Abstract
Coat Protein I (COPI) is one of the most intensely investigated coat complexes. Numerous studies have contributed to a general understanding of how coat proteins act to initiate intracellular vesicular transport. This review highlights key recent findings that have shaped our current understanding of how COPI vesicles are formed.
Keywords: ARF1, ARFGAP1, BARS, coatomer, COPI, GBF1
Introduction
COPI was originally discovered through an in vitro reconstitution system that sought to study vesicular transport among the Golgi stacks. Vesicles reconstituted from this approach were found to have a novel coating [1]. The initial components identified within this coating were found to exist as a seven-subunit (α, β, β', γ, δ, ε, ζ) complex [2–6], also known as coatomer [7]. Despite having been intensely investigated, the precise transport roles of COPI vesicles are still being debated, as summarized by a recent review [8]. The current review will focus instead on our understanding of how COPI vesicles are formed. It should also be noted that another recent review has detailed key controversies in the evolving understanding of COPI vesicle formation [9]. As such, the current review is meant to complement this previous review by covering a broader scope of recent advancements.
ADP-Ribosylation Factor 1 (ARF1) is a key small GTPase that regulates COPI vesicle formation by recruiting coatomer onto Golgi membrane to initiate its coat function [10]. Early reconstitution studies used pharmacologic inhibitors to gain insight into how the GTPase cycle of ARF1 regulates coatomer during vesicle biogenesis. Brefeldin-A (BFA), which inhibited ARF1 activation, was found to prevent the recruitment of coatomer onto Golgi membrane [11,12]. On the other hand, a non-hydrolyzable form of GTP (GTPγS) that inhibited ARF1 deactivation resulted in coatomer being locked on vesicular membrane, which led to the prediction that ARF1 deactivation would trigger the release of coatomer from membrane for vesicle uncoating [13]. Thus, these early observations led to a simple model for how ARF1 regulates coat function. In particular, the membrane distribution of the COPI complex would be directly coupled to the GTPase cycle of ARF1, such that ARF1 activation recruits the COPI complex onto membranes, while ARF1 deactivation causes the release of the coat complex to the cytosol.
As a small GTPase, activation of ARF1 is predicted to be catalyzed by a guanine nucleotide exchange factor (GEF), while its deactivation is predicted to be catalyzed by a GTPase-activating protein (GAP). Thus, another key prediction from the early observations is that at least a GEF and a GAP would also participate in COPI vesicle biogenesis. The identification of these ARF1 regulators and the subsequent characterization of they act have led the way in revealing the complexity of COPI vesicle formation. As detailed below, additional factors have been identified and unanticipated mechanisms have been uncovered.
The GAP
The first ARF1 regulator identified was the GAP, which was named ARFGAP1 (ADP-ribosylation factor GTPase activating protein 1) [14]. Early studies suggested that its GAP catalytic activity was regulated by interactions with coatomer and cargo proteins [15–17]. At the time, this regulation was interpreted to modulate the role of the GAP activity in COPI vesicle uncoating. Subsequently, this role has been reassessed when ARFGAP1 became available as a recombinant protein for further scrutiny by the COPI vesicle reconstitution system. When GTP, rather than GTPγS, was used to allow GAP activity to be manifested, ARFGAP1 was found to behave as a coat component [18,19], rather than as an uncoating factor as previously predicted [13]. It is also notable that Sec23p, the GAP that acts on the small GTPase Sar1p in COPII transport, had already been shown to act as a key component of the COPII complex [20]. Thus, GAPs that act on ARF-related small GTPases appear to behave in fundamentally similar ways, acting not only as negative regulators of the GTPase cycle, but also as key effectors by being coat components.
The newly appreciated role of ARFGAP1 has also helped to resolve a seeming mechanistic dilemma stemming from another key insight that the GAP activity is regulated by membrane curvature [21]. Positive curvature dominates during the early (budding) phase of vesicle formation, when a bud begins to emerge from the planar surface of compartmental membrane. In contrast, negative curvature dominates during the late (fission) phase of vesicle formation, at the bud neck where constriction occurs prior to the bud being released as a vesicle [22,23]. As the GAP activity of ARFGAP1 was found to be enhanced by positive membrane curvature [21], how the coat could be maintained on the budding membrane (when positive membrane curvature predominates) in the face of increasing GAP activity seemed enigmatic, if one were to assume that this activity triggered membrane uncoating. In contrast, if the GAP activity promotes vesicle formation rather than vesicle uncoating, then the regulation GAP activity by membrane curvature could be more readily reconciled with events of membrane coating that are needed for the early phase of vesicle formation.
It should be further noted that this revised role of how the GAP activity acts during COPI vesicle biogenesis is predicted to work in conjunction with other mechanisms known to promote the role of the COPI complex in vesicle formation. In particular, coatomer has been elucidated to interact with both protein and lipid components on the vesicular membrane [24–27]. Moreover, ARFGAP1 has been shown to possess functions other than the catalytic activity, including interactions with coatomer and cargo proteins that contribute to coat function [19]. As such, the GAP activity represents just one, albeit a critical, component of multiple mechanisms that dictate how the COPI complex acts in vesicle biogenesis.
Nevertheless, a role for ARFGAP1 in promoting COPI vesicle formation has been questioned by other studies. In contrast to the use of Golgi membrane, when a minimal membrane system (using purified lipids to generate liposomes) was used, the GAP activity was observed to trigger the release of coatomer from membrane association [21,28]. Likely reconciling explanations for this apparent contradictory result have been discussed in detail in a recent review [9]. Thus, they will only be summarized briefly here. Factors that exist on Golgi membrane but not on the liposomal membrane suggest multiple potential reconciling explanations, which need not be mutually exclusive. They include cargo proteins and lipids needed to stabilize the membrane recruitment of ARFGAP1, and additional proteins found recently to be critical for COPI vesicle formation, some of which are likely to promote the coat function of ARFGAP1 (such as BARS and endophilin B, which will be discussed further below).
Other recent studies have sought to understand the roles of ARFGAP2 and ARFGAP3, which exhibit significant sequence similarity to ARFGAP1. Like ARFGAP1, these other ARFGAPs also interact with coatomer [29,30]. Moreover, they can be detected on COPI vesicles reconstituted using either Golgi membrane [29] or liposomes [31]. However, further scrutiny of these other ARFGAPs has revealed functional differences as compared to ARFGAP1. Whereas membrane curvature plays a major role in regulating the GAP activity of ARFGAP1 [21], this mode of regulation appears to be less relevant for the GAP activity of ARFGAP2 and ARFGAP3. Instead, their interaction with coatomer plays the more significant role in regulating their GAP activity [30,31]. Further detailing this role, studies on ARFGAP3 reveals that it possesses distinct domains that interact with coatomer versus membrane. A region within a central portion of ARFGAP3 that contains a stretch of basic residues mediates its binding to coatomer, while a region near the carboxy terminus that contains amphipathic residues allows its binding to target membrane [30]. In light of these noted differences between ARFGAP1 and its close cousins, a future goal will be to elucidate how they may contribute to potentially distinct roles of different ARFGAPs in Golgi transport pathways.
It should also be noted that in the context of the minimal liposome-based membrane system, ARFGAP2 and ARFGAP3 behave similarly as ARFGAP1 in triggering the release of coatomer from such membrane [31]. However, as discussed above, the situation is likely to be more complex on Golgi membrane. Thus, it will be informative in the future to further scrutinize their potential role in vesicle formation versus uncoating, using a reconstitution system that contains native membrane.
The GEF
In contrast to the GAP, the initial identification of a GEF predicted to act in COPI vesicle formation has not been straightforward. Taking advantage of an ARF1 mutation predicted to stabilize interactions with the GEF, a two-hybrid screen first identified yeast GEFs (Gea1p and Gea2p) that acted on ARF1 [32]. On the other hand, the initially identified mammalian GEF, known as ARNO (ARF nucleotide site opener), did not exhibit sensitivity to BFA [33], as would be predicted for the relevant GEF that acts in COPI transport. Accumulated evidence now suggests that this GEF is GBF1 (Golgi-specific brefeldin A resistant guanine nucleotide exchange factor 1). However, evidence for its role has also led a tortuous path.
As its name suggests, the initial characterization of GBF1, based primarily on an in vitro GEF assay, led to the conclusion that it was insensitive to BFA [34]. However, further scrutiny through in vivo approaches suggested that GBF1 was in fact a BFA-sensitive GEF, and likely involved in regulating key components of COPI transport [35–37]. Recently, GBF1 has been discovered to interact with coatomer. Notably, this interaction was found to be independent of ARF1 activation [38]. Thus, it has been proposed that coatomer helps to dictate the Golgi localization of GBF1 to initiate COPI transport. As such, this proposed role further illustrates the complex mechanistic relationship between ARF regulators and effectors.
Fission proteins
The utility of the COPI vesicle reconstitution system has also been demonstrated in recent years with the identification of novel factors critical for COPI vesicle formation. When Golgi membrane was washed more stringently to release peripheral membrane proteins, the purified proteins used at the time (ARF1, coatomer and ARFGAP1) were found to be no longer sufficient in reconstituting COPI vesicles. Fractionation of the salt wash led to the identification of Brefeldin-A ADP-Ribosylated Substrate (BARS) as another critical component. Insight into how BARS acts in vesicle formation came from studies on mutant forms that induced the accumulation of buds in blocking COPI vesicle formation. Thus, BARS was concluded to be critical for the fission step of COPI vesicle formation [39].
Further characterization of how BARS acts in this role has suggested an explanation for a long-standing observation that has been puzzling. In an early study, palmitoyl-coenzyme A (p-CoA) was found to be critical for the fission step of COPI vesicle formation [40]. How p-CoA is required for this process has been unclear. As such lipid moieties have often been associated with protein acylation, one speculation has been that certain acylated proteins are needed for COPI vesicle fission. However, the elucidation of how BARS acts in COPI vesicle fission has revealed an unexpected mechanism. This role of BARS was found to be regulated by its binding to cofactors. When nicotinamide adenine dinucleotide (NAD) was bound to BARS, BARS was prevented from interacting with ARFGAP1 and participating in COPI vesicle fission. In contrast, when p-coA was bound to BARS, its interaction with ARFGAP1 was enhanced, which allowed its participation in vesicle fission. Thus, one role of p-CoA in COPI vesicle fission involved its binding to BARS [39], which was distinctly different from an assumed mechanism of protein acylation.
The newly elucidated role for BARS in COPI vesicle fission also suggested apparent contradictions. As background, earlier studies had found that BFA not only inhibited the GEF activity that activates ARF1 [11,12], but also activated an endogenous ADP-ribosylation reaction [41]. A prominent target was a 50 kd protein, which led the protein being named “Brefeldin-A ADP-Ribosylated Substrate” [42]. Upon its molecular identification, it became evident that BARS belonged to a family of proteins previously characterized as transcription co-repressors, which had been named C-terminal Binding Proteins (CtBPs) [42]. CtBP1 and CtBP2 are encoded by two distinct genes. BARS appears to be a splice variant of CtBP1, and hence it is also known as CtBP3 [42].
A perplexing issue arose from the observation that when all CtBP genes in mice were deleted, even though animal lethality resulted, viable cells could be generated [43]. Thus, as coatomer is critical for cell viability [44], why would a critical role for BARS in COPI vesicle formation still allow viable cells? It turned out that BARS acted mechanistically interchangeable with another factor for vesicle fission, which was subsequently identified to be endophilin B. As a further surprising twist, the use of endophilin B did not simply reflect developmental plasticity forced by the deletion of BARS. Instead, some cells derived from normal adult animals were found to use BARS, while other used endophilin B, for COPI vesicle fission [45]. Thus, unanticipated mechanistic flexibility was revealed for COPI vesicle formation.
The saga regarding BARS subsequently further deepened. An early study found that BARS could induce the fission of Golgi membrane [46]. This capability was linked to an acyltransferase activity detected in the recombinant protein, which catalyzed the conversion of lysophosphatidic acid to phosphatidic acid (PA). However, it was subsequently discovered that the acyltransferase activity was not intrinsic to BARS [47]. Moreover, this activity was not required for the role of BARS in COPI vesicle fission [39]. Thus, a key mechanistic question became: how could BARS induce membrane fission? Recently, this mystery was resolved by the finding that BARS can directly deform membrane [48].
Critical lipids
Further characterization of this newly discovered membrane-deforming ability of BARS has led to the identification of another critical component needed for COPI vesicle formation. It turns out that BARS could only induce membrane deformation in the presence of phosphatidic acid (PA). The role of PA in this case was not simply acting as a membrane platform to recruit BARS. Rather, PA was found to be an active participant that cooperated with BARS to “bend” membrane. Further studies revealed that PA generated specifically by phospholipase D type 2 (PLD2) was critical for a late stage of COPI vesicle fission, when constricted bud necks must undergo scission to release buds as vesicles from Golgi membrane [48].
Diacylglycerol (DAG) is another critical lipid recently discovered for COPI vesicle formation. Using pharmacologic inhibitors to target PA phosphohydrolases, which catalyzed the conversion of PA to DAG, a recent study found that such inhibition induced the accumulation of Golgi buds and released ARFGAP1 from Golgi membrane. Thus, DAG was concluded to recruit ARFGAP1 to act in COPI vesicle fission [49]. However, using similar pharmacologic inhibition, a later study concluded that DAG is critical for a different mechanistic step of COPI vesicle formation. Because the formation of Golgi buds was suppressed altogether, DAG was suggested to act in early (budding), rather than late (fission), vesicle formation. Furthermore, knocking down ARFGAP1 was found to induce the accumulation of Golgi buds. As such, it was further concluded that the critical role of DAG in early vesicle budding could not be attributed to the recruitment of ARFGAP1 to Golgi membrane [50]. How can the different conclusions on the role of DAG be reconciled? One potential explanation can be that, despite using similar pharmacologic inhibitors, different dosage has been used. Thus, different molecular targets may have been preferentially affected. As such, the future identification of specific molecular entities and their characterization seem likely to resolve this current uncertainty regarding the role of DAG.
Re-visiting ARF1 and coatomer
ARF1 is known to be post-translationally modified by myristoylation at its amino terminus. This myristoyl chain is predicted to be sequestered when ARF1 is in its inactive (GDP-bound) form, and exposed when ARF1 is activated to its GTP-bound form by the GEF activity. Moreover, when exposed, the myristoyl chain is predicted to stabilize membrane association of ARF1 by inserting into target membrane [51]. However, the details of how this acyl chain undergoes conformational changes during ARF1 activation could not be scrutinized for many years. Attempt to gain structural insight by x-ray crystallography was stymied, because this region of the ARF1 was too flexible. The recent pursuit of nuclear magnetic resonance has overcome this hurdle. Notably, the sequestered myristate in the inactive form of ARF1 was suggested to have a role in regulating the ability of the GEF to activate ARF1 [52]. Thus, the myristoyl chain of ARF1 acts in more complex ways than previously imagined
Besides in regulating the recruitment coatomer, ARF1 has been suggested recently to have another role in COPI vesicle formation. An initial clue came from studies in COPII transport, in which the small GTPase Sar1p regulates the recruitment of the COPII complex to the ER membrane in initiating COPII vesicle formation [20]. In addition to this role, recent studies have found that Sar1p has the intrinsic ability to deform membrane. When incubated with liposomes, Sar1p was found to constrict this membrane to produce tubular extensions. Further characterization suggested that this behavior was likely critical for the fission step of COPII vesicle formation [53,54]. When ARF1 was examined similarly, it was also noted to have an intrinsic ability to induce the tubulation of liposomes [55–57]. Thus, ARF1 may also have an unexpected role in COPI vesicle fission.
There has also been a refinement in our understanding of how coatomer deforms membrane. Liposomes have been used to study how lipids organize themselves into two phases, liquid-ordered and -disordered states. Further expanding on such characterization, coatomer has been shown recently to assemble onto the liquid-disordered domain of liposomes. Moreover, its ability of deform such liposomes is limited by membrane tension. Thus, besides specific lipid moieties, the organization of membrane domains at the Golgi is also suggested to modulate the formation of COPI vesicles [58].
Notably however, whereas studies have found ARF1 to possess an intrinsic ability to deform membrane [55–57], ARF1 did not appear to possess this ability in the study that sought to examine how liquid phases that generates membrane tension contribute to COPI vesicle biogenesis [58]. Rather, membrane deformation in this study was shown to require both ARF1 and coatomer [58]. As all studies have used liposomes, a reconciling explanation is likely to come from a closer inspection of specific lipid species used to generate liposomal membrane. Such future studies may reveal that ARF1 requires a particular set of lipids for its ability to induce membrane deformation.
Perspective
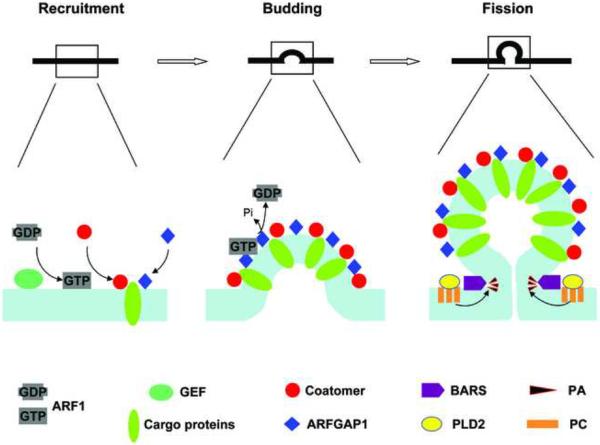
Mechanisms of COPI vesicle formation is now appreciated to be more complex than originally envisioned, both with respect to new factors identified and novel mechanisms elucidated. Besides ARF1 and coatomer, additional proteins critical for COPI vesicle formation include GBF1, ARFGAP1, and BARS (or endophilin B depending on the cell type). Vesicle formation also requires two critical lipids, PA and DAG. The identification of these additional key components have resulted in a more complex appreciation for how COPI vesicle formation is achieved (see Figure 1 for a working model).
Figure 1.
Key components acting in COPI vesicle formation. For the initial step that involves the recruitment of key factors, GBF1 activates ARF1 and this process is likely to be facilitated by additional interactions with coatomer and cargo proteins. Subsequently, coatomer and ARFGAP1 act as coat components to drive both the early (budding) and late (fission) stage of vesicle formation. Their roles involve interaction with cargo proteins, which also achieves cargo sorting and thereby coupling vesicle formation with cargo sorting. For the fission stage, BARS in cooperation with PA are needed additionally. Not shown is the role of DAG, because its precise function in COPI vesicle formation remains to be resolved.
Given the recent advancements that have led to multiple unexpected insights, it seems likely that further revision of this model will occur in the future. Along this line, key questions have arisen in light of recent findings. For instance, as the GAP activity catalyzes the deactivation of ARF1, it is not immediately obvious how this activity could explain why the GAP activity promotes COPI cargo sorting. Moreover, a role for the GAP activity in COPI vesicle formation predicts that other factor(s) would be involved in vesicle uncoating. Thus, a future goal will be to identify such predicted factor(s). It is also notable that PA can be generated through multiple distinct enzymatic activities. Thus, as BARS requires PA for membrane fission, and PA generated by PLD2 only acts at the late stage of vesicle fission, could other ways of generating PA be involved in promoting the early stage of COPI vesicle fission?
The proven utility of the COPI vesicle reconstitution system predicts that it will continue to spearhead future mechanistic discoveries. However, depending on how this reconstitution system is devised, for instance using minimal (liposomes) versus complex (Golgi) membrane, it is also apparent that the behavior of some key factors (such as the GAP) can be altered. Thus, an additional future goal will be generate a membrane system that is simpler than Golgi membrane but more sophisticated than the liposomes devised thus far, so that physiologic mechanisms can be further deduced.
Acknowledgements
This work is funded by the National Institutes of Health (GM058615). We apologize that all primary work could not be cited due to space limitation.
Glossary
Abbreviations
- ARF
ADP-Ribosylation Factor
- ARFGAP
ADP-ribosylation factor GTPase activating protein
- BARS
Brefeldin-A ADP-Ribosylated Substrate
- BFA
brefeldin A
- COP
Coat Protein
- CtBP
C-terminal Binding Protein
- DAG
diacylglycerol
- GAP
GTPase-activating protein
- GBF1
Golgi-specific brefeldin A resistant guanine nucleotide exchange factor 1
- GEF
guanine nucleotide exchange factor
- GTP
guanosine-5'-triphosphate
- NAD
nicotinamide adenine dinucleotide
- PA
phosphatidic acid
- p-CoA
palmitoyl coenzyme A
- PLD2
phospholipase D type 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Orci L, Glick BS, Rothman JE. A new type of coated vesicular carrier that appears not to contain clathrin: its possible role in protein transport within the Golgi stack. Cell. 1986;46:171–184. doi: 10.1016/0092-8674(86)90734-8. [DOI] [PubMed] [Google Scholar]
- [2].Malhotra V, Serafini T, Orci L, Shepherd JC, Rothman JE. Purification of a novel class of coated vesicles mediating biosynthetic protein transport through the Golgi stack. Cell. 1989;58:329–336. doi: 10.1016/0092-8674(89)90847-7. [DOI] [PubMed] [Google Scholar]
- [3].Serafini T, Stenbeck G, Brecht A, Lottspeich F, Orci L, Rothman JE, Wieland FT. A coat subunit of Golgi-derived non-clathrin-coated vesicles with homology to the clathrin-coated vesicle coat protein beta-adaptin. Nature. 1991;349:215–20. doi: 10.1038/349215a0. [DOI] [PubMed] [Google Scholar]
- [4].Duden R, Griffiths G, Frank R, Argos P, Kreis TE. Beta-COP, a 110 kd protein associated with non-clathrin-coated vesicles and the Golgi complex, shows homology to beta-adaptin. Cell. 1991;64:649–65. doi: 10.1016/0092-8674(91)90248-w. [DOI] [PubMed] [Google Scholar]
- [5].Stenbeck G, Harter C, Brecht A, Herrmann D, Lottspeich F, Oric L, Wieland FT. Beta prime COP, a novel subunit of coatomer. EMBO J. 1993;12:2841–2845. doi: 10.1002/j.1460-2075.1993.tb05945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Harrison-Lavoie KJ, Lewis VA, Hynes GM, Collison KS, Nutland E, Willison KR. A 102 kDa subunit of a Golgi-associated particle has homology to beta subunits of trimeric G proteins. EMBO Journal. 1993;12:2847–53. doi: 10.1002/j.1460-2075.1993.tb05946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Waters MG, Serafini T, Rothman JE. `Coatomer': a cytosolic protein complex containing subunits of non-clathrin-coated Golgi transport vesicles. Nature. 1991;349:248–51. doi: 10.1038/349248a0. [DOI] [PubMed] [Google Scholar]
- [8].Rabouille C, Klumperman J. Opinion: The maturing role of COPI vesicles in intra-Golgi transport. Nat Rev Mol Cell Biol. 2005;6:812–7. doi: 10.1038/nrm1735. [DOI] [PubMed] [Google Scholar]
- [9].Hsu VW, Lee SY, Yang JS. The evolving understanding of COPI vesicle formation. Nat Rev Mol Cell Biol. 2009;10:360–364. doi: 10.1038/nrm2663. [DOI] [PubMed] [Google Scholar]
- [10].Donaldson JG, Cassel D, Kahn RA, Klausner RD. ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein beta-COP to Golgi membranes. Proc Natl Acad Sci USA. 1992;89:6408–6412. doi: 10.1073/pnas.89.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Donaldson JG, Finazzi D, Klausner RD. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature. 1992;360:350–2. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- [12].Helms JB, Rothman JE. Inhibition by brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature. 1992;360:352–4. doi: 10.1038/360352a0. [DOI] [PubMed] [Google Scholar]
- [13].Tanigawa G, Orci L, Amherdt M, Ravazzola M, Helms JB, Rothman JE. Hydrolysis of bound GTP by ARF protein triggers uncoating of Golgi-derived COP-coated vesicles. J Cell Biol. 1993;123:1365–1371. doi: 10.1083/jcb.123.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cukierman E, Huber I, Rotman M, Cassel D. The ARF1-GTPase-Activating Protein: Zinc finger motif and Golgi complex localization. Science. 1995;270:1999–2002. doi: 10.1126/science.270.5244.1999. [DOI] [PubMed] [Google Scholar]
- [15].Goldberg J. Structural and functional analysis of the ARF1-ARFGAP complex reveals a role for coatomer in GTP hydrolysis. Cell. 1999;96:893–902. doi: 10.1016/s0092-8674(00)80598-x. [DOI] [PubMed] [Google Scholar]
- [16].Goldberg J. Decoding of sorting signals by coatomer through a GTPase switch in the COPI coat complex. Cell. 2000;100:671–9. doi: 10.1016/s0092-8674(00)80703-5. [DOI] [PubMed] [Google Scholar]
- [17].Lanoix J, Ouwendijk J, Stark A, Szafer E, Cassel D, Dejgaard K, Weiss M, Nilsson T. Sorting of Golgi resident proteins into different subpopulations of COPI vesicles: a role for ArfGAP1. J Cell Biol. 2001;155:1199–212. doi: 10.1083/jcb.200108017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yang JS, Lee SY, Gao M, Bourgoin S, Randazzo PA, Premont RT, Hsu VW. ARFGAP1 promotes the formation of COPI vesicles, suggesting function as a component of the coat. J Cell Biol. 2002;159:69–78. doi: 10.1083/jcb.200206015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lee SY, Yang JS, Hong W, Premont RT, Hsu VW. ARFGAP1 plays a central role in coupling COPI cargo sorting with vesicle formation. J Cell Biol. 2005;168:281–290. doi: 10.1083/jcb.200404008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Barlowe C, et al. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- [21].Bigay J, Gounon P, Robineau S, Antonny B. Lipid packing sensed by ArfGAP1 couples COPI coat disassembly to membrane bilayer curvature. Nature. 2003;426:563–6. doi: 10.1038/nature02108. [DOI] [PubMed] [Google Scholar]
- [22].McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–6. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- [23].Zimmerberg J, Kozlov MM. How proteins produce cellular membrane curvature. Nat Rev Mol Cell Biol. 2006;7:9–19. doi: 10.1038/nrm1784. [DOI] [PubMed] [Google Scholar]
- [24].Stamnes MA, Craighead MW, Hoe MH, Lampen N, Geromanos S, Tempst P, Rothman JE. An integral membrane component of coatomer-coated transport vesicles defines a family of proteins involved in budding. Proc Natl Acad Sci U S A. 1995;92:8011–5. doi: 10.1073/pnas.92.17.8011. published erratum appears in Proc Natl Acad Sci U S A 1995 Nov 7;92(23):10816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ktistakis NT, Brown HA, Waters MG, Sternweis PC, Roth MG. Evidence that phospholipase D mediates ADP ribosylation factor-dependent formation of Golgi coated vesicles. J Cell Biol. 1996;134:295–306. doi: 10.1083/jcb.134.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Spang A, Matsuoka K, Hamamoto S, Schekman R, Orci L. Coatomer, Arf1p, and nucleotide are required to bud coat protein complex I-coated vesicles from large synthetic liposomes. Proc Natl Acad Sci U S A. 1998;95:11199–204. doi: 10.1073/pnas.95.19.11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bremser M, et al. Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell. 1999;96:495–506. doi: 10.1016/s0092-8674(00)80654-6. [DOI] [PubMed] [Google Scholar]
- [28].Reinhard C, Schweikert M, Wieland FT, Nickel W. Functional reconstitution of COPI coat assembly and disassembly using chemically defined components. Proc Natl Acad Sci U S A. 2003;100:8253–7. doi: 10.1073/pnas.1432391100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Frigerio G, Grimsey N, Dale M, Majoul I, Duden R. Two Human ARFGAPs Associated with COP-I-Coated Vesicles. Traffic. 2007;8:1644–1655. doi: 10.1111/j.1600-0854.2007.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kliouchnikov L, Bigay J, Mesmin B, Parnis A, Rawet M, Goldfeder N, Antonny B, Cassel D. Discrete determinants in ArfGAP2/3 conferring Golgi localization and regulation by the COPI coat. Mol Biol Cell. 2009;20:859–69. doi: 10.1091/mbc.E08-10-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Weimer C, Beck R, Eckert P, Reckmann I, Moelleken J, Brugger B, Wieland F. Differential roles of ArfGAP1, ArfGAP2, and ArfGAP3 in COPI trafficking. J Cell Biol. 2008;183:725–35. doi: 10.1083/jcb.200806140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Peyroche A, Paris S, Jackson CL. Nucleotide exchange on ARF mediated by yeast Gea1 protein. Nature. 1996;384:479–481. doi: 10.1038/384479a0. [DOI] [PubMed] [Google Scholar]
- [33].Chardin P, Paris S, Antonny B, Robineau S, Beraud-Dufour S, Jackson CL, Chabre M. A human exchange factor for ARF contains Sec7- and pleckstrin-homology domains. Nature. 1996;384:481–484. doi: 10.1038/384481a0. [DOI] [PubMed] [Google Scholar]
- [34].Claude A, et al. GBF1: A novel Golgi-associated BFA-resistant guanine nucleotide exchange factor that displays specificity for ADP-ribosylation factor 5. J Cell Biol. 1999;146:71–84. [PMC free article] [PubMed] [Google Scholar]
- [35].Kawamoto K, Yoshida Y, Tamaki H, Torii S, Shinotsuka C, Yamashina S, Nakayama K. GBF1, a Guanine Nucleotide Exchange Factor for ADP-Ribosylation Factors, is Localized to the cis-Golgi and Involved in Membrane Association of the COPI Coat. Traffic. 2002;3:483–95. doi: 10.1034/j.1600-0854.2002.30705.x. [DOI] [PubMed] [Google Scholar]
- [36].Garcia-Mata R, Szul T, Alvarez C, Sztul E. ADP-ribosylation factor/COPI-dependent events at the endoplasmic reticulum-Golgi interface are regulated by the guanine nucleotide exchange factor GBF1. Mol Biol Cell. 2003;14:2250–61. doi: 10.1091/mbc.E02-11-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Niu TK, Pfeifer AC, Lippincott-Schwartz J, Jackson CL. Dynamics of GBF1, a Brefeldin A-sensitive Arf1 exchange factor at the Golgi. Mol Biol Cell. 2005;16:1213–22. doi: 10.1091/mbc.E04-07-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Deng Y, Golinelli-Cohen MP, Smirnova E, Jackson CL. A COPI coat subunit interacts directly with an early-Golgi localized Arf exchange factor. EMBO Rep. 2009;10:58–64. doi: 10.1038/embor.2008.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yang JS, et al. A role for BARS at the fission step of COPI vesicle formation from Golgi membrane. EMBO J. 2005;24:4133–43. doi: 10.1038/sj.emboj.7600873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ostermann J, Orci L, Tani K, Amherdt M, Ravazzola M, Elazar Z, Rothman JE. Stepwise assembly of functionally active transport vesicles. Cell. 1993;75:1015–25. doi: 10.1016/0092-8674(93)90545-2. [DOI] [PubMed] [Google Scholar]
- [41].De Matteis MA, et al. Stimulation of endogenous ADP-ribosylation by brefeldin A. Proceedings of the National Academy of Sciences of the United States of America; 1994. pp. 1114–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Spano S, et al. Molecular cloning and functional characterization of brefeldin AADP-ribosylated substrate. A novel protein involved in the maintenance of the Golgi structure. J Biol Chem. 1999;274:17705–10. doi: 10.1074/jbc.274.25.17705. [DOI] [PubMed] [Google Scholar]
- [43].Hildebrand JD, Soriano P. Overlapping and unique roles for C-terminal binding protein 1 (CtBP1) and CtBP2 during mouse development. Mol Cell Biol. 2002;22:5296–307. doi: 10.1128/MCB.22.15.5296-5307.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Guo Q, Vasile E, Krieger M. Disruptions in Golgi structure and membrane traffic in a conditional lethal mammalian cell mutant are corrected by epsilon-COP. J Cell Biol. 1994;125:1213–24. doi: 10.1083/jcb.125.6.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yang JS, Zhang L, Lee SY, Gad H, Luini A, Hsu VW. Key components of the fission machinery are interchangeable. Nat Cell Biol. 2006;8:1376–82. doi: 10.1038/ncb1503. [DOI] [PubMed] [Google Scholar]
- [46].Weigert R, et al. CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature. 1999;402:429–33. doi: 10.1038/46587. [DOI] [PubMed] [Google Scholar]
- [47].Gallop JL, Butler PJ, McMahon HT. Endophilin and CtBP/BARS are not acyl transferases in endocytosis or Golgi fission. Nature. 2005;438:675–8. doi: 10.1038/nature04136. [DOI] [PubMed] [Google Scholar]
- [48].Yang JS, et al. A role for phosphatidic acid in COPI vesicle fission yields insights into Golgi maintenance. Nat Cell Biol. 2008;10:1146–53. doi: 10.1038/ncb1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Fernandez-Ulibarri I, et al. Diacylglycerol is required for the formation of COPI vesicles in the Golgi-to-ER transport pathway. Mol Biol Cell. 2007;18:3250–63. doi: 10.1091/mbc.E07-04-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Asp L, et al. Early stages of Golgi vesicle and tubule formation require diacylglycerol. Mol Biol Cell. 2009;20:780–90. doi: 10.1091/mbc.E08-03-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Helms JB, Palmer DJ, Rothman JE. Two distinct populations of ARF bound to Golgi membranes. J Cell Biol. 1993;121:751–60. doi: 10.1083/jcb.121.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Liu Y, Kahn RA, Prestegard JH. Structure and membrane interaction of myristoylated ARF1. Structure. 2009;17:79–87. doi: 10.1016/j.str.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lee MC, Orci L, Hamamoto S, Futai E, Ravazzola M, Schekman R. Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell. 2005;122:605–17. doi: 10.1016/j.cell.2005.07.025. [DOI] [PubMed] [Google Scholar]
- [54].Bielli A, Haney CJ, Gabreski G, Watkins SC, Bannykh SI, Aridor M. Regulation of Sar1 NH2 terminus by GTP binding and hydrolysis promotes membrane deformation to control COPII vesicle fission. J Cell Biol. 2005;171:919–24. doi: 10.1083/jcb.200509095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Beck R, et al. Membrane curvature induced by Arf1-GTP is essential for vesicle formation. Proc Natl Acad Sci U S A. 2008;105:11731–6. doi: 10.1073/pnas.0805182105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Krauss M, Jia JY, Roux A, Beck R, Wieland FT, De Camilli P, Haucke V. Arf1-GTP-induced tubule formation suggests a function of Arf family proteins in curvature acquisition at sites of vesicle budding. J Biol Chem. 2008;283:27717–23. doi: 10.1074/jbc.M804528200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lundmark R, Doherty GJ, Vallis Y, Peter BJ, McMahon HT. Arf family GTP loading is activated by, and generates, positive membrane curvature. Biochem J. 2008;414:189–94. doi: 10.1042/BJ20081237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Manneville JB, et al. COPI coat assembly occurs on liquid-disordered domains and the associated membrane deformations are limited by membrane tension. Proc Natl Acad Sci U S A. 2008;105:16946–51. doi: 10.1073/pnas.0807102105. [DOI] [PMC free article] [PubMed] [Google Scholar]