Abstract
Placental development occurs under a low oxygen (2–8% O2) environment, which is critical for placental development and angiogenesis. In this study, we examined if hypoxia affected fibroblast growth factor 2 (FGF2)- and vascular endothelial growth factor (VEGF)-stimulated cell proliferation via the mitogen-activated protein kinase kinase 1/2 (MEK1/2)/extracellular signal-regulated kinases 1/2 (ERK1/2) and phosphatidylinositol-3 kinase (PI3K)/v-akt murine thymomaviral oncogene homologue (AKT1) pathways in human placental artery endothelial (HPAE) cells. We observed that under normoxia (~20% O2), FGF2 and VEGF dose-dependently stimulated cell proliferation. Hypoxia (3% O2) significantly promoted FGF2- and VEGF-stimulated cell proliferation as compared to normoxia. Under both normoxia and hypoxia, FGF2 rapidly induced ERK1/2 and AKT1 phosphorylation, while VEGF induced ERK1/2, but not AKT1 phosphorylation. However, hypoxia did not significantly alter FGF2- and VEGF-induced ERK1/2 and AKT1 phosphorylation as compared to normoxia. PD98059 (a MEK1/2 inhibitor) at 20 μM and LY294002 (a PI3K inhibitor) at 5 μM attenuated FGF2- and VEGF-induced phosphorylation of ERK1/2 and AKT1, respectively. PD98059, even at doses that drastically inhibited FGF2-induced ERK1/2 phosphorylation (20 μM) and caused cell loss (40 μM), did not affect FGF2-stimulated cell proliferation, which was confirmed by U0126 (another potent MEK1/2 inhibitor). PD98059, however, dose-dependently inhibited VEGF-stimulated cell proliferation. Conversely, LY294002 dose-dependently inhibited FGF2-, but not VEGF-stimulated cell proliferation. These data suggest that in the MEK1/2/ERK1/2 and PI3K/AKT1 pathways differentially mediate FGF2- and VEGF-stimulated HPAE cell proliferation. These results also indicate that hypoxia promotes FGF2- and VEGF-stimulated cell proliferation without further activation of the PI3K/AKT1 and MEK1/2/ERK1/2, respectively.
Keywords: hypoxia, placenta, endothelial cells, kinases, cell proliferation
INTRODUCTION
Placental development is associated with significant increases in both angiogenesis and vasodilatation, giving rise to a dramatic elevation of placental blood flow during pregnancy. This increased blood flow is directly correlated with fetal growth and survival as well as neonatal birth weights and survivability [1–3]. Placental development during normal pregnancy takes place within a low oxygen (~2–8 % O2) environment [4–6]. This physiological hypoxia is critical for early placental development and angiogenesis [4–6]. Fibroblast growth factor-2 (FGF2) and vascular endothelial growth factor (VEGF) are two potent angiogenic factors, both of which have been implicated to play important roles in placental angiogenesis [6–9].
The cells sense changes in oxygen concentrations by a family of oxygen sensitive transcriptional factors, hypoxia inducible factors (HIF) [5,10]. Numerous in vitro studies have shown that hypoxia (2–5% O2) promotes endothelial cell proliferation [11–15], migration [12], and formation of capillary-like tube structures [16]. These stimulatory actions of hypoxia are generally mediated via altering expression of angiogenic factors and their receptors [17]. For example, it is well illustrated that hypoxia increases expression of VEGF and its receptors VEGF receptor-1 and -2 in endothelial cells [17]. Hypoxia also increases expression of FGF receptors [18] and alters distribution of FGF2 inside different cellular compartments [16]. On the other hand, hypoxia could also enhance angiogenesis without involvement of angiogenic factors (e.g., VEGF), suggesting alternative mechanisms underlying hypoxia-regulated angiogenesis [13,19].
It is well established that FGF2- and VEGF-induced cellular responses are mediated via binding to their specific tyrosine kinase receptors and downstream activation of mitogen-activated protein kinase kinase 1/2 (MEK1/2)/extracellular signal-regulated kinases 1/2 (ERK1/2) and phosphatidylinositol-3 kinase (PI3K)/v-akt murine thymomaviral oncogene homologue (AKT1) pathways [20–22]. We have reported that FGF2- and VEGF-activated MEK1/2/ERK1/2 and PI3K/AKT1 pathways actively participate in FGF2- and VEGF-stimulated ovine placental endothelial cell responses including proliferation [23] and endothelial nitric oxide expression [24,25].
Hypoxia can directly activate the MEK1/2/ERK1/2 and PI3K/AKT1 pathways in many cell types [26–29]. Nuclear translocation of activated ERK1/2 and/or AKT1 leads to phosphorylation of several transcription factors (e.g. Egr-1, c-Jun, Elk-1, c-Fos, and Ets-1), which in turn regulate expression of some key genes involved in cell proliferation, migration, and survival [30,31]. For example, it has been shown that hypoxia (3% O2, 24 h) stimulates bovine pulmonary artery adventitial fibroblast proliferation via activation of ERK1/2 and PI3K/AKT1, which does not require other exogenous stimuli [28].
Little is known regarding the effects of hypoxia on FGF2- and VEGF-stimulated placental angiogenesis and the underlying signaling mechanisms. Herein, we tested a hypothesis that hypoxia enhanced FGF2- and VEGF-stimulated proliferation via increasing activation of the MEK1/2/ERK1/2 and PI3K/AKT1 pathways in human placental artery endothelial (HPAE) cells.
METHODS
HPAE Cell Preparation
Primary HPAE cells were isolated and cultured in our laboratory as previously described [10]. After isolation, these cells were expanded in MCDB131 media containing 5% FBS, 5% CS, 1% P/S under normoxia (37° C, 5% CO2, 95% air [~20% O2]). All HPAE cells used in this study were at passages 5–6. Protocols for placental artery collection and endothelial isolation were approved by the Institutional Review Boards, Meriter Hospital and University of Wisconsin-Madison, and followed the recommended guidelines for using human subjects.
Hypoxic Culture
Cells grown in 35-mm dishes were cultured in 1 ml of MCDB131 media containing serum in an incubator (Sanyo Electric Co., Japan) under hypoxia (37° C, 5% CO2, 3% O2, 92% N2). To confirm the hypoxic condition, O2 tension in media was real-time monitored using a SevenGo™ dissolved oxygen meter (Mettler Toledo, Columbus, OH). We found that once the cultures were placed in the hypoxic condition, O2 tension in media was rapidly reduced, reaching ~ 3% after 2 hr in culture, and maintained at this level afterward. We also verified hypoxia in cells by determining cellular HIF-1α protein levels using Western blot analysis as described [24,33,34]. Briefly, cells cultured under hypoxia were washed twice with cold PBS, harvested, and lyzed by sonication in buffer (20 mM imidazole-HCl, 2mM EGTA, 2mM EDTA, pH7.0, 0.1 mM PMSF, 0.01% Triton X-100, 5 mg/ml leupeptin, 5 mg/ml aprotinin). The lysates were centrifuged and protein concentrations of the supernatant were determined. Proteins (15 μg) were separated on 10% SDS-PAGE gels and electroblotted onto Immobilon-P membrane (Millipore, Bedford, MA), probed with an antibody against HIF-1α (1: 500; BD Biosciences, San Jose, CA). The proteins were detected using ECL-plus detection systems (Amersham Biosciences, Piscataway, NJ). The membranes were re-probed with β-actin (1: 5000; Ambion, Austin, TX). Changes in HIF-1α and β-actin protein levels were quantified by a scanning densitometer. Data on HIF-1α were normalized to β-actin.
Cell Proliferation
Cell proliferation was assayed as described [24,33,34]. Cells were cultured in 75 cm2 cell culture flasks in MCDB131 media under normoxia until reaching confluence. Cells were split into 96 well plates (5000–6000 cells/well) and cultured for 24 hr under hypoxia (37° C, 5% CO2, 3% O2, 92% N2) or normoxia (37° C, 5% CO2, 95% air). After serum starvation for another 24 hr, cells were treated with FGF2 or VEGF (0–100 ng/ml) for 72 hr under hypoxia or normoxia, followed by determining the number of cells using the crystal violet method. Briefly, wells were rinsed with PBS, fixed in methanol, air-dried, and stained with 0.1% (w/v) crystal violet. Wells were rinsed with distilled water, and air-dried again. Cells were lyzed with 2% (w/v) sodium deoxycholate solution with gentle agitation. Absorbance was measured at 570 nm on a microplate reader (Bio-TEK Instrument, Winooski, VT). Wells containing known cell numbers (0, 1000, 2000, 5000, 10000, 20000 or 40000 cells/well; 6 wells/cell density) were treated in the similar fashion to establish standard curves.
To determine the roles of the MEK1/2/ERK1/2 and PI3K/AKT1 pathways in FGF2- and VEGF-stimulated cell proliferation, additional cells were subjected to the cell proliferation assay under normoxia as described above. After deprivation from serum for 24 hr, cells were treated with 10 ng/ml of FGF2 or VEGF in the absence or presence of PD98059 (a selective MEK1/2 inhibitor, 1 hr pretreatment; CalBiochem, San Diego, CA), U0126 (a selective MEK1/2 inhibitor; 1 hr pretreatment; Promega, Madison, WI) or LY 294002 (a selective PI3K inhibitor, 1 hr pretreatment; Cell Signaling Technology, Beverly, MA). After an additional 72 hr of growth factor treatments, the numbers of cells were determined as described above.
Western Blot Analysis for ERK1/2 and AKT1
Western blot analysis was performed as described [24,33,34]. Cells were cultured as described above. After reaching confluence, cells were split into 3.5 cm culture dishes and cultured for 24 hr under hypoxia or normoxia. After serum starvation for additional 24 hr, FGF2 or VEGF was added to the dishes (final concentrations of FGF2 and VEGF were 10 ng/ml). Cells were cultured for 0, 5, 10, 30, or 60 min. For the hypoxia group, all cell treatments after cells were split into 3.5 cm dishes were carried out inside a specially designed heated hypoxic glove box (Coy Laboratory Products, Grass Lake, MI).
To verify activation of ERK1/2 and AKT1, additional cells were treated with FGF2 for 5 min or with VEGF for 10 min in the absence or presence of PD98059 (20 μM; 1 hr pretreatment) or LY294002 (5 μM; 1 hr pretreatment). Controls included cells cultured with the inhibitors alone. Cells were washed twice with cold PBS, and then harvested and lyzed by sonication in buffer (4 mM sodium pyrophosphate, 50 mM HEPES, pH 7.5, 100 mM NaCl, 10 mM EDTA, 10 mM sodium fluoride, 2 mM sodium orthovanadate, 1 mM PMSF, 1% Triton X-100, 5 mg/ml leupeptin, 5 mg/ml aprotinin). Proteins (10–15 μg) were subjected to Western blot analysis as described above. For each set of samples, at least two gels were run simultaneously. One membrane was blotted with a phospho-ERK1/2 antibody, followed by reblotting with a total ERK1/2 antibody (both were at 1: 2000). Another membrane was blotted with a phospho-AKT1 antibody (1:500) followed by reblotting with a total AKT1 antibody (1:2000). All four antibodies were purchased from Cell Signaling Technology, Beverly, MA. Changes in total and phospho-ERK1/2 and AKT1 protein levels were quantified. Data on phospho-ERK1/2 and AKT1 were normalized to total ERK1/2 and AKT1.
Statistics Procedures
Data were analyzed using one-way ANOVA (SigmaStat; Jandel Co., San Rafael, CA). When an F-test was significant, data were compared with their respective control by the Bonferroni’s multiple comparison test or Student t-test.
RESULTS
Hypoxia Increased HIF-1α Protein Levels in HPAE Cells
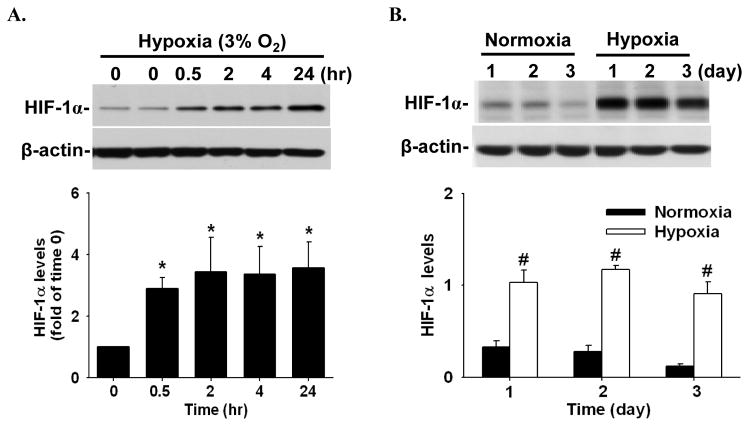
In HPAE cells cultured in media containing serum, hypoxia rapidly increased (≤ 0.5 hr; p < 0.05) HIF-1α protein levels (~ 2.9 fold of the time 0 control), which were maintained at relatively high levels up to 24 hr (~ 3.6 fold), without changing β-actin levels (Fig. 1A). When HPAE cells were cultured in serum free media, HIF-1α protein levels were increased (p < 0.05) in hypoxia as compared with normoxia (average ~ 5.6 fold) and these relatively high HIF-1α levels were maintained for at least three days (Fig. 1B).
Figure 1.
Hypoxia-increased HIF-1α levels in HPAE cells. Cells were cultured in media containing serum for 24 hr under normoxia, followed by culturing cells under hypoxia in serum containing media (A) or in serum free media (B). Proteins were subjected to Western blot analysis for HIF-1α. Representative images are shown. Data normalized to β-actin are expressed as means ± SEM of fold of the time 0 control (in A; n = 4) or normoxia controls (in B; n = 3). *differ (p < 0.05) from the time 0 control. #differ (p < 0.05) from normoxia on each corresponding day.
Hypoxia Enhanced FGF2- and VEGF-Stimulated Cell Proliferation
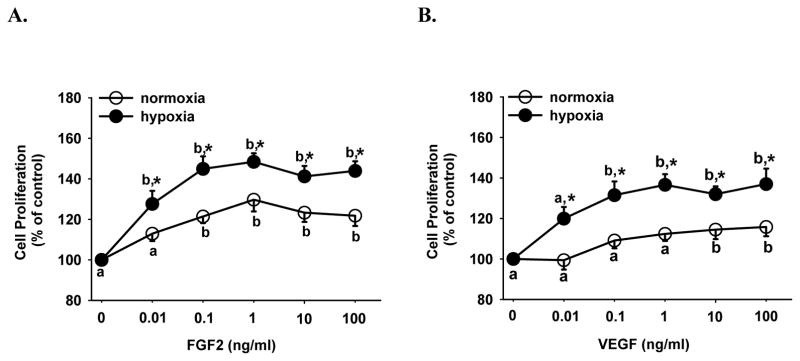
Both FGF2 and VEGF dose-dependently stimulated (p < 0.05) HPAE cell proliferation under normoxia and hypoxia (Fig. 2). However, hypoxia significantly enhanced (p < 0.05) both FGF2- and VEGF-stimulated HPAE cell proliferation by ~ 20% at all doses tested as compared with normoxia. Interestingly, the minimum concentration of each growth factor which stimulated cell proliferation under hypoxia (0.01 ng/ml FGF2 and 0.1 ng/ml VEGF) was at least 10 fold lower than those under normoxia (0.1 ng/ml FGF2 or 10 ng/ml VEGF). These data suggest that cells become more sensitive to FGF2 and VEGF stimulation under hypoxia (Fig. 2).
Figure 2.
Effects of hypoxia on FGF2- and VEGF-stimulated HPAE cell proliferation. Cells seeded in 96-well plates (5000 cells/well) were cultured in serum containing medium for 24 hr under normoxia and hypoxia. After 24 hr of serum starvation, cells were treated without or with FGF2 (A) or VEGF (B) for additional 72 hr. Cell numbers were then determined. Data are expressed as means ± SEM percent of the control (no growth factor). Numbers of cells per well in the controls after 72 hr were 5573 ± 633 and 4728 ± 327 under normoxia and hypoxia, respectively. a,b Within each growth factor treatment group, means with different letters differ (p < 0.05). *differs (p < 0.05) from the normoxia at the corresponding dose of growth factor.
Both FGF2 and VEGF Induced Phosphorylation of ERK1/2 and AKT1
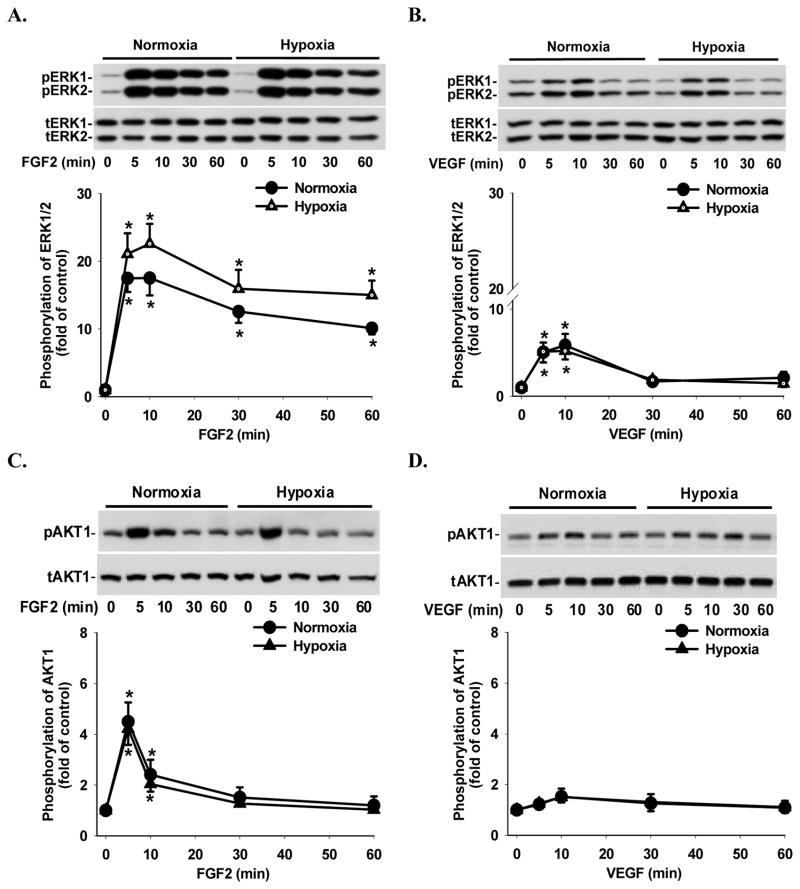
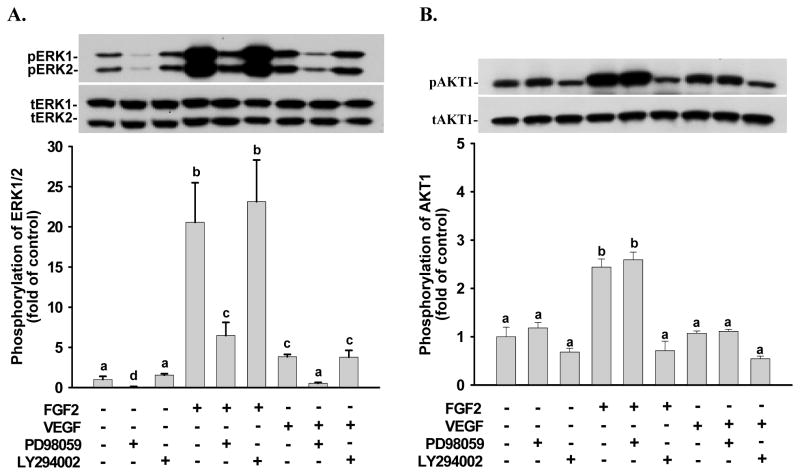
Under normoxia and hypoxia, FGF2 and VEGF rapidly induced (≤ 5 min; p < 0.05) ERK1/2 phosphorylation (Fig. 3A and B). FGF2-induced ERK1/2 phosphorylation was maintained at relatively high levels up to 60 min even though it declined slightly after 10 min of FGF2 treatments (Fig. 3A). VEGF-induced ERK1/2 phosphorylation began to decrease after 10 min and returned to the basal levels after 30 min of VEGF treatments (Fig. 3B). Under normoxia and hypoxia, FGF2 also rapidly induced (≤ 5 min; p < 0.05) AKT1 phosphorylation; however, this phosphorylation declined to the basal level after 30 min of FGF2 treatments (Fig. 3C). In contrast, VEGF did not significantly increase AKT1 phosphorylation at any time point examined (Fig. 3D). Hypoxia slightly enhanced FGF2-induced ERK1/2 phosphorylation as compared to normoxia; however, this stimulatory effect did not reach the statistical significance (Fig. 3A). Similarly, hypoxia also did not alter VEGF-induced ERK1/2 phosphorylation (Fig. 3B) as well as FGF2- and VEGF-induced AKT1 phosphorylation (Fig. 3C and D) as compared to normoxia. No significant change was observed for total and phospho-ERK1/2 or AKT1 levels between normoxia and hypoxia at the time 0 controls (Fig. 3). PD98059 at 20 μM significantly inhibited (p < 0.05) FGF2- and VEGF-induced ERK1/2 phosphorylation (Fig. 4A), while LY294002 at 5 μM blocked (p < 0.05) FGF2-induced AKT1 phosphorylation (Fig. 4B). PD98059 at 20 μM (Fig. 4A) and LY294002 at 5 μM (Fig. 4B) did not significantly affect FGF2-induced and VEGF-induced phosphorylation of AKT1 and ERK1/2, respectively, confirming the specificity of these kinase inhibitors and also suggesting that no cross-talk exists between these two pathways in HPAE cells.
Figure 3.
Effects of hypoxia on FGF2- and VEGF-induced ERK1/2 and AKT1 phosphorylation in HPAE cells. Cells plated in 6 cm culture dishes were cultured under normoxia until reaching 70–80% confluence, followed by normoxic or hypoxic culture for 24 hr and serum starvation for additional 24 hr. Cells were then treated with 10 ng/ml of FGF2 (A and C) or VEGF (B and D) for 0–60 min. Proteins were subjected to Western blot analysis for total ERK1/2 (tERK1/2) and total AKT1 (tAKT1) or phospho-ERK1/2 (pERK1/2) and phospho-AKT1 (pAKT1). Data normalized to tERK1/2 and tAKT1 are expressed as means ± SEM fold of the time 0 controls (n = 6). *differ (p < 0.05) from the corresponding time 0 control.
Figure 4.
Effects of PD98059 and LY294002 on FGF2- and VEGF-induced phosphorylation of ERK1/2 and AKT1 in HPAE cells. Cells were cultured under normoxia for 24 hr. After serum starvation, cells were treated with FGF2 (A) for 5 min or VEGF (B) for 10 min in the absence or presence of PD98059 (20 μM) or LY294002 (5 μM) (1 hr pretreatment). Proteins were subjected to Western blot analysis for total ERK1/2 (tERK1/2) and total AKT1 (tAKT1) or phospho-ERK1/2 (pERK1/2) and phospho-AKT1 (pAKT1). Data are expressed as means ± SEM fold of the control (no growth factor and kinase inhibitor; n = 4). a,b,c,d Means with different letters differ (p < 0.05).
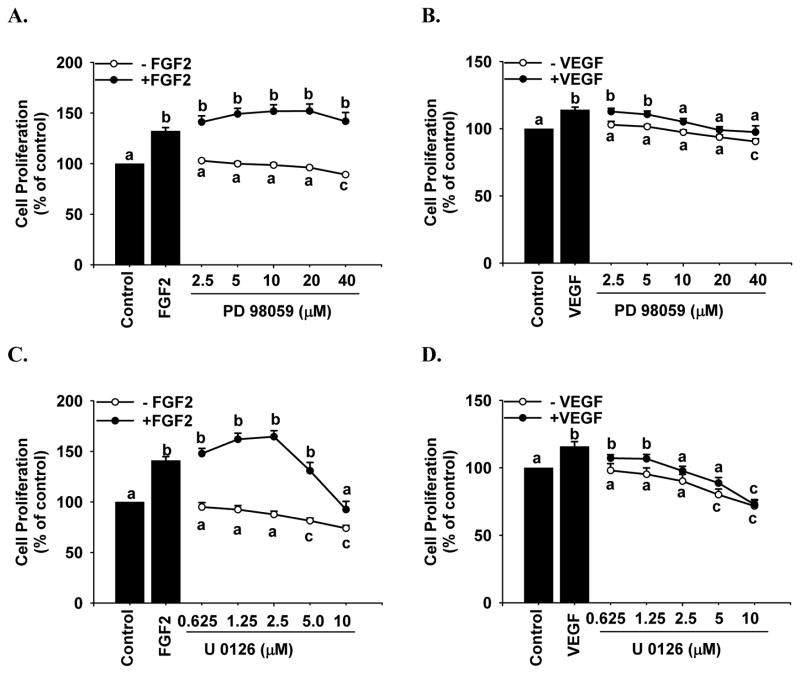
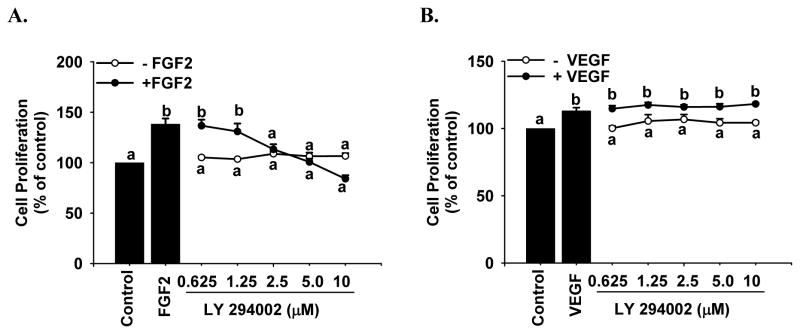
Effects of PD98059, U0126, and LY294002 on FGF2- and VEGF-Stimulated Cell Proliferation
Both PD98059 (Fig. 5A) and U0126 (Fig. 5C) at any dose studied did not affect FGF2-stimulated cell proliferation; however, they both dose-dependently inhibited (p < 0.05) VEGF-stimulated cell proliferation (Fig. 5B and 5D). In contrast, LY294002 dose-dependently inhibited (p < 0.05) FGF2- (Fig. 6A), but not VEGF-stimulated cell proliferation (Fig. 6B). In the absence of FGF2 and VEGF, PD98059 up to 20 μM and U0126 up to 2.5 μM (Fig. 5) as well as LY294002 at any dose studied (Fig. 6) did not significantly change basal cell numbers; PD98059 at 40 μM and U0126 at 5 and 10 μM decreased (p < 0.05) cell numbers (Fig. 5), indicating that these inhibitors at these relatively high concentrations were toxic to HPAE cells.
Figure 5.
Effects of PD98059 and U0126 on FGF2- and VEGF-stimulated HPAE cell proliferation. Cells were cultured under normoxia for 24 hr. After 24 hr of serum starvation, cells seeded in 96-well plates were treated with 10 ng/ml of FGF2 (A and C) or VEGF (B and D) in the absence or presence of PD98059 (2.5–40 μM) or U0126 (0.625–10μM; 1 hr pretreatment). Cells were counted after 72 hr of treatment. Data are expressed as means ± SEM percentage of the control (n = 4). The number of cells per well in the control was 6029 ± 457.4 and 4407 ± 139.6, respectively, for the PD98059 (A and B) and U0126 (C and D) treatments. a,b,c Means with different letters differ within each growth factor and kinase inhibitor treatment (p < 0.05).
Figure 6.
Effects of LY294002 on FGF2- and VEGF-stimulated HPAE cell proliferation. Cells were cultured under normoxia for 24 hr. After 24 hr of serum starvation, cells seeded in 96 well plates were treated with 10 ng/ml of FGF2 (A) or VEGF (B) in the absence or presence of LY294002 (0.625–10 μM; 1 hr pretreatment). Cells were counted after 72 hr of treatment. Data are expressed as means ± SEM percentage of the control (n = 5). The number of cells per well in the control was 6550 ± 467.8. a,b Means with different letters differ within each growth factor and kinase inhibitor treatment (p < 0.05).
DISCUSSION
It has become clear that the biological actions of FGF2 and VEGF are tightly mediated by a complex signaling network involving multiple protein kinases and phosphatases [20–23]. However, such a signaling network mediating FGF2- and VEGF-induced placental angiogenesis under hypoxia is poorly defined. Herein, using HPAE cells as a model, we have demonstrated that physiological hypoxia (3% O2) enhances FGF2- and VEGF-stimulated cell proliferation without altering FGF2- and VEGF-induced ERK1/2 and AKT1 activation, suggesting that hypoxia-enhanced FGF2- and VEGF-stimulated HPAE cell proliferation is independent of activation of these two signaling pathways. Moreover, we have also shown that the MEK1/2/ERK1/2 and PI3K/AKT1 pathways differentially mediate FGF2- and VEGF-stimulated HPAE cell proliferation.
Hypoxia alone is capable of promoting several major steps of angiogenesis including cell proliferation [11–15], migration [12], and formation of capillary-like tube structures [16]. Nonetheless, little is known about the effects of hypoxia on FGF2- and VEGF-regulated placental endothelial functions and needless to mention, the underlying signaling mechanisms. In one of a few relevant studies, it has been reported that hypoxia (3% O2) enhances PDGF- and FGF2-stimulated rat aortic endothelial proliferation and sprouting [14]. Consistent with this report, we also showed herein that 3% O2 enhanced FGF2-stimulated HPAE cell proliferation. Further more we found similar stimulatory effects of hypoxia on VEGF-stimulated HPAE cell proliferation. These data imply that the physiological hypoxia could enhance both FGF2- and VEGF-stimulated placental angiogenesis. However, it is noteworthy that opposite effects on angiogenic factor-induced angiogenesis might occur under more severe hypoxia conditions. For example, it has been reported that 1 % O2 (24 hr) greatly decreases VEGF-stimulated endothelial cell proliferation, migration, and tube formation in primary human coronary artery endothelial cells [35]. Thus, hypoxia might differentially modulate angiogenic factor-stimulated placental angiogenesis, possibly depending on the severity of hypoxia and the exposure duration.
A follow-up important question addressed in this study is what are the signaling mechanisms underlying these hypoxia-enhanced HPAE cell proliferation. Indeed, hypoxia (1–10% O2) can induce the activation of many protein kinases (e.g., jun-N-terminal kinase, ERK1/2, AKT1, and p38 MAPK) in endothelial cells [27–30,35]. This activation could lead to phosphorylation of several nuclear transcription factors (e.g., Egr-1, c-Jun, Elk-1, c-Fos, and Ets-1), which in turn activate many key genes involved in cell proliferation, migration and survival [30,31]. In the current study, we focused only on the MEK1/2/ERK1/2 and PI3K/AKT1 pathways since both are critical for mediating FGF2- and VEGF-stimulated placental endothelial function including cell proliferation under normoxia [23,24,36–38]. We found that FGF2 and VEGF induced ERK1/2 activation under both normoxia and hypoxia, in which the actions of FGF2 was more robust and sustained than VEGF in HPAE cells. Moreover, we observed that FGF2 significantly, but VEGF only slightly increased AKT1 activation in HPAE cells under normoxia and hypoxia. These observations are consistent with our previous reports in ovine fetoplacental artery endothelial (OFPAE) cells [24,33,34]. However, in the current study, hypoxia did not significantly increase basal levels of ERK1/2 and AKT1 activation as well as FGF2- and VEGF-induced activation of ERK1/2 and AKT1 as compared with normoxia. Thus, although hypoxia alone could upregulate expression of FGF2 and VEGF receptors in endothelial cells derived from vessels other than human placental arteries [18], hypoxia did not enhance activation of the receptor-coupled ERK1/2 and AKT1 in HPAE cells, implying that hypoxia did not increase expression of FGF2 and VEGF receptors in HPAE cells. Moreover, these data also indicate that the hypoxia-enhanced FGF2- and VEGF-stimulated HPAE cell proliferation is unlikely due to enhanced activation of MEK1/2/ERK1/2 and PI3K/AKT1 pathways. Further studies are needed for determining the alternative signaling pathways that are involved in such hypoxia-enhanced cell responses.
Another intriguing finding of the current study was that PD98059, as well as U0126, did not affect FGF2-stimulated HPAE cell proliferation although it greatly inhibited FGF2-induced activation of the MEK1/2/ERK1/2 pathway. Similar phenomenon was also reported by Willcocks and colleagues who demonstrated that in T cells PD98059 abolished interleukin-2 (IL-2)-induced ERK1/2 activation, but had no effects on IL-2 stimulated proliferation [40]. However, this finding differed from our recent report in which PD98059 significantly inhibited OFPAE cell proliferation [24]. It is noteworthy that even in OFPAE cells FGF2-stimulated cell proliferation is much less sensitive to PD98059 as compared to VEGF [24]. For example, in OFPAE cells the concentration of PD98059 required to inhibit FGF2-stimulated cell proliferation is ~ 4 fold higher than that required for inhibiting VEGF-stimulated cell proliferation, and PD98059, at a maximal concentration (20 μM) without toxic effect inhibits FGF2-stimulated cell proliferation only by approximately 38% while abolishing VEGF-induced cell proliferation [24]. Thus, together with our previous report [24] and current data that LY294002 completely abrogated FGF2-stimulated OFPAE and HPAE cells proliferation, it is concluded that the PI3K/AKT1 pathway is likely a major signaling pathway in FGF2-induced placental endothelial cell proliferation, whereas the MEK1/2/ERK1/2 plays a less important role in such FGF2-induced cell responses.
In contrast to FGF2, VEGF-stimulated HPAE cell proliferation is mediated primarily via the MEK1/2/ERK1/2 pathway. These findings suggest that the MEK1/2/ERK1/2 pathway is a major signaling pathway in VEGF-stimulated HPAE cell proliferation. This contradicts our recent reports, in which both of these two pathways actively mediate VEGF-stimulated OFPAE cell proliferation [24,26]. What causes this discrepancy between HUAE and OFPAE cells is still not clear. However, this discrepancy could be partially explained by the fact that VEGF induced AKT1 activation only in OFPAE, but not in HPAE cells.
Of note is that although activation of ERK1/2 and AKT1 does not have a critical role in mediating HPAE cell proliferation stimulated by FGF2 and VEGF, respectively, their activation may be required for mediating other function of HPAE cells such as endothelial migration and tube formation, and NOS expression as we have shown in OFPAE cells under normoxia [24,25,41], which is awaiting further investigation.
In summary, we have shown that hypoxia enhances FGF2- and VEGF-stimulated HPAE cell proliferation independent of AKT1 and ERK1/2 and the PI3K/AKT1 and MEK1/2/ERK1/2 pathways differentially mediate HPAE cell proliferation stimulated by FGF2 and VEGF, respectively. These studies might advance our understanding of the extremely complex signaling networks mediating placental angiogenesis under both normoxic and hypoxic conditions.
Acknowledgments
This work was supported in part by the National Institutes of Health grants HL64703 and HD38843 (JZ) and HL74947 & HL70562 (DBC).
Footnotes
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenfeld CR, Morriss FH, Makowski El, Meschia G, Battaglia FC. Circulatory changes in the reproductive tissues of ewes during pregnancy. Gynecol Invest. 1974;5:252–268. doi: 10.1159/000301658. [DOI] [PubMed] [Google Scholar]
- 2.Alexander G. Birth weight of lambs: influences and consequences. In: Elliott K, Knight J, editors. Ciba Foundation Symposium 27: Size at Birth. New York: Elsevier; 1974. pp. 215–245. [Google Scholar]
- 3.Reynolds LP, Redmer DA. Utero-placental vascular development and placental function. J Anim Sci. 1995;73:1839–1851. doi: 10.2527/1995.7361839x. [DOI] [PubMed] [Google Scholar]
- 4.Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- 5.Jauniaux E, Watson AL, Hempstock J, Bao Y-P, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress: a possible factor in human early pregnancy failure. Am J Pathol. 2000;157:2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: involvement of oxidative stress and implications in human evolution. Hum Reprod Update. 2006;12:747–755. doi: 10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zygmunt M, Herr F, Munstedt K, Lang U, Liang OD. Angiogenesis and vasculogenesis in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2003;110:S10–S18. doi: 10.1016/s0301-2115(03)00168-4. [DOI] [PubMed] [Google Scholar]
- 8.Magness RR, Zheng J. Circulatory changes during gestation. In: Gluckman PD, Heymann MA, editors. Scientific Basis of Pediatric and Perinatal Medicine. London: Edward Arnold Publishers; 1996. pp. 762–772. [Google Scholar]
- 9.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 10.Zheng J, Bird IM, Chen DB, Magness RR. Angiotensin II regulation of fetoplacental artery endothelial functions. J Physiol (London) 2005;565:59–69. doi: 10.1113/jphysiol.2004.082420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster M, Fisher SJ. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest. 2004;114:744–754. doi: 10.1172/JCI22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meininger CJ, Schelling ME, Granger HJ. Adenosine and hypoxia stimulate proliferation and migration of endothelial cells. Am J Physiol. 1988;255:H554–562. doi: 10.1152/ajpheart.1988.255.3.H554. [DOI] [PubMed] [Google Scholar]
- 13.Tucci M, Hammerman SI, Furfaro S, Saukonnen JJ, Conca TJ, Farber HW. Distinct effect of hypoxia on endothelial cell proliferation and cycling. Am J Physiol. 1997;272:C1700–1708. doi: 10.1152/ajpcell.1997.272.5.C1700. [DOI] [PubMed] [Google Scholar]
- 14.Schäfer M Ewald N, Schafer C, Stapler A, Piper HM, Noll T. Signaling of hypoxia-induced autonomous proliferation of endothelial cells. FASEB J. 2003;17:449–451. doi: 10.1096/fj.02-0398fje. [DOI] [PubMed] [Google Scholar]
- 15.Humar R, Kiefer FN, Berns H, Resink TJ, Battegay EJ. Hypoxia enhances vascular cell proliferation and angiogenesis in vitro via rapamycin (mTOR)-dependent signaling. FASEB J. 2002;16:771–780. doi: 10.1096/fj.01-0658com. [DOI] [PubMed] [Google Scholar]
- 16.Li W, Petrimpol M, Molle KD, Hall MN, Battegay EJ, Humar R. Hypoxia-induced endothelial proliferation requires both mTORC1 and mTORC2. Circ Res. 2007;100:79–87. doi: 10.1161/01.RES.0000253094.03023.3f. [DOI] [PubMed] [Google Scholar]
- 17.Phillips PG, Birnby LM, Narendran A. Hypoxia induces capillary network formation in cultured bovine pulmonary microvessel endothelial cells. Am J Physiol. 1995;268:L789–800. doi: 10.1152/ajplung.1995.268.5.L789. [DOI] [PubMed] [Google Scholar]
- 18.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 19.Shreeniwas R, Ogawa S, Cozzolino F, Torcia G, Braunstein N, Butura C, Brett J, Lieberman HB, Furie MB, Joseph-Silverstein J, et al. Macrovascular and microvascular endothelium during long-term hypoxia: alterations in cell growth, monolayer permeability, and cell surface coagulant properties. J Cell Physiol. 1991;146:8–17. doi: 10.1002/jcp.1041460103. [DOI] [PubMed] [Google Scholar]
- 20.Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, Van Dorpe J, Hellings P, Gorselink M, Heymans S, Theilmeier G, Dewerchin M, Laudenbach V, Vermylen P, Raat H, Acker T, Vleminckx V, Van Den Bosch L, Cashman N, Fujisawa H, Drost MR, Sciot R, Bruyninckx F, Hicklin DJ, Ince C, Gressens P, Lupu F, Plate KH, Robberecht W, Herbert JM, Collen D, Carmeliet P. Deletion of the hypoxiaresponse element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28:131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 21.Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF) J Cell Mol Med. 2005;9:777–794. doi: 10.1111/j.1582-4934.2005.tb00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dailey L, Ambrosetti D, Mansukhani A, Basilico C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev. 2005;16:233–247. doi: 10.1016/j.cytogfr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocrine-Related Cancer. 2000;7:165–197. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- 24.Zheng J, Wen Y, Song Y, Wang K, Chen DB, Magness RR. Activation of multiple signaling pathways is critical for fibroblast growth factor 2- and vascular endothelial growth factor-stimulated ovine fetoplacental endothelial cell proliferation. Biol Reprod. 2008;78:143–150. doi: 10.1095/biolreprod.107.064477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mata-Greenwood E, Liao WX, Zheng J, Chen DB. Differential activation of multiple signalling pathways dictates eNOS upregulation by FGF2 but not VEGF in placental artery endothelial cells. Placenta. 2008;29:708–717. doi: 10.1016/j.placenta.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng J, Bird IM, Melsaether AN, Magness RR. Activation of the mitogen-activated protein kinase cascade is necessary but not sufficient for basic fibroblast growth factor- and epidermal growth factor-stimulated expression of endothelial nitric oxide synthase in ovine fetoplacental artery endothelial cells. Endocrinology. 1999;140:1399–1407. doi: 10.1210/endo.140.3.6542. [DOI] [PubMed] [Google Scholar]
- 27.Risbud MV, Guttapalli A, Albert TJ, Shapiro IM. Hypoxia activates MAPK activity in rat nucleus pulposus cells: regulation of integrin expression and cell survival. Spine. 2005;30:2503–2509. doi: 10.1097/01.brs.0000186326.82747.13. [DOI] [PubMed] [Google Scholar]
- 28.Minet E, Arnould T, Michel G, Roland I, Mottet D, Raes M, Remacle J, Michiels C. ERK activation upon hypoxia: involvement in HIF-1 activation. FEBS Lett. 2000;468:53–58. doi: 10.1016/s0014-5793(00)01181-9. [DOI] [PubMed] [Google Scholar]
- 29.Gerasimovskaya EV, Tucker DA, Stenmark KR. Activation of phosphatidylinositol 3-kinase, Akt, and mammalian target of rapamycin is necessary for hypoxia-induced pulmonary artery adventitial fibroblast proliferation. J Appl Physiol. 2005;98:722–731. doi: 10.1152/japplphysiol.00715.2004. [DOI] [PubMed] [Google Scholar]
- 30.Das M, Bouchey DM, Moore MJ, Hopkins DC, Nemenoff RA, Stenmark KR. Hypoxia-induced proliferative response of vascular adventitial fibroblasts is dependent on G protein-mediated activation of mitogen-activated protein kinases. J Biol Chem. 2001;276:15631–15640. doi: 10.1074/jbc.M010690200. [DOI] [PubMed] [Google Scholar]
- 31.Campbell JS, Seger R, Graves JD, Graves LM, Jensen AM, Krebs EG. The MAP kinase cascade. Recent Prog Horm Res. 1995;50:131–159. doi: 10.1016/b978-0-12-571150-0.50011-1. [DOI] [PubMed] [Google Scholar]
- 32.L’Allemain G. Deciphering the MAP kinase pathway. Prog Growth Factor Res. 1994;5:291–334. doi: 10.1016/0955-2235(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 33.Zheng J, Wen YX, Austin JL, Chen DB. Exogenous nitric oxide stimulates cell proliferation via activation of a mitogen activated protein kinase pathway in ovine fetoplacental artery endothelial cells. Biol Reprod. 2006;74:375–382. doi: 10.1095/biolreprod.105.043190. [DOI] [PubMed] [Google Scholar]
- 34.Wang K, Song Y, Chen DB, Zheng J. Protein phosphatase 3 differentially modulates vascular endothelial growth factor- and fibroblast growth factor 2-stimulated cell proliferation and signaling in ovine fetoplacental artery endothelial cells. Biol Reprod. 2008;79:704–710. doi: 10.1095/biolreprod.108.068957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olszewska-Pazdrak B, Hein TW, Olszewska P, Carney DH. Chronic hypoxia attenuates VEGF signaling and angiogenic responses by downregulation of KDR in human endothelial cells. Am J Physiol Cell Physiol. 2009;296:C1162–1170. doi: 10.1152/ajpcell.00533.2008. [DOI] [PubMed] [Google Scholar]
- 36.Jin N, Hatton N, Swartz DR, Xia X, Harrington MA, Larsen SH, Rhoades RA. Hypoxia activates jun-N-terminal kinase, extracellular signal-regulated protein kinase, and p38 kinase in pulmonary arteries. Am J Respir Cell Mol Biol. 2000;23:593–601. doi: 10.1165/ajrcmb.23.5.3921. [DOI] [PubMed] [Google Scholar]
- 37.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 38.Zubilewicz A, Hecquet C, Jeanny JC, Soubrane G, Courtois Y, Mascarelli F. Two distinct signalling pathways are involved in FGF2-stimulated proliferation of choriocapillary endothelial cells: a comparative study with VEGF. Oncogene. 2001;20:1403–1413. doi: 10.1038/sj.onc.1204231. [DOI] [PubMed] [Google Scholar]
- 39.Eriksson K, Magnusson P, Dixelius J, Claesson-Welsh L, Cross MJ. Angiostatin and endostatin inhibit endothelial cell migration in response to FGF and VEGF without interfering with specific intracellular signal transduction pathways. FEBS Lett. 2003;536:19–24. doi: 10.1016/s0014-5793(03)00003-6. [DOI] [PubMed] [Google Scholar]
- 40.Crawley JB, Willcocks J, Foxwell BM. Interleukin-7 induces T cell proliferation in the absence of Erk/MAP kinase activity. Eur J Immunol. 1996;26:2717–2723. doi: 10.1002/eji.1830261125. [DOI] [PubMed] [Google Scholar]
- 41.Liao WX, Feng L, Zhang H, Zheng J, Moore TR, Chen DB. Compartmentalizing VEGF-induced ERK2/1 signaling in placental artery endothelial cell caveolae: a paradoxical role of caveolin-1 in placental angiogenesis in vitro. Mol Endocrinol. 2009;23:1428–1444. doi: 10.1210/me.2008-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]