Abstract
The Bordetella type III secretion system (T3SS) effector protein BteA is necessary and sufficient for rapid cytotoxicity in a wide range of mammalian cells. We show that BteA is highly conserved and functionally interchangeable between Bordetella bronchiseptica, Bordetella pertussis and Bordetella parapertussis. The identification of BteA sequences required for cytotoxicity allowed the construction of non-cytotoxic mutants for localization studies. BteA derivatives were targeted to lipid rafts and showed clear colocalization with cortical actin, ezrin and the lipid raft marker GM1. We hypothesized that BteA associates with the cytoplasmic face of lipid rafts to locally modulate host cell responses to Bordetella attachment. B. bronchiseptica adhered to host cells almost exclusively to GM1-enriched lipid raft microdomains and BteA colocalized to these same sites following T3SS-mediated translocation. Disruption of lipid rafts with methyl-β-cyclodextrin protected cells from T3SS-induced cytotoxicity. Localization to lipid rafts was mediated by a 130-amino-acid lipid raft targeting domain at the N-terminus of BteA, and homologous domains were identified in virulence factors from other bacterial species. Lipid raft targeting sequences from a T3SS effector (Plu4750) and an RTX-type toxin (Plu3217) from Photorhabdus luminescens directed fusion proteins to lipid rafts in a manner identical to the N-terminus of BteA.
Introduction
Lipid membrane rafts (rafts), which are prominent targets for bacterial, viral and protozoan pathogens (Manes et al., 2003; Lafont et al., 2004; Riff et al., 2005; Mejia et al., 2008), are specialized membrane microdomains enriched in cholesterol and sphingolipids that produce ordered structures distinct from surrounding areas of disordered, predominantly unsaturated lipid species (Simons and Ikonen, 1997, Laude and Prior, 2004). The clustering of signalling proteins and lipid cofactors within rafts suggests they function as platforms where close proximity increases efficiency and specificity of signalling cascades (Hanzal-Bayer and Hancock, 2007, Viola and Gupta, 2007). Although controversy exists regarding the size, function and precise physical nature of rafts (Edidin, 2003; Munro, 2003; Laude and Prior, 2004; Skwarek, 2004), considerable evidence supports their importance for attachment of bacterial pathogens (e.g. Mycoplasma, Shigella, enteropathogenic Escherichia coli, Porphyromonas, Yersinia) (Lafont et al., 2004; Riff et al., 2005; Allen-Vercoe et al., 2006; Hawkes and Mak, 2006, Mejia et al., 2008; Hayward et al., 2009), bacterial invasion (Shigella, Salmonella, Listeria, Mycobacterium, Chlamydia) (Manes et al., 2003; Lafont et al., 2004; Lafont and van der Goot, 2005a), oligomerization of pore-forming toxins (aerolysin, streptolysin O, listeriolysin O) (Gekara et al., 2005) and cell-surface binding by A-B toxins (cholera toxin, shiga toxin) (Takenouchi et al., 2004; Lencer and Saslowsky, 2005). Recent evidence suggests that association of Salmonella and Shigella type III secretion system (T3SS) translocon components with target cell membranes is cholesterol-dependent (Lafont and van der Goot, 2005b). Although interactions between bacterial components and the exofacial surface of lipid rafts have been extensively studied, virulence factor targeting events occurring on the cytoplasmic side of rafts are less well understood.
In this study we report an analysis of the activity and intracellular localization of BteA, a 658 amino acid (aa) effector protein secreted by the Bordetella bsc T3SS (Panina et al., 2005; Kuwae et al., 2006). The bsc T3SS is most extensively characterized in Bordetella bronchiseptica, the broad-host-range evolutionary progenitor of Bordetella pertussis and Bordetella parapertussis that cause acute respiratory diseases in humans (Yuk et al., 1998; 2000). Although nearly identical T3SS loci are encoded in the genomes of all three species, the ability to readily detect T3SS activity in vitro, and the availability of natural-host animal models to study respiratory infection have made B. bronchiseptica the species of choice for studying type III secretion by Bordetella. The loci encoding bteA and the bsc T3SS apparatus are co-regulated by the alternative sigma factor BtrS, which in turn is dependent on the BvgAS phosphorelay for expression (Mattoo et al., 2004). In addition to transcriptional control, the partner-switching proteins BtrU, BtrV and BtrW regulate secretion through a complex series of protein–protein interactions, serine-phosphorylation and serine-dephosphorylation events (Mattoo et al., 2004; Kozak et al., 2005). BteA is an unusually potent cytotoxin capable of inducing rapid, non-apoptotic death in a diverse array of cell types, and precise, multilevel control of expression and secretion may be essential for its role in promoting persistent colonization of the respiratory epithelium (Stockbauer et al., 2003; Panina et al., 2005; Shrivastava and Miller, 2009). BteA is the only Bordetella effector that has been identified and its central importance is illustrated by the observation that null mutations in bteA recapitulate phenotypes associated with mutations that eliminate type III secretion altogether (Panina et al., 2005). Despite its central role in pathogenesis, little is known regarding the events that occur following BteA injection into target cells. In the course of analysing the intracellular localization of BteA, we have identified a lipid raft targeting (LRT) motif found in virulence determinants expressed by diverse bacterial pathogens.
Results
BteA is functionally conserved among Bordetella species
The bsc T3SS loci encoded by B. pertussis, B. parapertussis and B. bronchiseptica are highly conserved (Yuk et al., 1998). bteA and btcA, which encode the BteA effector and its chaperone, respectively, are present in the genomes of all three species and are predicted to encode nearly identical proteins (Fig. 1A). As shown in Fig. 1B, bteA alleles from B. pertussis and B. parapertussis were able to complement a ΔbteA derivative of B. bronchiseptica strain RB50. Cytotoxicity against rat lung epithelial (L2), human epithelial (HeLa) and mouse lung epithelial (MLE12) cells was restored to levels comparable to those observed with the RB50 bteA allele, and the morphological characteristics of dying cells were identical (data not shown). The slightly lower levels of complementation observed with B. parapertussis BteA correspond to its greater degree of divergence. These results demonstrate that the BteA effector proteins encoded by Bordetella species are functionally conserved.
Fig. 1.
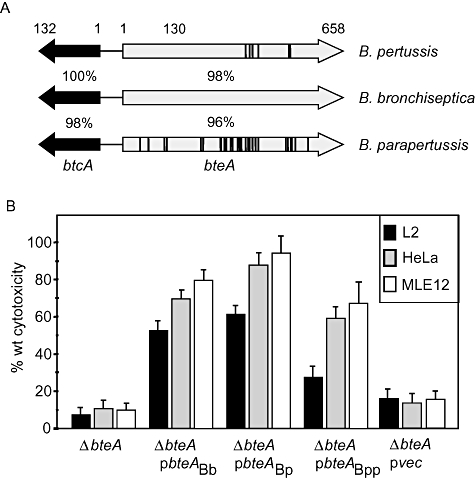
B. pertussis, B. parapertussis and B. bronchiseptica bteA alleles are functionally interchangeable. A. Schematic representation of bteA loci from three Bordetella species. The per cent amino acid identities between B. bronchiseptica proteins and their homologues in human-adapted B. pertussis and B. parapertussis are indicated. Positions of residues that differ between B. bronchiseptica and other species are shown by vertical bars. B. B. pertussis and B. parapertussis bteA alleles are functionally interchangeable with B. bronchiseptica bteA. HeLa, L2 and MLE-12 epithelial cell lines were infected with wild-type B. bronchiseptica RB50 (WT), a ΔbteA derivative (ΔbteA), the ΔbteA strain complemented with vector alone (pvec), or the ΔbteA strain complemented with bteA loci from B. bronchiseptica RB50 (pbteABb), B. pertussis Tohama 1 (pbteABp), or B. parapertussis 12822 (pbteABpp). Cytotoxicity was measured by LDH release assays performed 1 h post infection at a multiplicity of infection of 50:1.
Identification of non-cytotoxic BteA derivatives
A characteristic of the wild-type BteA protein is the induction of rapid, non-apoptotic cytotoxicity in a wide range of target cells (Stockbauer et al., 2003; Panina et al., 2005). In an attempt to identify a time interval where BteA might be detected and localized prior to the onset of cell death, we performed the time-course experiment shown in Fig. 2A.bteA was inserted downstream from the CMV promoter in plasmid pEGFP-N1, fusing EGFP to the C-terminus of BteA. Expression of the resulting fusion construct, BteA658-EGFP, was analysed by fluorescence microscopy at time intervals beginning 2 h after transfection. With the vector control, GFP-positive cells could be identified as early as 4 h., while no GFP-positive cells were found at any time point following transfection with the construct encoding BteA658-EGFP (Fig. 2B), and the fusion protein was not detectable by Western blot analysis (data not shown). Lactate dehydrogenase (LDH) release assays showed that expression of BteA with the C-terminal EGFP tag resulted in a level of cytotoxicity comparable to the native untagged protein (Fig. 2B). These data show that even trace amounts of BteA658-EGFP expression are sufficient to induce cytotoxicity and prevent detection of the fusion protein by fluorescence microscopy or Western blot analysis.
Fig. 2.
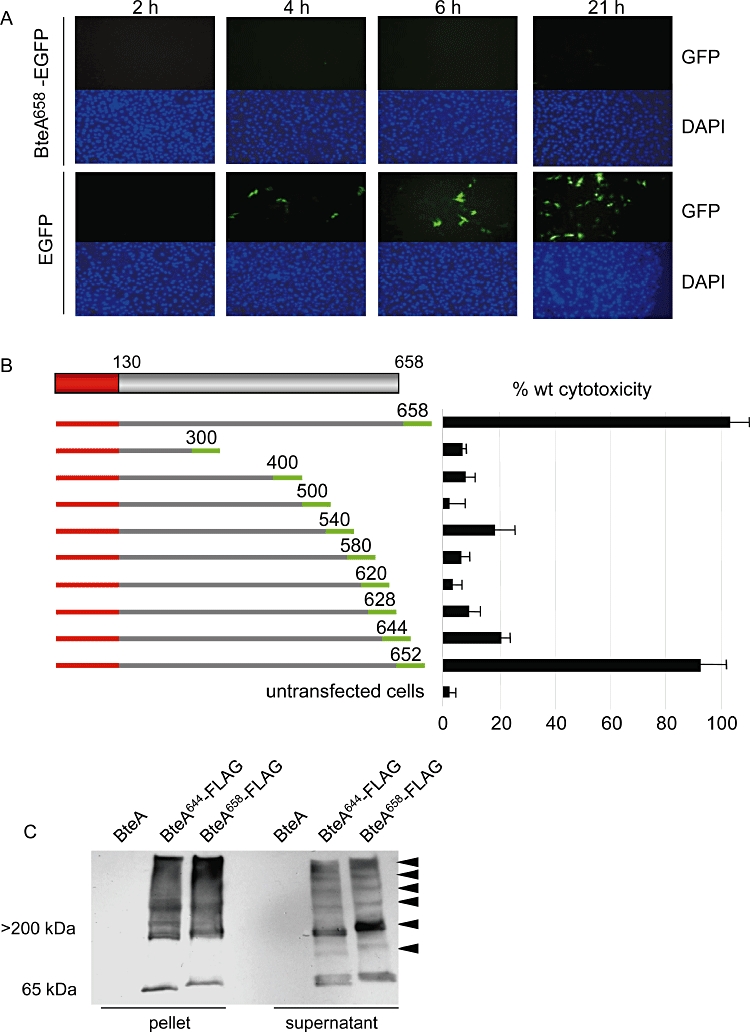
BteA is a potent cytotoxin for mammalian cells. A. Full-length BteA is not detected in eukaryotic cells by fluorescence microscopy. HeLa cells were transfected with BteA658-EGFP or EGFP alone for 2, 4, 6 or 21 h and visualized via fluorescence microscopy. Typical images of the same field showing green fluorescence and DAPI nuclear staining are shown. B. Full-length BteA (aa 1–658) and C-terminally truncated mutants were fused to EGFP (green bars) on the plasmid pEGFP-N1. The EGFP tag is not drawn to scale. The N-terminal BteA domain (aa 1–130) is shown in red and residues at EGFP fusion junctions are indicated. Constructs were transfected into HeLa cells and cytotoxicity was measured 21 h later by LDH release assays. Cytotoxicity measurements are expressed relative to untagged, wild-type BteA. C. Western blot analysis of complex formation by full-length BteA658-FLAG (658 aa) and the non-cytoxic derivative BteA644-FLAG (644 aa) using α-FLAG monoclonal antibody. Both proteins were expressed in a ΔbteA derivative of B. bronchiseptica strain RB50. RB50 expressing untagged full-length BteA was used as a negative control. High-molecular-weight complexes (arrowheads) were observed for both BteA658-FLAG and BteA644-FLAG in whole-cell extracts and culture supernatants. Images shown are representative of three independent experiments.
We reasoned that BteA derivatives with reduced cytotoxicity would be required for localization studies to proceed. A series of bteA fragments truncated at their 3′ termini were cloned into pEGFP-N1 to create C-terminal fusions with EGFP (Fig. 2B). Following transfection into HeLa cells, expression of BteA–EGFP derivatives was monitored by fluorescence microscopy and cytotoxicity was measured by LDH release assays (Fig. 2B). Although BteA652-EGFP retained full cytotoxicity, further truncations eliminated activity. The markedly reduced LDH release following transfection with BteA644-EGFP indicates that the last 14 aa of BteA are critical for full cytotoxicity. To determine if the BteA644 mutant retains structural features of the wild-type protein, we assessed its ability to self-associate, a characteristic of native BteA (Panina et al., 2005). Full-length BteA resolves as high-molecular-weight multimers on SDS-PAGE (Fig. 2C), a property that correlates with the formation of fibres in buffered aqueous solution that can be visualized by electron microscopy (data not shown). BteA644 retains this ability to form SDS-insoluble high-molecular-weight species, suggesting that it maintains its core structural scaffold despite a significant loss of cytotoxicity.
BteA644 localizes to ezrin- and GM1-rich membrane sites
We next examined the localization of BteA644-EGFP in HeLa and L2 cells by scanning confocal microscopy. As shown in Fig. 3A, BteA644-EGFP clearly colocalized with cortical actin. BteA was observed to associate with regions of the cell surface (Fig. 3A, arrowheads), but not actin stress fibres, suggesting that it targets proteins and/or lipids positioned in the vicinity of actin-associated membrane sites. Cortical actin participates in the formation of signalling platforms with membrane receptor cytoplasmic domains (Xavier et al., 2004; Gajate and Mollinedo, 2005; Halayko and Stelmack, 2005; Chichili and Rodgers, 2009). These signalling complexes are often located at lipid raft microdomains anchored to the actin cytoskeleton by members of the ERM (ezrin-radixin-moesin) family of adaptor proteins. (Bretscher et al., 2002; Hoeflich and Ikura, 2004). We were thus interested to determine whether BteA colocalizes with ezrin, an ERM protein prominently expressed by HeLa cells. Cells transfected with constructs expressing BteA644-EGFP or EGFP alone were stained using antibodies against ezrin. Confocal microscopy revealed a high degree of colocalization between BteA644-EGFP and ezrin, but not EGFP and ezrin, suggesting that BteA is specifically targeted to ezrin-enriched membrane sites (Fig. 3B and C).
Fig. 3.
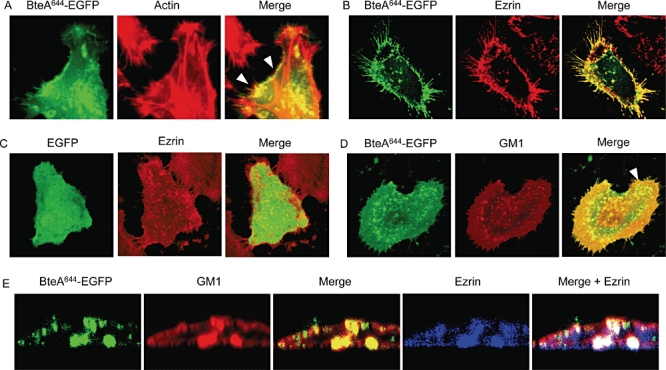
BteA localization. BteA644-EGFP was transfected into HeLa cells. Eighteen hours later cells were fixed, permeabilized and visualized by laser scanning confocal microscopy. Images represent average composite Z-stacks processed by Leica's LCS software. BteA644-EGFP colocalization was assessed using Alexa Fluor 568-conjugated phalloidin to stain actin (A), Alexa Fluor 633 α-ezrin (B and C) and Alexa Fluor 555-labelled CtxB to stain GM1 ganglioside (D). E. XZ scans of the region indicated by the arrow in (D). Colocalization appears as yellow for two-colour overlays, or white in three-colour overlay (4D), and is representative of a single Y section.
In many cells examined, BteA644-EGFP was particularly prominent in filopodia. Filopodia are rod-like projections on the cell surface that are supported by cortical actin and function in sampling and exploration of the environment (Fig. 3B) (Bryant, 1999; Small et al., 2002; Faix et al., 2009). BteA644 expression appeared to increase both the number and length of these structures in HeLa cells (Fig. 3B). In contrast, EGFP alone (Fig. 3C) did not localize to filopodia and did not result in abnormal filopodia formation. Due to their sensory and exploratory function, filopodia possess a large number of transmembrane receptors and raft-associated signalling complexes, which vary according to cell type and growth conditions (Lidke et al., 2005; Ladwein and Rottner, 2008). As filopodia usually contain high concentrations of lipid rafts, we next examined the colocalization of BteA with GM1 ganglioside, which serves as a marker for lipid rafts. Cells expressing BteA644-EGFP were stained using fluorescently labelled cholera toxin subunit B (CtxB), which binds with high avidity and specificity to ganglioside GM1 (Schon and Freire, 1989). Nearly complete colocalization was observed between BteA644-EGFP and GM1 (Fig. 3D). High-magnification XZ scans of the region indicated by the arrowhead in Fig. 3D further demonstrate that transfected BteA localizes precisely with GM1 in cell membranes (Fig. 3E).
BteA contains a conserved, multifunctional N-terminal domain
The BteA proteins expressed by Bordetella species are highly homologous, especially within an N-terminal 130 aa segment (Fig. 1A). Using iterative psi-blast, six homologues of this domain were found in Photorhabdus luminescens and two others in Vibrio splendidus and Photorhabdus asymbiotica (Fig. 4A and B). We had previously predicted that two of the P. luminescens proteins (Plu0822 and Plu4750) function as T3SS effectors on the basis of their close proximity to class I chaperones (Panina et al., 2005). The remaining four P. luminescens proteins (Plu1341, Plu1344, Plu3217, Plu3324) and the proteins from V. splendidus and P. asymbiotica are homologous to RtxA, an atypical RTX toxin with actin cross-linking activity (Lin et al., 1999; Fullner and Mekalanos, 2000).
Fig. 4.
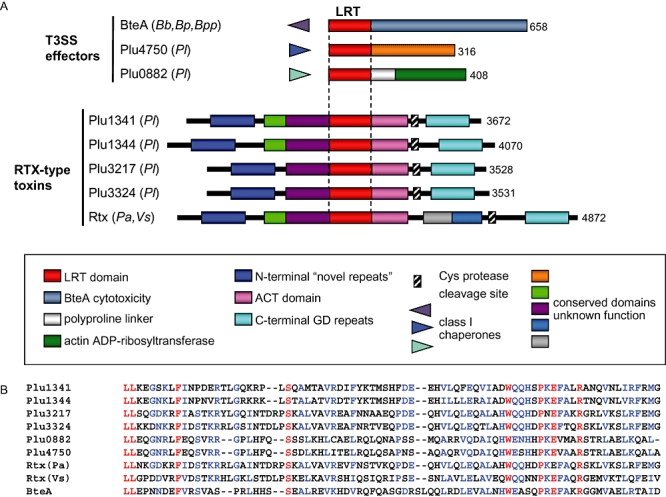
Distribution of LRT domains in putative bacterial virulence factors. A. Schematic representation of known and predicted proteins with LRT domains (red bars) and their closest homologues lacking LRT sequences. The schematic is not drawn to scale. Homologous domains are coloured as indicated. The cysteine protease domain in RtxA and related toxins, and a cleavage site identified in RtxA, are indicated by hatched boxes and an arrow respectively (Sheahan et al., 2007) (see Discussion). Species origin is indicated in parentheses by a two-letter code: Bb, B. bronchiseptica; Bp, B. pertussis; Bpp, B. parapertussis; Pl, P. luminescens; Pa, Photorhabdus asymbiotica, Vs, V. splendidus. B. Alignment of LRT domains in known and predicted virulence factors. The central region of the BteA LRT domain (aa 34–112) is shown, with identical amino acids present in all nine sequences in red and conserved residues in blue.
Given the presence of homologous sequences in known and putative virulence determinants from multiple species, we hypothesized that the N-terminal 130 aa segment of BteA may perform a conserved cellular function, possibly by directing localization. This was tested by fusing the first 130 aa of BteA to EGFP (BteA130-EGFP) and examining transfected cells by confocal microscopy. BteA130-EGFP localized to GM1-positive lipid rafts in a manner indistinguishable from BteA644-EGFP (Figs 3D and 5B respectively). Similarly, the localization patterns of all C-terminal truncated derivatives of BteA (Fig. 3B) were indistinguishable from BteA644-EGFP, while the first 30 aa of BteA was unable to direct EGFP to membrane sites (data not shown). When a construct lacking the N-terminal 130 aa region of BteA (BteA131-644-EGFP) was tested, it too failed to localize to GM1-stained raft microdomains (Fig. 5C). These observations demonstrate that an LRT signal of BteA is contained between aa 1 and 130. Although the BteA LRT motif is essential for localization, it is not required for cytotoxicity following transfection (Fig. 5D). Expression of BteA131-658-EGFP conferred a high level of cytotoxicity, even though there was no detectable localization to the cell membrane. Although overexpression of toxic domains may compensate for the lack of localization following transfection, membrane association is likely to be important during T3SS-mediated translocation (see Discussion).
Fig. 5.
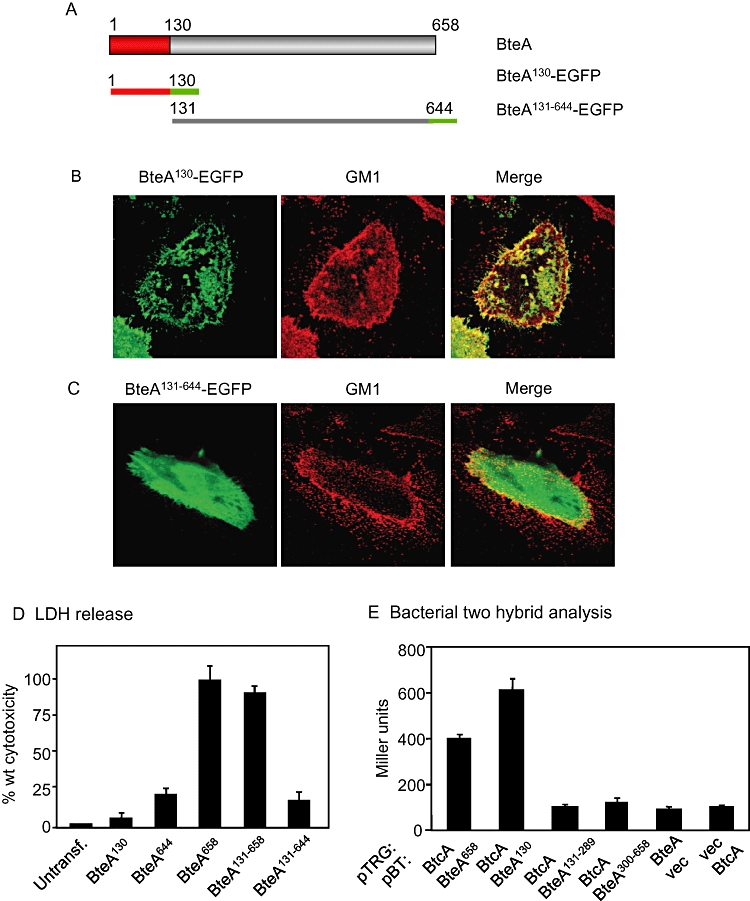
The N-terminal LRT domain of BteA mediates chaperone binding and host cell localization. A. Constructs used in this experiment. Notations are the same as in Fig. 3A. HeLa cells were transfected with BteA130-EGFP (B) or BteA130-644-EGFP (C). EGFP fusion proteins and Alexa Fluor 555-labelled CtxB bound to GM1 were visualized by confocal microscopy. Images are single Z sections. Colocalization between transfected proteins and GM1 appears as yellow on the overlay images. D. Cytotoxicity assays. Full-length (BteA658) or truncated BteA–EGFP fusion constructs were transfected into HeLa cells and LDH release was measured 21 h post transfection. Cytotoxicity is expressed as a percentage relative to wild-type BteA. E. Bacterial two-hybrid analysis of interactions between BtcA, BteA and BteA domains. The pTRG target vector alone (vec), or pTRG expressing full-length BtcA fused to the amino-terminal domain of the alpha subunit of RNA polymerase was transformed with the pBT bait vector (vec), or pBT expressing full-length BteA (BteA658), or fragments truncated at their N- and/or C-termini fused to the C-terminus of the lambda repressor.
N-terminal sequences of T3SS effectors contain secretion signals and often mediate chaperone–effector interactions (Harrington et al., 2003). bteA is directly adjacent to a divergently transcribed locus, btcA, which is predicted to encode a class I chaperone (Panina et al., 2005). As shown in Fig. 5E, full-length BteA associates with BtcA in a bacterial two-hybrid system (Dove and Hochschild, 2004) and the 130 aa LRT domain is both necessary and sufficient for this interaction. Taken together, our results show that the N-terminus of BteA is multifunctional, mediating chaperone interactions and directing the localization of BteA to lipid raft microdomains in eukaryotic cells.
BteA fractionates with detergent-resistant membranes
The relative insolubility of cholesterol in Triton X-100 facilitates the separation of cholesterol-enriched lipid rafts during cell fractionation (Ignoul et al., 2007). Detergent-resistant membrane (DRM) fractions were prepared from HeLa cells transfected with BteA–EGFP derivatives to further investigate the association between BteA and lipid rafts. HeLa cells expressing EGFP, BteA644-EGFP, BteA130-EGFP or BteA130-644-EGFP were fractionated by Triton X-100 solubilization followed by sucrose gradient density centrifugation. Cellular fractions were separated by SDS-PAGE and Western blots were probed with antibody to EGFP. As expected, fusion constructs containing the BteA LRT domain (BteA644- and BteA130-EGFP) were abundant in the low-density Triton X-100-insoluble DRM fractions, suggesting that BteA associates with rafts, raft proteins or associated protein complexes (Fig. 6). Notably, the distribution pattern of BteA in DRMs was nearly identical to that of ezrin and the lipid raft marker, caveolin-1. Although clearly associated with lipid rafts, the presence of detectable levels of LRT-containing BteA derivatives, ezrin and caveolin in dense cellular fractions is likely due to the presence of non-membrane-associated intracellular pools and/or disruption of complexes during fractionation. In contrast, BteA130-644-EGFP and the EGFP-negative control were exclusively present in dense, non-membrane-associated Triton X-100-soluble fractions. Thus, the LRT domain-dependent fractionation of BteA within DRMs coincides with results from colocalization studies.
Fig. 6.
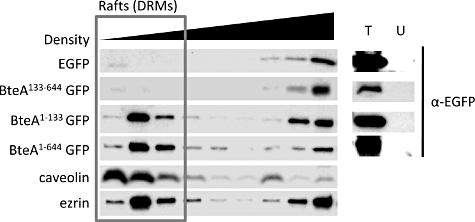
BteA is present in DRM fractions from transfected cells. HeLa cells were transfected with the constructs indicated (left sidebar), and fractionated as described in Experimental procedures. Western blots were probed with α-EGFP or antibodies to ezrin and caveolin as indicated in the left sidebar. Transfected (T) or untransfected (U) cell controls are shown in the right sidebar. Lanes within the red outline are low-density DRM fractions.
BteA is translocated to the cytoplasmic face of membrane raft locations that function as sites for bacterial attachment
The observation that BteA targets discrete locations on host cell membranes prompted us to examine whether a correlation exists with sites of Bordetella attachment. HeLa cells were infected with wild-type B. bronchiseptica strain RB50 for 15 min, a time point before cytotoxicity is observed. Cells were washed to remove unbound bacteria, fixed and stained with CtxB. Remarkably, almost every cell-associated bacterium was found to colocalize with GM1-positive rafts (Fig. 7A). We reasoned that B. bronchiseptica may specifically adhere to lipid rafts and that BteA functions locally following injection, or that BteA and/or other T3SS-associated factors actively recruit GM1-rich structures to sites of Bordetella attachment. To distinguish these possibilities we analysed the attachment pattern of a T3SS ATPase mutant, RB50 ΔbscN (Yuk et al., 1998), which adhered to GM1-enriched sites in a manner indistinguishable from wild-type RB50 (Fig. 7B). Association with lipid rafts therefore is independent of T3SS activity and is likely mediated by adhesin specificity.
Fig. 7.
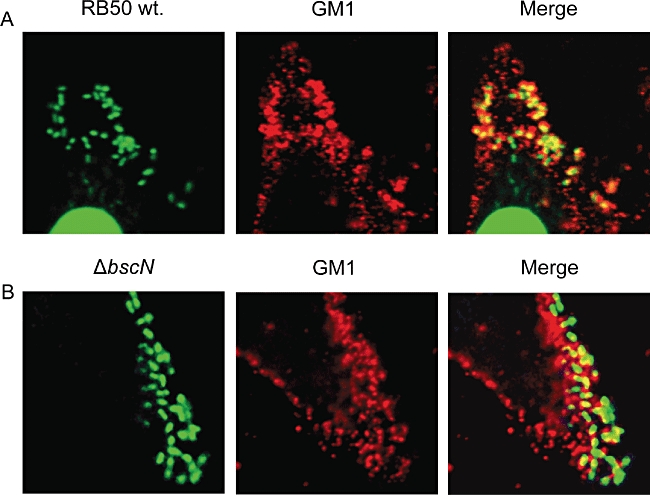
B. bronchiseptica RB50 adheres to GM1-containing lipid rafts. Images are single Z sections, and are representative of at least three separate experiments. HeLa cell monolayers were infected with B. bronchiseptica strain RB50 (A) or the T3SS-defective strain RB50 ΔbscN (B) for 25 min, washed, fixed and stained. DAPI staining (green) reveals bacteria adherent to HeLa cells. GM1 appears red, and overlap is yellow in the merged images.
We next determined if BteA localizes to lipid rafts following T3SS-mediated translocation by adherent Bordetella. HeLa cells were infected with RB50 derivatives expressing haemagglutinin (HA)-tagged BteA (BteA644-HA), permeabilized with Triton X-100 and stained with DAPI, CtxB and an α-HA monoclonal antibody. BteA644-HA closely associated with adherent bacteria (Fig. 8A) and colocalized with GM1-positive lipid rafts (Fig. 8B). XZ confocal scans of the region marked in Fig. 8B (arrow) show that BteA is translocated into lipid rafts that coincide with sites of bacterial attachment (arrowheads, Fig. 8C). T3SS-translocated BteA644-HA could not be visualized when the Triton X-100 membrane permeabilization step was omitted, indicating that BteA was not localized to the outer surface of the plasma membrane (data not shown). Deletions in loci encoding the BscN ATPase (RB50 ΔbscN), or the BopB translocon component (RB50 ΔbopB), eliminated the ability to detect raft-associated BteA644-HA, demonstrating a requirement for T3SS-mediated translocation (data not shown). These data demonstrate that translocated BteA associates with the cytoplasmic face of lipid rafts that serve as attachment sites for Bordetella, and suggest that BteA acts locally following bacterial attachment. It is interesting to note that only a subset of adherent bacteria are associated with translocated BteA at any given time (Fig. 8A). The mechanistic basis for this lack of synchrony is currently unknown.
Fig. 8.
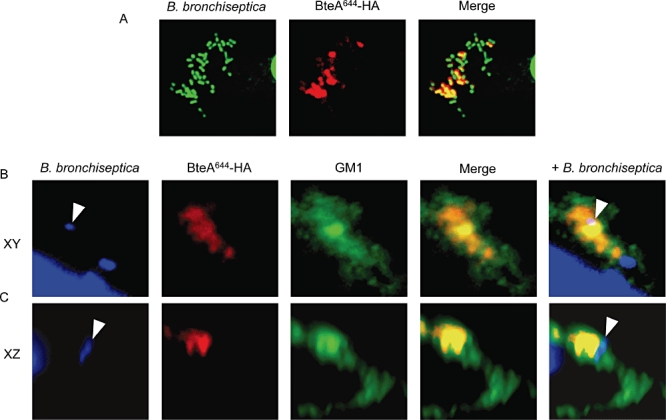
BteA is translocated by the bsc T3SS into lipid rafts that coincide with sites of bacterial attachment. A single HeLa cell is shown, infected with RB50 expressing an HA-tagged BteA644 (BteA644-HA) and stained using DAPI (bacteria) and antibody to HA. A. BteA644-HA (red) colocalizes with adherent bacteria (green). B. 100× magnification, XY confocal image of BteA644-HA stained with DAPI for bacteria (blue), α-HA (red) and GM1 ganglioside (green). C. 100× magnification XZ scan and merged images of the same portion of the cell as in (B). The arrows indicate the position of bacterial cells. Colocalization appears yellow in two colour overlays, and white in triple colour overlays.
Disruption of lipid rafts decreases T3SS-induced cytotoxicity
As both B. bronchiseptica and BteA preferentially localize to lipid rafts, we were curious to determine if the integrity of cholesterol-enriched membrane domains is important for attachment and/or T3SS-mediated cytotoxicity. Methyl-β-cyclodextrin (MBCD), a cyclic glucose oligomer that forms soluble inclusion complexes with cholesterol, was used to disrupt lipid rafts (Shaw, 2006). HeLa cells were pretreated with 2.5 mM MBCD or media alone for 30 min, washed and briefly infected with B. bronchiseptica RB50. Mild treatment conditions were employed to minimize the MBCD-dependent cytotoxicity observed with higher concentrations or longer exposure. As shown in Fig. 9A, a modest decrease in bacterial attachment was observed at 20 min, a time before T3SS-induced cytotoxicity could be detected. In the experiment in Fig. 9B, HeLa cells were first infected with B. bronchiseptica RB50 for 15 min, followed by a 30 min treatment with MBCD or media alone. In this case, no significant difference was observed between MBCD-treated cells and untreated controls, indicating that treatment with MBCD has little effect on previously attached Bordetella.
Fig. 9.
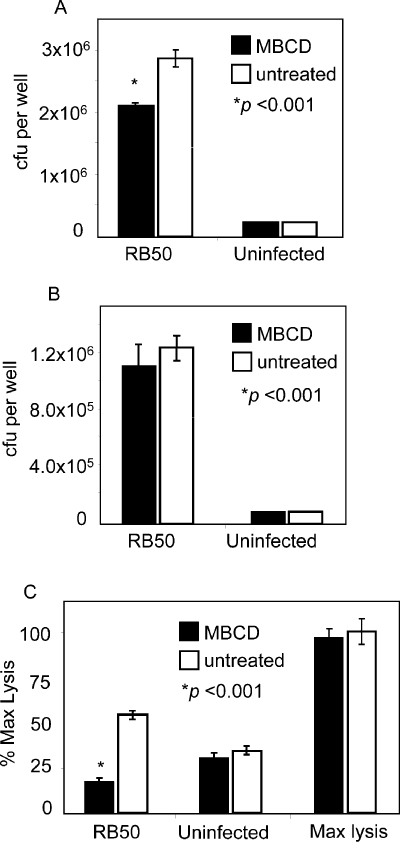
MBCD treatment interferes with bacterial attachment and protects cells from T3SS-mediated cytotoxicity. A. HeLa cells incubated with media alone (black bars) or 2.5 mM MBCD (grey bars) for 30 min were washed and infected with wild-type B. bronchiseptica RB50 for 15 min at a multiplicity of infection of 50:1. Following infection, cells were extensively washed, solubilized and adherent bacteria measured as colony forming units (cfu) per well. All statistical analyses were performed using the Student's t-test with data from three independent experiments. Asterisk indicates a P-value < 0.001. B. HeLa cells were first infected with wild-type B. bronchiseptica RB50 for 15 min, washed and incubated with media alone (black bars) or 2.5 mM MBCD (grey bars) for 30 min Cells were extensively washed, solubilized, and adherent bacteria measured as cfu per well. There was no statistically significant difference between untreated and MCDB-treated cells. C. HeLa cells infected with B. bronchiseptica RB50 for 15 min, or uninfected, were incubated with media alone (black bars) or 2.5 mM MBCD (striped bars) for 30 min as described in (B). Cells were then washed and incubated in fresh media for 2 h. Bars indicate cytotoxicity measured by LDH release as a percentage of maximum cell lysis. Wells designated as ‘Max lysis’ were treated with MBCD as described above and lysed with 0.9% Triton-X100 to generate maximum values for LDH release.
Having identified a MBCD treatment protocol that was non-toxic and did not disrupt bacterial attachment, we next determined the effects of cholesterol depletion on T3SS-induced cytotoxicity. HeLa cells were infected with B. bronchiseptica RB50 for 15 min, treated with MBCD or media alone for 30 min, washed extensively and the infection was allowed to proceed for a total of 2 h. As shown in Fig. 9C, cytotoxicity was reduced to background levels in cells subjected to mild treatment with MBCD. These results suggest that BteA-mediated cytotoxicity following Bordetella infection is dependent on the integrity of lipid rafts.
Functional conservation of LRT domains in T3SS effectors and RTX toxins
The relative positions of LRT domains differ according to the known or predicted nature of the protein; T3SS effectors bear them near their N-termini while RTX-type toxins encode them near the middle of their 3500- to 4900-aa-long sequences (Fig. 4A). Having demonstrated that the BteA LRT domain is necessary and sufficient for membrane localization, we determined whether putative LRT sequences from two P. luminescens ORFs, one predicted to encode a T3SS effector (Plu4750) and the other an RTX toxin (Plu3217), were capable of directing EGFP fusion constructs to lipid rafts. Sequences encoding the P. luminescens LRT domains were fused to EGFP at their C-termini and transfected into HeLa cells. As predicted, both LRT sequences localized the EGFP fusion constructs to ezrin-rich sites, despite their presence in otherwise unrelated proteins (Fig. 10A–C). Moreover, the same localization pattern was observed when full-length Plu4750 was fused to EGFP, confirming that the LRT motif functions as predicted in the context of the entire protein (Fig. 10C). On the basis of these observations we conclude that the N-terminal LRT motif of BteA defines a newly identified family of localization domains that target diverse bacterial proteins to membrane rafts in eukaryotic cells.
Fig. 10.
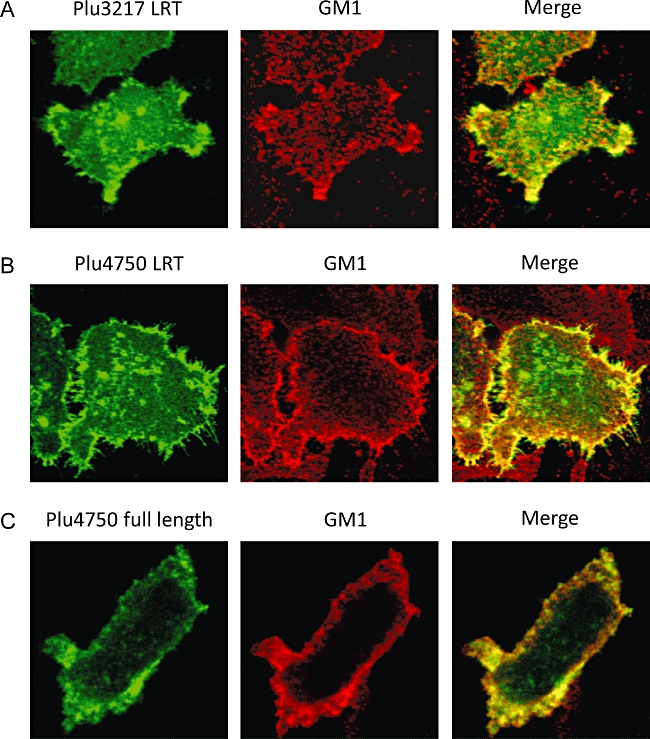
Functional conservation of LRT domains from T3SS effectors and RTX-type toxins. Colocalization of ezrin with the isolated LRT domains from Plu3217 (A) and Plu4750 (B) fused to EGFP, and with the full-length Plu4750 protein fused to EGFP (C). HeLa cells were transfected with expression plasmids for EGFP fusion proteins, stained for GM1 and visualized by confocal microscopy. Colocalization between transfected proteins and GM1 appears as yellow on the merged images. Images are single Z sections.
Discussion
BteA is the only Bordetella T3SS effector protein described to date (Panina et al., 2005). This stands in marked contrast to other T3SS-expressing species where numerous effectors have been identified by proteomic, bioinformatic or translocation screens (Fouts et al., 2002; Chang et al., 2005). BteA is both necessary and sufficient for the induction of rapid cell death in a remarkably broad range of cell types (Panina et al., 2005). Although BteA has no full-length homologues among previously characterized proteins, it is highly conserved and functionally interchangeable between Bordetella species. Despite its central role in virulence, the molecular function and intracellular targets of BteA have remained elusive.
The potent cytotoxicity of BteA precludes the ability to detect even trace amounts of full-length protein in mammalian cells. A deletion of 14 aa from the C-terminus was sufficient to eliminate cytotoxicity. This derivative, BteA644, retained the ability to form high-molecular-weight, detergent-insoluble complexes that were similar to full-length BteA, suggesting that the structural scaffold of the protein remains intact. Most importantly, the reduction of cytotoxicity permitted the efficient detection of intracellular protein. Following transfection or T3SS-mediated translocation, BteA644 clearly localized to ezrin- and GM1-rich membrane microdomains in HeLa cells as observed by confocal microscopy. Localization was dependent on the 130 aa LRT motif present at the N-terminus of BteA. Identical patterns of localization were observed with BteA derivatives that retain the LRT sequence, and its removal disrupted normal targeting. As predicted, BteA associated with low-density, detergent-insoluble membrane fractions from transfected cells, indicating direct or indirect association with lipid rafts. Importantly, BteA displayed a pattern of localization following T3SS-mediated translocation that was identical to that observed following transfection. When sites of Bordetella attachment were examined, nearly every cell-associated bacterium was found to colocalize with GM1 and there was a striking correlation between the position of extracellular bacteria and the location of translocated BteA. These observations tempt the hypothesis that BteA modulates localized cellular responses to Bordetella infection, and we speculate that this may be especially relevant to interactions with phagocytic antigen-presenting cells. This is consistent with previous results showing T3SS-dependent modulation of T cell- and B cell-mediated responses during B. bronchiseptica infections (Skinner et al., 2005; Pilione and Harvill, 2006; Siciliano et al., 2006).
Although lipid raft localization is likely to be essential under physiologically relevant conditions, a BteA130-658 construct lacking LRT sequences displayed cytotoxic activity following transfection. This contrasts with observations that transfected and translocated BteA behave in an otherwise identical manner and that depletion of membrane cholesterol with MBCD abrogates BteA-dependent cell death following T3SS-mediated delivery. A likely explanation is that the LRT domain plays an essential role in facilitating interactions between BteA and host cell targets during T3SS-mediated translocation, and overexpression of BteA following transfection eliminates the need for LRT-dependent localization. In addition to its role in membrane association, the BteA LRT domain also contains sequences that mediate secretion and chaperone binding. The identification of mutations that disrupt targeting without affecting secretion or translocation will facilitate further studies on the importance of LRT for BteA function. Experiments aimed at identifying BteA binding partners responsible for localization and/or cytotoxicity are also underway. On the basis of results presented here, we expect that BteA targets will be associated with the host cell membrane and cholesterol-rich domains. Likely candidates include components of membrane-associated signalling complexes that are found in lipid rafts and are known to be associated with the cortical actin cytoskeleton.
The BteA LRT domain represents a newly identified motif capable of directing proteins to lipid rafts. Although the identification of similar sequences at the N-termini of other T3SS effectors was not unexpected, we were intrigued to find homologous motifs in RTX-type toxins. A model has been recently proposed for Vibrio cholerae RtxA, which postulates that both N-terminal and C-terminal sequences associate with the plasma membrane and mediate translocation of the central portion of the protein into the cytosol. A cysteine protease domain then cleaves the toxin between L3428 and A3429 (Fig. 4A) and additional cleavage events liberate the actin cross-linking and Rho-inactivation domains from their membrane anchors (Sheahan et al., 2007; Prochazkova and Satchell, 2008). As homologous cysteine protease domains are found in all of the RTX-type toxins shown in Fig. 4A, proteolytic processing following cytosolic delivery is likely to be a common feature (Sheahan et al., 2007). For the P. luminescens and V. splendidus RTX-type toxins, we propose that adjacent LRT motifs target activity domains released by proteolysis to GM1-rich lipid rafts. The relative positions of cleavage sites would determine exactly which domains colocalize with the LRT. Although LRT-containing proteins represent distinct classes of virulence determinants with completely different translocation mechanisms, the ability of the LRT domains from a Photorhabdus effector and an RTX-type toxin to guide fusion proteins to lipid rafts clearly demonstrates conserved function.
Photorhabdus luminescens is a bacterial symbiont of entomopathogenic nematodes. It is transported to the hemocoel of an insect prey, which is then killed by an arsenal of toxins and T3SS effectors (Derzelle et al., 2004). V. splendidus is a dominant Vibrio in coastal sediments and seawater, which is pathogenic for bivalves and fish and shares an ecological niche with V. cholerae (Lacoste et al. 2001, Gay et al., 2004). B. bronchiseptica infects the respiratory epithelia of a wide range of mammals, including humans, and appears to have an environmental reservoir as well (Akerley et al., 1995). The evolutionary journey the LRT domain has taken between pathogens of insects, fish and mammals provides a poignant example of the conservation of virulence mechanisms in nature and exemplifies the ancient origins of bacterial–host interactions.
Experimental procedures
Bacterial strains and cell culture
HeLa cells were maintained in DMEM (Gibco) supplemented with 10% Fetal Bovine Serum (FBS, Hyclone) and penicillin-streptomycin. L2 cells were maintained in DMEM or F12K media (Invitrogen) supplemented with 10% FBS and pen-strep. Both cell lines were grown at 37°C in a 5% CO2 atmosphere. B. bronchiseptica strains RB50, RB50 ΔbscN, ΔbteA andΔbopB have been previously described (Cotter and Miller, 1994; Yuk et al., 1998; Panina et al., 2005). Full-length or truncated bteA loci from B. bronchiseptica, B. pertussis Tohama I or B. parapertussis strain 12822 (Parkhill et al., 2003) were cloned into pBBR1MCS-5 for expression in Bordetella. All B. bronchiseptica-derived strains were cultured in Stainer-Scholte liquid medium or on Bordet-Gengou agar medium (Becton Dickinson Microbiology Systems, San Jose, CA) containing 7.5% defibrinated sheep blood (Mission Laboratories, Rosemead, CA) and appropriate antibiotics for plasmid selection.
Construction of BteA–EGFP and LRT-EGFP fusions
Genes encoding full-length BteA (BteA658) and BteA derivatives with C-terminal deletions (BteA30, BteA300, BteA400, BteA500, BteA540, BteA580, BteA620, BteA628, BteA644 and BteA652) were cloned into the NheI and HindIII restriction sites in pEGFP-N1 (Clontech), in-frame with the C-terminal EGFP tag. bteA fragments were PCR-amplified from B. bronchiseptica RB50 using Herculase (Stratagene). All fragments include the first codon of BteA and terminate at the codon indicated by their superscript designation (e.g. BteA300 includes codons 1–300). EGFP-tagged mutants containing N-terminal BteA deletions (BteA131-644, BteA131-658) were constructed by cloning BteA fragments into the same sites on pEGFP-N1 and adding ATG start codons for translation initiation. LRT domains from Plu4750 (codons 1–132), Plu3217 (codons 1808–1907) and the full-length Plu4750 protein (codons 1–316) were cloned from P. luminescens sp. luminescens (ATCC) into pEGFP-N1 to construct EGFP fusions. An N-terminal ATG codon was added immediately upstream of LRT sequences from Plu3217.
Cytotoxicity assays, transfection and MBCD treatments
Bordetella bronchiseptica strains expressing or bteABb, bteABp or bteABpp alleles grown to mid-log phase were added to cell cultures at a multiplicity of infection of 50:1 and centrifuged onto cultured cells at 640 g for 5 min. DMEM + 1% FBS was added to cells following a thorough wash with PBS, and incubated at 37°C for indicated periods of time. Cytotoxicity was assayed with the CytoTox 96 LDH release assay kit (Promega) (Lappalainen et al., 1994) following the manufacturer's suggested procedures. To analyse cytotoxicity from transfected constructs, HeLa or L2 cells grown in 12-well plates were transfected with 1.6 μg of plasmid DNA using Lipofectamine LTX (Invitrogen). Transfected cell cultures were briefly centrifuged to remove cell debris, and 50 μl of media was removed and assayed for LDH release at various times post transfection. For MBCD protection or attachment assays, HeLa cells were washed with PBS and incubated in DMEM + 2.5 mM MBCD (Sigma Aldrich) for 30 min. MBCD-containing medium was removed, cells were washed with PBS and cultured in reduced serum DMEM (1.0% FBS) lacking MBCD. Fifteen minutes after centrifugation, colony-forming units of attached bacteria were assayed by spreading serial dilutions of the cell lysate on BG agar medium following washing and solubilization of the cell monolayer in 0.2% Triton X-100. Bordetella-induced cytotoxicity was measured as described above at 2 h post infection.
Confocal microscopy
Cells were grown in multiwell plates on glass coverslips and transfected as described above. Eighteen hours post transfection, cells were washed and fixed with 4% paraformaldehyde in 1× PBS for 30 min at room temperature. To visualize EGFP fusion proteins, glass slides were mounted using Vectashield mounting media (Vector Laboratories) and sealed with clear nail polish. For actin and ezrin staining, cells were permeabilized with 0.1% Triton X-100 for 10 min, and stained with either Alexa Fluor-conjugated phalloidin (Invitrogen) or α-ezrin primary antibody (BD Biosciences) followed by fluorescently labelled secondary antibody (Invitrogen). GM1 staining using Alexa Fluor 555-labelled CtxB was performed as described (Chen et al., 2007). HeLa cells infected with B. bronchiseptica were washed twice with PBS, fixed as described above, and B. bronchiseptica was visualized using Vectashield mounting media with DAPI (Vector Laboratories). All images were acquired with a Leica SP2-AOBS laser scanning confocal microscope set-up. Images shown in the figures are representative of at least three independent experiments and were produced with Leica's LCS confocal software.
Western blot analysis
Bordetella bronchiseptica strains were grown overnight in Stainer-Scholte medium and cell cultures were separated into pellet and supernatant fractions. Whole-cell extracts were prepared by dissolving pellets in Laemmli buffer (Laemmli, 1970). Proteins from supernatant fractions were precipitated with 10% trichloroacetic acid for 6 h at 4°C. OD equivalents of B. bronchiseptica whole-cell lysates and supernatants were separated on 10% SDS-PAGE gels and transferred to PVDF membranes (Millipore). Membranes were incubated with mouse α-FLAG antibody (Sigma Aldrich) and antigen–antibody complexes were detected with horseradish peroxidase-conjugated α-mouse immunoglobulin (Amersham Biosciences) and visualized by chemiluminescence using SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL).
Bacterial two-hybrid analysis
Bordetella bronchiseptica sequences were cloned into the BacterioMatch (Stratagene) two-hybrid ‘bait’ vector pBT, creating fusions with the C terminus of the lambda cI protein, and/or into the cognate ‘target’ vector pTRG, creating fusions with the amino-terminal domain of the alpha subunit of RNA polymerase. Clones were confirmed by sequence analysis and cotransformed into the BacterioMatch I reporter strain (XL1Blue MRF′laqIqbla lacZ Kanr). To assay β-galactosidase activity, overnight cultures were subcultured to an optical density (OD600) of 0.07 in LB medium containing tetracycline (15 μg ml−1) and chloramphenicol (34 μg ml−1). Cultures were incubated at 30°C until the OD600 reached 0.4, at which point isopropyl β-D-thiogalactoside was added to a final concentration of 10 μM. After 2 h of isopropyl β-D-thiogalactoside induction, cells were harvested and permeabilized with SDS and CHCl3, and β-galactosidase activity was measured using the method described by Miller (Sommer et al., 1978).
DRM fractionation
The DRM fractions were prepared from HeLa cells as described (Ignoul et al., 2007). Approximately 18 h after transfection with BteA- and LRT-fusion derivates, 1–2 × 107 cells were washed with PBS, scraped into 1 ml of lysis buffer containing 1.0% Triton X-100 and protease inhibitor cocktail (Roche), and adjusted to a final sucrose concentration of 45%. The cell lysate was overlaid with a 30–5% sucrose step gradient, and separated for 16 h at 38 000 r.p.m. by isopycnic floatation in a Beckman SW-41 rotor. 1 ml fractions were collected and sedimented in a Ti70.1 rotor at 40 000 r.p.m. for 1 h. The pellets were resuspended in 1× Laemmli buffer (Laemmli, 1970) and electrophoresed on 4–16% gradient SDS-PAGE gels (Bio-Rad). Proteins were transferred to PVDF-Plus membranes (Amersham) and probed with rabbit α-EGFP (Abcam), mouse α-ezrin (BD-Biosciences) or mouse α-caveolin-1 (Santa Cruz). Chemiluminescent detection was performed using Amersham's ECL reagents and Kodak BioMax MS film.
Bacterial attachment assays
HeLa cells pre-incubated with MBCD or media alone as described above were infected with B. bronchiseptica RB50 at a multiplicity of infection of 50:1 for 25 min. Infected cultures were briefly centrifuged at 640 g for 5 min followed by a thorough wash with PBS. Cells were solubilized in 0.2% Triton X-100, and aliquots assayed for colony-forming units on BG agar medium. In parallel, attached B. bronchiseptica were stained, visualized by light microscopy, and manually counted.
Acknowledgments
We would like to thank Laurent Bentolila and Matt Schibler at the CNSI Advanced Light Microscopy and Spectroscopy Facility at UCLA, which is supported by an NIH-NCRR Shared Resources Grant (CJX1-443835-WS-29646) and NSF Major Research Instrumentation Grant (CHE-0722519). Asher Hodes assisted in editing the manuscript. C.T.F. is supported by the National Institutes of Health under the Ruth L. Kirschstein National Research Service Award GM07185. This work was supported by NIH Grants AI 38417 and AI 61598 to J.F.M.
References
- Akerley BJ, Cotter PA, Miller JF. Ectopic expression of the flagellar regulon alters development of the Bordetella–host interaction. Cell. 1995;80:611–620. doi: 10.1016/0092-8674(95)90515-4. [DOI] [PubMed] [Google Scholar]
- Allen-Vercoe E, Waddell B, Livingstone S, Deans J, DeVinney R. Enteropathogenic Escherichia coli Tir translocation and pedestal formation requires membrane cholesterol in the absence of bundle-forming pili. Cell Microbiol. 2006;8:613–624. doi: 10.1111/j.1462-5822.2005.00654.x. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- Bryant PJ. Filopodia: fickle fingers of cell fate? Curr Biol. 1999;9:R655–R657. doi: 10.1016/s0960-9822(99)80416-3. [DOI] [PubMed] [Google Scholar]
- Chang JH, Urbach JM, Law TF, Arnold LW, Hu A, Gombar S, et al. A high-throughput, near-saturating screen for type III effector genes from Pseudomonas syringae. Proc Natl Acad Sci USA. 2005;102:2549–2554. doi: 10.1073/pnas.0409660102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Chen L, Wang G, Tang H. Cholesterol-dependent and – independent CD40 internalization and signaling activation in cardiovascular endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27:2005–2013. doi: 10.1161/ATVBAHA.107.145961. [DOI] [PubMed] [Google Scholar]
- Chichili GR, Rodgers W. Cytoskeleton–membrane interactions in membrane raft structure. Cell Mol Life Sci. 2009;66:2319–2328. doi: 10.1007/s00018-009-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter PA, Miller JF. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect Immun. 1994;62:3381–3390. doi: 10.1128/iai.62.8.3381-3390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derzelle S, Turlin E, Duchaud E, Pages S, Kunst F, Givaudan A, Danchin A. The PhoP-PhoQ two-component regulatory system of Photorhabdus luminescens is essential for virulence in insects. J Bacteriol. 2004;186:1270–1279. doi: 10.1128/JB.186.5.1270-1279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove SL, Hochschild A. A bacterial two-hybrid system based on transcription activation. Meth Mol Biol. 2004;261:231–246. doi: 10.1385/1-59259-762-9:231. [DOI] [PubMed] [Google Scholar]
- Edidin M. The state of lipid rafts: from model membranes to cells. Annu Rev Biophys Biomol Struct. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- Faix J, Breitsprecher D, Stradal TE, Rottner K. Filopodia: Complex models for simple rods. Int J Biochem Cell Biol. 2009;41:1656–1664. doi: 10.1016/j.biocel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Fouts DE, Abramovitch RB, Alfano JR, Baldo AM, Buell CR, Cartinhour S, et al. Genomewide identification of Pseudomonas syringae pv. tomato DC3000 promoters controlled by the HrpL alternative sigma factor. Proc Natl Acad Sci USA. 2002;99:2275–2280. doi: 10.1073/pnas.032514099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullner KJ, Mekalanos JJ. In vivo covalent cross-linking of cellular actin by the Vibrio cholerae RTX toxin. EMBO J. 2000;19:5315–5323. doi: 10.1093/emboj/19.20.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajate C, Mollinedo F. Cytoskeleton-mediated death receptor and ligand concentration in lipid rafts forms apoptosis-promoting clusters in cancer chemotherapy. J Biol Chem. 2005;280:11641–11647. doi: 10.1074/jbc.M411781200. [DOI] [PubMed] [Google Scholar]
- Gay M, Renault T, Pons AM, Le Roux F. Two vibrio splendidus related strains collaborate to kill Crassostrea gigas: taxonomy and host alterations. Dis Aquat Organ. 2004;62:65–74. doi: 10.3354/dao062065. [DOI] [PubMed] [Google Scholar]
- Gekara NO, Jacobs T, Chakraborty T, Weiss S. The cholesterol-dependent cytolysin listeriolysin O aggregates rafts via oligomerization. Cell Microbiol. 2005;7:1345–1356. doi: 10.1111/j.1462-5822.2005.00561.x. [DOI] [PubMed] [Google Scholar]
- Halayko AJ, Stelmack GL. The association of caveolae, actin, and the dystrophin-glycoprotein complex: a role in smooth muscle phenotype and function? Can J Physiol Pharmacol. 2005;83:877–891. doi: 10.1139/y05-107. [DOI] [PubMed] [Google Scholar]
- Hanzal-Bayer MF, Hancock JF. Lipid rafts and membrane traffic. FEBS Lett. 2007;581:2098–2104. doi: 10.1016/j.febslet.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Harrington AT, Hearn PD, Picking WL, Barker JR, Wessel A, Picking WD. Structural characterization of the N terminus of IpaC from Shigella flexneri. Infect Immun. 2003;71:1255–1264. doi: 10.1128/IAI.71.3.1255-1264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes DJ, Mak J. Lipid membrane; a novel target for viral and bacterial pathogens. Curr Drug Targets. 2006;7:1615–1621. doi: 10.2174/138945006779025455. [DOI] [PubMed] [Google Scholar]
- Hayward RD, Hume PJ, Humphreys D, Phillips N, Smith K, Koronakis V. Clustering transfers the translocated Escherichia coli receptor into lipid rafts to stimulate reversible activation of c-Fyn. Cell Microbiol. 2009;11:433–441. doi: 10.1111/j.1462-5822.2008.01265.x. [DOI] [PubMed] [Google Scholar]
- Hoeflich KP, Ikura M. Radixin: cytoskeletal adopter and signaling protein. Int J Biochem Cell Biol. 2004;36:2131–2136. doi: 10.1016/j.biocel.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Ignoul S, Simaels J, Hermans D, Annaert W, Eggermont J. Human ClC-6 is a late endosomal glycoprotein that associates with detergent-resistant lipid domains. PLoS ONE. 2007;2:e474. doi: 10.1371/journal.pone.0000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak NA, Mattoo S, Foreman-Wykert AK, Whitelegge JP, Miller JF. Interactions between partner switcher orthologs BtrW and BtrV regulate type III secretion in Bordetella. J Bacteriol. 2005;187:5665–5676. doi: 10.1128/JB.187.16.5665-5676.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwae A, Matsuzawa T, Ishikawa N, Abe H, Nonaka T, Fukuda H, et al. BopC is a novel type III effector secreted by Bordetella bronchiseptica and has a critical role in type III-dependent necrotic cell death. J Biol Chem. 2006;281:6589–6600. doi: 10.1074/jbc.M512711200. [DOI] [PubMed] [Google Scholar]
- Lacoste A, Jalabert F, Malham SK, Cueff A, Poulet SA. Stress and stress-induced neuroendocrine changes increase the susceptibility of juvenile oysters (Crassostrea gigas) to Vibrio splendidus. Appl Environ Microbiol. 2001;67:2304–2309. doi: 10.1128/AEM.67.5.2304-2309.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladwein M, Rottner K. On the Rho'd: the regulation of membrane protrusions by Rho-GTPases. FEBS Lett. 2008;582:2066–2074. doi: 10.1016/j.febslet.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lafont F, Abrami L, van der Goot FG. Bacterial subversion of lipid rafts. Curr Opin Microbiol. 2004;7:4–10. doi: 10.1016/j.mib.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Lafont F, van der Goot FG. Bacterial invasion via lipid rafts. Cell Microbiol. 2005a;7:613–620. doi: 10.1111/j.1462-5822.2005.00515.x. [DOI] [PubMed] [Google Scholar]
- Lafont F, van der Goot FG. Oiling the key hole. Mol Microbiol. 2005b;56:575–577. doi: 10.1111/j.1365-2958.2005.04570.x. [DOI] [PubMed] [Google Scholar]
- Lappalainen K, Jaaskelainen I, Syrjanen K, Urtti A, Syrjanen S. Comparison of cell proliferation and toxicity assays using two cationic liposomes. Pharm Res. 1994;11:1127–1131. doi: 10.1023/a:1018932714745. [DOI] [PubMed] [Google Scholar]
- Laude AJ, Prior IA. Plasma membrane microdomains: organization, function and trafficking. Mol Membr Biol. 2004;21:193–205. doi: 10.1080/09687680410001700517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencer WI, Saslowsky D. Raft trafficking of AB5 subunit bacterial toxins. Biochim Biophys Acta. 2005;1746:314–321. doi: 10.1016/j.bbamcr.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Lidke DS, Lidke KA, Rieger B, Jovin TM, Arndt-Jovin DJ. Reaching out for signals: filopodia sense EGF and respond by directed retrograde transport of activated receptors. J Cell Biol. 2005;170:619–626. doi: 10.1083/jcb.200503140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Fullner KJ, Clayton R, Sexton JA, Rogers MB, Calia KE, et al. Identification of a Vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc Natl Acad Sci USA. 1999;96:1071–1076. doi: 10.1073/pnas.96.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes S, del Real G, Martinez AC. Pathogens: raft hijackers. Nat Rev Immunol. 2003;3:557–568. doi: 10.1038/nri1129. [DOI] [PubMed] [Google Scholar]
- Mattoo S, Yuk MH, Huang LL, Miller JF. Regulation of type III secretion in Bordetella. Mol Microbiol. 2004;52:1201–1214. doi: 10.1111/j.1365-2958.2004.04053.x. [DOI] [PubMed] [Google Scholar]
- Mejia E, Bliska JB, Viboud GI. Yersinia controls type III effector delivery into host cells by modulating Rho activity. PLoS Pathog. 2008;4:e3. doi: 10.1371/journal.ppat.0040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- Panina EM, Mattoo S, Griffith N, Kozak NA, Yuk MH, Miller JF. A genome-wide screen identifies a Bordetella type III secretion effector and candidate effectors in other species. Mol Microbiol. 2005;58:267–279. doi: 10.1111/j.1365-2958.2005.04823.x. [DOI] [PubMed] [Google Scholar]
- Parkhill J, Sebaihia M, Preston A, Murphy LD, Thomson N, Harris DE, et al. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat Genet. 2003;35:32–40. doi: 10.1038/ng1227. [DOI] [PubMed] [Google Scholar]
- Pilione MR, Harvill ET. The Bordetella bronchiseptica type III secretion system inhibits gamma interferon production that is required for efficient antibody-mediated bacterial clearance. Infect Immun. 2006;74:1043–1049. doi: 10.1128/IAI.74.2.1043-1049.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazkova K, Satchell KJ. Structure-function analysis of inositol hexakisphosphate-induced autoprocessing of the Vibrio cholerae multifunctional autoprocessing RTX toxin. J Biol Chem. 2008;283:23656–23664. doi: 10.1074/jbc.M803334200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riff JD, Callahan JW, Sherman PM. Cholesterol-enriched membrane microdomains are required for inducing host cell cytoskeleton rearrangements in response to attaching-effacing Escherichia coli. Infect Immun. 2005;73:7113–7125. doi: 10.1128/IAI.73.11.7113-7125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon A, Freire E. Thermodynamics of intersubunit interactions in cholera toxin upon binding to the oligosaccharide portion of its cell surface receptor, ganglioside GM1. Biochemistry. 1989;28:5019–5024. doi: 10.1021/bi00438a017. [DOI] [PubMed] [Google Scholar]
- Shaw AS. Lipid rafts: now you see them, now you don't. Nat Immunol. 2006;7:1139–1142. doi: 10.1038/ni1405. [DOI] [PubMed] [Google Scholar]
- Sheahan KL, Cordero CL, Satchell KJ. Autoprocessing of the Vibrio cholerae RTX toxin by the cysteine protease domain. EMBO J. 2007;26:2552–2561. doi: 10.1038/sj.emboj.7601700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava R, Miller JF. Virulence factor secretion and translocation by Bordetella species. Curr Opin Microbiol. 2009;12:88–93. doi: 10.1016/j.mib.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano NA, Skinner JA, Yuk MH. Bordetella bronchiseptica modulates macrophage phenotype leading to the inhibition of CD4+ T cell proliferation and the initiation of a Th17 immune response. J Immunol. 2006;177:7131–7138. doi: 10.4049/jimmunol.177.10.7131. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Skinner JA, Pilione MR, Shen H, Harvill ET, Yuk MH. Bordetella type III secretion modulates dendritic cell migration resulting in immunosuppression and bacterial persistence. J Immunol. 2005;175:4647–4652. doi: 10.4049/jimmunol.175.7.4647. [DOI] [PubMed] [Google Scholar]
- Skwarek M. Recent controversy surrounding lipid rafts. Arch Immunol Ther Exp (Warsz) 2004;52:427–431. [PubMed] [Google Scholar]
- Small JV, Stradal T, Vignal E, Rottner K. The lamellipodium: where motility begins. Trends Cell Biol. 2002;12:112–120. doi: 10.1016/s0962-8924(01)02237-1. [DOI] [PubMed] [Google Scholar]
- Sommer H, Schmitz A, Schmeissner U, Miller JH. Genetic studies of the lac repressor. VI. The B116 repressor: an altered lac repressor containing amino acid specified by both the trp and lacI leader regions. J Mol Biol. 1978;123:457–469. [PubMed] [Google Scholar]
- Stockbauer KE, Foreman-Wykert AK, Miller JF. Bordetella type III secretion induces caspase 1-independent necrosis. Cell Microbiol. 2003;5:123–132. doi: 10.1046/j.1462-5822.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- Takenouchi H, Kiyokawa N, Taguchi T, Matsui J, Katagiri YU, Okita H, et al. Shiga toxin binding to globotriaosyl ceramide induces intracellular signals that mediate cytoskeleton remodeling in human renal carcinoma-derived cells. J Cell Sci. 2004;117:3911–3922. doi: 10.1242/jcs.01246. [DOI] [PubMed] [Google Scholar]
- Viola A, Gupta N. Tether and trap: regulation of membrane-raft dynamics by actin-binding proteins. Nat Rev Immunol. 2007;7:889–896. doi: 10.1038/nri2193. [DOI] [PubMed] [Google Scholar]
- Xavier R, Rabizadeh S, Ishiguro K, Andre N, Ortiz JB, Wachtel H, et al. Discs large (Dlg1) complexes in lymphocyte activation. J Cell Biol. 2004;166:173–178. doi: 10.1083/jcb.200309044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuk MH, Harvill ET, Miller JF. The BvgAS virulence control system regulates type III secretion in Bordetella bronchiseptica. Mol Microbiol. 1998;28:945–959. doi: 10.1046/j.1365-2958.1998.00850.x. [DOI] [PubMed] [Google Scholar]
- Yuk MH, Harvill ET, Cotter PA, Miller JF. Modulation of host immune responses, induction of apoptosis and inhibition of NF-kappaB activation by the Bordetella type III secretion system. Mol Microbiol. 2000;35:991–1004. doi: 10.1046/j.1365-2958.2000.01785.x. [DOI] [PubMed] [Google Scholar]


