Abstract
Summary
This primary care database survey evaluated whether osteoporotic women treated with bisphosphonates were more adherent to monthly than to weekly treatment. Both compliance (medication possession ratio [MPR]) and persistence (time to discontinuation) were superior in the monthly ibandronate treatment group. Better control of fracture risk may thus be achieved using monthly treatment regimens.
Introduction
Treatment adherence in osteoporosis is poor. The objective of this study was to evaluate whether monthly bisphosphonate treatment provided superior adherence than weekly treatment.
Methods
We analysed medical claims from a national prescription database (Thales). All women aged >45 years receiving a first prescription of monthly ibandronate or weekly bisphosphonates in 2007 were included. Treatment adherence was monitored from initial prescription until January 2008. Compliance was measured by the MPR and persistence by the time from treatment initiation to discontinuation. Multivariate analysis was used to identify variables independently associated with adherence.
Results
Twelve-month persistence rates were 47.5% for monthly ibandronate and 30.4% for weekly bisphosphonates. Compliance was significantly higher in the monthly cohort (MPR = 84.5%) than in the weekly cohort (MPR = 79.4%). After adjustment for potential confounding variables, women with monthly regimens were 37% less likely to be non-persistent (HR = 0.63 [0.56–0.72]) and presented a 5% higher mean MPR (84.5% versus 79.3%, p < 0.001) than women with weekly regimens. Other major factors associated with improved adherence were previous densitometry and calcium or vitamin D supplementation (p < 0.01).
Conclusions
Adherence to bisphosphonates may be superior for monthly treatment than for weekly treatment and may thus provide improved fracture protection.
Keywords: Adherence, Bisphosphonates, Compliance, Osteoporosis, Persistence, Treatment regimen
Introduction
Low long-term adherence to drugs by asymptomatic patients with chronic diseases is an important public health issue. Indeed, a report published by the World Health Organisation in 2003 noted that, in developed countries, only 50% of patients with chronic diseases adhered to their recommended treatment regimens [1]. A community survey performed in Canada has indicated that nonadherence to medication in general was associated with adverse health outcomes such as hospitalisation, emergency department visits or death [2].
One field of medicine in which treatment adherence is a major issue is antiresorptive therapy to prevent osteoporotic fractures [3]. A recent expert consensus group in osteoporosis [4] described adherence as a general term encompassing both compliance and persistence. Compliance was defined as the extent to which a patient acts in accordance with the prescribed interval and dose of a given treatment regimen, whereas persistence was defined as the cumulative time from initiation to discontinuation of therapy. Compliance is frequently assessed by measuring the medication possession ratio (MPR), defined as the ratio between the actual interval between prescription refills and the anticipated interval assuming full compliance [5].
Oral bisphosphonates are effective treatments of osteoporosis, and several large randomised clinical trials have shown that they can reduce the risk of osteoporotic fractures by an average of 50% [6]. However, the effectiveness of bisphosphonates is compromised by poor adherence to treatment, since a significant proportion of patients abandon their treatment within 6 months of initiation [7] and more than half stop treatment within the first year [8–10]. Low adherence reduces the effectiveness of treatment and, in consequence, increases the risk of fracture [10–13] and resulting healthcare use and costs [14]. A recent Belgian database analysis [15] showed that the relative reduction in the risk of hip fracture was 60% for women who were persistent with bisphosphonate treatment compared to those who were non-persistent. In addition, for each incremental decrease of 1% in compliance, as measured by the MPR, the risk of hip fracture increased by 0.4%.
For antiresorptive treatments for osteoporosis, public awareness of the risks associated with osteoporosis, the absence of a simple ‘read-out’ of the efficacy of medication, gastrointestinal side effects and the constraints associated with treatment may all contribute to suboptimal adherence [13, 16, 17]. In particular, the regimen recommended for bisphosphonates, which requires overnight fasting before medication and the necessity of remaining upright for at least 30 min after having taken the medication, is a major limitation to the acceptability of treatment, especially when treatments need to be taken daily. For this reason, formulations of bisphosphonates allowing weekly and, subsequently, monthly administration have been developed with the aim of reducing the constraints associated with dosing.
Indeed, a number of studies have shown that adherence to weekly administration is superior to that of daily dosing. For example, a study of US prescriptions claims demonstrated a significantly higher MPR (69.2% versus 57.6%; p < 0.0001) and persistence rate (44.2% versus 31.7%; p < 0.0001) associated with the use of once-weekly alendronate compared to once-daily alendronate or risedronate over the 12 months following the initial prescription [18]. A pharmacy database study in the US also reported that only around one-third of patients taking daily bisphosphonates and around one-half using weekly administration achieved adequate adherence. Such findings have been reiterated in other healthcare systems such as France and the UK [19, 20].
More recently, monthly administration of ibandronate has been developed with the aim of increasing adherence further [21]. However, to date, there is little published information on whether adherence to a monthly regimen is indeed superior. The PERSIST study [22] has compared 6-month persistence rates in women randomised either to monthly ibandronate together with a patient support programme or to weekly alendronate and reported higher persistence rates in the former group (56.6% versus 38.6%; p < 0.0001). However, the relative contributions of the dosing regimen and the patient support programme in improving persistence cannot be identified in this study. On the other hand, a study in the US reported poorer adherence in women receiving monthly ibandronate than in a historical control group treated with weekly risedronate [23]. This study is difficult to interpret since the two groups were not compared at the same time using the same protocol and because the follow-up period did not start when treatment was initiated. Given the limited amount of comparative data on adherence to monthly bisphosphonate treatment, we have undertaken a pharmacoepidemiological study whose objective was to compare adherence to weekly and monthly bisphosphonate therapy in a cohort of post-menopausal women.
Materials and methods
This was a retrospective pharmacoepidemiological study conducted within the context of primary healthcare in France during 2007 using medical claims data from a national prescription database. We examined the data collected during the year preceding and the year following the introduction of ibandronate in France (January 2007).
Data source
We used medical claims from the Thales longitudinal prescription database. Thales is a computerised network of 1,200 general practitioners (GPs) who contribute exhaustive anonymous data on patient consultations and treatment to a centralised electronic database, allowing subsequent follow-up of outcomes. Analyses performed using this database have been approved by the Commission Nationale de l'Informatique et des Libertés.
GPs participating in the Thales network are selected to be representative of the French GP population according to three main criteria, namely, geographical area, age and gender. Activity and prescription habits of the panel have also been compared a posteriori with national data and shown to be representative [24]. The database includes routinely collected records for >1.6 million patients. Since 2005, each patient is required by law to have a single referent GP in order to limit medical roaming and multiple consultations for the same reason. Patient data collected by GPs since 2005 can thus be considered exhaustive and non-redundant.
For each patient, information on disease status and medication prescription is entered directly into the database by the physician at the time of the consultation. No information as to the reasons for making individual diagnostic or prescription choices is, however, provided. The disease status is encoded using terms from a specific thesaurus of symptoms and disease entities adapted from the International Classification of Diseases (ICD-10) system. Prescription data contain the dispensed drug name (commercial and international common denomination), the Anatomical Therapeutic Chemical (ATC) classification category, dose regimens and prescription duration.
Study population
We identified all female patients in the Thales database, aged over 45 years who had received a first prescription of either a weekly or a monthly bisphosphonate treatment between January 2007 (date of introduction of ibandronate in France) and the end of 2007. The index date for the analysis was the date of the initial prescription. These patients were followed up prospectively until January 2008 to evaluate treatment adherence. A retrospective analysis was also performed covering the period from January 2006 to January 2007 in order to identify subjects who had been prescribed any other osteoporosis treatment (bisphosphonates, selective oestrogen receptor modulators or strontium ranelate) during the 12-month period prior to the index prescription, who were excluded. In order to ensure completeness of data, patients were also required to have consulted their GP at least twice a year for any reason during the retrospective and prospective follow-up periods (January 2006–January 2008). In order to restrict the analysis to patients who discontinued treatment definitively, we excluded any women who subsequently switched treatment from one bisphosphonate to another during the follow-up period. Study subjects were then assigned to one of two cohorts on the basis of their treatment administration regimen, namely, a weekly (risedronate 35 mg or alendronate 70 mg with or without vitamin D) or a monthly (ibandronate 150 mg) cohort. Within the weekly cohort, women receiving alendronate and those receiving risedronate were pooled, on the basis that the two bisphosphonates present side effect profiles and risks of discontinuation [25].
Data collection
Data were collected on demographic and clinical variables at the time of the index prescription. Information on comorbidities and other medication use or clinical examinations at the time of the index prescription and during the follow-up period were recorded for each patient. All prescriptions for bisphosphonates during the follow-up period were identified. Information on fracture history at inclusion and incident fractures during the follow-up period was also retrieved from the database. Information on fracture site and radiological evaluation was, however, not systematically available.
Outcome measures
The outcome measures of the study were MPR and persistence. MPR was defined as the duration of all filled prescriptions divided by the follow-up period. Persistence was measured by the time from initiation of therapy to discontinuation. As required for persistence analysis, a limit on the number of days allowed between refills, the permissible gap (PG), was prespecified. Patients who stopped their treatment for a duration longer than the PG were considered to have discontinued, even if they subsequently restarted treatment. In many previous studies, the PG applied to weekly bisphosphonates was specified empirically at 30 days [9, 26–28]. Cramer et al. [5] recently proposed a less arbitrary method based on the pharmacological properties of the drug and the treatment situation in which the PG definition should take into account the maximum allowable period for which patients could go untreated without anticipating reduced or suboptimal outcomes. As specified in the product labelling, the recommended acceptable dosing window for monthly ibandronate (21 days) is 15 days longer than that of weekly bisphosphonates (6 days). For this reason, a prespecified PG of 45 days for the monthly regimen and of 30 days for the weekly regimen was considered acceptable, as previously implemented in a US database analysis [29]. We also performed a sensitivity analysis in order to test the influence of the definition of PG on the persistence results in which an identical PG of 30, 45 or 60 days was allowed for both formulations.
Statistical analysis
The demographic and clinical characteristics of patients included in the two cohorts were compared using the χ 2 test or Fisher’s exact test for categorical variables and the Kruskal–Wallis test for continuous variables.
Persistence rates were evaluated using Kaplan–Meier survival analysis and compared between the two cohorts using the log-rank test in a Cox proportional hazards model. For MPR, the two cohorts were described by mean MPR values and by distribution of patients across MPR classes. This analysis was performed on the entire study population.
Since the profiles of patients in the weekly and monthly cohorts were potentially different and confounding factors could thus contribute to the difference in persistence and in MPR between the two cohorts, these were taken into account by constructing a propensity score [30]. This score included all demographic, clinical and treatment variables recorded in the database and was calculated using multivariate logistic regression. Each patient was attributed a propensity score that represented the probability of receiving monthly rather than weekly bisphosphonate treatment with respect to the pattern of potential confounding factors presented. The cohorts were compared by means of hazard ratios for persistence and least squares means for compliance. These parameters are presented with their 95% confidence intervals (95%CI), both unadjusted and after adjustment by the propensity score. With respect to persistence, a sensitivity analysis was performed in order to determine the influence of the definition of the permissible gap on the results obtained.
All demographic and clinical variables were tested for their association with MPR and persistence using multivariate logistic regression analysis. This analysis was restricted to women for whom at least 6 months' follow-up was available since the initial prescription of a bisphosphonate. For persistence, the dependent variable to be explained was reaching a persistence of at least 6 months, and for MPR, reaching an MPR of at least 68%. These thresholds were chosen since they had been identified as the best predictors of fracture risk in a previous case–control analysis of women treated with bisphosphonates in the Thalès database [31]. Variables were selected serially in an ascending manner, with a cut-off probability threshold of 0.05 at each step. The variables retained in the stepwise model were then entered into a final multivariate logistic regression in order to compute odds ratios.
All analyses were performed using SAS® software version 8.2 (SAS, Cary, USA) on Windows.
Results
Participating investigators
In the Thales database, 1,073 physicians provided patients to the study, of whom 541 prescribed both monthly and weekly regimens, 123 only monthly regimens and 409 only weekly regimens. These three groups of physicians did not differ with respect to age, gender or place of practice in France (data not shown).
Study sample
A total of 3,157 women were prescribed a weekly or monthly bisphosphonate treatment for the first time during the reference period (January 2007 to January 2008). Of these, 63 women were under 45 years and were excluded. In addition, 104 subjects (82 in the weekly group and 22 in the monthly group) subsequently switched to another bisphosphonate treatment and were also excluded from the study sample (Fig. 1). The analysis was thus performed on the remaining 2,990 women, of whom 1,989 received weekly bisphosphonate (581 alendronate and 1,408 risedronate) and 1,001 monthly ibandronate. Given that the demographic and clinical characteristics of women receiving alendronate and risedronate were comparable (data not shown), these two groups were not analysed separately but pooled in a single weekly regimen group.
Fig. 1.
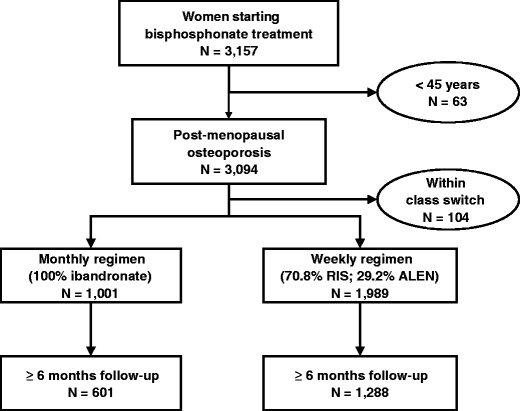
Flowchart illustrating selection of patients evaluated in the database. RIS risedronate, ALEN alendronate
In the two cohorts, data was available over at least 6 months of follow-up since the initial prescription of a bisphosphonate for a total of 1,889 women. This subgroup was used for the analysis of variables associated with good adherence.
Patient characteristics in the two treatment cohorts
The demographic and clinical characteristics of the study sample at the time of the index prescription are presented in Table 1. Patients receiving monthly ibandronate were younger than patients in the weekly cohort and had less frequent osteoporotic fractures before treatment initiation. At initiation, bone densitometry had been performed more frequently in the monthly cohort than in the weekly cohort (p = 0.003), but there was no difference in the two cohorts for bone mass densitometry (BMD) assessments during the follow-up.
Table 1.
Demographic and clinical variables in the study sample
| Monthly ibandronate (N = 1,001) | Weekly bisphosphonates (N = 1,989) | p value | |
|---|---|---|---|
| Age (years) | 68.8 ± 10.3 | 70.4 ± 10.3 | <0.001* |
| BMI (kg/m2) | 24.9 ± 4.4 | 24.9 ± 4.8 | 0.890 |
| Height (cm) | 158 ± 7 | 158 ± 6 | 0.128 |
| Weight (kg) | 62.5 ± 11.6 | 62.2 ± 12.3 | 0.375 |
| Known smoker, n (%) | 35 (3.5) | 74 (3.7) | 0.836 |
| Known alcohol problem, n (%) | 26 (2.6) | 52 (2.6) | 1.000 |
| Previous osteoporotic fracture, n (%) | 325 (32.5) | 810 (40.7) | <0.001* |
| BMD availability, n (%) | |||
| Before treatment initiation | 186 (18.6) | 288 (14.5) | 0.003* |
| After treatment initiation | 32 (3.2) | 61 (3.1) | 0.845 |
| Comorbidities, n (%) | |||
| Any | 875 (87.4) | 1,729 (86.9) | 0.481 |
| ≥4 comorbidities | 173 (17.3) | 368 (18.5) | 0.421 |
| Comedicationsa | 0.041* | ||
| Number of ATC classes | 7.7 ± 4.5 | 7.3 ± 4.2 | |
| ≤7 classes, n (%) | 538 (53.7) | 1,130 (56.8) | |
| >7 classes, n (%) | 463 (46.3) | 859 (43.2) |
Quantitative variables are presented as mean values±standard deviations and categorical variables as absolute patient numbers (percent)
BMI body mass index, BMD bone mass densitometry
*p < 0.10, significant differences between the two treatment regimens
aBased on osteoporosis treatment initiation and prior 6 months
The most common comorbidities were arterial hypertension (44.5%), other rheumatic diseases (31.5%), malignant neoplasms (28.0%) and neurological diseases (27.1%). The only condition whose distribution differed significantly between the monthly and weekly cohorts was rheumatoid arthritis (1.6% versus 2.7%, respectively), although this was only reported in 70 patients overall.
The most frequently prescribed comedication classes were tranquillisers (34.7%), anti-inflammatory and anti-rheumatic drugs (31.8%) and lipid-reducing agents (29.5%). No difference in prescription rates between cohorts was observed for these medication classes. However, the prescription of 13 other comedication classes did differ significantly between the two cohorts, notably drugs used for functional gastrointestinal disorders (19.3% in the monthly group and 16.3% in the weekly group), systemic antibacterial drugs (23.9% and 19.3%, respectively) and antineoplastic drugs (0.3% and 1.2%, respectively). In addition, calcium or vitamin D supplementation (53.0% in the monthly group versus 57.6% in the weekly group) and other mineral supplementation (56.1% in the monthly group versus 60.9% in the weekly group) were more frequently used in the weekly regimen group (p = 0.017 and p = 0.013, respectively).
Patient follow-up
Following the index prescription, patients treated with monthly ibandronate were followed up for an average of 189 days and those treated with weekly bisphosphonates for an average of 197 days. In the weekly group, 14.0% of patients had been followed up for less than 3 months, compared to 17.9% in the monthly group. The corresponding proportions of patients followed up for more than 9 months were 38.0% for weekly treatment and 34.0% for monthly treatment. The mean treatment cover of an individual prescription was 69 days in the weekly cohort and 75 days in the monthly cohort, with 39.5% and 46.9%, respectively, of prescriptions covering at least 3 months.
Adherence to bisphosphonate treatment
Survival analysis demonstrated treatment persistence to be significantly longer (p < 0.0001) in the monthly bisphosphonate cohort than in the weekly bisphosphonate cohort (Fig. 2). Persistence rates at 6 months in the two cohorts were 57.3% and 45.7% and fell to 47.5% and 30.4%, respectively, at 12 months. After propensity score adjustment, women in the monthly group were 37% more likely to persist than those in the weekly group (Table 2).
Fig. 2.
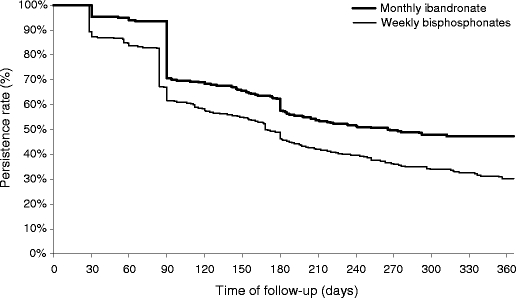
Kaplan–Meier analysis of treatment discontinuation with bisphosphonate. Thick line monthly ibandronate cohort, thin line weekly bisphosphonates. A permissible gap of 45 days for monthly ibandronate and 30 days for weekly bisphosphonates was allowed in this analysis
Table 2.
Median persistence duration and associated hazard ratios with bisphosphonate treatments for the base case analysis and for different definitions of the permissible gap
| Persistence models | Median persistence duration (days) | Hazard ratios (95%CI) | ||
|---|---|---|---|---|
| Monthly ibandronate (N = 1,001) | Weekly BP (N = 1,989) | Unadjusted | Adjusteda | |
| Base case | 265 | 169 | 0.63* (0.56–0.71) | 0.63* (0.56–0.72) |
| Monthly regimen: PG = 45 days | ||||
| Weekly regimen: PG = 30 days | ||||
| Sensitivity analyses | ||||
| Both PG of 30 days | 184 | 169 | 0.76* (0.68–0.85) | 0.77* (0.69–0.86) |
| Both PG of 45 days | 265 | 211 | 0.77* (0.68–0.87) | 0.78* (0.69–0.89) |
PG permissible gap
*p < 0.0001
aCox proportional hazard model adjusted by propensity score
Sensitivity analyses were performed to assess the impact of the attributed PG on the persistence rates obtained. If an identical PG was allowed for both treatment regimens, the difference in persistence at 1 year was reduced but remained significantly higher (p < 0.0001) for the monthly regimen. If a PG of 30 days was allowed, persistence rates over 12 months were 38.0% for the monthly regimen and 30.4% for the weekly regimen (adjusted HR = 0.77, 95%CI = 0.69–0.86, p < 0.0001). If 45 days were allowed for both regimens, the rates were 47.5% and 40.5%, respectively (adjusted HR = 0.78, 95%CI = 0.69–0.89, p < 0.0001).
Of the non-persistent patients, certain women discontinued treatment definitively whilst others resumed their treatment at a later date following a ‘drug holiday’. In the base case scenario, 29.8% [95%CI = 25.5% to 34.1%] of non-persistent women in the monthly ibandronate cohort (13.0% of all women in the cohort) and 31.3% [95%CI = 28.5% to 34.1%] in the weekly bisphosphonate cohort (16.2% of the entire cohort) resumed treatment after a ‘drug holiday’ which extended beyond the permissible gap. These proportions were not significantly different between the two cohorts.
Similarly, compliance as measured by the mean MPR was significantly lower (p < 0.001) in the weekly cohort (Table 3), with 65.8% of subjects presenting an MPR of ≥80% compared to 74.1% in the monthly ibandronate cohort.
Table 3.
Compliance to bisphosphonate treatments over 12 months
| MPR | Monthly ibandronate (N = 1,001) | Weekly bisphosphonates (N = 1,989) | p value |
|---|---|---|---|
| Mean±SD (95% CI) | 84.5 ± 23.0 (83.1–85.9) | 79.4 ± 26.7 (78.2–80.5) | <0.001 |
| Adjusteda mean±SD (95%CI) | 84.5 ± 25.9 (82.9–86.2) | 79.3 ± 25.7 (78.2–80.4) | <0.001 |
| <20% | 20 (2.0%) | 98 (4.9%) | <0.001 |
| 20–<40% | 61 (6.1%) | 169 (8.5%) | |
| 40–<60% | 85 (8.5%) | 179 (9.0%) | |
| 60–<80% | 93 (9.3%) | 234 (11.8%) | |
| ≥80% | 742 (74.1%) | 1,309 (65.8%) |
MPR medication possession ratio
aGeneral linear model adjusted by propensity score
Determinants of persistence and compliance to bisphosphonate treatment
Variables independently associated with persistence and compliance with bisphosphonate treatment were identified using stepwise logistic regression (Table 4). Each regression retained five variables, of which four were common to both models. Availability of baseline BMD data, monthly treatment regimen and use of calcium or vitamin D supplementation were associated with better persistence and higher compliance, whereas a diagnosis of rheumatoid arthritis was associated with worse persistence and compliance. A diagnosis of neurological disease was associated with better persistence and the use of topical products for joint and muscular pain (ATC class: M02) with poor compliance only.
Table 4.
Determinants of persistence (≥6 months) and compliance (MPR ≥68%)
| Odds ratio | 95%CI | |
|---|---|---|
| Determinants of persistence | ||
| BMD available | 1.84* | 1.43–2.37 |
| Monthly regimen | 1.57* | 1.29–1.91 |
| Neurological disorder | 1.30*** | 1.06–1.59 |
| Calcium or vitamin D intake | 1.28** | 1.06–1.54 |
| Rheumatoid arthritis | 0.37** | 0.19–0.73 |
| Determinants of compliance | ||
| Bone mass densitometry available | 1.55** | 1.18–2.04 |
| Calcium or vitamin D intake | 1.36** | 1.12–1.65 |
| Monthly regimen | 1.28*** | 1.04–1.58 |
| Topical products for joint and muscular pain | 0.73** | 0.58–0.92 |
| Rheumatoid arthritis | 0.45** | 0.25–0.81 |
Data are presented as odds ratios with their 95%CI determined by stepwise logistic regression
*p < 0.0001; **p < 0.01; ***p < 0.05
Fracture incidence
During the follow-up period, a lower proportion of patients in the monthly cohort (20 women; 2.0%) reported an incident fracture than in the weekly cohort (125 women; 6.3%). This difference remained significant after adjustment for the propensity score, which included major known risk factors for fracture, such as age and prior fracture (HR = 0.69, 95%CI = 0.54–0.89, p = 0.0043).
Discussion
This retrospective pharmacoepidemiological study using a large primary care database showed that adherence to a monthly bisphosphonate treatment regimen is higher than that to weekly regimens in post-menopausal women. This association could be detected after adjustments for all other available confounding factors. We observed that patients treated with a monthly regimen were 37% less likely to be non-persistent and were more compliant, with a 5% higher absolute MPR, than women treated with weekly regimens.
Optimising treatment adherence to bisphosphonates is crucial to minimising fracture risk [32]. Indeed, several studies have shown that adherence to treatment is the major determinant of its efficacy. For example, Siris et al. [33] reported that patients with an MPR >80% who were persistent (no permissible gap in refills for >30 days over 24 months) presented a reduction in fracture risk of 20% to 45% compared to patients who did not meet these adherence goals. A patient registry study in The Netherlands [13] revealed that non-compliant bisphosphonate use (MPR <80%) was associated with a 45% increase in fracture risk compared to compliant use and that patients with an MPR <20% presented an increased fracture risk of 80% compared to those with an MPR ≥90%. Similarly, in a Canadian healthcare claims database [34], women with an MPR <80% presented a relative risk of hip fracture of 1.28 compared with more compliant women. In these studies, the thresholds for optimal MPR were defined a priori. In a recent case–control study, we attempted to determine empirically the thresholds of persistence and MPR associated with optimal protection against fracture [31] and found that a threshold MPR of 68% was the most discriminant for fracture protection. Fracture risk was reduced by 51% in women who achieved this threshold compared to less compliant women. Concerning persistence, the optimal threshold was at least 6 months of drug therapy.
In this context, it is possible that the increased compliance and persistence associated with the use of monthly administration observed in the present study could provide a clinically relevant reduction in the risk of fracture. Indeed, the observed fracture rates were significantly lower (p = 0.0043) in the monthly treatment group (2% versus 6.3% in the weekly treatment group) and this remained significant after adjustment for the propensity score. This score included many important fracture risk factors, such as BMI, previous fracture history and age, but not all of these (for example, family history of osteoporotic fracture and bone mass density were not included). Nonetheless, prospective randomised comparative trials would be useful to quantify any correlation between adherence and fracture outcome for different bisphosphonate treatment regimens, and the observation of the current study should only be regarded as hypothesis-generating.
Even with monthly administration, adherence to bisphosphonate treatment remains largely suboptimal, and strategies are needed to improve this. A number of methods to improve persistence to oral bisphosphonates have indeed been suggested. For example, offering bone densitometry to women treated with bisphosphonates has been found to be associated with a lower probability of discontinuation [35], although there is no evidence that the BMD change, if any, is directly related to anti-fracture effectiveness. Moreover, the impact of offering densitometry may be limited, since the largest loss of patients to treatment occurs within the first 6 months of prescription, an interval in which bone densitometry is neither recommended nor proposed. Others have suggested the utility of biochemical markers to provide patients with feedback on treatment effectiveness [36], but such markers are not determined in routine clinical practice. Improving patient communication on the importance of treatment and use of reminder systems is clearly important. For example, Briot et al. [37] reported that osteoporotic women starting therapy with a parathyroid hormone analogue who enrolled in an education and follow-up programme could achieve 15-month persistence rates >80%.
It should be noted that non-persistence, as defined in this and other studies, is not necessarily equivalent to treatment discontinuation, as patients may lapse and then resume treatment after a ‘drug holiday’ of variable duration. Given the long half-life of bisphosphonates in bone tissue, such women may continue to gain some benefit from their treatment even if they go on ‘drug holidays’. Although such behaviour was not studied in detail here and would merit evaluation in a study with considerably longer follow-up duration, it is unlikely that the differences in persistence observed in our study could be accounted for by ‘drug holidays’, as the proportion of women who did this was relatively low and similar between the two cohorts.
An important potential confounding factor in any comparison of adherence between different treatment regimens is that patients prescribed one or other regimen may be different. Indeed, in the present study, we found, for example, that women prescribed monthly bisphosphonates tended to be younger and less likely to have already experienced an osteoporotic fracture. In contrast, they were more likely to have undergone bone densitometry. This probably relates to the fact that the women could either receive a diagnosis on the basis of BMD or on the basis of fracture. Since the proportion of women with previous fractures was lower, they were de facto more likely to have received a diagnosis on the basis of low BMD, accounting for the higher use of bone densitometry in this group. Women in the monthly group were also more frequently receiving multiple comedications, which may have been an incentive for their physicians to prescribe them less frequently administered bisphosphonates.
These factors may themselves influence treatment adherence and it is important that they be taken into account in any adherence study. For this reason, we performed multivariate analysis of our adherence data in order to determine the influence of the treatment regimen independently of that of such confounding factors. Both for MPR and persistence rates, we found that the treatment regimen was a highly significant independent determinant of both MPR and persistence.
With respect to other variables independently associated with persistence or compliance, our findings were broadly consistent with previous reports. The influence of bone densitometry on reinforcing adherence has been a consistent finding of previous studies [16, 35], but is difficult to interpret here as the outcome of the evaluation (T-score) was not available. Calcium or vitamin D supplementation has previously been reported to be associated with better compliance and improved fracture outcome in the ICARO study [38]. Such dietary supplementation may also be indicative of higher motivation. Likewise, patients with a neurological disorder (notably epilepsy, Alzheimer or Parkinson’s disease) were more persistent than others, which may reflect awareness of physicians about the high risk of fracture in such patients [39–41], as well as, for patients with epilepsy, a history of treatment for which good adherence is critical. Topical products for joint and muscular pain mainly correspond to non-steroidal anti-inflammatory drugs. Those drugs could be prescribed for their analgesic effects on pain related to fractures, such as back pain with vertebral fractures. Relief of these symptoms may also lead patients to be less adherent to treatment of osteoporosis. Even though the absolute number of patients was low (70 patients in all), a significant association between a diagnosis of comorbid rheumatoid arthritis and low MPR and poor persistence was observed. The interpretation of this finding is unclear, but in the absence of further information on rheumatoid pathology, it merits exploration in a dedicated study. It is noteworthy that it has been previously reported that patients with rheumatoid arthritis taking oral glucocorticoids did not routinely undergo bone densitometry or receive prescription medications for osteoporosis [42].
An important determinant of the validity of our findings is the representativity of the source data. The Thales database has been demonstrated to be a reliable source of information in numerous previous studies in rheumatology [19, 24] and in other fields of medicine [43–46]. In addition, the proportions of patients with various comorbidities in our study sample are consistent with the known prevalence of these diseases in women over 45.
This study has several limitations. Some of these are linked to the use of a primary care registry as the data source. The use of such databases for pharmacoepidemiological studies has become popular of recent years, since it allows access to information on a large number of patients gathered in real-world conditions [3, 47]. However, it is not possible to ascertain ascribed diagnoses and to ensure that these are exhaustive. Moreover, data on many important variables that may influence the risk of fracture and the uptake of treatment, such as family history and lifestyle factors, are not available. In our study, patients who switched treatment have been excluded from the analysis, and this may limit the extent to which the findings can be generalised to all women starting an antiresorptive therapy with bisphosphonates. Such women may switch to a treatment that they consider more acceptable, with which they may be more compliant. In our study, the proportion of women who switched treatments within the following year was 3%, lower than switch rates reported in previous studies [35] and is unlikely to have introduced significant bias. However, the adherence of switchers to their new treatment merits a dedicated study. Finally, the definition of an acceptable prescription refill gap for determining persistence rates in the study was arbitrary, even though this definition is known to exert a crucial influence on the observed persistence. We have attempted to control for the influence of confounders on the observed differences between the monthly and weekly regimens by using propensity scoring, but it is clearly possible that unidentified confounders for which data were not collected may play a role. It should be noted that a criterion for inclusion was that women should have consulted their GP during the reference period, which may de facto enriched the study population in more adherent patients. However, such a bias is in principle non-differential between the two groups.
The study also presents a number of strengths. These include the representativity of the study sample with respect to primary care in France. In addition, multivariate analysis was performed to take into account the influence of potential confounding factors on the relationship between treatment regimen and adherence. The fact that the confounding factors identified were consistent with known determinants of adherence supports the face validity of the model. In addition, sensitivity analyses were performed to determine the influence of the definition of the permissible gap on the findings. A significant relationship between treatment regimen and adherence was found with all hypotheses, supporting the robustness of this relationship.
In conclusion, this study suggests that adherence to bisphosphonates is superior using a monthly treatment regimen than using a weekly one. This difference would be expected to have major repercussions on fracture protection in osteoporotic women using such treatments. However, adherence remains suboptimal and other interventions to improve adherence need to be identified and implemented.
Acknowledgements
This study was funded by Laboratoire GlaxoSmithKline and Laboratoire Roche, purveyors of ibandronate, an osteoporosis treatment. FEC and AFG are employees of Laboratoire GlaxoSmithKline. The study was initiated by Laboratoire GlaxoSmithKline who designated two independent academic advisors (CR and PF) to advise on the scientific and strategic direction of the study. The advisors assisted in developing the study protocol and the statistical analysis plan, made recommendations on the analysis and exploitation of the study results and contributed to the writing of the present article. Both received honoraria from the sponsor in return for their participation. Data analysis was performed by Stat-Process, an independent data analysis company working in the field of healthcare, which was responsible for the extraction of the source data from the Thalès database, contributed to the statistical analysis plan and produced the statistical report. Stat-Process received fees from Laboratoire GlaxoSmithKline for its involvement in the study. Laboratoire GlaxoSmithKline also funded the editorial support for the preparation of the present article. The authors thanks Adam Doble (Foxymed, Paris, France) for help in preparing the manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Conflicts of interest
C. Roux has received research grants and/or speaker’s fees from Alliance, Amgen, Lilly, MSD, Novartis, GSK-Roche, Servier and Wyeth.
References
- 1.World Health Organization . Adherence to long-term therapies: evidence for action. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 2.Vik SA, Hogan DB, Patten SB, Johnson JA, Romonko-Slack L, Maxwell CJ. Medication nonadherence and subsequent risk of hospitalisation and mortality among older adults. Drugs Aging. 2006;23:345–356. doi: 10.2165/00002512-200623040-00007. [DOI] [PubMed] [Google Scholar]
- 3.Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18:1023–1031. doi: 10.1007/s00198-006-0322-8. [DOI] [PubMed] [Google Scholar]
- 4.Lekkerkerker F, Kanis JA, Alsayed N, Bouvenot G, Burlet N, Cahall D, Chines A, Delmas P, Dreiser RL, Ethgen D, Hughes N, Kaufman JM, Korte S, Kreutz G, Laslop A, Mitlak B, Rabenda V, Rizzoli R, Santora A, Schimmer R, Tsouderos Y, Viethel P, Reginster JY. Adherence to treatment of osteoporosis: a need for study. Osteoporos Int. 2007;18:1311–1317. doi: 10.1007/s00198-007-0410-4. [DOI] [PubMed] [Google Scholar]
- 5.Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 6.Geusens PP, Roux CH, Reid DM, Lems WF, Adami S, Adachi JD, Sambrook PN, Saag KG, Lane NE, Hochberg MC. Drug insight: choosing a drug treatment strategy for women with osteoporosis—an evidence-based clinical perspective. Nat Clin Pract Rheumatol. 2008;4:240–248. doi: 10.1038/ncprheum0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tosteson AN, Grove MR, Hammond CS, Moncur MM, Ray GT, Hebert GM, Pressman AR, Ettinger B. Early discontinuation of treatment for osteoporosis. Am J Med. 2003;115:209–216. doi: 10.1016/S0002-9343(03)00362-0. [DOI] [PubMed] [Google Scholar]
- 8.Caro JJ, Ishak KJ, Huybrechts KF, Raggio G, Naujoks C. The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int. 2004;15:1003–1008. doi: 10.1007/s00198-004-1652-z. [DOI] [PubMed] [Google Scholar]
- 9.Huybrechts KF, Ishak KJ, Caro JJ. Assessment of compliance with osteoporosis treatment and its consequences in a managed care population. Bone. 2006;38:922–928. doi: 10.1016/j.bone.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Gehlbach SH, Avrunin JS, Puleo E, Spaeth R. Fracture risk and antiresorptive medication use in older women in the USA. Osteoporos Int. 2007;18:805–810. doi: 10.1007/s00198-006-0310-z. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher AM, Rietbrock S, Olson M, van Staa TP. Fracture outcomes related to persistence and compliance with oral bisphosphonates. J Bone Miner Res. 2008;23:1569–1575. doi: 10.1359/jbmr.080510. [DOI] [PubMed] [Google Scholar]
- 12.Gold DT, Martin BC, Frytak JR, Amonkar MM, Cosman F. A claims database analysis of persistence with alendronate therapy and fracture risk in post-menopausal women with osteoporosis. Curr Med Res Opin. 2007;23:585–594. doi: 10.1185/030079906X167615. [DOI] [PubMed] [Google Scholar]
- 13.Penning-van Beest FJ, Erkens JA, Olson M, Herings RM. Loss of treatment benefit due to low compliance with bisphosphonate therapy. Osteoporos Int. 2008;19:511–517. doi: 10.1007/s00198-007-0466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sunyecz JA, Mucha L, Baser O, Barr CE, Amonkar MM. Impact of compliance and persistence with bisphosphonate therapy on health care costs and utilization. Osteoporos Int. 2008;19:1421–1429. doi: 10.1007/s00198-008-0586-2. [DOI] [PubMed] [Google Scholar]
- 15.Rabenda V, Mertens R, Fabri V, Vanoverloop J, Sumkay F, Vannecke C, Deswaef A, Verpooten GA, Reginster JY. Adherence to bisphosphonates therapy and hip fracture risk in osteoporotic women. Osteoporos Int. 2008;19:811–818. doi: 10.1007/s00198-007-0506-x. [DOI] [PubMed] [Google Scholar]
- 16.Rossini M, Bianchi G, Di Munno O, Giannini S, Minisola S, Sinigaglia L, Adami S. Determinants of adherence to osteoporosis treatment in clinical practice. Osteoporos Int. 2006;17:914–921. doi: 10.1007/s00198-006-0073-6. [DOI] [PubMed] [Google Scholar]
- 17.Carr AJ, Thompson PW, Cooper C. Factors associated with adherence and persistence to bisphosphonate therapy in osteoporosis: a cross-sectional survey. Osteoporos Int. 2006;17:1638–1644. doi: 10.1007/s00198-006-0166-2. [DOI] [PubMed] [Google Scholar]
- 18.Cramer JA, Amonkar MM, Hebborn A, Altman R. Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin. 2005;21:1453–1460. doi: 10.1185/030079905X61875. [DOI] [PubMed] [Google Scholar]
- 19.Cramer JA, Lynch NO, Gaudin AF, Walker M, Cowell W. The effect of dosing frequency on compliance and persistence with bisphosphonate therapy in postmenopausal women: a comparison of studies in the United States, the United Kingdom, and France. Clin Ther. 2006;28:1686–1694. doi: 10.1016/j.clinthera.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Fardellone P, Gaudin A, Cotte F, Lafuma A, Marchand C, El Hasnaoui A. Comparison of the persistence of daily and weekly bisphosphonates in French female patients treated for osteoporosis. J Bone Miner Res. 2005;20:S285–S286. [Google Scholar]
- 21.Bauss F, Schimmer RC. Ibandronate: the first once-monthly oral bisphosphonate for treatment of postmenopausal osteoporosis. Ther Clin Risk Manag. 2006;2:3–18. [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper A, Drake J, Brankin E. Treatment persistence with once-monthly ibandronate and patient support vs. once-weekly alendronate: results from the PERSIST study. Int J Clin Pract. 2006;60:896–905. doi: 10.1111/j.1742-1241.2006.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gold DT, Safi W, Trinh H. Patient preference and adherence: comparative US studies between two bisphosphonates, weekly risedronate and monthly ibandronate. Curr Med Res Opin. 2006;22:2383–2391. doi: 10.1185/030079906X154042. [DOI] [PubMed] [Google Scholar]
- 24.Bouee S, Charlemagne A, Fagnani F, Le Jeunne P, Sermet C, Naudin F, Lancry PJ. Changes in osteoarthritis management by general practitioners in the COX2-inhibitor era-concomitant gastroprotective therapy. Joint Bone Spine. 2004;71:214–220. doi: 10.1016/S1297-319X(03)00158-1. [DOI] [PubMed] [Google Scholar]
- 25.Rosen CJ, Hochberg MC, Bonnick SL, McClung M, Miller P, Broy S, Kagan R, Chen E, Petruschke RA, Thompson DE, de Papp AE. Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res. 2005;20:141–151. doi: 10.1359/JBMR.040920. [DOI] [PubMed] [Google Scholar]
- 26.McCombs JS, Thiebaud P, McLaughlin-Miley C, Shi J. Compliance with drug therapies for the treatment and prevention of osteoporosis. Maturitas. 2004;48:271–287. doi: 10.1016/j.maturitas.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Brankin E, Walker M, Lynch N, Aspray T, Lis Y, Cowell W. The impact of dosing frequency on compliance and persistence with bisphosphonates among postmenopausal women in the UK: evidence from three databases. Curr Med Res Opin. 2006;22:1249–1256. doi: 10.1185/030079906X112688. [DOI] [PubMed] [Google Scholar]
- 28.Lynch N, Walker M, Cowell W, Suppapanya N, Hammerschmidt T, Rigney U. An international comparison of the impact of dosing frequency on adherence with bisphosphonate therapy among postmenopausal women in the UK and Germany. J Bone Miner Res. 2005;21:S180. [Google Scholar]
- 29.Silverman S, Chesnut C, Amonkar M, Cziraky M, Barr C. Improved persistence in women treated with once-monthly ibandronate versus weekly biphosphonates: a first look. J Bone Miner Res. 2006;22:SU355. [Google Scholar]
- 30.Rosenbaum P, Rubin D. The central role of the propensity score in observational studied for causal effects. Biometrika. 1983;70:41–55. doi: 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 31.Cotte FE, Mercier F, De Pouvourville G. Relationship between compliance and persistence with osteoporosis medications and fracture risk in primary health care in France: a retrospective case–control analysis. Clin Ther. 2008;30:2410–2422. doi: 10.1016/j.clinthera.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 32.Adachi J, Lynch N, Middelhoven H, Hunjan M, Cowell W. The association between compliance and persistence with bisphosphonate therapy and fracture risk: a review. BMC Musculoskelet Disord. 2007;8:97. doi: 10.1186/1471-2474-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siris ES, Harris ST, Rosen CJ, Barr CE, Arvesen JN, Abbott TA, Silverman S. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006;81:1013–1022. doi: 10.4065/81.8.1013. [DOI] [PubMed] [Google Scholar]
- 34.Blouin J, Dragomir A, Moride Y, Ste-Marie LG, Fernandes JC, Perreault S. Impact of noncompliance with alendronate and risedronate on the incidence of nonvertebral osteoporotic fractures in elderly women. Br J Clin Pharmacol. 2008;66:117–127. doi: 10.1111/j.1365-2125.2008.03178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lo JC, Pressman AR, Omar MA, Ettinger B. Persistence with weekly alendronate therapy among postmenopausal women. Osteoporos Int. 2006;17:922–928. doi: 10.1007/s00198-006-0085-2. [DOI] [PubMed] [Google Scholar]
- 36.Delmas PD, Vrijens B, Eastell R, Roux C, Pols HA, Ringe JD, Grauer A, Cahall D, Watts NB. Effect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab. 2007;92:1296–1304. doi: 10.1210/jc.2006-1526. [DOI] [PubMed] [Google Scholar]
- 37.Briot K, Ravaud P, Dargent-Molina P, Zylberman M, Liu-Leage S, Roux C. Persistence with teriparatide in postmenopausal osteoporosis; impact of a patient education and follow-up program: the French experience. Osteoporos Int. 2009;20:625–630. doi: 10.1007/s00198-008-0698-8. [DOI] [PubMed] [Google Scholar]
- 38.Adami S, Isaia G, Luisetto G, Minisola S, Sinigaglia L, Gentilella R, Agnusdei D, Iori N, Nuti R. Fracture incidence and characterization in patients on osteoporosis treatment: the ICARO study. J Bone Miner Res. 2006;21:1565–1570. doi: 10.1359/jbmr.060715. [DOI] [PubMed] [Google Scholar]
- 39.Schneider JL, Fink HA, Ewing SK, Ensrud KE, Cummings SR. The association of Parkinson's disease with bone mineral density and fracture in older women. Osteoporos Int. 2008;19:1093–1097. doi: 10.1007/s00198-008-0583-5. [DOI] [PubMed] [Google Scholar]
- 40.Tsiropoulos I, Andersen M, Nymark T, Lauritsen J, Gaist D, Hallas J. Exposure to antiepileptic drugs and the risk of hip fracture: a case–control study. Epilepsia. 2008;49:2092–2099. doi: 10.1111/j.1528-1167.2008.01640.x. [DOI] [PubMed] [Google Scholar]
- 41.Formiga F, Navarro M, Duaso E, Chivite D, Ruiz D, Perez-Castejon JM, Lopez-Soto A, Pujol R. Factors associated with hip fracture-related falls among patients with a history of recurrent falling. Bone. 2008;43:941–944. doi: 10.1016/j.bone.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 42.Solomon DH, Katz JN, Jacobs JP, La Tourette AM, Coblyn J. Management of glucocorticoid-induced osteoporosis in patients with rheumatoid arthritis: rates and predictors of care in an academic rheumatology practice. Arthritis Rheum. 2002;46:3136–3142. doi: 10.1002/art.10613. [DOI] [PubMed] [Google Scholar]
- 43.Crochard A, El Hasnaoui A, Pouchain D, Huas D, Arnulf I, Krieger J, Lainey E, Le Jeunne P, Leger D, Schuck S, Texier N, Tison F, Montplaisir J. Diagnostic indicators of restless legs syndrome in primary care consultations: the DESYR study. Mov Disord. 2007;22:791–797. doi: 10.1002/mds.21325. [DOI] [PubMed] [Google Scholar]
- 44.Chassany O, Le-Jeunne P, Duracinsky M, Schwalm MS, Mathieu M. Discrepancies between patient-reported outcomes and clinician-reported outcomes in chronic venous disease, irritable bowel syndrome, and peripheral arterial occlusive disease. Value Health. 2006;9:39–46. doi: 10.1111/j.1524-4733.2006.00079.x. [DOI] [PubMed] [Google Scholar]
- 45.Van Ganse E, Laforest L, Alemao E, Davies G, Gutkin S, Yin D. Lipid-modifying therapy and attainment of cholesterol goals in Europe: the Return on Expenditure Achieved for Lipid Therapy (REALITY) study. Curr Med Res Opin. 2005;21:1389–1399. doi: 10.1185/030079905X59139. [DOI] [PubMed] [Google Scholar]
- 46.Fagnani F, German-Fattal M. Antibiotic prescribing patterns of French GPs for upper respiratory tract infections: impact of fusafungine on rates of prescription of systemic antibiotics. Am J Respir Med. 2003;2:491–498. doi: 10.1007/BF03256676. [DOI] [PubMed] [Google Scholar]
- 47.Cramer JA, Silverman SL, Gold DT. Methodological considerations in using claims databases to evaluate persistence with bisphosphonates for osteoporosis. Curr Med Res Opin. 2007;23:2369–2377. doi: 10.1185/030079907X226311. [DOI] [PubMed] [Google Scholar]


