Abstract
Maternal immunity undergoes subtle adjustment in order to tolerate the semi-allogeneic embryo and maintain the host defense against potential pathogens. Concomitantly, coagulation systems change from an anti-coagulant state to a pro-coagulant state to meet the hemostatic challenge of placentation and delivery. Innate immunity and blood coagulation systems are the first line of defense to protect a host against exogenous challenges, including alloantigens and mechanical insults, and preserve the integrity of an organism. The interactions between coagulation and immune systems have been extensively studied. Immune cells play a pivotal role in the initiation of the coagulation cascade, whereas coagulation proteases display substantial immunomodulatory effects. Upon exogenous challenges, the immune and coagulation systems are capable of potentiating each other leading to a vicious cycle. Natural killer (NK) cells, macrophages (Mϕs) and dendritic cells (DCs) are three major innate immune cells that have been demonstrated to play essential roles in early pregnancy. However, immune maladaptation and hemostatic imbalance have been suggested to be responsible for adverse pregnant outcomes, such as preeclampsia (PE), miscarriage, recurrent spontaneous abortion (RSA) and intrauterine growth restriction (IUGR). In this review, we will summarize the mutual regulation between blood coagulation and innate immune systems as well as their roles in the maintenance of normal pregnancy and in the pathogenesis of adverse pregnancy outcomes.
Introduction
Inflammatory response and hemostasis are multi-factorial host defensive processes to infectious or noxious insults. When an organism is exposed to microbial invasion or trauma, innate immune cells will be recruited to the foci and initiate a series of immune responses to confine the damage. The host response to immune challenges requires coordination between innate and adaptive elements. As the first line of defense against exogenous challenges, the innate immune system generates an initial, nearly instantaneous, relatively non-specific response to potential pathogens [1]. The subsequent highly specific, albeit slower, adaptive immune response provides a far more efficient and long-term suppression of pathogens. While the innate immunity does not require the presence of an adaptive arm, the latter requires an intact innate immune system.
Mechanical trauma not only induces tissue damage but also generates vascular injury. Over the past two decades, an increasing body of evidence shows that the activation of coagulation is an integral part of inflammatory response. In addition to innate immune cells, the coagulation system also participate host defense and wound healing processes. Although immune system and coagulation system are not usually associated in time, emerging evidence indicates that there are extensive interactions between these two systems throughout vertebrae evolution [2]. Immune cells and inflammatory mediators are capable of modifying hemostasis, while molecules in coagulation pathway have considerable immuno-modulatory effects.
Pregnancy is a complex and well-regulated process, which leads to systemic changes, including hormonal homeostasis, cardiovascular kinetics and metabolism. The placenta is the first organ that develops immediately after implantation. Abnormal development of a placenta will result in adverse pregnancy outcomes. Preeclampsia is a multi-system disorder characterized by maternal hypertension, proteinuria and edema that complicates 5% to 10% of pregnancies [3]. It may be associated with IUGR and preterm delivery, and is a leading cause of maternal and fetal morbidity and mortality worldwide [4]. In addition to PE, preterm birth and abortion are common abnormal pregnancy outcomes. Preterm birth occurs in approximately 13% of all pregnancies, accounts for 75% of neonatal [5]. In all human conceptions, only 30% of fetuses are viable and more than 50% of which are lost prior to the first missed menstruation [6]. Furthermore, the risk of subsequent abortion is increased as the frequency of failed pregnancy increases; for example, the approximate failure rates in the index pregnancy are 24% after two, 30% after three and 50% after four spontaneous abortions [7]. RSA is defined as three or more consecutive spontaneous abortions, which affects about 1% of the childbearing women. Although the pathogenesis of PE, preterm birth and spontaneous abortion is thought to be related to the placenta, the exact mechanisms of these adverse pregnancy outcomes remain unclear. An increasing body of evidence indicates that the foundations of lifelong health are established in utero [8-11]. Prematurity contributes significantly to such major long-term morbidity as chronic lung disease, hearing and visual impairment, developmental delay and cerebral palsy. Prematurity and IUGR are also associated with subtle intellectual and behavioral problems during development [12, 13]. Thus, placenta-related abnormal pregnancy is a source of stress and financial burden for affected families and society. While numerous factors, such as gene polymorphisms, dysregulated immune responses, aberrant angiogenesis and excessive coagulopathy have been postulated to be associated with adverse pregnancy outcomes, this review will highlight the role of immunity in conjunction with coagulation during the pathogenesis of placenta-related adverse pregnancy outcomes.
Regulation of coagulation by the immune system
The host response to outside challenges requires coordination between innate and adaptive immunity. When pathogens successfully invade an organism, innate immune system is the first line of host defense to encounter these intruders. The pathogens are first recognized and engulfed by professional phagocytes (e.g. Mϕs, DCs and neutrophils), among which DCs and Mϕs are major antigen-presenting cells (APCs). Chemokines are then secreted by pathogen-stimulated APCs and chemoattract more innate immune cells to the foci. Subsequently, the adaptive immunity takes place in the peripheral lymph nodes that is characterized by APC-mediated activation of naïve T- and B-lymphocytes, the key cell types of the adaptive immune system. Eventually, activated T- and B-lymphocytes are recruited back to the site of inflammation that augments the host defense.
In addition to immune system, coagulation pathway serves as another survival mechanism for an organism. Coagulation immediately fills up the wound to preserve the integrity of the tissues and plugs up the opening of the circulatory system to prevent further loss of blood. Coagulation involves both platelets and coagulation factors and is activated instantaneously after an injury. The coagulation cascade is classified to tissue factor (TF) (extrinsic) and contact activation (intrinsic) pathways that activate a final common pathway of factor X, thrombin and fibrin. Platelets immediately aggregate at the site of injury. Simultaneously, a complex cascade mediated by coagulation factors is initiated to form a fibrin meshwork, which reinforces the platelet plug. Interestingly, most of the inflammatory signals also trigger pro-coagulant signals to the coagulation cascade. Phylogenetically, many of the inflammatory cytokines of innate immune system and coagulation molecules, such as CD40 ligand from platelets versus tumor necrosis factor (TNF) family members [14] and TF versus cytokine receptors [15], share structural homologies, indicating the crosstalk between innate immune system and coagulation. Based on the observation of septic shock, the actions of the innate system and coagulation system are remarkably homologous.
When pathogens trigger innate immune response, inflammatory cytokines are secreted by innate immune cells. Microbial components and pro-inflammatory cytokines, including endotoxin, IL-6, TNF-α, IL-1, IL-2 and C5a, can induce the de novo synthesis of TF in endothelial cells and leukocytes, especially monocytes [16-19]. The coagulation cascade is then activated by exposure of TF to the blood. The coagulation can also be enhanced by complement-activated formation of plasma membrane enriched in negatively charged phospholipids [20]. Furthermore, platelet activating factor, L- and P-selectin as well as Mac-1 integrin production in endothelial cells is up-regulated, that either synergistically increase the monocyte TF response or potentially amplify the pro-coagulant response [21-24]. Meanwhile, the chemotaxis and activation of neutrophils and monocytes are promoted via the generation of thrombin and fibrinogen/fibrin degradation product [25, 26]. Thus, a vicious cycle between immune response and coagulation is established. Coagulation is counterbalanced by natural anti-coagulant pathways, including protein C anti-coagulant pathways, anti-thrombin-heparin and tissue factor pathway inhibitor (TFPI) [27]. During acute inflammatory response, anti-thrombin is consumed and inactivated [28]. The vascular heparin-like molecules and protein C pathway appear to be inhibited by endotoxin and inflammatory cytokines, such as IL-1β and TNF-α, through down-regulation of thrombomodulin and the endothelial protein C receptor (EPCR) [29-31]. Fibrinolysis is also suppressed by increased production of plasminogen activator inhibitor-1 (PAI-1) [32, 33].
Regulation of immune system by coagulation
Fibrin/platelet deposition is one of the characteristics of inflammation that helps an organism to wall off infection and accelerate wound healing. Several molecules in the coagulation cascade, such as thrombin and factor Xa have been shown to be pro-inflammatory [34, 35]. Both molecules activate mast cells to induce degranulation with the secretion of bioamines [34, 36]. Thrombin is a serine protease, which cleaves fibrinogen to fibrin and promotes platelet aggregation and degranulation for clot formation. However, thrombin also mediates different cellular events in a catalytic activity-independent manner [15, 37]. After binding to its receptor, protease-activated receptors (PARs), thrombin is able to induce cytokine expression, including IL-6, IL-8, MCP-1 and MIF production in various cell types [38-42]. Acting through NFκB, thrombin, factor Xa and TF-VIIa complex enhance the expression of adhesion molecules in leukocytes [43-45]. Binding of TF-VIIa complex to PAR-2 also affects neutrophils infiltration and TNF-α and IL-1β expression [46]. Besides, thrombin and fibrin/fibrinogen degradation products also exhibit chemotactic activity for neutrophils and monocytes [37, 47].
On the contrary, anti-thrombin down-regulates factor X receptor, CD11b/CD18, on leukocytes and leads to decrease in leukocyte adhesion [27]. The treatment of endothelial cells with anti-thrombin increases prostacyclin formation [48] and decreases NFκB signaling and IL-6 expression [49, 50]. Both thrombomodulin and activated protein C attenuate NFκB signaling [51, 52]. In recent years, the role of activated protein C in anti-inflammatory response has been inspired by the studies in human sepsis. Activated protein C was demonstrated to reduce leukocyte infiltration [51-54], suppress TNF-α and NFκB expression, inhibit cytokine signaling, interfere with cytokine-induced up-regulation of leukocyte adhesion molecules as well as inflammation- and apoptosis-related genes [55-57].
Immediately after a blood vessel injury, platelets form a hemostatic plug at the site of injury and secrete factors to initiate inflammatory response that chemoattracts and activates leukocytes. Due to their early role in coagulation in response to injury, platelets can act as a sentinel cell and provide information transfer in host defense similar to such innate immune cells as mast cells, Mϕs and DCs. Interestingly, the interaction between platelets and innate defensive cells, including polymorphonuclear cells, monocytes, mast cells, Mϕs and DCs, has been well-recognized. Activated platelets release inflammatory and immune-modulating factors, which can affect the cells of the innate and adaptive immune systems [58-60]. For instance, CXCL4 and CXCL7 secreted by platelets [58] in the early response to injury are recognized by neutrophils and modulate their activities [61]. In addition to CXC chemokines, platelets also secrete such CC chemokines as CCL3, CCL5 and CCL7 displaying similar diverse functions in regulating monocyte activities [62, 63]. Platelets also produce pro-inflammatory lipids and cytokines, such as cyclooxygenase-1 (COX-1), COX-2, thromboxane A2 (TXA2) [64] and IL-1β [65]. Besides the ability to modulate immune cell activity via secreted molecules, platelets also interact with target cells through cell-cell adhesion. P-selectin on the surface of platelets mediates the induction of chemokine and urokinase plasminogen activator receptor expression in neutrophils and monocytes by binding P-selectin glycoprotein-1 (PSGL-1) [66, 67]. Furthermore, binding of CD40 ligand on platelets and CD40 on B cells, monocytes, Mϕs, DCs and endothelial cells induces multiple inflammatory responses [68]. Thus, platelets can be considered as one of the cell types bridging inflammation and coagulation.
Dendritic cells are the major antigen-presenting cell links innate and adaptive immune systems. Normally, platelets and tissue DCs do not interact with each other. However, the CD40 ligand and IL-1β produced by activated platelets in bleeding tissue can serve as an endogenous danger signal to DCs and quickly initiate DC maturation [1, 68]. DC recruitment can also be facilitated by platelets [69]. Platelets form tight aggregates with monocytes. P-selectin on activated platelets may induce differentiation of DCs from monocytes [70, 71]. In a recent report, coagulation was shown to amplify inflammation through PAR1 signaling in DCs [72]. Conversely, activated platelets may suppress secretion of pro-inflammatory cytokines from DCs [73].
Coagulation and placenta-related abnormal pregnancy
During normal pregnancy, there is a marked increase in the pro-coagulant activity and a down-regulation in levels of physiological anti-coagulants. Such changes meet the hemostatic challenge of placentation and delivery but may account for adverse pregnancy outcomes in women [74]. Blood coagulation and platelet abnormalities may lead to hemorrhagic and thrombotic defects. Thrombophilic defects are extremely common in RSA compared to hemorrhagic defects [75]. The insufficient fibrin formation leads to hemorrhage, thus precluding adequate implantation of the fertilized ovum into the uterus. Patients having hemorrhagic defects, including factor XIII, XII, X, VII, V, II deficiency, von Willebrand disease, carriers of hemophilia and fibrinogen defects, are susceptible to RSA [76-79]. Inherited thrombophilic conditions have been associated a variety of pregnancy complications including fetal loss, preeclampsia, abruption and intrauterine growth restriction [80, 81], although other studies have not confirmed the association [82, 83]. While initial case control studies have confirmed a positive association between inherited thrombophilic conditions and pregnancy complications [84], larger prospective evaluations have not confirmed the association [85], suggesting that larger prospective studies are still needed to confirm the association between inherited thrombophilias and pregnancy complications [86].
Thrombosis associated with fetal wastage is most common in the first trimester. The earlier this presents in pregnancy, the smaller the placental and uterine vessels will be, therefore, the propensity to undergo partial or total occlusion by thrombus formation will be greater [75]. Despite the strong association between thrombosis and adverse pregnancy outcomes, its pathogenic role in the development of adverse pregnant has not been elucidated. Moreover, it is not clear why adverse pregnancies only occur in some of the women having thrombophilia. The involvement of additional factors in the regulation of coagulation and immune response in placenta need to be further scrutinized.
Recent data suggest that trophoblasts produce endothelial regulators of hemostasis, such as thrombomodulin (TM), endothelial protein C receptor (EPCR) and tissue factor pathway inhibitor (TFPI) [87, 88]. Sood, et al. identified some candidate fetal genes expressed by trophoblasts that were potential promoters of thrombophilia-associated pregnancy complications [89].
Innate immunity and placenta-related abnormal pregnancy
In non-pregnant women, the allogeneic tissues will be rejected by maternal immune system. However, the semi-allogeneic fetus survives in normal pregnancy. Robertson et al. [3] proposed that pregnancy was a state of “altered immune competence”. Indeed, peripheral blood mononuclear cells isolated from pregnant women were found to elicit stronger Th-2 immune response compared to non-pregnant women [90, 91]. High levels of estradiol, HCG and progesterone during the pregnancy can inhibit secretion of such NFkB-induced inflammatory cytokines as IL-1, -2, -3, -5, -8, interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) by Mϕs and T cells, while enhance the secretion of the monocyte-deactivating cytokine, IL-10 [92-94]. Decidual cells, syncytiotrophoblasts and cytotrophoblasts can secrete an array of pregnancy-associated molecules to suppress pro-inflammatory activation of decidual Mϕs and NK cells [95]. Therefore, there must be a balance between Th-1 and Th-2 immune responses during normal pregnancy.
After implantation, extravillous cytotrophoblasts penetrate and traverse the underlying decidua and myometrium [96]. During this invasive process, cytotrophoblasts surround, breach and transform the smooth muscle and endothelium of endometrial spiral arteries and arterioles to produce large-bore, low-resistance vessels [97]. The resulting increased uterine blood flow to the intervillous space is required for growth, development and survival of the fetal-placental unit [98, 99].
Shallow cytotrophoblast invasion [98-100] will lead to incomplete uterine vascular conversion, an inadequate fetal blood supply, and a pathological hypoxic milieu [101]. This placental defect is associated with persistence of a pro-inflammatory environment and is considered as a failure of maternal immune tolerance required for normal cytotrophoblast invasion [91, 100].
Mϕs and DCs initiate innate and adaptive immunity and are involved in developing immune tolerance. These antigen-presenting cells are uniquely capable of modulating the balance between the innate and adaptive immune systems to provide protection against pathogens yet confer immune tolerance to the semi-allogeneic embryo [102, 103]. A marked excess of Mϕs in the preeclamptic decidua has been observed [104]. Other than Mϕs, mature and immature DC infiltration in preeclamptic decidua was also reported [105]. Although uterine NK (uNK) cells have been suggested to be required for the success of pregnancy and shown to be the main immune cells in the implantation site [106, 107], an increase of uNK cells has been shown in decidual parietalis in patients with miscarriage [108]. Interestingly, an increase of Mϕs, tissue factor and coagulation factor IX around anchoring villi in recurrent abortion was demonstrated [109], suggesting the role of Mϕs and coagulopathy as well as their potential relationship during the pathogenesis of miscarriage. The link between abnormal pregnancy and aberrant maternal immune responses [107] and the versatility exhibited by uNK cells, Mϕs and DCs in mediating both immune activation and tolerance reveal the importance of the study of the regulation of uNK cell, Mϕ and DC infiltration and activation in the decidua of placenta-related pregnant complications.
The role of NK cells in adverse pregnancy outcomes
NK cells play a fundamental role in the innate immune response through their ability to secrete cytokines and kill target cells without prior sensitization. uNK cells constitute approximately 70 - 75% of the decidual immune cells during early pregnancy but undergo extensive apoptosis upon the onset of trophoblast infiltration and are virtually absent at term [110, 111]. Uterine NK cells are thought to support remodeling of the uterine spiral arteries and to facilitate successful placentation through the regulation of trophoblast invasion [106, 112]. Mouse studies demonstrated that mice deficient in uNK cells displayed incomplete widening of uterine vessels and significant decidual pathology [113]. In peripheral blood, 95% of NK cells are CD56dimCD16 bright and approximately 5% are CD56brightCD16dim, however, the majority of uNK cells are CD56brightCD16dim [114, 115]. Basically, CD56dimCD16 bright NK cells are highly cytotoxic whereas the CD56brightCD16dim uNK cells have low cytotoxic capacity but effectively secrete cytokines, such as IFN-γ, vascular endothelial growth factor (VEGF), angiopoietin-2 (Ang-2) and placental growth factor (PlGF) which may facilitate vascular remolding and normal endometrial decidualization [116-118].
NK cell functions, specifically cytokine production and cytotoxicity, are tightly regulated by the inhibitory and excitatory receptors which can recognize the ubiquitously expressed major histocompatibility complex class I (MHC-I) proteins on target cells [119]. Functionally, inhibitory receptors can block NK cell activation signals originated from excitatory receptors and leave NK cells in a quiescent state. In early development, the blastocyst divides into two cell lineages. The inner cells give rise to the embryo while the outer cells develop into the fetal portion of the placenta, including trophoblasts, some of which have direct contact with the maternal immune system [120]. Human and murine trophoblasts are devoid of MHC-II molecules and only express limited MHC-I [110] which renders them the potential target of an allogeneic immune response. In humans, the studies of maternal KIRs (on NK cells) and fetal HLA-C genes (on extravillous trophoblasts) pairing in PE indicate that strong KIR-HLA-C inhibitory signals predispose women to PE by inhibiting the expression of growth factors. This inhibition in turn impairs trophoblast invasion, spiral artery remodeling and thus, the overall quality of placentation. By contrast, in women with unexplained RSA, the relatively lower inhibitory receptor expression or higher MHC-I-specific excitatory receptor expression results in hyperactivation and cytotoxicity of uNK cells [121].
The role of macrophages in adverse pregnancy outcomes
Mϕs comprise 20 – 25% of decidual leukocytes in early pregnancy. Unlike uNK cells, the number of Mϕs remains relatively constant throughout gestation [122]. They are recruited to the cytotrophoblast shell in close association with invading extravillous trophoblasts through expression of a variety of receptor-ligand pairs. During placentation, apoptotic bodies are removed by Mϕs [123-125]. Ingestion of low levels of apoptotic debris has been shown to reinforce the secretion of Th2 cytokines by Mϕs [126]. Although apoptosis is required for normal placentation, excess apoptosis and insufficient clearance of apoptotic bodies enhance the secretion of pro-inflammatory cytokines, such as TNF-α and IFN-γ, by activated Mϕs [127]. Consequently, these pro-inflammatory cytokines will induce excess trophoblast apoptosis and lead to adverse pregnant outcomes, including PE, IUGR and abortion [128, 129].
Shallow trophoblast invasion and impaired spiral artery remodeling as well as decreased placental vascularity [130] were found in pregnancies complicated by PE and in some cases of IUGR and abortion [131-133]. Excess inflammation is postulated to be associated with PE [134], IUGR and abortion, suggesting that altered behavior of uterine leukocytes may account for the defective placentation.
An increased infiltration of Mϕs was found in decidua complicated by PE and IUGR. Mϕs were also shown to mediate fetal demise in a mouse model of abortion [135]. In addition, the expression of such cytokines involved in the recruitment and development of Mϕs as M-CSF, GM-CSF, IL-8, MCP-1, MIP-1b, RANTES and MCP-3 are increased in preeclamptic decidua [95, 105]. The production of TNF-α [136], plasminogen activator inhibitor 1 (PAI-1) [137]and inducible nitric oxide synthase (iNOS) [138] by activated Mϕs may result in impaired trophoblast invasion and spiral artery remodeling. In addition, Mϕs are key mediators of all steps during angiogenesis due to their ability to secrete VEGF, matrix metalloproteinases (MMPs), fibroblast growth factor, fibronectin and collagen [139]. Girardi et al. found that decreased levels of free VEGF in a mouse model of IUGR and abortion coincided with increased levels of sFlt-1 and an influx of Mϕs in the decidua [140]. However, the role of Mϕs in the impaired placental angiogenesis remains unclear.
The role of dendritic cells in adverse pregnancy outcomes
DCs are specialized antigen-presenting and phagocytic cells that play important role in mediating both innate and subsequent adaptive immune responses. The maternal–fetal interface is an immunologically privileged site that provides immune tolerance to the semi-allogeneic fetus while maintains host defense against possible pathogens. Immature myeloid DCs are found in tissues and may maintain tolerance via induction of T-cell anergy or regulatory T-cells, whereas mature DCs may promote a Th1 immune response. Gardner suggested that decidual DCs in the proximity of extravillous trophoblasts at the implantation site are HLA-DR+, CD11c+, DEC-205+, CD40+, DC-SIGN+, CD1a+, CD123+, indicating that the majority of the decidual DCs in normal pregnancy are immature DCs [141]. Normal pregnant mice show an expansion of CD4+CD25+ and IL-10+ Treg cells at the periphery compared to non-pregnant mice [142]. Significantly decreased CD4+CD25bright T cells and elevated mature DCs in RSA or PE indicate that mature DCs may induce inflammatory response in decidua. Our study demonstrated that the recruitment of DCs as well as the expression of their recruiting-chemokines was significantly higher in preeclamptic decidua compared to gestational age-matched control [105]. However, unlike Mϕs, DCs did not directly impede trophoblast invasion. Plaks et al. suggested that DCs might regulate tissue remodeling and angiogenesis via such important blood vessel maturing factors as sFlt1 and TGF-β [143].
Conclusions
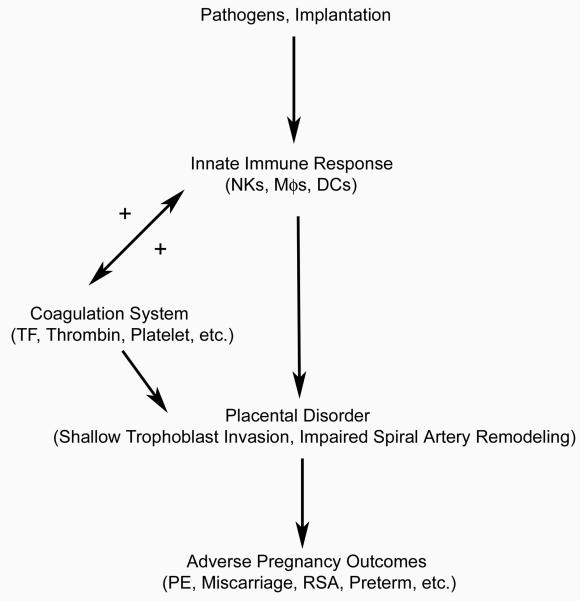
Normal pregnancy is a mild inflammatory state and the adverse pregnancy outcomes seem to be an exaggeration of the norm. This deteriorated inflammatory state is associated with maternal leukocyte activation, especially as related to local uterine innate immunity (Fig. 1). In addition, the release of cytokines from immune cells and uteroplacental tissues, endothelial cell activation as well as immune/coagulation interaction are also observed. As indicated, the inflammatory signals may induce the synthesis of TF in endothelial cells and monocytes to trigger the coagulation cascade [16-19]. The coagulation system then in turn enhances the chemoattraction and activation of the leukocytes by thrombin or factors released from activated platelets. Through this vicious cycle between immune response and coagulation, the inflammatory state is therefore worsened. The enhanced expression of such molecules as TNF-α, IL-1, IFN-γ, sFlt1 and iNOS by activated immune cells was shown to be important during the formation of a dysfunctional placenta in complications of pregnancy through the induction of excessive trophoblast apoptosis, shallow trophoblast invasion, and impaired spiral artery remodeling [127, 136, 138, 144]. Consequently, this leads to various adverse pregnancy outcomes. Moreover, Lockwood reported that thrombin, a critical coagulation factor activated by tissue factor, could block the angiogenic effects of VEGF and placental growth factor by enhancing the expression of sFlt-1 in first trimester decidual cells that might result in incomplete remodeling of the spiral arteries [144]. Given the pivotal roles of the coagulation and inflammatory cascades in the etiopathogenesis of PE, IUGR, abruption, fetal loss, and preterm delivery, their precise interaction in this placenta mediated complications still requires further scrutinization.
Fig. 1.
Hypothesized effects of immune-coagulation interaction on pregnancy
Acknowledgments
Funding support: National Institutes of Health 5R01HD056123-02 (S. J. Huang)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 2.Opal SM. Phylogenetic and functional relationships between coagulation and the innate immune response. Crit Care Med. 2000;28:S77–80. doi: 10.1097/00003246-200009001-00017. [DOI] [PubMed] [Google Scholar]
- 3.Robertson SA, Redman CW, McCracken SA, Hunt JS, Dimitriadis E, Moffett-King A, Chamley L. Immune modulators of implantation and placental development--a workshop report. Placenta. 2003;24(Suppl A):S16–20. doi: 10.1053/plac.2002.0937. [DOI] [PubMed] [Google Scholar]
- 4.Duley L. Maternal mortality associated with hypertensive disorders of pregnancy in Africa, Asia, Latin America and the Caribbean. Br J Obstet Gynaecol. 1992;99:547–553. doi: 10.1111/j.1471-0528.1992.tb13818.x. [DOI] [PubMed] [Google Scholar]
- 5.Challis JRG. Mechanism of parturition and preterm labor. Obstet Gynecol Surv. 2000;55:650–660. doi: 10.1097/00006254-200010000-00025. [DOI] [PubMed] [Google Scholar]
- 6.Lockwood CJ. Prediction of pregnancy loss. Lancet. 2000;355:1292–1293. doi: 10.1016/S0140-6736(00)02108-5. [DOI] [PubMed] [Google Scholar]
- 7.Regan L, Braude PR, Trembath PL. Influence of past reproductive performance on risk of spontaneous abortion. BMJ. 1989;299:541–545. doi: 10.1136/bmj.299.6698.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander BT. Fetal programming of hypertension. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1–R10. doi: 10.1152/ajpregu.00417.2005. [DOI] [PubMed] [Google Scholar]
- 9.Barker DJ. The fetal and infant origins of adult disease. Bmj. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker DJ. Fetal nutrition and cardiovascular disease in later life. Br Med Bull. 1997;53:96–108. doi: 10.1093/oxfordjournals.bmb.a011609. [DOI] [PubMed] [Google Scholar]
- 11.Lucas A, Fewtrell MS, Cole TJ. Fetal origins of adult disease-the hypothesis revisited. Bmj. 1999;319:245–249. doi: 10.1136/bmj.319.7204.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hack M, Taylor HG, Klein N, Eiben R, Schatschneider C, Mercuri-Minich N. School-age outcomes in children with birth weights under 750 g. N Engl J Med. 1994;331:753–759. doi: 10.1056/NEJM199409223311201. [DOI] [PubMed] [Google Scholar]
- 13.Halsey CL, Collin MF, Anderson CL. Extremely low birth weight children and their peers: a comparison of preschool performance. Pediatrics. 1993;91:807–811. [PubMed] [Google Scholar]
- 14.Peitsch MC, Jongeneel CV. A 3-D model for the CD40 ligand predicts that it is a compact trimer similar to the tumor necrosis factors. Int Immunol. 1993;5:233–238. doi: 10.1093/intimm/5.2.233. [DOI] [PubMed] [Google Scholar]
- 15.Bazan JF. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990;87:6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baars JW, de Boer JP, Wagstaff J, Roem D, Eerenberg-Belmer AJ, Nauta J, Pinedo HM, Hack CE. Interleukin-2 induces activation of coagulation and fibrinolysis: resemblance to the changes seen during experimental endotoxaemia. Br J Haematol. 1992;82:295–301. doi: 10.1111/j.1365-2141.1992.tb06421.x. [DOI] [PubMed] [Google Scholar]
- 17.Jansen PM, Boermeester MA, Fischer E, de Jong IW, van der Poll T, Moldawer LL, Hack CE, Lowry SF. Contribution of interleukin-1 to activation of coagulation and fibrinolysis, neutrophil degranulation, and the release of secretory-type phospholipase A2 in sepsis: studies in nonhuman primates after interleukin-1 alpha administration and during lethal bacteremia. Blood. 1995;86:1027–1034. [PubMed] [Google Scholar]
- 18.Rauch U, Bonderman D, Bohrmann B, Badimon JJ, Himber J, Riederer MA, Nemerson Y. Transfer of tissue factor from leukocytes to platelets is mediated by CD15 and tissue factor. Blood. 2000;96:170–175. [PubMed] [Google Scholar]
- 19.van der Poll T, Levi M, Hack CE, ten Cate H, van Deventer SJ, Eerenberg AJ, de Groot ER, Jansen J, Gallati H, Buller HR, et al. Elimination of interleukin 6 attenuates coagulation activation in experimental endotoxemia in chimpanzees. J Exp Med. 1994;179:1253–1259. doi: 10.1084/jem.179.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bevers EM, Comfurius P, Dekkers DW, Harmsma M, Zwaal RF. Transmembrane phospholipid distribution in blood cells: control mechanisms and pathophysiological significance. Biol Chem. 1998;379:973–986. [PubMed] [Google Scholar]
- 21.Altieri DC, Morrissey JH, Edgington TS. Adhesive receptor Mac-1 coordinates the activation of factor X on stimulated cells of monocytic and myeloid differentiation: an alternative initiation of the coagulation protease cascade. Proc Natl Acad Sci U S A. 1988;85:7462–7466. doi: 10.1073/pnas.85.20.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Celi A, Pellegrini G, Lorenzet R, De Blasi A, Ready N, Furie BC, Furie B. P-selectin induces the expression of tissue factor on monocytes. Proc Natl Acad Sci U S A. 1994;91:8767–8771. doi: 10.1073/pnas.91.19.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kucey DS, Kubicki EI, Rotstein OD. Platelet-activating factor primes endotoxin-stimulated macrophage procoagulant activity. J Surg Res. 1991;50:436–441. doi: 10.1016/0022-4804(91)90021-d. [DOI] [PubMed] [Google Scholar]
- 24.Lo SK, Cheung A, Zheng Q, Silverstein RL. Induction of tissue factor on monocytes by adhesion to endothelial cells. J Immunol. 1995;154:4768–4777. [PubMed] [Google Scholar]
- 25.Bar-Shavit R, Kahn A, Mudd MS, Wilner GD, Mann KG, Fenton JW., 2nd Localization of a chemotactic domain in human thrombin. Biochemistry. 1984;23:397–400. doi: 10.1021/bi00298a001. [DOI] [PubMed] [Google Scholar]
- 26.Stecher VJ, Sorkin E. The chemotactic activity of fibrin lysis products. Int Arch Allergy Appl Immunol. 1972;43:879–886. doi: 10.1159/000230905. [DOI] [PubMed] [Google Scholar]
- 27.Esmon CT. Interactions between the innate immune and blood coagulation systems. Trends Immunol. 2004;25:536–542. doi: 10.1016/j.it.2004.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341:586–592. doi: 10.1056/NEJM199908193410807. [DOI] [PubMed] [Google Scholar]
- 29.Conway EM, Rosenberg RD. Tumor necrosis factor suppresses transcription of the thrombomodulin gene in endothelial cells. Mol Cell Biol. 1988;8:5588–5592. doi: 10.1128/mcb.8.12.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukudome K, Esmon CT. Identification, cloning, and regulation of a novel endothelial cell protein C/activated protein C receptor. J Biol Chem. 1994;269:26486–26491. [PubMed] [Google Scholar]
- 31.Klein NJ, Ison CA, Peakman M, Levin M, Hammerschmidt S, Frosch M, Heyderman RS. The influence of capsulation and lipooligosaccharide structure on neutrophil adhesion molecule expression and endothelial injury by Neisseria meningitidis. J Infect Dis. 1996;173:172–179. doi: 10.1093/infdis/173.1.172. [DOI] [PubMed] [Google Scholar]
- 32.Sakamoto T, Woodcock-Mitchell J, Marutsuka K, Mitchell JJ, Sobel BE, Fujii S. TNF-alpha and insulin, alone and synergistically, induce plasminogen activator inhibitor-1 expression in adipocytes. Am J Physiol. 1999;276:C1391–1397. doi: 10.1152/ajpcell.1999.276.6.C1391. [DOI] [PubMed] [Google Scholar]
- 33.Seki T, Healy AM, Fletcher DS, Noguchi T, Gelehrter TD. IL-1beta mediates induction of hepatic type 1 plasminogen activator inhibitor in response to local tissue injury. Am J Physiol. 1999;277:G801–809. doi: 10.1152/ajpgi.1999.277.4.G801. [DOI] [PubMed] [Google Scholar]
- 34.Cirino G, Cicala C, Bucci M, Sorrentino L, Ambrosini G, DeDominicis G, Altieri DC. Factor Xa as an interface between coagulation and inflammation. Molecular mimicry of factor Xa association with effector cell protease receptor-1 induces acute inflammation in vivo. J Clin Invest. 1997;99:2446–2451. doi: 10.1172/JCI119428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cirino G, Cicala C, Bucci MR, Sorrentino L, Maraganore JM, Stone SR. Thrombin functions as an inflammatory mediator through activation of its receptor. J Exp Med. 1996;183:821–827. doi: 10.1084/jem.183.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strukova SM, Dugina TN, Khlgatian SV, Redkozubov AE, Redkozubova GP, Pinelis VG. Thrombin-mediated events implicated in mast cell activation. Semin Thromb Hemost. 1996;22:145–150. doi: 10.1055/s-2007-999002. [DOI] [PubMed] [Google Scholar]
- 37.Bar-Shavit R, Kahn A, Wilner GD, Fenton JW., 2nd Monocyte chemotaxis: stimulation by specific exosite region in thrombin. Science. 1983;220:728–731. doi: 10.1126/science.6836310. [DOI] [PubMed] [Google Scholar]
- 38.Connolly TM, Condra C, Feng DM, Cook JJ, Stranieri MT, Reilly CF, Nutt RF, Gould RJ. Species variability in platelet and other cellular responsiveness to thrombin receptor-derived peptides. Thromb Haemost. 1994;72:627–633. [PubMed] [Google Scholar]
- 39.Shimizu T, Nishihira J, Watanabe H, Abe R, Honda A, Ishibashi T, Shimizu H. Macrophage migration inhibitory factor is induced by thrombin and factor Xa in endothelial cells. J Biol Chem. 2004;279:13729–13737. doi: 10.1074/jbc.M400150200. [DOI] [PubMed] [Google Scholar]
- 40.Sower LE, Froelich CJ, Carney DH, Fenton JW, 2nd, Klimpel GR. Thrombin induces IL-6 production in fibroblasts and epithelial cells. Evidence for the involvement of the seven-transmembrane domain (STD) receptor for alpha-thrombin. J Immunol. 1995;155:895–901. [PubMed] [Google Scholar]
- 41.Ueno A, Murakami K, Yamanouchi K, Watanabe M, Kondo T. Thrombin stimulates production of interleukin-8 in human umbilical vein endothelial cells. Immunology. 1996;88:76–81. doi: 10.1046/j.1365-2567.1996.d01-635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wenzel UO, Fouqueray B, Grandaliano G, Kim YS, Karamitsos C, Valente AJ, Abboud HE. Thrombin regulates expression of monocyte chemoattractant protein-1 in vascular smooth muscle cells. Circ Res. 1995;77:503–509. doi: 10.1161/01.res.77.3.503. [DOI] [PubMed] [Google Scholar]
- 43.Camerer E, Huang W, Coughlin SR. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc Natl Acad Sci U S A. 2000;97:5255–5260. doi: 10.1073/pnas.97.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rahman A, Anwar KN, True AL, Malik AB. Thrombin-induced p65 homodimer binding to downstream NF-kappa B site of the promoter mediates endothelial ICAM-1 expression and neutrophil adhesion. J Immunol. 1999;162:5466–5476. [PubMed] [Google Scholar]
- 45.Riewald M, Kravchenko VV, Petrovan RJ, O'Brien PJ, Brass LF, Ulevitch RJ, Ruf W. Gene induction by coagulation factor Xa is mediated by activation of protease-activated receptor 1. Blood. 2001;97:3109–3116. doi: 10.1182/blood.v97.10.3109. [DOI] [PubMed] [Google Scholar]
- 46.Cunningham MA, Romas P, Hutchinson P, Holdsworth SR, Tipping PG. Tissue factor and factor VIIa receptor/ligand interactions induce proinflammatory effects in macrophages. Blood. 1999;94:3413–3420. [PubMed] [Google Scholar]
- 47.Bizios R, Lai L, Fenton JW, 2nd, Malik AB. Thrombin-induced chemotaxis and aggregation of neutrophils. J Cell Physiol. 1986;128:485–490. doi: 10.1002/jcp.1041280318. [DOI] [PubMed] [Google Scholar]
- 48.Yamauchi T, Umeda F, Inoguchi T, Nawata H. Antithrombin III stimulates prostacyclin production by cultured aortic endothelial cells. Biochem Biophys Res Commun. 1989;163:1404–1411. doi: 10.1016/0006-291x(89)91135-2. [DOI] [PubMed] [Google Scholar]
- 49.Oelschlager C, Romisch J, Staubitz A, Stauss H, Leithauser B, Tillmanns H, Holschermann H. Antithrombin III inhibits nuclear factor kappaB activation in human monocytes and vascular endothelial cells. Blood. 2002;99:4015–4020. doi: 10.1182/blood.v99.11.4015. [DOI] [PubMed] [Google Scholar]
- 50.Souter PJ, Thomas S, Hubbard AR, Poole S, Romisch J, Gray E. Antithrombin inhibits lipopolysaccharide-induced tissue factor and interleukin-6 production by mononuclear cells, human umbilical vein endothelial cells, and whole blood. Crit Care Med. 2001;29:134–139. doi: 10.1097/00003246-200101000-00027. [DOI] [PubMed] [Google Scholar]
- 51.Conway EM, Van de Wouwer M, Pollefeyt S, Jurk K, Van Aken H, De Vriese A, Weitz JI, Weiler H, Hellings PW, Schaeffer P, Herbert JM, Collen D, Theilmeier G. The lectin-like domain of thrombomodulin confers protection from neutrophil-mediated tissue damage by suppressing adhesion molecule expression via nuclear factor kappaB and mitogen-activated protein kinase pathways. J Exp Med. 2002;196:565–577. doi: 10.1084/jem.20020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuksel M, Okajima K, Uchiba M, Horiuchi S, Okabe H. Activated protein C inhibits lipopolysaccharide-induced tumor necrosis factor-alpha production by inhibiting activation of both nuclear factor-kappa B and activator protein-1 in human monocytes. Thromb Haemost. 2002;88:267–273. [PubMed] [Google Scholar]
- 53.Iba T, Kidokoro A, Fukunaga M, Nagakari K, Shirahama A, Ida Y. Activated protein C improves the visceral microcirculation by attenuating the leukocyte-endothelial interaction in a rat lipopolysaccharide model. Crit Care Med. 2005;33:368–372. doi: 10.1097/01.ccm.0000153415.04995.88. [DOI] [PubMed] [Google Scholar]
- 54.Nick JA, Coldren CD, Geraci MW, Poch KR, Fouty BW, O'Brien J, Gruber M, Zarini S, Murphy RC, Kuhn K, Richter D, Kast KR, Abraham E. Recombinant human activated protein C reduces human endotoxin-induced pulmonary inflammation via inhibition of neutrophil chemotaxis. Blood. 2004;104:3878–3885. doi: 10.1182/blood-2004-06-2140. [DOI] [PubMed] [Google Scholar]
- 55.Grey ST, Tsuchida A, Hau H, Orthner CL, Salem HH, Hancock WW. Selective inhibitory effects of the anticoagulant activated protein C on the responses of human mononuclear phagocytes to LPS, IFN-gamma, or phorbol ester. J Immunol. 1994;153:3664–3672. [PubMed] [Google Scholar]
- 56.Joyce DE, Gelbert L, Ciaccia A, DeHoff B, Grinnell BW. Gene expression profile of antithrombotic protein c defines new mechanisms modulating inflammation and apoptosis. J Biol Chem. 2001;276:11199–11203. doi: 10.1074/jbc.C100017200. [DOI] [PubMed] [Google Scholar]
- 57.Joyce DE, Grinnell BW. Recombinant human activated protein C attenuates the inflammatory response in endothelium and monocytes by modulating nuclear factor-kappaB. Crit Care Med. 2002;30:S288–293. doi: 10.1097/00003246-200205001-00019. [DOI] [PubMed] [Google Scholar]
- 58.Brandt E, Ludwig A, Petersen F, Flad HD. Platelet-derived CXC chemokines: old players in new games. Immunol Rev. 2000;177:204–216. doi: 10.1034/j.1600-065x.2000.17705.x. [DOI] [PubMed] [Google Scholar]
- 59.Krijgsveld J, Zaat SA, Meeldijk J, van Veelen PA, Fang G, Poolman B, Brandt E, Ehlert JE, Kuijpers AJ, Engbers GH, Feijen J, Dankert J. Thrombocidins, microbicidal proteins from human blood platelets, are C-terminal deletion products of CXC chemokines. J Biol Chem. 2000;275:20374–20381. doi: 10.1074/jbc.275.27.20374. [DOI] [PubMed] [Google Scholar]
- 60.Rouhiainen A, Imai S, Rauvala H, Parkkinen J. Occurrence of amphoterin (HMG1) as an endogenous protein of human platelets that is exported to the cell surface upon platelet activation. Thromb Haemost. 2000;84:1087–1094. [PubMed] [Google Scholar]
- 61.Schenk BI, Petersen F, Flad HD, Brandt E. Platelet-derived chemokines CXC chemokine ligand (CXCL)7, connective tissue-activating peptide III, and CXCL4 differentially affect and cross-regulate neutrophil adhesion and transendothelial migration. J Immunol. 2002;169:2602–2610. doi: 10.4049/jimmunol.169.5.2602. [DOI] [PubMed] [Google Scholar]
- 62.Boehlen F, Clemetson KJ. Platelet chemokines and their receptors: what is their relevance to platelet storage and transfusion practice? Transfus Med. 2001;11:403–417. doi: 10.1046/j.1365-3148.2001.00340.x. [DOI] [PubMed] [Google Scholar]
- 63.Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19:568–574. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 64.Riondino S, Trifiro E, Principessa L, Mascioletti S, Di Renzo L, Gaudio C, Biasucci LM, Crea F, Pulcinelli FM. Lack of biological relevance of platelet cyclooxygenase-2 dependent thromboxane A2 production. Thromb Res. 2008;122:359–365. doi: 10.1016/j.thromres.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 65.Lindemann S, Tolley ND, Eyre JR, Kraiss LW, Mahoney TM, Weyrich AS. Integrins regulate the intracellular distribution of eukaryotic initiation factor 4E in platelets. A checkpoint for translational control. J Biol Chem. 2001;276:33947–33951. doi: 10.1074/jbc.M104281200. [DOI] [PubMed] [Google Scholar]
- 66.Bournazos S, Rennie J, Hart SP, Fox KA, Dransfield I. Monocyte functional responsiveness after PSGL-1-mediated platelet adhesion is dependent on platelet activation status. Arterioscler Thromb Vasc Biol. 2008;28:1491–1498. doi: 10.1161/ATVBAHA.108.167601. [DOI] [PubMed] [Google Scholar]
- 67.Itoh S, Takeshita K, Susuki C, Shige-Eda K, Tsuji T. Redistribution of P-selectin ligands on neutrophil cell membranes and the formation of platelet-neutrophil complex induced by hemodialysis membranes. Biomaterials. 2008;29:3084–3090. doi: 10.1016/j.biomaterials.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 68.Weyrich AS, Zimmerman GA. Platelets: signaling cells in the immune continuum. Trends Immunol. 2004;25:489–495. doi: 10.1016/j.it.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 69.Langer HF, Daub K, Braun G, Schonberger T, May AE, Schaller M, Stein GM, Stellos K, Bueltmann A, Siegel-Axel D, Wendel HP, Aebert H, Roecken M, Seizer P, Santoso S, Wesselborg S, Brossart P, Gawaz M. Platelets recruit human dendritic cells via Mac-1/JAM-C interaction and modulate dendritic cell function in vitro. Arterioscler Thromb Vasc Biol. 2007;27:1463–1470. doi: 10.1161/ATVBAHA.107.141515. [DOI] [PubMed] [Google Scholar]
- 70.Li G, Kim YJ, Mantel C, Broxmeyer HE. P-selectin enhances generation of CD14+CD16+ dendritic-like cells and inhibits macrophage maturation from human peripheral blood monocytes. J Immunol. 2003;171:669–677. doi: 10.4049/jimmunol.171.2.669. [DOI] [PubMed] [Google Scholar]
- 71.Nguyen XD, Muller-Berghaus J, Kalsch T, Schadendorf D, Borggrefe M, Kluter H. Differentiation of monocyte-derived dendritic cells under the influence of platelets. Cytotherapy. 2008;10:720–729. doi: 10.1080/14653240802378912. [DOI] [PubMed] [Google Scholar]
- 72.Niessen F, Schaffner F, Furlan-Freguia C, Pawlinski R, Bhattacharjee G, Chun J, Derian CK, Andrade-Gordon P, Rosen H, Ruf W. Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature. 2008;452:654–658. doi: 10.1038/nature06663. [DOI] [PubMed] [Google Scholar]
- 73.Hilf N, Singh-Jasuja H, Schwarzmaier P, Gouttefangeas C, Rammensee HG, Schild H. Human platelets express heat shock protein receptors and regulate dendritic cell maturation. Blood. 2002;99:3676–3682. doi: 10.1182/blood.v99.10.3676. [DOI] [PubMed] [Google Scholar]
- 74.Brenner B. Haemostatic changes in pregnancy. Thromb Res. 2004;114:409–414. doi: 10.1016/j.thromres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 75.Bick RL. Recurrent miscarriage syndrome and infertility caused by blood coagulation protein or platelet defects. Hematol Oncol Clin North Am. 2000;14:1117–1131. doi: 10.1016/s0889-8588(05)70174-x. [DOI] [PubMed] [Google Scholar]
- 76.Kumar M, Mehta P. Congenital coagulopathies and pregnancy: report of four pregnancies in a factor X-deficient woman. Am J Hematol. 1994;46:241–244. doi: 10.1002/ajh.2830460315. [DOI] [PubMed] [Google Scholar]
- 77.Nelson DB, Ness RB, Grisso JA, Cushman M. Influence of hemostatic factors on spontaneous abortion. Am J Perinatol. 2001;18:195–201. doi: 10.1055/s-2001-15503. [DOI] [PubMed] [Google Scholar]
- 78.Pauer HU, Burfeind P, Kostering H, Emons G, Hinney B. Factor XII deficiency is strongly associated with primary recurrent abortions. Fertil Steril. 2003;80:590–594. doi: 10.1016/s0015-0282(03)00788-x. [DOI] [PubMed] [Google Scholar]
- 79.Sugi T, Makino T. Antiphospholipid antibodies and kininogens in pathologic pregnancies: a review. Am J Reprod Immunol. 2002;47:283–288. doi: 10.1034/j.1600-0897.2002.01103.x. [DOI] [PubMed] [Google Scholar]
- 80.Alfirevic Z, Roberts D, Martlew V. How strong is the association between maternal thrombophilia and adverse pregnancy outcome? A systematic review. Eur J Obstet Gynecol Reprod Biol. 2002;101:6–14. doi: 10.1016/s0301-2115(01)00496-1. [DOI] [PubMed] [Google Scholar]
- 81.Rey E, Kahn SR, David M, Shrier I. Thrombophilic disorders and fetal loss: a meta-analysis. Lancet. 2003;361:901–908. doi: 10.1016/S0140-6736(03)12771-7. [DOI] [PubMed] [Google Scholar]
- 82.Hefler L, Jirecek S, Heim K, Grimm C, Antensteiner G, Zeillinger R, Husslein P, Tempfer C. Genetic polymorphisms associated with thrombophilia and vascular disease in women with unexplained late intrauterine fetal death: a multicenter study. J Soc Gynecol Investig. 2004;11:42–44. doi: 10.1016/j.jsgi.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 83.Kahn SR, Platt R, McNamara H, Rozen R, Chen MF, Genest J, Jr., Goulet L, Lydon J, Seguin L, Dassa C, Masse A, Asselin G, Benjamin A, Miner L, Ghanem A, Kramer MS. Inherited thrombophilia and preeclampsia within a multicenter cohort: the Montreal Preeclampsia Study. Am J Obstet Gynecol. 2009;200:e151–159. doi: 10.1016/j.ajog.2008.09.023. 151. discussion e151-155. [DOI] [PubMed] [Google Scholar]
- 84.Kupferminc MJ, Eldor A, Steinman N, Many A, Bar-Am A, Jaffa A, Fait G, Lessing JB. Increased frequency of genetic thrombophilia in women with complications of pregnancy. N Engl J Med. 1999;340:9–13. doi: 10.1056/NEJM199901073400102. [DOI] [PubMed] [Google Scholar]
- 85.Infante-Rivard C, Rivard GE, Yotov WV, Genin E, Guiguet M, Weinberg C, Gauthier R, Feoli-Fonseca JC. Absence of association of thrombophilia polymorphisms with intrauterine growth restriction. N Engl J Med. 2002;347:19–25. doi: 10.1056/NEJM200207043470105. [DOI] [PubMed] [Google Scholar]
- 86.Rodger MA, Paidas M, McLintock C, Middeldorp S, Kahn S, Martinelli I, Hague W, Rosene Montella K, Greer I. Inherited thrombophilia and pregnancy complications revisited. Obstet Gynecol. 2008;112:320–324. doi: 10.1097/AOG.0b013e31817e8acc. [DOI] [PubMed] [Google Scholar]
- 87.Weiler-Guettler H, Aird WC, Rayburn H, Husain M, Rosenberg RD. Developmentally regulated gene expression of thrombomodulin in postimplantation mouse embryos. Development. 1996;122:2271–2281. doi: 10.1242/dev.122.7.2271. [DOI] [PubMed] [Google Scholar]
- 88.Edstrom CS, Calhoun DA, Christensen RD. Expression of tissue factor pathway inhibitor in human fetal and placental tissues. Early Hum Dev. 2000;59:77–84. doi: 10.1016/s0378-3782(00)00084-0. [DOI] [PubMed] [Google Scholar]
- 89.Sood R, Kalloway S, Mast AE, Hillard CJ, Weiler H. Fetomaternal cross talk in the placental vascular bed: control of coagulation by trophoblast cells. Blood. 2006;107:3173–3180. doi: 10.1182/blood-2005-10-4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McCracken SA, Drury CL, Lee HS, Morris JM. Pregnancy is associated with suppression of the nuclear factor kappaB/IkappaB activation pathway in peripheral blood mononuclear cells. J Reprod Immunol. 2003;58:27–47. doi: 10.1016/s0165-0378(02)00081-5. [DOI] [PubMed] [Google Scholar]
- 91.Saito S, Sakai M, Sasaki Y, Tanebe K, Tsuda H, Michimata T. Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1:Th2 cell ratio during normal human pregnancy and preeclampsia. Clin Exp Immunol. 1999;117:550–555. doi: 10.1046/j.1365-2249.1999.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hardy DB, Janowski BA, Corey DR, Mendelson CR. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression. Mol Endocrinol. 2006;20:2724–2733. doi: 10.1210/me.2006-0112. [DOI] [PubMed] [Google Scholar]
- 93.Ramirez F, Fowell DJ, Puklavec M, Simmonds S, Mason D. Glucocorticoids promote a TH2 cytokine response by CD4+ T cells in vitro. J Immunol. 1996;156:2406–2412. [PubMed] [Google Scholar]
- 94.Song XY, Zeng L, Jin W, Pilo CM, Frank ME, Wahl SM. Suppression of streptococcal cell wall-induced arthritis by human chorionic gonadotropin. Arthritis Rheum. 2000;43:2064–2072. doi: 10.1002/1529-0131(200009)43:9<2064::AID-ANR18>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 95.Huang SJ, Schatz F, Masch R, Rahman M, Buchwalder L, Niven-Fairchild T, Tang C, Abrahams VM, Krikun G, Lockwood CJ. Regulation of chemokine production in response to pro-inflammatory cytokines in first trimester decidual cells. J Reprod Immunol. 2006;72:60–73. doi: 10.1016/j.jri.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 96.Brosens I, Robertson WB, Dixon HG. The physiological response of the vessels of the placental bed to normal pregnancy. J Pathol Bacteriol. 1967;93:569–579. doi: 10.1002/path.1700930218. [DOI] [PubMed] [Google Scholar]
- 97.Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta. 1983;4:397–413. doi: 10.1016/s0143-4004(83)80043-5. [DOI] [PubMed] [Google Scholar]
- 98.Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69:1–7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- 99.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 100.van den Brule F, Berndt S, Simon N, Coulon C, Le Goarant J, Munaut C, Noel A, Frankenne F, Foidart JM. Trophoblast invasion and placentation: molecular mechanisms and regulation. Chem Immunol Allergy. 2005;88:163–180. doi: 10.1159/000087833. [DOI] [PubMed] [Google Scholar]
- 101.Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, Post M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3) J Clin Invest. 2000;105:577–587. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Piccinni MP, Romagnani S. Regulation of fetal allograft survival by a hormone-controlled Th1- and Th2-type cytokines. Immunol Res. 1996;15:141–150. doi: 10.1007/BF02918503. [DOI] [PubMed] [Google Scholar]
- 103.Rescigno M, Granucci F, Ricciardi-Castagnoli P. Dendritic cells at the end of the millennium. Immunol Cell Biol. 1999;77:404–410. doi: 10.1046/j.1440-1711.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- 104.Lockwood CJ, Matta P, Krikun G, Koopman LA, Masch R, Toti P, Arcuri F, Huang SJ, Funai EF, Schatz F. Regulation of Monocyte Chemoattractant Protein-1 Expression by Tumor Necrosis Factor-{alpha} and Interleukin-1{beta} in First Trimester Human Decidual Cells: Implications for Preeclampsia. Am J Pathol. 2006;168:445–452. doi: 10.2353/ajpath.2006.050082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huang SJ, Chen CP, Schatz F, Rahman M, Abrahams VM, Lockwood CJ. Preeclampsia is associated with dendritic cell recruitment into the uterine decidua. J Pathol. 2008;214:328–336. doi: 10.1002/path.2257. [DOI] [PubMed] [Google Scholar]
- 106.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, NatansonYaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 107.Parham P. NK cells and trophoblasts: partners in pregnancy. J Exp Med. 2004;200:951–955. doi: 10.1084/jem.20041783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Plaisier M, Dennert I, Rost E, Koolwijk P, van Hinsbergh VW, Helmerhorst FM. Decidual vascularization and the expression of angiogenic growth factors and proteases in first trimester spontaneous abortions. Hum Reprod. 2008 doi: 10.1093/humrep/den296. [DOI] [PubMed] [Google Scholar]
- 109.Labarrere CA, Faulk WP. Anchoring villi in human placental basal plate: lymphocytes, macrophages and coagulation. Placenta. 1991;12:173–182. doi: 10.1016/0143-4004(91)90021-7. [DOI] [PubMed] [Google Scholar]
- 110.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 111.Trundley A, Moffett A. Human uterine leukocytes and pregnancy. Tissue Antigens. 2004;63:1–12. doi: 10.1111/j.1399-0039.2004.00170.x. [DOI] [PubMed] [Google Scholar]
- 112.Hiby SE, Walker JJ, O'Shaughnessy KM, Redman CW, Carrington M, Trowsdale J, Moffett A. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200:957–965. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Greenwood JD, Minhas K, di Santo JP, Makita M, Kiso Y, Croy BA. Ultrastructural studies of implantation sites from mice deficient in uterine natural killer cells. Placenta. 2000;21:693–702. doi: 10.1053/plac.2000.0556. [DOI] [PubMed] [Google Scholar]
- 114.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 115.Ferry BL, Starkey PM, Sargent IL, Watt GM, Jackson M, Redman CW. Cell populations in the human early pregnancy decidua: natural killer activity and response to interleukin-2 of CD56-positive large granular lymphocytes. Immunology. 1990;70:446–452. [PMC free article] [PubMed] [Google Scholar]
- 116.Ashkar AA, Croy BA. Functions of uterine natural killer cells are mediated by interferon gamma production during murine pregnancy. Semin Immunol. 2001;13:235–241. doi: 10.1006/smim.2000.0319. [DOI] [PubMed] [Google Scholar]
- 117.Clark DE, Smith SK, Licence D, Evans AL, Charnock-Jones DS. Comparison of expression patterns for placenta growth factor, vascular endothelial growth factor (VEGF), VEGF-B and VEGF-C in the human placenta throughout gestation. J Endocrinol. 1998;159:459–467. doi: 10.1677/joe.0.1590459. [DOI] [PubMed] [Google Scholar]
- 118.Lash GE, Schiessl B, Kirkley M, Innes BA, Cooper A, Searle RF, Robson SC, Bulmer JN. Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J Leukoc Biol. 2006;80:572–580. doi: 10.1189/jlb.0406250. [DOI] [PubMed] [Google Scholar]
- 119.Trowsdale J, Betz AG. Mother's little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol. 2006;7:241–246. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- 120.Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- 121.Riley JK, Yokoyama WM. NK cell tolerance and the maternal-fetal interface. Am J Reprod Immunol. 2008;59:371–387. doi: 10.1111/j.1600-0897.2008.00593.x. [DOI] [PubMed] [Google Scholar]
- 122.Vince GS, Starkey PM, Jackson MC, Sargent IL, Redman CW. Flow cytometric characterisation of cell populations in human pregnancy decidua and isolation of decidual macrophages. J Immunol Methods. 1990;132:181–189. doi: 10.1016/0022-1759(90)90028-t. [DOI] [PubMed] [Google Scholar]
- 123.He YY, Du MR, Guo PF, He XJ, Zhou WH, Zhu XY, Li DJ. Regulation of C-C motif chemokine ligand 2 and its receptor in human decidual stromal cells by pregnancy-associated hormones in early gestation. Hum Reprod. 2007;22:2733–2742. doi: 10.1093/humrep/dem208. [DOI] [PubMed] [Google Scholar]
- 124.Petroff MG, Chen L, Phillips TA, Azzola D, Sedlmayr P, Hunt JS. B7 family molecules are favorably positioned at the human maternal-fetal interface. Biol Reprod. 2003;68:1496–1504. doi: 10.1095/biolreprod.102.010058. [DOI] [PubMed] [Google Scholar]
- 125.Sharkey AM, Charnock-Jones DS, Boocock CA, Brown KD, Smith SK. Expression of mRNA for vascular endothelial growth factor in human placenta. J Reprod Fertil. 1993;99:609–615. doi: 10.1530/jrf.0.0990609. [DOI] [PubMed] [Google Scholar]
- 126.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yui J, Garcia-Lloret M, Wegmann TG, Guilbert LJ. Cytotoxicity of tumour necrosis factor-alpha and gamma-interferon against primary human placental trophoblasts. Placenta. 1994;15:819–835. doi: 10.1016/s0143-4004(05)80184-5. [DOI] [PubMed] [Google Scholar]
- 128.Hunt JS. Cytokine networks in the uteroplacental unit: macrophages as pivotal regulatory cells. J Reprod Immunol. 1989;16:1–17. doi: 10.1016/0165-0378(89)90002-8. [DOI] [PubMed] [Google Scholar]
- 129.Straszewski-Chavez SL, Abrahams VM, Mor G. The role of apoptosis in the regulation of trophoblast survival and differentiation during pregnancy. Endocr Rev. 2005;26:877–897. doi: 10.1210/er.2005-0003. [DOI] [PubMed] [Google Scholar]
- 130.Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11:342–352. doi: 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 131.Haeger M, Unander M, Norder-Hansson B, Tylman M, Bengtsson A. Complement, neutrophil, and macrophage activation in women with severe preeclampsia and the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol. 1992;79:19–26. [PubMed] [Google Scholar]
- 132.Katabuchi H, Yih S, Ohba T, Matsui K, Takahashi K, Takeya M, Okamura H. Characterization of macrophages in the decidual atherotic spiral artery with special reference to the cytology of foam cells. Med Electron Microsc. 2003;36:253–262. doi: 10.1007/s00795-003-0223-2. [DOI] [PubMed] [Google Scholar]
- 133.Reister F, Frank HG, Heyl W, Kosanke G, Huppertz B, Schroder W, Kaufmann P, Rath W. The distribution of macrophages in spiral arteries of the placental bed in pre-eclampsia differs from that in healthy patients. Placenta. 1999;20:229–233. doi: 10.1053/plac.1998.0373. [DOI] [PubMed] [Google Scholar]
- 134.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 135.Wang YY, Tawfik O, Wood GW. Endotoxin-induced abortion in mice is mediated by activated fetal macrophages. J Leukoc Biol. 1998;63:40–50. doi: 10.1002/jlb.63.1.40. [DOI] [PubMed] [Google Scholar]
- 136.Reister F, Frank HG, Kingdom JC, Heyl W, Kaufmann P, Rath W, Huppertz B. Macrophage-induced apoptosis limits endovascular trophoblast invasion in the uterine wall of preeclamptic women. Lab Invest. 2001;81:1143–1152. doi: 10.1038/labinvest.3780326. [DOI] [PubMed] [Google Scholar]
- 137.Bauer S, Pollheimer J, Hartmann J, Husslein P, Aplin JD, Knofler M. Tumor necrosis factor-alpha inhibits trophoblast migration through elevation of plasminogen activator inhibitor-1 in first-trimester villous explant cultures. J Clin Endocrinol Metab. 2004;89:812–822. doi: 10.1210/jc.2003-031351. [DOI] [PubMed] [Google Scholar]
- 138.Pathak N, Sawhney H, Vasishta K, Majumdar S. Estimation of oxidative products of nitric oxide (nitrates, nitrites) in preeclampsia. Aust N Z J Obstet Gynaecol. 1999;39:484–487. doi: 10.1111/j.1479-828x.1999.tb03139.x. [DOI] [PubMed] [Google Scholar]
- 139.Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukoc Biol. 1994;55:410–422. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- 140.Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med. 2006;203:2165–2175. doi: 10.1084/jem.20061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gardner L, Moffett A. Dendritic cells in the human decidua. Biol Reprod. 2003;69:1438–1446. doi: 10.1095/biolreprod.103.017574. [DOI] [PubMed] [Google Scholar]
- 142.Zenclussen AC. CD4(+)CD25+ T regulatory cells in murine pregnancy. J Reprod Immunol. 2005;65:101–110. doi: 10.1016/j.jri.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 143.Plaks V, Birnberg T, Berkutzki T, Sela S, Benyashar A, Kalchenko V, Mor G, Keshet E, Dekel N, Neeman M, Jung S. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Invest. 2008 doi: 10.1172/JCI36682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lockwood CJ, Krikun G, Caze R, Rahman M, Buchwalder LF, Schatz F. Decidual cell-expressed tissue factor in human pregnancy and its involvement in hemostasis and preeclampsia-related angiogenesis. Ann N Y Acad Sci. 2008;1127:67–72. doi: 10.1196/annals.1434.013. [DOI] [PubMed] [Google Scholar]