To the Editor:
Endogenous adenosine is an important mediator of ischaemic preconditioning and postconditioning. The protective effect of ischaemic preconditioning is dependent on activation of adenosine A1 receptors in the first few minutes of reperfusion [1]. In contrast, the infarct size-limiting effect of ischaemic postconditioning is mediated by the activation of adenosine A2A and A2B receptors at the time of reperfusion [2, 3]. Paradoxically, although the cardioprotective effect of preconditioning and postconditioning are critically dependent on adenosine receptor stimulation immediately after reperfusion, several research groups could not demonstrate an infarct size-limiting effect of administration of exogenous adenosine at the moment of reperfusion [4–6]. A possible explanation is that the pre- and postconditioning stimuli increase the sensitivity of a specific adenosine receptor sub-type. Indeed, this mechanism was recently proposed for the adenosine A2B receptor [7]. The A2B receptor has a K m value for adenosine that is much higher than the endogenous adenosine concentration reached during ischaemia. Kuno et al have recently shown in the rabbit heart that brief preconditioning ischaemia markedly lowered the threshold for an adenosine A2B agonist to increase the phosphorylation of the kinases, Akt and Erk1/2 [7].
In this study, we test the hypothesis that ischaemic preconditioning also increases the sensitivity of the adenosine A1 receptor at the time of myocardial reperfusion, and that ischaemic postconditioning increases the sensitivity of the adenosine A2A receptor, using an isolated perfused rat heart model. As adenosine A1 receptor stimulation has a negative inotropic effect and adenosine A2A receptor stimulation induces coronary vasodilation, we used heart rate and coronary flow as read-out parameters for adenosine A1 and A2A receptor sensitivity, respectively. Male Sprague-Dawley rats were obtained from the Biological Services Unit of University College London (United Kingdom). All experiments were conducted in accordance with UK Home Office Guide on the Operation of Animal (Scientific Procedures) Act of 1986. After anaesthesia with pentobarbital, hearts were excised and mounted on a constant pressure (80 mmHg) Langendorff-apparatus and perfused with modified Krebs–Henseleit bicarbonate buffer (gassed with 95% O2 / 5% CO2, pH 7.35–7.5). Heart rate and coronary flow were monitored at regular intervals. To study the sensitivity of the adenosine A1 receptor, hearts were perfused with incremental concentrations of the selective A1 receptor agonist 2-chloro-N6-cyclopentyl-adenosine (CCPA) (10−10 to 10−6 mmol/l) for 5 min per concentration. Heart rate was measured in the last minute of perfusion of each concentration and compared to the baseline heart rate. To study the sensitivity of the A2A receptor, the hearts were perfused with the selective A2A receptor agonist CGS21680 (10−10 to 10−6 mmol/l) and coronary flow rate was measured in the last minute of each concentration. These measurements were performed at baseline (after 30 min of equilibration), at 20 min reperfusion after 35 min of regional ischaemia in control hearts and hearts treated with a standard ischaemic preconditioning protocol (two cycles of 5 min of global ischaemia and reperfusion) or a standard ischaemic postconditioning protocol (6 cycles of 10 s of global ischaemia and reperfusion). The measurements were performed 20 min after reperfusion to allow the heart rate and coronary flow to return to baseline values after the ischaemic insult.
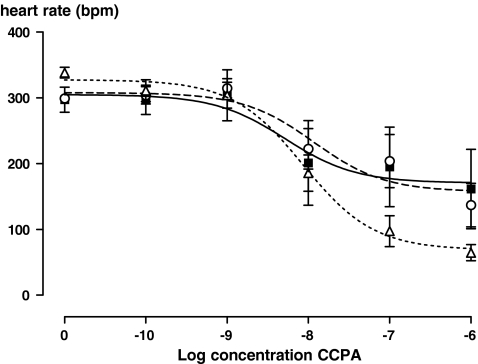
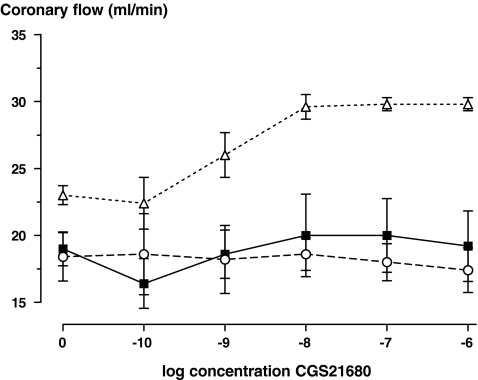
Perfusion with CCPA dose-dependently lowered heart rate (Fig. 1). This reduction in heart rate was similar at baseline and at 20 min myocardial reperfusion (data not shown), and was not affected by preconditioning (P = 0.9 and P = 0.4 for comparison of EC50 and Vmax values, respectively, univariate analysis of variance). Perfusion with CGS21680 dose-dependently increased coronary flow at baseline (Fig. 2). However, after ischaemia, this effect was blunted, and did not differ between the postconditioning and control groups (P = 0.8, ANOVA for repeated measures on absolute flow values).
Fig. 1.
The heart rate response to perfusion with incremental concentrations of CCPA at baseline (before any intervention, ∆, n = 5), after 35 min of regional ischaemia and 20 min of myocardial reperfusion (■, n = 5), after ischaemic preconditioning, 35 min of regional ischaemia and 20 min of myocardial reperfusion, ○, n = 5)
Fig. 2.
The coronary flow rate response to perfusion with incremental concentration of CGS21680 at baseline (before any intervention, ∆, n = 5), after 35 min of regional ischaemia and 20 min reperfusion (■, n = 5), and after 35 min of ischaemia and 20 min of reperfusion incorporating the ischaemic postconditioning protocol, ○, n = 5)
In conclusion, the present study demonstrates that ischaemic preconditioning and postconditioning do not increase the sensitivity of the adenosine A1 and A2A receptor, respectively. Therefore, this mechanism does not explain the apparent discrepancy that the cardioprotective effect of ischaemic pre-, and postconditioning is critically dependent on adenosine receptor sub-type stimulation during reperfusion, whereas administration of exogenous adenosine during reperfusion does not reduce infarct size. An alternative explanation could relate to the role of the coronary endothelium in the metabolism of adenosine. As the endothelium acts as a strong metabolic barrier for circulating adenosine by its rapid uptake and intracellular degradation, intravascular administration of adenosine does not affect the myocardial interstitial adenosine concentration, whereas ischaemic pre- and postconditioning do also increase the interstitial concentration [8]. Additional studies are needed to test this hypothesis.
Acknowledgements
Niels P. Riksen is a recipient of a Rubicon fellowship from the Netherlands Organisation of Scientific Research (ZonMW) and by the Excellence-3 Award from the Novartis Foundation of Cardiovascular Excellence. We thank the British Heart Foundation for continuous support.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Solenkova NV, Solodushko V, Cohen MV, Downey JM. Endogenous adenosine protects preconditioned heart during early minutes of reperfusion by activating Akt. Am J Physiol Heart Circ Physiol. 2006;290:H441–H449. doi: 10.1152/ajpheart.00589.2005. [DOI] [PubMed] [Google Scholar]
- 2.Morrison RR, Tan XL, Ledent C, Mustafa SJ, Hofmann PA. Targeted deletion of A2A adenosine receptors attenuates the protective effects of myocardial postconditioning. Am J Physiol Heart Circ Physiol. 2007;293:H2523–9. doi: 10.1152/ajpheart.00612.2007. [DOI] [PubMed] [Google Scholar]
- 3.Kin H, Zatta AJ, Lofye MT, Amerson BS, Halkos ME, Kerendi F, et al. Postconditioning reduces infarct size via adenosine receptor activation by endogenous adenosine. Cardiovasc Res. 2005;67:124–33. doi: 10.1016/j.cardiores.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Goto M, Miura T, Iliodoromitis EK, et al. Adenosine infusion during early reperfusion failed to limit myocardial infarct size in a collateral deficient species. Cardiovasc Res. 1991;25:943–9. doi: 10.1093/cvr/25.11.943. [DOI] [PubMed] [Google Scholar]
- 5.Vander Heide RS, Reimer KA. Effect of adenosine therapy at reperfusion on myocardial infarct size in dogs. Cardiovasc Res. 1996;31:711–8. doi: 10.1016/S0008-6363(95)00235-9. [DOI] [PubMed] [Google Scholar]
- 6.Budde JM, Velez DA, Zhao Z, et al. Comparative study of AMP579 and adenosine in inhibition of neutrophil-mediated vascular and myocardial injury during 24 h of reperfusion. Cardiovasc Res. 2000;47:294–305. doi: 10.1016/S0008-6363(00)00115-2. [DOI] [PubMed] [Google Scholar]
- 7.Kuno A, Critz SD, Cui L, et al. Protein kinase C protects preconditioned rabbit hearts by increasing sensitivity of adenosine A2B-dependent signaling during early reperfusion. J Mol Cell Cardiol. 2007;43:262–71. doi: 10.1016/j.yjmcc.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gamboa A, Ertl AC, Costa F, et al. Blockade of nucleoside transport is required for delivery of intraarterial adenosine into the interstitium: relevance to therapeutic preconditioning in humans. Circulation. 2003;108:2631–5. doi: 10.1161/01.CIR.0000101927.70100.41. [DOI] [PubMed] [Google Scholar]