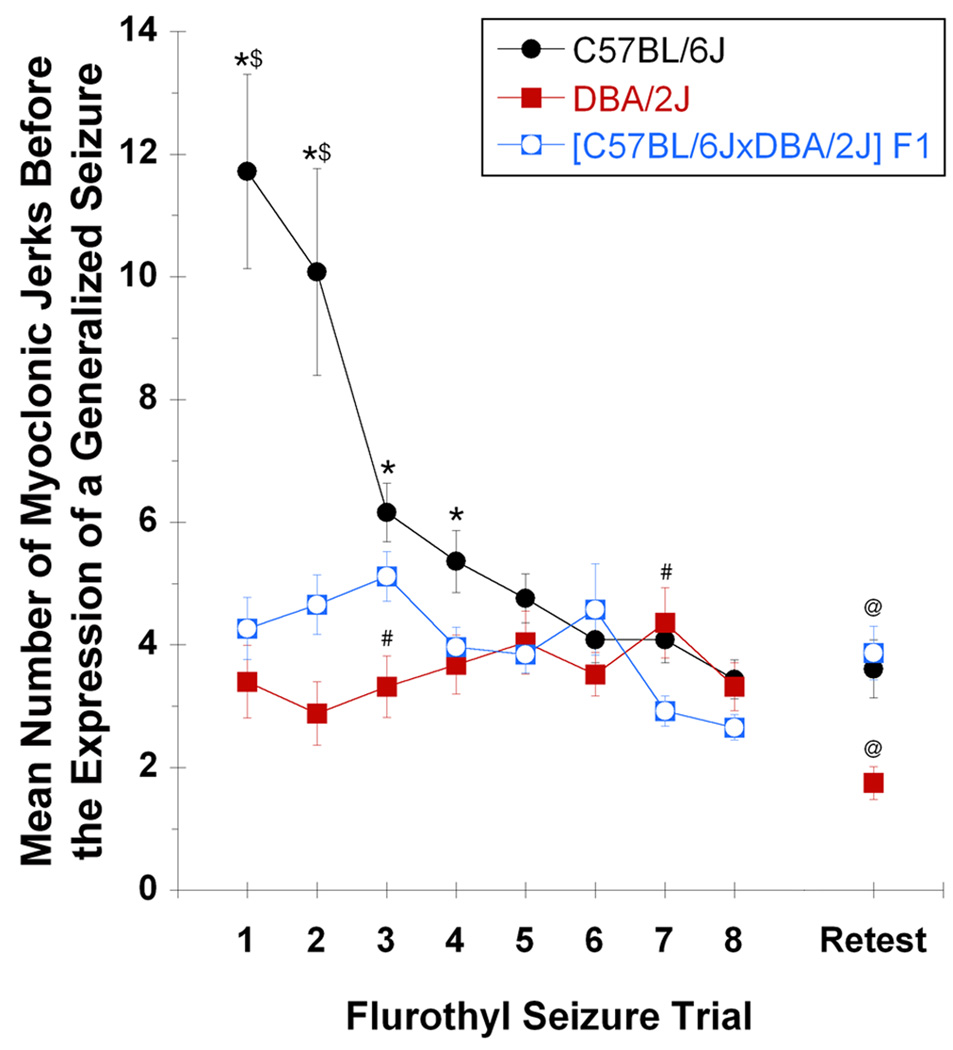
[C57BL/6J × DBA/2J] F1 hybrid mice were tested to determine the mode of inheritance for initial myoclonic jerk threshold and initial myoclonic jerk number in C57BL/6J and DBA/2J mice. Significant differences were observed among C57BL/6J, DBA/2J, and [C57BL/6J × DBA/2J] F1 hybrid mice for both myoclonic jerk threshold (F2,73 = 27.4, P < 0.0001; Figure 4) and myoclonic jerk number (F2,73 = 19.8, P < 0.0001; Figure 5). More specifically, [C57BL/6J × DBA/2J] F1 hybrid mice showed a trial 1 myoclonic jerk threshold that was more similar to the threshold for C57BL/6J mice (Figure 4: not significant; Tukey HSD) than to the threshold for DBA/2J mice (Figure 4: P < 0.0002; Tukey HSD). Conversely, the number of myoclonic jerks expressed by [C57BL/6J × DBA/2J] F1 hybrid mice on trial 1 was more similar to the number for DBA/2J mice (Figure 5: not significant; Tukey HSD) than to the number for C57BL/6J mice (Figure 5: P < 0.0002; Tukey HSD).
Figure 4.
Independent segregation for the traits of myoclonic jerk threshold in [C57BL/6J × DBA/2J] F1 hybrid mice. The mean latency to the first myoclonic jerk, per flurothyl trial throughout the repeated flurothyl model, for C57BL/6J (black circles), DBA/2J (red squares), and [C57BL/6J × DBA/2J] F1 hybrid (blue open squares) mice (data from C57BL/6J and DBA/2J mice have been replotted from Figure 1 for this comparison). Specific comparisons between C57BL/6J and DBA/2J mice on the myoclonic jerk threshold, demonstrated a significant group by trial interaction (F7,336 = 12.7, P < 0.0001), and significant differences between groups (F1,48 = 8.0, P < 0.007) and between trials (F7,336 = 8.6, P < 0.0001). * denotes significantly different from C57BL/6J (P < 0.04); # denotes significantly different from DBA/2J (P < 0.03); $ denotes significantly different from C57BL/6J (P < 0.03).
Figure 5.
Independent segregation for the traits of myoclonic jerk number in [C57BL/6J × DBA/2J] F1 hybrid mice. The mean total number of myoclonic jerks preceding a generalized seizure per flurothyl trial, throughout the repeated flurothyl model, for C57BL/6J (black circles), DBA/2J (red squares), and [C57BL/6J × DBA/2J] F1 hybrid (blue open squares) mice (data from C57BL/6J and DBA/2J mice have been replotted from Figure 3 for this comparison). A significant group by trial interaction (F7,336 = 11.2, P < 0.0001), in addition to a significant between-group difference (F1,48 = 32.8, P < 0.007) and between-trial difference (F7,336 = 8.1, P < 0.0001) was noted. * denotes significantly different from DBA/2J (P < 0.03); # denotes significantly different from [C57BL/6J × DBA/2J] F1 hybrid (P < 0.05); $ denotes significantly different from [C57BL/6J × DBA/2J] F1 hybrid (P < 0.0002); @ denotes significantly different within the strain from trial 8 (P < 0.02).
[C57BL/6J × DBA/2J] F1 hybrid mice demonstrated a trend of a decrease in myoclonic jerk threshold across the eight trials that was similar to the trend for C57BL/6J mice; however, the trend was intermediate as compared to the parental lines (Figure 4). C57BL/6J, DBA/2J, and [C57BL/6J × DBA/2J] F1 hybrid mice demonstrated significant between-group (F2,73 = 41.2, P < 0.0001) and between-trial (F7,511 = 12.7, P < 0.0001) differences, in addition to a significant group by trial interaction (F14,511 = 6.3, P < 0.0001). C57BL/6J mice had significantly shorter latencies to the expression of a myoclonic jerk than did [C57BL/6J × DBA/2J] F1 hybrid mice, for all flurothyl trials except trial 1 (P < 0.03; Tukey HSD). The myoclonic jerk threshold for DBA/2J mice was significantly lower than for [C57BL/6J × DBA/2J] F1 hybrid mice, for every flurothyl trial except trial 6 (P < 0.03; Tukey HSD).
The number of myoclonic jerks observed in [C57BL/6J × DBA/2J] F1 hybrid mice before the expression of a generalized clonic seizure closely resembled the number for DBA/2J mice (Figure 5). Comparisons of C57BL/6J, DBA/2J, and [C57BL/6J × DBA/2J] F1 hybrid mice demonstrated significant differences among these groups (F2,73 = 25.7, P < 0.0001) and between trials (F7,511 = 9.4, P < 0.0001), in addition to a significant group by trial interaction (F14,511 = 8.5, P < 0.0001). For number of myoclonic jerks observed, DBA/2J mice were comparable to that for [C57BL/6J × DBA/2J] F1 hybrid mice, in both the total number and the consistency of that number across trials (difference was not significant except for trials 3 and 7 (P < 0.05; Tukey HSD)). For C57BL/6J mice, statistically significant differences from [C57BL/6J × DBA/2J] F1 hybrid mice were observed for only trials 1–2; in these, C57BL6J mice had more myoclonic jerks than did the [C57BL/6J × DBA/2J] F1 hybrid mice (P < 0.0002; Tukey HSD).
When C57BL/6J, DBA/2J, and [C57BL/6J × DBA/2J] F1 hybrid mice were retested with flurothyl 28-days after the last induction-phase seizure, the by-strain latencies to the first myoclonic jerk were not significantly different between trial 8 and retest (Figure 4). Similar results were obtained for myoclonic jerk number in C57BL/6J and [C57BL/6J × DBA/2J] F1 hybrid mice; however, DBA/2J mice had significantly fewer myoclonic jerks after flurothyl retest (DBA/2J: t15 = 2.32, P < 0.04; Figure 5).
4. Discussion
Current knowledge on the genetics of epileptic myoclonus is based mainly on disorders that exhibit myoclonus as a symptom (Kaneko et al., 2002; Grisar et al., 2006). In addition, animal models are being developed, to enable further study of these debilitating disorders (Vaarmann et al., 2006; Wang et al., 2007). However, few studies have examined the genetics of myoclonus. In the current study, we have begun to dissect the genetic contribution to the expression of myoclonus by examining myoclonic behavior in genetically diverse inbred strains of mice. Overall, our results document the potential for genetic heterogeneity of myoclonus in mice in addition to independent segregation for the traits of myoclonic jerk threshold and number in [C57BL/6J × DBA/2J] F1 hybrid mice from the C57BL/6J and DBA/2J parental strains. Our results suggest that [C57BL/6J × DBA/2J] F1 hybrid mice have inherited the myoclonic jerk threshold profile from the C57BL/6J parental strain, but the myoclonic jerk number preceding a generalized seizure profile from the DBA/2J parental strain. Moreover, there was no correlation of initial myoclonic jerk threshold with myoclonic jerk number in the strains examined. Therefore, these two traits may be controlled by distinct genetic loci. Further dissection of the contribution of different genes to these behaviors is warranted for elucidating the regulation of threshold and recurrence of myoclonus. Evaluation of the genetic loci that contribute to these complex traits could reveal novel pathways that mediate myoclonus in humans, with implications for the development of therapies for this epilepsy spectrum disorder.
Myoclonic jerk thresholds decreased as a result of induction of multiple flurothyl-induced generalized seizures in C3H/HeJ, C57BLKS/J, and C57BL/6J strains. Induction of only one myoclonic jerk per flurothyl trial (with no subsequent generalized seizure) was not sufficient to produce a significant change in myoclonic jerk threshold over the eight trials in C57BL/6J mice. These findings demonstrate that the changes in myoclonic threshold following repeated flurothyl exposures are a result of generalized seizure expression, rather than induction of a single myoclonic jerk.
The correlation between myoclonic jerk threshold and GST on trial 1 for the inbred strains tested suggested an interaction between these two variables. Interestingly, the myoclonus trends in C57BL/6J and DBA/2J mice correlated with previously observed decreases in generalized seizure threshold (GST) (Papandrea et al., 2009). That is, for C57BL/6J mice, the trend of high myoclonic jerk thresholds and numbers, which decreased and then plateaued across flurothyl trials was comparable to the decreases in GST that have been shown to occur in the repeated flurothyl model (Papandrea et al., 2009). Similarly, low myoclonic jerk latencies and numbers of myoclonic jerks in DBA/2J mice remained constant across flurothyl trials, paralleling the strain’s GST trend (Papandrea et al., 2009). However, when taking into account all of the strains, there was no correlation between myoclonic jerk threshold and GST on trials 2–8. This lack of correlation between multiple genetically diverse strains indicates that although generalized seizures themselves are necessary to cause changes in myoclonus in our model, changes in myoclonic jerk threshold are not dependent on changes in GST. This is suggestive of two separate processes occurring in the brain resulting in either decreases in myoclonic jerk threshold or GST.
The high number of initial myoclonic jerks in C57BL/6J mice, which decreased over multiple flurothyl-induced seizures could be due to the paralleling decrease in the latency to the expression of a generalized seizure. That is, since the duration from the exposure to flurothyl to the beginning of a generalized seizure decreases with multiple seizures in some strains, could the limited time available for having more myoclonic jerks just be decreased resulting in few myoclonic jerks. This is probably unlikely, given that there was not a significant correlation between myoclonic jerk threshold and myoclonic jerk number in the strains tested. Also, [C57BL/6J × DBA/2J] F1 hybrid mice, which have an initial high seizure threshold, display a low number of myoclonic jerks. Although the [C57BL/6J × DBA/2J] F1 hybrid mice have decreasing myoclonic jerk thresholds following repeated flurothyl exposures, the number of myoclonic jerks does not continue to decrease, but remains relatively fixed. Therefore, the number of myoclonic jerks does not seem to be affected by the length of time before the onset of a generalized seizure.
C57BLKS/J mice have been characterized as having a mixed genetic background principally from C57BL/6J (71%), but with the remainder from DBA/2J (25%) and a mixture of C57BL/10J, 129S1/SvlmJ, and unknown sources (4%) (Mao et al., 2006). Interestingly, C57BLKS/J mice showed initial low myoclonic jerk thresholds (similar to the thresholds for DBA/2J mice), but demonstrated a significant decrease in myoclonic jerk thresholds across successive flurothyl trials (similar to the decreases seen for C57BL/6J and 129S1/SvlmJ mice). Moreover, the myoclonic jerk threshold for C57BLKS/J mice at the end of the eight trials was actually lower than for DBA/2J mice. These patterns suggest that the decrease in myoclonic jerk threshold across flurothyl trials is not dependent on baseline myoclonic jerk susceptibility and that the MJ threshold has not reached a floor effect in DBA/2J mice since C57BLKS/J mice can actually have even lower myoclonic jerk thresholds after 8 flurothyl trials. Taken together, the genotype as well as the observed phenotype of C57BLKS/J mice suggests that the myoclonic behavior observed in the repeated flurothyl model is dependent on genetic background, with specific genes from the parental inbred strains contributing to the different traits. Thus, C57BLKS/J mice could represent a powerful genetic model in which to identify QTLs associated with myoclonic jerk susceptibility in the repeated flurothyl model.
Flurothyl-induced generalized seizures result from neuronal activity in several brain regions, including (but not limited to) the cerebral cortex, amygdala, hippocampus, and hypothalamus (Samoriski et al., 1998). Myoclonus is believed to originate in the cerebral cortex, subcortical regions (i.e., thalamus), brainstem, cerebellum, or spinal cord, depending on the type of myoclonus (Tassinari et al., 1998; Fahn, 2002). The effects of generalized seizures on myoclonus and the overlap in neuroanatomical regions thought to be responsible for these two distinct seizure types suggests that the activation of these regions during a generalized seizure leads to a reorganization of the areas, resulting not only in decreased GST, but also in decreased myoclonic jerk threshold.
Acknowledgements
This work was partly supported by NIH grants K01MH71801 (to RJF) and R01MH065400 (to BJH). The authors wish to thank Dr. Adriana Verschoor (Wadsworth Center) for critical reading of and input to the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors have any conflict of interest to disclose.
References
- Applegate CD, Samoriski GM, Ozduman K. Effects of valproate, phenytoin, and MK-801 in a novel model of epileptogenesis. Epilepsia. 1997;38:631–636. doi: 10.1111/j.1528-1157.1997.tb01231.x. [DOI] [PubMed] [Google Scholar]
- Cassim F, Houdayer E. Neurophysiology of myoclonus. Neurophysiol Clin. 2006;36:281–291. doi: 10.1016/j.neucli.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Caviness JN, Evidente VG. Cortical myoclonus during lithium exposure. Arch Neurol. 2003;60:401–404. doi: 10.1001/archneur.60.3.401. [DOI] [PubMed] [Google Scholar]
- Duron RM, Medina MT, Martinez-Juarez IE, Bailey JN, Perez-Gosiengfiao KT, Ramos-Ramirez R, Lopez-Ruiz M, Alonso ME, Ortega RH, Pascual-Castroviejo I, Machado-Salas J, Mija L, Delgado-Escueta AV. Seizures of idiopathic generalized epilepsies. Epilepsia. 2005;46 Suppl 9:34–47. doi: 10.1111/j.1528-1167.2005.00312.x. [DOI] [PubMed] [Google Scholar]
- Edwards HE, Burnham WM, Mendonca A, Bowlby DA, MacLusky NJ. Steroid hormones affect limbic afterdischarge thresholds and kindling rates in adult female rats. Brain Res. 1999;838:136–150. doi: 10.1016/s0006-8993(99)01619-4. [DOI] [PubMed] [Google Scholar]
- Fahn S. Overview, history, and classification of myoclonus. Adv Neurol. 2002;89:13–17. [PubMed] [Google Scholar]
- Ferland RJ, Applegate CD. Bidirectional transfer between electrical and flurothyl kindling in mice: evidence for common processes in epileptogenesis. Epilepsia. 1999;40:144–152. doi: 10.1111/j.1528-1157.1999.tb02067.x. [DOI] [PubMed] [Google Scholar]
- Grisar T, de Nijs L, Chanas G, Leon C, Coumans B, Foidart A, Lakaye B. Some genetic and biochemical aspects of myoclonus. Neurophysiol Clin. 2006;36:271–279. doi: 10.1016/j.neucli.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Jansen AC, Andermann E, Niel F, Creveaux I, Boespflug-Tanguy O, Andermann F. Leucoencephalopathy with vanishing white matter may cause progressive myoclonus epilepsy. Epilepsia. 2008;49:910–913. doi: 10.1111/j.1528-1167.2008.01542.x. [DOI] [PubMed] [Google Scholar]
- Joho RH, Street C, Matsushita S, Knopfel T. Behavioral motor dysfunction in Kv3-type potassium channel-deficient mice. Genes Brain Behav. 2006;5:472–482. doi: 10.1111/j.1601-183X.2005.00184.x. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Okada M, Iwasa H, Yamakawa K, Hirose S. Genetics of epilepsy: current status and perspectives. Neurosci Res. 2002;44:11–30. doi: 10.1016/s0168-0102(02)00065-2. [DOI] [PubMed] [Google Scholar]
- Lewin E, Bleck V. Effect of inosine on seizures induced with pentylenetetrazole, bicuculline, or picrotoxin. Epilepsia. 1985;26:258–261. doi: 10.1111/j.1528-1157.1985.tb05415.x. [DOI] [PubMed] [Google Scholar]
- Mao HZ, Roussos ET, Peterfy M. Genetic analysis of the diabetes-prone C57BLKS/J mouse strain reveals genetic contribution from multiple strains. Biochim Biophys Acta. 2006;1762:440–446. doi: 10.1016/j.bbadis.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Mathis C, Ungerer A. Comparative analysis of seizures induced by intracerebroventricular administration of NMDA, kainate and quisqualate in mice. Exp Brain Res. 1992;88:277–282. doi: 10.1007/BF02259102. [DOI] [PubMed] [Google Scholar]
- Papandrea D, Anderson TM, Herron BJ, Ferland RJ. Dissociation of seizure traits in inbred strains of mice using the flurothyl kindling model of epileptogenesis. Exp Neurol. 2009;215:60–68. doi: 10.1016/j.expneurol.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov PM, Ding Y, Cassell MA, Zhang W, Wagner G, Sargent EE, Asquith S, Crew V, Johnson KA, Robinson P, Scott VE, Wiles MV. An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res. 2004;14:1806–1811. doi: 10.1101/gr.2825804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoriski GM, Applegate CD. Repeated generalized seizures induce time-dependent changes in the behavioral seizure response independent of continued seizure induction. J Neurosci. 1997;17:5581–5590. doi: 10.1523/JNEUROSCI.17-14-05581.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoriski GM, Piekut DT, Applegate CD. Differential spatial patterns of Fos induction following generalized clonic and generalized tonic seizures. Exp Neurol. 1997;143:255–268. doi: 10.1006/exnr.1996.6368. [DOI] [PubMed] [Google Scholar]
- Samoriski GM, Piekut DT, Applegate CD. Regional analysis of the spatial patterns of Fos induction in brain following flurothyl kindling. Neuroscience. 1998;84:1209–1222. doi: 10.1016/s0306-4522(97)00571-x. [DOI] [PubMed] [Google Scholar]
- Tagliabracci VS, Girard JM, Segvich D, Meyer C, Turnbull J, Zhao X, Minassian BA, Depaoli-Roach AA, Roach PJ. Abnormal metabolism of glycogen phosphate as a cause for Lafora disease. J Biol Chem. 2008;283:33816–33825. doi: 10.1074/jbc.M807428200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A, Hirate M, Oku N. Elimination of zinc-65 from the brain under kainate-induced seizures. Biometals. 2004;17:141–144. doi: 10.1023/b:biom.0000018378.55494.d2. [DOI] [PubMed] [Google Scholar]
- Tassinari CA, Rubboli G, Shibasaki H. Neurophysiology of positive and negative myoclonus. Electroencephalogr Clin Neurophysiol. 1998;107:181–195. doi: 10.1016/s0013-4694(98)00058-3. [DOI] [PubMed] [Google Scholar]
- Uzbay TI, Kayir H, Ceyhan M. Effects of tianeptine on onset time of pentylenetetrazole-induced seizures in mice: possible role of adenosine A1 receptors. Neuropsychopharmacology. 2007;32:412–416. doi: 10.1038/sj.npp.1301143. [DOI] [PubMed] [Google Scholar]
- Vaarmann A, Kaasik A, Zharkovsky A. Altered tryptophan metabolism in the brain of cystatin B-deficient mice: a model system for progressive myoclonus epilepsy. Epilepsia. 2006;47:1650–1654. doi: 10.1111/j.1528-1167.2006.00638.x. [DOI] [PubMed] [Google Scholar]
- Wang W, Lohi H, Skurat AV, DePaoli-Roach AA, Minassian BA, Roach PJ. Glycogen metabolism in tissues from a mouse model of Lafora disease. Arch Biochem Biophys. 2007;457:264–269. doi: 10.1016/j.abb.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Schwartzkroin PA. Hormonal effects on the brain. Epilepsia. 1998;39 Suppl 8:S2–S8. doi: 10.1111/j.1528-1157.1998.tb02601.x. [DOI] [PubMed] [Google Scholar]