Abstract
Background
Anemia management in hemodialysis patients poses significant challenges. The present study explored the hypothesis that computerized dosing of intravenous erythropoietin (EPO) would increase the percentage of hemoglobin (Hb) values within the target range and reduce staff time spent on anemia management.
Study Design
Retrospective cohort
Setting and Participants
In-center hemodialysis patients who received EPO at Dialysis Clinic Inc dialysis units for at least 3 months between Oct 1, 2005 and April 30, 2006
Quality Improvement Plan
Computerized decision support (CDS) for EPO dosing is compared with manual physician-directed dosing.
Outcomes and Measurements
Achieved monthly Hb values, quantity of EPO administered, and time spent by dialysis unit personnel
Measurements
Monthly Hb values and the quantity of EPO administered to 1118 patients from 18 dialysis units treated by CDS and 7823 patients from 125 dialysis units treated by manual dosing.
Results
There was no difference in the likelihood of a monthly Hb of 11–12 g/dl or 10–12 g/dl with CDS as compared with manual dosing. The likelihood of Hb > 12 g/dL decreased and the likelihood of Hb < 10g/dl increased with CDS. EPO use was 4% lower with CDS, although the difference was not statistically significant. CDS was associated with a nearly 50% reduction (p<0.001) in the time spent by dialysis unit staff on anemia management.
Limitations
Retrospective, non-randomized
Conclusion
The number of monthly Hb values in a 11 (and 10)–12 g/dl target range and EPO use did not differ with EPO dosing by a CDS as compared with manual dosing. The staff resources devoted to anemia management declined significantly with CDS.
Text words: Anemia, hemoglobin, hemodialysis, ESRD, erythropoietin, quality improvement, decision support, computer
Despite considerable time spent by nephrologists and dialysis units staff in adjusting erythropoietin (EPO) doses, a minority of hemodialysis patients maintain hemoglobin (Hb) values within the target range over 6 months [1]. Fluctuations in Hb despite a constant EPO dose are common and result from a number of factors including changes in EPO responsiveness due to illness or inflammation, changes in iron stores or its availability, blood loss, or changes in extracellular fluid volume at the time of Hb measurement.
Decision support systems have been designed for dosing medications such as heparin, warfarin, theophylline, and anesthetic agents [2]. Their use has been shown to reduce medication errors, increase the number of patients in target and reduce the time practitioners spend on medication prescribing [3]. A computerized decision support (CDS) for EPO dosing would be expected to reduce the time spent by dialysis unit staff on anemia management but it is unclear whether the number of patients meeting the target Hb each month would remain the same. If some of the variability in Hb is due to overly aggressive dose changes or inadequate time for a Hb response to be seen before the next dose change is made, a protocol that is uniform in detecting a need or not for a dose change and that uses a uniform dose ladder, might prove better than manual physician-directed dosing, particularly in reducing overshooting of the target. EPO use would be hypothesized to be less as well. On the other hand, given the current state of clinical information systems, a computer would not be able to take account of many of the clinical events surrounding a change in Hb. To the extent that these events influence fluctuations in Hb and decision making, CDS for EPO dosing might not perform as well as manual dosing.
This study compares Hb values and EPO doses in 18 dialysis units from a national dialysis provider that adopted a CDS for EPO dosing with 125 units that continued to dose EPO manually, using a combination of individualized physician-directed dosing and nurses’ application of paper-based treatment protocols.
METHODS
Study Population
The study population consisted of incident and prevalent patients receiving in-center hemodialysis treatments at facilities operated by Dialysis Clinic, Inc. (DCI) The CDS was introduced in 2005 and adopted by 18 units at the time of this analysis. This analysis includes all patients with at least 3 monthly Hb values between November 1, 2005 and April 30, 2006 and who were treated with intravenous epoetin alfa throughout the study period. Patients less than 18 years of age and those who had fewer than three monthly Hb levels were excluded (n=1,851). Dialysis units with fewer than 20 patients (n=176) and units that switched to the CDS after October 1, 2005 (n=2,043) were excluded. At the start of the study, the CDS had been used at 18 units a median of 61 days (quartile [Q] 1 and Q3, 42 and 99 days). The ratio of included/potential candidate patients was not different in CDS vs. manual dosing (85.6 vs. 84.4, respectively). The study was conducted on a limited dataset, i.e. a dataset containing dates but no other personal health information, and was approved by the Tufts Medical Center Institutional Review Board.
Medical Information Systems
All DCI dialysis units use an electronic medical information system (Darwin) that has largely replaced the paper medical record. Information about comorbid conditions, hospitalization dates, oral and intravenous medications, the delivered dialysis prescription as well as physician, nurse, dietician and social worker progress notes are entered into Darwin. Hb is measured monthly by some facilities and twice monthly by others. When more than one Hb was available, the first value for that month was used. Samples for Hb measurement are processed at DCI Laboratory in Nashville, TN, a central facility accredited by the College of American Pathologists. New Hb results populate the Darwin database as soon as they are available. The EPO dose administered at each dialysis treatment is recorded in Darwin by the nurse administering the medication.
Description of the Computerized EPO Dosing Algorithm
The CDS is shown in the appendix. It takes into consideration the most recent Hb value, the change in Hb from the month prior, the most recent EPO dose and the change in EPO dose from the prior month. The algorithm was derived from paper-based protocols that had been used with success by individual DCI units. The interval between dose changes is no less than 4 weeks unless a) Hb exceeds 13 g/dl, in which case EPO is stopped and Hb is subsequently measured weekly; b) Hb falls below 11 at 2 weeks following an EPO dose reduction, in which case EPO may be increased; or c) Hb exceeds 12.5 g/dl 2 weeks after an EPO increase, in which case EPO may be reduced. Entry of Hb results into Darwin triggers the calculation of a new EPO prescription, which may be accepted or overridden by the physician. The new prescription appears on the hemodialysis treatment sheet.
Units that did not use the CDS represent usual care, in which EPO was dosed by physician decision or according to paper protocols that were devised by the dialysis unit physicians and nurses and administered by the nurse.
Survey of Dialysis Units
A survey was sent to the nurse managers of all DCI units that asked “What is the number of hours per month you and your staff spend managing EPO dosing?” (respondents were asked to round off to the nearest half hour). Results were compared for facilities that adopted the CDS with facilities that continued with MD.
Statistical Analyses
Baseline demographics, clinical characteristics and the frequency of achieving the Hb target range were compared for patients treated by CDS and manual dosing. Student t test and Pearson Chi-square test were used to compare continuous and categorical variables respectively. A three-level mixed effects model with two independent normally distributed random intercepts for patients and dialysis centers that accounts for correlated longitudinal measurements within patient over time and between patients within the same dialysis center was used to compare Hb values and log-transformed weekly EPO dose between patients by CDS and manual dosing in unadjusted and adjusted models. The unadjusted models accounts for dialysis facility only. The adjusted models account for all of the characteristics listed in Table 1 as well as time. SAS PROC MIXED was used for the continuous Hb levels and the log-transformed weekly EPO doses, and, SAS PROC GLIMMIX (SAS 9.1.3, Cary, NC) was used for binary variables (e.g. Hb 10–12 g/dL). Within and between patient variability in Hb levels was estimated from the mixed effect models. Sampling methods was used to calculate the adjusted monthly marginal population average probabilities for the binary Hb responses for patients treated with CDS vs. manual dosing from the above mixed random effect model. This method consists of simulating two independent vectors of random effects of size 20,000 for the patient and dialysis centers intercept from a normal distribution with variance components derived from the multivariable model. We then calculated the patient-specific probability of having the binary Hb response through the logistic regression model. Finally, we calculated the population-average probability of having the binary Hb response by averaging each participant-specific probability.
Table 1.
Baseline characteristics of hemodialysis patients treated with computerized decision support versus manual dosing of EPO
| Patient Characteristics | Manual Dosing (n = 7,823) |
Computerized Decision Support (n = 1,118) |
Total Patients (N = 8,941) |
P-value | |
|---|---|---|---|---|---|
| Age (years) | |||||
| 18-<45 | 15.0% (1170/7823) | 13.7% (153/1118) | 14.8% (1323/8941) | <0.001 | |
| 45-<65 | 41.3% (3229/7823) | 36.8% (411/1118) | 40.7% (3640/8941) | ||
| 65-<75 | 23.1% (1805/7823) | 23.3% (260/1118) | 23.1% (2065/8941) | ||
| ≥75 | 20.7% (1619/7823) | 26.3% (294/1118) | 21.4% (1913/8941) | ||
| Women | 46.2% (3611/7823) | 44.9% (502/1118) | 46.0% (4113/8941) | ||
| Race | |||||
| White | 49.5% (3869/7823) | 59.5% (665/1118) | 50.7% (4534/8941) | <0.001 | |
| African American | 42.5% (3322/7823) | 31.8% (355/1118) | 41.1% (3677/8941) | ||
| Other | 8.1% (632/7823) | 8.8% (98/1118) | 8.2% (730/8941) | ||
| Cause of ESRD | |||||
| GN | 11.6% (905/7807) | 15.2% (169/1115) | 12.0% (1074/8922) | <0.001 | |
| Hypertension | 28.6% (2233/7807) | 24.2% (270/1115) | 28.1% (2503/8922) | ||
| Diabetes | 42.9% (3347/7807) | 43.3% (483/1115) | 42.9% (3830/8922) | ||
| Other | 13.4% (1047/7807) | 12.2% (136/1115) | 13.3% (1183/8922) | ||
| Cystic/Hereditary | 3.5% (275/7807) | 5.1% (57/1115) | 3.7% (332/8922) | ||
| Incident to Dialysis& | 11.6 (907/7802) | 12.5 (140/1118) | 11.7 (1047/8920) | 0.4 | |
| Months on Dialysis~ | 28.4 (9.3, 58.1) | 23.9 (7.4, 54.9) | 27.5 (9.0, 57.8) | 0.03 | |
| Center Characteristics | |||||
| Facility Size (mean, SD) | 79.0 (36.9) | 81.2 (37.2) | 79.2(37.0) | 0.06 | |
| Serum Phosphate 3.5–5.5 mg/dL^ (%) | 53.2 (4164/7823) | 57.6 (644/1118) | 53.8 (4808/8941) | 0.006 | |
| Kt/V >1.3^ (%) | 87.0 (6794/7814) | 88.1 (984/1117) | 87.1 (7778/8931) | 0.3 | |
| AVF# (%) | 39.5(3093/7823) | 46.1 (515/1118) | 40.3 (3608/8941) | <0.001 | |
| Serum Albumin >3.5 g/dL^ (%) | 75.6 (5910/7823) | 75.0 (839/1118) | 75.5 (6749/8941) | 0.7 | |
| Serum Ferritin ng/mL ^ | 669.1 +/− 347.7 | 588.1 +/− 364.3 | 661.6 +/−350.3 | <0.001 | |
| Iron deficient## for ≥1 mo (%) | 13.8 (1077/7823) | 17.8 (199/1118) | 14.4 (1276/8941) | <0.001 | |
|
IV Iron (mg) administered per patient during study period mean(SD) |
1152.8 (899.1) | 892.4 (771.5) | 1143.3 (887.5) | <0.001 | |
| Hospitalization in 6 months % | 0.5 | ||||
| 0 | 54.7 (4280/7823) | 56.6 (633/1811) | 55.0 (4913/8941) | ||
| 1 | 22.6 (1769/7823) | 21.7 (243/1811) | 22.5 (2012/8941) | ||
| ≥2 | 22.7 (1774/7823) | 21.7 (242/1811) | 22.6 (201/8941) | ||
| Deaths in 6 months (%) | 4.7 (367/7823) | 5.7 (64/1118) | 4.8 (431/8941) | 0.1 | |
| Loss to Follow-up in 6 months | 3.3 (259/7823) | 2.3 (26/1118) | 3.2 (285/8941) | 0.08 | |
Note: Conversion factors for units: phosphate in mg/dL to mmol/L, x0.323; serum albumin in g/dL to g/L, x10, serum ferritin in ng/mL to mmol/L, x2.247
Abbreviations: ESRD, end-stage renal disease; GN, glomerulonephritis; AVF, arteriovenous fistula; IV, intravenous.
Based on the average for an individual over the 6-month observation period
AVF is used for greater than 70% of dialysis treatments over the 6-month observation period
Incident defined as started dialysis 60 days before the start of the observation period or during the observation period.
Median (Q1, Q3)
Iron deficient defined as Tsat (transferrin saturation)<20% and ferritin <200 ng/mL
RESULTS
During the six-month period, 13,011 patients were treated with EPO and had at least one Hb value, and 8,941 met the entry criteria. The 18 dialysis units that used CDS throughout the six months contributed 1,118 patients, and the 125 units that used manual dosing contributed 7,823. Patients treated in units using CDS were slightly older, less likely to be African American, more likely to have glomerulonephritis and less likely to have hypertension as the cause of kidney failure (Table 1). With regards to parameters reflecting quality of care, there was no difference in the proportion of patients with albumin>3.5 g/dl and Kt/V>1.3 among facilities that used CDS as compared with manual dosing. Facilities using the CDS had a lower proportion of patients with serum phosphate in the targeted range but a higher arteriovenous fistula rate. Patient case-mix, as reflected in serum albumin, hospitalization rates, or death rates, was not different between CDS and manual dosing. The rates of transfer out of the facility did not differ between patients treated by CDS and manual dosing. The observation time was six months for 79.2% of the population, five months for 7.4%, four months for 6.4% and three months for 7.0% of the study population, and there was no difference in observation time between those treated by CDS and manual dosing (p=0.6). In the units using CDS, physicians altered fewer than 10% of computer-generated prescriptions.
Achieved Hemoglobin Values
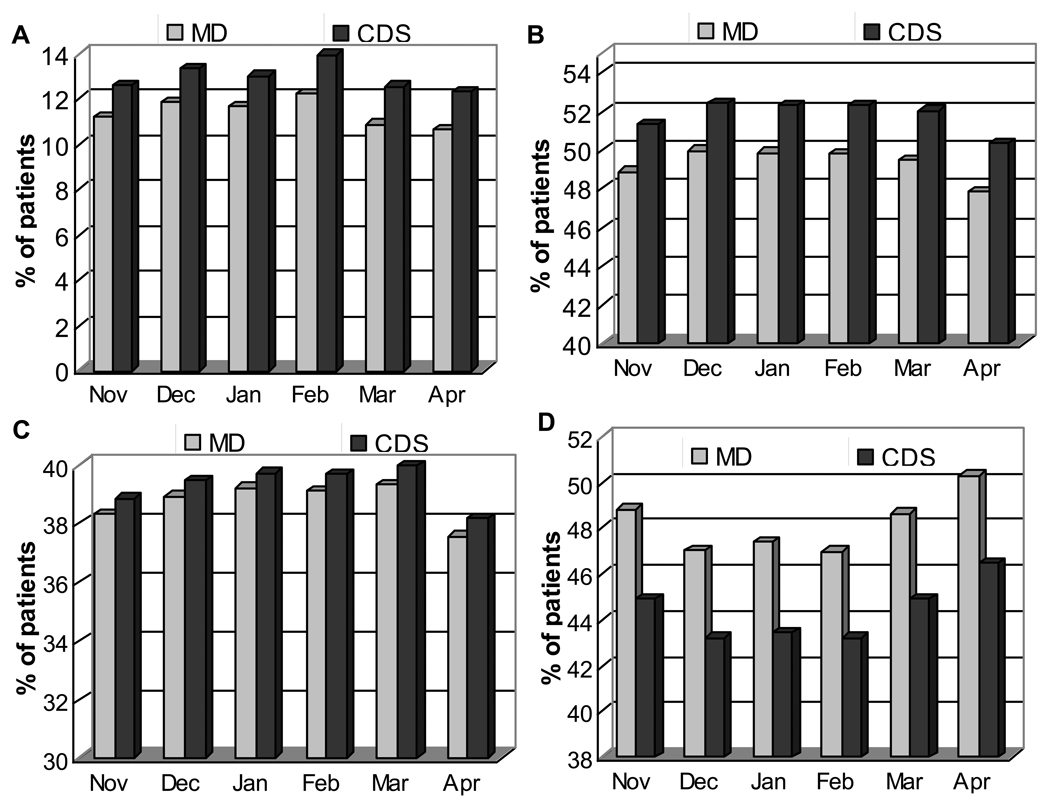
In the model adjusted only for center, the average Hb in patients treated by manual dosing was 11.8 (SE, 0.2) g/dL and was 0.11 (SE, 0.04) g/dL lower (p<0.001) in those treated by CDS. After adjusting for differences in baseline characteristics shown in Table 1, the Hb in patients treated by CDS was −0.14 (SE, 0.05) lower than patients treated by manual dosing. As shown in Table 2, there was a trend towards an increased likelihood of a monthly Hb of 10–12 g/dl (odds ratio (OR), 1.12; 95% confidence interval (CI), 0.97–1.28) and of 11–12 g/dl (OR, 1.03; 95% CI, 0.89–1.18) with CDS, but neither was statistically significant. As compared to manual dosing, CDS was less frequently associated with monthly Hb values exceeding 12 g/dl (OR, 0.85; 95%CI, 0.73–0.99) or 13 g/dL, (OR, 0.67; 95% CI, 0.53–0.84) but more frequently associated with monthly Hb values below 11 g/dL (OR, 1.25; 95% CI, 1.04–1.49) and 10 g/dL (OR, 1.26; 95% CI, 1.01–1.56)). Figure 1 shows the number of patients each month treated by CDS vs. manual dosing with a Hb value of 10–12g/dL, 11–12 g/dL, <10 g/dL or >12 g/dL each month, after accounting for patient and center differences in the CDS and manual dosing groups. As compared to patients treated by manual dosing, there is no difference in the frequency of Hb 10–12 g/dl (p=0.1) or 11–12 g/dl (p=0.7), a decrease in Hb >13 g/dL and an increase in Hb<10 g/dL with the CDS.
Table 2.
Likelihood of an achieved hemoglobin value within, below or above target with computerized decision support versus manual dosing of EPO
| Achieved Hemoglobin |
Unadjusted Odds | p value |
Adjusted# Odds |
P value |
|---|---|---|---|---|
| <10 g/dL | 1.14 (0.92–1.40) | 0.2 | 1.23 (1.06–1.42) 1.26 (1.01–1.56) |
0.03 |
| <11 g/dL | 1.19 (1.00–1.41) | 0.05 | 1.25 (1.04–1.49) | 0.01 |
| 10–12 g/dL | 1.11 (0.97–1.28) | 0.1 | 1.12 (0.97–1.28) | 0.1 |
| 11–12 g/dL | 1.03 (0.90–1.18) | 0.7 | 1.03 (0.89–1.18) | 0.7 |
| >12 g/dL | 0.87 (0.84–1.02) | 0.08 | 0.85 (0.73–0.99) | 0.04 |
| >13 g/dL | 0.68 (0.54–0.86) | 0.001 | 0.67 (0.53–0.84) | 0.001 |
Note: Conversion factor for hemoglobin in g/dL to g/L, x10.
Results are presented as odds ratio (95% confidence interval). Each result represents separate models. hemoglobin values falling outside the selected range serve as the reference group for the model.
Models are adjusted for age, race, cause of Eend-stage renal disease, sex, dialysis vintage, Kt/v, serum phosphate, albumin and ferritin, hospitalization count, and arteriovenous fistula use during the observation period.
Figures 1.
The frequency of Hemoglobin (Hb) measurements within the listed ranges over a 6-month period in patients treated by computerized decision support (CDS) and as per manual physician-directed dosing (manual dosing). A: (Hb)<10 g/dL; B: Hb 10–12 g/dL; C: Hb 11–12 g/dL; D: >12 g/dL. Statistically significant differences between patients treated by CDS and manual dosing were found for Hb<10 (P = 0.03) and Hb >12 g/dL (P=0.04). There were no differences for 10–12 g/dL (P=0.1) and 11–12 g/dL (P=0.7).
Hemoglobin Variability
The within-patient variability in Hb values was similar among patients treated by computerized protocol (σ2 = 1.30) and manual dosing (σ2 =1.30). The frequencies of larger Hb excursions (defined by a change in Hb level ≥1.5 g/dL) over 2-, 3- and 6-month intervals were 16% versus 17% (p=0.08), 33% versus 37% (p<0.001), and 67% versus 72% (p=0.003) for patients dosed using computerized and manual dosing, respectively.
EPO Use
The median weekly EPO dose was higher for patients treated by manual dosing [11,400 (Q1–Q3, 4,500–23,400) units] as compared with patients treated by the CDS [9,600 (Q1–Q3, 4,200–19,200) units]. After adjustment for center and baseline differences, the average log weekly EPO dose in patients treated by CDS was 4% less than those dosed manually (RR, 0.96; 95% CI, 0.77–1.18 ) but the difference was not statistically significant. The frequency of extreme EPO dose changes, defined as a dose change of ≥10,000 units per treatment from one 2-week period to the next, was 3.1 per 1,000 EPO doses administered by computerized protocol and 7.0 per 1000 EPO doses (p<0.001) with manual prescribing. The mean +/− SD cumulative intravenous iron administered during the study period to patients treated by CDS was less than to patients treated by manual dosing (892 +/−772 vs. 1173 +/− 899 mg, respectively p<0.001).
Time Spent on Anemia Management as Reported by the Dialysis Unit Nurse Manager
Nurse managers of 92% of dialysis units responded to the survey question. Units using the computerized protocol reported spending a median of 3 hours monthly on anemia management (Q1–Q3, 2.0–4.5), whereas units using manual dosing reported spending a median 6.5 hours (Q1–Q3, 4.0–9.5). The difference was statistically significant (p<0.001).
DISCUSSION
When compared to manual dosing, whether based on the physician’s decision or on paper-based protocols, EPO dosing using an algorithm integrated into a medical information system was associated with the same proportion of Hb values between 10 and 12 g/dL and 11 and 12 g/dL, while the time spent by dialysis unit staff on anemia management was reduced by nearly one half. The computerized protocol was associated with a smaller proportion of monthly values exceeding 12 g/dL, and with a modest increase in the proportion of Hb values below 10 g/dL. There was no difference in EPO doses or Hb variability in patients treated with the CDS as compared with manual dosing.
Several smaller, single center studies have shown benefits of protocol-based anemia management in the dialysis unit. Patterson et al. reported an increase from 27 to 61% of patients meeting the targeted hematocrit (Hct) range (of 31 to 36%) with use of a nurse-administered EPO and iron dosing protocol in 30 patients [4]. Iron use increased and there was a significant reduction in the average weekly EPO dose from 11,200 ± 1,400 to 9,400 ± 1,200 units (p =0.06). The average Hct among 49 patients treated by a pharmacist-administered anemia management protocol was not different than a physician-directed dosing [5]. In this same study iron use increased and there was a trend towards a reduction in EPO use although it was not significant. In another study of 278 incident dialysis patients (86% African American) a pharmacist- managed EPO and iron dosing protocol maintained Hb, while the average weekly EPO dose was 46% less [6]. In the only randomized clinical trial to compare protocolized EPO dosing vs. physician-directed dosing, more Hb values were within the targeted range by protocol, but the difference was not significant [7]. In this trial, however, study arms may have been cross-contaminated as dialysis staff at the same unit treated both groups. Together, these studies are smaller, single center studies that predate the global improvements in anemia management and increased attention that has been paid to quality monitoring within recent years [8, 9]. Our contemporary results show that, even present day where anemia is a major focus of care in the dialysis unit, protocolized EPO dosing performs as well as individual physician decision or by paper-based protocol and significantly reduces time devoted by dialysis unit staff to anemia management.
Experience with computerized anemia management decision support is limited. A computerized dosing algorithm similar to the one described in this study, with a direct interface between the laboratory and the electronic medical record in conjunction with an electronic order entry system, has been used at a dialysis center in Leeds, UK for more than 8 years and, has recently been adopted by other units [10]. EPO dosing is considerably more complicated than dosing most medications, such as antibiotics or antihypertensive agents, where dose adjustments are based on one or two factors such as creatinine clearance or blood pressure. A number of factors, including the current Hb value, the trend in Hb over the past 30 days, whether there was a recent EPO dose change and is so, the response to it, whether iron stores are adequate, and whether the patient is inflamed, among others, weigh in to deciding which EPO dose to administer. Because of the economic and regulatory importance of EPO, the role of “anemia manager” often falls to one of the most experienced nurses in a dialysis facility, competing with other aspects of quality improvement and patient care. Computerized decision support seems particularly well suited to EPO prescribing given the large amount of information that must be retrieved and processed and the time that is being spent by highly trained staff on anemia management present day.
The protocols used in this and past studies are based on a finite number of rules (20 in this study) and give ‘one-size fits all’ recommendations. Individualized models that describe Hb and/or EPO responsiveness on a continuous scale and incorporate parameters that predict a change in EPO responsiveness before it occurs, may further increase the proportion of patients that are within the target each month. Our observation that computerized dosing was equivalent to manual EPO dosing in achieving a Hb target might be taken to suggest that the clinical circumstances which can influence physicians and nurses in adjusting EPO doses do not convey information beyond that summarized in the hemoglobin value itself. Alternatively, it is possible that if the computerized algorithm took account of clinical factors, such as the occurrence of infection, or changes in serum albumin, it might do even better. Models developed to date [11–14] have been tested in small, relatively stable populations and results are promising, but their accuracy in larger populations, including unstable patients, needs to be demonstrated before they can be used in practice.
The lack of incorporating iron administration in conjunction with EPO is a weakness of the CDS used in this study. Indeed a negative effect of using the CDS was lower intravenous iron use than units dosing EPO manually. It is plausible that clinicians using the CDS may have been less aware of iron stores. However, there was a trend towards less EPO use, despite the lower iron use. This raises the possibility that a CDS that incorporates both iron and EPO dosing may increase the numbers achieving the targeted Hb range and use less EPO.
The strength of this study is the use of the computerized decision support in a large, representative hemodialysis population. There was no possibility for cross-contamination of the two different methods of EPO prescribing, because CDS was adopted at the dialysis unit level. The main limitation is that the use of CDS was neither blinded nor randomized by dialysis unit. While it is possible that patients from the units that voluntarily adopted the computerized protocol had less EPO resistance and more stable Hb values than those that were dosed manually, there are no data to support this hypothesis. Case-mix, as reflected in serum albumin, hospitalization and death rates was the same. CDS units had a higher rate of arteriovenous fistulas (AVF) and serum phosphates in range, but more patients were iron deficient and less iron was administered, which would argue that CDS units were not more likely to have processes in place (other than the CDS) promoting anemia management. Spolter et al has also shown a lack of concordance between a unit’s performance on one quality indicator and another [15]. We believe there is no strong evidence to suggest that CDS units were biased towards achieving better results in anemia management. Nonetheless, these analyses are adjusted for center, which should reduces the potential for bias if there were differences in patient factors or practices across facilities.
In summary, a computerized EPO dosing protocol was associated with no difference in the proportion of monthly Hb values between 10 and 12 g/dL and 11 and 12 g/dL, fewer Hb values exceeding 12 g/dL, and more Hb values below 10 mg/dL. The CDS was associated with a nearly 50% reduction in nursing staff time spent on anemia management. We conclude that computerized decision support maintains anemia management and reduces its burden on dialysis staff.
ACKNOWLEDGEMENTS
The Medical Directors of DCI include ♦♦♦♦♦♦♦♦
We wish to acknowledge the essential contributions of dialysis unit staff who administered and recorded the EPO doses and who responded to our survey, and of the DCI and Keane staff who developed and implemented the computerized EPO treatment protocol, as well as the information system which made its implementation possible.
Support: Drs Miskulin and Weiner are supported by National Institutes of Health grants K23 DK066273 and DK 071636, respectively. Drs Meyer, Weiner and Zager receive salary support from DCI as medical directors at DCI units. Drs Miskulin, Meyer, and Zager receive research support from DCI.
APPENDIX
DCI EPO Management Protocol (pre-May 2006)
Note: This protocol is expressed in terms of calculated Hct, a value equal to three times the measured Hb concentration.
EPO doses are based on estimated dry weight.
- The protocol will change the EPO dose no more often than every 4 weeks, except in the following cases:
- Discontinue EPO when the calculated Hct is greater than or equal to 39%.
- Increase the EPO dose when the calculated Hct is less than 33% and the previous dose change was a reduction in EPO.
- Decrease the EPO dose when the calculated Hct is greater than 37.4% and the previous dose change was an increase in EPO.
If the calculated Hct is greater than or equal to 39%, then discontinue EPO and check the calculated Hct weekly. Resume EPO at 25% less than the previous dose as soon as the calculated Hct is less than or equal to 37.4%.
For established patients who have had no EPO order within the last 3 months and have calculated Hct < 33%, start EPO 375 units/kg/week; for Hct 33–35.9%, start EPO 225 units/kg/week; for Hct 36–37.4%, start EPO 150 units/kg/week.
For new patients without EPO orders who have calculated Hct < 33%, start EPO 375 units/kg/week; for Hct 33–35.9%, start EPO 225 units/kg/week; for Hct > 35.9%, start no EPO, but check calculated Hct every two weeks.
The weekly doses described in (4) and (5) will be equally divided into IV dosing for each hemodialysis treatment.
Increase EPO dose by protocol no higher than 900 units/kg/week.
- For patients who have an EPO order and the order was not changed with the last 4 weeks or cases described in (2):
- If calculated Hct < 28%, then increase EPO 50% but not less then 375 units/kg/week.
- If calculated Hct between 28 – 29.9%, then increase EPO 50%.
- If calculated Hct between 30 – 32.9%, then increase EPO 20%.
- If calculated Hct between 33 – 35.9% and Hct decreased 1.5 or more since last dose change, then increase EPO 10%.
- If calculated Hct between 33 – 35.9% and Hct increased/decreased less than 1.5 since last dose change, then do not change EPO dose.
- If calculated Hct between 33 – 35.4% and Hct increased 1.5 or more since last dose change, then do not change EPO dose.
- If calculated Hct between 35.5 – 35.9% and Hct increased 1.5 or more since last dose change, then decrease EPO 10%.
- If calculated Hct between 36 – 37.4% and Hct decreased 1.5 or more since last dose change, then do not change EPO dose.
- If calculated Hct between 36 – 37.4% and Hct increased/decreased less than 1.5 since last dose change, then decrease EPO 10%.
- If calculated Hct between 36 – 37.4% and Hct increased 1.5 or more since last dose change, then decrease EPO 20%.
- If calculated Hct between 37.5 – 38.9% and Hct decreased 1.5 or more since last dose change, then decrease EPO 10%.
- If calculated Hct between 37.5 – 38.9% and Hct increased/decreased less than 1.5 since last dose change, then decrease EPO 20%.
- If calculated Hct between 37.5 – 38.9% and Hct increased 1.5 or more since last dose change then, decrease EPO 20%.
- If calculated Hct is greater than or equal to 39%, then stop EPO and check calculated Hct weekly.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
For N section:
A list of the Medical Directors of Dialysis Clinic Inc appears at the end of this article.
Because an author of this manuscript is an editor for AJKD, the peer-review and decision-making processes were handled entirely by an Associate Editor (Michel Jadoul, MD, Cliniques Universitaires St-Luc) who served as Acting Editor-in-Chief. Details of the journal’s procedures for potential editor conflicts are given in the Editorial Policies section of the AJKD website.
REFERENCES
- 1.Ebben JP, Gilbertson DG, Foley RN, et al. Hemoglobin Level Variability: Associations with Comorbid Conditions, Intercurrent Events and Hospitalizations. Clin J Am Soc Nephrol. 2006;1:1205–1210. doi: 10.2215/CJN.01110306. [DOI] [PubMed] [Google Scholar]
- 2.Kuperman GJ, Bobb A, Payne TH, et al. Medication-related clinical decision support in computerized provider order entry systems: a review. J Am Med Inform Assoc. 2007;14:29–40. doi: 10.1197/jamia.M2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawamoto K, Houlihan CA, Balas EA, et al. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330:765. doi: 10.1136/bmj.38398.500764.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patterson P. M A: Prospective evaluation of an anemia treatment algorithm in hemodialysis patients. Am J Kidney Dis. 1998;32:635–641. doi: 10.1016/s0272-6386(98)70028-9. [DOI] [PubMed] [Google Scholar]
- 5.To LL, Stoner CP, Stolley SN, et al. Effectiveness of a pharmacist-implemented anemia management protocol in an outpatient hemodialysis unit. American Society of Health-System Pharmacists. 2001;58:2061–2065. doi: 10.1093/ajhp/58.21.2061. [DOI] [PubMed] [Google Scholar]
- 6.Walton T, Holloway K, Knauss M. Pharmacist-Managed Anemia Program in an Outpatient Hemodialysis Population. Hospital Pharmacy. 2005:40. [Google Scholar]
- 7.Brimble K, Rabbat C, McKenna P, et al. Protocolized anemia management with erythropoietin in hemodialysis patients: a randomized controlled trial. J Am Soc Nephrol. 2003;14:2654–2661. doi: 10.1097/01.asn.0000088026.88074.20. [DOI] [PubMed] [Google Scholar]
- 8.Department of Health and, Human Services, Centers for Medicare & Medicaid Services, Office of Clinical Standards & Quality. Maryland: Baltimore; 2007. Dec, Centers for Medicare & Medicaid Services 2007 Annual Report, End Stage Renal Disease Clinical Performance Measures Project. [Google Scholar]
- 9.National Kidney Foundation. [accessed Sept 14 2008];KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. 2006 doi: 10.1053/j.ajkd.2006.03.010. Available at http://www.kidney.org/Professionals/kdoqi/guidelines_anemia/index.htm. [DOI] [PubMed]
- 10.Will EJ, Richardson D, Tolman C, et al. Development and exploitation of a clinical decision support system for the management of renal anaemia. Nephrol Dial Transplant. 2007;22 Suppl 4:iv31–iv36. doi: 10.1093/ndt/gfm163. [DOI] [PubMed] [Google Scholar]
- 11.Gaweda AE, Jacobs AA, Aronoff GR, et al. Model predictive control of erythropoietin administration in the anemia of ESRD. American Journal of Kidney Diseases. 2008;51:71–79. doi: 10.1053/j.ajkd.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Martin-Guerrero JD, Camps-Valls G, Soria-Olivas E, et al. Dosage individualization of erythropoietin using a profile-dependent support vector regression. IEEE Transactions on Biomedical Engineering. 2003;50:1136–1142. doi: 10.1109/TBME.2003.816084. [DOI] [PubMed] [Google Scholar]
- 13.Uehlinger DE, Gotch FA, Sheiner LB. A pharmacodynamic model of erythropoietin therapy for uremic anemia. Clin Pharmacol Ther. 1992;51:76–89. doi: 10.1038/clpt.1992.10. [DOI] [PubMed] [Google Scholar]
- 14.West RM, Harris K, Gilthorpe MS, et al. Functional data analysis applied to a randomized controlled clinical trial in hemodialysis patients describes the variability of patient responses in the control of renal anemia. J Am Soc Nephrol. 2007;18:2371–2376. doi: 10.1681/ASN.2006050436. [DOI] [PubMed] [Google Scholar]
- 15.Spolter YS, Seliger S, Zhan M, et al. The relationship between dialysis performance measures: adequacy and anemia management. Am J Kidney Dis. 2007;50:774–781. doi: 10.1053/j.ajkd.2007.08.006. [DOI] [PubMed] [Google Scholar]