Abstract
Opioid-induced proinflammatory glial activation modulates wide-ranging aspects of opioid pharmacology including: opposition of acute and chronic opioid analgesia, opioid analgesic tolerance, opioid-induced hyperalgesia, development of opioid dependence, opioid reward, and opioid respiratory depression. However, the mechanism(s) contributing to opioid-induced proinflammatory actions remains unresolved. The potential involvement of toll like receptor 4 (TLR4) was examined using in vitro, in vivo, and in silico techniques. Morphine non-stereoselectively induced TLR4 signaling in vitro, blocked by a classical TLR4 antagonist and non-stereoselectively by naloxone. Pharmacological blockade of TLR4 signaling in vivo potentiated acute intrathecal morphine analgesia, attenuated development of analgesic tolerance, hyperalgesia, and opioid withdrawal behaviors. TLR4 opposition to opioid actions was supported by morphine treatment of TLR4 knockout mice, which revealed a significant threefold leftward shift in the analgesia dose response function, versus wildtype mice. A range of structurally diverse clinically employed opioid analgesics was found to be capable of activating TLR4 signaling in vitro. Selectivity in the response was identified since morphine-3-glucuronide, a morphine metabolite with no opioid receptor activity, displayed significant TLR4 activity, whilst the opioid receptor active metabolite, morphine-6-glucuronide, was devoid of such properties. In silico docking simulations revealed ligands bound preferentially to the LPS binding pocket of MD-2 rather than TLR4. An in silico to in vitro prediction model was built and tested with substantial accuracy. These data provide evidence that select opioids may non-stereoselectively influence TLR4 signaling and have behavioral consequences resulting, in part, via TLR4 signaling.
Keywords: toll like receptor 4, knockout, opioid, analgesia, dependence, glia, microglia
INTRODUCTION
Recent evidence indicates that glia can exhibit proinflammatory responses to opioids, contributing to a reduced opioid analgesia and development of tolerance and dependence, albeit by a previously uncharacterized mechanism (Hutchinson et al., 2007; Watkins et al., 2005). In vivo opioid-induced proinflammatory glial activation has been inferred from: (a) morphine-induced upregulation of microglial (Cui et al., 2008; Hutchinson et al., 2009) and astrocytic (Hutchinson et al., 2009; Song and Zhao, 2001) activation markers, (b) morphine-induced upregulation and/or release of proinflammatory cytokines (Hutchinson et al., 2008; Hutchinson et al., 2009; Johnston et al., 2004; Raghavendra et al., 2002; Raghavendra et al., 2004), (c) enhanced morphine analgesia by coadministering the microglial attenuators minocycline (Cui et al., 2008; Hutchinson et al., 2008) or AV411 (Hutchinson et al., 2009), and the astrocyte inhibitor fluorocitrate (Song and Zhao, 2001), (d) enhanced morphine analgesia by blocking proinflammatory cytokine actions (Hutchinson et al., 2008; Shavit et al., 2005), and (e) opioid-induced selective activation of microglial p38 MAPK and associated enhanced morphine analgesia (Cui et al., 2006). As such, opioid-induced proinflammatory glial activation is characterized by a cellular phenotype of enhanced reactivity and propensity to proinflammation in response to exposure of glia to opioids.
In vitro studies support that opioids can alter the function of microglia and astrocytes (Dobrenis et al., 1995; El-Hage et al., 2005; Horvath and DeLeo, 2009; Hutchinson et al., 2008; Lipovsky et al., 1998; Narita et al., 2006; Peterson et al., 1998; Stefano, 1998; Takayama and Ueda, 2005). Also, morphine can sensitize (“prime”) microglia in vitro to over-respond to subsequent stimuli, thereby generating exaggerated release of neuroexcitatory substances (Chao et al., 1994).
As microglia and astrocytes can express mRNA for mu, delta, and kappa opioid receptors (Ruzicka and Akil, 1997), opioids have been thought to exclusively influence glia via these receptors. However, opioid receptor knockout mouse studies of opioid-induced peripheral immune function modulations reveal both opioid receptor dependent (Gaveriaux-Ruff et al., 1998) and independent actions (Gaveriaux-Ruff et al., 2001).
Opioids may potentially activate glia through mechanisms distinct from classical opioid receptors. While classical opioid receptors are stereoselective, as they bind (−)-opioid isomers but not (+), several studies report (+)-isomer glial effects for both opioid agonists and antagonists. For example, (+)-opioid agonists suppress (−)-opioid analgesia (Wu et al., 2007), an effect attributed to glial activation based on propentofylline blockade (Wu et al., 2005) and independent of classical μ-opioid receptors in knockout mice studies (Wu et al., 2006). It has also been reported that morphine administered to triple opioid receptor knockouts can induce hyperalgesia (Juni et al., 2007), supporting the studies reviewed above that suggest that a non-classical opioid receptor may exist that opposes analgesia.
Intriguingly, it has recently been reported that (+)-opioid antagonists attenuate the reduction in opioid analgesia that occurs in response to glial activation by lipopolysaccharide (LPS) (Wu et al., 2006). This is exciting because it suggests the novel possibility that opioid agonists may actually signal, not only via classical opioid receptors, but also through the LPS receptor, toll-like receptor 4 (TLR4). TLR4 is an innate immune receptor, also capable of recognizing endogenous danger signals, whose signaling via the Toll/Interleukin-1 receptor (TIR) domain results in a profound proinflammatory signal. Thus, opioid effects via TLR4 could potentially provide an explanation for opioid-induced proinflammatory glial activation (Hutchinson et al., 2008; Hutchinson et al., 2009; Johnston et al., 2004; Raghavendra et al., 2002; Song and Zhao, 2001). The present series of in vivo, in vitro, and in silico studies were designed to provide an initial exploration of this issue.
MATERIALS AND METHODS
Subjects
Pathogen-free adult male Sprague–Dawley rats (n = 6 rats/group for each experiment; 300–375 g; Harlan Labs, Madison, WI, USA) were used. Pathogen-free male Balb/c wild-type and TLR4 knockout mice, back crossed onto Balb/c 10 times were used for the TLR4 knockout studies (n = 6 mice/group for each experiment; 24–32 g; kindly gifted by Dr. Simon Phipps and sourced from Prof. Akira). This TLR4 knockout strain has an established track record in the TLR4 literature (Hoshino et al., 1999). Mice and rats were housed in temperature (23 ± 3 °C) and light (12 h:12 h light:dark cycle; lights on at 0700) controlled rooms with standard rodent chow and water available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Colorado at Boulder (rat) and the Animal Ethics Committee of the University of Adelaide (mouse).
Drugs
Endotoxin-free morphine sulfate was kindly gifted by Mallinckrodt, Inc. (St. Louis, MO, USA). Endotoxin-free (+)-naloxone, (+)-naltrexone, (+)-nalmefene and (+)-morphine were kindly gifted by Dr. Kenner Rice (Chemical Biology Research Branch, National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Rockville, MD, USA). Endotoxin-free (−)-naloxone, (−)-naltrexone, morphine-3-glucuronide, morphine-6-glucuronide, pethidine, and oxycodone, were purchased from Sigma (St. Louis, MO, USA) as was lipopolysaccharide. Lipopolysaccharide from msbB E. coli mutant (a TLR4 antagonist due to its lack of the myristoyl fatty acid moiety of lipid A) and LPS-RS (a TLR4 antagonist naturally produced by R. sphaeroides) were purchased from Invivogen (San Diego, CA, USA). TIRAP inhibitor peptide and the control peptide were obtained from IMGENEX (San Diego, CA, USA). Complement 5a was sourced from R&D Systems (Minneapolis, MN, USA). Where applicable, drugs were prepared and are reported as free base concentrations. Where applicable, endotoxin-free status of compounds was confirmed by LAL assay (see below). Drugs were dissolved in endotoxin-free physiological saline and handled using aseptic procedures.
Real time microsocopy of TLR4 signaling in a stably transfected RAW264.7 mouse macrophage cell line
TLR4 signaling leads to the simultaneous activation of three parallel intra-cellular signaling pathways. Two of these (through NF-κB and MAPK) are principally responsible for the proinflammatory responses induced by TLR4 activation, while the third parallel pathway (PI3K/Akt1) is more related to cell survival, apoptosis, and cell motility (Dauphinee and Karsan, 2006; Laird et al., 2009). As all three are activated by agonism at TLR4, any can be used as a reflection of TLR4 activation. Given the availability of a RAW264.7 mouse macrophage cell line stably transfected to express green fluorescent protein (GFP)-tagged Akt1 (Evans and Falke, 2007), mobilization and cytosolic clearance of GFP-Akt1 was used as an indicator of TLR4 activation. Cells were grown up in growth media and then were plated at 2 × 105 cells/mL density in growth media on 35 mm MatTek Glass Bottom Dishes (Ashland, MA, USA) for 18 h prior to imaging. Just prior to imaging the growth media was removed from the plates, washed two times with HBSS supplemented with 25 mM HEPES buffered to pH 7.4 and replaced with 1 mL warmed conditioned imaging Hanks Buffer media (media was conditioned by a 24 h incubation with RAW264.7 cells). Imaging was carried out on a Nikon inverted microscope (Melville, NY, USA) with a 60X oil immersion objective, GFP/RFP dichroic mirror with corresponding single band excitation and emission filters (Chroma Technology), and CoolSNAP ES camera (Photometrics, Tucson, AZ, USA). A mercury lamp provided excitation. Images were captured every 7.5 s. Baseline fluorescence was captured for 5 frames, following which vehicle or antagonist was added in 200 μl. Imaging continued for a further 20 frames at which time LPS or test agonist (200 μl) were applied and monitored for a further 20 frames. If no visual response was obtained C5a (200 μl) was added to the plates to confirm if the cells were responsive. GFP-Akt1 cytosolic clearance was quantified using ImageJ and expressed as a normalized change in cytoplasmic fluorescence over time.
In vivo TLR4 blockade and behavior: intrathecal and systemic administration experiments
Catheter implantation
The method of constructing and implanting the indwelling intrathecal catheters in rats was based on that described previously (Hutchinson et al., 2008). Briefly, intrathecal catheters were implanted under anesthesia (isoflurane; Phoenix Pharmaceuticals, St. Joseph, MO, USA) by threading sterile polyethylene-10 tubing (PE-10 Intramedic Tubing; Becton Dickinson Primary Care Diagnostics, Sparks, MD, USA) guided by an 18-gauge needle between the L5 and L6 vertebrae. The catheter was threaded rostrally such that the proximal catheter tip lay over the lumbosacral enlargement. The needle was removed and the catheter was sutured to the superficial musculature of the lower back and the exterior end led subcutaneously to exit through a small incision at the nape of the neck.
For acute intrathecal drug experiments, the catheters were preloaded with drugs at the distal end in a total volume of no greater than 25 μl. The catheters were 90 cm in length, allowing remote drug delivery without touching or otherwise disturbing the rats during the testing. Acute intrathecal drug administration began 2 h after surgery.
For the subcutaneous catheter, a 90 cm length of PE-10 tubing was sutured to the superficial musculature of the lower back at the same time as the intrathecal catheter was implanted in these animals. The exterior end of the subcutaneous catheter paralleled the intrathecal catheter out of the same incision in the nape of the neck, allowing for remote subcutaneous administration without disturbance of the animals.
Minipump implantation
For chronic drug delivery experiments rats were baselined on Hargreaves short latency (analgesia) and long latency (hyperalgesia) prior to surgery and then were subcutaneously implanted with 2 subcutaneous lumbar osmotic minipumps (model 2001, Alzet). Each delivered 1 μl/hr, with one delivering 6 mg/day morphine and the other 12 mg/day (+)-naloxone or saline.
Hargreaves tests for analgesia and hyperalgesia
Rats received at least three 60 min habituations over successive days to the test environment prior to behavioral testing. Latencies for behavioral response to radiant heat stimuli applied to the plantar surface of each hind-paw and tail were assessed using a modified Hargreaves test (Hargreaves et al., 1988). All testing was conducted blind with respect to group assignment. Pilot studies determined that intrathecal catheter surgery did not affect baseline responses after 2 h recovery from surgery, compared to latencies recorded prior to surgery. Briefly, baseline withdrawal values were calculated from an average of 2 consecutive withdrawal latencies of the tail and the left and the right hind-paws, measured at 15 min intervals. The intensity of the radiant heat stimuli were adjusted across tests so to obtain either short or long baseline latencies. This allowed quantification of analgesia (lengthening of the latency, relative to baseline, in response to pain suppressive procedures) and hyperalgesia (shortening of the latency, relative to baseline, in response to pain enhancing procedures), respectively. Latencies for the short baseline latency Hargreaves stimuli at baseline ranged from 2 to 3 s, and a cut-off time of 10 s was imposed to avoid tissue damage. Latencies for the long baseline latency Hargreaves stimuli at baseline ranged from 8 to 10 s, and a cut-off time of 20 s was imposed to avoid tissue damage. While it is possible that these two stimuli may activate different sensory afferents, the need for two different stimuli is due to the type and direction of the anticipated behavioral response, and are standardly used for this purpose in pain research. The order of paw and tail testing varied randomly. Nociceptive assessments for acute administration experiments were then made at 0 (immediately following remote drug delivery), 5 min, 15 min and every 10 min thereafter until completion of the experiment only using the short baseline latency Hargreaves stimuli. For the chronic drug delivery experiments rats were tested on days 1, 3, 5 and 7 after pump implant.
Opioid withdrawal assessment
The rats in the chronic administration dosing regimen were also assessed for opioid withdrawal. After nociceptive testing on day 7 the animals received subcutaneous administration of 100 mg/kg (−)-naltrexone to precipitate opioid withdrawal. Withdrawal behaviors were then scored over 60 min by 3 independent observers blinded to treatment groups. Observers scored 2–3 rats each rotating scoring positions every 10 min. Withdrawal behaviors that were scored included: jumping, rearing, exploration (movement greater than one body length), teeth chattering, wet dog shakes, abnormal posture, escape attempts, ptosis of the eyes, diarrhea, penis licking, pica, paw chewing, cleaning, salivation, vocalization, chewing (large jaw movements including masseter muscle contraction) and fidgeting (a writhing type of behavior involving small shifts in body position). Counts of each of these behaviors were made upon their presentation with the total number of withdrawal scores calculated across the 60 min observation for each rat.
TLR4 knockout and wildtype mice opioid behavioral assessment
Hotplate analgesia assessment
Mice received at least three 5 min habituations over successive days to the test environment prior to behavioral testing. Latencies for behavioral responses to the 50°C hotplate were assessed. All testing was conducted blind to group assignment. A cut-off time of 60 s was imposed to avoid tissue damage. Latencies for the hotplate response ranged from 18 to 27 s. Baseline response latencies were recorded prior to drug administration. For the construction of the morphine dose response saline vehicle, 1, 2.5, 10, 25 and 50 mg/kg morphine was administered i.p. in a dose volume of 10 ml/kg and the hotplate response latency recorded 20 min following drug administration. Mice (wildtype and knockout) were randomized in an incomplete crossover design with one week washout between treatments and tested on four occasions (total of 9 animals used per group for the six doses of the dose response).
Examination of the effect of pharmacological TLR4 signaling blockade and microglial attenuation on morphine analgesia in wildtype and knockout mice was conducted by giving 60 mg/kg (+)-naloxone i.p. 10 min prior to 2.5 mg/kg morphine i.p. and 50 mg/kg minocycline i.p. 60 min prior to 2.5 mg/kg morphine i.p. with hotplate latencies recorded prior to the (+)-naloxone and morphine doses and 20 min after the morphine dose. Mice in this study received a two week washout between the two treatments.
In vitro HEK293-hTLR4 agonist and antagonist assay
As we have previously described (Hutchinson et al., 2008) a human embryonic kidney-293 (HEK293) cell line stably transfected to express human TLR4 was used to assess opioid TLR4 activity. This HEK293 cell line expresses high levels of TLR4, the required TLR4 co-signaling molecules (MD-2 and CD14) and an optimized alkaline phosphatase reporter gene under the control of a promoter inducible by several transcription factors such as NF-kB and AP-1 (Invivogen, San Diego, CA USA; 293-htlr4a-md2cd14). TLR4 activity in the cells is assessed by measuring the expression of secreted alkaline phosphatase (SEAP) protein that is produced as a consequence of TLR4 activation.
in silico docking simulations
The complexed human TLR4 and MD-2 pdb file was obtained from RCSB Protein Data Bank database (PDBID: 3fxi). All ligands were extracted via Molegro Molecular Viewer to eliminate exgogenous water moleculues and artifacts from crystallization. Modified pdb files were inputted into AutoDock 4.0 (http://autodock.scripps.edu), hydrogens added, and resaved in pdbqt format. Drug ligands for docking were gathered using PubChem isomeric SMILES then converted to .pdb using a structure file generator (http://cactus.nci.nih.gov/services/translate/). Ligand torsion tree roots were generated automatically by choosing ‘detect root’, and number torsions set at zero for all compounds except oxycodone, (±)-methadone and pethidine, which were allowed their natural rotations. Initially, the in silico docking of ligands to the entire TLR4 MD-2 dimer complex was conducted (AutoGrid center set 3.438, −7.805, 2.034; 126 grid points expanding in all directions; GA running number of 100, Max Evals 5 × 106 and 1.0 Å spacing). These data demonstrated that the great majority of the ligands docked with human MD-2 independent of human TLR4 interactions. Therefore, all the ligands were docked to MD-2 alone with greater resolution (AutoGrid center set 27.991, 0.851, 19.625; 126 grid points expanding in all directions; GA running number of 100, Max Evals 5 × 106 and 0.375 Å spacing). All dockings were executed with Lamarkian genetic algorithms on Apple desktop computers running OS X 10.5.6. The lowest energy and highest interaction docking conformation was visualized and Binding Energy, Ligand Efficacy, Inhibitory Constant, Intermolecular Energy, van der Waals + Hydrogen Bonds + Disolvation Energy, Electrostatic Energy, Total Internal Energy, Torsional Energy, Unbound Energy, Lowest Energy, Docking Frequency, Rank and the amino acid residues of MD-2 that the ligand conformation interacted with were collected (coded +1 for interaction and −1 for no interaction). These parameters constitute the in silico portion of the data. The two sets of in vitro data were then integrated to produce the sum of the percent above control (set to 0%) of the activity of individual ligands in the in vitro agonist assay (when tested alone at 100 μM); and the maximal inhibition of LPS signaling (when tested at 100 μM) expressed as a percent of the LPS control. Therefore, positive values equaled TLR4 signaling activators and negative values represented TLR4 signaling inhibitors. All the data was then standardized (mean = 0 and standard deviation = 1) to remove the inherent variability between the scores reported thereby removing numerical magnitude bias from correlations. A linear regression was then applied to the sum of all of the in silico parameters plotted against the integrated in vitro data, with variable multiplication values applied to each in silico parameter which were then varied by an iteration process to maximize the correlation between the sum of the in silico parameters and the integrated in vitro data. This process also provided the opportunity to ascertain which parameters contributed most to the in vitro prediction as their multiplication factors is inherently larger. The starting values for the multiplication values were trained based on the individual correlations of each in silico parameter to the in vitro data, such that the multiplication factor was set to −1 for negative correlations and +1 for positive correlations. This process was conducted using the in silico and in vitro data from 10 opioids (M3G, oxycodone, (−)-methadone, (+)-methadone, dextrorphan, buprenorphine, M6G, (+)-nalmefene, (−)-naloxone and (+)-naloxone) enabling a model of in silico to in vitro prediction to be built that was subsequently tested using 7 different opioids ((−)-morphine, (+)morphine, (−)-naltrexone, (+)-naltrexone, fentanyl, pethadine, levorphanol) and 3 non-opioid compounds that docked successfully to the same MD-2 pocket (minocycline, propentofylline and dextromethophan). The final model was then refined further by the inclusion of all the data points and tested again on an unrelated, structurally diverse ligand, AV411 (ibudilast), demonstrated to have TLR4 signaling inhibitory action from hTLR4-HEK293 results.
Statistics and data analysis
Graphpad Prism 5.0 was used for all statistical analysis. A two-way ANOVA with Bonferroni post-hoc was used to test the agonism and antagonism results obtained from the GFP-Akt RAW264.7 cells and in vivo Hargreaves and hotplate analgesia data. A one-way ANOVA with Bonferroni post-hoc was used to assess TLR4 activity in the HEK293-hTLR4 cell line. Non-linear regressions were used to examine the dose response data to obtain EC50 and Hill Slope values. The maximum response was constrained to 100 owing to the cutoff latency set in the behavioral test. Area under the analgesia curve was calculated using Prism 5.0 with the statistical differences in chronic analgesia and opioid withdrawal assessed using a Student’s t-test. Error bars on graphs represent standard error of the mean.
RESULTS
Experiment 1. In vitro studies of TLR4 signaling in RAW264.7 macrophages
As noted above, we have recently reported that naloxone can non-stereoselectively inhibit TLR4-mediated LPS signaling in vitro, using a human TLR4 transfected human embryonic kidney (HEK293-hTLR4) cell line that generates secreted alkaline phosphatase (SEAP) as a reporter protein in response to human TLR4 activation (Hutchinson et al., 2008). (+)- and (−)-Naloxone appears to be capable of acting as TLR4 antagonists based on their dose-dependent block of TLR4 activation by the classical TLR4 agonist, LPS, as indicated by suppression of LPS-induced SEAP (Hutchinson et al., 2008). Further, using a microglial cell line, (+)- and (−)-naloxone each blocked TLR4 activation (LPS) induced gene expression of both proinflammatory cytokines (interleukin-1 and -6) and a microglial activation marker (CD11b) (Hutchinson et al., 2008).
However, these data provide no indication as to where in the signaling cascade this non-stereoselective blockade by naloxone is occurring, especially given the high lipophilicity of naloxone granting it ready access to intracellular compartments. There is precedence for other drugs interfering with TLR4 signaling through non-receptor mediated mechanisms (Cuschieri et al., 2007). To begin to approach this issue, we utilized a RAW264.7 mouse macrophage cell line that stably expresses green fluorescent protein (GFP)-tagged Akt1. The PI3K/Akt1 pathway is one of 3 parallel intracellular signaling pathways which are all activated upon TLR4 signaling. The other 2 (NF-κB and MAPK) are the predominant pathways leading to proinflammatory responses resulting from TLR4 activation, while the PI3K pathway is predominantly associated with cell survival vs. apoptosis, and cell motility effects (Laird et al 2009; Dauphinee and Karsan, 2006). The advantages of this cell line are that: (a) activation of Akt1, like NF-κB and MAPK, occurs in response to the activation of TLR4 and (b) within the TLR4 signaling cascade, activation of Akt1 by PI3 kinase is very early step, quite close to TLR4 (Dauphinee and Karsan, 2006; Laird et al., 2009). Thus, if Akt1 activation is affected by the drugs under test, an interaction must be occurring at or close to the TLR4 complex, if the drugs are activating TLR4. Under basal conditions, Akt1 is diffusely distributed in the cytosol but, upon activation, rapidly moves to the membrane site where an Akt1 activating event is occurring. In the case of LPS-induced TLR4 signaling, activated Akt1 moves out of the cytosol to the TLR4 lipid raft (Ojaniemi et al., 2003). As expected, the classical TLR4 agonist LPS (200 ng/mL in 1.2 mL total dish volume) reliably induced robust, rapid clearance of GFP-Akt1 from the cytosol to the cell membrane (Figure 1). In contrast, preincubation with the established TLR4 competitive antagonist LPS-RS (200 ng/mL, Figure 1A), (+)-naloxone (100 μM, Figure 1B), or (−)-naloxone (100 μM, Figure 1C) shortly before addition of LPS significantly (P < 0.001) attenuated the Akt1 response.
Figure 1. (+)-Naloxone, like (−)-Naloxone and the classic TLR competitive inhibitor LPS-RS, inhibits TLR4 Akt1 signaling.
LPS (200 ng/ml) plus vehicle (■) causes significant membrane localization of GFP-Akt1 quantified by cytosolic clearance of GFP-Akt1 in a stably expressing RAW264.7 cell line. Pretreatment with the competitive TLR4 antagonist LPS-RS (200 ng/ml, ●, panel A) or the novel TLR4 inhibitors (+)-naloxone (200 μM, ▼, panel B) and (−)-naloxone (200 μM, ▲, panel C) all significantly attenuate subsequent LPS-induced GFP-Akt1 membrane localization. To ensure the TLR4-selectivity of the inhibition the Akt1 blockade, cells were then stimulated with C5a (25 ng/ml), which utilizes a non-TLR4 pathway to activate Akt1. Notably, C5a triggers significant GFP-Akt1 membrane localization. Given how early Akt1 is activated in the TLR4 cascade, this reveals a non-classical opioid effect at or very close to TLR4 itself. n = 10 cells/group from a minimum of 4 separate plates.
Since Akt1 activation is one of the earliest events in the TLR4 intracellular signaling cascade (Dauphinee and Karsan, 2006; Laird et al., 2009), this raises the possibility that (+)- and (−)-naloxone may be blocking either LPS activation of TLR4, TLR4 interaction/activation of the TRIF/TRAM complex, the subsequent activation of PI3K, or PI3K phosphorylation of Akt1. To clarify whether the blockade of the Akt1 by naloxone response was specific to the TLR4 signaling pathway, rather than a general suppression of Akt1 itself, complement 5a (C5a; 25 ng/mL) was applied to the LPS-RS, (+)-naloxone and (−)-naloxone-TLR4 blocked cells. C5a immediately precipitated significant (P < 0.001) Akt1 membrane localization quantified by cytosolic clearance (Figure 1A,B,C). Thus, these data suggest that naloxone may be disrupting the TLR4 signaling cascade, either at the level of LPS binding or TLR4 interaction/activation of the TRIF/TRAM complex or this method of activation of PI3K, but not via an alteration of the action of PI3K itself.
Experiment 2. In vitro studies of TLR4 signaling induced by opioids in RAW264.7 macrophages
The μ opioid receptor agonist (−)-morphine has previously been reported to induce Akt1 signaling (Gupta et al., 2002; Horvath and DeLeo, 2009). Mediation of this effect was concluded to be by a classical opioid receptor, but the results of Experiment 1 suggest further exploration whether TLR4 may potentially have a contributing role. We wished to determine if (a) opioid-induced TLR4 signaling was stereoselective, (b) it could be blocked by the TLR4 selective competitive antagonist LPS-RS, and (c) it could be blocked non-stereoselectively by naloxone.
Using the same GFP-Akt1 RAW264.7 cell line as used for Experiment 1, (+)- and (−)-morphine (200 μM) were both found to cause significant (P < 0.05) and prolonged clearance of GFP-Akt1 from the cytosol (Figure 2) resulting from membrane localization of the GFP-Akt1. Thus the effect of morphine is non-stereoselective, unlike its actions at classical opioid receptors. This non-stereoselective morphine-induced Akt1 response was significantly (P < 0.05) blocked by the TLR4 receptor competitive antagonist LPS-RS (200 ng/mL, Figure 2A,B), (+)-naloxone (200 μM; Figure 2C,D) and (−)-naloxone (200 μM, Figure 2E,F).
Figure 2. Morphine non-stereoselectively activates (+)- and (−)-naloxone sensitive TLR4 Akt1 signaling.
(−)-Morphine (200 μM; A,C,E) and (+)-morphine (200 μM; B,D,F) plus vehicle (■) causes significant membrane localization of GFP-Akt1 quantified by cytosolic clearance of GFP-Akt1 in a stably expressing RAW264.7 cell line. Pretreatment with characterized TLR4 antagonist LPS-RS (200 ng/ml, ●, A,B) or the novel TLR4 inhibitors (+)-naloxone (200 μM, ▼, C,D) and (−)-naloxone (200 μM, ▲, E,F) all significantly attenuate subsequent non-stereoselective morphine-induced GFP-Akt1 membrane localization. To ensure the TLR4-selectivity of the inhibition the blockaded cells were then stimulated with C5a (25 ng/ml), which triggers significant GFP-Akt1 membrane localization. n = 10 cells/group from a minimum of 4 separate plates.
RAW264.7 macrophages can express μ-opioid receptors (Hwang et al., 2004), and morphine has been shown to activate PI3K in neurons (Narita et al., 2004) presumably by classical μ-opioid receptors since neurons, except in rare instances, do not express TLR4. While morphine can activate PI3K/Akt in monocyte-derived cells (Horvath and DeLeo, 2009), the results of the present study suggest that TLR4 is responsible for the current activation of PI3K/Akt as the TLR4 antagonist LPS-R/S (Figures 2A) and (+)-naloxone (Figure 2C) completely blocked the (−)-morphine-induced Akt1 signal. Co-activation of classical μ-opioid receptors does not appear to be required for the effects obtained in the present study. As will be reported in Experiment 5 (below), HEK-TLR4 over-expressing cells do not express classical opioid receptors so TLR4 activation and downstream signaling do not require co-activation of classical opioid receptors.
Experiment 3. In vivo studies of the impact of TLR4 blockade on analgesia and withdrawal
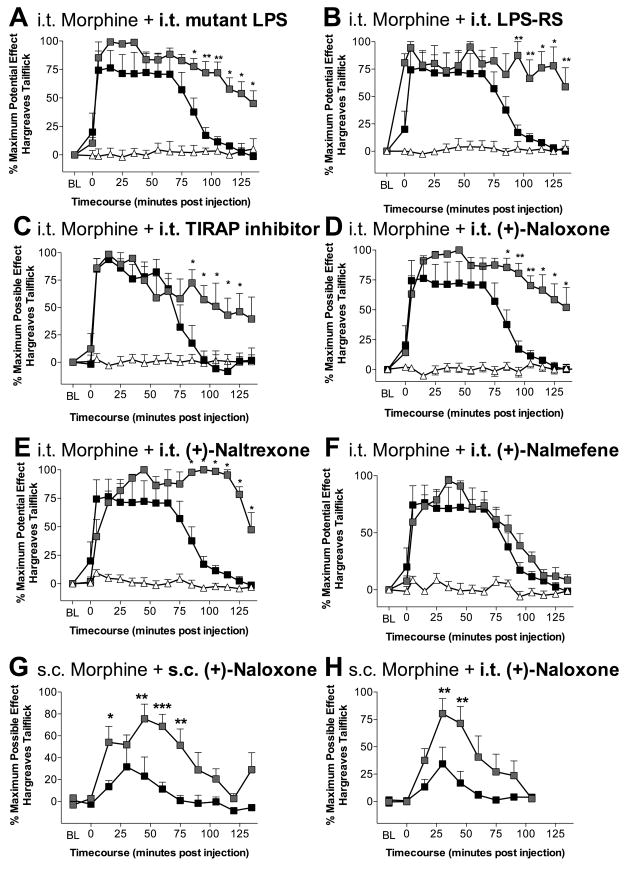
While morphine produces proinflammatory responses by glia (Hutchinson et al., 2008; Watkins et al., 2005), the mechanism underlying this effect is unknown. The in vitro studies, above, suggest that, in vivo, one may be able to potentiate morphine analgesia by selectively blocking TLR4 signaling and its known downstream proinflammatory effects. Here, intrathecal morphine (15 μg plus vehicles; open squares, Figure 3A–F) produced significant (P < 0.01 compared to vehicle) analgesia to radiant heat which dissipated by ~100 min, whereas vehicle alone had no effect on escape latencies (▵; Figure 3A–F). In all experiments, both hindpaw and tail withdrawal thresholds produced consistent results, as we have previously reported, so only tailflick results are presented here for simplicity (Hutchinson et al., 2008). Intrathecal morphine analgesia was significantly (P < 0.05 compared to morphine) prolonged when co-administered intrathecally with either of 2 LPS variants that have each been previously characterized as TLR4 competitive receptor antagonists (msbB E. coli LPS mutant, 20 μg, Figure 3A; or LPS-RS, 40 μg; Figure 3B), with an inhibitor of Toll-Interleukin-1 receptor domain containing adaptor protein (TIRAP) which is a key component of one of the intracellular signaling cascades activated by TLR4 (TIRAP inhibitor peptide; 50 μM in 1 μl; Figure 3C), or with (+)-isomers of either naloxone (20 μg; Figure 3D) or naltrexone (20 μg; Figure 3E). Like PI3K/Akt, TIRAP is one of the earliest components of the TLR4 signaling pathway (indeed several steps earlier than PI3K) (Dauphinee and Karsan, 2006; Laird et al., 2009), suggesting actions at or very close to TLR4. There is selectivity in the effect as intrathecal coadministration of the opioid inactive isomer of the opioid antagonist nalmefene (20 μg) failed to potentiate morphine analgesia (Figure 3F, P > 0.05). Systemic morphine analgesia also appears to be opposed by TLR4 signaling, since coadministration of systemic (+)-naloxone (8 mg/kg) with systemic morphine (4 mg/kg s.c.; Figure 3G) significantly (P < 0.05 compared to morphine) potentiated acute morphine analgesia. Notably, the spinal cord is a key site for this effect as coadministration of intrathecal (+)-naloxone (20 μg) with systemic morphine (4 mg/kg s.c.; Figure 3H) also significantly (P < 0.05 compared to morphine) potentiating analgesia.
Figure 3. TLR4 blockade potentiates morphine analgesia.
Intrathecal (i.t.) co-administration of morphine (15 μg) with LPS antagonist (A; 20 μg; msbB E. coli mutant), LPS-RS (B; 40 μg), a TIRAP inhibitory peptide (C; 50 μM; TIRAP is a secondary signaling molecule required to for a TLR4 signaling complex), (+)-naloxone (D; 20 μg), or (+)-naltrexone (E; 20 μg) all lead to significant potentiation of morphine tailflick analgesia (morphine + vehicle black; morphine in combination with TLR4 signaling attenuators in grey). Coadministration with (+)-nalmefene (F: 20 μg) failed to potentiate morphine analgesia. Systemic morphine (4 mg/kg) is also potentiated by coadministration of systemic (G; 8 mg/kg) or intrathecal (H; 20 μg) (+)-naloxone. n = 6/group * = P < 0.05, ** = P < 0.01, *** = P < 0.001; n = 6/group
Chronic systemic administration of morphine can lead to the development of analgesic tolerance, “paradoxical” hyperalgesia, and dependence (Ossipov et al., 2005). It has been recently proposed that progressive activation of glia with repeated opioid exposure may contribute to these phenomena (Hutchinson et al., 2008; Watkins et al., 2005). Here, each phenomenon was examined under chronic (+)-naloxone. Continuous s.c. (−)-morphine plus vehicle (6 mg morphine/day for 7 days delivered via osmotic minipump) produced significant (P < 0.05 compared to vehicle) analgesia to radiant heat over the first 24 h following osmotic minipump implant, followed by development of analgesic tolerance by the third day such that no significant analgesia was detected thereafter (P > 0.05). Continuous s.c. (+)-naloxone (12 mg/day for 7 days delivered via osmotic minipump) attenuated the development of analgesic tolerance to this morphine regimen, demonstrated by the larger area under the analgesic curve (morphine + vehicle: 28.2 ± 24.2 vs. morphine + (+)-naloxone: 112.4 ± 20.9; P < 0.05). In addition, continuous (+)-naloxone significantly attenuated the development of morphine-induced paradoxical hyperalgesia (morphine + vehicle: −171.5 ± 76.4 vs. morphine + (+)-naloxone: +30.8 ± 37.4; P < 0.05; negative values are representative of hyperalgesia as escape latencies to the radiant heat stimulus now occur faster than pre-drug baseline) and attenuated the dampening effect of continuous morphine on weight gain across days (morphine + vehicle: 21.5 ± 1.4 g vs. morphine+(+)-naloxone; 28.2 ± 2.5 g; P < 0.05). Lastly, continuous (+)-naloxone throughout this morphine regimen significantly attenuated precipitated withdrawal in these same morphine-dependent rats induced by the opioid receptor antagonist (−)-naloxone (total withdrawal scores; morphine+(+)-naloxone: 90.0 ± 3.5 vs. morphine + vehicle 140.3 ± 9.3; P < 0.05). As (+)-naloxone and (−)-naloxone can each block TLR4, the addition of (−)-naloxone would at most continue the TLR4 blockade by (+)-naloxone. The action of (−)-naloxone relevant here to withdrawal is antagonism of morphine binding to classical opioid receptors.
Experiment 4. In vivo studies of the impact of TLR4 knockout on morphine analgesia
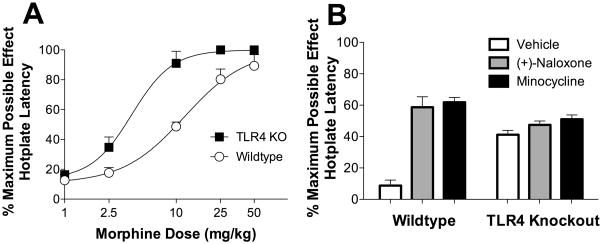
In order to extend the results of Experiment 3, a morphine hotplate latency dose response was generated in TLR4 knockout and wildtype animals. There was no difference between the baseline hotplate nociceptive responsiveness of the wildtype and TLR4 knockout strains (P > 0.05). However, upon acute morphine administration a significant difference was observed with TLR4 knockout mice showing nearly a three fold leftward shift in the analgesia dose response (EC50 TLR4 knockout 4.0 mg/kg versus wildtype 11.9 mg/kg; P = 0.003) compared to wildtype mice (Figure 4A).
Figure 4. Morphine is a more effective analgesic in TLR4 knockout mice compared to wildtype controls.
A: A morphine dose response in TLR4 knockout is shifted significantly to the left (EC50 4.0 mg/kg) compared to the wildtype mice (EC50 11.9 mg/kg) assessed by hotplate latency. B: Pharmacological blockade of TLR4 with (+)-naloxone (60 mg/kg i.p.) or microglial activation attenuation with minocycline (50 mg/k i.p) only potentiates morphine analgesia in wildtype but not TLR4 knockout animals. n = 6/group
(+)-Naloxone was then tested in TLR4 knockout and wildtype mice. (+)-Naloxone (60 mg/kg i.p.) caused a significant potentiation of morphine (2.5 mg/kg i.p; P < 0.001) analgesia in wildtype, but not TLR4 knockout mice (Figure 4B; P > 0.05), despite analgesia being sub-maximal. In addition, co-administration of minocycline (50 mg/kg), a drug previously reported to suppress microglial activation by opioids, significantly (P < 0.001) potentiated morphine analgesia in wildtype but not TLR4 knockout mice (Figure 4B; P > 0.05).
Experiment 5. In vitro studies of TLR4 signaling in HEK293-TLR4 cells
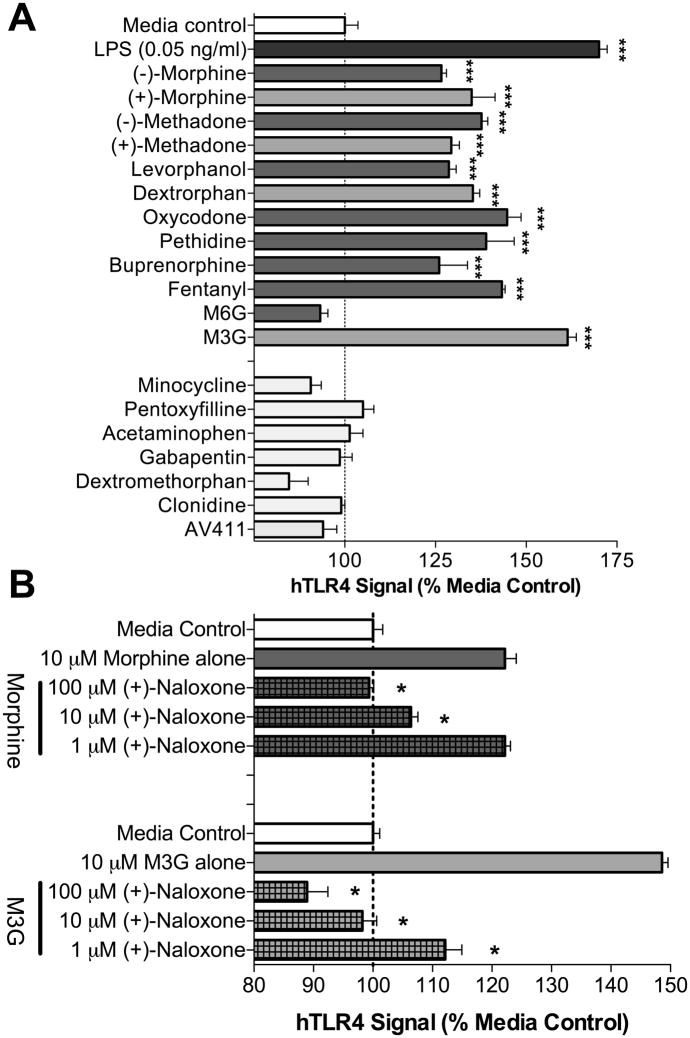
To define whether opioids may potentially affect TLR4 signaling, a series of endotoxin-free, structurally diverse clinically-employed opioid agonists, opioid antagonists, and morphine metabolites (0.001–100 μM) were tested for TLR4 activity in vitro under CNS conditions. Where available, opioid receptor inactive stereoisomers were also tested. Other endotoxin-free pharmacological agents including glial attenuators, typical and atypical analgesics were also examined for TLR4 agonist activity. Morphine, methadone, and levorphanol/dextrorphan (10–100 μM) non-stereoselectively induced TLR4 signaling (Figure 5A; P < 0.05). Pethidine (meperidine), buprenorphine, fentanyl and oxycodone produced significant TLR4 agonism as well (10–100 μM; P < 0.05). In contrast, the opioid receptor antagonists (−)-naloxone and (−)-naltrexone and their opioid receptor inactive stereoisomers (+)-naloxone and (+)-naltrexone were devoid of any TLR4 agonist activity (P > 0.05) as was (+)-nalmefene (data not shown). Importantly, to ensure the responses were TLR4 specific, each compound was tested on a cell line only expressing the reporter gene and not TLR4. No significant non-TLR4 signaling was observed in these cells (data not shown; P > 0.05). While we have previously documented that naloxone and naltrexone non-stereoselectively antagonize LPS-induced TLR4 signaling (Hutchinson et al., 2008), here we add that, in agreement with the failure of (+)-nalmefene to potentiate morphine analgesia in Experiment 3, (+)-nalmefene failed to attenuate LPS-induced TLR4 signaling (data not shown; P > 0.05). The major morphine metabolites morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G) were also examined for TLR4 activity with M3G producing significant (P < 0.01) TLR4 signaling whilst M6G did not (Figure 5A; P > 0.05). This profile is consistent with M3G but not M6G producing mechanical allodynia and thermal hyperalgesia, which we have recently observed to likely be mediated by TLR4 activation (Unpub. Obs.). The positive TLR4 responses produced by opioid agonists at the doses tested were typically less than for 0.5 ng/mL LPS under these conditions, with the exception of M3G which exerted more profound effects. Other analgesics, atypical analgesics and glial attenuators were also devoid of any activation of the TLR4 signaling cascade over the concentration range tested (Figure 5A; P > 0.05). Importantly, stock solutions of each compound were negative for endotoxin contamination using a non-TLR4 dependent endotoxin test (LAL assay). Morphine and M3G TLR4 responses were further examined in vitro and found to be dose dependently inhibited by (+)-naloxone (Figure 5B; P < 0.05). These data suggest that opioid agonists and antagonists will likely be found in future studies to modulate the production of classical products of TLR4 activation, such as proinflammatory cytokines. This is in keeping with initial explorations of this issue in vitro in studies of microglia (Hutchinson et al., 2008) and in peripheral immune studies (Greeneltch et al., 2004; Liu et al., 2006). These data do not preclude morphine interaction with other TLRs, as TLR2 and TLR9 signaling have been documented previously to be affected by opioids (Li et al., 2009; Wang et al., 2008).
Figure 5. Non-stereoselective TLR4 agonism by opioid agonists and antagonism by (+)-naloxone.
A: Opioid agonists (10 μM) non-stereoselectively produced significant TLR4 signaling in the HEK293-hTLR4 cell line, except for morphine-6-glucuronide (M6G), which failed to produce any significant TLR4 response. Notably, TLR4 agonism was observed for representative members of every clinically relevant class of opioids (4,5-epoxymorphinans: morphine, oxycodone, buprenorphine; 3,3,-diphenylpropylamine: methadone; 4-phenylpiperidine: pethadine/meperadine; 4-amilinopiperidine: fentanyl). Other glial attenuators (minocycline, propentofylline and AV411) and non-opioid typical/atypical analgesics (acetaminophen, gabapentin, dextromethorphan and clonidine) had no TLR4 agonist activity. B: (−)-Morphine and morphine-3-glucuronide (M3G) TLR4 signaling is dose dependently inhibited by (+)-naloxone. * = P < 0.05, n = 3 experiments/group.
Experiment 6: In silico studies of human MD-2
An in silico docking analysis was conducted using the recently published high-resolution crystalline structure of the dimer of human TLR4 and MD-2 (Park et al., 2009) and the in silico docking software suite AutoDock 4. Using AutoDock 4, 100 independent docking simulations were run for each ligand with the entire dimer of human TLR4 and MD-2. It was observed that the majority of the ligands docked with greater preference to the LPS binding cleft of MD-2, independent of interactions with TLR4. Current docking simulations have higher confidence in predicting the binding site than calculating the binding energies. Therefore, the in silico docking was repeated with MD-2 alone at higher resolution (0.375 Å), with the resulting ligand docking conformations, frequency of each ligand docking conformation and the estimated energy for each docking conformation being generated. In each case, the optimal conformation with the lowest energy and greatest frequency was selected as the docking conformation of preference for each ligand and the protein residues this conformation interacted with were collected.
All of the opioids docked with varying success to the LPS binding pocket of MD-2 of LPS with an average of 35 out of 100 dockings to this location with as many as 84 (dextrorphan) and as few as 7 (M6G) dockings for this choice conformation. Importantly, in each case, despite the quality of the docking the best conformation for each ligand docked to the same MD-2 pocket. Agonists and antagonists are predicted to dock similarly, as the software does not allow for global conformation change. Conformational change upon agonist binding is necessary to facilitate signal transduction (agonism) versus inhibition (antagonism). Notably, a recently solved TLR4/ligand complex crystal structure showed that agonists and antagonists occupy the same binding site in the TLR4/MD-2 association (Park et al., 2009). Moreover, there was non-stereoselectivity in the docking with both isomers of morphine, methadone, naloxone and naltrexone docking to MD-2. There were specific residues that ligands interacted that were shared with the majority of the opioids screened. Specifically phenylalanine 147 and 76, and isoleucine 63 were prominent. Valine 48 appeared to be of particular importance in opioid binding as this is the only residue differing between the in silico docking of the successful TLR4 inhibitor (+)-naltrexone, which does not interact with valine 48, and the TLR4 inactive (+)-nalmefene, which does interact with residue 48.
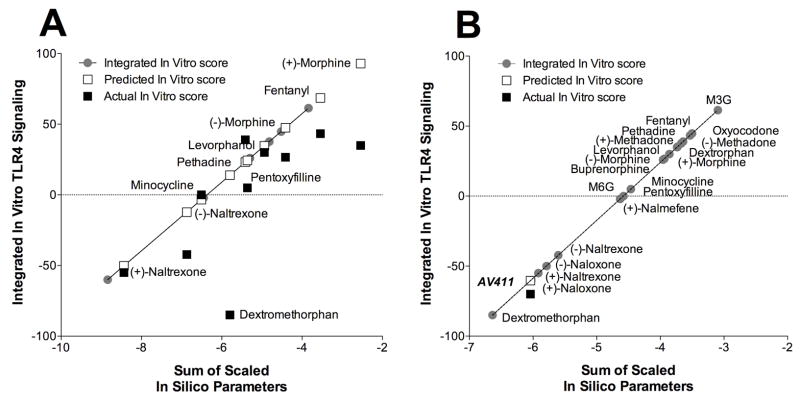
In order for this in silico data to have any validity it should be relatable to in vitro results. Given MD-2 is pivotal to classical TLR4 signaling, alterations in MD-2 function would be expected to impact on TLR4 signaling, possibly changing TLR4 activation or inhibition. Therefore, it is possible that the in silico predictions may have in vitro consequences. As such, an in silico to in vitro prediction model was built using 10 opioid ligands and subsequently tested on 7 different opioid ligands spanning both TLR4 activators and inhibitors (Figure 6A). Three non-opioid compounds that also docked successfully to MD-2 were also tested. The in silico to in vitro prediction model successfully determined stimulation and inhibition categorical assignment of all 7 the small molecule opioids and 2 out of 3 of the non-opioid compounds tested on TLR4 signaling. The ability to predict the extent of activation and inhibition of TLR4 signaling has more error associated with it, but is likely due to the relatively small number of ligands examined thus far. The model successfully built when all twenty ligands were included in the additional adaptation (Figure 6B). This complete model was then used to test the non-opioid, structurally diverse ligand, AV411 (ibudilast), which was found to have TLR4 signaling inhibitory properties using the human TLR4 cell line. The in silico to in vitro prediction proved to be very effective, establishing that based on the MD-2 in silico docking, AV411 was predicted to be a TLR4 signaling inhibitor with an integrated prediction score of −60 versus the actual in vitro score of −70.
Figure 6. in silico MD-2 docking to in vitro TLR4 signaling prediction model.
A: 10 opioid ligands were used to build the in silico to in vitro prediction model which was subsequently tested on 7 opioid and 3 non-opioid ligands, with the predicted and actual in vitro scores displayed. B: The modified complete 20 ligands in silico to in vitro model retested on the structurally diverse TLR4 signaling inhibitor AV411, with the predicted and actual in vitro scores displayed.
Analysis of the multiplication factors demonstrates that binding energy and torsional energy significantly positively contributed, whilst disolvation energy and ligand efficacy parameters significantly negatively contributed to the in vitro response prediction. Interestingly, these same factors contribute in similar fashions in the full twenty ligand model.
DISCUSSION
The present series of studies utilized a combination of in vitro, in vivo, and in silico techniques to explore whether opioids may potentially influence TLR4 signaling. Evidence was found suggestive that TLR4 signaling can occur in response to clinically-employed opioid agonists, their non-opioid (+)-isomers, and the opioid-inactive metabolite morphine-3-glucuronide, but not other classes of typical/atypical analgesics or glial attenuators. Also, the opioid-inactive (+)-naloxone and opioid-active (−)-naloxone block activation of TLR4 signaling by opioids and LPS. Due to the LPS-RS sensitivity of opioid-induced TLR4 responses, and in silico to in vitro correlations, it appears that these xenobiotics (chemicals not endogenous to the organism) can potentially have TLR4 action possibly via interaction with MD-2. The in vivo consequence of xenobiotic induced-MD-2/TLR4 signaling appears to be opposition of acute and chronic opioid analgesia, and contribution to development of hyperalgesia and dependence. Each of these opioid-induced effects has previously been hypothesized to be mediated, in part, via proinflammatory responses induced by unknown mechanisms. Whilst glia appear a likely cellular candidate as the cellular source of opioid-induced proinflammation, the contributory and/or permissive role of other cell types and cofactors within the CNS remains to be elucidated. Together, these data suggest a departure from classical views of opioid pharmacology, and also may provide a novel explanation for several key phenomena, such as effects of M3G.
Prior publications support our conclusion of opioid-induced MD-2/TLR4 signaling and non-stereoselective MD-2/TLR4 signaling inhibition. Firstly, (+)-opioid antagonists block suppression of morphine analgesia by LPS-induced proinflammatory glial activation (Wu et al., 2006). Secondly, naloxone nonstereoselectively prevents LPS-induced microglial activation (Liu et al., 2000). Thirdly, naloxone non-stereoselectively decreases microglial LPS binding (Liu et al., 2000). Last, TLR2 and MyD88-dependent apoptosis in HEK293 cells is induced by morphine (Li et al., 2009). Given TLR4/MD-2 is the endogenous receptor complex for LPS, this suggests a role of TLR4/MD-2 in the non-stereoselective action of both opioid agonists and antagonists. The activity of morphine in TLR2-expressing cells demonstrates that small molecules can have TLR activity, with the possibility that MD-2 may facilitate this for both TLR4 and TLR2, owing to the role of MD-2 in also enabling TLR2 signaling (Dziarski et al., 2001). An alternative hypothesis of (+)-isomer inhibition of a disparate enzyme has been postulated (Liu et al., 2000), but this line of evidence cannot explain the proinflammatory glial activation caused by opioid agonists, as it is unlikely opioid agonists cause activation of the enzyme postulated by Liu et al. (2000).
Acute blockade of the TLR4 receptor, genetic knockout of TLR4, or blockade of TLR4 downstream signaling, was observed to potentiate the magnitude and duration of (−)morphine analgesia. These data suggest a rapid (within minutes) TLR4-dependent opposition of analgesia. A spinal site of action exists since systemic morphine analgesia is potentiated by intrathecal (+)-naloxone. A role for TLR4 in pain enhancement following chronic opioids is suggested since (+)-naloxone blunts development of analgesic tolerance and blocks opioid-induced hyperalgesia. These findings extend previous reports that opioid-induced proinflammatory cytokines and chemokines oppose acute and chronic opioid analgesia (Hutchinson et al., 2007; Hutchinson et al., 2008; Hutchinson et al., 2008; Johnston et al., 2004; Shavit et al., 2005; Watkins et al., 2005). Moreover, evidence was obtained that opioid-TLR4-induced signaling may contribute to opioid dependence, in agreement with previous findings that attenuation of opioid-induced glial activation blunts opioid dependence and withdrawal-induced allodynia (Hutchinson et al., 2009; Hutchinson et al., 2008; Johnston et al., 2004). Previous studies of opioids have used the spontaneous TLR4 mutant, LPS-nonresponsive mouse strain C3H/HeJ (Liang et al., 2006; Rady and Fujimoto, 2001). Differences in opioid action in this strain have been observed, although conclusions are clouded by design issues and lack of control strains, unlike in the present study where knockouts and wildtypes were Balb/c. C3H.HeJ mice are insensitive to dynorphin induced anti-analgesia (Rady and Fujimoto, 2001) and develop less morphine tolerance (Liang et al., 2006). Our direct examination of morphine dose responses, and lack of effect of (+)-naloxone, in TLR4 knockout versus wildtype animals are supportive of a potential importance of morphine-induced TLR4 signaling in morphine’s actions.
The structure activity relationship of xenobiotic MD-2/TLR4 signaling activation differs from that of opioid receptors. Firstly, the similarities are that opioid agonists are MD-2/TLR4 signaling activators, with the exception of M6G; and opioid antagonists are MD-2/TLR4 signaling inhibitors. However, the similarities end here, as the MD-2/TLR4 activity of these xenobiotics is non-stereoselective. Both opioid-active (−)-isomer agonists and opioid-inactive (+)-isomer agonists display MD-2/TLR4 activity. For MD-2/TLR4 activity, there is a significant departure from reliance on the 3′OH of the 4,5-epoxymorphinan, which is required for opioid receptor activity (Chen et al., 1991). This is typified by the MD-2/TLR4 signaling activation of M3G and lack of activity of M6G. Interestingly, the 6′ position of the 4,5-epoxymorphinan is important as (+)-nalmefene loses all activity whilst (+)-naltrexone is TLR4 active. In silico docking suggests that the valine 48 residue of MD-2 is important in this disparity, although the significance of this is unknown. The disparity in the opioid receptor agonist and antagonist structure activity relationship versus that of MD-2/TLR4 is a fortuitous and pharmacologically exploitable one. Importantly, not all xenobiotics display MD-2/TLR4 activity, with a range of other glial attenuators, typical and atypical analgesics being devoid of any such TLR4 responses. Therefore, TLR4 activity is a property of select xenobiotics. Given this, in silico to in vitro prediction tools would be useful. Here, such models were implemented with sizable success of predicting in vitro activity of opioids and non-opioids based on MD-2 in silico docking data. Further development and refinement of this model is underway.
Opioid induced Akt1 phosphorylation has been demonstrated previously using both small molecule (Gupta et al., 2002) and peptide (Polakiewicz et al., 1998) opioid agonists. This action was thought to be mediated exclusively via classical μ opioid receptors. However, the previous non-classical opioid literature, plus the data newly presented here, are consistent with a role of TLR4 in mediating such responses, as well. Moreover, MD-2/TLR4 activity is not limited to neuronally active opioids since the opioid receptor-inactive (+)-isomers also possess the same properties as their opioid active (−)-isomer counterparts. Rather a range of small molecule xenobiotics more broadly may also act at MD-2/TLR4 in either activator or inhibitor fashions (Hutchinson et al., 2007).
Tentative speculations about the mechanism of action at MD-2/TLR4 of small molecules are possible, owing to the diverse techniques employed. Firstly, it appears that the TLR4 signaling activation of (−)-morphine may involve recruitment of MyD88-dependent and MyD88-independent signaling cascades since (−)-morphine induced cytosolic Akt1 clearance and the TIRAP inhibitor potentiated (−)-morphine analgesia. TIRAP is a pivotal adapter protein that interacts with the TIR domain of TLR4 (Schilling et al., 2002) that enables recruitment of MyD88 and the activation of the Toll-Interleukin-1 signaling cascade. In contrast, Akt1 is recruited by TLR4 activation via the TRIF/TRAM complex activation of PI3K (Okun et al., 2008). Since the common component to these two signaling cascades is TLR4, morphine may plausibly be exerting an action at or near TLR4 or at parallel multi-site actions within multiple downstream signaling cascades, which would appear a far less likely possibility than a direct action at or near TLR4. The blockade of morphine’s actions by LPS-RS is in agreement with this possibility as it is a selective TLR4 competitive antagonist. Secondly, a similar conclusion can be made for the TLR4 signaling blockade by (+)-naloxone. (+)-Naloxone blocks (−)- and (+)-morphine, and LPS-induced Akt1 cytosolic clearance, TLR4-induced SEAP expression, and TLR4-mediated actions in vivo. Given this, it appears plausible that (+)-naloxone may inhibit above Akt1 in the TLR4 signaling cascade as discussed previously and blocks MyD88-dependent TLR4 signaling as complete inhibition of LPS and (−)-morphine-induced hTLR4 SEAP expression was observed. The in silico data pointing toward MD-2 are consistent with these hypotheses.
In conclusion, these in vitro, in vivo, and in silico data suggest a potential for non-stereoselective TLR4 agonist and antagonist activity of opioids. The consequence of such MD-2/TLR4 signaling includes opposition of acute opioid analgesia, and development of opioid tolerance, hyperalgesia and dependence, all previously associated with opioid-induced proinflammatory glial activation. If substantiated by future investigations, such non-classical opioid action of MD-2/TLR4 signaling and identification of novel inhibitors of this xenobiotic-induced signaling may suggest new pharmacological avenues to separate the beneficial neuronal analgesic actions of opioids, from the detrimental MD-2/TLR4-mediated glial unwanted side effects.
Acknowledgments
This work was supported by an International Association for the Study of Pain International Collaborative grant, American Australian Association Merck Company Foundation Fellowship, National Health and Medical Research Council CJ Martin Fellowship (ID 465423; M.R.H.) and NIH Grants DA015642, DA017670, DA024044, DE017782, T32 GM-065103, and DE017782. This work was partially supported by the by the NIH Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism. H.Y. thanks the Council on Research and Creative Work at the University of Colorado at Boulder and the Kimmel Foundation (SKF-08-101) for financial support of the work. M.S., T.X.Z. and M.M.B are grateful for HHMI undergraduate grants for biomedical research provided by the Undergraduate Research Opportunities Program at the University of Colorado at Boulder. P.F.S. thanks for the University of Colorado Pre-doctoral Training program in Molecular Biophysics (T32 GM-065103). We thank Avigen (Alameda, CA, USA) for the gift of the HEK293-TLR4 cell line and AV411. Thanks to the Akira Research group, Osaka University, Osaka, Japan for permission to use the TLR4 knockout mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chao CC, Gekker G, Sheng WS, Hu S, Tsang M, Peterson PK. Priming effect of morphine on the production of tumor necrosis factor-alpha by microglia: implications in respiratory burst activity and human immunodeficiency virus-1 expression. J Pharmacol Exp Ther. 1994;269:198–203. [PubMed] [Google Scholar]
- Chen ZR, Irvine RJ, Somogyi AA, Bochner F. Mu receptor binding of some commonly used opioids and their metabolites. Life Sci. 1991;48:2165–71. doi: 10.1016/0024-3205(91)90150-a. [DOI] [PubMed] [Google Scholar]
- Cui Y, Chen Y, Zhi JL, Guo RX, Feng JQ, Chen PX. Activation of p38 mitogen-activated protein kinase in spinal microglia mediates morphine antinociceptive tolerance. Brain Res. 2006;1069:235–43. doi: 10.1016/j.brainres.2005.11.066. [DOI] [PubMed] [Google Scholar]
- Cui Y, Liao XX, Liu W, Guo RX, Wu ZZ, Zhao CM, Chen PX, Feng JQ. A novel role of minocycline: Attenuating morphine antinociceptive tolerance by inhibition of p38 MAPK in the activated spinal microglia. Brain Behav Immun. 2008;22:114–23. doi: 10.1016/j.bbi.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Cuschieri J, Bulger E, Billgrin J, Garcia I, Maier RV. Acid sphingomyelinase is required for lipid Raft TLR4 complex formation. Surgical infections. 2007;8:91–106. doi: 10.1089/sur.2006.050. [DOI] [PubMed] [Google Scholar]
- Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Lab Invest. 2006;86:9–22. doi: 10.1038/labinvest.3700366. [DOI] [PubMed] [Google Scholar]
- Dobrenis K, Makman MH, Stefano GB. Occurrence of the opiate alkaloid-selective mu3 receptor in mammalian microglia, astrocytes and Kupffer cells. Brain Res. 1995;686:239–48. doi: 10.1016/0006-8993(95)00452-v. [DOI] [PubMed] [Google Scholar]
- Dziarski R, Wang Q, Miyake K, Kirschning CJ, Gupta D. MD-2 enables Toll-like receptor 2 (TLR2)-mediated responses to lipopolysaccharide and enhances TLR2-mediated responses to Gram-positive and Gram-negative bacteria and their cell wall components. J Immunol. 2001;166:1938–44. doi: 10.4049/jimmunol.166.3.1938. [DOI] [PubMed] [Google Scholar]
- El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia. 2005;50:91–106. doi: 10.1002/glia.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JH, Falke JJ. Ca2+ influx is an essential component of the positive-feedback loop that maintains leading-edge structure and activity in macrophages. Proc Natl Acad Sci USA. 2007;104:16176–81. doi: 10.1073/pnas.0707719104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Filliol D, Simonin F, Matthes HW, Kieffer BL. Immunosuppression by delta-opioid antagonist naltrindole: delta- and triple mu/delta/kappa-opioid receptor knockout mice reveal a nonopioid activity. Journal of Pharmacology and Experimental Therapeutics. 2001;298:1193–8. [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Matthes HW, Peluso J, Kieffer BL. Abolition of morphine-immunosuppression in mice lacking the mu-opioid receptor gene. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:6326–30. doi: 10.1073/pnas.95.11.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeneltch KM, Haudenschild CC, Keegan AD, Shi Y. The opioid antagonist naltrexone blocks acute endotoxic shock by inhibiting tumor necrosis factor-alpha production. Brain Behav Immun. 2004;18:476–84. doi: 10.1016/j.bbi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Gupta K, Kshirsagar S, Chang L, Schwartz R, Law PY, Yee D, Hebbel RP. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62:4491–8. [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Horvath RJ, DeLeo JA. Morphine enhances microglial migration through modulation of P2X4 receptor signaling. J Neurosci. 2009;29:998–1005. doi: 10.1523/JNEUROSCI.4595-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence and reward. TheScientificWorldJOURNAL. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Coats BD, Lewis SS, Zhang Y, Sprunger DB, Rezvani N, Baker EM, Jekich BM, Wieseler JL, Somogyi AA, Martin D, Poole S, Judd CM, Maier SF, Watkins LR. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav Immun. 2008;22:1178–1189. doi: 10.1016/j.bbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL, Brzeski A, Northcutt A, Vietz CM, Judd CM, Maier SF, Watkins LR, Johnson KW. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast) Brain Behav Immun. 2009;23:240–50. doi: 10.1016/j.bbi.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Northcutt AL, Chao LW, Kearney JJ, Zhang Y, Berkelhammer DL, Loram LC, Rozeske RR, Bland ST, Maier SF, Gleeson TT, Watkins LR. Minocycline suppresses morphine-induced respiratory depression, suppresses morphine-induced reward, and enhances systemic morphine-induced analgesia. Brain Behav Immun. 2008;22:1248–1256. doi: 10.1016/j.bbi.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4) Eur J Neurosci. 2008;28:20–9. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CK, Kim CS, Choi HS, McKercher SR, Loh HH. Transcriptional regulation of mouse mu opioid receptor gene by PU.1. J Biol Chem. 2004;279:19764–74. doi: 10.1074/jbc.M400755200. [DOI] [PubMed] [Google Scholar]
- Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, Leinwand L, Maier SF, Watkins LR. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004;24:7353–65. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni A, Klein G, Pintar JE, Kest B. Nociception increases during opioid infusion in opioid receptor triple knock-out mice. Neuroscience. 2007;147:439–44. doi: 10.1016/j.neuroscience.2007.04.030. [DOI] [PubMed] [Google Scholar]
- Laird MH, Rhee SH, Perkins DJ, Medvedev AE, Piao W, Fenton MJ, Vogel SN. TLR4/MyD88/PI3K interactions regulate TLR4 signaling. J Leukoc Biol. 2009 doi: 10.1189/jlb.1208763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Sun X, Zhang Y, Huang J, Hanley G, Ferslew KE, Peng Y, Yin D. Morphine promotes apoptosis via TLR2, and this is negatively regulated by beta-arrestin 2. Biochem Biophys Res Commun. 2009;378:857–61. doi: 10.1016/j.bbrc.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Liang DY, Guo T, Liao G, Kingery WS, Peltz G, Clark JD. Chronic pain and genetic background interact and influence opioid analgesia, tolerance, and physical dependence. Pain. 2006;121:232–40. doi: 10.1016/j.pain.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Lipovsky MM, Gekker G, Hu S, Hoepelman AI, Peterson PK. Morphine enhances complement receptor-mediated phagocytosis of Cryptococcus neoformans by human microglia. Clin Immunol Immunopathol. 1998;87:163–7. doi: 10.1006/clin.1998.4518. [DOI] [PubMed] [Google Scholar]
- Liu B, Du L, Hong JS. Naloxone protects rat dopaminergic neurons against inflammatory damage through inhibition of microglia activation and superoxide generation. J Pharmacol Exp Ther. 2000;293:607–17. [PubMed] [Google Scholar]
- Liu SL, Li YH, Shi GY, Chen YH, Huang CW, Hong JS, Wu HL. A novel inhibitory effect of naloxone on macrophage activation and atherosclerosis formation in mice. J Am Coll Cardiol. 2006;48:1871–9. doi: 10.1016/j.jacc.2006.07.036. [DOI] [PubMed] [Google Scholar]
- Narita M, Imai S, Kasukawa A, Yajima Y, Suzuki T. Increased level of neuronal phosphoinositide 3-kinase gamma by the activation of mu-opioid receptor in the mouse periaqueductal gray matter: further evidence for the implication in morphine-induced antinociception. Neuroscience. 2004;124:515–21. doi: 10.1016/j.neuroscience.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Narita M, Miyatake M, Narita M, Shibasaski M, Shindo K, Nakamura A, Kuzumaki N, Nagumo Y, Suzuki T. Direct evidence of astrocytic modulation in the development of rewarding effects induced by drugs of abuse. Neuropsychopharmacol. 2006;31:2476–2488. doi: 10.1038/sj.npp.1301007. [DOI] [PubMed] [Google Scholar]
- Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J Immunol. 2003;33:597–605. doi: 10.1002/eji.200323376. [DOI] [PubMed] [Google Scholar]
- Okun E, Griffioen K, Lathia J, Tang S, Mattson M, Arumugam T. Toll-Like Receptors in Neurodegeneration. Brain research reviews. 2008;59:278–292. doi: 10.1016/j.brainresrev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, King T, Vanderah TW, Porreca F. Underlying mechanisms of pronociceptive consequences of prolonged morphine exposure. Biopolymers. 2005;80:319–24. doi: 10.1002/bip.20254. [DOI] [PubMed] [Google Scholar]
- Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Molitor TW, Chao CC. The opioid-cytokine connection. J Neuroimmunol. 1998;83:63–69. doi: 10.1016/s0165-5728(97)00222-1. [DOI] [PubMed] [Google Scholar]
- Polakiewicz RD, Schieferl SM, Gingras AC, Sonenberg N, Comb MJ. mu-Opioid receptor activates signaling pathways implicated in cell survival and translational control. J Biol Chem. 1998;273:23534–41. doi: 10.1074/jbc.273.36.23534. [DOI] [PubMed] [Google Scholar]
- Rady JJ, Fujimoto JM. Confluence of antianalgesic action of diverse agents through brain interleukin(1beta) in mice. J Pharmacol Exp Ther. 2001;299:659–65. [PubMed] [Google Scholar]
- Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci. 2002;22:9980–9. doi: 10.1523/JNEUROSCI.22-22-09980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Attenuation of morphine tolerance, withdrawal-induced hyperalgesia, and associated spinal inflammatory immune responses by propentofylline in rats. Neuropsychopharmacology. 2004;29:327–34. doi: 10.1038/sj.npp.1300315. [DOI] [PubMed] [Google Scholar]
- Ruzicka BB, Akil H. The interleukin-1beta-mediated regulation of proenkephalin and opioid receptor messenger RNA in primary astrocyte-enriched cultures. Neuroscience. 1997;79:517–24. doi: 10.1016/s0306-4522(96)00669-0. [DOI] [PubMed] [Google Scholar]
- Schilling D, Thomas K, Nixdorff K, Vogel SN, Fenton MJ. Toll-like receptor 4 and Toll-IL-1 receptor domain-containing adapter protein (TIRAP)/myeloid differentiation protein 88 adapter-like (Mal) contribute to maximal IL-6 expression in macrophages. J Immunol. 2002;169:5874–80. doi: 10.4049/jimmunol.169.10.5874. [DOI] [PubMed] [Google Scholar]
- Shavit Y, Wolf G, Goshen I, Livshits D, Yirmiya R. Interleukin-1 antagonizes morphine analgesia and underlies morphine tolerance. Pain. 2005;115:50–59. doi: 10.1016/j.pain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001;39:281–6. doi: 10.1016/s0168-0102(00)00226-1. [DOI] [PubMed] [Google Scholar]
- Stefano GB. Autoimmunovascular regulation: morphine and anandamide and ancodamide stimulated nitric oxide release. J Neuroimmuno. 1998;83:70–76. doi: 10.1016/s0165-5728(97)00223-3. [DOI] [PubMed] [Google Scholar]
- Takayama N, Ueda H. Morphine-induced chemotaxis and brain-derived neurotrophic factor expression in microglia. J Neurosci. 2005;25:430–5. doi: 10.1523/JNEUROSCI.3170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Barke RA, Charboneau R, Schwendener R, Roy S. Morphine induces defects in early response of alveolar macrophages to Streptococcus pneumoniae by modulating TLR9-NF-kappa B signaling. J Immunol. 2008;180:3594–600. doi: 10.4049/jimmunol.180.5.3594. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 2005;28:661–9. doi: 10.1016/j.tins.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Wu HE, Hong JS, Tseng LF. Stereoselective action of (+)-morphine over (−)morphine in attenuating the (−)-morphine-produced antinociception via the naloxone-sensitive sigma receptor in the mouse. Eur J Pharmacol. 2007;571:145–51. doi: 10.1016/j.ejphar.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HE, Sun HS, Cheng CW, Terashvili M, Tseng LF. dextro-Naloxone or levo-naloxone reverses the attenuation of morphine antinociception induced by lipopolysaccharide in the mouse spinal cord via a non-opioid mechanism. Eur J Neurosci. 2006;24:2575–80. doi: 10.1111/j.1460-9568.2006.05144.x. [DOI] [PubMed] [Google Scholar]
- Wu HE, Sun HS, Terashivili M, Schwasinger E, Sora I, Hall FS, Uhl GR, Tseng LF. dextro- and levo-morphine attenuate opioid delta and kappa receptor agonist produced analgesia in mu-opioid receptor knockout mice. Eur J Pharmacol. 2006;531:103–7. doi: 10.1016/j.ejphar.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Wu HE, Thompson J, Sun HS, Terashvili M, Tseng LF. Antianalgesia: stereoselective action of dextro-morphine over levo-morphine on glia in the mouse spinal cord. J Pharmacol Exp Ther. 2005;314:1101–8. doi: 10.1124/jpet.105.087130. [DOI] [PubMed] [Google Scholar]