SUMMARY
Objectives
The relationship between community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) nasal colonization and subsequent infection in children is unknown. We sought to define risk factors for skin and soft tissue infection (SSTI) in community children.
Methods
A prior study measured S. aureus nasal colonization prevalence for 1300 community children. To detect subsequent SSTI in these children or a household member, surveys were administered 6 and 12 months following enrollment.
Results
SSTIs were reported by 56/708 (8.1%) respondents during the initial 6-month interval. SSTI developed in 6/26 (23%) initially colonized with MRSA, 16/194 (8%) with methicillin-sensitive S. aureus colonization, and 34/474 (7%) not colonized with S. aureus (MRSA vs. not MRSA, univariate analysis, p=0.014). In multivariable analysis, factors associated with SSTI included history of SSTI in the child during the year preceding enrollment (p<0.01) and SSTI in household contacts during the follow-up interval (p<0.01); MRSA nasal colonization approached statistical significance (p=0.08).
Conclusions
In the current era of community MRSA transmission, SSTI is a disease of households, with recurrences in index cases and occurrences among household contacts. Children with MRSA colonization may be at risk for subsequent SSTI. Further study of MRSA transmission dynamics in households and preventive strategies should receive high priority.
Keywords: Community-associated Staphylococcus aureus, Methicillin resistance, Practice-based research network, Skin and soft tissue infection
INTRODUCTION
Strains of methicillin-resistant Staphylococcus aureus that cause community-associated infections (CA-MRSA) have become widespread in many communities and are distinct from the MRSA strains associated with healthcare-related infections (1, 2). Although CA-MRSA strains are capable of causing severe, invasive infections, they most frequently cause skin and soft tissue infections (SSTI) (1–8).
The anterior nares represent the most common site of staphylococcal colonization (9, 10). S. aureus nasal carriage has previously been shown to be a risk factor for nosocomial staphylococcal infections, for infections in patients undergoing surgery or hemodialysis, and for skin infections in otherwise normal individuals in the adult population (10–13). More recently, increased rates of SSTI have been described among United States soldiers colonized with CA-MRSA strains (14). Several reports have documented increasing prevalence of MRSA nasal colonization in healthy children (15, 16) and rising rates of CA-MRSA SSTI in the pediatric population (1). However, to date, the relationship between CA-MRSA nasal colonization and subsequent SSTI has not been investigated in children.
We recently completed a prevalence study measuring S. aureus nasal colonization in 1300 children visiting pediatric primary care offices in the St. Louis metropolitan area (17). The rates of colonization with MRSA and methicillin-sensitive Staphylococcus aureus (MSSA) were 2.5% (95% confidence interval [CI] 1.7, 3.5) and 25.5% (95% CI 23.2, 27.9), respectively. Risk factors for MRSA nasal colonization included African-American race, enrollment in the Medicaid insurance program, household crowding (defined as greater than 2 people per bedroom per household), and contact with the healthcare system (e.g., recent emergency department visit, antibiotic use, or hospitalization; taking a daily medication; or living with someone working in a healthcare setting). SSTI during the year prior to study enrollment in the participant or a household member was not a risk factor for MRSA colonization (17). The objectives of the present longitudinal study were to measure the one-year incidence of SSTI in this cohort of children and their household members and to identify risk factors associated with the development of SSTI. Specifically, we investigated whether nasal colonization with CA-MRSA is associated with increased risk of SSTI, and whether household transmission contributes to the contemporary epidemiology of SSTI.
PATIENTS AND METHODS
Participant recruitment
From October 2005 to June 2006, 1300 children from birth to 18 years of age presenting for well or sick visits were enrolled from pediatric practices affiliated with the Washington University Pediatric and Adolescent Ambulatory Research Consortium, a practice-based research network of community pediatricians. Only one child per household was enrolled. Methods of participant enrollment, practice characteristics, and other aspects of study design have been described previously (17). Written, informed parental consent was obtained and written assent was provided by children of developmentally appropriate age (typically ≥7 years). This study was approved by the Washington University Human Research Protection Office.
Data collection and follow-up
At the time of initial enrollment, an anterior nasal swab was obtained. Participants were considered “colonized” if S. aureus (MRSA or MSSA) was recovered from the nasal swab. Additionally, a questionnaire was administered to identify epidemiologic risk factors associated with S. aureus nasal colonization. Participants were followed for one year to detect development of SSTI, of any cause, in the child or a household member. A household member was defined as anyone living in the household during the follow-up interval. During this period, investigators and study participants were blinded to each participant’s nasal colonization status. Follow-up surveys were administered by mail or telephone 6 and 12 months following study enrollment (see below) and inquired about interval development of skin abscesses, impetigo, cellulitis, and spider bites (because CA-MRSA abscesses are often mistaken for spider bites (8)). Conditions being sought were described in detail in both lay and medical language to maximize the capture and correct classification of each entity. In the final analysis, these 4 entities were combined as “SSTI” to account for any misclassified reports. Participants were also asked about the development of more severe infections, such as bone, joint, or bloodstream infections. The 6-month follow-up surveys were returned by the participants between April 2006 and March 2007 and the 12-month surveys were returned between October 2006 and October 2007.
Mode of survey administration
A 6-month follow-up survey was mailed to 1195 of the 1300 participants. A 12-month survey was sent only to families who returned the 6-month survey. Those who did not return the 6-month survey were considered lost to follow-up. At each time point, if the survey was not returned, a second survey was mailed 1–2 months after the first survey. As part of a nested natural history study (not reported here), 105 participants were contacted by telephone to administer 6 and 12-month follow-up surveys identical to the questionnaires mailed to the remaining 1195 participants. The questions were asked in a standardized manner; answers were documented per the respondent and no interpretation was made by the interviewer. This group included all of the participants colonized with MRSA (n=32) and matched participants with MSSA colonization (n=37) and not colonized with S. aureus (n=36) (selected on the basis of age group, pediatric practice, and date of enrollment).
Medical chart review
For each participant reporting an interval infection, the provider’s medical record was reviewed by study personnel and compared with participant reports. Data collected included the presence or absence of a recorded visit to the pediatrician or an emergency department for this infection, the physician’s diagnosis, whether or not a drainage procedure was performed, and, where available, culture results.
Statistical analysis
Confidence intervals for prevalence estimates were calculated with Confidence Interval Analysis version 2.1.2 (BMJ books, London) (18). The remaining statistical analyses were performed using SPSS for Windows 15.0 (SPSS, Chicago, IL). Initial nasal colonization status and epidemiologic risk factors identified in the original prevalence survey were compared between children who did and did not develop at least one interval SSTI using chi-square or Fisher’s exact test for categorical variables and Student’s t-test for continuous variables. Only participants returning surveys for both time intervals (0–6 months and 7–12 months) were evaluated in the 0–12 month analysis.
Multivariable analyses were performed by backward logistic regression. Variables included in the model were factors significant in the univariate analysis (SSTI in the child or household member in the year prior to study enrollment and interval SSTI in a household member during the follow-up study period) as well as factors thought a priori to be associated with skin infections (initial nasal colonization status, race, and household crowding [>2 people per bedroom]). Interactions were also tested in this model and included: race by household crowding; SSTI in the child in the year prior to enrollment by initial colonization status; and SSTI in the child in the year prior to enrollment by household crowding. A p-value of ≤ 0.05 was considered significant. All tests of significance were two tailed.
RESULTS
Survey response
Of the initial cohort of 1300 participants, 708 (55%) completed the 6-month survey (82% of those contacted by telephone and 52% of those contacted by mail, p<0.001). Of these, 557 (43% of the original 1300 participants) also completed the 12-month survey. Differences between responders and nonresponders to the 6-month survey are displayed in Table 1. These differences were similar for the 12-month survey, with the exception that the response rate at 12 months did not differ between children colonized at baseline with MRSA in comparison to those colonized with MSSA or not colonized with S. aureus.
Table 1.
Demographic Differences between Survey Responders and Non-Responders to the 6-month Follow-up Survey (n= 1300)
| Factor | Responders N (%) | Non-responders N, (%) | P Value |
|---|---|---|---|
| Gender | 0.47 | ||
| Male (n=671) | 372 (55.4) | 299 (44.6) | |
| Female (n=629) | 336 (53.4) | 293 (46.6) | |
| Age: Mean years (± SD) | 5.3 (± 5.1) | 5.6 (± 5.3) | 0.32 |
| Race | <0.001 | ||
| Caucasian (n=774) | 548 (70.8) | 226 (29.2) | |
| African-American (n=426) | 115 (27.2) | 311 (72.8) | |
| Other (n=95) | 42 (44.2) | 53 (55.8) | |
| Health insurance | <0.001 | ||
| Private (n=825) | 549 (66.5) | 276 (33.5) | |
| Medicaid or none (n=469) | 156 (33.3) | 313 (66.7) | |
| Initial colonization | <0.001 | ||
| MRSA (n=32) | 26 (81.2) | 6 (18.8) | |
| MSSA (n=331) | 201 (60.7) | 130 (39.3) | |
| Not colonized (n=937) | 481 (51.3) | 456 (48.7) | |
| Prior SSTI in participant (n=73) | 39 (53.4) | 34 (46.6) | 0.90 |
| Prior SSTI in household member (n=113) | 55 (48.7) | 58 (51.3) | 0.20 |
Entries in table represent percentage of variable in row header, unless otherwise indicated.
Interval SSTI rate, 0–6 months
During the 6-month follow-up interval, 56 participants (8.1%) reported the development of SSTI. These included 66 total episodes (abscess 38%, “spider bite” 24%, cellulitis 17%, and impetigo 21%) at a total of 76 body sites (head and neck 22%, upper extremity 14%, trunk 12%, lower extremity 20%, groin 4%, buttock 16%, and unspecified 12%). No respondents reported severe or invasive infections. During the 6-month interval, SSTI in a household member was reported by 59 participants (8.6%).
Risk factors for SSTI, univariate analysis, 0–6 months
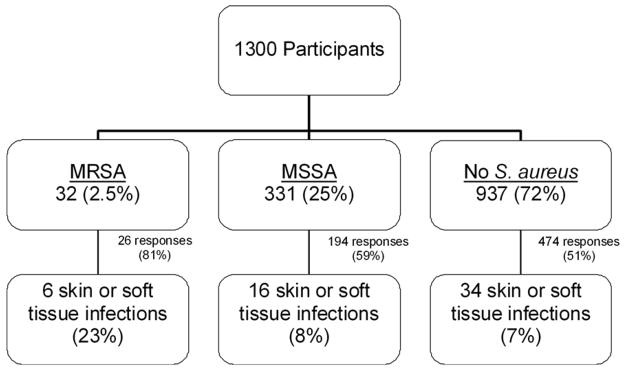
SSTI developed in 6 (23%) of 26 children initially colonized with MRSA, 16 (8%) of 194 children with MSSA colonization, and 34 (7%) of 474 children not colonized with S. aureus (Figure 1). Colonization with MRSA was associated with an increased risk of SSTI (OR 3.7, 95% CI 1.4–9.7) compared to those colonized with MSSA or not colonized with any form of S. aureus (collectively referred to as “Not MRSA”). A similar trend was apparent when the analysis was restricted only to participants who were administered the survey by telephone. In this group, 23% of those initially colonized with MRSA reported SSTI, compared to 6% of participants with initial MSSA-colonization and 11% of those not colonized with S. aureus (MRSA vs. Not MRSA: OR 3.3, 95% CI 0.9, 12.0). Baseline nasal colonization with MSSA was not a significant risk factor for the development of subsequent SSTI in comparison to children with baseline MRSA colonization or those not colonized with S. aureus.
Figure 1.
Initial nasal colonization status and 6-month incidence of skin and soft tissue infection. The incidence of SSTI in participants initially colonized with MRSA was significantly greater than rates of infection in participants colonized at baseline with MSSA or not colonized with S. aureus, p = 0.014.
Children who developed SSTI were older than those who did not (mean age 6.6 ± 5.2 y vs. 5.2 ± 5.0 y, p=0.04). SSTI in the year prior to enrollment in the participant (OR 7.4, 95% CI 3.5, 15.5) or a household member (OR 3.6, 95% CI 1.7, 7.5) were also statistically significant risk factors for SSTI in the study participant. Children with a household member who developed SSTI during the 6-month follow-up interval were also at increased risk for SSTI (OR 9.3, 95% CI 4.9, 17.7). Other factors evaluated in the univariate analysis for SSTI during the 6-month interval are shown in Table 2. The development of SSTI in a household member during the 0–6 month follow-up interval was associated with baseline MRSA nasal colonization in the child participant (OR 5.3, 95% CI 2.2, 12.9).
Table 2.
Significant Risk Factors in Univariate Analysis for Development of a Skin or Soft Tissue Infection in the Child Participant during the 0–6 Month Follow-up Interval (n= 708)
| Risk Factor | Participants with Risk Factor Who Developed Skin Infection N, (%) | Participants without Risk Factor Who Developed Skin Infection N, (%) | Odds Ratio (95% CI) |
|---|---|---|---|
| MRSA nasal colonizationa | 6 (23.1) | 50 (7.5) | 3.7 (1.4, 9.7) |
| Male gender | 35 (9.6) | 21 (6.4) | 1.5 (0.9, 2.7) |
| African-American raceb | 15 (12.9) | 41 (7.1) | 1.9 (1.0, 3.6) |
| Medicaid Insurance | 17 (11) | 39 (7.3) | 1.6 (0.9, 2.9) |
| Household crowding (more than 2 people per bedroom) | 3 (13) | 49 (7.9) | 1.8 (0.5, 6.1) |
| Skin infection in year prior to enrollment | 13 (33.3) | 40 (6.3) | 7.4 (3.5, 15.5) |
| Household member with skin infection in year prior to enrollment | 11 (20.8) | 42 (6.7) | 3.6 (1.7, 7.5) |
| Skin infection in a household member during follow-up interval | 20 (35.1) | 34 (5.5) | 9.3 (4.9, 17.7) |
| Chronic health problemc | 35 (11.2) | 21 (5.7) | 2.1 (1.2, 3.7) |
| Antibiotic use in year prior to enrollment | 34 (9.8) | 19 (6.1) | 1.7 (0.9, 3.0) |
| Sports participation (in children ≥ 4 years of age) | 24 (10.1) | 10 (11.0) | 0.9 (0.4, 2.0) |
| Bathes less than once a day | 26 (6.5) | 30 (10.3) | 0.6 (0.4, 1.1) |
| Nail biter | 24 (16.0) | 32 (5.9) | 3.0 (1.7, 5.3) |
MRSA, methicillin-resistant Staphylococcus aureus; CI, confidence interval. Other than “Skin infection in a household member during the follow-up interval,” all factors were identified at the time of study enrollment.
Comparing MRSA colonization vs. MSSA colonization and no S. aureus colonization.
Race was self-reported.
Chronic health problems included in the questionnaire were asthma, allergies, eczema, seizure disorder, heart disease, cancer, diabetes, sickle cell disease, cystic fibrosis, and ear tubes.
Factors included in the questionnaire but not significantly associated with SSTI and not listed in the table included: MSSA nasal colonization, taking a daily medication, hospitalization or surgery in the past year, emergency department or urgent care visit in the prior 6 months, history of a systemic infection, immunodeficiency, pet ownership, frequency of changing bed linens, healthcare or corrections worker in household, recently visiting a patient in a hospital or nursing home, recently visiting a prison inmate or having contact with someone released from prison in the previous year, daycare or school attendance, breastfeeding, thumb-sucking, pacifier use, piercings, tattoos, shaving, waxing, using alcohol-based hand sanitizers, sharing a bed, bath, face cloth or bath towel, and receiving an antibiotic during the follow-up interval.
Multivariable analysis, 0–6 months
Factors independently associated with SSTI during the initial 6-month interval were history of SSTI in the child during the year prior to study enrollment (OR 5.3, 95% CI 2.2, 13.1) and interval SSTI in a household member over the 6-month period (OR 7.6, 95% CI 3.6, 16.4). Nasal MRSA colonization approached significance for risk of SSTI (OR 2.8, 95% CI 0.9, 8.6) (Table 3).
Table 3.
Multivariate Analyses of Factors Associated with Skin and Soft Tissue Infection in the Child Participant
| 0–6 Month Intervala (n= 595) | 0–12 Month Intervalb (n= 448) | |||
|---|---|---|---|---|
| Risk Factor | aOR (95% CI) | P Value | aOR (95% CI) | P Value |
| Skin infection in a household member during the follow-up interval | 7.6 (3.6, 16.4) | <0.001 | 5.3 (2.5, 11.0) | <0.001 |
| Skin infection in child during year prior to enrollment | 5.3 (2.2, 13.1) | <0.001 | 4.1 (1.5, 11.0) | 0.005 |
| MRSA nasal colonization | 2.8 (0.9, 8.6) | 0.076 | 3.0 (0.9, 9.4) | 0.063 |
aOR, adjusted odds ratio; CI, confidence interval. None of the interactions included in the model were significant in the multivariable analysis.
Model Parameters: model chi square = 47.1, p <0.001; −2 log likelihood = 276.8; Hosmer and Lemeshow test significance = 0.81.
Model Parameters: model chi square = 41.7, p <0.001; −2 log likelihood = 250.5; Hosmer and Lemeshow test significance = 0.86.
Interval SSTI rate, 0–12 months
Participants returning both the 6 and 12-month surveys and completing the questions about SSTI development (n=535) were evaluated. Of these, there were a total of 72 children reporting at least one SSTI over the 12-month study period; 56 children reported SSTI during the 0–6 month interval and 25 reported SSTI during the 7–12 month interval. Nine children reported at least one SSTI during both intervals. Children who developed SSTI during the first 6-month interval were at significantly increased risk for SSTI during the second (7–12 month) interval (OR 9.3, 95% CI 3.8, 23.0).
Risk factors for SSTI, univariate analysis, 0–12 months
During the overall 12-month study period, SSTI developed in 7 (31.8%) of 22 children initially colonized with MRSA, 14 (9.9%) of 142 children with MSSA colonization, and 33 (8.9%) of 370 children not colonized with S. aureus at the time of study enrollment (MRSA vs. Not MRSA: OR 4.6, 95% CI 1.8, 11.9). Age did not differ between children who developed SSTI and those without infection (mean age 5.9 ± 5.4 y vs. 5.1 ± 4.9 y, p=0.25). SSTI in the year prior to enrollment in the participant (OR 4.2, 95% CI 1.8, 9.7) or a household member (OR 2.9, 95% CI 1.2, 6.8), as well as SSTI in a household member during the 12-month follow-up interval (OR 6.4, 95% CI 3.4, 12.2), were significant risk factors for SSTI in the study participant. Other factors significant for SSTI over the 12-month study period in the univariate analysis included a chronic health problem (specific conditions are listed in the footnote of Table 2) in the participant (OR 1.8, 95% CI 1.0, 3.2) and African-American race (OR 3.1, 95% CI 1.5, 6.4).
Multivariable analysis, 0–12 months
Factors independently associated with SSTI included history of SSTI in the child during the year prior to study enrollment (OR 4.1, 95% CI 1.5, 11.0) and an interval skin infection over the 12-month period in a household member (OR 5.3, 95% CI 2.5, 11.0). The increased risk for subsequent SSTI conferred by nasal MRSA colonization approached statistical significance (OR 3.0, 95% CI 0.9, 9.4) (Table 3).
Medical chart review
A total of 66 episodes of SSTI were reported over the first 6-month follow-up period by 56 participants. Of these, 25 episodes (38%) had records at the pediatrician’s office documenting the episode. For 22 of the 25 SSTI episodes (88%), the physician’s diagnosis corroborated the patient’s report. Participants who reported SSTI but did not have a record of the episode in their medical record at the primary pediatrician’s office either did not seek medical care or may have sought care at an emergency department or urgent care center but the record was not present in the pediatrician’s chart.
S. aureus molecular risk factors for SSTI
In the original prevalence study of MRSA nasal colonization, the MRSA isolates underwent molecular typing analysis including presence of genes encoding Panton-Valentine leukocidin (PVL, a toxin associated with severe SSTI and necrotizing pneumonia (3, 19)) and typing of the staphylococcal cassette chromosome mec (SCCmec) (17). In the longitudinal cohort, there was not a significant difference in incidence of SSTI between children colonized with PVL-positive strains (3 SSTIs out of 11 children carrying PVL-positive MRSA strains) and those with PVL-negative strains (3 SSTIs out of 15 children carrying PVL-negative MRSA strains) (p=1.00). The rate of SSTI in participants colonized with SCCmec IV strains (classified as CA-MRSA) (5 SSTIs out of 15 children with type IV strains) was higher than the rate in those colonized with SCCmec II strains (classified as healthcare-associated MRSA) (0 SSTIs out of 9 children with type II strains), but this difference did not reach statistical significance (p=0.12).
DISCUSSION
This is the first longitudinal, community-based study to track the development of SSTI in children with MRSA and MSSA nasal carriage. Among this large pediatric cohort, this study identified important risk factors that are important for devising interventions to reduce future infections. History of participant SSTI (of any cause) prior to study enrollment and living with a household member who experienced a SSTI (of any cause) during the follow-up interval posed an increased risk of SSTI in the study participant. The picture that emerges is consistent with the clinical observation that CA-MRSA is a disease of households, characterized by recurrences in index cases and in household members (8, 20–25). This is likely because the transmission of infection is a dynamic phenomenon among household members likely due to frequent person-to-person contact, contamination of household surfaces, and sharing of personal hygiene items.
This study is the first to quantify the risk of SSTI in children with CA-MRSA nasal colonization. Over a 6-month period, 23% (95% CI 11.0, 42.1) of participants colonized with MRSA in the baseline prevalence study developed SSTI, a 3-fold higher burden than experienced by participants with MSSA or those without S. aureus nasal colonization. In contrast, the rate of SSTI in those with MSSA colonization did not differ from that of participants who were not colonized with S. aureus. Interestingly, no invasive infections related to MRSA occurred in participants during the study period. The positive association between baseline MRSA nasal colonization and subsequent SSTI was also seen in the multivariable analysis (OR 2.8), although when adjusting for race, household crowding, previous SSTI, and SSTI in a household member, the 95% confidence limits on the odds ratio included 1.0 (0.9–8.6). Consistent with the findings of household transmission of SSTI, not only were children with MRSA nasal colonization themselves at increased risk for subsequent SSTI, but their household members were also more likely to develop SSTI during the follow-up interval.
While univariate analysis identified baseline MRSA colonization as a significant risk factor for subsequent SSTI in the study participant, it was of borderline significance in multivariate analysis. There are several factors that may have weakened the relationship between MRSA nasal colonization and subsequent SSTI. Although baseline colonization status was defined in a large number of children, the proportion of participants with MRSA nasal colonization was relatively small (2.5%), thereby limiting the statistical power of the study. It is also likely that a single culture of the nares is insensitive for defining MRSA colonization status, as some S. aureus carriers are intermittently colonized (10). Finally, CA-MRSA isolates may colonize other body sites in the absence of colonization of the anterior nares. For example, specimens from the groin or axilla have revealed MRSA colonization in some individuals with negative nares cultures (26, 27). In the present study, children colonized at sites other than the nose would not have been categorized as “colonized” and the association between MRSA colonization and infection would have been underestimated. Thus, we suggest that MRSA colonization is a risk factor for subsequent SSTI, but in this study colonization status was inadequately defined by a single nasal culture.
Participants of African-American race and Medicaid enrollees were more likely to be colonized with MRSA in our prevalence study (17) and were significantly less likely to respond to the follow-up surveys. If bias were introduced by this difference, it may have weakened the association between MRSA nasal colonization and risk of SSTI. Another bias may be related to the fact that participants who developed interval SSTI were more likely to return surveys compared to participants without SSTI. This would have led us to overestimate the incidence of SSTI in our study population. However, since there was no difference in the rate of survey return among participants with a personal or household history of SSTI prior to study enrollment, compared with those without a history of prior SSTI, this bias was unlikely to affect our results.
During the follow-up period, we relied on participants and their families to report the occurrence of skin and soft tissue infection. The accuracy of self-reporting SSTI has not been verified. However, the fact that most reports from participants who sought medical care for their skin condition were corroborated by the child’s medical record at the pediatrician’s office is reassuring. Although we do not have culture data from the participants with infection, it is likely that many of the SSTI were caused by MRSA, since current epidemiology supports that the majority (>80%) of community-acquired skin abscesses in our community are caused by MRSA (28). Finally, there is no reason to suspect that subjects with MRSA baseline colonization would be more likely to falsely report SSTI than subjects with baseline MSSA colonization or subjects whose nares cultures were negative for staphylococci.
Response rates for the 6-month survey were higher among participants initially colonized with MRSA, likely due to the fact that all MRSA-colonized participants (compared to only a portion of subjects not initially colonized with MRSA) were contacted by telephone as part of a nested study. However, we would not expect the method of survey administration to affect the reported infection rate, especially because the telephone surveys were conducted in a standardized manner without interviewer interpretation. Participants and investigators were blinded to initial colonization status, eliminating the possibility of ascertainment bias resulting from a tendency to over-report events in those initially colonized with MRSA. Most importantly, in the nested study in which all participants were contacted by telephone, the increased risk of SSTI in participants initially colonized with MRSA in comparison to those colonized with MSSA and those initially not colonized with S. aureus were very similar to the increased risk found in the entire study population.
The present study suggests that children colonized with MRSA may be an important reservoir and source for transmission of skin and soft tissue infections in the household. While intrafamilial carriage and exchange are likely important components of persistence of CA- MRSA in the community, further study of these transmission dynamics is needed. In addition, in demonstrating a risk of SSTI in children colonized with MRSA, we provide a rationale for studies evaluating interventions designed to reduce risk of recurrence. Our study supports the concept that preventive interventions, such as decolonization, might best be directed at the household unit, rather than only at the index case.
Acknowledgments
This research was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award 5 T32 HD007507 from the National Institute of Child Health and Human Development. In addition, this publication was made possible by Grant Number UL1 RR024992 and K30 RR022251 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
We appreciate the participation of the children and their families to make this study possible. We thank the Washington University Pediatric and Adolescent Ambulatory Research Consortium physicians and staff for allowing us to enroll patients in their offices: Blue Fish Pediatrics, Children’s Clinic, Crystal City Pediatrics, Esse Health – Creve Coeur, Esse Health – Webster Groves, Grace Hill – Soulard, Healthcare for Kids, Northwest Pediatrics, St. Louis Pediatric Practitioners, Southwest Pediatrics, and Tots thru Teens Pediatrics. We also thank Victoria Fraser, M.D., David Hunstad, M.D., Michael DeBaun, M.D., M.P.H., and Alexis Elward, M.D., M.P.H. for their thoughtful reviews of this manuscript. We appreciate the assistance of Yu Tao, M.D., M.S. with our statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaplan SL, Hulten KG, Gonzalez BE, Hammerman WA, Lamberth L, Versalovic J, et al. Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin Infect Dis. 2005;40:1785–1791. doi: 10.1086/430312. [DOI] [PubMed] [Google Scholar]
- 2.Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, Etienne J, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290:2976–2984. doi: 10.1001/jama.290.22.2976. [DOI] [PubMed] [Google Scholar]
- 3.Lina G, Piemont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–1132. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez BE, Hulten KG, Dishop MK, Lamberth LB, Hammerman WA, Mason EO, Jr, et al. Pulmonary manifestations in children with invasive community-acquired Staphylococcus aureus infection. Clin Infect Dis. 2005;41:583–590. doi: 10.1086/432475. [DOI] [PubMed] [Google Scholar]
- 5.Pannaraj PS, Hulten KG, Gonzalez BE, Mason EO, Jr, Kaplan SL. Infective pyomyositis and myositis in children in the era of community-acquired, methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2006;43:953–960. doi: 10.1086/507637. [DOI] [PubMed] [Google Scholar]
- 6.Herold BC, Immergluck LC, Maranan MC, Lauderdale DS, Gaskin RE, Boyle-Vavra S, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593–598. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 7.Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como-Sabetti K, Jernigan JA, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436–1444. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- 8.Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 9.Williams REO. Healthy carriage of Staphylococcus aureus: its prevalence and importance. Bacteriol Rev. 1963;27:56–71. doi: 10.1128/br.27.1.56-71.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505–520. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group N Engl J Med. 2001;344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 12.Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 13.Toshkova K, Annemuller C, Akineden O, Lammler C. The significance of nasal carriage of Staphylococcus aureus as risk factor for human skin infections. FEMS Microbiol Lett. 2001;202:17–24. doi: 10.1111/j.1574-6968.2001.tb10774.x. [DOI] [PubMed] [Google Scholar]
- 14.Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis. 2004;39:971–979. doi: 10.1086/423965. [DOI] [PubMed] [Google Scholar]
- 15.Creech CB, 2nd, Kernodle DS, Alsentzer A, Wilson C, Edwards KM. Increasing rates of nasal carriage of methicillin-resistant Staphylococcus aureus in healthy children. Pediatr Infect Dis J. 2005;24:617–621. doi: 10.1097/01.inf.0000168746.62226.a4. [DOI] [PubMed] [Google Scholar]
- 16.Gorwitz RJ, Kruszon-Moran D, McAllister SK, McQuillan G, McDougal LK, Fosheim GE, et al. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J Infect Dis. 2008;197:1226–1234. doi: 10.1086/533494. [DOI] [PubMed] [Google Scholar]
- 17.Fritz SA, Garbutt J, Elward A, Shannon W, Storch GA. Prevalence of and risk factors for community-acquired methicillin-resistant and methicillin-sensitive Staphylococcus aureus colonization in children seen in a practice-based research network. Pediatrics. 2008;121:1090–1098. doi: 10.1542/peds.2007-2104. [DOI] [PubMed] [Google Scholar]
- 18.Altman DG, Machin D, Bryant TN, Gardner MJ. Statistics with Confidence. 2. BMJ Books; 2000. pp. 45–47. [Google Scholar]
- 19.Gillet Y, Issartel B, Vanhems P, Fournet JC, Lina G, Bes M, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359:753–759. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan SL. Community-acquired methicillin-resistant Staphylococcus aureus infections in children. Semin Pediatr Infect Dis. 2006;17:113–119. doi: 10.1053/j.spid.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Dietrich DW, Auld DB, Mermel LA. Community-acquired methicillin-resistant Staphylococcus aureus in southern New England children. Pediatrics. 2004;113:e347–352. doi: 10.1542/peds.113.4.e347. [DOI] [PubMed] [Google Scholar]
- 22.Johansson PJ, Gustafsson EB, Ringberg H. High prevalence of MRSA in household contacts. Scand J Infect Dis. 2007;39:764–768. doi: 10.1080/00365540701302501. [DOI] [PubMed] [Google Scholar]
- 23.Huijsdens XW, van Santen-Verheuvel MG, Spalburg E, Heck ME, Pluister GN, Eijkelkamp BA, et al. Multiple cases of familial transmission of community-acquired methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2006;44:2994–6. doi: 10.1128/JCM.00846-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones TF, Creech CB, Erwin P, Baird SG, Woron AM, Schaffner W. Family outbreaks of invasive community-associated methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2006;42:e76–78. doi: 10.1086/503265. [DOI] [PubMed] [Google Scholar]
- 25.Faden H, Ferguson S. Community-acquired methicillin-resistant Staphylococcus aureus and intrafamily spread of pustular disease. Pediatr Infect Dis J. 2001;20:554–5. doi: 10.1097/00006454-200105000-00023. [DOI] [PubMed] [Google Scholar]
- 26.Fritz SA, Fritz JM, Mitchell K, Storch GA, Orscheln RC, Wessman B, et al. Sites of community-acquired Staphylococcus aureus colonization in patients presenting with skin and soft tissue infections. Infectious Diseases Society of America 46th Annual Meeting; Washington, D.C. 2008. [Google Scholar]
- 27.Yang ES, Tan J, Rieg G, Miller LG. Body site colonization prevalence in patients with community-associated methicillin-resistant Staphylococcus aureus infections. Infectious Diseases Society of America 45th Annual Meeting; San Diego, CA. 2007. [DOI] [PubMed] [Google Scholar]
- 28.Orscheln RC, Hunstad DA, Fritz SA, Loughman JA, Mitchell K, Storch EK, et al. Contribution of genetically restricted, methicillin-susceptible strains to the ongoing epidemic of community-acquired Staphylococcus aureus infections. Clin Infect Dis. 2009;49:536–42. doi: 10.1086/600881. [DOI] [PMC free article] [PubMed] [Google Scholar]