Abstract
The major pharmacological ingredient in tobacco smoke is nicotine, a mild stimulant known to alter brain electrical activity. The object of this study was to determine if tobacco smoking in humans produces localized or widespread neocortical dominant alpha electroencephalographic (EEG) frequency increases consistent with nicotine stimulation of the brainstem activating system in animals. Twenty-two male volunteer non-deprived tobacco smokers were studied. They were asked not to smoke for at least 1 hr before the experiment in mid-morning as part of their usual smoking schedule. In the laboratory, they sham smoked and then smoked their favorite tobacco cigarette. Two experimental sessions (#1 and #2) were conducted, separated by a one to two month interval. In both sessions, there were minor statistically significant increases in the dominant alpha frequencies after sham smoking. In both sessions, after the subjects smoked a favorite tobacco cigarette there was a significant generalized increase in dominant alpha EEG frequencies in most scalp recording sites. This study demonstrates that tobacco smoking produces widespread bilateral neocortical increases in dominant alpha EEG frequencies consistent with the stimulant effects of nicotine on the brainstem reticular activating system.
Keywords: Dominant Alpha EEG frequencies, Carbon monoxide (CO), Heart rate, Sham smoking, Tobacco smoking
1. Introduction
Both behavioral and electroencephalographic (EEG) studies indicate that nicotine in tobacco smoking amounts is a mild stimulant. Tobacco smoking/nicotine improves performance on some, but not all, cognitive tasks (Swan and Lessov-Schlaggar, 2007). Selective attention, arousal, recognition memory, and working memory have been shown to improve after acute nicotine administration in some studies (Foulds et al., 1996; Ernst et al., 2001; Smith et al., 2002; Kumari et al., 2003). There is little to no effect of nicotine on verbal memory, calculation (arithmetic), or mental association (letter fluency; Sakuri and Kanazawa, 2002). However, prenatal and adolescent exposure to nicotine appears to be correlated with hippocampal changes and a decline in cholinergic activity, resulting in altered visuospatial memory (Jacobsen et al., 2006). An increasing number of studies have associated tobacco smoking with a decline in older adults’ verbal memory, visual search speeds (Richards et al., 2003), Mini Mental State Examination scores (Ott et al., 2004), memory (Reitz et al., 2005), and executive functioning (Starr et al., 2007). It should be noted that in many EEG studies the degree of tobacco/nicotine dependence and withdrawal is a critical issue seldom prominently addressed.
EEG bandwidth frequency analysis from different scalp recording sites and their abundance or power have has been used by many investigators to study the effects of tobacco smoking because these EEG parameters reflect the state of arousal/sedation. Church (1989) reviewed earlier qualitative studies on the effects of cigarette smoking on the EEG. The immediate effect of smoking produces an ‘arousal’ or ‘activated’ EEG profile. Smoking one or two cigarettes causes a decrease in the delta (1-4 Hz) and theta (4-8 Hz) bands, an increase in the beta (14-30 Hz) band, either an increase or decrease in the alpha (8-13 Hz) band, and a shift to a higher dominant alpha frequency. Computer advances have resulted in more objective quantitation analyses of EEG activity. EEG topographic displays indicate important regional spatial distributions of brain wave changes induced by tobacco smoking.
Some of the major changes in the effects of tobacco smoking are in the alpha EEG range. The largest increases occur in the occipital cortex (Kadoya et al., 1994; Domino et al., 1995). It is known that all neocortical recording sites show some alpha activity. Monitoring dominant alpha activity allows one to determine if only the occipital or if all neocortical recording sites are affected after smoking. In animals given nicotine, all cortical areas show EEG arousal due to its stimulant action on the brainstem activating system (Knapp and Domino, 1962). Research has also shown decreases in alpha frequencies during smoking abstinence with increased cigarette craving, decreased arousal, and worsened mood (Teneggi et al., 2004). Additional research is needed to clearly distinguish between nicotine effects in nonsmokers, non-deprived, and deprived smokers. The recent study by Knott et al. (2009) is a step in that direction. It is important to clarify if the EEG changes are due to 1) smoking, 2) arousal from a normal resting state, or 3) a return to a normal resting state after generalized EEG slowing because of tobacco abstinence. For example, in the brief above list of studies the following types of volunteers were studied: 1) deprived smokers (Ulett and Itil, 1969; Knott and Venables, 1977; Pickworth, 1986 (gum), 1989; Kadoya et al., 1994; Cook, 1995; Domino et al., 1995; Teneggi, 2004), 2) non-deprived smokers (Ulett and Itil, 1969; Golding and Mangan, 1982a,b; Cinciripini et al., 1986; Knott, 1988, 1989a,b; Pritchard et al., 1992; Hasenfratz et al., 1993; Pickworth et al., 2003; Teneggi, 2004), 3) sham/denicotinized cigarettes (Golding and Mangan, 1982a,b; Golding, 1988; Pickworth et al., 2003), 4) different levels of tar/nicotine cigarettes (Knott, 1989a,b; Golding and Mangan, 1982b; Hazenfratz, 1993; Pickworth et al., 1986; Domino et al., 1995), 5) nonsmokers given nicotine (Foulds et al., 1994; Knott et al., 2009a,b on evoked potentials).
Separating the various components of tobacco smoking, especially psychological vs pharmacological, is very important. Cook et al. (1995) studied the EEG effects of sham smoking and actual smoking of a cigarette of the subject’s preferred brand. Denicotinized cigarettes have other substances including tar and a very small amount of nicotine. Sham cigarette inhalation only involves room air. The EEG changes due to tobacco smoking varied with differences in male smokers’ arousal. EEG theta power was decreased when the subjects smoked their cigarette in an arousal avoidance state. Beta 2 power increased when the subjects smoked their cigarette in an arousal seeking state. Compared to sham, tobacco smoking increased the peak alpha frequencies in both genders but more in males in an arousal avoidance state. Disagreements in the literature exist which may be explained by the types of smokers, whether or not they are deprived, what they smoke, set and setting differences, etc.. The reports in both rats and humans by Caggiula et al. (2001, 2002), Rose et al. (2003), Rose and Behm (2004), Rose (2006), and Donny et al. (2006) are especially pertinent in better defining the role of nicotine tobacco smoking. Many of these studies highlight the importance of non-nicotine reinforcers of smoking/nicotine. Cues associated with smoking/nicotine administration are powerful reinforcers in rats and humans (Caggiula et al., 2001, 2002; Rose and Behm, 2004). Denicotinzed cigarettes have been shown to reduce craving (Donny et al., 2006) and even reduce ad libitum smoking more than i.v. nicotine (Rose et al., 2003). Nicotine Replacement Therapies (NRT) often fall short of expectations because the importance of non-nicotine components of smoking is ignored (Rose, 2006).
The hypothesis tested in this study was that favorite brand tobacco smoking would have widespread cortical effects on dominant alpha frequencies. To our knowledge, these have never previously been tested and reported in the literature. As predicted, smoking produced widespread bilateral cortical dominant EEG frequency effects. In contrast, there were significant small right hand hemisphere EEG changes in dominant alpha frequency activity with sham smoking. Throughout this manuscript, dominant alpha frequency means the one peak frequency ± 0.2 Hz and not a change in the amount (abundance or power) of the alpha frequency band.
2. Materials and methods
2.1. Subjects and experimental design
Twenty-two subject volunteers in this study were selected from a group of healthy, right handed male Japanese volunteers recruited from the Tobacco Science Research Center in Yokohama, Japan. The inclusion criteria were adult, mentally and physically healthy males from 21 to 40 years of age who were chronic daily tobacco smokers for at least 5 or more years. They were all habitual tobacco smokers but consumed a wide range of from 5 to 40 cigarettes per day. They were asked to smoke as usual so they would not be craving to smoke prior to coming to the laboratory. The only restriction was the subjects were asked not to smoke at least one hr before arrival to the experimental facility (mean smoking interval time ± SD, 5.6 ± 10.9 hr) for both sessions.
The data from the 22 smokers were grouped together for purposes of dominant EEG frequency analyses. Exclusion criteria included any neurologic or psychiatric diseases and drug or substance abuse including alcohol. Caffeinated beverages were permitted with a light breakfast. Their mean ±SD age was 27.6 ± 2.6 yr. This narrow age range with at least five years of smoking was used since onset was controlled. Each subject smoked his own brand of commercial cigarettes (mean nicotine ± SD, 0.91 ± 0.24 mg; mean tar ± SD, 10.7 ± 2.7 mg; mean daily cigarette consumption ± SD, 24.1 ± 17.6 cigarettes). In view of the hypothesis to be tested, this lack of control for duration since the last cigarette was deemed not critical. The subjects were studied twice, experiment 1 was in winter/spring. An EEG only replicate experiment 2 was done in the spring/summer. There were four dropouts and two replacements of similar demographic characteristics. Hence, the same subjects were studied twice, separated by a month or more. This replication was necessary to be confident we could confirm our first findings. During the month plus interval, the smokers conducted their usual work schedule and behaviors. Subjects were each asked to close their eyes and try to relax but not sleep. The experiments began at 9:00 or 10:30 a.m., about 1/2 hr after reporting to the laboratory. A counterbalanced design was not used because the effects of smoking the tobacco cigarette first could confound the sham condition. Hence, sham smoking always preceded the smoking condition. The volunteers were always tested in the morning. The schedule phases were: 1) pre-sham EEG, 2) sham smoking, 3) post-sham EEG, 4) pre-tobacco EEG, 5) tobacco smoking, 6) post-tobacco EEG. Different baselines were used for the analyses of sham and tobacco smoking
2.2. Cigarettes
The cigarettes used in this study were commercial brands available in Japan. The subjects selected each brand according to their preference. The mean ± SD nicotine and tar yield of the cigarettes were 0.9 ± 0.3 mg and 10.5 ± 3.3, respectively, for the subjects of this report.
2.3. Procedures
The entire experiment was carried out in a soundproof, electrically shielded room over a period of about one hr. The subjects sat relaxed in an adjustable armchair. Room illumination was 45 lux. The EEGs were recorded during the eyes closed quiet resting state for about 5-10 min per each experimental phase. Subjects were requested to perform two kinds of smoking: sham (simulated) smoking and real smoking. In sham smoking, the subject ‘smoked’ an unlit pseudocigarette made of a cellulose acetate filter plug and cigarette wrapping paper. The sham cigarettes purposefully differed from the usual obvious control of which the subject would sham-smoke an unlighted preferred brand. The latter would give the smoker strong psychological and sensory smoking cues (own cigarette from own cigarette pack, “flavor” of the taste and smell of tobacco, etc.) and are worthy of a future study. The sham procedure chosen was done to examine only the effects of smoking behavior such as deep inhalation, expiration and cigarette smoking type manipulations. During real tobacco smoking, the subjects smoked their favorite brand of cigarettes in their accustomed manner.
2.4. Physiological recordings
EEGs were recorded on a 16 channel electroencephalograph from 20 spaced electrodes placed on the surface of the scalp and head in accordance with the standard positions of the International EEG 10/20 system. Ag/AgCl electrodes were applied with conductive paste at scalp sites Fp1, Fp2, F3, F4, C3, C4, P3, P4, O1, O2, F7, F8, Fz, Pz, T5 and T6. Monopolar recording was used with a linked-mastoid reference. An electrode placed on the forehead was used as a ground. The impedances of all the electrodes were kept below 5k Ω and tested only at the beginning of each study. The EEGs were recorded at a 5.0-60 Hz band pass to minimize extraneous artifacts for different analyses, of which the present study reports only the alpha frequency effects. When artifacts were detected, those portions of the recordings were deleted. Two methods were used. The first was the San-ei automatic programmed artifact detection system which has its own notch filter and slope filter settings with a sampling rate to reduce aliasing artifact in the data. The second was visual inspection of EEG data included for analyses. The electrocardiogram (EKG) was recorded from an electrode placed on the left shoulder with a ground electrode of the forehead. Acquired EEG and EKG data were stored on a VHS-magnetic tape for further off-line analysis. Only clean artifact free data were used in the analyses.
2.5. Data reduction
The stored EEG data were transferred into a data processing computer (DP 1100, NEC San-ei Instrument Inc.). EEG epochs that contained excessive artifacts induced by blinking or other body movements were omitted from further analysis. A fast Fourier transform cosine algorithm processed a five-sec EEG epoch (1,024 points) for all 16 channels, which computed a 0.2-30 Hz power spectrum with a 0.2 Hz frequency resolution of 1 min for each phase for a variable artifact free dominant alpha. Edge effects during FFT conversion were reduced. The dominant alpha frequency in the 8-12.8 Hz range was determined for each of 16 EEG channels for each subject and each phase condition and separate baselines. Artifact-free EEG epochs before and after sham smoking and after tobacco smoking were obtained for the different conditions blind to the subjects’ experimental phase.
2.6. Statistical analysis
Statistical computations were carried out on each condition (baseline, sham and cigarette smoking) for both experiments 1 and 2. The two way analysis of variance (ANOVA) with repeated measures was performed to test for significant differences among different conditions and between the two experiments. A post hoc test was run to indicate the locations of the differences. Since there were multiple tests for each channel of the EEG, a conservative method was chosen. The Bonferonni correction for multiple testsing was used to generate the p-value cut point (alpha = .05/7 = .00714) for the multiple comparisons. Univariate test degrees of freedom using Greenhouse Geisser Epsilon were also used to prevent the violation of sphericity. Sham or cigarette smoking 1 refers to the first experiment and 2 refers to the second experiment done a month plus later. Multiple analyses for the two experiments were performed: 1) Control baseline 1 vs control baseline 2. 2) Sham 1 vs sham 2. 3) Cigarette smoking 1 vs cigarette smoking 2. 4) Baseline 1 vs sham 1. 5) Baseline 2 vs sham 2. 6) Baseline 1 vs cigarette smoking 1. 7) Baseline 2 vs cigarette smoking 2. 8) Interaction effect experiments 1 and 2. Inasmuch as only one group of subjects served for all levels, it reduced but did not eliminate the error component. Subjects were still likely to respond differently over repeated measures due to changes in motivation, practice effects, etc.
3. Results
3.1. Smoking behavior, expiratory CO level, and heart rate
These were measured only in experiment 1. The chronic daily smokers did not change their habits during the period between the two experiments. The results were similar to those previously described by Shikata et al. (1995a,b). The mean ± SD number of puffs was 7.7 ± 2.4 and mean ± SD butt length was 5.3 ± 0.7 cm during the accustomed tobacco smoking mode for the subjects. The mean ± SD CO level in subjects’ expiratory air was 20.3 ± 9.7 ppm before the experiment, and after the experiment 24.6 ± 8.2 ppm. Heart rate was 63.2 ± 7.4 (presham), 63.0 ± 7.5 (postsham), and 71.1 ± 10.1 (post tobacco smoking).
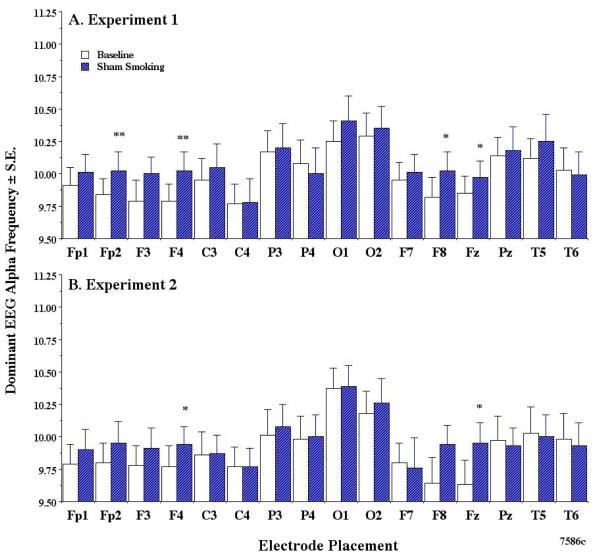
3.2. Effects of sham smoking on dominant alpha EEG frequency
For purposes of clarity, dominant EEG alpha results refer to the EEG frequency and not the EEG abundance or power. Fig. 1 represents the effects of sham smoking on the dominant EEG alpha frequency in experiment 1 and replicated in experiment 2. The two way ANOVA with repeated measures (Pillai’s trace test) indicated that sham smoking in experiment 1 produced an increase in alpha in Fp2 (F (2, 20) = 10.85, p < .001, the post hoc test p = .002, F4 (F (2, 20) = 18.42, p < .0001, post hoc test p = .008), F8 (F (2, 20) = 14.87, p < .0001, the post hoc test p = .022), and Fz (F (2, 20) = 21.12, p < .0001, the post hoc test p = .050). Some of these recording sites are in the right hemisphere. In experiment 2, sham smoking produced an increase in dominant alpha in F4 (F (2, 20) = 18.42, p < .0001, post hoc test p = 0.49) and Fz (F (2, 20) = 21.11, p < .0001, post hoc test p = 0.41).
Fig 1.
Effects of sham smoking on dominant EEG alpha frequency.
In this and the subsequent figures, the scalp electrode placements are shown on the x-axis and the mean ± SE dominant alpha frequency (in Hz) on the y-axis. A. Data obtained from experiment 1. B. Data obtained from experiment 2. In this and subsequent figures: *p <.05, **p<.01, ***p<.001 indicated the significant alpha level that is produced by the two way ANOVA with repeated measures. Note that sham smoking increases selective right hemisphere neocortical dominant alpha frequencies. Slide file numbers are listed in the lower right hand corner.
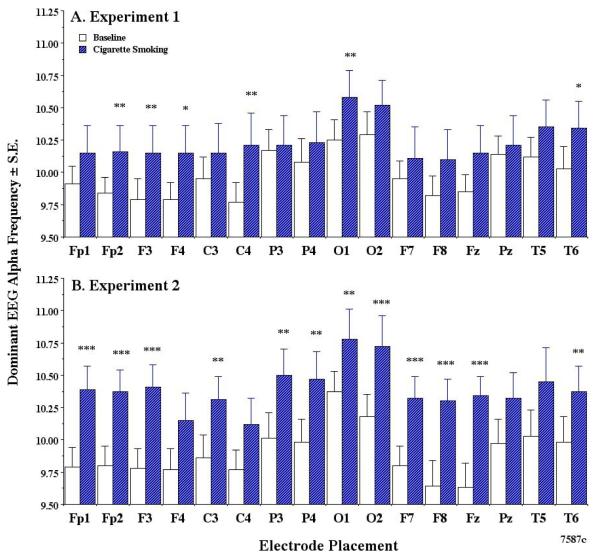
3.3. Effects of tobacco smoking on dominant alpha frequency
The effects of smoking each volunteer’s favorite cigarettes are illustrated in Fig. 2. There was a much greater increase in the dominant alpha frequency in many neocortical brain areas. In experiment 1, cigarette smoking produced a large increase in electrodes Fp2 (F (2, 20) = 10.85, p < .001, post hoc test p < .001, F3 (F (2, 20) = 19.84, p < .001, post hoc test p = .005), F4 (F (2, 20) = 18.42, p < .001, post hoc test p = .015), C4 (F (2, 20) = 2.91, p > .05, but post hoc test p = .010), O1 (F (2, 20) = 7.04, p < .01, post hoc test p = .012), and T6 (F (2, 20) = 8.39, p < .01, post hoc test p = .051).
Fig 2.
Effects of smoking ones favorite brand cigarette on dominant EEG alpha frequency.
Note that tobacco smoking increases dominant alpha frequencies in most neocortical areas in contrast to sham air smoking.
In experiment 2, all electrode sites except F4, C4, P2, and T5 showed a significant increase (see Fig. 2). The magnitude and variability of the frequency shifts produced by sham and regular smoking are given in both figures as mean ± S.E. The two way ANOVA with repeated measures analysis also indicated that cigarette smoking produced more dominant alpha frequency changes in experiment 2 than in experiment 1. Interaction effects of cigarette smoking between experiments 1 and 2 were not quite statistically significant, although 14 of 16 cortical sites of experiment 2 showed an interaction. The major dominant alpha frequencies were obtained from the occipital brain sensors in both experiments 1 and 2, confirming what is well described in the literature.
In experiment 1, there were no significant correlations between Δ dominant alpha frequency and times from last smoking, nicotine content, and tar content. There were slight correlations between Δ dominant alpha frequency at frontal sites and daily consumption of cigarettes (F8; r= −0.280, p < 0.05), between Δ dominant alpha frequency at electrode O2 and Δ heart (r=0.422, p < 0.01, but not between Δ dominant alpha frequency at electrode O2 and estimated (Kadoya et al., 1994) Δ plasma nicotine (r=0.245, p < 0.1). In both experiments, there were no differences in the nicotine and tar content of cigarettes between smoking groups.
4. Discussion
Sham smoking slightly increased dominant alpha frequency in Fp2, Fz, F4, F8 sensors. In contrast, cigarette smoking increased dominant alpha EEG frequencies throughout many of the cerebral cortical sensors, indicating a more general effect. Dominant alpha waves are of particular interest in regard to their changes with brain activity.
Over the years, the role of Pavlovian (classical) and Skinnerian (operant instrumental) conditioning in tobacco smoking has been studied. After all, smoking a pack of 20 cigarettes per day for 365 days per year involves 7,300 reinforcing sessions per year multiplied by the number of years smoked. Clearly, tobacco smoking is an overlearned drug taking behavior. Bindra (1974), and Robinson and Berridge (1993, 2000, 2001, 2003, 2008) have proposed an incentive salience theory involving Pavlovian contingencies between specific stimuli and drug reinforcement. Incentive salience learning in human tobacco smoking is well documented by the data of Hogarth et al. (2003) as well as their review of the literature. The very act of mimicking smoking behavior by inhaling only environmental air is apparently a sufficient conditioned stimulus to have physiological consequences.
The present research findings regarding both heart rate and EEG alpha frequency are both unexpected and expected. The fact that the mean heart rate after sham smoking air did not change is expected. However, the slight but significant increase in the dominant alpha frequency in the right brain hemisphere (Fp2, Fz, F4, and F8) was not expected. The subjects obviously knew they were simply inhaling air during the sham pseudosmoking session. This is not a true placebo effect because it lacks a cognitive basis. It is probably is due to a conditioned brain response to motor smoking behavior such as holding the sham cigarette, sucking, and inhaling air. The EEG effects were obtained after sham smoking when the motor movements ceased. This finding was replicated a month plus later in experiment 2. The predominant parietal-occipital distribution of dominant alpha frequency is consistent with a large literature that alpha EEG abundance predominates in these brain regions. The data for cigarette smoking minus the baseline controls indicate that tobacco smoking increases the dominant alpha frequency in almost all scalp recording sites. Nicotinic cholinergic receptors are located in both the thalamus and cortex. Hence, one would predict that the thalamocortical generators of alpha activity projecting to different cortical sites would be altered, as described in this report.
The neurophysiological basis of the alpha rhythm, its possible source, and modulation in the thalamus have been described (Andersen and Andersson, 1968; Schreckenberger et al., 2004). Although the alpha rhythms have been well studied, their genesis is still unknown. Two hypotheses have been proposed. The first involves endogenous cortical or thalamic neurons that fire rhythmically to recruit additional partners. The second hypothesis is that synaptic coupling of various populations of neurons oscillate because of their time constants and nonlinear gain properties of the system. A limit cycle, chaos, or noise are involved in all brain rhythms, not only alpha. The present research measuring only the dominant alpha frequency in 16 channels with 20 electrodes spaced bilaterally on the scalp surface supports previous research on the existence of different neuronal generators. Whether the alpha frequency shifts are due to an increased number of neuronal generators is unknown. What has been demonstrated in this study are differences between conditions in which a greater number of scalp sensors recorded changes compared to sham smoking. Their brain source localizations have not been identified. Most of the neocortical areas were affected by real tobacco smoking to increase their peak alpha oscillating frequency or increase their abundance so as to be better detected. In contrast, sham inhalation of air produces relatively minor changes. Tobacco smoke must be affecting most of the alpha generators by stimulating nicotinic cholinergic receptors that, in turn, may release many different substances throughout the diffuse corticothalamic networks. It should be stressed that sham smoking air is very different from smoking a denicotinized versus an average nicotine tobacco cigarette. In the former, the smoker is aware that he is or she is inhaling only air, whereas in the latter the smoker is unsure whether denicotinized or nicotine cigarettes are smoked. After smoking denicotinized cigarettes, overnight abstinent smokers show greater increases in heart rate in contrast to the negligible blood levels of nicotine measured (Scott et al., 2007). Behavioral effects of smoking depend, in part, on the time interval or degree of abstinence since the last smoked cigarette as described by Fant et al. (1995) Schuh and Stitzer (1995). Robinson et al. (1992) found no EEG effects of smoking denicotinized cigarettes in overnight abstinent smokers. Similarly, Pickworth et al. (1989) found 3 hr abstinent smokers had no EEG effects after denicotinized cigarettes. Pickworth et al. (2003) subsequently compared the effects of average nicotine to denicotinized cigarettes in a spaced smoking schedule. In contrast to smoking average nicotine cigarettes which increased spectral edge EEG frequency, smoking denicotinized cigarettes decreased it. The EEG spectral edge refers to the highest frequency measured in the entire spectral range. Hasenfratz et al. (1993) raised the question whether the tar content of denicotinized cigarettes was responsible for some of the effects. An important difference in results is that the present study used a 0.2 Hz measure of a shift in the dominant alpha frequency.
Humans normally show EEG brain asymmetry (both abundance and frequency) related to individual differences in emotional responses. Functional asymmetry in mentally normal persons also occurs with emotional interventions (Davidson et al., 1990, 1999, 2000, 2002; Sobotka et al., 1992; Sutton and Davidson, 1997; Sutton et al., 1997). It is also present in persons with anxiety and depressed mood (Bruder et al., 1997; Davidson, 2004; Arranus and Pavlov, 2005; Blackhart et al., 2006). Tobacco smokers have EEG hemispheric asymmetry in relation to depressed mood and its normalization by nicotine/tobacco smoking (Gilbert et al., 1994, 1999). The present study indicates that just mimicking smoking behavior causes a slight predominant right hemisphere increase in dominant alpha frequency. Artifactual lateralization may be a factor, but this appears to be unlikely.
Arousal/sedation components to the EEG effects of tobacco smoking have been described by Ashton et al. (1973) and Golding and Mangan (1982a,b). The EEG analysis herein did not separate tobacco smokers into different types. Instead, it is concerned only with the regional brain generators of the dominant alpha EEG frequency with awake smokers with eyes closed. After sham smoking, some right frontal neocortical alpha frequency sensors detected a higher frequency, whereas after tobacco smoking most neocortical alpha sensor frequency increased. Clearly, sham smoking behavior like gum chewing, pencil chewing, lip smacking, etc. involves physiological as well as psychological components.
The U.S. Surgeon General’s 1988 report emphasized that tobacco smoking involves both psychological as well as pharmacological components. Additional published studies have shown that smoking denicotinized cigarettes has significant psychological and physiological effects. Butschky et al. (1995) found that smoking without nicotine temporarily reduces withdrawal. Rose et al. (2000) showed that smoker craving for a cigarette is reduced after smoking a denicotinized cigarette. Individual smoker types, as described by Shikata et al. (1995a,b) are another factor that makes the relationship between tobacco smoking and many resulting EEG changes complicated. It is of note that Cook et al. (1995) reported that tobacco smoking resulted in greater alpha peak frequencies in men who were goal oriented.
This study has several limitations. The first is that only Japanese males were studied. Although a greater percentage of Japanese smokers are male, obviously females as well as different races must be studied. Another limitation is the potential for order effects. A counterbalanced design was not used. Counterbalancing could have confounded the sham condition in the design used. Subjects could have been tested on two consecutive days (one sham, one actual) in counterbalanced order. Inasmuch as the smokers were specifically requested to abstain from smoking for at least 1 hr prior to the experiment, this would require at least 1 hr and preferably much longer between the sham and favorite nicotine cigarette in a counterbalanced design. A future study using a counterbalanced design on separate days for sham and favorite cigarette smoking in male vs. females should be done. A factor not well controlled in this study was the large average period since the last cigarette (5.6 ± 10.9 hr). This suggests that some of the smokers were experiencing nicotine withdrawal at the time of the study. However, for some this was their usual time interval for smoking. Although the present results were statistically significant, variability could have also been reduced by controlling the manner of puffing and inhalation. Because sham smoking was a control for cigarette smoking, and the findings associated with sham smoking were less expected, there was no control condition for it including the psychological factors of one’s own non-smoked personal choice cigarette. Without such controls, it is unclear whether the observed results would occur with similar movements unassociated with smoking (e.g., deep inhalation alone) or in nonsmokers. Smoking behavior, CO, and heart rate were not measured at the second session. EEG alpha power measures would also have been very useful. However, in spite of these limitations, resting baseline measures compared to smoking conditions provided positive results.
Conclusions
Sham smoking by inhaling air increased the dominant EEG alpha frequency slightly more in the right brain hemisphere.
Smoking a favorite brand of tobacco cigarette significantly increased dominant alpha frequency more generally in both brain hemispheres, especially when replicated 1-2 months later.
The results in human tobacco smokers are consistent with known effects of nicotine on the brainstem activating system in animals to produce generalized cortical activations.
Acknowledgements
This research was supported in part by Japan Tobacco Inc., the University of Michigan Psychopharmacology Research Fund, the University of Michigan Education and Research Development Fund, and NIDA grant RO1 DA 018974-03. The authors do not envision any conflict of interest in conducting or reporting the results of this research sponsored in part by Japan Tobacco Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen P, Andersson SA. Physiological Basis of the Alpha Rhythm. Appleton-Century-Crofts; New York: 1968. [Google Scholar]
- Arranas L, Pavlov S. Trait anxiety impact on posterior activation asymmetries at rest and during evoked negative emotions: EEG investigation. Int, J. Psychiat. 2005;55:85–94. doi: 10.1016/j.ijpsycho.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Ashton H, Millman JE, Telford R, Thompson JW. Stimulant and depressant effects of cigarette smoking on brain activity in man. Brit. J. Pharmacol. 1973;48(4):715–717. doi: 10.1111/j.1476-5381.1973.tb08260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindra D. A motivational view of learning, performance, and behavior modification. Psychol. Rev. 1974;81:199–213. doi: 10.1037/h0036330. [DOI] [PubMed] [Google Scholar]
- Blackhart G, Minnex J, Kline J. Can EEG asymmetry patterns predict future development of anxiety and depression? A preliminary study. Biol. Psychol. 2006;72:46–50. doi: 10.1016/j.biopsycho.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Bruder G, Fong R, Tenke C, et al. Regional brain asymmetries in major depression with or without an anxiety disorder: A quantitative electroencephalographic study. Biol. Psychiat. 1997;41:939–948. doi: 10.1016/S0006-3223(96)00260-0. [DOI] [PubMed] [Google Scholar]
- Butschky MF, Bailey D, Henningfield JE, Pickworth WB. Smoking without nicotine delivery decreases withdrawal in 12-hour abstinent smokers. Pharmacol. Biochem. Behav. 1995;50:91–96. doi: 10.1016/0091-3057(94)00269-o. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiol. Behav. 2002;77:683–687. doi: 10.1016/s0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol. Biochem. Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Church RE. Smoking and the human EEG. In: Ney T, Gale A, editors. Smoking and Human Behavior. John Wiley and Sons Ltd; 1989. pp. 115–140. [Google Scholar]
- Cinciripini PM. The effects of smoking on electrocortical arousal in coronary prone (type A) and non-coronary prone (type B) subjects. Psychopharmacol. 1986;90:522–527. doi: 10.1007/BF00174072. [DOI] [PubMed] [Google Scholar]
- Cook MR, Gerkovich MM, Hoffman SJ, McClernon FJ, Cohen HD, Oakleaf KL, O’Connell FJ. Smoking and EEG power spectra: Effects of differences in arousal seeking. Int. J. Psychophysiol. 1995;19:247–256. doi: 10.1016/0167-8760(95)00016-l. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. What does the prefrontal cortex “do” in affect perspectives on frontal EEG asymmetry research. Biol. Psychol. 2004;67:219–233. doi: 10.1016/j.biopsycho.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Davidson R, Ekman P, Saron C, et al. Approach/withdrawal and cerebral asymmetry: Emotional expression and brain physiology. J. Person. Soc. Psychol. 1990;58:330–341. [PubMed] [Google Scholar]
- Davidson R, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn. Sci. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Davidson R, Jackson D, Kalin N. Emotion, plasticity, context and regulation: Perspectives from affective neuroscience. Psychol. Bull. 2000;126:890–906. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- Davidson R, Pizzagalli D, Nitschke J, et al. Depression: Perspectives from affective neuroscience. Ann. Rev. Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Domino EF, Kadoya C, Matsuoka S. Effects of tobacco smoking on the topographic electroencephalogram. In: Domino EF, editor. Brain Imaging of Nicotine and Tobacco smoking. NPP Books; Ann Arbor, MI: 1995. pp. 253–262. [Google Scholar]
- Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction. 2006;102:324–334. doi: 10.1111/j.1360-0443.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- Ernst M, Heishman SJ, Spurgeon L, London ED. Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacol. 2001;25:313–319. doi: 10.1016/S0893-133X(01)00257-3. [DOI] [PubMed] [Google Scholar]
- Fant RV, Schuh KJ, Stitzer ML. Response to smoking as a function of prior smoking amounts. Psychopharmacol. 1995;119:385–390. doi: 10.1007/BF02245853. [DOI] [PubMed] [Google Scholar]
- Foulds J, McSorley K, Sneddon J, Feyerabend C, Jarvis MJ, Russell MAH. Effect of subcutaneous nicotine injections on EEG alpha frequency in non-smokers: a placebo-controlled pilot study. Psychopharmacol. 1994;115:163–166. doi: 10.1007/BF02244767. [DOI] [PubMed] [Google Scholar]
- Foulds J, Stapleton J, Swettenham J, Bell N, McSorley K, Russell MA. Cognitive performance effects of subcutaneous nicotine in smokers and never-smokers. Psychopharmacol. 1996;127:31–38. doi: 10.1007/BF02805972. [DOI] [PubMed] [Google Scholar]
- Gilbert D, McClernon F, Rabinovich M, et al. EEG, physiology, and task-related mood fail to resolve across 31 days of smoking abstinence: Relations to depressive traits, nicotine exposure, and dependence. Exp. Clin. Psychopharmacol. 1999;7:427–443. doi: 10.1037//1064-1297.7.4.427. [DOI] [PubMed] [Google Scholar]
- Gilbert D, Meliska C, Wesler R, et al. Depression, personality, and gender influence EEG, cortisol, beta-endorphin, heart rate, and subjective responses to smoking multiple cigarettes. Person. Ind. Diffs. 1994;16:247–264. [Google Scholar]
- Golding JF. Effects of cigarette smoking on resting EEG, visual evoked potentials and photic driving. Pharmacol Biochem Behav. 1988;29:23–32. doi: 10.1016/0091-3057(88)90268-7. [DOI] [PubMed] [Google Scholar]
- Golding J, Mangan G. Effects of cigarette smoking on measures of arousal, response suppression and excitation/inhibition balance. Int. J. Addict. 1982a;17:793–804. doi: 10.3109/10826088209056327. [DOI] [PubMed] [Google Scholar]
- Golding J, Mangan G. Arousing and de-arousing effects of cigarette smoking under conditions of stress and mild sensory isolation. Psychophysiol. 1982b;19:449–456. doi: 10.1111/j.1469-8986.1982.tb02504.x. [DOI] [PubMed] [Google Scholar]
- Hasenfratz M, Baldinger B, Bättig K. Nicotine or tar titration in cigarette smoking behavior? Psychopharmacol. 1993;112:253–258. doi: 10.1007/BF02244919. [DOI] [PubMed] [Google Scholar]
- Hogarth L, Dickinson A, Duka T. Discriminative stimuli that control instrumental tobacco-seeking by human subjects also command selective attention. Psychopharmacol. 2003;168:435–445. doi: 10.1007/s00213-003-1456-4. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Slotkin TA, Westerveld M, Menci WE, Pugh KR. Visuospatial memory deficits emerging during nicotine withdrawal in adolescents with prenatal exposure to active maternal smoking. Neuropsychopharmacol. 2006;31:1550–1561. doi: 10.1038/sj.npp.1300981. [DOI] [PubMed] [Google Scholar]
- Kadoya C, Domino EF, Matsuoka S. Relationship of electroencephalographic and cardiovascular changes to plasma nicotine levels in tobacco smokers. Clin. Pharmacol. Ther. 1994;55:370–377. doi: 10.1038/clpt.1994.44. [DOI] [PubMed] [Google Scholar]
- Knapp DE, Domino EF. Action of nicotine on the ascending reticular activating system. Int. J. Neuropharmacol. 1962;1:333–351. [Google Scholar]
- Knott VJ. Dynamic EEG changes during cigarette smoking. Neuropsychobiol. 1988;19:54–60. doi: 10.1159/000118434. [DOI] [PubMed] [Google Scholar]
- Knott VJ. Brain electrical imaging: The dose response effects of cigarette smoking. Neuropharmacol. 1989a;22:236–242. doi: 10.1159/000118623. [DOI] [PubMed] [Google Scholar]
- Knott VJ. Effects of low-yield cigarettes on electroencephalographic dynamics. Neuropsychobiol. 1989b;21:216–222. doi: 10.1159/000118580. [DOI] [PubMed] [Google Scholar]
- Knott VJ. Electroencephalographic characterization of cigarette smoking behavior. Alcohol. 2001;24:95–97. doi: 10.1016/s0741-8329(00)00140-3. [DOI] [PubMed] [Google Scholar]
- Knott VJ, Bolton K, Heeman A, Shah D, Fisher DJ, Villeneuve C. Effects of acute nicotine on event-related potential and performance indices of auditory distraction in nonsmokers. Nic Tobac Res. 2009a;11(5):519–530. doi: 10.1093/ntr/ntp044. [DOI] [PubMed] [Google Scholar]
- Knott VJ, Shah D, Fisher D, Millar A, Prise S, Scott TL, Thompson M. Nicotine and attention: event-related potential investigators in nonsmokers. Clin EEG Neurosci. 2009b;40(1):11–20. doi: 10.1177/155005940904000108. [DOI] [PubMed] [Google Scholar]
- Knott VJ, Venables PR. EEG alpha correlates of non-smokers, smokers, smoking and smoking deprivation. Psychophysiol. 1977;14:153–156. doi: 10.1111/j.1469-8986.1977.tb03367.x. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA, Ffytche DH, Mitterschiffthaler MT, Das M, Zacariah E, et al. Cognitive effects of nicotine in humans. An fMRI study. NeuroImage. 2003;19:1002–1013. doi: 10.1016/s1053-8119(03)00110-1. [DOI] [PubMed] [Google Scholar]
- Ott A, Andersen K, Dewey ME, Letenneur L, Brayne C, Copland JRM, et al. Effect of smoking on global cognitive function in non-demented elderly. Neurol. 2004;62:920–924. doi: 10.1212/01.wnl.0000115110.35610.80. [DOI] [PubMed] [Google Scholar]
- Pickworth WB, Heishman SJ, Henningfield JE. Relationships between EEG and performance during nicotine withdrawal and administration. In: Domino EF, editor. Brain Imaging of Nicotine and Tobacco Smoking. NPP Books; Ann Arbor, MI: 1995. pp. 275–287. [Google Scholar]
- Pickworth WB, Herning RI, Henningfield JE. Electroencephalographic effects of nicotine chewing gum in humans. Pharmacol. Biochem. Behav. 1986;25:879–882. doi: 10.1016/0091-3057(86)90401-6. [DOI] [PubMed] [Google Scholar]
- Pickworth WB, Herning RI, Henningfield JE. Spontaneous EEG changes during tobacco abstinence and nicotine substitution in human volunteers. J. Pharmacol. Exper. Ther. 1989;251:976–982. [PubMed] [Google Scholar]
- Pickworth WB, O’Hare ED, Fant RV, Moolchan ET. EEG effects of conventional and denicotinized cigarettes in a spaced smoking paradigm. Brain Cogn. 2003;53:75–81. doi: 10.1016/s0278-2626(03)00205-7. [DOI] [PubMed] [Google Scholar]
- Pritchard WS, Robinson JH, Guy TD. Enhancement of continuous performance task reaction time by smoking in non-deprived smokers. Psychopharmacol. 1992;108:437–442. doi: 10.1007/BF02247417. [DOI] [PubMed] [Google Scholar]
- Reitz C, Luchsinger J, Tang M-X, Mayeux R. The effect of smoking and time on cognitive function in the elderly without dementia. Neurol. 2005;65:879–875. doi: 10.1212/01.wnl.0000176057.22827.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M, Jarvis MJ, Thompson N, Wadsworth MEJ. Cigarette smoking and cognitive decline in midlife: Evidence from a prospective birth cohort study. Amer. J. Pub. Hlth. 2003;93:994–998. doi: 10.2105/ajph.93.6.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JH, Pritchard WS, Davis RA. Psychopharmacological effects of smoking a cigarette with typical “tar” and carbon monoxide yields but minimal nicotine. Psychopharmacol. 1992;108:466–472. doi: 10.1007/BF02247423. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95:S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Ann. Rev. Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philos. Trans. R. Soc. B. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacol. 2006;184:274–285. doi: 10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Extinguishing the rewarding value of smoke cues: pharmacological and behavioral treatments. Nicotine Tob. Res. 2004;6:523–532. doi: 10.1080/14622200410001696501. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Bates JE, Salley A. Pharmacologic and sensorimotor components of satiation in cigarette smoking. Pharmacol. Biochem. Behav. 2003;76:243–250. doi: 10.1016/j.pbb.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Johnson M. Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacol. Biochem. Behav. 2000;67:71–81. doi: 10.1016/s0091-3057(00)00301-4. [DOI] [PubMed] [Google Scholar]
- Sakuri Y, Kanazawa I. Acute effects of cigarettes in non-deprived smokers on memory, calculation and executive functions. Human Psychopharmacol. Clin. Experiment. 2002;17:369–373. doi: 10.1002/hup.424. [DOI] [PubMed] [Google Scholar]
- Schreckenberger M, Lange-Asschenfeld C, Lochmann M, Mann K, Siessmeier T, Buchholtz H-G, Bartenstein P, Gründer G. The thalamus as the generator and modulator of EEG alpha rhythm: combined PET/EEG study with lorazepam challenge in humans. NeuroImage. 2004;22:637–644. doi: 10.1016/j.neuroimage.2004.01.047. [DOI] [PubMed] [Google Scholar]
- Schuh KJ, Stitzer ML. Desire to smoke during spaced smoking intervals. Psychopharmacol. 1995;120:289–295. doi: 10.1007/BF02311176. [DOI] [PubMed] [Google Scholar]
- Scott D, Domino EF, Heitzeg MM, Koeppe RA, Ni L, Guthrie S, Zubieta J-K. Smoking modulation of μ-opioid and dopamine D2 receptor mediated neurotransmission in humans. Neuropsychopharmacol. 2007;32:450–57. doi: 10.1038/sj.npp.1301238. [DOI] [PubMed] [Google Scholar]
- Shikata H, Fukai H, Ohya I, Sakaki T. Characterization of topographic EEG changes when smoking a cigarette. Psychopharmacol. 1995a;119:361–367. doi: 10.1007/BF02245850. [DOI] [PubMed] [Google Scholar]
- Shikata H, Fukai H, Sakaki T. Pattern recognition study in topographic EEG changes when smoking a cigarette. In: Domino EF, editor. Brain Imaging of Nicotine and Tobacco Smoking. NPP Books; Ann Arbor, MI: 1995b. pp. 235–252. [Google Scholar]
- Smith RC, Singh A, Infante M, Khandat A, Kloos A. Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacol. 2002;27:479–497. doi: 10.1016/S0893-133X(02)00324-X. [DOI] [PubMed] [Google Scholar]
- Sobotka S, Davidson R, Senulis J. Anterior brain electrical asymmetries in response to reward and punishment. EEG Clin. Neurophysiol. 1992;83:236–247. doi: 10.1016/0013-4694(92)90117-z. [DOI] [PubMed] [Google Scholar]
- Starr JM, Dreary IJ, Fox HC, Whalley LJ. Smoking in cognitive change from age 11 to 66 years: A confirmatory investigation. Addict. Behav. 2007;32:63–68. doi: 10.1016/j.addbeh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Sutton S, Davidson R, Donzella B, et al. Manipulating affective state using extended picture presentation. Psychophysiol. 1997;34:217–226. doi: 10.1111/j.1469-8986.1997.tb02135.x. [DOI] [PubMed] [Google Scholar]
- Sutton S, Davidson R. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychol. Sci. 1997;8:204–210. [Google Scholar]
- Swan GE, Lessov-Schlaggar CN. The effects of tobacco smoke and nicotine on cognition and brain. Neuropsychol. Rev. 2007;17:259–273. doi: 10.1007/s11065-007-9035-9. [DOI] [PubMed] [Google Scholar]
- Teneggi V, Squassante L, Milleri S, Polo A, Lanteri P, Ziviani L, Bye A. EEG power spectra and auditory P300 during free smoking and enforced smoking abstinence. Pharmacol Biochem Behav. 2004;77:103–9. doi: 10.1016/j.pbb.2003.10.002. [DOI] [PubMed] [Google Scholar]
- The Health Consequences of Smoking. Nicotine Addiction; 1988. U.S. Surgeon General Report; pp. 1–643. [Google Scholar]
- Ulett GA. Alterations in quantitative EEG and behavior during smoking deprivation. EEG Clin. Neurophysiol. 1969;27:658. doi: 10.1016/0013-4694(69)91224-3. [DOI] [PubMed] [Google Scholar]
- Ulett JA, Itil TM. Quantitative electroencephalogram in smoking and smoking deprivation. Science. 1969;164:969–970. doi: 10.1126/science.164.3882.969. [DOI] [PubMed] [Google Scholar]