Abstract
Background
Behaviorally inhibited (BI) children who also exhibit enhanced response monitoring might be at particularly high risk for anxiety disorders. The current study tests the hypothesis that response monitoring, as manifest in the error-related negativity (ERN), moderates the association between BI and anxiety.
Methods
Participants (n = 113; 73 male) assessed for early-childhood BI were re-assessed as adolescents with a clinical interview and a flanker paradigm that generated behavioral data and event-related potentials (ERPs). Risk for anxiety disorders in adolescents was examined as a function of childhood-BI status and adolescent performance on the flanker paradigm.
Results
Adolescents with childhood BI displayed ERP evidence of enhanced response monitoring, manifest as large ERNs. The ERN moderated the relationship between early BI and later clinically significant disorders.
Conclusions
Physiological measures of response monitoring might moderate associations between early-childhood BI and risk for psychopathology. The subset of children with BI and enhanced response monitoring might face greater risk for later-life clinical anxiety than children with either BI or enhanced response monitoring alone.
Keywords: Anxiety, attention, behavioral inhibition, ERP, flanker, response monitoring
Early appearing behavioral inhibition (BI) predicts heightened emotional reactivity throughout childhood (see 1 for a review) and increased risk for clinically significant anxiety (2-5). Nevertheless, only a subset of BI children actually develops anxiety disorders. Little research to date has identified the mechanisms by which some children with BI develop anxiety disorders and others do not.
Theories on moderators of the BI/anxiety association suggest that both biological attributes of the child (6) as well as parental traits (7,8) might influence initial temperamental dispositions, leading to increased vigilance toward threat particularly in novel or unfamiliar social situations. This heightened vigilance is thought to increase a child’s monitoring of their interactions with the environment, which in turn might enhance the risk for anxiety in BI children (see 9).
Consistent with this possibility, data have demonstrated links between physiological measures of response monitoring, manifest as the error-related negativity (ERN) (10) and anxiety (11-13). These studies suggest that anxious individuals display enhanced response monitoring and heightened ERNs compared with non-anxious subjects. However, to date, there has been no examination of error monitoring in BI children. And most of the studies linking anxiety and response monitoring have used cross-sectional designs. The current, prospective, longitudinal study examines the degree to which response monitoring moderates the association between early-childhood BI and adolescent anxiety.
Methods and Materials
Participants
Participants (n = 153, 73 male) were assessed during infancy and early childhood for BI. The mean of children’s BI scores at four time points (14 and 24 months, 4 and 7 years of age) indexed a BI composite. Participants were seen in adolescence (mean = 15.1 ± 1 years), at which time parents/adolescents provided written informed consent/assent, and the flanker task (14) was completed (n = 113, 50 male subjects). No significant demographic differences emerged between participants who did or did not complete the flanker task. Participants with only one BI assessment (n = 8) or with incomplete (n = 10) or inaccurate (n = 13) flanker data were excluded. Demographic and BI data were similar in the final included (n = 82, 37 male) and excluded subjects (Supplements 1).
Procedures
Flanker Task
Equal numbers of congruent (HHHHH or SSSSS) and incongruent (SSHSS or HHSHH) trials were presented, and participants were told to use a button-box to identify the middle letter (14). Hand-letter mappings were counterbalanced; participants were encouraged to respond quickly and accurately. A practice block (20 trials) was administered, followed by three test blocks (160 trials each) during which reaction time (RT) and accuracy were recorded. Trials with RTs ≤ 200 msec were excluded.
Electroencephalogram Data
Electroencephalogram was recorded from 15 sites (F3, Fz, F4, F7, F8, Fz, C3, C4, P3, P4, Pz, O1, O2, T7, and T8; Cz reference, AFz ground). Impedance was 10 kΩ or below. Electroencephalogram was time-locked to participant response to create ERPs for both correct and incorrect trials. Only errors of commission (average number of trials = 47 ± 34.2) were classified as incorrect and were used to generate the ERN, scored as the negative most deflection in a −20–120 msec window. The Pe was scored as the positive most peak 100 – 400 msec after response. Both the ERN and Pe were scored for minimal and maximal amplitudes, and mean amplitudes, respectively, as well as mean amplitude at Fz, Cz, and Pz (12). Results did not differ between approaches; thus only peak amplitude data are presented.
Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version Diagnostic Interview
Adolescents and parents completed semi-structured diagnostic interviews, conducted by advanced clinical psychology doctoral students under the supervision of diagnostic experts, all of whom were blind to BI data (15). Final diagnoses were discussed by the clinical team and made by expert consensus. Audio-taped interviews (n = 59) also were reviewed by experts to maintain reliability (κ > .80 for all diagnostic categories). The current study focused on the lifetime presence or absence of any clinically significant anxiety disorder (including generalized anxiety, separation anxiety, and social phobia).
Data Analysis
Flanker performance was examined with two separate repeated-measures analyses of variance (ANOVAs) with trial type (congruent vs. incongruent) and accuracy (correct or incorrect) as within-subjects variables and BI (median split to create high vs. low groups) served as the between-subjects factor. Gender and age served as covariates. Response monitoring was first investigated with separate repeated-measures ANOVAs for the ERN and Pe, with site as a within-subjects factor, BI group as the between-subjects factor, and gender and age as covariates. Of note, correct-trial-amplitude (CRN) did not differ as a function of BI, verifying that group differences did not reflect general task responding. Response monitoring was then further examined in relation to anxiety outcomes, with ANOVAs and logistic regression.
Results
Behavioral Performance
The main effects for trial type indicated that all participants were more accurate [F(1,78) = 76.03, p < .01] and had faster reaction times [F(1,78) = 144.86, p < .01] on congruent as compared with incongruent trials (see Table 1). These findings demonstrate the expected pattern of behavioral performance on the flanker task.
Table 1.
Flanker Interference Effects as Assessed by Accuracy Rate and Average Reaction Time
| % Accuracy Rate (SD) | Average RT in msec (SD) | |
|---|---|---|
| Congruent Trials | ||
| Total sample | 95.7 (7.8)a | 448 (65)b |
| Low BI | 94.6 (9.5) | 453 (57) |
| High BI | 96.9 (5.3) | 442 (72) |
| Incongruent Trials | ||
| Total sample | 87.2 (10.5)a | 492 (71)b |
| Low BI | 86.3 (12.1) | 500 (69) |
| High BI | 88.2 (8.7) | 484 (73) |
Typical flanker effects were present across all participants regardless of behaviorally inhibited (BI) group, such that faster and more accurate responses emerged for congruent as compared with incongruent trials. Note: matching superscripts indicate significant differences (p values < .01).
Response Monitoring
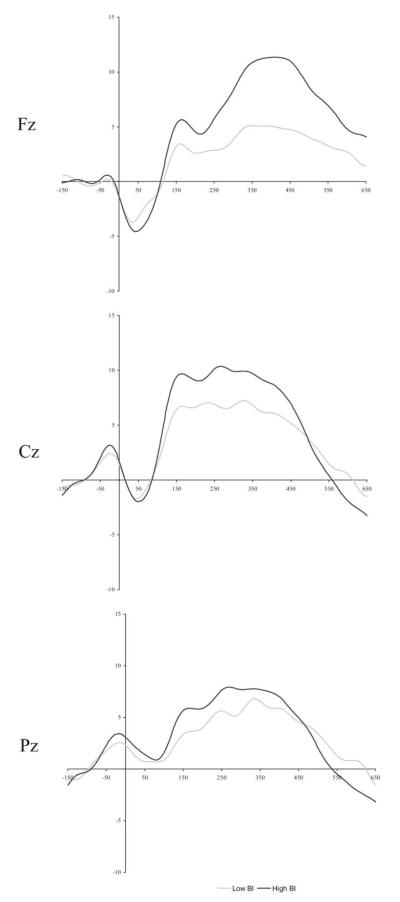
For the ERN, a two-way interaction emerged between BI Group and Site [F (2,156) = 3.15, p < .05]. Specifically, adolescents high in childhood BI exhibited greater ERN amplitude at site Fz than low childhood BI adolescents [t (80) = 2.84, p < .05; see Figure 1]. No effects with group emerged for the Pe.
Figure 1.
Average event related potential amplitudes for incorrect trials across the midline sites of Fz, Cz, and Pz. Low and high behaviorally inhibited (BI) groups are represented by gray and black lines, respectively. The window for the error-related negativity response is −20–120 msec, whereas the positivity window is from 100 to 400 msec.
BI, Response Monitoring, and Anxiety
The two-way interaction between frontal ERN and BI predicted anxiety diagnosis (odds ratio = 1.3; 95% confidence interval = 1.02–1.6; Wald χ2 = 4.4; p < .05; see Figure 2). This interaction reflected the fact that the relations between ERN and anxiety varied by BI classification. In the high BI group, smaller ERN responses were related to lower risk for anxiety diagnosis at a trend level (odds ratio = .82; 95% CI = .66 –1.01; Wald χ2 = 3.39, p = .06). For the low BI group, there was no relation between ERN response and anxiety diagnosis (odds ratio = 1.2; 95% CI = .92–1.5; Wald χ2 = 1.7, p = .19).
Figure 2.
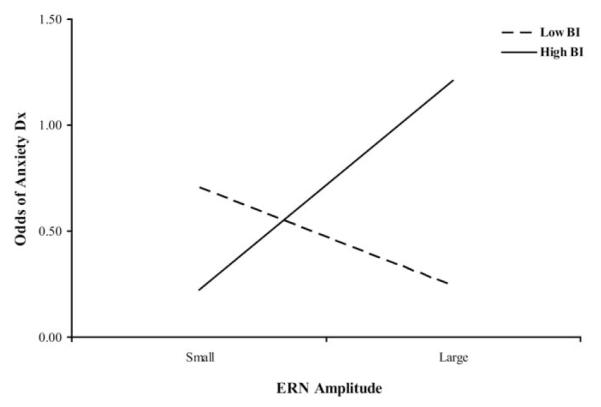
The relation between behavioral inhibition (BI) and frontal error-related negativity (ERN) with respect to risk of anxiety diagnosis. High BI children with large ERN responses are at greater risk of an anxiety diagnosis, as are low BI children with small ERN responses.
Discussion
Two main findings emerged from this study. First, children with BI exhibited an enhanced ERN response as adolescents. Second, those BI children with enhanced ERN responses were at particularly high risk for clinically significant anxiety. These findings correspond to work with BI adults (16), which suggests that an enhanced cholinergic system in highly inhibited individuals contributes to enhanced ERNs by initiating phasic decreases in the dopaminergic system. According to Gray (17,18), the cholinergic system is also involved in the expression of heightened anxiety via inhibited behavior.
Although previous work links early childhood BI and anxiety disorders, major questions have remained concerning which BI children face particularly high risk for anxiety. The current study provides evidence within a longitudinal framework that variability in the ERN response moderates individual differences in vulnerability to anxiety disorders among BI and non-BI children. Due to the sample sizes and trend-level significance of the key post hoc test (ERN-by-anxiety relation in the high anxiety group) as well as the use of concurrent measures of anxiety and response monitoring, replication of these relations is needed.
Interestingly, there were no significant findings for the Pe despite the appearance of a large group difference at Fz. This result is similar to the work of Ladouceur et al. (13) in which Pe amplitude was visibly distinct but not significantly different among clinically anxious and non-anxious adolescents. Although a number of functions have been ascribed to the Pe (19,20), this component is not well-studied yet, especially within populations at-risk for clinical issues (16). Thus, future work should extend the investigation of this sample of children and aim to further clarify the basic neural processes within the frontal region that are linked to heightened response monitoring and self-evaluation at the level of regional specification and function.
In sum, this study highlights the role of individual differences in response monitoring patterns among adolescents and implicates monitoring as an important cognitive process involved in modulating outcomes associated with anxious behavior. Whereas other studies have found that adults exhibiting negative affect or anxiety disorders show heightened self-monitoring, this is the first study to demonstrate these associations in a sample of children selected as at-risk for anxiety disorders.
Supplementary Material
Acknowledgments
This project was supported by grants from the National Institutes of Health (MH074454 and HD17899) to NAF. We would like to thank the children and their families for their continued participation in our studies.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: Linking biology and behavior within a developmental framework. Ann Rev Psychol. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- 2.Biederman J, Hirshfeld-Becker DR, Rosenbaum JF, Herot C, Friedman D, Snidman N, et al. Further evidence of association between behavioral inhibition and social anxiety in children. Am J Psychiatry. 2001;158:1673–1679. doi: 10.1176/appi.ajp.158.10.1673. [DOI] [PubMed] [Google Scholar]
- 3.Gladstone GL, Parker GB, Mitchell PB, Wilhelm KA, Malhi GS. Relationship between self-reported childhood behavioral inhibition and lifetime anxiety disorders in a clinical sample. Depression and Anxiety. 2005;22:103–113. doi: 10.1002/da.20082. [DOI] [PubMed] [Google Scholar]
- 4.Hirshfeld DR, Rosenbaum JF, Biederman J, Bolduc EA, Faraone SV, Snidman N, et al. Stable behavioral inhibition and its association with anxiety disorder. J Am Acad Child Adolesc Pscychiatry. 1992;31:103–111. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Edgar K, Fox NA. Temperament and anxiety disorders. Child Adolesc Psychiatry Clin North Am. 2005;14:681–706. doi: 10.1016/j.chc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Biederman J, Hirshfeld-Becker DR, Rosenbaum JF, Herot C, Friedman D, Snidman N, et al. Further evidence of association between behavioral inhibition and social anxiety in children. Am J Psychiatry. 2001;158:1673–1679. doi: 10.1176/appi.ajp.158.10.1673. [DOI] [PubMed] [Google Scholar]
- 7.Barrett P, Rapee R, Dadds M, Ryan S. Family enhancement of cognitive style in anxious and aggressive children. J Abnorm Child Psychol. 1996;24:187–203. doi: 10.1007/BF01441484. [DOI] [PubMed] [Google Scholar]
- 8.Moore PS, Whaley SE, Sigman M. Interactions between mothers and children: Impacts of maternal and child anxiety. J Abnorm Child Psychol. 2004;113:471–476. doi: 10.1037/0021-843X.113.3.471. [DOI] [PubMed] [Google Scholar]
- 9.Rapee RM, Heimberg RG. A cognitive-behavioral model of anxiety in social phobia. Behav Res Ther. 1997;35:741–756. doi: 10.1016/s0005-7967(97)00022-3. [DOI] [PubMed] [Google Scholar]
- 10.Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- 11.Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychol Sci. 2000;11:1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- 12.Hajcak G, McDonald N, Simons RF. Anxiety and error-related brain activity. Biol Psychol. 2003;64:77–90. doi: 10.1016/s0301-0511(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 13.Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, Ryan ND. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. J Child Psychol Psychiatry. 2006;47:1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- 14.Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception and Psychophysics. 1974;16:143–149. [Google Scholar]
- 15.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS_PL): Initial reliability and validity data. J Am Acad Child Adolesc Pscychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Boksem MAS, Tops M, Wester AE, Meijman TF, Lorist MM. Error-related ERP components and individual differences in punishment and reward sensitivity. Brain Res. 2006;1101:92–101. doi: 10.1016/j.brainres.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Gray JA. The neuropsychology of emotion and personality. In: Stahl SM, Iverson SD, Goodman EC, editors. Cognitive Neurochemistry. Oxford University Press; Oxford: 1987. pp. 171–190. [Google Scholar]
- 18.Gray JA. Fundamental systems of emotion in the mammalian brain. In: Palermo DS, editor. Coping with Uncertainty: Behavioral and Developmental Perspectives. Lawrence Erlbaum; Hillsdale, New Jersey: 1989. pp. 173–195. [Google Scholar]
- 19.Falkenstein M. ERP correlates of erroneous performance. In: Ullsperger M, Falkenstein M, editors. Errors, Conflicts, and the Brain. Current Opinions on Performance Monitoring. Max Planck Institute of Cognitive Neuroscience; Leipzig, Germany: 2004. [Google Scholar]
- 20.Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GPH, Kok A. Error-related brain potentials are differentially related to awareness of response errors: Evidence from an anti-saccade task. Psychophysiology. 2001;38:752–760. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.