Abstract
Purpose
To evaluate HER2 status in residual tumor identified at the time of surgery in patients not achieving a pathologic complete response (pCR) and to determine the impact of alterations in HER2 status on recurrence-free survival (RFS).
Experimental Design
Clinicopathologic data for patients with HER2-overexpressing breast cancer receiving neoadjuvant therapy with a taxane, anthracycline and concomitant trastuzumab between 2004 and 2007 were reviewed. Surgical specimens for patients achieving less than a pCR were assessed to determine if there was enough residual tissue to evaluate post-treatment HER2 status. RFS was determined using the Kaplan-Meier method and compared by the log rank statistic.
Results
A pCR was achieved in 72 (50.7%) of the 142 patients. Residual tumor was sufficient to assess post-treatment HER2 status in 25 patients. FISH performed on pre-treatment specimens confirmed HER2-amplification prior to beginning therapy. Eight (32.0%) post-treatment tumors were found to be HER2-negative by FISH. At a median follow-up of 37 months (range 8–56 months), the RFS was significantly better for patients with tumors that retained HER2 amplification (87.5% vs. 50%, p=0.04).
Conclusion
High pCR rates are achieved in patients with HER2-positive breast cancer treated with neoadjuvant trastuzumab in combination with anthracyclines and taxanes. One-third of patients with significant residual disease lose HER2 amplification and this change is associated with poor RFS. Residual tumor identified at the time of surgery should be reassessed for HER2 status and novel adjuvant therapy strategies need to be studied in this population.
Keywords: breast cancer, HER2/neu, neoadjuvant chemotherapy, trastuzumab
INTRODUCTION
The HER2/neu (HER2) gene is amplified in approximately 25% of breast cancers (1). Gene amplification results in overexpression of the HER2 protein which is associated with an aggressive clinical course to include a shorter disease-free interval after adjuvant therapy and decreased overall survival (OS) (2–4). The natural history of HER2-overexpressing breast cancer has been altered however by the routine use of trastuzumab, a monoclonal antibody targeting the extracellular domain of the HER2 protein. Trastuzumab has been shown to improve survival in patients with metastatic HER2-positive breast cancer (5,6) as well as in patients with earlier stage disease. Several large, multicenter adjuvant therapy trials demonstrated that the addition of trastuzumab to systemic chemotherapy reduces recurrence by approximately 50% and improves OS by 30% (7,8). Trastuzumab has also been demonstrated to be efficacious when administered in the neoadjuvant setting with pathologic complete response (pCR) rates ranging from 7% to as high as 65% in patients with both early and locally advanced breast cancer (LABC) (9–14). Despite these successes with trastuzumab therapy, not all HER2-positive tumors respond and some patients whose tumors do respond will experience disease recurrence. Investigators from our group recently reported a case of a patient with HER2-positive breast cancer who received adjuvant trastuzumab, but relapsed with HER2-negative metastatic disease (15). In a study conducted to evaluate changes in HER2 status in metastatic lesions of patients previously treated with trastuzumab, Pectasides et al showed that 37% of patients no longer had HER2 expression/amplification, and these patients had significantly shorter time to tumor progression than the group who remained HER2-positive (16).
The purpose of the current study was to evaluate HER2 gene amplification status using fluorescence in situ hybridization (FISH) in the residual tumors of patients who received neoadjuvant systemic therapy with paclitaxel and FEC (5-flourouracil, epirubicin and cyclophosphamide) with concomitant weekly trastuzumab. We also sought to determine the impact of changes in HER2 status on recurrence-free survival (RFS).
MATERIALS AND METHODS
Cell lines and treatments
The BT-474 cell line was purchased from the American Type Culture Collection (Rockville, MD). Cells were maintained in Dulbecco’s modified Eagle medium/Ham F12 1:1 (DMEM/F12) supplemented with 10% fetal bovine serum (FBS) and 2 mM L-glutamine (Life Technologies, Inc. Ltd., Paisley, UK) at 37°C in 5% CO2. Trastuzumab (Herceptin®; kindly provided by F. Hoffmann-La Roche, Basel, Switzerland) was dissolved in sterile apyrogen water and stored at 4 °C. Trastuzumab resistant BT-474 (BT-474R) cells were obtained by culturing the parental BT-474 cells in the presence of increasing concentrations of trastuzumab (up to 500nM) for more than 18 months. Genetic analysis was performed using SNP arrays on the clones and parental cell lines. Protein extraction, western blot and IHC were performed as previously described (17).
Patient Selection
The Department of Breast Medical Oncology database was queried to identify patients with histologically confirmed, HER2-overexpressing (defined as immunohistochemical 3+) or amplified (fluorescence in situ hybridization [FISH]-positive), nonmetastatic, invasive breast cancer who received the neoadjuvant systemic chemotherapy-based regimen with concomitant trastuzumab described below. Patient and tumor characteristics including age at diagnosis, presenting clinical stage, histology, nuclear grade, estrogen (ER) and progesterone (PR) receptor status, presence or absence of lymphovascular invasion, type of surgery and pathologic response in the breast and axilla were recorded. Follow-up data was updated through January 2009. The University of Texas M. D. Anderson Cancer Center Institutional Review Board approved this study.
Pathology
The breast cancer diagnosis was confirmed by review of core biopsy material by dedicated breast pathologists. The histologic subtype of all tumors was defined according to the WHO classification system (18) and the modified Black’s nuclear grading system was used (19). Immunohistochemical analysis was performed to determine ER and PR status. Nuclear staining ≥ 10% was considered positive. HER2 status was evaluated by immunohistochemistry (IHC) and further confirmed by fluorescence in situ hybridization (FISH) in tissue obtained before initiation of neoadjuvant chemotherapy. Interpretations of these assays were based on the most recent American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines (20).
FISH analysis of breast carcinoma was performed using the PathVysion HER-2 DNA probe kit (Vysis, Inc., Downer Grove, IL). Briefly, this assay uses two directly labeled fluorescent DNA probes that specifically target the HER2 locus and CEP17, the alpha-satellite DNA sequence at the centromeric region of the chromosome. For the pretreatment biopsy specimens, all areas of invasive tumor were screened under a fluorescent microscope to evaluate the possibility of heterogeneity among tumor cells. No heterogeneity was identified. Sixty tumor cells (vs 20 cells as per the manufacturer’s recommendation) in each case were then scored for HER2 and CEP17 signals. Among the post-treatment specimens, we scored all tumors cells identified up to 60 when present. For cases with reduced residual tumor cell density due to treatment response, we scored a minimum of 20 tumor cells for HER2 and CEP 17 signals. A FISH ratio (HER2 gene signals to chromosome 17 signals) was determined and if greater than 2.2 was considered positive.
A pathologic complete response (pCR) was defined as no residual invasive disease in the breast and axilla on final pathologic assessment. For patients achieving less than a pCR who had enough residual tumor tissue, a dedicated breast pathologist (YW) reassessed HER2 status in the pretreatment biopsy specimen and in the post-treatment residual tumor using FISH (described above) to determine if HER2 gene amplification was present.
Treatment
Paclitaxel was administered weekly for 12 weeks at a dose of 80 mg/m2/week intravenously. This was followed by 4 cycles of FEC75 (fluorouracil 500 mg/m2 epirubicin 75 mg/m2, cyclophosphamide 500 mg/m2 intravenously, given the first day of each cycle) administered every 3 weeks. Trastuzumab was administered as a loading dose of 4 mg/kg intravenously on the first day and then subsequently given weekly at a dose of 2 mg/kg concomitantly with both the anthracycline and taxane chemotherapy. After completion of neoadjuvant systemic therapy, patients underwent appropriate surgery with either a segmental or total mastectomy. The axillary lymph nodes were assessed with sentinel lymph node biopsy for patients who presented initially with node negative disease and with axillary lymph node dissection for patients who were documented to have axillary lymph node metastasis prior to beginning neoadjuvant systemic therapy. Surgery was followed by radiation therapy when indicated and appropriate endocrine therapy for patients with hormone receptor positive disease. Trastuzumab was continued to complete one year of therapy.
Statistical Analysis
Patient characteristics were tabulated or described by their median and range overall, by pCR group, and by post-neoadjuvant chemotherapy HER2 status group. The Chi square test, or Wilcoxon rank sum test was used as appropriate to determine associations between patient characteristics. Median follow-up time was calculated as the median observation time among all patients. Recurrence was defined as recurrence of disease in either local, regional or distant sites. Recurrence-free survival was defined as the time from diagnosis to the time of first recurrence or last follow-up. Survival distributions were estimated with the Kaplan-Meier method, and the log-rank statistic was used to compare the differences between groups.
RESULTS
Between June 2003 and May 2007, 142 HER2-positive patients were treated with the concomitant trastuzumab and neoadjuvant systemic therapy regimen. Table 1 lists patient characteristics overall and by whether they experienced a pCR. Seventy-two (50.7%) patients achieved a pCR. From the 70 patients with residual disease, 61 (43.0%) had a partial response to neoadjuvant chemotherapy, 6 (4.2%) had stable disease, and three (2.1%) had progression of disease. Compared to patients with residual disease, patients who had a pCR were more likely to have ductal histology (versus lobular or mixed ductal/lobular) (p<0.0001), absence of lymphovascular invasion (p=0.005) and hormone receptor-negative tumors (p=0.045 for ER; p=0.046 for PR).
Table 1.
Patient Characteristics Overall and by pCR
| Overall | pCR | |||||
|---|---|---|---|---|---|---|
| No | Yes | p-value | ||||
| N | N | % | N | % | ||
| 142 | 70 | 72 | ||||
| Race | ||||||
| Black | 23 | 11 | 15.7% | 12 | 16.7% | |
| Spanish/Hispanic | 29 | 15 | 21.4% | 14 | 19.4% | |
| White | 84 | 43 | 61.4% | 41 | 56.9% | |
| Asian/Pacific Islander | 6 | 1 | 1.4% | 5 | 6.9% | 0.480 |
| Age at diagnosis, years | ||||||
| Median (range) | 50 (21–81) | 48 | (25–74) | 52 | (21–81) | 0.0954 |
| Histology | ||||||
| Ductal | 133 | 64 | 91.4% | 69 | 95.8% | |
| Other | 9 | 6 | 8.6% | 3 | 4.2% | <0.0001 |
| Clinical T stage | ||||||
| T1 | 23 | 7 | 10.0% | 16 | 20.8% | |
| T2 | 71 | 34 | 48.6% | 37 | 51.4% | |
| T3 | 23 | 15 | 21.4% | 8 | 11.1% | |
| T4 | 25 | 14 | 20.0% | 11 | 15.3% | 0.138 |
| Clinical N stage | ||||||
| N0 | 45 | 20 | 28.6% | 25 | 34.7% | |
| N1 | 60 | 32 | 45.7% | 28 | 38.9% | |
| N2 | 4 | 1 | 1.4% | 3 | 4.2% | |
| N3 | 33 | 17 | 24.3% | 16 | 22.2% | 0.633 |
| Clinical stage | ||||||
| I | 5 | 1 | 1.4% | 4 | 5.6% | |
| II | 75 | 38 | 54.3% | 37 | 51.4% | |
| III | 62 | 31 | 44.3% | 31 | 43.0% | 0.513 |
| Nuclear grade | ||||||
| II | 33 | 20 | 28.6% | 13 | 18.1% | |
| III | 106 | 49 | 70.0% | 57 | 79.2% | |
| Not reported | 3 | 1 | 1.4% | 2 | 2.8% | 0.214 |
| LVI | ||||||
| Positive | 28 | 21 | 30.0% | 7 | 9.7% | |
| Negative | 114 | 49 | 70.0% | 65 | 90.3% | 0.005 |
| ER | ||||||
| Positive | 68 | 40 | 57.1% | 28 | 38.9% | |
| Negative | 74 | 30 | 42.9% | 44 | 61.1% | 0.045 |
| PR | ||||||
| Positive | 50 | 31 | 44.3% | 19 | 26.4% | |
| Negative | 91 | 39 | 55.7% | 52 | 72.2% | |
| Not reported | 1 | 0 | 1 | 1.4% | 0.046 | |
Abbreviations: pCR, pathologic complete response; LVI, lymphovascular invasion; ER, estrogen receptor; PR, progesterone receptor.
other includes lobular (n=3) and mixed ductal/lobular (n=6) histology
The majority of patients who did not achieve a pCR had a near complete response with only minimal residual disease, such as scattered tumor cells in the primary tumor site or lymph node or minimal cellularity in the surgical specimens. In these patients, HER2 status could not be reassessed. However, in 25 patients achieving less than a pCR, enough residual tissue was available at the time of surgery to reassess HER2 status by FISH. Eight (32.0%) of these patients had tumors that lost HER2 amplification. To confirm that these patients had HER2 gene amplified tumors prior to receiving the concomitant trastuzumab and neoadjuvant chemotherapy regimen, FISH was repeated on their pre-treatment biopsy specimens and homogeneous HER2 amplification was confirmed in all cases (Table 2, Fig. 1). Twenty patients had enough residual disease to reassess ER status in order to compare to pre-treatment ER status. Four (20%) patients had tumors that converted from ER-negative to ER-positive disease. When comparing patients with tumors that lost HER2 gene amplification (n=8) to those with tumors that remained HER2-amplified (n=17), there were no significant differences in clinicopathologic features associated with conversion of HER2 status (Table 3).
Table 2.
HER2 Gene Amplification and Hormone Receptor Status Following Trastuzumab Containing Neoadjuvant Chemotherapy in Patients with Enough Residual Disease Identified at the Time of Surgery to Reassess HER2 Status
| Patient Number | HER2 FISH Ratio Pre-Treatment | HER2 FISH Ratio Post-Treatment | ER* Pre-Treatment | ER Post-Treatment |
|---|---|---|---|---|
| 1 | 3.17 | 1.96 | NEG | NEG |
| 2 | 6.06 | 4.78 | POS | POS |
| 3 | POS (Aneuploid)† | POS (Aneuploid)† | POS | POS |
| 4 | 7.19 | 6.22 | NEG | POS |
| 5 | 3.70 | 1.94 | NEG | NEG |
| 6 | 2.88 | 1.24 | POS | N/A |
| 7 | 5.06 | 5.02 | POS | POS |
| 8 | 5.26 | 5.26 | POS | POS |
| 9 | 5.48 | 4.46 | POS | POS |
| 10 | 5.41 | 1.32 | NEG | POS |
| 11 | 5.04 | 1.26 | NEG | N/A |
| 12 | 13.79 | 6.23 | POS | POS |
| 13 | 4.70 | 4.25 | NEG | POS |
| 14 | 11.63 | 9.63 | POS | POS |
| 15 | 2.39 | 2.42 | POS | POS |
| 16 | 6.22 | 1.23 | NEG | NEG |
| 17 | 4.26 | 4.22 | NEG | NEG |
| 18 | 11.65 | 1.28 | POS | N/A |
| 19 | 8.74 | 6.56 | POS | POS |
| 20 | 6.52 | 4.26 | POS | N/A |
| 21 | 3.87 | 3.56 | NEG | NEG |
| 22 | 2.56 | 2.61 | NEG | POS |
| 23 | 6.82 | 7.12 | POS | POS |
| 24 | 2.96 | 1.29 | NEG | NEG |
| 25 | 2.78 | 2.38 | POS | N/A |
ER status was determined by immunohistochemical analysis. Nuclear staining ≥ was considered positive.
Due to marked aneuploidy of tumor cells and clustering of signals, HER2/neu and CEP17 signals could not be accurately counted however there was at least a 2-fold increase in the number of signals for HER2/neu compared to CEP17.
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; POS, positive; NEG, negative; N/A = not enough residual tumor available to assess.
Patients who lost HER2 amplification are identified in bold.
Fig 1.

FISH was performed to assess HER2 status. (A) FISH performed on biopsy specimen prior to treatment with a trastuzumab containing neoadjuvant chemotherapy regimen. Red = HER2 gene, green = centromere of chromosome 17 (CEP17). HER2/CEP17 = 6.22. Due to the intensity of HER2 staining, merged images were not obtained. (B) FISH performed on residual disease identified at the time of surgery from the same patient following completion of neoadjuvant chemotherapy. Image shown is a merged image of staining for HER2 and CEP 17. HER2/CEP17 = 1.1.
Table 3.
Patient Characteristics by HER2 Status Following Trastuzumab Containing Primary Chemotherapy
| HER2 not Amplified | HER2 Amplified | p-value | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| 8 | 17 | ||||
| Race | |||||
| Black | 1 | 12.5% | 3 | 17.7% | |
| Spanish/Hispanic | 2 | 25.0% | 3 | 17.7% | |
| White | 5 | 62.5% | 11 | 64.7% | 1 |
| Age at diagnosis, years | |||||
| Min | 40 | -- | 30 | -- | |
| Median | 49 | -- | 50 | -- | |
| Max | 67 | -- | 61 | -- | 0.777 |
| Histology | |||||
| Ductal | 7 | 87.5% | 14 | 82.3% | |
| Other | 1 | 12.5% | 3 | 17.7% | 1 |
| Clinical T Stage | |||||
| T1 | 1 | 12.5% | 3 | 17.7% | |
| T2 | 3 | 37.5% | 7 | 41.2% | |
| T3 | 0 | 0.0% | 5 | 29.4% | |
| T4 | 4 | 50.0% | 2 | 11.8% | 0.143 |
| Clinical N Stage | |||||
| N0 | 2 | 25.0% | 6 | 35.3% | |
| N1 | 2 | 25.0% | 9 | 52.9% | |
| N3 | 4 | 50.0% | 2 | 11.8% | 0.186 |
| Clinical Stage | |||||
| I | 0 | 0.0% | 1 | 5.9% | |
| II | 3 | 37.5% | 9 | 52.9% | |
| III | 5 | 62.5% | 7 | 41.2% | 0.774 |
| Nuclear Grade | |||||
| II | 2 | 25.0% | 5 | 29.4% | |
| III | 6 | 75.0% | 12 | 70.6% | 1 |
| LVI | |||||
| Positive | 3 | 37.5% | 5 | 29.4% | |
| Negative | 5 | 62.5% | 12 | 70.6% | 1 |
| ER | |||||
| Positive | 2 | 25.0% | 12 | 70.6% | |
| Negative | 6 | 75.0% | 5 | 29.4% | 0.081 |
| PR | |||||
| Positive | 2 | 25.0% | 11 | 64.7% | |
| Negative | 6 | 75.0% | 6 | 35.3% | 0.097 |
Abbreviations: LVI, lymphovascular invasion; ER, estrogen receptor; PR, progesterone receptor.
The median follow-up for the entire population was 33.5 months (range 8 – 65 months). Patients achieving a pCR had significantly better RFS compared with patients who did not achieve a pCR (p=0.0175; Fig. 2A). The 3- and 5-year RFS estimate for all patients and the 3-year RFS estimate for those who achieved a pCR versus those who did not achieve a pCR are listed in table 4. The median follow-up for the patients who achieved less than a pCR and had enough residual tumor tissue to reassess HER2 status was 37 months (range 8–56 months). Analysis of these patients demonstrated that patients who retained HER2 gene amplification had significantly better RFS compared with patients whose tumors lost HER2 gene amplification (p=0.041; Fig. 2B). The 3-year RFS estimates for patients whose tumors retained HER2 amplification was 87.5% (95% CI: 72.7% – 100%) versus 50.0% (95% CI: 25.0% – 100%) for those that did not (Table 4).
Fig 2.
Kaplan-Meier plots of recurrence-free survival (RFS) by (A) pathologic complete response (pCR), and (B) status of HER2 gene amplification in patients with residual tissue identified at the time of surgery.
Table 4.
Kaplan-Meier Estimates of RFS Among All Patients by pCR and by HER2 Status in Patients with Residual Tissue Identified at the Time of Surgery
| Status | No. of Patients | No. of Events | Median Follow-Up | 3 - Year Estimates | 5 - Year Estimates | p-value | ||
|---|---|---|---|---|---|---|---|---|
| Time (months) | % | 95% CI | % | 95% CI | ||||
| Overall | 142 | 17 | 33.5 | 87.8 | 82.4 to 93.6 | 86.20 | 80.1 to 92.8 | |
| pCR | 33.5 | |||||||
| Yes | 72 | 4 | 95.7 | 91.0 to 100 | 92.90 | 86.0 to 100 | ||
| No | 70 | 13 | 80.1 | 70.8 to 90.5 | - | 0.0175 | ||
| HER2 Status in Residual Tissue | 25 | 6 | 37.0 | 74.9 | 59.4 to 94.5 | - | - | |
| Amplified | 17 | 2 | 87.5 | 72.7 to 100 | - | - | ||
| Not Amplified | 8 | 4 | 50.0 | 25.0 to 100 | - | - | 0.041 | |
There have been 8 deaths in the entire cohort, 2 in the group of patients who achieved a pCR versus 6 in the group achieving less than a pCR. (p=0.137). In the group of 25 patients that had enough residual disease to reassess HER2 status, there has been one death which occurred in a patient whose tumor had lost HER2 amplification.
To investigate the hypothesis that trastuzumab treatment could play a causative role in selecting HER2 negative (without gene amplification) cells within a population of HER2 positive (with gene amplification) cells we cultivated HER2 positive BT-474 breast cancer cells in the presence of increasing concentrations of trastuzumab for more than 18 months isolating several independent subclones. After this period of time, we found that two independent clones treated continuously with trastuzumab (BT-474R) had lost both HER2 overexpression and HER2 gene amplification (Fig. 3) and had acquired resistance to the antiproliferative activity of trastuzumab in vitro (data not shown).
Fig 3.
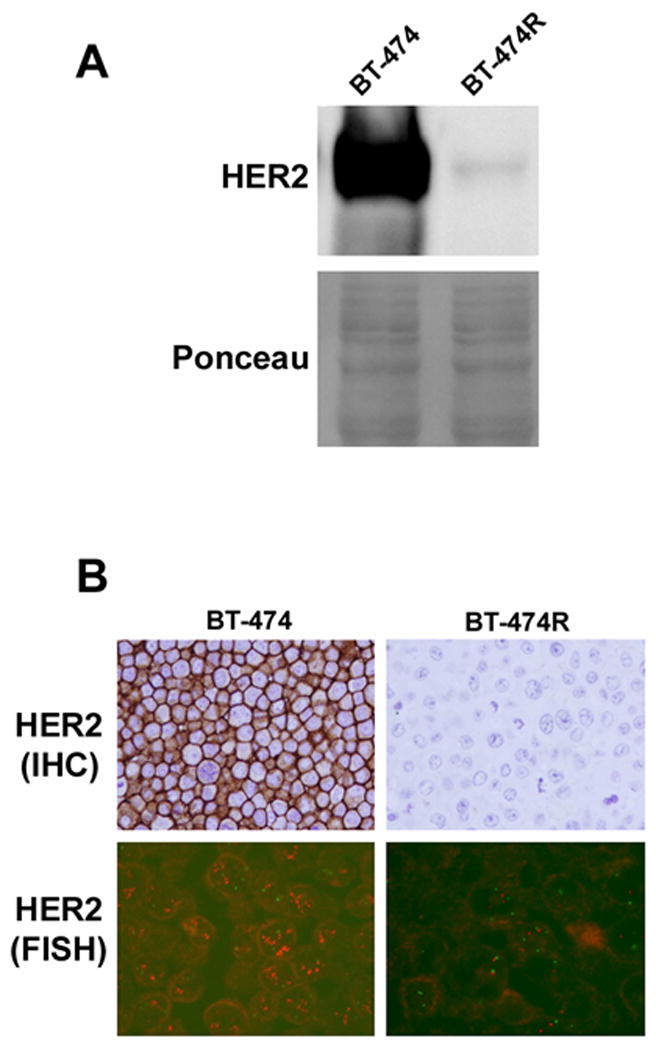
Loss of HER2 overexpression and amplification in BT-474R cells. (A) Western blot showing loss of HER2 overexpression in a representative clone of BT-474R cells. Ponceau staining serves as the loading control. (B) Loss of HER2 overexpression by IHC and loss of HER2 gene amplification by FISH (red = HER2 gene, green = CEP17) of a representative clone of BT-474R cells.
DISCUSSION
Patients with HER2-overexpressing breast cancer treated with trastuzumab-based neoadjuvant systemic therapy achieve a high rate of pCR. In the current study, the pCR rate was 51% following treatment with a neoadjuvant regimen that included taxane and anthracycline-based chemotherapy used concurrently with weekly trastuzumab for 24 weeks. The majority of patients not achieving a pCR had very minimal residual disease (near complete response) with only a third of patients having enough tumor tissue identified at the time of surgery to reassess HER2 status. Importantly, one third of patients who had enough residual disease to repeat HER2 testing had lost amplification of the HER2 gene. Patients who had enough residual disease to reassess HER2 status and had lost HER2 gene amplification had a significantly decreased RFS compared with patients whose tumors remained HER2 amplified.
Other investigators have evaluated HER2 expression in paired samples of pre- and post-treatment tissue from patients treated with trastuzumab in the neoadjuvant setting. Burstein et al reported on HER2 status in patients with residual tumor after treatment with 12 weeks of paclitaxel and trastuzumab (9). Their trial enrolled 40 patients, 23 of whom had residual tissue available for HER2 testing by IHC. In six (26.1%) cases, all of whom were IHC 3+ prior to treatment, the HER2 status changed; to 2+ in two patients and 0 in four patients. In a phase II study of 48 patients treated with 12 weeks of neoadjuvant trastuzumab and vinorelbine, Harris et al reported a HER2 conversion rate of 12% in 18 patients with enough residual tissue to repeat HER2 testing by IHC (12). Although the concordance between HER2 overexpression detected by IHC and HER2 gene amplification by FISH has been demonstrated to be statistically significant, (21–23) there are issues regarding consistency in IHC testing that may impact results including variable fixation, antigen retrieval methods and observer analysis (24). In addition, FISH has been demonstrated to be more reproducible than IHC between central and peripheral laboratories (22,25). Since we utilized FISH to determine HER2 gene amplification status pre- and post-treatment, we are confident that the changes in HER2 status are not due to artifact or inconsistent testing. Consistent with our findings, Hurley et al showed that 43% of tumors that had HER2 gene amplification by FISH before treatment with neoadjuvant trastuzumab, docetaxel and cisplatin, became FISH-negative after therapy (13).
It is unclear whether this change reflects response to therapy or a mechanism of resistance. It is possible that a change in HER2 status could reflect the heterogeneity of HER2 expression within the tumor, suggesting that trastuzumab eliminated HER2-overexpressing clones leaving only HER2-negative tumor cells upon completion of therapy. The results obtained with our preclinical model based on BT-474 cells that acquired resistance to trastuzumab, support this possibility. It seems likely that the change in HER2 status reflects treatment of HER2-overexpressing clones and one could speculate that the trastuzumab therapy was effective in treating the HER2-amplified cells in over 65% of tumors; the 50% that achieved a pCR as well as the 15% that became HER2-negative.
Another interesting finding from our analysis is that four patients whose tumors were ER-negative pretreatment were found to be ER-positive when residual tumor tissue was examined. Previous reports have described cross-talk between the ER and the HER2 pathways and studies have suggested an association between HER2 signaling and resistance to anti-estrogens in human breast cancer. (26–28) While we acknowledge that the current study reports a small number of patients, the findings suggest that, in some patients with HER2-overexpressing, ER-negative breast cancer, treatment with trastuzumab may facilitate sensitivity to anti-estrogen therapy by up-regulating ER expression. This finding requires further confirmation in a larger cohort of patients but given the potential therapeutic implications, we recommend that residual tumor tissue identified in patients treated with concurrent trastuzumab and neoadjuvant chemotherapy be reassessed for HER2 and ER status.
Studies incorporating trastuzumab into neoadjuvant chemotherapy regimens have reported pCR rates ranging from 17% to 65% (9–14). One explanation for the high pCR rates using such regimens is the use of two potentially non-cross resistant chemotherapy agents administered sequentially in combination with trastuzumab. This concept is supported by data from the NOAH (NeOAdjuvant Herceptin) trial which randomized women with HER2-overexpressing locally advanced breast cancer (LABC) or inflammatory breast cancer to receive doxorubicin, paclitaxel and CMF-based neoadjuvant systemic therapy with or without concomitant trastuzumab. This trial enrolled 327 women and the pCR rates were significantly higher in trastuzumab treated patients (39% v. 20%; p=.002) (14). This lower pCR rate compared with our patient cohort may be due to differences in presenting disease stage. An earlier study also focusing on locally advanced and inflammatory HER2-positive disease administered 12 weeks of docetaxel, cisplatin and trastuzumab in 48 patients and reported a pCR rate of 23% in the breast and 17% in the breast and axilla (13). It is difficult to compare pCR rates between trials due to differences in presenting clinical stage, regimens used and duration of therapy as well as differing definitions of pCR. However, because the NOAH trial and the trial reported by Hurley and colleagues enrolled similar patient populations, the differences in the pCR rates are interesting and suggest that the duration of therapy and the use of an anthracycline may be important determining factors for pCR. Currently, the American College of Surgeons Oncology Group is leading a large, multicenter trial (ACOSOG Z1041) comparing a neoadjuvant regimen of FEC75 followed by paclitaxel plus trastuzumab with a neoadjuvant regimen of paclitaxel plus trastuzumab followed by FEC75 plus trastuzumab in patients with HER2-overexpressing breast cancer. Results from this trial should provide conclusive data regarding the utility of administering trastuzumab concurrently with an anthracycline in the neoadjuvant setting.
Achieving a pCR is an important endpoint for patients receiving neoadjuvant systemic therapy as it has been demonstrated to correlate with long-term outcomes (29,13). In the current study, we again show that achieving a pCR is associated with improved RFS. There was a trend towards improvement in OS although this did not reach statistical significance, which we attribute to the relative short median follow up time of 33.5 months. Importantly, a novel finding in the current study is the effect on RFS of loss of HER2 gene amplification in patients with measurable residual disease following administration of trastuzumab. Patients whose tumors lost HER2 gene amplification as determined by FISH analysis had a significantly worse RFS than those whose tumors remained HER2 amplified.
In conclusion, we observed that approximately one third of patients with measurable residual disease following administration of a neoadjuvant systemic therapy regimen that included taxane/anthracycline-based chemotherapy used concurrently with weekly trastuzumab for 24 weeks, lost HER2 gene amplification. Our data demonstrate that this change impacts RFS. Patients who had measurable residual disease and converted to HER2-negative disease had a significantly shorter RFS than patients who had measurable residual tumor but retained HER2 gene amplification. This finding could have implications regarding additional adjuvant therapy. Currently, our practice is to administer trastuzumab post-operatively to complete one year of therapy, based on data from the multicenter adjuvant trials (7,8). If conversion of HER2 status reflects response to therapy such that only HER2-negative clones remain, the need to complete one year of trastuzumab in the adjuvant setting comes into question. Furthermore, all patients with early stage HER2-positive disease who relapse after adjuvant or neoadjuvant trastuzumab therapy should have biopsies of their recurrent disease and re-assessment of their marker status as we have demonstrated that a change in marker status correlates with outcome in patients who develop metastatic disease (30). These data suggest that there may be utility in assessing HER2 status in residual disease identified at the time of surgery and that future clinical trials should be designed to investigate the most appropriate strategy for adjuvant therapy in these patients.
Acknowledgments
A.M.G. is supported in part by an ASCO Career Development Award and NCI 1K23CA121994–01. E.A.M. is supported in part by NCI 1K99CA133244–01. This work was supported in part by the Nellie B. Connally Breast Cancer Research Fund.
Footnotes
Presented at the American Society of Clinical Oncology Breast Cancer Symposium, Washington, DC, September 4-7, 2008.
STATEMENT OF TRANSLATIONAL RELEVANCE
This study confirmed that patients with HER2-overexpressing breast cancer treated in the neoadjuvant setting with trastuzumab-based systemic therapy achieve a high rate (approximately 50%) of pathologic complete response (pCR). Importantly, in patients not achieving a pCR who had significant residual disease, fluorescence in situ hybridization demonstrated that the tumors from one third of these patients no longer had amplification of the HER2 gene. Those patients with tumors that were no longer HER2 amplified had a significantly worse recurrence free survival than those with tumors that retained HER2 amplification. Taken together, these data suggest that residual tumor identified at the time of surgery in patients receiving trastuzumab-based neoadjuvant therapy should be reassessed for HER2 status and that novel adjuvant therapy strategies need to be studied in this population.
References
- 1.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–12. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 2.Paik S, Hazan R, Fisher ER, et al. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: prognostic significance of erbB-2 protein overexpression in primary breast cancer. J Clin Oncol. 1990;8:103–12. doi: 10.1200/JCO.1990.8.1.103. [DOI] [PubMed] [Google Scholar]
- 3.Press MF, Bernstein L, Thomas PA, et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol. 1997;15:2894–904. doi: 10.1200/JCO.1997.15.8.2894. [DOI] [PubMed] [Google Scholar]
- 4.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 5.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–48. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 7.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 8.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 9.Burstein HJ, Harris LN, Gelman R, et al. Preoperative therapy with trastuzumab and paclitaxel followed by sequential adjuvant doxorubicin/cyclophosphamide for HER2 overexpressing stage II or III breast cancer: a pilot study. J Clin Oncol. 2003;21:46–53. doi: 10.1200/JCO.2003.03.124. [DOI] [PubMed] [Google Scholar]
- 10.Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–85. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 11.Buzdar AU, Valero V, Ibrahim NK, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007;13:228–33. doi: 10.1158/1078-0432.CCR-06-1345. [DOI] [PubMed] [Google Scholar]
- 12.Harris LN, You F, Schnitt SJ, et al. Predictors of resistance to preoperative trastuzumab and vinorelbine for HER2-positive early breast cancer. Clin Cancer Res. 2007;13:1198–207. doi: 10.1158/1078-0432.CCR-06-1304. [DOI] [PubMed] [Google Scholar]
- 13.Hurley J, Doliny P, Reis I, et al. Docetaxel, cisplatin, and trastuzumab as primary systemic therapy for human epidermal growth factor receptor 2-positive locally advanced breast cancer. J Clin Oncol. 2006;24:1831–8. doi: 10.1200/JCO.2005.02.8886. [DOI] [PubMed] [Google Scholar]
- 14.Gianni L, Semiglazov V, Manikhas GM, et al. Neoadjuvant trastuzumab in locally advanced breast cancer (NOAH trial): Feasibility safety and antitumor effects. 2007 Breast Cancer Symposium Proceedings; 2007. p. 131. [Google Scholar]
- 15.Dawood S, Resetkova E, Gonzalez-Angulo AM. Trastuzumab administration associated with change in HER2 status. Clin Breast Cancer. 2008;8:366–9. doi: 10.3816/CBC.2008.n.044. [DOI] [PubMed] [Google Scholar]
- 16.Pectasides D, Gaglia A, Arapantoni-Dadioti P, et al. HER-2/neu status of primary breast cancer and corresponding metastatic sites in patients with advanced breast cancer treated with trastuzumab-based therapy. Anticancer Res. 2006;26:647–53. [PubMed] [Google Scholar]
- 17.Scaltriti M, Verma C, Guzman M, et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28:803–14. doi: 10.1038/onc.2008.432. [DOI] [PubMed] [Google Scholar]
- 18.The World Health Organization. Histological Typing of Breast Tumors--Second Edition. The World Organization. Am J Clin Pathol. 1982;78:806–16. doi: 10.1093/ajcp/78.6.806. [DOI] [PubMed] [Google Scholar]
- 19.Black MM, Speer FD. Nuclear structure in cancer tissues. Surg Gynecol Obstet. 1957;105:97–102. [PubMed] [Google Scholar]
- 20.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 21.Dybdal N, Leiberman G, Anderson S, et al. Determination of HER2 gene amplification by fluorescence in situ hybridization and concordance with the clinical trials immunohistochemical assay in women with metastatic breast cancer evaluated for treatment with trastuzumab. Breast Cancer Res Treat. 2005;93:3–11. doi: 10.1007/s10549-004-6275-8. [DOI] [PubMed] [Google Scholar]
- 22.Perez EA, Suman VJ, Davidson NE, et al. HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. J Clin Oncol. 2006;24:3032–8. doi: 10.1200/JCO.2005.03.4744. [DOI] [PubMed] [Google Scholar]
- 23.Press MF, Sauter G, Bernstein L, et al. Diagnostic evaluation of HER-2 as a molecular target: an assessment of accuracy and reproducibility of laboratory testing in large, prospective, randomized clinical trials. Clin Cancer Res. 2005;11:6598–607. doi: 10.1158/1078-0432.CCR-05-0636. [DOI] [PubMed] [Google Scholar]
- 24.Sauter G, Lee J, Bartlett JM, et al. Guidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerations. J Clin Oncol. 2009;27:1323–33. doi: 10.1200/JCO.2007.14.8197. [DOI] [PubMed] [Google Scholar]
- 25.Paik S, Bryant J, Tan-Chiu E, et al. Real-world performance of HER2 testing--National Surgical Adjuvant Breast and Bowel Project experience. J Natl Cancer Inst. 2002;94:852–4. doi: 10.1093/jnci/94.11.852. [DOI] [PubMed] [Google Scholar]
- 26.Kurokawa H, Arteaga CL. Inhibition of erbB receptor (HER) tyrosine kinases as a strategy to abrogate antiestrogen resistance in human breast cancer. Clin Cancer Res. 2001;7:4436s–4442s. discussion 4411s–4412s. [PubMed] [Google Scholar]
- 27.Kurokawa H, Arteaga CL. ErbB (HER) receptors can abrogate antiestrogen action in human breast cancer by multiple signaling mechanisms. Clin Cancer Res. 2003;9:511S–5S. [PubMed] [Google Scholar]
- 28.Liu Y, el-Ashry D, Chen D, et al. MCF-7 breast cancer cells overexpressing transfected c-erbB-2 have an in vitro growth advantage in estrogen-depleted conditions and reduced estrogen-dependence and tamoxifen-sensitivity in vivo. Breast Cancer Res Treat. 1995;34:97–117. doi: 10.1007/BF00665783. [DOI] [PubMed] [Google Scholar]
- 29.Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–9. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- 30.Liedtke C, Broglio K, Moulder S, et al. Prognostic impact of discordance between triple receptor measurements in primary and recurrent breast cancer. Annals of Oncology. 2009 doi: 10.1093/annonc/mdp263. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]



