Abstract
As the potential for bioterrorism has appeared to increase, the need for simple systems for identifying potential inhibitors of the binding of such agents to cell membranes has increased. In this work, surface plasmon resonance (SPR) was used to monitor binding of ricin, a ribosome inactivating protein, to the plasma membranes of NIH 3T3 cells. Once conditions were established, efficacy of the system for monitoring effectiveness of compounds at inhibiting ricin binding was ascertained by determining the IC50s for asialofetuin and for bovine serum albumin derivatized with an average of 34 lactosyl moieties (BSA-Lac34). Results indicated that SPR is an efficient method for measuring adherence of a toxin to isolated cell plasma membranes. SPR can also indicate whether a compound that is an effective inhibitor of binding when a single ligand such as ASF is used will be as effective when used in studies with cells that may express multiple cell surface ligands for ricin and/or the inhibitor.
Keywords: ricin, plasma membranes, surface plasmon resonance, asialofetuin, lactose-derivatized bovine serum albumin
Introduction
In recent years, the possibility of exposure to a biological agent has gone from being an obscure threat to military personnel stationed abroad, to a perceived threat to the public at large as well as to those using them for medical purposes. With the increased awareness of the possibility of exposure, interest in the development of methods to identify potential inhibitors of their binding to cells has escalated. This perceived need is periodically highlighted by news articles describing occurrences such as the finding of ricin in a Las Vegas motel room in February of 2007 [1] and the discovery, in November of 2004, of ricin-containing letters addressed to government officials [2]. Two things that make ricin difficult to deal with are the ease with which it can be isolated from castor beans [3], and the lack of an effective treatment for people exposed to the toxin.
In order for ricin to act on cells, it must first bind to terminal galactose moieties on cell surface glycoproteins and/or glycolipids. This binding is mediated by the binding subunit that has two defined binding sites about 70Å apart [4]. In previous work we found that when several galactose moieties were spaced appropriately far apart, ricin exhibited a greater binding affinity than when it bound to a single galactose moiety [5]. These observations agreed with the findings that 1) the individual affinities of ricin’s carbohydrate binding sites for lactose were 3.5×10−4M and 0.28×10−4M [6] and 2) ricin adhered to ASF ~1,000 times better than to monovalent galactose [7]. This coupled with the fact that HeLa cells have between 1–3 × 107 ricin binding sites per cell [8] supports the hypothesis that ricin might bind to the cell surface in an avidity driven manner. Therefore, monitoring the effectiveness of a potential inhibitor at blocking toxin adherence to sites presented on the cell surface, should provide a good indication of inhibitor efficacy. To test this, surface plasmon resonance (SPR) was used to monitor the effectiveness of two multivalent compounds at inhibiting adherence of ricin toxin to isolated plasma membranes. The results indicated that plasma membranes immobilized on the surface of a L1 sensor chip could be used for repetitive analyses of ricin binding and for monitoring the effectiveness of asialofetuin (ASF), an excellent ligand for ricin [8], and lactose-derivatized bovine serum albumin (BSA-Lac34) as inhibitors of that binding.
Materials and Methods
Materials
Ricin (RCAII, RCA60) having a molecular weight of 60,000 daltons, was purchased from Vector Laboratories (Burlingame, CA). Vector indicates that it is presumably Ricin D, a point supported by our observation that the amino acid sequence of the B chain obtained from Vector Laboratories was that of ricin D. 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphorylcholine (POPC) was obtained from Matreya (Pleasant Gap, PA). Polyvinylidene difluoride (PVDF) transfer membranes were purchased from Millipore (Bedford, MA). Anti-Na+/K+ATPase mouse monoclonal and prohibitin rabbit polyclonal antibodies were from Abcam (Cambridge, MA), anti-calreticulin mouse monoclonal antibody from Transduction Labs (San Jose, CA), anti-GAPDH mouse monoclonal antibody from Imgenex (San Diego, CA), and sheep anti-bovine asialofetuin IgG from AbD Serotec (Raleigh, NC). Dilutions of the primary antibodies were 1:5000 for the anti-Na+/K+ATPase, 1:400 for the anti-prohibitin, and 1:1000 for both the anti-GAPDH and anti-calreticulin antibodies. Horse radish peroxidase (HRP)-conjugated goat anti-mouse (1:2500) and HRP-conjugated donkey anti-sheep IgG (1:2000) were from Sigma (St. Louis, MO) while HRP-conjugated goat anti-rabbit IgG (1:2000) was from Zymed Laboratories, Inc. (San Francisco, CA). Protein standards were obtained from BioRad (Hercules, CA). NIH 3T3 cells were from the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, from Drs. Thomas D. Martin and Vineet N. KewalRamani [9]. Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum in an atmosphere of 5% CO2/95% air and 90% humidity. Bovine serum albumin (BSA), lactose, ASF, and all other reagents and materials were purchased from either Sigma Aldrich or VWR International. BSA derivatized with an average of approximately 34 lactose residues (BSA-Lac34) was prepared using reductive amination as described by Roy et al. [10] and characterized as previously described [5]. Surface plasmon resonance (SPR) was performed using a Biacore 3000 [Biacore, part of GE Healthcare (Uppsala, Sweden)]. HBS-EP buffer (10 mM HEPES, 150 mM NaCl, 3mM EDTA, and 0.005% surfactant P20, pH 7.4) was purchased from Biacore, or freshly prepared and degassed just prior to use. All other reagents used during the immobilization procedure as well as surfactant P20 were obtained from Biacore. PBS-P (10mM phosphate, 2.7mM KCl, 137mM NaCl, and 0.005% surfactant P20, pH 7.4) and PBS (prepared without surfactant P20) were freshly prepared, degassed, and used as both running and sample buffer for all toxin binding experiments.
Isolation of plasma membranes from cultured mammalian cells
Conditions for the reproducible isolation of plasma membranes from NIH 3T3 cells were adapted from those described by Graham and Sandall [11]. In brief, after cells were harvested by scraping near the end of the log phase of growth, they were exposed to 1.2M glycerol in 5mM Tris•HCl, pH7.4, containing 0.2mM MgCl2 prior to centrifugation. Cells were then taken up in 0.25M sucrose in the same Tris buffer and homogenized. After centrifugation to first remove unbroken cells and nuclei (600 × g, 10 min) and then to remove mitochondria, lysosomes, and peroxisomes (7500 × g, 10 min), a fraction enriched in plasma membranes was obtained by centrifuging the supernatant (100,000 × g, 60 min). To obtain further enrichment of plasma membranes, the pellet was taken up in 50% sucrose in 5mM Tris•HCl, pH 7.4, and overlaid with a step gradient of sucrose. After centrifugation (100,000 × g, 16 hr) samples recovered in the 20 and 30% sucrose fractions were combined, diluted with PBS and centrifuged (100,000 × g, 60 min) to pellet the plasma membranes. The pellet was taken up in PBS and aliquots analyzed for protein (Bio-Rad DC™ protein assay) and membrane markers, or used for surface plasmon resonance (SPR) experiments. To assay for membrane markers, proteins were separated by SDS-PAGE on 10% gels and blotted to PVDF membranes and western blot analyses used to visualize proteins associated with specific subcellular organelles. The results indicated the presence of the plasma membrane marker Na+/K+ ATPase in the plasma membrane fraction, a marked reduction in the ER marker calreticulin, and the absence of detectable prohibitin and GAPDH (mitochondrial and cytosolic markers, respectively) that were present in the whole cell homogenate (see Fig. 1). ASF was also not detected in the plasma membrane fraction (Fig. 2).
Figure 1.
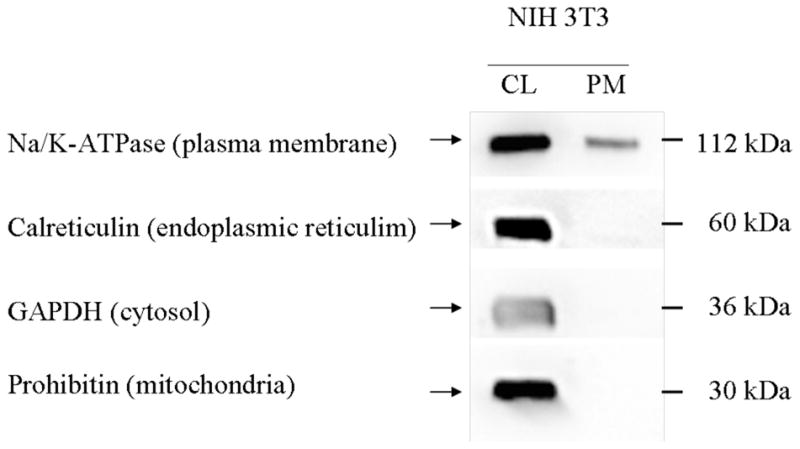
Distribution of subcellular markers. Cell lysates (CL, 15μg) and plasma membrane enriched fractions (PM, 5μg) were separated by SDS-PAGE on a 10% gel and transferred to a PVDF membrane. Subcellular markers were detected using specific antibodies and an HRP-conjugated secondary antibody. Bands were visualized using Super-Signal West Femto Chemiluminescent Substrate and exposure in the SynGene Gnome. CL, cell lysate, PM, plasma membrane fraction.
Figure 2.
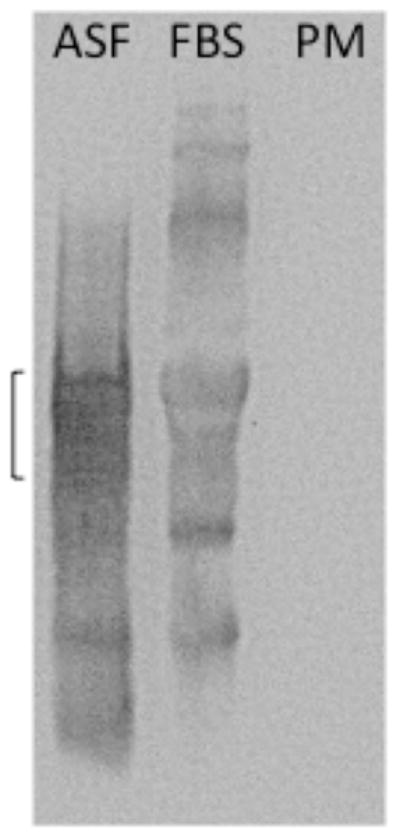
Absence of detectable levels of ASF in the plasma membrane fraction. ASF (10ug), FBS (20ul) and plama membrane proteins (PM, 5ug) were separated by SDS-PAGE on a 10% gel and transferred to a PVDF membrane. ASF was detected using a sheep anti-bovine ASF IgG polyclonal antibody (1:1000) and a HRP-conjugated donkey anti-sheep IgG secondary antibody (1:2000). Bands were visualized using Super-Signal West Pico Chemiluminescent Substrate and exposure in the SynGene Gnome. The bracket indicates the molecular weight region (~ 61kDa) in which ASF is found [17]. Because ASF is glycosylated it is not found as a tight band.
Immobilization of ligands on the surface of L1 and CM5 sensor chips
An L1 sensor chip was used for SPR experiments to monitor binding of toxin to immobilized plasma membranes. The surface of the L1 sensor chip is coated with dextran that is modified with alkyl chains to increase lipophilicity [12]. POPC liposomes, used as a negative control, were prepared as described previously [13]. Briefly, POPC was placed into a glass test tube and dried under nitrogen to form a thin film. The lipid film was hydrated with HBS for 20 min at 37°C, sonicated for 1 minute, and then extruded through a 100nm filter 21 times. Liposome preparations were used immediately after preparation.
For lipid binding experiments flow cell 1 was coated with POPC liposomes and used as a negative control, additional flow cells were coated with plasma membranes. Prior to coating the flow cells, contaminants were removed by washing flow cell surfaces with a 5 min injection of 20mM CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfon-ate) at a flow rate of 5μL/min. Plasma membranes isolated from NIH 3T3 cells were diluted with PBS to a protein concentration of 0.15 mg/mL and the solution sonicated in a bath sonicator for 1 min prior to immobilization. Depending upon the plasma membrane preparation used, a 55 minute injection (at a flow rate of 2μL/min) followed by a 20μL injection of 50mM NaOH (at a flow rate of 100μL/min) to remove loosely bound structures [BIAtechnology note 106] gave an increase of between 1000 and 5000 RUs. The flow cell was then washed for 2 minutes with PBS at a flow rate of 100μL/min prior to a 30 min injection of POPC liposomes (2μL/min) and a 5 min injection of 0.1mg/mL BSA (at a flow rate of 10μL/min) to block nonspecific binding sites.
When using SPR to monitor the binding of toxin to ASF, a CM5 sensor chip was used. Because the surface of the CM5 chip is coated with dextran containing carboxyl groups, functional groups on the ligand to be immobilized (i.e., -NH2) can be covalently coupled to the surface. ASF was covalently immobilized directly to the surface of the CM5 sensor chip using the amine coupling method provided with the control software. Throughout the immobilization procedure, a flow rate of 5μL/min was maintained using HBS-EP as the running buffer. The sensor chip surface was activated with a 7 min injection of a mixture (1:1 v/v) of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC, 11.5 mg/mL) and N-hydroxysuccinimide (NHS, 75 mg/mL). Short pulses of 10mM acetate buffer, pH 5.5, containing 50nM ASF were injected across the appropriate flow cell surfaces until approximately 300RUs of protein were immobilized. Any activated carboxyl groups remaining after protein immobilization were blocked using a 7 min (5μL/min) injection of 1M ethanolamine. Similar RU levels of BSA were immobilized on the reference flow cell using the amine coupling procedure described for immobilization of the ASF.
SPR analysis of toxin binding
Either PBS (for plasma membrane binding experiments) or PBS-P (for ASF binding experiments) was used as both the running and sample buffer in experiments to monitor binding of toxin to all flow cell surfaces. Toxin binding was monitored while ricin (500nM) was injected across the flow cell surface at a flow rate of 75μL/min. Toxin binding to lipid and protein surfaces was monitored for 2 min and 3 min, respectively. For inhibition experiments, serial dilutions of the potential inhibitor were prepared and added to 1,000nM ricin (1:1 by volume, to yield a final ricin concentration of 500nM) prior to injection, in a random order, over both a reference and test surface in parallel. Maximum binding by ricin alone was taken as 100%. After allowing ricin to dissociate by exposure to running buffer at a flow rate of 10μL/min. for 2.5 min, remaining ricin was removed using a 1 min injection of regeneration solution (200mM lactose, 1M NaCl) at a flow rate of 30μL/min. For plasma membrane surfaces, POPC alone was used as the reference surface, while BSA was use as the reference surface for ASF. To account for any non-specific binding, sensorgrams from the respective reference surface were subtracted from those obtained using the test surface. Percent of maximum binding was calculated for each concentration of potential inhibitor tested. Experiments for each sample were repeated at least three times and the mean and SEM calculated.
Results and Discussion
Isolation of plasma membranes
Western blot analyses (Fig. 1) indicated that the plasma membrane fraction obtained using the procedure described, had a barely detectable level of ER contamination and undetectable levels of cytosolic and mitochondrial contaminants. It also confirmed that the plasma membranes immobilized on the flow cells lacked detectable ASF (Fig. 2). It was necessary to show that ASF was not a detectable component of the plasma membrane fraction to ensure that binding observed was to cell surface components and not to contamination by a component found in fetal bovine serum. Isolation of plasma membranes from ~8 × 107 3T3 cells routinely provided more than a 100 μg of protein
Use of the L1 sensor chip for capturing plasma membranes
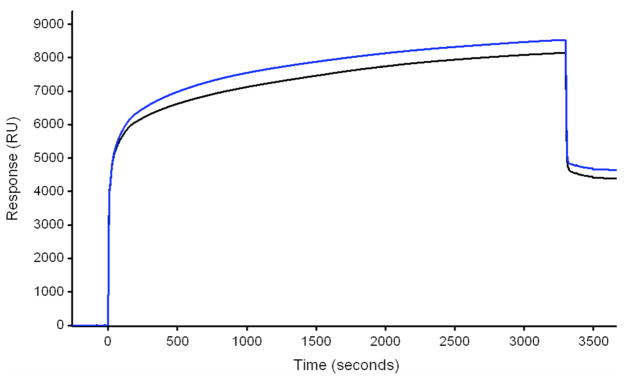
The approach used for immobilizing isolated plasma membranes on the surface of the L1 chip were those found to optimize the reproducibility of the RUs obtained. Initially, attempts to make uniformly sized liposomes through extrusion were unsuccessful as they clogged the 100nm filter. Therefore, small vesicles were formed by sonicating the membranes for one min in a bath sonicator and immediately captured on the L1 sensor chip surface (Fig. 3). In agreement with observations made by Klimka et al (14), we observed more adherence to flow cells by sonicated membrane preparations than to those not sonicated. Use of different membrane protein concentrations (21–0.021μg/mL) increased the number of RUs obtained at the end of the 55 min injection by 0.002% while a change from 5 to 590μg/mL resulted in a 3% increase in RUs at the end of a 45 min injection. These observations indicated that the concentration of plasma membranes used was not a major factor in their adherence to a L1 flow cell. While different plasma membrane preparations gave different amounts of adherence to the surface of the L1 sensor chip, repetitive use of the same preparation gave similar results.
Figure 3.
SPR sensorgrams obtained during the coating procedure of two different flow cells on a L1 chip with two different plasma membrane preparations. Note that the difference in base line from start to the end of the runs increased by 4400 (upper line) and 4600 (lower line) RUs. Protein concentration of the respective samples was 0.7 and 0.4 mg/mL.
Validation of reproducible toxin binding to immobilized plasma membranes using SPR
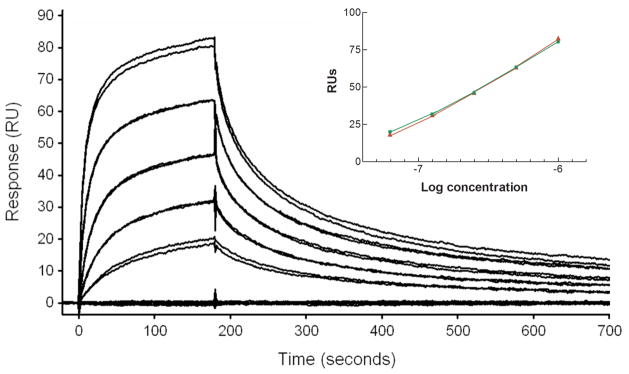
To validate the use of SPR for analysis of ricin binding to immobilized plasma membranes, a series of concentrations of ricin (1μM-62.5nM, 2-fold dilutions) were injected across the captured membrane surface. A dose response curve was generated by plotting the binding response (RUs) of each injection against the concentration of ricin toxin used (Fig. 4 inset). To confirm that the regeneration step did not adversely affect the binding capacity of the plasma membrane surface, each concentration of ricin was injected twice, and the order of the injections was randomized to avoid masking any drift in surface capacity. Visual inspection of the dose response curves (Fig. 4) reveals that ricin binding occurred in a dose dependent manner, and that variability between replicate injections of the same concentration was low.
Figure 4.
Dose-response curves demonstrating that replicate injections of various plasma membrane concentrations, randomly run, gave similar binding responses. Inset shows a plot of the replicate values obtained for each concentration of ricin used (1000, 500, 250, 125, and 62.5 nM). It can be seen that adherence of ricin to immobilized membranes did not change over time, or with repeated regeneration steps.
Inhibition of ricin binding to immobilized ASF and to plasma membranes
ASF has been used as a model for studying the binding of ricin to its cell surface receptors [15,16] so in this study its effectiveness at inhibiting the binding of ricin to both immobilized ASF and to immobilized plasma membranes was compared with that of BSA derivatized with an average of 34 lactosyl moieties (BSA-Lac34). The results (Fig. 5) indicated that the ASF was a more effective inhibitor of ricin binding to both immobilized ASF and plasma membranes. Analyses of inhibition of ricin binding to plasma membranes were carried out using the same surface for both inhibitors. Similarly, the same ASF surface was used to monitor the effectiveness of ASF and BSA-Lac34 at blocking ricin binding. While inhibition by BSA-Lac34 followed the same initial path for both ligands, inhibition by ASF of binding to plasma membranes differed (IC50=291nM) from its inhibition of binding to immobilized ASF (IC50=62.5nM). These observations may reflect the presence of more receptors on flow cells coated with plasma membranes, spacing of potential ligands on the membrane-coated flow cells, or the binding of terminal galactosyl moieties on ASF by galactose binding proteins on the membranes. Until more is known about the subtleties of ricin binding a conclusive reason cannot be given. In contrast to ASF, the much poorer inhibitor, BSA-Lac34, appeared to be equally weak at inhibiting ricin binding to both immobilized ASF and plasma membranes. Note that the IC50 for inhibition of each by BSA-Lac34 is/would be greater than the concentration of ASF needed to completely inhibit ricin adherence to either immobilized ASF or plasma membranes with its respective IC50s ~45-fold and >10-fold more than those for ASF.
Figure 5.
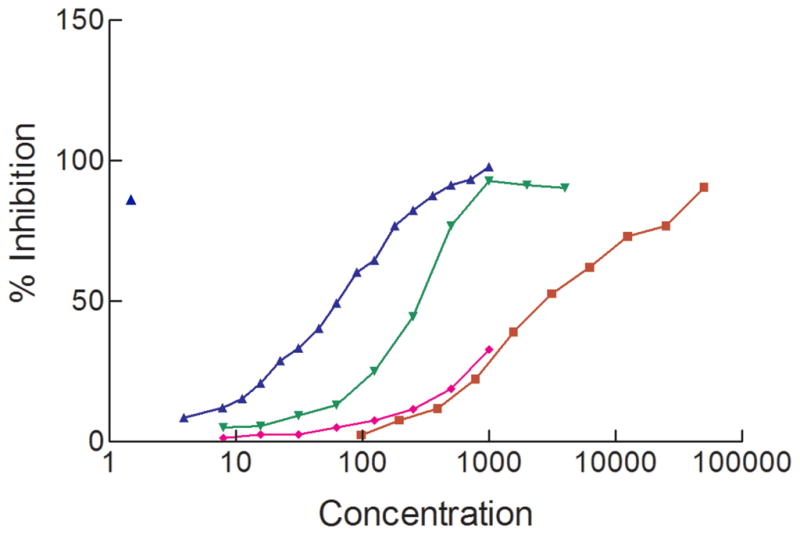
IC50s obtained for inhibition of ricin binding to either immobilized ASF or plasma membranes. Inhibition of ricin binding to immobilized ASF by ASF is indicated by the right side up triangles (blue) and to immobilized plasma membranes by the upside down triangles (green). Inhibition of ricin binding to immobilized ASF by BSA(Lac34) to immobilized plasma membranes is indicated by the diamonds (pink) and to immobilized ASF by the squares (red). Values obtained are an average of 3 or more replicate assays.
In conclusion, the results presented indicate that 1) reproducible observations can be obtained when a series of binding analyses are carried out using small concentrations of plasma membranes immobilized on an L1 chip, 2) an indication of the effectiveness of a single ligand for monitoring binding can be obtained by comparing those results to results obtained using immobilized plasma membranes that may express a number of different ligands, and 3) most importantly may indicate whether a compound that appears to be an effective inhibitor of binding using a single ligand such as ASF might be effective when used in studies with cells.
Acknowledgments
This work was supported in part by Public Health Service grant R21 AI71877 and a grant from the Pennsylvania Department of Health’s Health Research Formula Funding Program (State of Pennsylvania, Act 2001-77-PA Tobacco Settlement Legislation). The Department specifically disclaims responsibility for any analyses, interpretations, or conclusions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friess S. Man in critical condition in ricin case. New York Times; 2008. http://www.nytimes.com/2008/02/29/us/31cnd-ricin.html?hp. [Google Scholar]
- 2.Johnston D, Hulse C. Finding of ricin in office disrupts Senate. New York Times; 2004. http://www.nytimes.com/2004/02/04/national/04POIS.html? [Google Scholar]
- 3.Nicolson GI, Blaustein J. The interaction of Ricinus communis agglutinin with normal and tumor cell surfaces. Biochim Biophys Acta. 1972;266:543–547. doi: 10.1016/0005-2736(72)90109-5. [DOI] [PubMed] [Google Scholar]
- 4.Rutenber E, Robertus JD. Structure of ricin B-chain at 2.5 Å resolution. Proteins. 1991;10:260–269. doi: 10.1002/prot.340100310. [DOI] [PubMed] [Google Scholar]
- 5.Blome MC, Schengrund CL. Multivalent binding of ricin to bovine serum albumin based neoglycoconjugates. Toxicon. 2008;51:1214–1224. doi: 10.1016/j.toxicon.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Zentz C, Frenoy JP, Bourrillon R. Binding of galactose and lactose to ricin. Equilibrium studies. Biochim Biophys Acta. 1978;536:18–26. doi: 10.1016/0005-2795(78)90047-8. [DOI] [PubMed] [Google Scholar]
- 7.Baenziger JU, Fiete D. Structural determinants of Ricinus communis agglutinin and toxin specificity for oligosaccharides. J Biol Chem. 1979;254:99795–9799. [PubMed] [Google Scholar]
- 8.Sandvig K, Olsnes S, Pihl A. Kinetics of binding of the toxic lectins abrin and ricin to surface receptors of human cells. J Biol Chem. 1976;251:3977–3984. [PubMed] [Google Scholar]
- 9.Wu L, Martin TD, Vazeux R, Unutmaz D, KewalRamani VN. Functional evaluation of DC-SIGN monoclonal antibodies reveals DC-SIGN interactions with ICAM-3 do not promote human immunodeficiency virus type 1 transmission. J Virol. 2002;76:5905–5914. doi: 10.1128/JVI.76.12.5905-5914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy R, Katzenellenbogen E, Jennings HJ. Improved procedures for the conjugation of oligosaccharides to protein by reductive amination. Can J Biochem Cell Biol. 1984;62:270–275. doi: 10.1139/o84-037. [DOI] [PubMed] [Google Scholar]
- 11.Graham JM, Sandall JK. Tissue-culture cell fractionation. Fractionation of membranes from tissue-culture cells homogenized by glycerol-induced lysis. Biochem J. 1979;182:157–164. doi: 10.1042/bj1820157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper MA, Hansson A, Lofas S, Williams DH. A vesicle capture sensor chip for kinetic analysis of interactions with membrane-bound receptors. Anal Biochem. 2000;277:196–205. doi: 10.1006/abio.1999.4389. [DOI] [PubMed] [Google Scholar]
- 13.Yowler BC, Schengrund CL. Botulinum neurotoxin A changes conformation upon binding to ganglioside GT1b. Biochemistry. 2004;43:9725–9731. doi: 10.1021/bi0494673. [DOI] [PubMed] [Google Scholar]
- 14.Klimka A, Tur MK, Huhn M, Bierfreund U, Terrada E, Fischer R, Finnern R, Barth S. Measurement of antibody-membrane interactions by surface plasmon resonance. Int J Molec Med. 2004;14:765–768. [PubMed] [Google Scholar]
- 15.Dawson RM, Alderton MR, Wells D, Hartley PG. Monovalent and polyvalent carbohydrate inhibitors of ricin binding to a model of the cell-surface receptor. J Appl Toxicol. 2006;26:247–252. doi: 10.1002/jat.1136. [DOI] [PubMed] [Google Scholar]
- 16.Wu JH, Singh T, Herp A, Wu AM. Carbohydrate recognition factors of the lectin domains present in the Ricinus communis toxic protein (ricin) Biochimie. 2006;88:201–217. doi: 10.1016/j.biochi.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Chia JS, Chang LY, Shun CT, Chang YY, Chen JY. A 60 kilodalton immunodominant glycoprotein is essential for cell wall integrity and the maintenance of cell shape in Streptococcus mutans. Infect Immun. 2001;69:6987–6998. doi: 10.1128/IAI.69.11.6987-6998.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]