Abstract
In contrast to chronic/long-term stress that suppresses/dysregulates immune function, an acute/short-term fight-or-flight stress response experienced during immune activation can enhance innate and adaptive immunity. Moderate ultraviolet-B (UV) exposure provides a non-invasive system for studying the naturalistic emergence, progression and regression of squamous cell carcinoma (SCC). Because SCC is an immunoresponsive cancer, we hypothesized that short-term stress experienced before UV exposure would enhance protective immunity and increase resistance to SCC. Control and short-term stress groups were treated identically except that the short-term stress group was restrained (2.5h) before each of nine UV-exposure sessions (minimum erythemal dose, 3-times/week) during weeks 4-6 of the ten-week UV-exposure protocol. Tumors were measured weekly, and tissue collected at weeks 7, 20, & 32. Chemokine and cytokine gene expression was quantified by real-time PCR, and CD4+ and CD8+ T cells by flow cytometry and immunohistochemistry. Compared to controls, the short-term stress group showed greater cutaneous-T-cell-attracting-chemokine (CTACK)/CCL27, RANTES, IL-12, and IFN-γ gene expression at weeks 7, 20, & 32, higher skin infiltrating T cell numbers (weeks 7 & 20), lower tumor incidence (weeks 11-20) and fewer tumors (weeks 11-26). These results suggest that activation of short-term stress physiology increased chemokine expression and T cell trafficking and/or function during/following UV exposure, and enhanced Type 1 cytokine-driven cell-mediated immunity that is crucial for resistance to SCC. Therefore, the physiological fight-or-flight stress response and its adjuvant-like immuno-enhancing effects, may provide a novel and important mechanism for enhancing immune system mediated tumor-detection/elimination that merits further investigation.
Keywords: skin cancer/tumor, psychological stress, cell-mediated immunity, immune cells, Th1-Th2 cytokines, Cutaneous T-cell Attracting Chemokine (CTACK)
INTRODUCTION
Fighting disease by augmenting endogenous biological defenses in addition to using pharmacological and surgical interventions is likely to be highly advantageous. The fight-or-flight stress response is one of nature’s under-appreciated defense mechanisms that activates multiple psycho-physiological systems to promote survival. We have proposed that it is important to identify biological mechanisms by which a fight-or-flight stress response promotes survival because such mechanisms could be clinically harnessed to fight disease (Dhabhar, 2009; Dhabhar and McEwen, 2001; Dhabhar and Viswanathan, 2005). Stress can be defined as a constellation of events that begins with a stimulus (stressor), that precipitates a reaction in the brain (stress perception), which activates physiologic fight-or-flight systems in the body (stress response) (Dhabhar and McEwen, 2001). The idea that a stress response may be harnessed to fight disease seems counter-intuitive at first glance because numerous studies have shown that stress can suppress or dysregulate immune function (Glaser and Kiecolt-Glaser, 2005) and increase susceptibility to some forms of infection (Cohen et al., 1991) and cancer (Antoni et al., 2006; Ben-Eliyahu, 2003; Ben-Eliyahu et al., 2007; Ben-Eliyahu et al., 1991; Saul et al., 2005; Thaker et al., 2006). However, in contrast to chronic or long-term (weeks to months in duration) stress which is generally harmful, acute or short-term (minutes to hours in duration) stress experienced at the time of immune activation or antigen exposure induces a redistribution of circulating immune cells to organs like the skin, subcutaneous tissues, and sentinel lymph nodes (Dhabhar and McEwen, 1997; Dhabhar et al., 1995b, 1996), increases leukocyte trafficking to sites of wounding or immune activation (Dhabhar, 1998; Viswanathan and Dhabhar, 2005), and enhances innate and adaptive immune responses (Blecha et al., 1982; Dhabhar, 1998; Dhabhar and McEwen, 1996, 1999, 2001; Dhabhar et al., 2000; Dhabhar and Viswanathan, 2005; Saint-Mezard et al., 2003; Viswanathan et al., 2005; Wood et al., 1993; Zalcman and Anisman, 1993). The studies described here were designed to investigate whether enhancement of immunity induced by short-term stress might delay/suppress the emergence or progression of an immunoresponsive cancer such as squamous cell carcinoma (SCC), and to examine the effects of short-term stress on specific chemokines (CTACK and RANTES), cytokines (IL-12, IFN-γ, IL-4 and IL-10), and helper (CD4+) and cytolytic (CD8+) T cell subpopulations, that are likely to mediate resistance to SCC.
Skin cancer is the most common form of cancer in the United States and is primarily induced by exposure to UV radiation (NIH, 2007; Oberyszyn, 2008). Over 2-3 million new cases of non-melanoma skin cancer (NMSC) occur each year worldwide (WHO, 2005). The ultraviolet B (UVB) component of sunlight is a complete carcinogen and is responsible for most NMSCs (Armstrong and Kricker, 2001). Moderate UVB exposure (minimum erythemal dose, 3-times/week, 10 weeks) provides a non-invasive, naturalistic model for SCC that enables the study of tumor emergence, progression and regression (Saul et al., 2005; Thomas-Ahner et al., 2007). Immediate effects of UVB include DNA damage, epidermal hyperplasia and inflammation, all of which contribute to the development of skin cancer (Berton et al., 2001; Oberyszyn, 2008). Skin tumors such as SCCs are susceptible to elimination by endogenous cell-mediated immunity (Fisher and Kripke, 1977; Kripke, 1994). However, SCC can progress and metastasize, particularly in immunocompromised hosts (Boukamp, 2005; Karagas et al., 2001). UV exposure also increases susceptibility to skin cancer by suppressing lymphocyte trafficking and T cell and natural killer (NK) cell function (Morison et al., 1979), promoting IL-10-induced systemic and local immunosuppression (Nishigori et al., 1996; Sieffert and Granstein, 2002; Toichi et al., 2008), and suppressing cell-mediated immunity that is crucial for protection against NMSC (Clydesdale et al., 2001; Kripke, 1994; Nghiem et al., 2002).
To our knowledge, the results described here show for the first time that activation of acute or short-term stress physiology may mobilize mechanisms that mediate anti-tumor immune responses. Given the importance of cell-mediated immunity for protection against infectious agents and cancers like SCC, it is crucial to elucidate mechanisms by which the body may endogenously enhance cell-mediated immunity. It is hoped that these studies will begin to provide insight into how biobehavioral or pharmacological activation of short-term stress physiology might bolster cell-mediated immunity, and enhance the detection and elimination of tumors that are either naturally immunogenic, or made immuno-responsive via tumor immunotherapy. Such insight may provide the means for optimizing a patient’s response to treatment.
MATERIALS AND METHODS
Mice
Young adult (7-8 weeks) female SKH1 mice (Charles River, Wilmington, Massachusetts) were housed in AAALAC-accredited facilities. SKH1 hairless mice are widely used in studies involving UVB exposure and skin cancer, and UVB-induced lesions in this strain resemble human squamous cell carcinoma (Ortonne, 2002). The animal room was maintained on a 12-hour light-dark cycle (lights on at 6 am). Mice were given food and water ad libitum. All experiments were conducted according to protocol approved by the Institutional Laboratory Animal Care and Use Committee.
Experimental Design
Mice were exposed to UVB three times per week (~10 minutes per session, every Monday, Wednesday, and Friday, during weeks 1 to 10). During each exposure session, mice were placed in plastic cages that were rotated (between sessions) with respect to location under the light banks to ensure homogeneous UVB exposure. Mice were exposed dorsally to UVB from Phillips FS40UVB lamps (American Ultraviolet Company, Murray Hill, New Jersey) fitted with Kodacel filters (Eastman Kodak, Rochester, New York) to ensure the emission of UVB light (290-320nm). One minimal erythemal dose (MED) of UVB (2240 J/m2, ~10 min exposure), as measured by a UVX digital radiometer (UVP, Inc., San Gabriel, California), was administered. The MED was previously determined and is periodically validated by T.M.O. The control and short-term stress groups each included 30 mice with the aim of collecting tissues from 10 mice per treatment group per time point. Ten mice were euthanized per treatment group per time point. Cages housing 5 mice each were randomly assigned to each treatment group and time point at the beginning of the study. One mouse died in the control group (week 20) and 2 mice died in the short-term stress group (week 32). Their data was not included in any of the analyses for this study.
Short-term stress was administered for 2.5 h before each ten minute UV exposure session during weeks 4-6 by placing each mouse in a well-ventilated plastic tube restrainer while ensuring that the mouse was not squeezed or compressed. Previous studies have shown that 2 to 2.5 h of restraint is optimal for observing the leukocyte redistribution as well as skin immuno-enhancing effects of short-term stress (Dhabhar and McEwen, 1997; Dhabhar et al., 2000; Dhabhar and Viswanathan, 2005; Viswanathan et al., 2005). This procedure mimics stress that is largely psychological in nature because of the perception of confinement experienced by the mice (Glavin et al., 1996). It activates the sympathetic nervous system (Kvetnansky et al., 1993), and hypothalamic-pituitary-adrenal axis (Dhabhar et al., 1994), resulting in activation of adrenal steroid receptors in tissues throughout the body (Dhabhar et al., 1995a). Stress was administered before each of nine UV exposure sessions during weeks 4-6. This time period was chosen as the midpoint of the ten-week UV exposure paradigm, and we have previously shown that chronic stress administered during this time period has immunosuppressive effects and increases susceptibility to SCC (Saul et al., 2005). Mice were monitored for tumor development from weeks 11 through 31. A short-term stress group without UV exposure was not included in this study because we have not observed the development of tumors when short-term stress is administered in the absence of UV. Mice in each treatment group were euthanized at each tissue collection time point (weeks 7, 20, and 32). These time points permitted examination of the immediate effects of short-term stress during UV exposure before any tumors had developed (week 7), as well as to examine longer term effects of short-term stress on both early (week 20) and later (week 32) phases of tumor development after the cessation of UV exposure. Mice were euthanized using CO2 at each time point. Tissues were immediately dissected, frozen on dry ice, and stored at -70 °C.
Tumor Counts and Measurements
None of the mice had tumors at baseline (week 10, or earlier). Tumor development was quantified weekly (by A.N.S.) beginning at week 11. The two longest measurements (mm) in perpendicular directions were made using a Cen-Tech digital caliper (Harbor Freight Tools, Camarillo, California) and were multiplied to obtain a representation of tumor area (expressed in mm2). All lesions that had a diameter of at least 1 mm were counted and almost all of these lesions were slightly raised. Some tumors regressed which registered as a decrease in tumor numbers. At the time of tissue collection, tumors were collected as individual samples with only one lesion being included in each collected sample.
Histopathology
Skin lesions, identified grossly as tumors by the prosector, were analyzed in 3 mice/group/time point, by our board-certified veterinary pathologist (D.M.B.). Briefly, tumors were excised, placed in 10% neutral-buffered formalin for 2 hours, washed in phosphate-buffered saline, processed, and embedded in paraffin blocks. Sections (5 μm) were mounted onto Superfrost Plus microscope slides (Fisher Scientific, Pittsburgh, Pennsylvania), and stained with hematoxylin and eosin (H&E). Tumors were analyzed in a blinded manner for characteristics of epidermal hyperplasia (EH), dysplastic epidermal hyperplasia (DEH), papilloma, microinvasive squamous cell carcinoma (MICA), and well-differentiated SCC.
Immunohistochemistry
Tumors for immunhistochemistry were collected by the prosector, embedded in TBS Tissue Freezing Medium (Triangle Biomedical Sciences, Durham, North Carolina), sectioned (10 μm) on a cryostat, and mounted on Superfrost Plus microscope slides (Fisher Scientific). Skin specimens were sectioned, processed, and stained for CD4 and CD8 as described previously (Saul et al., 2005). Briefly, sections were quenched for endogeneous peroxidase, blocked in 10% normal goat serum, and incubated with primary antibodies (BD-Pharmingen, San Diego, California): rat anti-mouse CD4 (1:25), and rat anti-mouse CD8 (1:50), biotin blocked for 40 min (Biotin Blocking System, Dako, Carpinteria, California), and incubated with mouse-adsorbed biotinylated rabbit anti-rat immunoglobulin (1:200) (Vector, Burlingame, California). 3,3-Diaminobenzidine-tetrahydrochloride (DAB, Dako, Carpinteria, California) was used as the substrate chromogen and hematoxylin as the counter-stain. Murine spleen tissue was used as a positive control, and no primary antibody served as the negative control. CD4+ and CD8+ T cell infiltration was measured in dorsal skin at weeks 7 and 20. At week 32, T cell infiltration was measured in dorsal skin that included thickened areas of epidermis containing lesions (diameter < 2 mm), which represent a transition from focal epidermal hyperplasia to true papilloma and are part of the spectrum of lesions leading ultimately to squamous cell carcinoma (Canfield et al., 1986; Kligman and Kligman, 1981). The number of positive cells per standardized field (16,085 μm2) were counted by an investigator (A.N.S.) who was blinded to the treatment groups. Ten fields at 60x magnification were analyzed per skin section, 10 mice per treatment group (week 7 and week 20) and 5 mice per treatment group (week 32).
Measurement of Gene Expression
Gene expression was measured in dorsal skin isolated from each animal in each treatment group euthanized at weeks 7 and 20. In groups that were euthanized at week 32, gene expression was measured in dorsal skin that included lesions (diameter < 2 mm) that represent a transition from focal epidermal hyperplasia to true papilloma and are part of the spectrum of lesions leading ultimately to SCC (Canfield et al., 1986; Kligman and Kligman, 1981). Areas of skin where it was evident that the mice had been scratching were not collected. Lesions were collected as individual samples (including dermis and panniculus carnosus) with only one lesion being collected per sample, and only one sample per animal being analyzed for gene expression. In order to further standardize the amount of analysis substrate, mRNA was isolated from the same quantity (20 μg) of total RNA for every sample. Moreover, GAPDH gene expression was measured in each well as a duplex analysis for every gene that was measured and results were normalized across samples using GAPDH expression as an internal standard. Gene expression for cutaneous T cell attracting chemokine (CTACK)/CCL27, interleukin (IL)-12p40, interferon (IFN)-γ, interleukin (IL)-4, and interleukin (IL)-10 was measured using quantitative PCR as described previously (Saul et al., 2005).
Detection of CD4+ and CD8+ T cells in Peripheral Blood
Numbers of helper (CD3+CD4+) and cytolytic (CD3+CD8+) T cells were measured in peripheral blood using flow cytometry (FACSCalibur, Becton Dickinson, San Jose, California). Directly conjugated rat anti-mouse CD3, CD4, and CD8, (BD-Pharmingen, San Diego, California) antibodies were used to identify specific subpopulations. Leukocytes were stained and analyzed as described previously (Saul et al., 2005).
Statistical Analysis
Generalized linear mixed-effects and non-linear mixed-effect models (McCulloch and Searle, 2001) were employed respectively to estimate mean tumor count and mean of logarithmically-transformed tumor area as a function of time since randomization, study arm, and their interaction. These regression models accommodated repeated measurements via choice of random components and correlation structure. To allow slope to change as a function of time and to approximate curvature in temporal response, modeling used linear splines with knots evenly placed at 16, 21 and 26 weeks (Neter et al., 1996). Even placement of knots distributes flexibility in curve fitting over the entire time span covered by the data. For tumor area, mice that developed tumors had positive values for area, but those that did not were assigned an area of zero. The non-linear mixed effect model for these data was structured to simultaneously model a mixture of zeros and positive values (Berk and Lachenbruch, 2002). Back-transformation from the logarithmic scale followed Finney (Finney, 1941). Mixed models fit a regression line to the data for each mouse.
We also performed a Bayesian changepoint analysis (Carlin et al., 1992) to identify empirically if tumor progression occurred in phases. Changepoint analysis examines time-series data to assess if and where one or more changes in slope occur. For tumor area and tumor count, we subtracted the observation at the prior time point from each time point. This type of “differencing” can create statistically independent change values from correlated time-series data. If a change in slope occurs, the average of these differences across both arms would change. Data were pooled across both arms to ensure that any phases detected were generic to tumor progression and not specific to one treatment condition or the other, which could bias results. For these analyses, tumor count data were transformed as log(x + 1) and area data as log(x + 0.01) to reduce skewness. For presence or absence of any tumors, data were binary (0/1); so instead of taking differences between successive observations, we used Bayesian methods to fit the transition models detailed in (Diggle et al., 1994) which model the probability of either staying within or transitioning from the current state (tumor present or tumor absent) between consecutive time points. Analysis looked for changepoints in these transition probabilities. For all three outcomes, mice that were removed after 19 weeks were not included in any changepoint analysis so as to avoid detecting removal as a changepoint. Each time series was then broken into phases defined by any detected changepoints. These analyses revealed that changes in tumor counts and areas over time occurred in three phases: Weeks 11-16, weeks 17-26, and weeks 27-31. Changes in % of mice with tumors occurred in two phases: Weeks 11-20 and weeks 21-31. Using results from the mixed-model regression analysis described in the preceding paragraph, we averaged its predicted values for each outcome within each of these phases for each mouse (including those mice removed after 19 weeks). We then compared the mean of the per-mouse averages between treatments using one-tailed two-sample t-tests for each of the three outcomes (tumor count, tumor area, and presence of tumor).
Kaplan-Meier estimates of distributions for time to first tumor were obtained, and the difference between arms tested via the log-rank test. Differences between means for gene expression and helper and cytolytic T cell numbers were analyzed using the unpaired Student’s t-test. In order to examine whether the presence or absence of tumors affected gene expression and T cell quantification data, we estimated the partial point-biserial correlation between treatment group and each variable, adjusting for presence of tumor. Data are reported as means + one standard error of the mean (SEM). Attained significance levels of P < 0.05 are reported as “statistically significant.” Analyses were conducted in SAS v.9.1.3 and v.9.2 (SAS Institute, Cary, NC), S-PLUS v 8.0 (Insightful Corporation, Seattle, WA), R v.2.8.1 (The R Foundation for Statistical Computing, Vienna, Austria) and Mathematica v.6.0.1 (Wolfram Research, Champaign, IL).
RESULTS
Effects of Short-term Stress on Tumor Emergence and Development
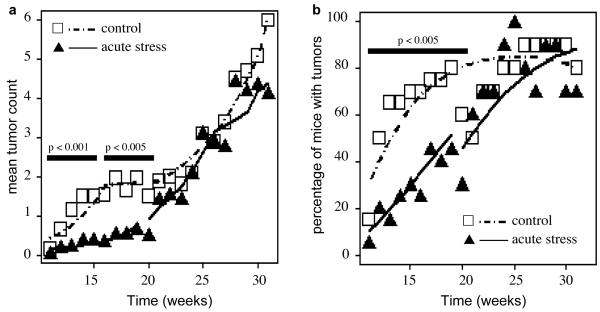
Figure 1 shows the effects of short-term stress on the time course of tumor counts (Fig. 1a) and percentage of mice with tumors (Fig. 1b). Sample means (symbols) as well as mean predictions from the regression model (lines) are shown. Since tumor progression is known to consist of different biological phases, we performed a Bayesian changepoint analysis to identify empirically if tumor progression is phased. This analysis revealed that changes in tumor counts and areas over time occurred in three phases: Weeks 11-16, weeks 17-26, and weeks 27-31 which roughly overlap with the phases of papilloma emergence and regression, transition from papilloma to SCC, and SCC progression, respectively. Changes in % of mice with tumors occurred in two phases: Weeks 11-20 and weeks 21-31. To identify whether there were differences in tumor burden between control and short-term stressed mice, we compared the mean of the per-mouse averages between treatments using one-tailed two-sample t-tests for each of the three outcomes (tumor count, tumor area, and presence of tumor) during each of the empirically determined phases. Compared to controls, acutely stressed mice showed a lower mean tumor count during weeks 11-16 (p < 0.001) and weeks 17-26 (p < 0.005) but not weeks 27-31 (p > 0.23). The percentage of mice with tumors in the short-term stress group was lower than that in controls during weeks 11-20 (p < 0.005) but not after week 21 (p > 0.21).
Fig. 1. Effects of short-term stress on tumor emergence.
Mean tumor counts (a) and percentage of mice with tumors (b) are shown. Tumors were counted weekly after they began to emerge at week 11. The short-term/acute stress group was stressed before each of nine UV exposure sessions during weeks 4-6 of the ten week UV exposure protocol. Since tumor progression is known to consist of different biological phases, a Bayesian change-point analysis was performed to identify empirically if tumor progression is phased. The means of the per-mouse averages between treatments were compared using one-tailed two-sample t-tests for tumor count (a) and percentage of mice with tumors (b) during each of the empirically determined phases. Compared to controls, acutely stressed mice showed a lower mean tumor count during weeks 11-16 (p < 0.001) and weeks 17-26 (p < 0.005) but not weeks 27-31 (p > 0.23). The percentage of mice with tumors in the short-term stress group was lower than that in controls during weeks 11-20 (p < 0.005) but not after week 21 (p > 0.21). Statistically significant differences between phases are indicated by black horizontal bars. Symbols denote observed weekly means by treatment group (control = open squares; short-term stress = filled triangles) and lines represent the fitted model (control = dashed line; short-term stress = solid line). Mixed models fit a separate curve to each mouse. Plotted lines are the averages of these curves by group and week.
Figure 2 shows Kaplan-Meier estimates of the distribution of tumor-free survival by group. Tumors are estimated to have appeared earlier in the control group which reached 50% incidence in 12 weeks versus 17.5 weeks for the short-term stress group. Because of the “crossing” of the tumor-free survival curves, the distribution of time to first tumor did not differ significantly between the two arms (log-rank p > 0.3). However, application of Peto’s test, which is weighted toward detecting short-term risks, gave p < 0.07 for the difference between the two curves suggesting that short-term stress affected early but not late phases of tumor development. Both groups reached approximately 90% incidence at 22 weeks.
Fig. 2. Kaplan-Meier estimates of distribution of proportions of tumor-free mice.
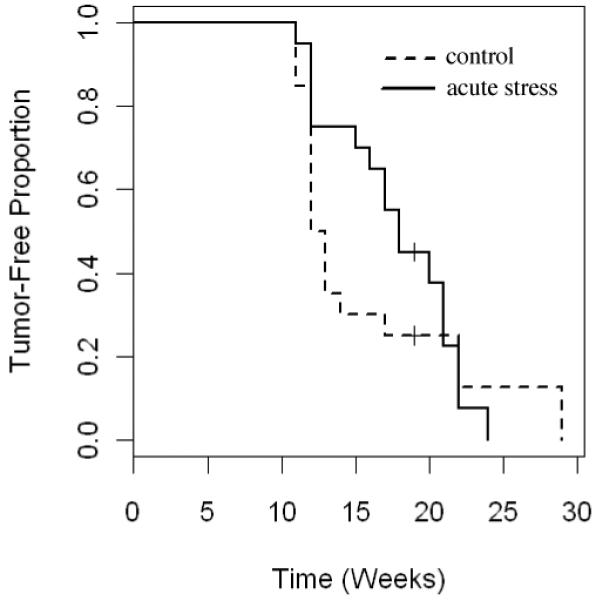
Kaplan-Meier estimates of the distribution of proportions of tumor-free mice (control = dashed line; short-term/acute stress = solid line) are shown. Tumors are estimated to have appeared earlier in the control group which reached 50% incidence in 12 weeks (versus 17.5 weeks for the short-term stress group). Both groups reached approximately 90% incidence at 22 weeks. The distribution of time to first tumor did not differ significantly between the two arms (log-rank p > 0.3). However, application of Peto’s test, which is weighted toward detecting short-term risks, gave p < 0.07 for the difference between the two curves.
The quantitative assessment of tumors described above was complimented with histopathological examination and identification of tissue characteristics. At week 7, among the control group, skin sections from 3 of 3 mice were characterized as having mild dysplastic epidermal hyperplasia (DEH); among the short-term stress group, 1 mouse had mild DEH, 1 had mild multifocal hyperplasia, and 1 had moderate multifocal hyperplasia. Additionally, all lesions from all animals showed leukocyte infiltration. At week 20, among the control group, 1 mouse had mild DEH, and 2 mice showed microinvasive squamous cell carcinoma (MICA); among the short-term stress group, 1 mouse had mild DEH, 1 mouse had a well-differentiated SCC, and 1 lesion could not be positively identified as an SCC but had moderate diffuse hyperplasia. Additionally, all lesions from all animals showed leukocyte infiltration. At week 32, among the control group, 2 mice had papillomas and 1 had MICA; among the short-term stress group, 2 mice had SCC, and 1 had a very small area of MICA.
Effects of Short-term Stress on Chemokine and Cytokine Gene Expression
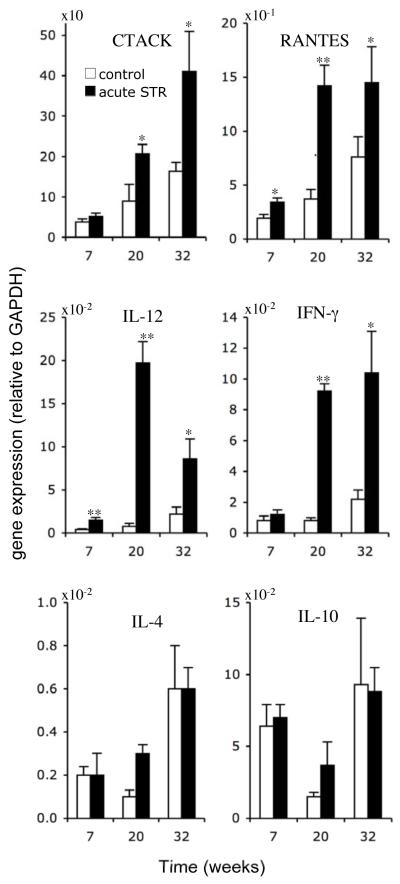
Gene expression of CTACK, RANTES, IL-12, IFN-γ, IL-10 and IL-4 was measured in skin of the control and short-term stress groups, at weeks 7, 20, and 32 (Fig. 3). At week 7, acutely stressed mice showed higher levels of RANTES (P < 0.05) and IL-12 (P < 0.01) gene expression. At week 20, acutely stressed mice showed higher levels of CTACK/CCL27 (P < 0.05), RANTES (P < 0.01), IL-12 (P < 0.0001), and IFN-γ (P < 0.0001) gene expression. The magnitude of the increase in gene expression induced by short-term stress was greater at week 20 than week 7, which indicates greater activation of specific cytokines and chemokines at this midpoint of tumor development and growth. At week 32, acutely stressed mice continued to show higher levels of CTACK (P < 0.05), RANTES (P < 0.05), IL-12 (P < 0.05), and IFN-γ (P < 0.05) gene expression in skin containing pre-malignant lesions compared to similar lesions from controls. Although estimates for average IL-4 and IL-10 levels were higher in acutely stressed mice at week 20, differences in means between groups were not statistically significant at any time point. In order to examine whether the presence or absence of tumors affected gene expression data at week 20, we estimated the partial point-biserial correlation between treatment group and gene expression in skin, adjusting for presence of tumor, and observed a statistically significant effect of short-term stress for each of the parameters examined (P < 0.05) except for IL-10. We did not run a similar analysis at week 7, because none of the animals had tumors before that time, and week 32, because all animals had tumors at some point prior to week 32.
Fig. 3. Effects of short-term stress on chemokine and cytokine gene expression.
Gene expression was measured by quantitative PCR at weeks 7 and 20 in dorsal skin from control and acutely stressed mice. At week 32, gene expression was measured in regions of dorsal skin that included thickened areas of epidermis containing lesions (< 2 mm) that represent a transition from focal epidermal hyperplasia to true papilloma and are part of the spectrum of lesions leading ultimately to SCC. Levels of mRNA expression normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA are shown. Data are expressed as mean + SEM. *P<0.05 and ** P<0.005 for between-group means comparison within a time point by unpaired Student’s t-test.
Effects of Short-term Stress on Circulating and Skin Infiltrating T cells
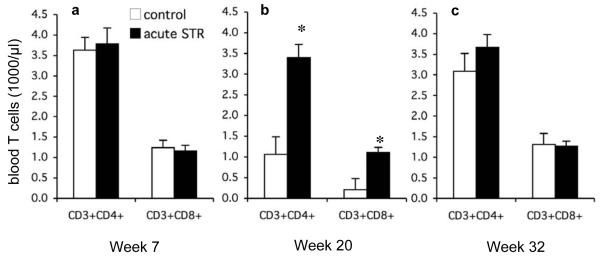
It is likely that the changes in gene expression described above would shift the Type 1/Type 2 cytokine balance towards Type 1 cytokine driven responses and promote cell-mediated immunity. Since T cells are crucial mediators of cell-mediated immunity, the observed stress-induced increase in T cell attracting chemokines and Type 1 cytokines suggested that short-term stress may induce an increase in T cell number/activity. Therefore, we quantified absolute numbers of peripheral blood T cells. At week 20, acutely stressed mice showed significantly higher numbers of circulating helper (CD3+CD4+, P<0.0001) and cytolytic (CD3+CD8+, P<0.0001) T cells compared to controls (Fig. 4b). No differences in circulating T cell numbers were observed between the two groups at weeks 7 or 32 (Fig. 4a and Fig. 4c).
Fig. 4. Helper (CD3+CD4+) and cytolytic (CD3+CD8+) T cells in peripheral blood.
Flow cytometry was used to quantify helper (CD3+CD4+) and cytolytic (CD3+CD8+) T cells in peripheral blood of the control and short-term/acute stress groups at week 7 (a), week 20 (b), and week 32 (c). Data are expressed as mean + SEM. *P<0.05 for between-group means comparison within a time point and T cell subset by unpaired Student’s t-test.
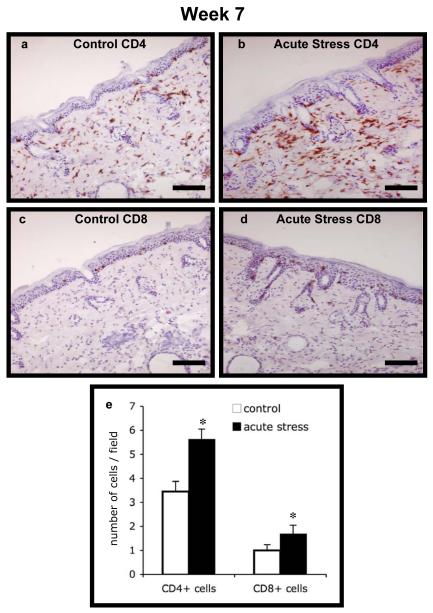
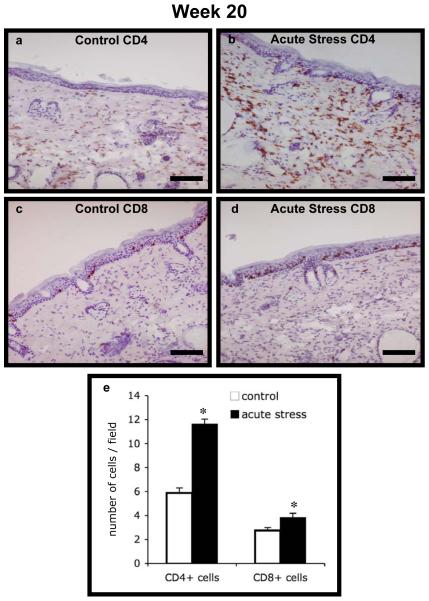
We also quantified CD4+ and CD8+ T cell infiltration into UV-exposed skin at weeks 7, 20 and 32 in order to examine whether there were differences in T cell infiltration in control versus acutely stressed mice. Photomicrographs of representative sections and quantified cell numbers are shown in Fig. 5 (week 7) and Fig. 6 (week 20). At week 7 and week 20, respectively, acutely stressed mice showed significantly higher numbers of CD4+ (P < 0.0001) and CD8+ (P = 0.003 and 0.016) T cell infiltration compared to controls. At week 32, acutely stressed mice showed only slightly higher CD4+ T cell numbers, and no statistically significant differences CD8+ T cell numbers (data not shown). In order to examine whether the presence or absence of tumors affected the observed effects on CD4 or CD8 numbers at week 20, we estimated the partial point-biserial correlation between treatment group and cell numbers, adjusting for presence of tumor. We observed a statistically significant effect of short-term stress for each of the parameters examined (P < 0.05). We did not run a similar analysis at week 7, because none of the animals had tumors before that time, and week 32, because all animals had tumors at some point prior to week 32.
Fig. 5. CD4+ and CD8+ T cell infiltration at week 7.
Immunohistochemical staining for CD4 and CD8 was used to enumerate T cell infiltration into dorsal skin from the control and short-term/acute stress groups at week 7. Representative photomicrographs are shown for: (a) Control CD4+, (b) Acute Stress CD4+, (c) Control CD8+, and (d) Acute Stress CD8+ cells. Scale bar denotes 100 μm. Counts of CD4+ and CD8+ cells in the two treatment groups are shown in (e). Data are expressed as mean + SEM. *P<0.05 for between-group means comparison within T cell subset by unpaired Student’s t-test.
Fig. 6. CD4+ and CD8+ T cell infiltration at week 20.
Immunohistochemical staining for CD4 and CD8 was used to enumerate T cell infiltration into dorsal skin from the control and short-term/acute stress groups at week 20. Representative photomicrographs are shown for: (a) Control CD4+, (b) Acute Stress CD4+, (c) Control CD8+, and (d) Acute Stress CD8+ cells. Scale bar denotes 100 μm. Counts of CD4+ and CD8+ cells in the two treatment groups are shown in (e). Data are expressed as mean + 1 SEM. *P<0.05 for between-group means comparison within a T cell subset by unpaired Student’s t-test.
DISCUSSION
The studies described here confirm the hypothesis that acute or short-term stress experienced at the time of UV exposure may enhance cellular immunity and increase resistance to UV-induced SCC. This hypothesis was based on studies showing that short-term stress experienced at the time of immune activation enhances innate and adaptive immunity (Blecha et al., 1982; Dhabhar, 2009; Dhabhar and McEwen, 1996; Dhabhar and Viswanathan, 2005; Saint-Mezard et al., 2003; Viswanathan et al., 2005; Wood et al., 1993; Zalcman and Anisman, 1993). Given the potential importance of these findings for revealing ways in which endogenous anti-tumor responses may be mobilized, the mechanisms by which the activation of short-term stress physiology during UV exposure reduces subsequent tumor burden need to be further identified. The findings described here also call for further investigation of the biological mediators by which activation of the short-term physiological stress response during specific stages of tumor emergence and/or progression may decrease tumor burden through immune and non-immune mechanisms.
Short-term stress decreased tumor burden during the early phases of tumor development
This study revealed an interesting temporal relationship between activation of short-term stress physiology during UV exposure and subsequent phases of tumor development. Short-term stress was administered immediately prior to each of nine UV sessions only during weeks 4 to 6 of the ten-week UV exposure period. Compared to controls, the short-term stress group showed lower tumor numbers (Fig. 1a) and percentage of mice bearing tumors (Fig. 1b) during the early phases (weeks 11-16 and 17-26 for tumor counts; and weeks 11-20 for tumor incidence) of tumor development and progression. The fact that the effects of short-term stress were observed from one to twenty six weeks after the last stress session suggests that activation of short-term stress physiology at the time of UV exposure had long-lasting effects and delayed the emergence of tumors for weeks after the cessation of stress.
Several potential proximal and distal mechanisms, all of which require further investigation, may mediate these seemingly immuno-protective effects of short-term stress: First, previous studies have shown that short-term stress induces leukocyte trafficking to the skin and sentinel lymph nodes (Dhabhar et al., 1995b; Viswanathan and Dhabhar, 2005), which is accompanied by an increase in innate and adaptive cutaneous immunity (Dhabhar and McEwen, 1996; Dhabhar et al., 2000; Dhabhar and Viswanathan, 2005; Viswanathan et al., 2005). In the present study, it is likely that each short-term stress-plus-UV session increased leukocyte trafficking and function at the site of UV exposure (Figs. 3, 5). This may have induced a more robust immune response against UV-damaged pre-cancerous cells in acutely stressed mice resulting in more efficient elimination which may account for the lower tumor burden observed weeks later. Second, in addition to increased elimination of pre-cancerous cells, close to the time of stress exposure, it is also possible that short-term stress induced a long-lasting enhancement of tumor specific immunity similar to that which has been shown using a model vaccine antigen (Dhabhar and Viswanathan, 2005). Third, exposure to repeated short-term stress during weeks 4 to 6 may have behaviorally sensitized the animals to disturbances in their environment. Such stress sensitization may have resulted in their mounting robust acute stress responses during husbandry-related procedures (e.g. cage checks and changes) which might have given them the benefits of adjuvant-like immuno-enhancing effects of short-term stress even after experimental stress exposure was discontinued at week 6. Clearly, further studies are required to elucidate behavioral and biological mechanisms mediating these immuno-protective effects of short-term stress in the context of skin cancer.
Given the potential importance of these findings for revealing ways in which endogenous anti-tumor responses may be mobilized, it is also important to consider mechanisms that may explain why the protective effects of short-term stress, in terms of decreased tumor burden, did not extend beyond week 26 even though protective effects in terms of a Type-1 cytokine gene expression profile persisted until the end of the study: First, it is possible that UV-damaged cells were eliminated with greater efficiency during weeks 4 to 6 when short-term stress was administered prior to each UV exposure session, but damaged cells that escaped elimination, or cells that were damaged during subsequent UV exposure in the absence of short-term stress (weeks 7-10) may have developed into tumors over a delayed time course. Second, it is also possible that the immunoenhancing effects of short-term stress do extend beyond the period of stressor administration but may be unable to protect animals once tumor number and/or size exceed a certain threshold. Interestingly, even at week 32, mice that had been acutely stressed during weeks 4-6 continued to show significantly higher CTACK, RANTES, IL-12, and IFN-γ gene expression compared to controls. This indicates an immuno-protective gene expression profile in acutely stressed animals even during late stages of tumor development. In spite of this, we found no differences in T cell infiltration or tumor burden at week 32.
Acute/short-term versus chronic/long-term stress
At first glance, these findings may seem counter-intuitive because chronic stress is known to dysregulate immune function and increase susceptibility to infection and cancer (Ben-Eliyahu, 2003; Ben-Eliyahu et al., 2007; Ben-Eliyahu et al., 1991; Cohen et al., 1991; Glaser and Kiecolt-Glaser, 2005; Parker et al., 2004; Saul et al., 2005; Thaker et al., 2006). In fact, using this same model of UVB-induced SCC, we showed that chronic or long-term stress (administered daily for 21 days) significantly increases susceptibility to SCC (Saul et al., 2005). Therefore, an important question that needs to be addressed is: What is the difference between chronic stress and repeated short-term stress? While repeated stressors can be administered in a manner that induces chronic stress (Saul et al., 2005), the stress-exposure paradigm used in this study was designed to induce a short-term, fight-or-flight time course of physiological activation during each stress session and is not akin to chronic stress: 1) Short-term stress was administered only three times per week (Monday, Wednesday, and Friday) with at least two days of recovery between sessions. Chronic stress models generally involve daily exposure without extensive recovery periods (Dhabhar and McEwen, 1997; Dhabhar et al., 1997; Saul et al., 2005). 2) Each of the nine short-term stress session lasted for 2.5 h, while most chronic stress models involve stress sessions of a longer duration and/or a larger number of repeated stress sessions (McEwen, 1998).
Importance of compartment specificity of stress-induced changes in immune cell distribution and function
Although much work remains to be done, this study demonstrates the potential for inducing cancer-related immuno-protection by harnessing the short-term stress response as an endogenous adjuvant. Such protection, in case of immuno-responsive tumors like SCC, is in agreement with studies showing that short-term stress enhances immune cell traffic to, and function within, the skin and sentinel lymph nodes (Dhabhar, 1998; Dhabhar and McEwen, 1996; Dhabhar and Viswanathan, 2005; Viswanathan and Dhabhar, 2005). However, in cases involving surgical removal of tumors, it is important to carefully and specifically consider the effects of stress on immune cell trafficking and function within the specific organs that are affected by the tumor and/or surgery. In contrast to the skin and sentinel lymph nodes, the blood and spleen are temporarily “depleted” of immune cells during short-term stress, which would decrease the potential for mounting immune responses against tumor cells released in the circulation during surgery. For example, it has been shown that surgery stress simulated by laparotomy, when followed by intravenous injection of syngeneic MADB106 tumor cells, results in increased tumor retention in the lungs (Benish et al., 2008). In such cases, measures to counter immuno-suppression of specific cell-types and/or specific compartments may be beneficial as shown by IL-12 pre-treatment which significantly reduces the laparotomy-induced increase in metastases of intravenously injected tumor cells (Schwartz et al., 2008). Therefore, understanding the effects of stress on immune cell numbers and function in a compartment-specific manner and in an in vivo context is critical for maximizing the potential positive effects and minimizing the negative effects of short- and long-term stress.
Effects of short-term stress on chemokine & cytokine gene expression
Compared to controls, acutely stressed mice showed higher levels of CTACK/CCL27, RANTES, IL-12, and IFN-γ, but not IL-4 or IL-10 gene expression in UV-exposed skin during early and late phases of tumor development (Fig. 3). CTACK is crucial for recruitment of skin-homing T cells (Homey et al., 2002; Morales et al., 1999; Reiss et al., 2001). RANTES is involved in recruiting T cells and monocytes to sites of immune activation (Schall, 1991). Therefore, the enhancement of CTACK and RANTES gene expression by short-term stress may have induced recruitment of higher numbers of T cells to the site of UV exposure (as seen in Figs. 5 & 6), and enhanced early effector function against damaged cells that were likely to develop into tumors. Moreover, short-term stress also increased gene expression of IL-12 and IFN-γ, cytokines that are critical for the initiation and maintenance of cell mediated immune responses (Boehm et al., 1997; Perussia et al., 1983; Trinchieri, 2003; Zhang et al., 1997). Increased expression of IL-12 and IFN-γ in acutely stressed mice (Fig. 3) may have further enhanced T cell infiltration (Figs. 5 & 6) and function. Importantly, both IL-12 and IFN-γ have been shown to exert anti-tumor effects. IL-12 administration can eliminate UV-induced immunosuppression (Schwarz et al., 1996) and IL-12 therapy retards the growth of murine mammary tumors by increasing the number of infiltrating leukocytes (Shi et al., 2004). IFN-γ has been shown to promote tumor recognition and elimination (Dighe et al., 1994), is a critical mediator of the anti-tumor effects of IL-12 (Voest et al., 1995), and has been shown to suppress tumor emergence (Shankaran et al., 2001). Interestingly, acutely stressed mice continued to show higher levels of CTACK, RANTES, IL-12 and IFN-γ gene expression at week 32. However, infiltrating T cell numbers and tumor burdens were not statistically different between groups at this time point. Importantly, control and acutely stress mice showed similar levels of IL-4 and IL-10 gene expression at virtually all time points examined. Taking into account the overall patterns and magnitude of chemokine and cytokine gene expression at the three time points, these results indicate that exposure to short-term stress shifted the cytokine balance towards conditions that favor the development and maintenance of cell-mediated immunity, which is known to confer protection against skin cancer (Clydesdale et al., 2001; Kripke, 1994; Nghiem et al., 2002).
Effects of short-term stress on T cell infiltration
To assess enhanced T cell infiltration as a potential mechanism mediating enhanced anti-tumor immunity, we quantified T cell numbers in skin and peripheral blood. At weeks 7 and 20, acutely stressed mice showed significantly greater CD4+ and CD8+ T cell infiltration into the skin compared to controls (Figs. 5 & 6). This indicates that short-term stress may have enhanced T cell effector action against pre-cancerous cells. Whereas CD8+ T cells could directly lyse pre-cancerous cells, CD4+ T cells may recruit and activate CD8+ T cells and other leukocytes to the site of immune activation. In addition, CD4+ T cells, observed here in significantly higher numbers in acutely stressed mice, have also been shown to control tumor growth by mechanisms independent of CD8+ T cells (Greenberg, 1991), and to mediate regression of non-melanoma skin cancers (Halliday et al., 1995). Interestingly, IFN-γ gene expression, which was also higher in acutely stressed mice, has been found to be involved in the CD4+ T cell mediated tumor elimination (Toes et al., 1991).
Limitations
These results must be viewed in the context of several potential limitations. First, the effects of stress were examined only during one period of UV-induced damage, (weeks 4-6) which represents the middle period of ten weeks of UV exposure. Examination of the effects of stress during other critical periods of tumor emergence and progression are warranted. Second, it is possible that short-term stressors administered throughout the ten weeks of UV exposure, would be more effective at reducing tumor burden. Therefore, future studies will attempt to examine the effects of short-term stress sessions administered during the 10-week UV exposure period. Third, further studies examining the sequence, quantities, concentrations, and activity of the cellular and molecular factors that mediate the effects of short-term stress are warranted. Clearly, a significant amount of work remains to be done to elucidate the endocrine and immune mediators of these adjuvant-like, immuno-enhancing effects of short-term stress.
Conclusion
The acute or short-term stress response is one of nature’s fundamental, adaptive, psycho-physiological survival mechanisms, i.e., without a fight-or-flight stress response neither predator nor prey could survive. Studies have shown that just as the short-term stress response prepares the cardiovascular and musculoskeletal systems for fight or flight, it also prepares the immune system for challenges that may arise from the stress-inducing agent (e.g. wound/infection inflicted by an aggressor) (Dhabhar and McEwen, 1997, 1999; Dhabhar et al., 1995b, 1996; Dhabhar et al., 2000; Dhabhar and Viswanathan, 2005; Viswanathan et al., 2005; Viswanathan and Dhabhar, 2005). While, the adjuvant-like effects of the physiological response activated during short-term stress may enhance wound healing and resistance to infection, such immunoenhancement may also increase protection against immuno-responsive cancers, during tumor immunotherapy, and/or during cancer surgery. By elucidating the biological mechanisms of endogenous immunoenhancement, we hope to facilitate the development of biobehavioral and/or pharmacological interventions designed to endogenously enhance protective immunity. To our knowledge these results show for the first time that activation of acute or short-term stress physiology may mobilize mechanisms that mediate anti-tumor immune responses. These findings merit further investigation because mobilizing protective immune responses is crucial during cancer therapy, both, for eliminating immuno-responsive tumors, and for general protection against infection and wounding, and these studies lay the groundwork for understanding how immunoprotective mechanisms may be enhanced by manipulating physiological mediators related to the fight-or-flight stress response.
ACKNOWLEDGMENTS
We thank Susie Jones for conducting immunohistochemistry, and Jean Tillie, Kanika Ghai, and Cynthia Walter, of the Dhabhar Laboratory for valuable assistance with these studies. Grant support: NIH RO1 CA107498 (FSD).
Abbreviations
- UVB
Ultraviolet-B
- SCC
Squamous cell carcinoma
- CTACK
Cutaneous T-cell Attracting Chemokine
- RANTES
Regulated upon Activation Normal T cell Expressed and Secreted
- IFN-γ
Interferon-gamma
- IL-12
Interleukin-12
- IL-4
Interleukin-4
- IL-10
Interleukin-10
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors state no conflict of interest
REFERENCES
- Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, Sood AK. The influence of bio-behavioral factors on tumour biology: pathways and mechanisms. Nature Reviews Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J. Photochem. Photobiol. B. 2001;63:8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- Ben-Eliyahu S. The promotion of tumor metastasis by surgery and stress: immunological basis and implications for psychoneuroimmunology. Brain, behavior, and immunity. 2003;17(Suppl 1):S27–36. doi: 10.1016/s0889-1591(02)00063-6. [DOI] [PubMed] [Google Scholar]
- Ben-Eliyahu S, Page GG, Schleifer SJ. Stress, NK cells, and cancer: Still a promissory note. Brain, behavior, and immunity. 2007;21:881–887. doi: 10.1016/j.bbi.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Ben-Eliyahu S, Yirmiya R, Liebeskind JC, Taylor AN, Gale RP. Stress increases metastatic spread of a mammary tumor in rats: evidence for mediation by the immune system. Brain, Behav., Immun. 1991;5:193–205. doi: 10.1016/0889-1591(91)90016-4. [DOI] [PubMed] [Google Scholar]
- Benish M, Bartal I, Goldfarb Y, Levi B, Avraham R, Raz A, Ben-Eliyahu S. Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Annals of surgical oncology. 2008;15:2042–2052. doi: 10.1245/s10434-008-9890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk KN, Lachenbruch PA. Repeated measures with zeros. Statistical methods in medical research. 2002;11:303–316. doi: 10.1191/0962280202sm293ra. [DOI] [PubMed] [Google Scholar]
- Berton TR, Pavone A, Fischer SM. Ultraviolet-B irradiation alters the cell cycle machinery in murine epidermis in vivo. J Invest Dermatol. 2001;117:1171–1178. doi: 10.1046/j.0022-202x.2001.01536.x. [DOI] [PubMed] [Google Scholar]
- Blecha F, Barry RA, Kelley KW. Stress-induced alterations in delayed-type hypersensitivity to SRBC and contact sensitivity to DNFB in mice. Proc. Soc. Exp. Biol. Med. 1982;169:239–246. doi: 10.3181/00379727-169-41338. [DOI] [PubMed] [Google Scholar]
- Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis. 2005;26:1657–1667. doi: 10.1093/carcin/bgi123. [DOI] [PubMed] [Google Scholar]
- Canfield PJ, Xu FN, Greenoak GE, Reeve VE, Gallagher CH, Wilkinson F. Ultrastructure of ultraviolet radiation-induced hairless mouse skin carcinogenesis, with special reference to the epidermal-dermal junction. Pathology. 1986;18:337–344. doi: 10.3109/00313028609059487. [DOI] [PubMed] [Google Scholar]
- Carlin BP, Gelfand AE, Smith AFM. Hierarchical Bayesian analysis of changepoint problems. Applied Statistics. 1992;41:389–405. [Google Scholar]
- Clydesdale GJ, Dandie GW, Muller HK. Ultraviolet light induced injury: immunological and inflammatory effects. Immunol Cell Biol. 2001;79:547–568. doi: 10.1046/j.1440-1711.2001.01047.x. [DOI] [PubMed] [Google Scholar]
- Cohen S, Tyrrell DAJ, Smith AP. Psychological stress and susceptibility to the common cold. New Engl J. Med. 1991;325:606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Stress-induced enhancement of cell-mediated immunity**. Annals of the New York Academy of Sciences. 1998;840:359–372. doi: 10.1111/j.1749-6632.1998.tb09575.x. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–317. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Stress-induced enhancement of antigen-specific cell-mediated immunity. The Journal of Immunology. 1996;156:2608. [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses immune function in vivo: A potential role for leukocyte trafficking. Brain, behavior, and immunity. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. PNAS, USA. 1999;96:1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Bidirectional Effects Of Stress & Glucocorticoid Hormones On Immune Function: Possible Explanations For Paradoxical Observations. In: Ader R, Felten DL, Cohen N, editors. Psychoneuroimmunology - Third Edition. Academic Press; San Diego: 2001. pp. 301–338. [Google Scholar]
- Dhabhar FS, McEwen BS, Spencer RL. Adaptation to prolonged or repeated stress -- Comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology. 1997;65:360–368. doi: 10.1159/000127196. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, McEwen BS, Spencer RI. Differential activation of adrenal steroid receptors in neural and immune tissues of Sprague Dawley, Fischer 344, and Lewis rats. J Neuroimmunol. 1995a;56:77–90. doi: 10.1016/0165-5728(94)00135-b. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Effects of stress on immune cell distribution -- dynamics and hormonal mechanisms. J. Immunology. 1995b;154:5511–5527. [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Stress-induced changes in blood leukocyte distribution--role of adrenal steroid hormones. J. Immunology. 1996;154:5511–5527. [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, Stein M, McEwen BS, Spencer RL. Diurnal and stress-induced changes in distribution of peripheral blood leukocyte subpopulations. Brain Behav. Immun. 1994;8:66–79. doi: 10.1006/brbi.1994.1006. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Satoskar AR, Bluethmann H, David JR, McEwen BS. Stress-Induced Enhancement of Skin Immune Function: A Role For IFNγ. PNAS, USA. 2000;97:2846–2851. doi: 10.1073/pnas.050569397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, Viswanathan K. Short-term stress experienced at time of immunization induces a long-lasting increase in immunologic memory. Am J Physiol Regul Integr Comp Physiol. 2005;289:R738–R744. doi: 10.1152/ajpregu.00145.2005. [DOI] [PubMed] [Google Scholar]
- Diggle PJ, Liang K, Zeger SL. Analysis of longitudinal data. Clarendon Press; Oxford: 1994. [Google Scholar]
- Dighe AS, Richards E, Old LJ, Schreiber RD. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1:447–456. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Finney DJ. On the distribution of a variate whose logarithm is normally distributed. Journal of the Royal Statistical Society (Supplement) 1941;7:155–161. [Google Scholar]
- Fisher MS, Kripke ML. Systemic alteration induced in mice by ultraviolet light irradiation and its relationship to ultraviolet carcinogenesis. Proc.Natl.Acad.Sci.USA. 1977;74:1688–1692. doi: 10.1073/pnas.74.4.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nature Reviews Immunology. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Glavin GB, Pare WP, Sandbak T, Bakke HK, Murison R. Restraint stress in biomedical research: an update. Neurosci Biobehav Rev. 1996;18:223–249. doi: 10.1016/0149-7634(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Greenberg PD. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv. Immunol. 1991;49:281–355. doi: 10.1016/s0065-2776(08)60778-6. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Patel A, Hunt MJ, Tefany FJ, Barnetson RS. Spontaneous regression of human melanoma/nonmelanoma skin cancer: association with infiltrating CD4+ T cells. World J Surg. 1995;19:352–358. doi: 10.1007/BF00299157. [DOI] [PubMed] [Google Scholar]
- Homey B, Alenius H, Muller A, Soto H, Bowman EP, Yuan W, McEvoy L, Lauerma AI, Assmann T, Bunemann E, Lehto M, Wolff H, Yen D, Marxhausen H, To W, Sedgwick J, Ruzicka T, Lehmann P, Zlotnick A. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat.Med. 2002;8:157–165. doi: 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Cushing GL, Greenberg ER, Mott LA, Spencer SK, Nierenberg DW. Non-melanoma skin cancers and gluocorticoid therapy. Br J Cancer. 2001;85:683–686. doi: 10.1054/bjoc.2001.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kligman LH, Kligman AM. Histogenesis and progression in ultraviolet light-induced tumors in hairless mice. J Natl Cancer Inst. 1981;67:1289–1293. [PubMed] [Google Scholar]
- Kripke ML. Ultraviolet radiation and immunology: something new under the sun--presidential address. Cancer research. 1994;54:6102–6105. [PubMed] [Google Scholar]
- Kvetnansky R, Fukuhara K, Pacak K, Cizza G, Goldstein DS, Kopin IJ. Endogeneous glucocorticoids restrain catecholamine synthesis and release at rest and during immobilization stress in rats. Endocrinology. 1993;133:1411–1419. doi: 10.1210/endo.133.3.8396019. [DOI] [PubMed] [Google Scholar]
- McCulloch CE, Searle SR. Generalized, linear, and mixed models. John Wiley & Sons, Inc.; New York: 2001. [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators: allostasis and allostatic load. NEJM. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Morales J, Homey B, Vicari AP, Hudak S, Oldham E, Hedrick J, Orozco R. CTACK, a skin associated chemokine that preferentially attracts skin-homing memory T-cells. Proc Natl Acad Sci USA. 1999;25:14470–14475. doi: 10.1073/pnas.96.25.14470. al., e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morison WL, Parrish JA, Bloch KJ, Krugler JI. In-vivo effect of UV-B on lymphocyte function. Br J Dermatol. 1979;101:513–519. doi: 10.1111/j.1365-2133.1979.tb11880.x. [DOI] [PubMed] [Google Scholar]
- Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied linear statistical models. WCB McGraw-Hill; Boston: 1996. [Google Scholar]
- Nghiem DX, Kazimi N, Mitchell DL, Vink AA, Ananthaswamy HN, Kripke ML, Ullrich SE. Mechanisms underlying the suppression of established immune responses by ultraviolet radiation. J Invest Dermatol. 2002;119:600–608. doi: 10.1046/j.1523-1747.2002.01845.x. [DOI] [PubMed] [Google Scholar]
- NIH 6 Common Cancers - Skin Caner. NIH Medline Plus. 2007;2:12. [Google Scholar]
- Nishigori C, Yarosh DB, Ullrich SE, Vink AA, Bucana CD, Roza L, Kripke ML. Evidence that DNA damage triggers interleukin 10 cytokine production in UV-irradiated murine keratinocytes. Proc Natl Acad Sci USA. 1996;93:10354–10359. doi: 10.1073/pnas.93.19.10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberyszyn TM. Non-melanoma skin cancer: importance of gender, immunosuppressive status and vitamin D. Cancer letters. 2008;261:127–136. doi: 10.1016/j.canlet.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Ortonne JP. From actinic keratosis to squamous cell carcinoma. Br J Dermatol. 2002;146(Suppl 61):20–23. doi: 10.1046/j.1365-2133.146.s61.6.x. [DOI] [PubMed] [Google Scholar]
- Parker J, Klein SL, McClintock MK, Morison WJ, Ye X, Conti CJ, Peterson N, Nousari CH, Tausk FA. Chronic stress accelerates ultraviolet-induced cutaneous carcinogenesis. J Am Acad Dermatol. 2004;51:919–922. doi: 10.1016/j.jaad.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Perussia B, Dayton ET, Fanning V, Thiagarajan P, Hoxie J, Trinchieri G. Immune interferon and leukocyte-conditioned medium induce normal and leukemic myeloid cells to differentiate along the monocytic pathway. J Exp Med. 1983;158:2058–2080. doi: 10.1084/jem.158.6.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss Y, Proudfoot AE, Power CA, Campbell JJ, Butcher EC. CC Chemokine Receptor (CCR)4 and the CCR10 Ligand Cutaneous T Cell-Attracting Chemokine (CTACK) in Lymphocyte Trafficking to Inflamed Skin. J. Exp.Med. 2001;194:1541–1547. doi: 10.1084/jem.194.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Mezard P, Chavagnac C, Bosset S, Ionescu M, Peyron E, Kaiserlian D, Nicolas J, Berard F. Psychological stress exerts an adjuvant effect on skin dendritic cell functions in vivo. J Immunol. 2003;171:4073–4080. doi: 10.4049/jimmunol.171.8.4073. [DOI] [PubMed] [Google Scholar]
- Saul AN, Oberyszyn TM, Daugherty C, Kusewitt D, Jones S, Jewell S, Malarkey WB, Lehman A, Lemeshow S, Dhabhar FS. Chronic stress and susceptibility to skin cancer. Journal of the National Cancer Institute. 2005;97:1760–1767. doi: 10.1093/jnci/dji401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall TJ. Biology of the RANTES/SIS cytokine family. Cytokine. 1991;3:165–183. doi: 10.1016/1043-4666(91)90013-4. [DOI] [PubMed] [Google Scholar]
- Schwartz Y, Avraham R, Benish M, Rosenne E, Ben-Eliyahu S. Prophylactic IL-12 treatment reduces postoperative metastasis: mediation by increased numbers but not cytotoxicity of NK cells. Breast cancer research and treatment. 2008;107:211–223. doi: 10.1007/s10549-007-9540-9. [DOI] [PubMed] [Google Scholar]
- Schwarz A, Grabbe S, Aragane Y, Sandkuhl K, Riemann H, Luger TA, Kubin M, Trinchieri G, Schwarz T. Interleukin-12 Prevents Ultraviolet B-Induced Local Immunosuppression and Overcomes UVB-Induced Tolerance. J Investig Dermatol. 1996;106:1187–1191. doi: 10.1111/1523-1747.ep12347944. [DOI] [PubMed] [Google Scholar]
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- Shi X, Liu J, Xiang Z, Mitsuhashi M, Wu RS, Ma X. Gene expression analysis in interleukin-12-induced suppression of mouse mammary carcinoma. Int. J. Cancer. 2004;110:570–578. doi: 10.1002/ijc.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieffert K, Granstein RD. Neuropeptides and neuroendocrine hormones in ultraviolet radiation-induced immunosuppression. Methods. 2002;28:97–103. doi: 10.1016/s1046-2023(02)00214-1. [DOI] [PubMed] [Google Scholar]
- Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, Merritt WM, Lin YG, Mangala LS, Kim TJ, Coleman RL, Landen CN, Li Y, Felix E, Sanguino AM, Newman RA, Lloyd M, Gershenson DM, Kundra V, Lopez-Berestein G, Lutgendorf SK, Cole SW, Sood AK. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- Thomas-Ahner JM, Wulff BC, Tober KL, Kusewitt DF, Riggenbach JA, Oberyszyn TM. Gender differences in UVB-induced skin carcinogenesis, inflammation, and DNA damage. Cancer research. 2007;67:3468–3474. doi: 10.1158/0008-5472.CAN-06-3798. [DOI] [PubMed] [Google Scholar]
- Toes REM, Ossendorp F, Offringa R, Melief CJM. CD4 T cells and their role in antitumor immune responses. J. Exp. Med. 1991;189:753–756. doi: 10.1084/jem.189.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toichi E, Lu KQ, Swick AR, McCormick TS, Cooper KD. Skin-Infiltrating Monocytes//Macrophages Migrate to Draining Lymph Nodes and Produce IL-10 After Contact Sensitizer Exposure to UV-Irradiated Skin. J Invest Dermatol. 2008 doi: 10.1038/jid.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- Viswanathan K, Daugherty C, Dhabhar FS. Stress as an endogenous adjuvant: augmentation of the immunization phase of cell-mediated immunity. Int Immunol. 2005;17:1059–1069. doi: 10.1093/intimm/dxh286. [DOI] [PubMed] [Google Scholar]
- Viswanathan K, Dhabhar FS. Stress-induced enhancement of leukocyte trafficking into sites of surgery or immune activation. PNAS, USA. 2005;102:5808–5813. doi: 10.1073/pnas.0501650102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voest EE, Kenyon BM, O’Reilly MS, Truitt G, D’Amato RJ, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst. 1995;87:581–586. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]
- WHO World Health Organization: Ultraviolet radiation. 2005 www.who.int/uv.
- Wood PG, Karol MH, Kusnecov AW, Rabin BS. Enhancement of antigen-specific humoral and cell-mediated immunity by electric footshock stress in rats. Brain, Behav., Immun. 1993;7:121–134. doi: 10.1006/brbi.1993.1014. [DOI] [PubMed] [Google Scholar]
- Zalcman S, Anisman H. Acute and chronic stressor effects on the antibody response to sheep red blood cells. Pharmacology, Biochemistry, and Behavior. 1993;46:445–452. doi: 10.1016/0091-3057(93)90377-6. [DOI] [PubMed] [Google Scholar]
- Zhang X, Brunner T, Carter L, Dutton RW, Rogers P, Bradley L, Sato T, Reed JC, Green D, Swain SL. Unequal death in T helper cell (Th1) and Th2 effectors: Th1, but not Th2, effectors undergo rapid fas/fasL-mediated apoptosis. J Exp Med. 1997;185:1837–1849. doi: 10.1084/jem.185.10.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]